Investigation of Fe-Ni Battery/Module for Grid Service Duty Cycles
Abstract
1. Introduction
2. Experimental
2.1. Module Specification
2.2. Capacity Test Protocols
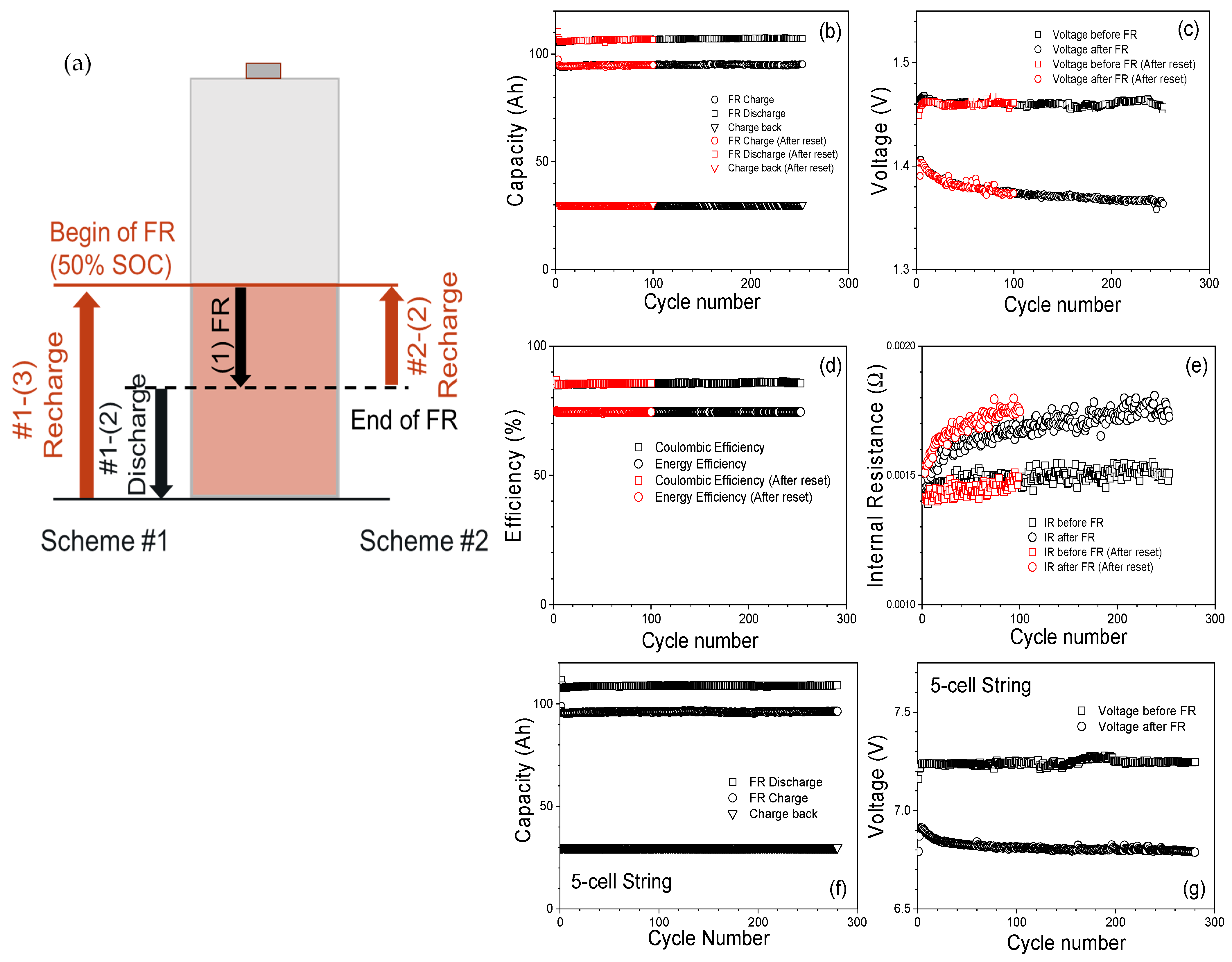
2.3. Frequency Regulation Testing
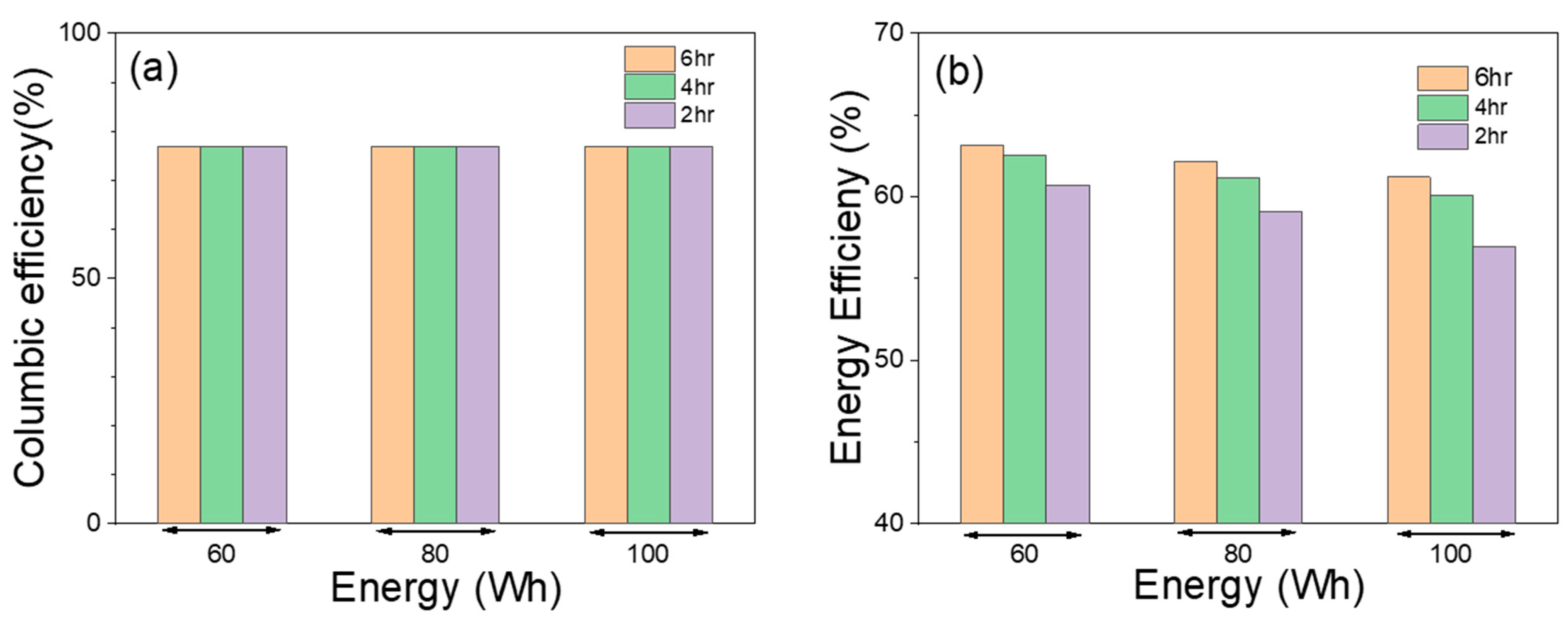
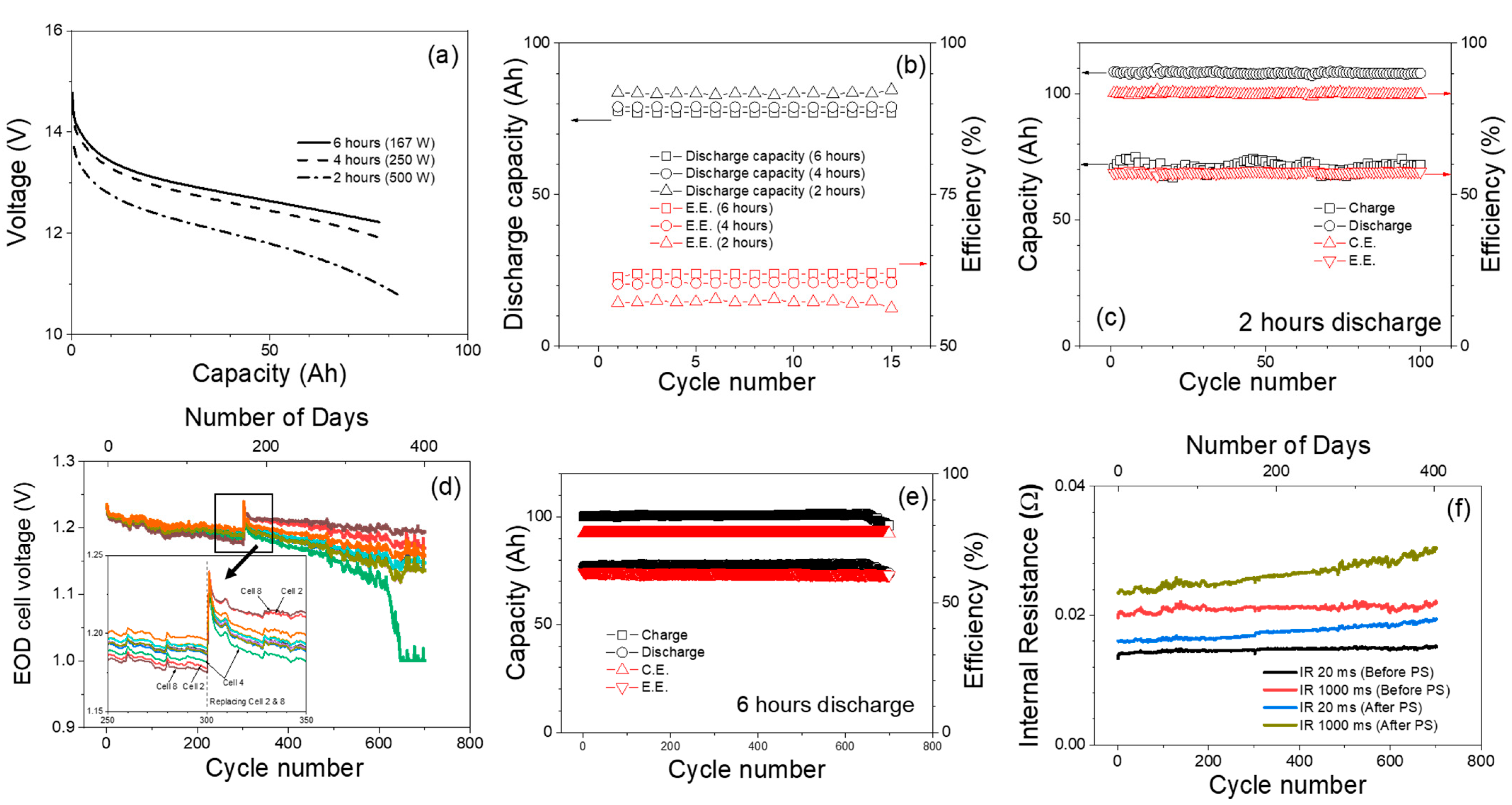
2.4. Peak Shaving Test Protocols
2.5. Resistance Measurement
3. Results and Discussion
3.1. Capacity Test Results
3.2. Frequency Regulation Test Result
3.2.1. Frequency Regulation at 100% SOC
3.2.2. Frequency Regulation at Initial 50% SOC
3.2.3. Frequency Regulation at 25% SOC
3.3. Peak Shaving Duty Cycle Test Results
Long-Term Peak Shaving Duty Cycle
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kebede, A.A.; Kalogiannis, T.; Van Mierlo, J.; Berecibar, M. A comprehensive review of stationary energy storage devices for large scale renewable energy sources grid integration. Renew. Sustain. Energy Rev. 2022, 159, 112213. [Google Scholar] [CrossRef]
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of current development in electrical energy storage technologies and the application potential in power system operation. Appl. Energy 2015, 137, 511–536. [Google Scholar] [CrossRef]
- Subburaj, A.S.; Pushpakaran, B.N.; Bayne, S.B. Overview of grid connected renewable energy based battery projects in USA. Renew. Sustain. Energy Rev. 2015, 45, 219–234. [Google Scholar] [CrossRef]
- Chen, T.; Jin, Y.; Lv, H.; Yang, A.; Liu, M.; Chen, B.; Xie, Y.; Chen, Q. Applications of Lithium-Ion Batteries in Grid-Scale Energy Storage Systems. Trans. Tianjin Univ. 2020, 26, 208–217. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Chakkaravarthy, C.; Periasamy, P.; Jegannathan, S.; Vasu, K.I. The nickel/iron battery. J. Power Sources 1991, 35, 21–35. [Google Scholar] [CrossRef]
- Shukla, A.K.; Ravikumar, M.K.; Balasubramanian, T.S. Nickel/iron batteries. J. Power Sources 1994, 51, 29–36. [Google Scholar] [CrossRef]
- Periasamy, P.; Ramesh Babu, B.; Venkatakrishna Iyer, S. Performance characterization of sintered iron electrodes in nickel/iron alkaline batteries. J. Power Sources 1996, 62, 9–14. [Google Scholar] [CrossRef]
- Halpert, G. Past developments and the future of nickel electrode cell technology. J. Power Sources 1984, 12, 177–192. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Wang, Z.; Wang, Z.; Zhang, Q.; He, B.; Zhang, T.; Gong, W.; Chen, M.; Qi, M.; et al. High-Capacity Iron-Based Anodes for Aqueous Secondary Nickel−Iron Batteries: Recent Progress and Prospects. ChemElectroChem 2021, 8, 274–290. [Google Scholar] [CrossRef]
- Iron Edison Battery Company. Nickel Iron Battery for Renewable Energy & Backup Power Applications; Iron Edison Battery Company: Denver, CO, USA, 2017. [Google Scholar]
- Jackovitz, J.F.; Bayles, G.A.; Brodd, R. Iron electrode batteries. In Handbook of Batteries; Linden, D., Reddy, T.B., Eds.; McGraw-Hill Professional: New York, NY, USA, 2002; pp. 720–746. [Google Scholar]
- Salkind, A.J.; Venuto, C.J.; Falk, S.U. The Reaction at the Iron Alkaline Electrode. J. Electrochem. Soc. 1964, 111, 493–495. [Google Scholar] [CrossRef]
- Liu, B.; Liu, X.; Fan, X.; Ding, J.; Hu, W.; Zhong, C. 120 Years of nickel-based cathodes for alkaline batteries. J. Alloys Compd. 2020, 834, 155185. [Google Scholar] [CrossRef]
- Posada, J.O.G.; Hall, P.J. Controlling hydrogen evolution on iron electrodes. Int. J. Hydrogen Energy 2016, 41, 20807–20817. [Google Scholar] [CrossRef]
- Lee, D.-C.; Lei, D.; Yushin, G. Morphology and Phase Changes in Iron Anodes Affecting their Capacity and Stability in Rechargeable Alkaline Batteries. ACS Energy Lett. 2018, 3, 794–801. [Google Scholar] [CrossRef]
- Beard, K.W. Linden’s Handbook of Batteries, 5th ed.; McGraw Hill: New York, NY, USA, 2019. [Google Scholar]
- Lei, D.; Lee, D.C.; Magasinski, A.; Zhao, E.B.; Steingart, D.; Yushin, G. Performance Enhancement and Side Reactions in Rechargeable Nickel-Iron Batteries with Nanostructured Electrodes. ACS Appl. Mater. Inter. 2016, 8, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, T.S.; Shukla, A.K. Effect of metal-sulfide additives on charge/discharge reactions of the alkaline iron electrode. J. Power Sources 1993, 41, 99–105. [Google Scholar] [CrossRef]
- Posada, J.O.G.; Hall, P.J. Towards the development of safe and commercially viable nickel-iron batteries: Improvements to Coulombic efficiency at high iron sulphide electrode formulations. J. Appl. Electrochem. 2016, 46, 451–458. [Google Scholar] [CrossRef]
- Mylad, C.; Björn, S.; Hilmar, V.; Ronald, I.S.; Stephen, H.; Martynas, L.; Darius, M.; Dag, N. Stannate Increases Hydrogen Evolution Overpotential on Rechargeable Alkaline Iron Electrodes. J. Electrochem. Soc. 2017, 164, A1251. [Google Scholar] [CrossRef]
- Hariprakash, B.; Martha, S.K.; Hegde, M.S.; Shukla, A.K. A sealed, starved-electrolyte nickel-iron battery. J. Appl. Electrochem. 2005, 35, 27–32. [Google Scholar] [CrossRef]
- Huang, L.X.; Zhu, D.; Chen, Y.G. W2C/porous graphene nanocomposite as an efficient non-noble metal catalyst for hydrogen and oxygen recombination in nickel-iron battery. Ionics 2019, 25, 3749–3759. [Google Scholar] [CrossRef]
- Yang, C.; Manohar, A.K.; Narayanan, S.R. A High-Performance Sintered Iron Electrode for Rechargeable Alkaline Batteries to Enable Large-Scale Energy Storage. J. Electrochem. Soc. 2017, 164, A418. [Google Scholar] [CrossRef]
- McKerracher, R.D.; Figueredo-Rodriguez, H.A.; Dimogiannis, K.; Alegre, C.; Villanueva-Martinez, N.I.; Lázaro, M.J.; Baglio, V.; Aricò, A.S.; Ponce de Leόn, C. Effect of 1-octanethiol as an electrolyte additive on the performance of the iron-air battery electrodes. J. Solid State Electrochem. 2021, 25, 225–230. [Google Scholar] [CrossRef]
- Weinrich, H.; Durmus, Y.E.; Tempel, H.; Kungl, H.; Eichel, R.A. Silicon and Iron as Resource-Efficient Anode Materials for Ambient-Temperature Metal-Air Batteries: A Review. Materials 2019, 12, 2134. [Google Scholar] [CrossRef]
- Sui, Y.; Ji, X. Anticatalytic Strategies to Suppress Water Electrolysis in Aqueous Batteries. Chem. Rev. 2021, 121, 6654–6695. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, J.; Jiang, M.; Hou, J.; Zhu, Y.; Zhang, J.; Ba, Z.; Li, L. A more economical choice for the cathode material of Ni-MH batteries with high electrochemical performances: 3D flower-like Ni–Fe LDHs. Int. J. Hydrog. Energy 2022, 47, 41087–41096. [Google Scholar] [CrossRef]
- Li, X.; Guo, Y.; Gao, T.; Li, P.; Jin, Z.; Xiao, D. Interconnecting 3D conductive networks with nanostructured iron/iron oxide enables a high-performance flexible battery. ACS Appl. Mater. Inter. 2021, 13, 57411–57421. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, L. Influence of nanosized Ni(OH)2 addition on the electrochemical performance of nickel hydroxide electrode. J. Power Sources 2004, 128, 326–330. [Google Scholar] [CrossRef]
- Li, J.; Guo, L.; Shangguan, E.; Yue, M.; Xu, M.; Wang, D.; Chang, Z.; Li, Q. Synthesis of novel spherical Fe3O4@ Ni3S2 composite as improved anode material for rechargeable nickel-iron batteries. Electrochim. Acta 2017, 240, 456–465. [Google Scholar] [CrossRef]
- Li, X.; Gao, T.; Liu, Q.; Xu, Y.; Li, J.; Xiao, D. Designing a high-performance anode composed of carbon nanotubes and Fe–Fe 3 C nanoparticles for quasi-solid-state fibrous Ni/Fe batteries. Mater. Chem. Front. 2021, 5, 3636–3645. [Google Scholar] [CrossRef]
- Lai, C.; Cheng, L.; Sun, Y.; Lee, K.; Lin, B. Alkaline aqueous rechargeable Ni-Fe batteries with high-performance based on flower-like hierarchical NiCo2O4 microspheres and vines-grapes-like Fe3O4-NGC composites. Appl. Surf. Sci. 2021, 563, 150411. [Google Scholar] [CrossRef]
- Xue, L.; Li, S.; Shen, T.; Ni, M.; Qiu, C.; Sun, S.; Geng, H.; Zhu, X.; Xia, H. Two-dimensional metal (oxy) hydroxide and oxide ultrathin nanosheets via liquid phase epitaxy. Energy Storage Mater. 2020, 32, 272–280. [Google Scholar] [CrossRef]
- Kiani, M.; Mousavi, M.; Ghasemi, S. Size effect investigation on battery performance: Comparison between micro-and nano-particles of β-Ni(OH)2 as nickel battery cathode material. J. Power Sources 2010, 195, 5794–5800. [Google Scholar] [CrossRef]
- Hu, W.-K.; Gao, X.-P.; Noréus, D.; Burchardt, T.; Nakstad, N.K. Evaluation of nano-crystal sized α-nickel hydroxide as an electrode material for alkaline rechargeable cells. J. Power Sources 2006, 160, 704–710. [Google Scholar] [CrossRef]
- Song, Q.; Chiu, C.; Chan, S. Effects of ball milling on the physical and electrochemical characteristics of nickel hydroxide powder. J. Appl. Electrochem. 2006, 36, 97–103. [Google Scholar] [CrossRef]
- Kamath, P.V.; Dixit, M.; Indira, L.; Shukla, A.; Kumar, V.G.; Munichandraiah, N. Stabilized α-Ni (OH) 2 as electrode material for alkaline secondary cells. J. Electrochem. Soc. 1994, 141, 2956. [Google Scholar] [CrossRef]
- Raventos, A.M.; Kortlever, R. Effect of different alkali metal cations on the oxygen evolution activity and battery capacity of nickel electrodes in concentrated hydroxide electrolytes. Electrochim. Acta 2022, 415, 140255. [Google Scholar] [CrossRef]
- Ying, T. Surface modification of nickel hydroxide particles by micro-sized cobalt oxide hydroxide and properties as electrode materials. Surf. Coat. Technol. 2005, 200, 2376–2379. [Google Scholar] [CrossRef]
- He, X.; Pu, W.; Cheng, H.; Jiang, C.; Wan, C. Granulation of nano-scale Ni(OH)2 cathode materials for high power Ni-MH batteries. Energy Convers. Manag. 2006, 47, 1879–1883. [Google Scholar] [CrossRef]
- Hong, W.; Tang, Z.-Y.; Liu, Y.-G.; Lee, C.-S. Synthesis and behavior of Al-stabilized α-Ni(OH)2. Trans. Nonferrous Met. Soc. China 2009, 19, 170–175. [Google Scholar]
- Conover, D.R.; Ferreira, S.R.; Crawford, A.J.; Schoenwald, D.A.; Fuller, J.; Rosewater, D.M.; Gourisetti, S.N.; Viswanathan, V. Protocol for Uniformly Measuring and Expressing the Performance of Energy Storage Systems. 2016. Available online: https://energystorage.pnnl.gov/pdf/PNNL-22010Rev2.pdf (accessed on 3 February 2023).
- Villanueva-Martínez, N.I.; Alegre, C.; Rubín, J.; McKerracher, R.; de León, C.P.; Rodríguez, H.A.F.; Lázaro, M.J. Investigation of the properties influencing the deactivation of iron electrodes in iron-air batteries. Electrochim. Acta 2023, 465, 142964. [Google Scholar] [CrossRef]
- Weinrich, H.; Gehring, M.; Tempel, H.; Kungl, H.; Eichel, R.-A. Impact of the charging conditions on the discharge performance of rechargeable iron-anodes for alkaline iron–air batteries. J. Appl. Electrochem. 2018, 48, 451–462. [Google Scholar] [CrossRef]
- Narayanan, S.R.; Surampudi, S.; Attia, A.I.; Bankston, C.P. Analysis of Redox Additive-Based Overcharge Protection for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1991, 138, 2224. [Google Scholar] [CrossRef]
- Abraham, K.M.; Brummer, S.B.; Gabano, J.P. (Eds.) Lithium Batteries; Academic Press: New York, NY, USA, 1983. [Google Scholar]
- Abarro, J.M.E.; Gavan, J.N.L.; Loresca, D.E.D.; Ortega, M.A.A.; Esparcia, E.A.; Paraggua, J.A.D.R. A Tale of Nickel-Iron Batteries: Its Resurgence in the Age of Modern Batteries. Batteries 2023, 9, 383. [Google Scholar] [CrossRef]
- Helivig, A.; Ahfock, T. Long-life Nickel Iron Battery Functionality/Cost Comparison for Peak Demand SWER Network Voltage Support Application. In 2013 Australasian Universities Power Engineering Conference (AUPEC); IEEE: Hobart, TAS, Australia, 2013. [Google Scholar]
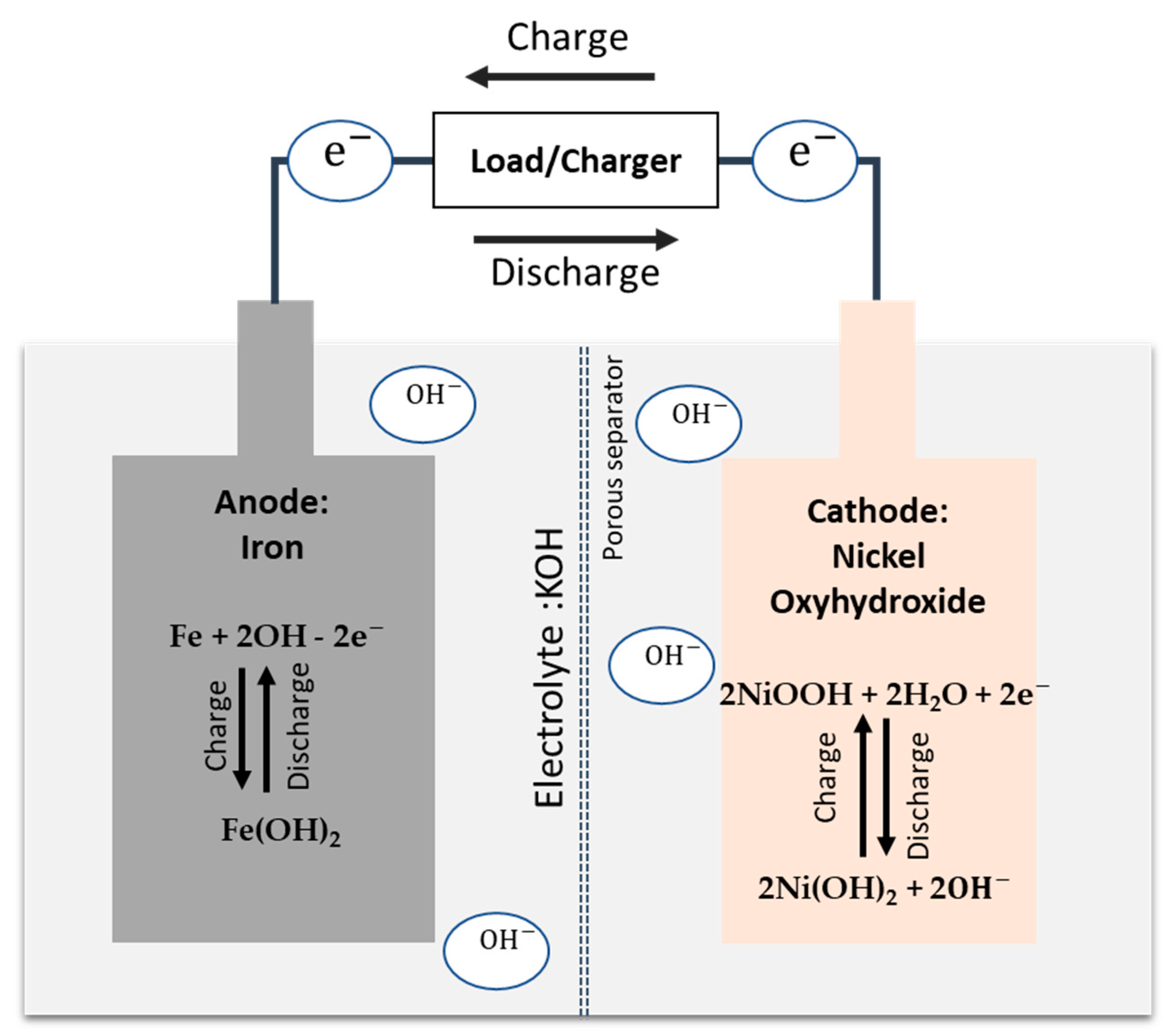

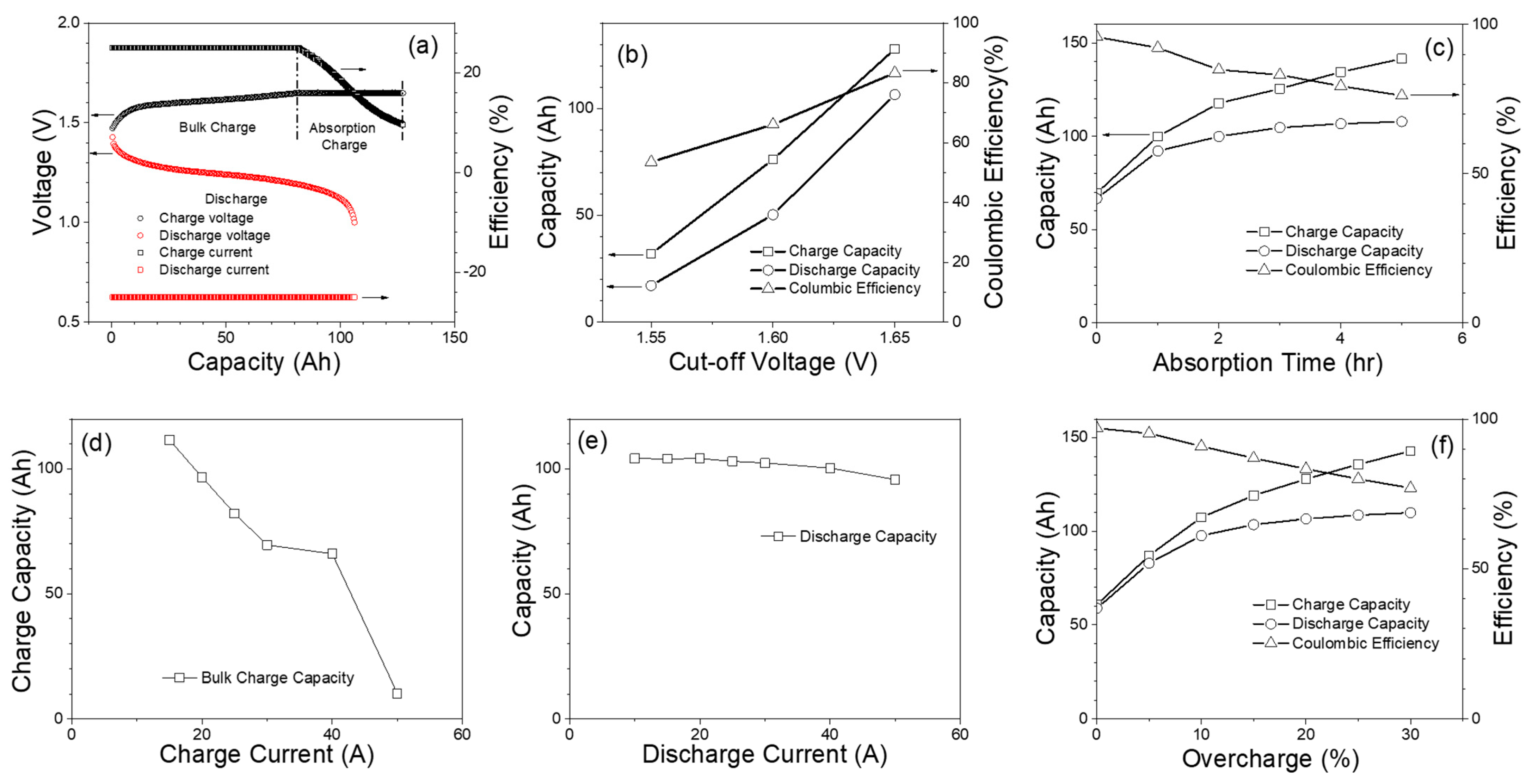

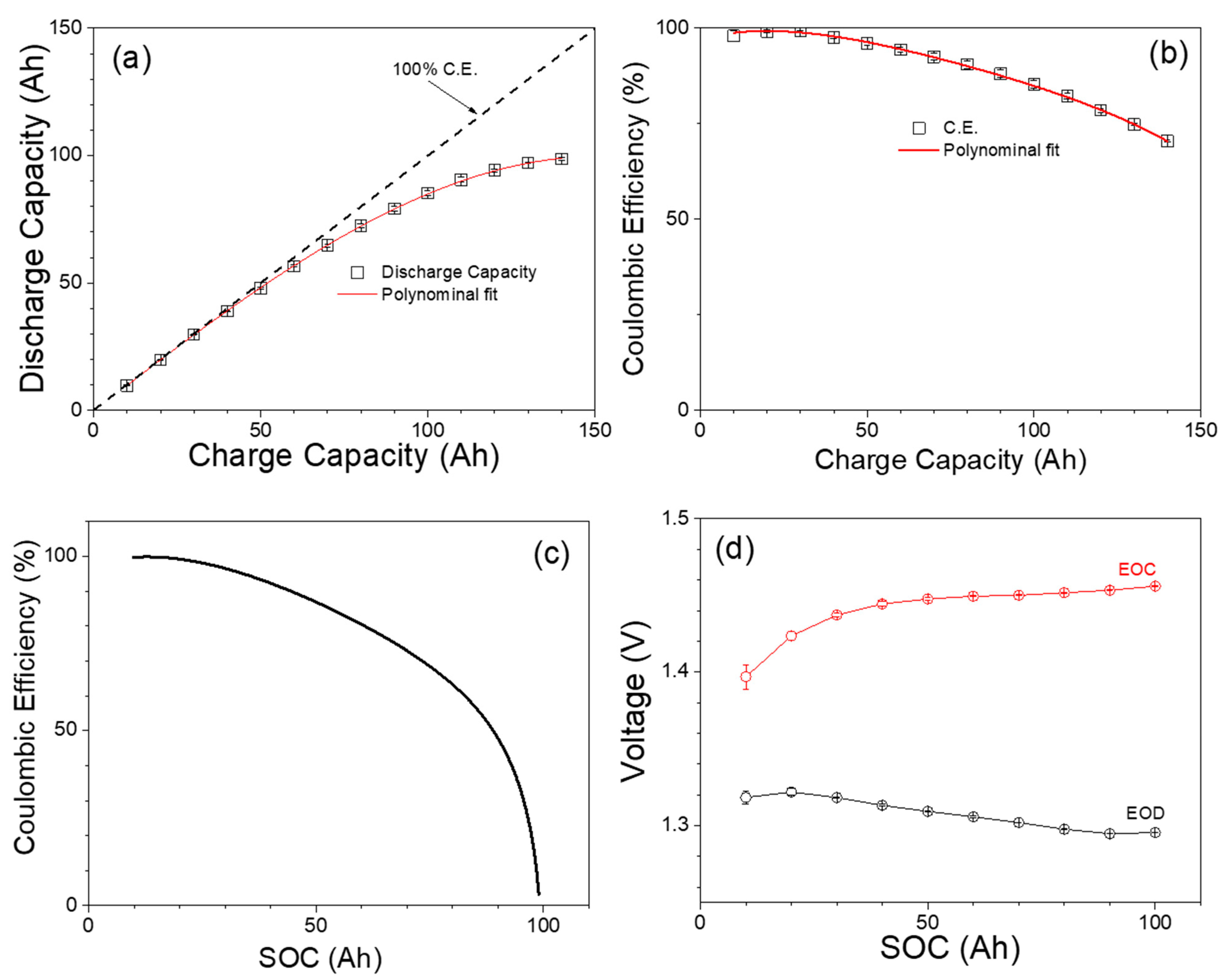
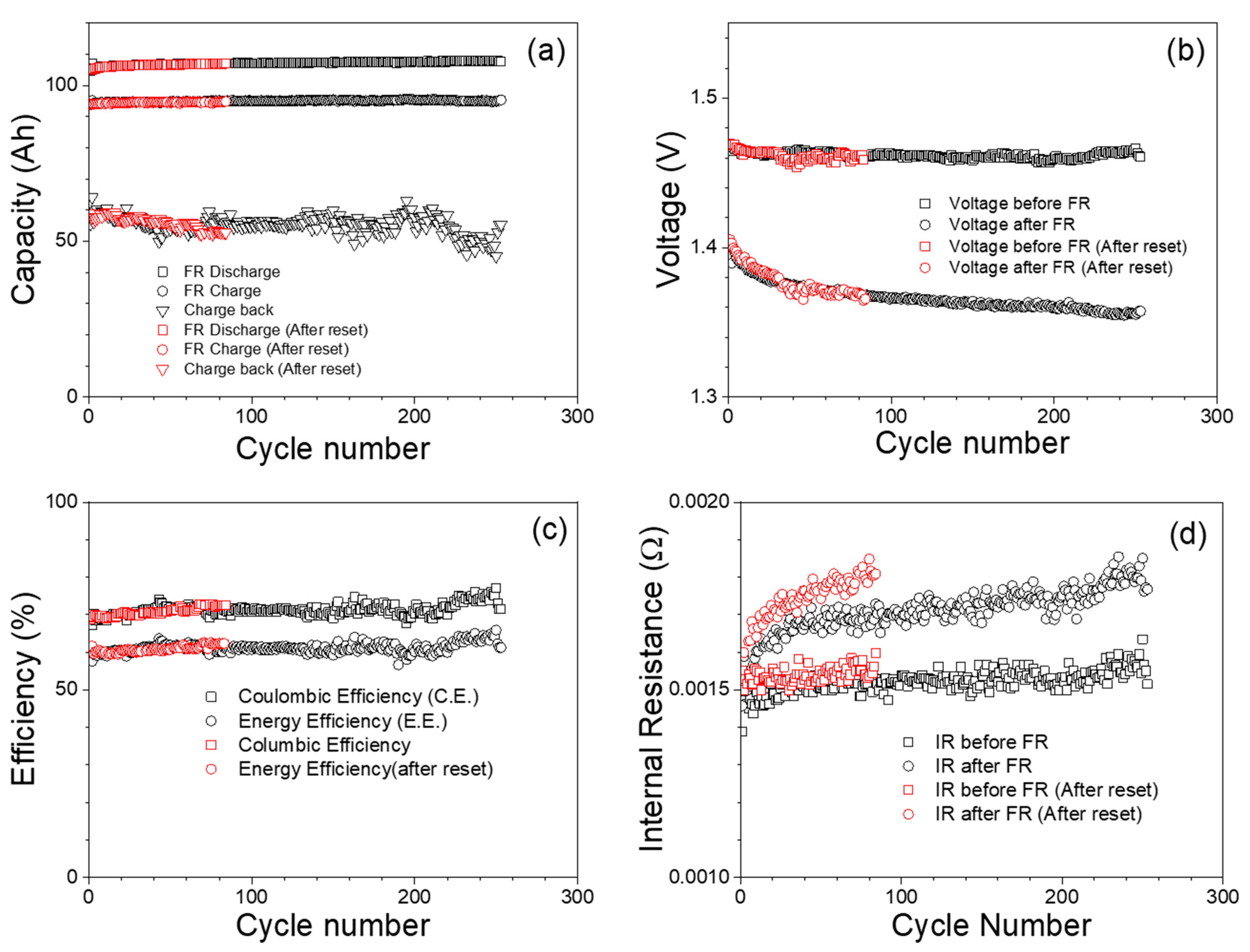

| Nominal Voltage, V | 12 V (1.2 V/cell) |
| Energy, kWh | 1.2 kWh, 5 h rate |
| Cell Capacity, Ah | 100 Ah, 5 h rate |
| Weight (filled), kg | 6.4/cell |
| Electrolyte Vol. liter | 1.5/cell |
| Dimension, cm | 8.1 (w) × 14.2 (d) × 36.8 (h)/cell |
| Operating Temperature, °C | −30 °C To + 60 °C |
| Initial SOC for Starting FR | Charge/Discharge Capability | Energy Efficiency | Coulombic Efficiency | Stability |
|---|---|---|---|---|
| 100% | More discharging capability | 50–60% | 70% | Stable |
| 50% | Has room for both charge and discharge | 70–75% | 85–90% | Stable |
| 25% | More charging capability | 85% | 99% | Unstable |
| Discharge Energy (Wh) | Discharge Duration (h) | Discharge Power (Watt) | Average Discharge Current (A) | Discharge Capacity (Ah) | Bulk Charge Current (Amp) | Charge Duration (h) |
|---|---|---|---|---|---|---|
| 60 | 6 | 10 | 7.5 | 45.4 | 25 | 4.42 |
| 80 | 6 | 13.3 | 10.2 | 61.4 | 25 | 6.10 |
| 100 | 6 | 16.7 | 12.9 | 77.8 | 25 | 7.69 |
| 60 | 4 | 15 | 11.4 | 45.9 | 25 | 4.44 |
| 80 | 4 | 20 | 15.6 | 62.4 | 25 | 6.26 |
| 100 | 4 | 25 | 19.8 | 79.4 | 25 | 7.96 |
| 60 | 2 | 30 | 23.7 | 47.4 | 25 | 4.64 |
| 80 | 2 | 40 | 32.5 | 65 | 40 | 6.44 |
| 100 | 2 | 50 | 42.05 | 84.1 | 40 | 8.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamim, N.; Thomsen, E.C.; Crawford, A.J.; Viswanathan, V.V.; Reed, D.M.; Sprenkle, V.L.; Li, G. Investigation of Fe-Ni Battery/Module for Grid Service Duty Cycles. Materials 2024, 17, 2935. https://doi.org/10.3390/ma17122935
Shamim N, Thomsen EC, Crawford AJ, Viswanathan VV, Reed DM, Sprenkle VL, Li G. Investigation of Fe-Ni Battery/Module for Grid Service Duty Cycles. Materials. 2024; 17(12):2935. https://doi.org/10.3390/ma17122935
Chicago/Turabian StyleShamim, Nimat, Edwin C. Thomsen, Alasdair J. Crawford, Vilayanur V. Viswanathan, David M. Reed, Vincent L. Sprenkle, and Guosheng Li. 2024. "Investigation of Fe-Ni Battery/Module for Grid Service Duty Cycles" Materials 17, no. 12: 2935. https://doi.org/10.3390/ma17122935
APA StyleShamim, N., Thomsen, E. C., Crawford, A. J., Viswanathan, V. V., Reed, D. M., Sprenkle, V. L., & Li, G. (2024). Investigation of Fe-Ni Battery/Module for Grid Service Duty Cycles. Materials, 17(12), 2935. https://doi.org/10.3390/ma17122935








