Synthesis of a Flaky CeO2 with Nanocrystals Used for Polishing
Abstract
1. Introduction
2. Methods
2.1. Preparation of Flaky CeO2 with Nanocrystals
2.2. Chemical Mechanical Polishing (CMP)
2.3. Characterization
3. Results and Discussion
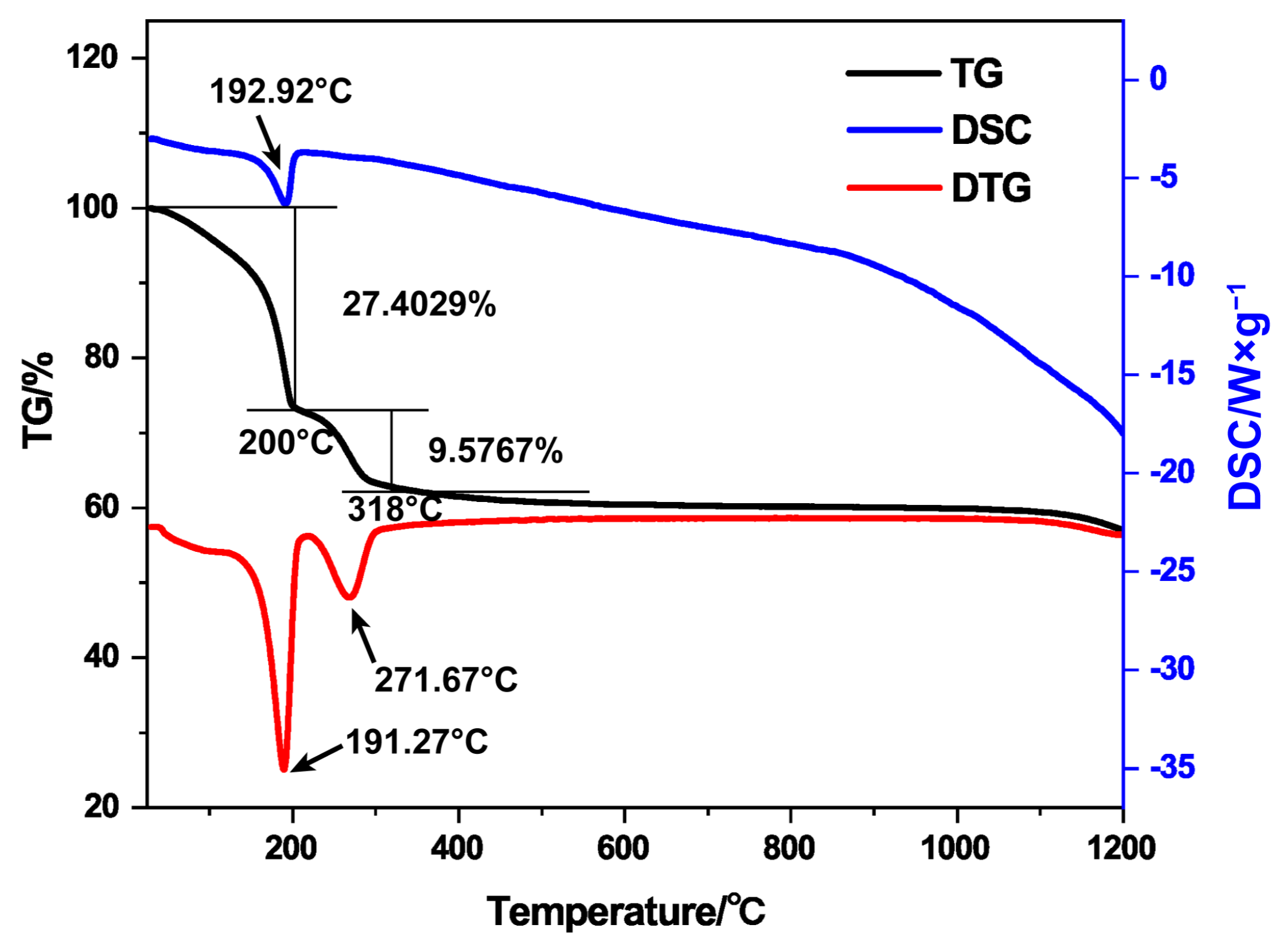
3.1. Thermal Analysis and Composition
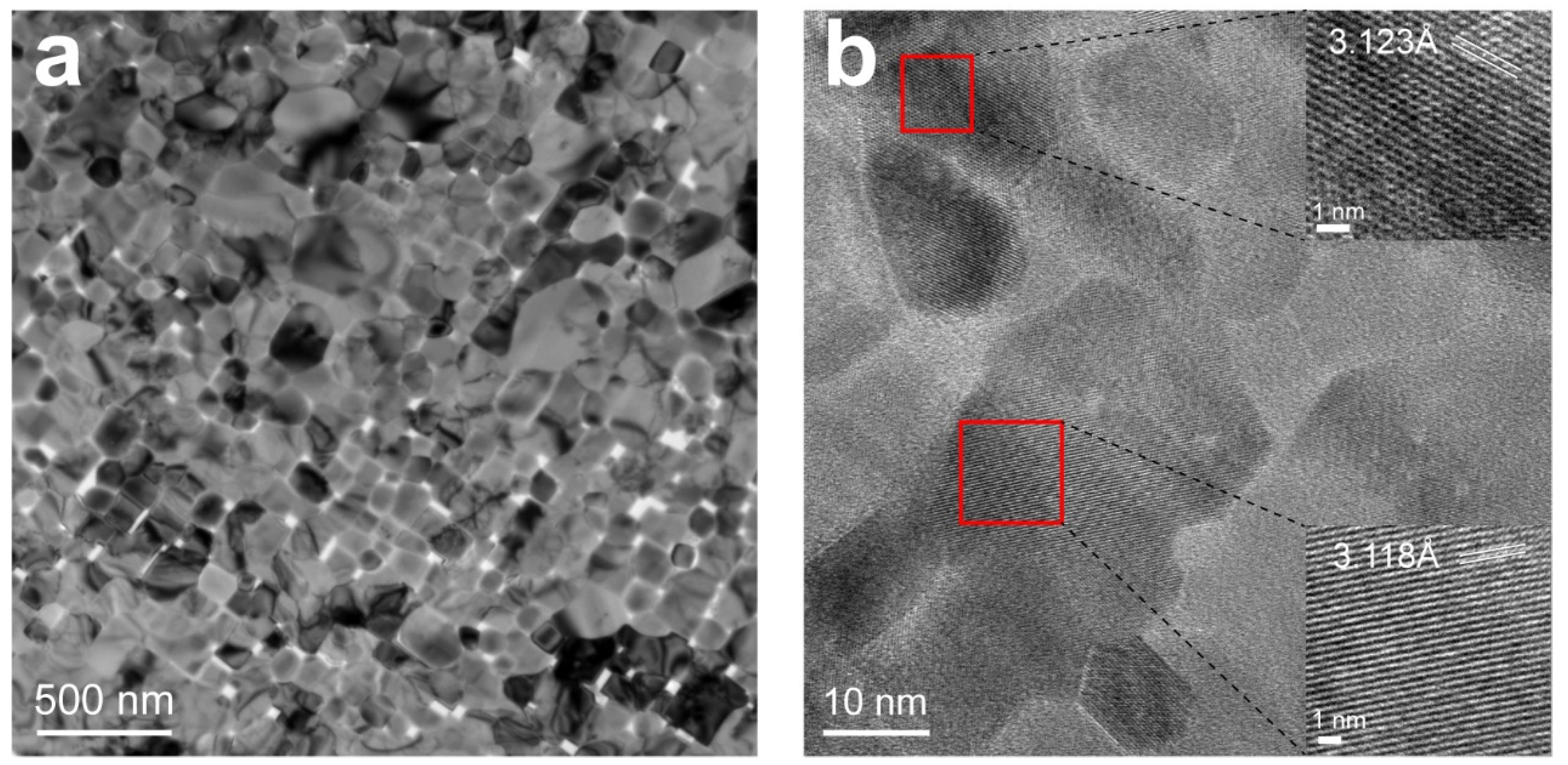
3.2. Morphology and Microstructure
3.3. Chemical State of Ce
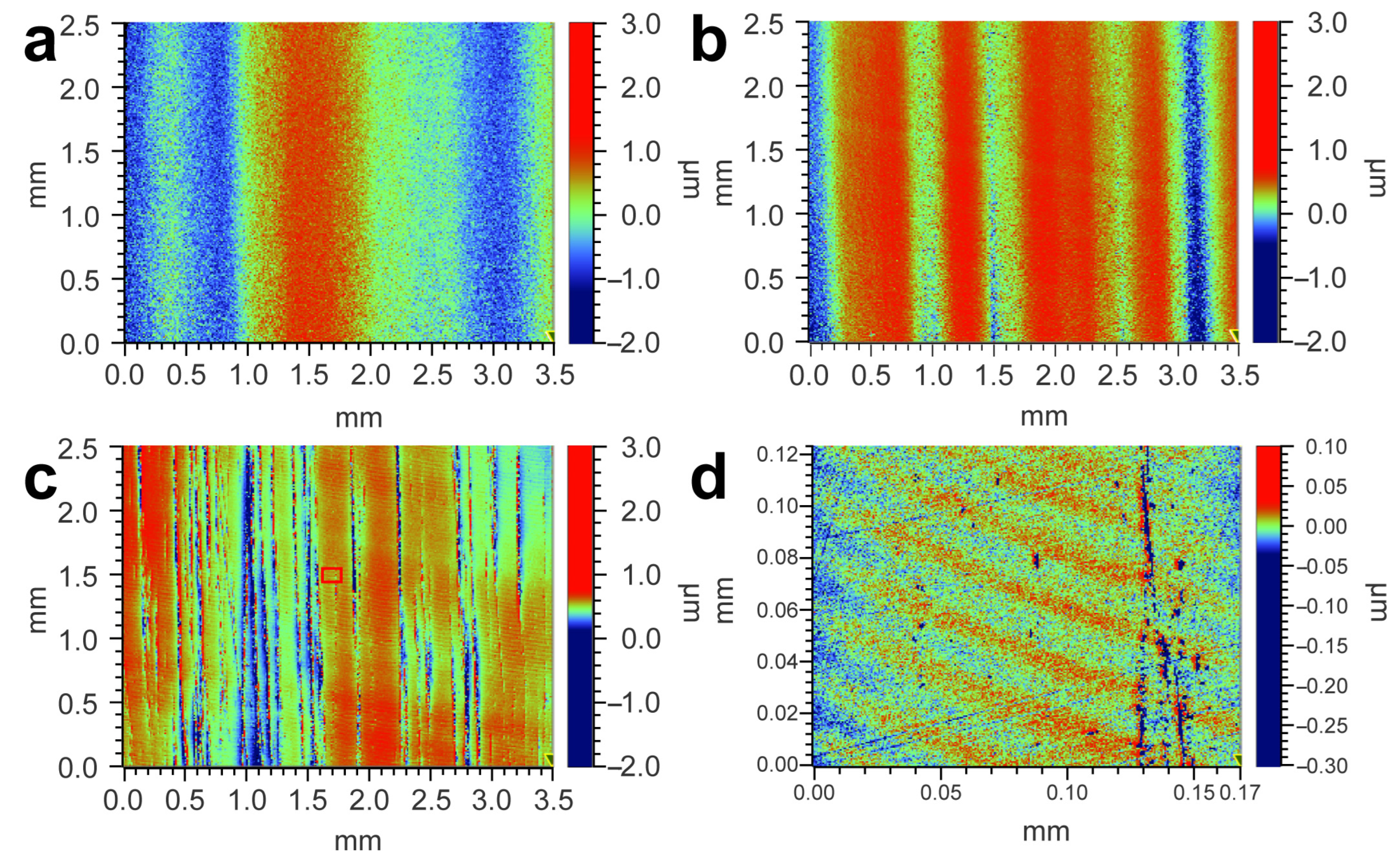
3.4. SiC Surface Polished by CeO2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nolan, M. Formation of Ce3+ at the cerium dioxide (110) surface by doping. Chem. Phys. Lett. 2010, 492, 115–118. [Google Scholar] [CrossRef]
- Kong, J.; Xiang, Z.; Li, G.; An, T. Introduce oxygen vacancies into CeO2 catalyst for enhanced coke resistance during photothermocatalytic oxidation of typical VOCs. Appl. Catal. B Environ. 2020, 269, 118755. [Google Scholar] [CrossRef]
- Chang, K.; Zhang, H.; Cheng, M.-J.; Lu, Q. Application of Ceria in CO2 Conversion Catalysis. ACS Catal. 2020, 10, 613–631. [Google Scholar] [CrossRef]
- Yin, D.; Zhao, F.; Zhang, L.; Zhang, X.; Liu, Y.; Zhang, T.; Wu, C.; Chen, D.; Chen, Z. Greatly enhanced photocatalytic activity of semiconductor CeO2 by integrating with upconversion nanocrystals and graphene. RSC Adv. 2016, 6, 103795–103802. [Google Scholar] [CrossRef]
- Kim, S.K.; Chang, H.; Jang, H.D. Synthesis of micron-sized porous CeO2-SiO2 composite particles for ultraviolet absorption. Adv. Powder Technol. 2017, 28, 406–410. [Google Scholar] [CrossRef]
- Abid, S.A.; Taha, A.A.; Ismail, R.A.; Mohsin, M.H. Antibacterial and cytotoxic activities of cerium oxide nanoparticles prepared by laser ablation in liquid. Environ. Sci. Pollut. Res. 2020, 27, 30479–30489. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, Y.; Liu, M.; Gao, Y.; Ye, F. CeO2 Protective Material against CMAS Attack for Thermal–Environmental Barrier Coating Applications. Coatings 2023, 13, 1119. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, S.; Li, Y.; Wang, T.; Xie, L.; Lu, X. RE (La, Nd and Yb) doped CeO2 abrasive particles for chemical mechanical polishing of dielectric materials: Experimental and computational analysis. Appl. Surf. Sci. 2020, 506, 144668. [Google Scholar] [CrossRef]
- Lu, C.-H.; Wang, H.-C. Formation and microstructural variation of cerium carbonate hydroxide prepared by the hydrothermal process. Mater. Sci. Eng. B 2002, 90, 138–141. [Google Scholar] [CrossRef]
- Chen, L.; Xie, H.; Liu, Y.; Huang, K. Synthesis of Bundle- and Flake-Like CeO2 Powders Via a Precursor-Pyrolysis Approach. J. Am. Ceram. Soc. 2007, 90, 1232–1236. [Google Scholar] [CrossRef]
- Gong, J.; Meng, F.; Yang, X.; Fan, Z.; Li, H. Controlled hydrothermal synthesis of triangular CeO2 nanosheets and their formation mechanism and optical properties. J. Alloys Compd. 2016, 689, 606–616. [Google Scholar] [CrossRef]
- Gai, W.Z.; Zhang, S.H.; Yang, Y.; Zhang, X.H.; Deng, Z.Y. Separation of Excess Fluoride from Water Using Amorphous and Crystalline AlOOH Adsorbents. ACS Omega 2016, 6, 16488–16497. [Google Scholar] [CrossRef] [PubMed]
- Baccarella, A.M.; Garrard, R.; Beauvais, M.L.; Bednarksi, U.; Fischer, S.; Abeykoon, A.M.; Chapman, K.W.; Phillips, B.L.; Parise, J.B.; Simonson, J.W. Cluster mediated conversion of amorphous Al(OH)3 to γ-AlOOH. J. Solid State Chem. 2021, 301, 122340. [Google Scholar] [CrossRef]
- Poggio, E.; Jobbágy, M.; Moreno, M.; Laborde, M.; Mariño, F.; Baronetti, G. Influence of the calcination temperature on the structure and reducibility of nanoceria obtained from crystalline Ce(OH)CO3 precursor. Int. J. Hydrogen Energy 2011, 36, 15899–15905. [Google Scholar] [CrossRef]
- Nylund, A.; Olefjord, I. Surface analysis of oxidized aluminium. 1. Hydration of Al2O3 and decomposition of Al(OH)3 in a vacuum as studied by ESCA. Surf. Interface Anal. 1994, 21, 283–289. [Google Scholar] [CrossRef]
- Majumder, D.; Chakraborty, I.; Mandal, K.; Roy, S. Facet-Dependent Photodegradation of Methylene Blue Using Pristine CeO2 Nanostructures. ACS Omega 2019, 4, 4243–4251. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Deng, J.; Chen, J.; Xing, X. Topochemical molten salt synthesis for functional perovskite compounds. Chem. Sci. 2016, 7, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-Y.; Kim, G.; Sun, H.; Hwang, U.; Kim, J.; Kwak, D.; Park, I.-K.; Kim, T.; Suhr, J.; Nam, J.-D. A nanoclustered ceria abrasives with low crystallinity and high Ce3+/Ce4+ ratio for scratch reduction and high oxide removal rates in the chemical mechanical planarization. J. Mater. Sci. 2022, 57, 12318–12328. [Google Scholar] [CrossRef]





| No. | Ce 3d5/2 | Ce 3d3/2 | Ce3+ (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| v0 | v1 | v2 | v3 | v4 | u0 | u1 | u2 | u3 | u4 | ||
| Ce3+ | Ce4+ | Ce3+ | Ce4+ | Ce4+ | Ce3+ | Ce4+ | Ce3+ | Ce4+ | Ce4+ | ||
| 1 | 1.56 | 10.07 | 13.14 | 14.47 | 20.3 | 0.61 | 6.67 | 10.69 | 9.02 | 13.6 | 26.00 |
| 2 | 0.39 | 10.32 | 13.89 | 15.77 | 19.63 | 0.59 | 6.84 | 10.09 | 9.45 | 13.02 | 24.96 |
| 3 | 2.05 | 9.26 | 15.63 | 14.62 | 20.02 | 2.41 | 6.14 | 8.39 | 8.2 | 13.27 | 28.48 |
| 4 | 1.37 | 9.39 | 17.23 | 14.32 | 18.96 | 10.51 | 6.22 | 2.01 | 7.41 | 12.57 | 31.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Gou, L. Synthesis of a Flaky CeO2 with Nanocrystals Used for Polishing. Materials 2024, 17, 2859. https://doi.org/10.3390/ma17122859
Zhang Y, Gou L. Synthesis of a Flaky CeO2 with Nanocrystals Used for Polishing. Materials. 2024; 17(12):2859. https://doi.org/10.3390/ma17122859
Chicago/Turabian StyleZhang, Yiming, and Li Gou. 2024. "Synthesis of a Flaky CeO2 with Nanocrystals Used for Polishing" Materials 17, no. 12: 2859. https://doi.org/10.3390/ma17122859
APA StyleZhang, Y., & Gou, L. (2024). Synthesis of a Flaky CeO2 with Nanocrystals Used for Polishing. Materials, 17(12), 2859. https://doi.org/10.3390/ma17122859





