Simultaneously Regulating Electrochemical Corrosion Behavior and Wettability of Magnesium–Neodymium Alloy by Self-Layered Chemical Conversion Coating
Abstract
1. Introduction
2. Materials and Methods
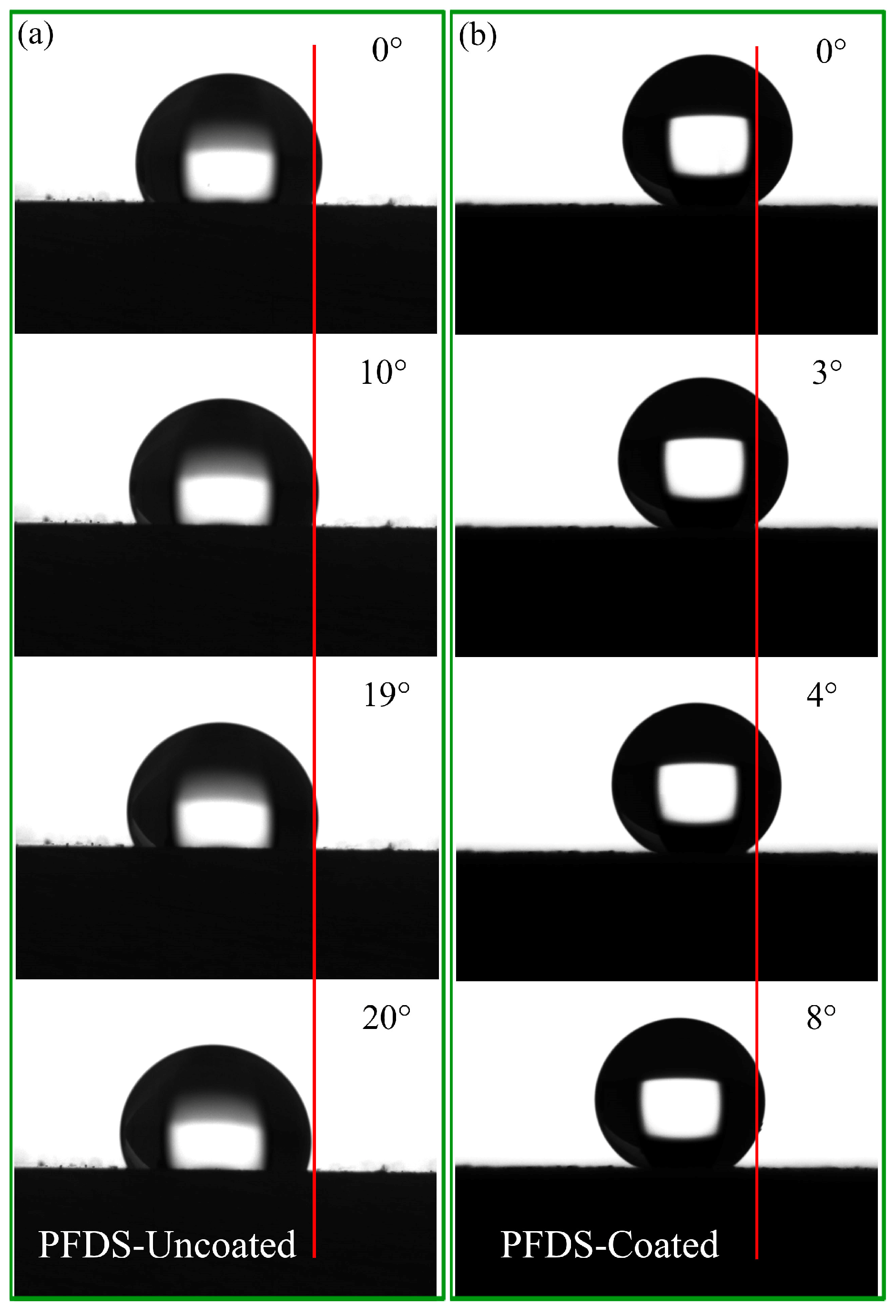
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, J.; Chen, J.; Xiong, X.; Peng, X.; Chen, D.; Pan, F. Research advances of magnesium and magnesium alloys worldwide in 2021. J. Magnes. Alloys 2022, 10, 863–898. [Google Scholar] [CrossRef]
- Luo, A.A. Magnesium casting technology for structural applications. J. Magnes. Alloys 2013, 1, 2–22. [Google Scholar] [CrossRef]
- Ishizaki, T.; Miyashita, T.; Inamura, M.; Nagashima, Y.; Serizawa, A. Effect of Al Content in the Mg-Based Alloys on the Composition and Corrosion Resistance of Composite Hydroxide Films Formed by Steam Coating. Materials 2019, 12, 1188. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Mahato, A.; De, M.; Bhattacharjee, P.; Kumar, V.; Mukherjee, P.; Singh, G.; Kundu, B.; Balla, V.K.; Nandi, S.K. Role of calcium phosphate and bioactive glass coating on in vivo bone healing of new Mg-Zn-Ca implant. J. Mater. Sci. Mater. Med. 2021, 32, 55. [Google Scholar] [CrossRef]
- Song, K.; Pan, F.S.; Chen, X.H.; Zhang, Z.H.; Tang, A.T.; She, J.; Yu, Z.W.; Pan, H.C.; Xu, X.Y. Effect of texture on the electromagnetic shielding property of magnesium alloy. Mater. Lett. 2015, 157, 73–76. [Google Scholar] [CrossRef]
- Bai, J.; Yang, Y.; Wen, C.; Chen, J.; Zhou, G.; Jiang, B.; Peng, X.; Pan, F. Applications of magnesium alloys for aerospace: A review. J. Magnes. Alloys 2023, 11, 3609–3619. [Google Scholar] [CrossRef]
- Wang, G.G.; Weiler, J.P. Recent developments in high-pressure die-cast magnesium alloys for automotive and future applications. J. Magnes. Alloys 2023, 11, 78–87. [Google Scholar] [CrossRef]
- Rahman, M.; Li, Y.; Wen, C. HA coating on Mg alloys for biomedical applications: A review. J. Magnes. Alloys 2020, 8, 929–943. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, H.; Liao, K.; Li, X. Improvement on corrosion resistance of micro-arc oxidized AZ91D magnesium alloy by a pore-sealing coating. J. Alloys Compd. 2021, 889, 161460. [Google Scholar] [CrossRef]
- Gao, J.; Su, Y.; Qin, Y.X. Calcium phosphate coatings enhance biocompatibility and degradation resistance of magnesium alloy: Correlating in vitro and in vivo studies. Bioact. Mater. 2021, 6, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Wu, J.; Jiang, H.; Zhang, L.; Zhao, J.; He, J. Effect of micro-alloying element La on corrosion behavior of Al-Mg-Si alloys. Corros. Sci. 2021, 179, 109113. [Google Scholar] [CrossRef]
- Liu, F.; Xin, R.; Wang, C.; Song, B.; Liu, Q. Regulating precipitate orientation in Mg-Al alloys by coupling twinning, aging and detwinning processes. Scr. Mater. 2019, 158, 131–135. [Google Scholar] [CrossRef]
- Hua, L.; Sun, J.; Wu, G. Enhancing corrosion resistance of hydrothermally-treated magnesium-aluminum alloys by preprocessed metallurgical microstructure. Thin Solid Film. 2022, 752, 139247. [Google Scholar] [CrossRef]
- Zahedi Asl, V.; Kazemzad, M.; Zhao, J.; Ramezanzadeh, B.; Anjum, M.J. An eco-friendly CaCe and CaY based LDH coating on AZ31 Mg alloy: Surface modification and its corrosion studies in simulated body fluid (SBF). Surf. Coat. Technol. 2022, 440, 128458. [Google Scholar] [CrossRef]
- Huang, J.; Dun, Y.; Wan, Q.; Wu, Z.; Zhao, X.; Tang, Y.; Zhang, X.; Zuo, Y. Improved corrosion resistance of MAO coating on Mg-Li alloy by RGO modified silanization. J. Alloys Compd. 2022, 929, 167283. [Google Scholar] [CrossRef]
- Qi, J.; Ye, Z.; Gong, N.; Qu, X.; Mercier, D.; Światowska, J.; Skeldon, P.; Marcus, P. Formation of a trivalent chromium conversion coating on AZ91D magnesium alloy. Corros. Sci. 2021, 186, 109459. [Google Scholar] [CrossRef]
- Liao, S.; Yu, B.; Zhang, X.; Lu, X.; Zhou, P.; Zhang, C.; Chen, X.; Zhang, T.; Wang, F. New design principles for the bath towards chromate- and crack-free conversion coatings on magnesium alloys. J. Magnes. Alloys 2021, 9, 505–519. [Google Scholar] [CrossRef]
- Saran, D.; Kumar, A.; Bathula, S.; Klaumünzer, D.; Sahu, K.K. Review on the phosphate-based conversion coatings of magnesium and its alloys. Int. J. Miner. Metall. Mater. 2022, 29, 1435–1452. [Google Scholar] [CrossRef]
- Wang, Y.; You, Z.; Ma, K.; Dai, C.; Wang, D.; Wang, J. Corrosion resistance of a superhydrophobic calcium carbonate coating on magnesium alloy by ultrasonic cavitation-assisted chemical conversion. Corros. Sci. 2023, 211, 110841. [Google Scholar] [CrossRef]
- Xue, K.; Li, Y.J.; Ma, T.H.; Cui, L.Y.; Liu, C.b.; Zou, Y.H.; Li, S.Q.; Zhang, F.; Zeng, R.C. In vitro corrosion resistance and dual antibacterial ability of curcumin loaded composite coatings on AZ31 alloy: Effect of amorphous calcium carbonate. J. Colloid Interface Sci. 2023, 649, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Lu, X.; Cheng, H.; Feng, X.; Zhao, Z.; Ding, Y.; Shen, Y.; Shi, X. Investigation of the surface modification of magnesium particles with stannate on the corrosion resistance of a Mg-rich epoxy coating on AZ91D magnesium alloy. Prog. Org. Coat. 2019, 135, 591–600. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, X.; Wan, L.; Yang, J.; Gao, Y. Preparation of Antimony-doped Stannate Chemical Conversion Coating on AZ31B Mg Alloy. Trans. Indian Inst. Met. 2020, 73, 1891–1898. [Google Scholar] [CrossRef]
- Jian, S.Y.; Chang, K.L. Effect of cerium ion on the microstructure and properties of permanganate conversion coating on LZ91 magnesium alloy. Appl. Surf. Sci. 2020, 509, 144767. [Google Scholar] [CrossRef]
- Hernandez-Alvarado, L.A.; Salvador Hernandez, L.; Lomeli, M.A.; Miranda-Vidales, J.M.; Narvaez, L.; Escudero, M.L. Corrosion rates of reabsorbable Mg-based materials coated with phytic acid. Corros. Eng. Sci. Technol. 2021, 56, 714–727. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, C. Electrochemical studies on ammonium magnesium carbonate tetrahydrate/calcium carbonate composite coating on AZ91D magnesium alloy. Mater. Chem. Phys. 2022, 292, 126787. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, G.; Peng, X.; Li, L.; Feng, H.; Gao, B.; Huo, K.; Chu, P.K. Mitigation of Corrosion on Magnesium Alloy by Predesigned Surface Corrosion. Sci. Rep. 2015, 5, 17399. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Kim, D.; Moon, H.-S.; Kim, K.; Lee, J. Durable anti-corrosive oil-impregnated porous surface of magnesium alloy by plasma electrolytic oxidation with hydrothermal treatment. Appl. Surf. Sci. 2020, 509, 145361. [Google Scholar] [CrossRef]
- Prabhu, D.B.; Gopalakrishnan, P.; Ravi, K.R. Morphological studies on the development of chemical conversion coating on surface of Mg–4Zn alloy and its corrosion and bio mineralisation behaviour in simulated body fluid. J. Alloys Compd. 2020, 812, 152146. [Google Scholar] [CrossRef]
- Yao, X.; Yang, Y.; Zheng, M.; Wang, J.; Liu, C.; Sun, J.; Wu, G. Enhanced corrosion resistance of magnesium-neodymium alloy in simulated concrete pore solution by predesigned corrosion product. Mater. Today Commun. 2022, 32, 104027. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, G.; Xu, C.; Yang, Z.; Sun, J. Revealing anti-corrosion behavior of magnesium alloy in simulated concrete pore solution. Mater. Lett. 2021, 285, 129047. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Yu, J.; Lu, Z.; Chen, Y. Effects of simulated seawater on static and fatigue performance of GFRP bar–concrete bond. J. Build. Eng. 2023, 68, 105985. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Understanding Magnesium Corrosion—A Framework for Improved Alloy Performance. Adv. Eng. Mater. 2004, 5, 837–858. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, W.; Ma, K.; Dai, C.; Wang, D.; Wang, J. Enhancing corrosion resistance of the CaCO3/MgO coating via ultrasound-assisted chemical conversion with addition of ethylenediamine tetra-acetic acid (EDTA) on AZ41 Mg alloy. Surf. Coat. Technol. 2023, 467, 129687. [Google Scholar] [CrossRef]
- Li, Y.; Kang, Z.; Zhang, X.; Pan, J.; Ren, Y.; Zhou, G. Fabricating an anti-corrosion carbonate coating on Mg Li alloy by low-temperature plasma. Surf. Coat. Technol. 2022, 439, 128418. [Google Scholar] [CrossRef]
- Chubar, N. XPS determined mechanism of selenite (HSeO3−) sorption in absence/presence of sulfate (SO42−) on Mg-Al-CO3 Layered double hydroxides (LDHs): Solid phase speciation focus. J. Environ. Chem. Eng. 2023, 11, 109669. [Google Scholar] [CrossRef]
- Popa, M.; Stefan, L.M.; Prelipcean, A.M.; Drob, S.I.; Anastasescu, M.; Calderon Moreno, J.M. Inhibition of Mg corrosion in physiological fluids by carbonate coating. Corros. Sci. 2022, 209, 110775. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, W.; Ma, K.; Dai, C.; Wang, D.; Wang, J. A new design strategy for the crack-free composite CaHPO4·2H2O/CaCO3 coating on AZ41 Mg alloy for magnesium concrete formwork. Surf. Coat. Technol. 2023, 468, 129784. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, C.; Wang, F.; Li, W. Electrochemical behavior of anodized Mg alloy AZ91D in chloride containing aqueous solution. Corros. Sci. 2005, 47, 2816–2831. [Google Scholar] [CrossRef]
- Wu, H.; Shi, Z.; Zhang, X.; Qasim, A.M.; Xiao, S.; Zhang, F.; Wu, Z.; Wu, G.; Ding, K.; Chu, P.K. Achieving an acid resistant surface on magnesium alloy via bio-inspired design. Appl. Surf. Sci. 2019, 478, 150–161. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, P.; Zhang, Y.; Zhang, T.; Wang, F. Bridge for the thermodynamics and kinetics of electrochemical corrosion: Designing of the high corrosion-resistant magnesium alloy. Corros. Sci. 2023, 222, 111428. [Google Scholar] [CrossRef]
- Saji, V.S. Recent progress in superhydrophobic and superamphiphobic coatings for magnesium and its alloys. J. Magnes. Alloys 2021, 9, 748–778. [Google Scholar] [CrossRef]
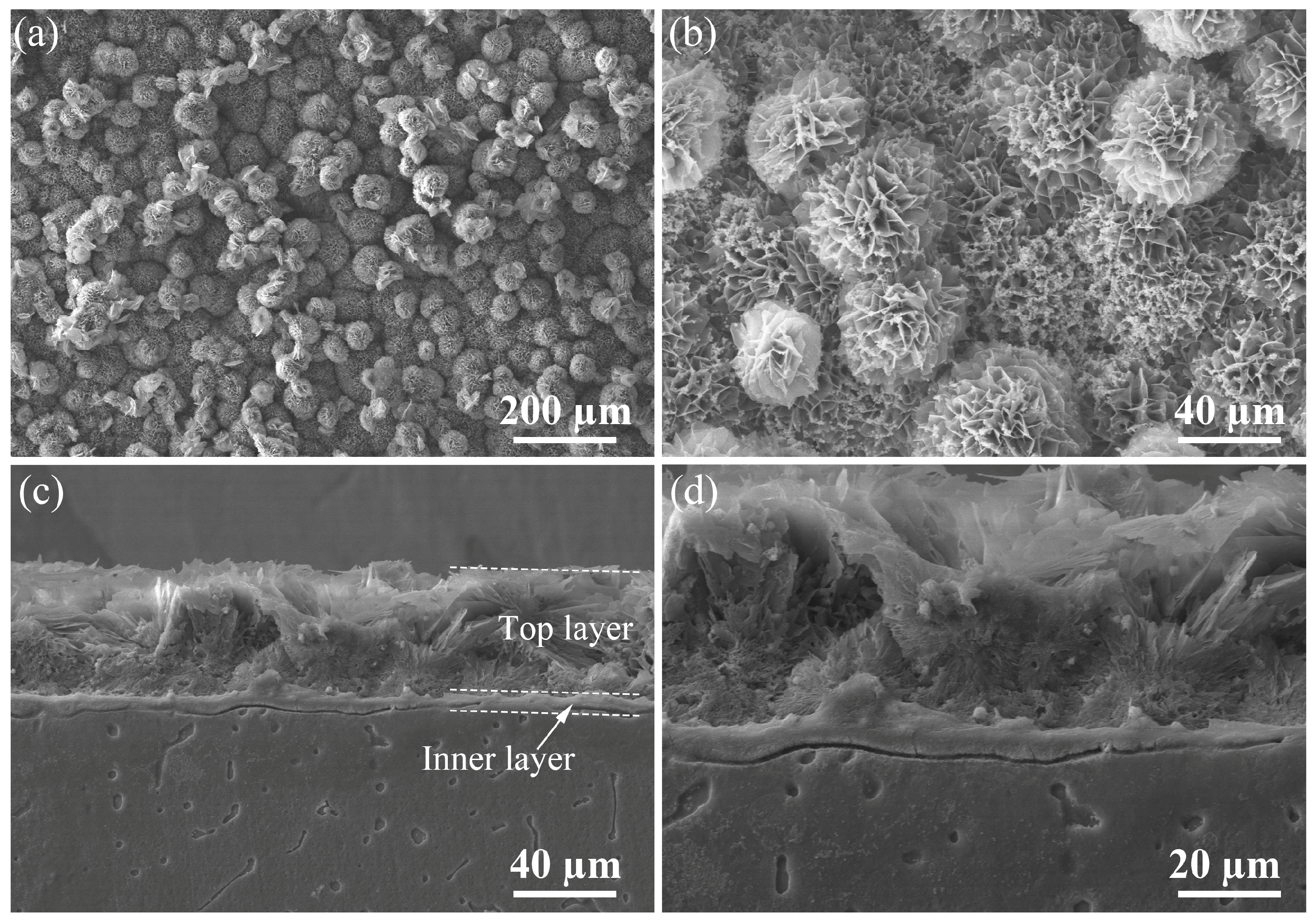
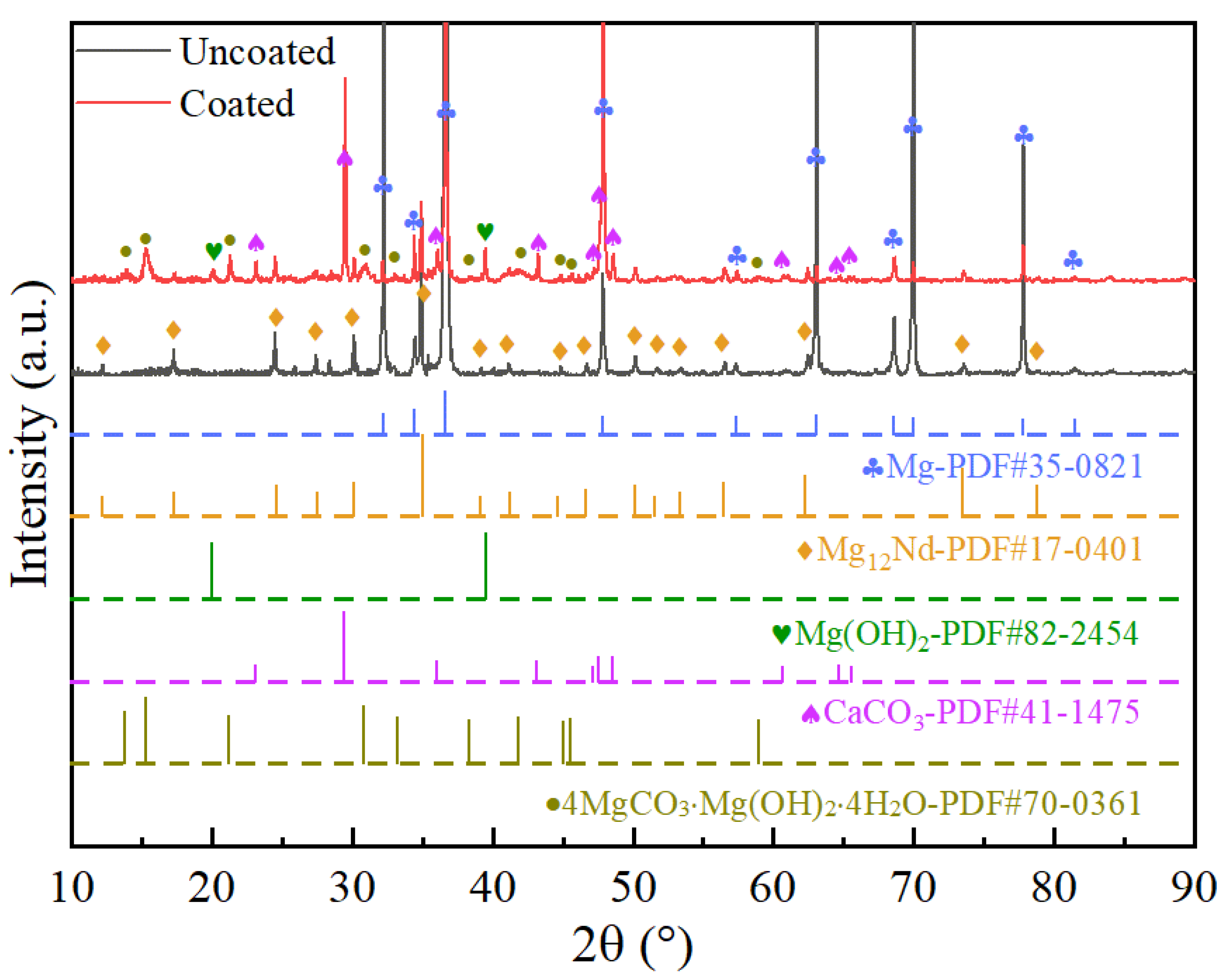

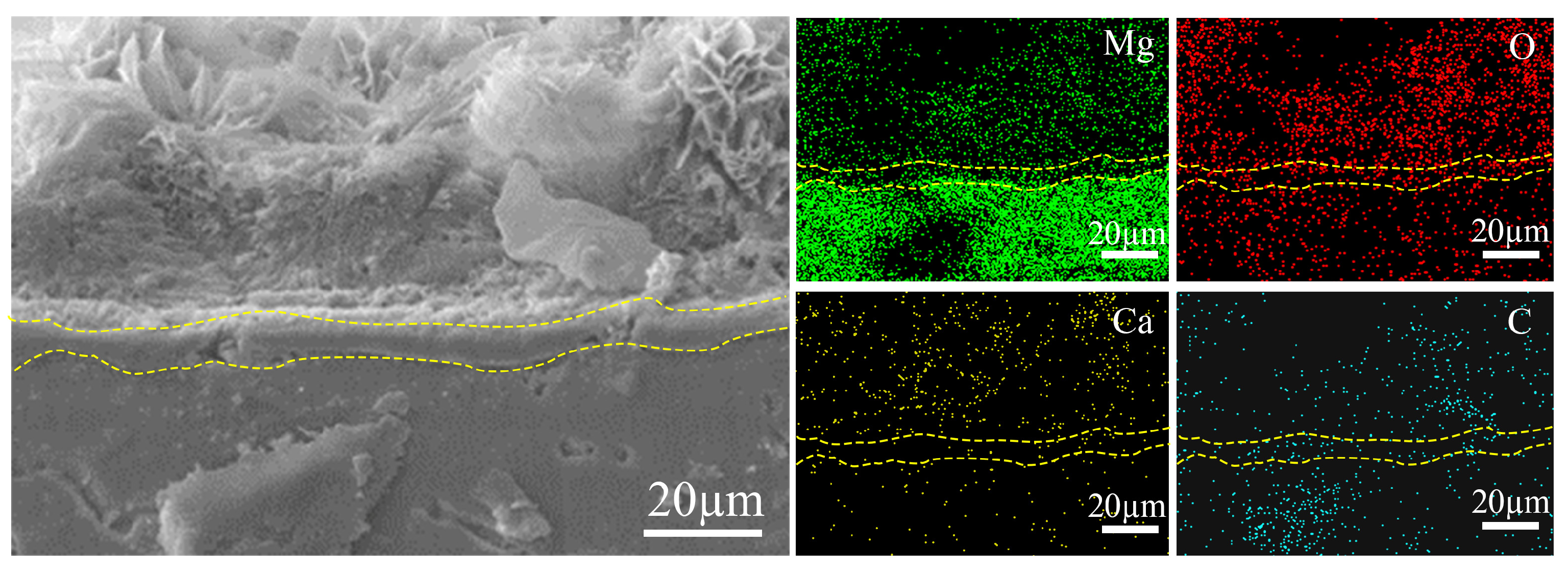
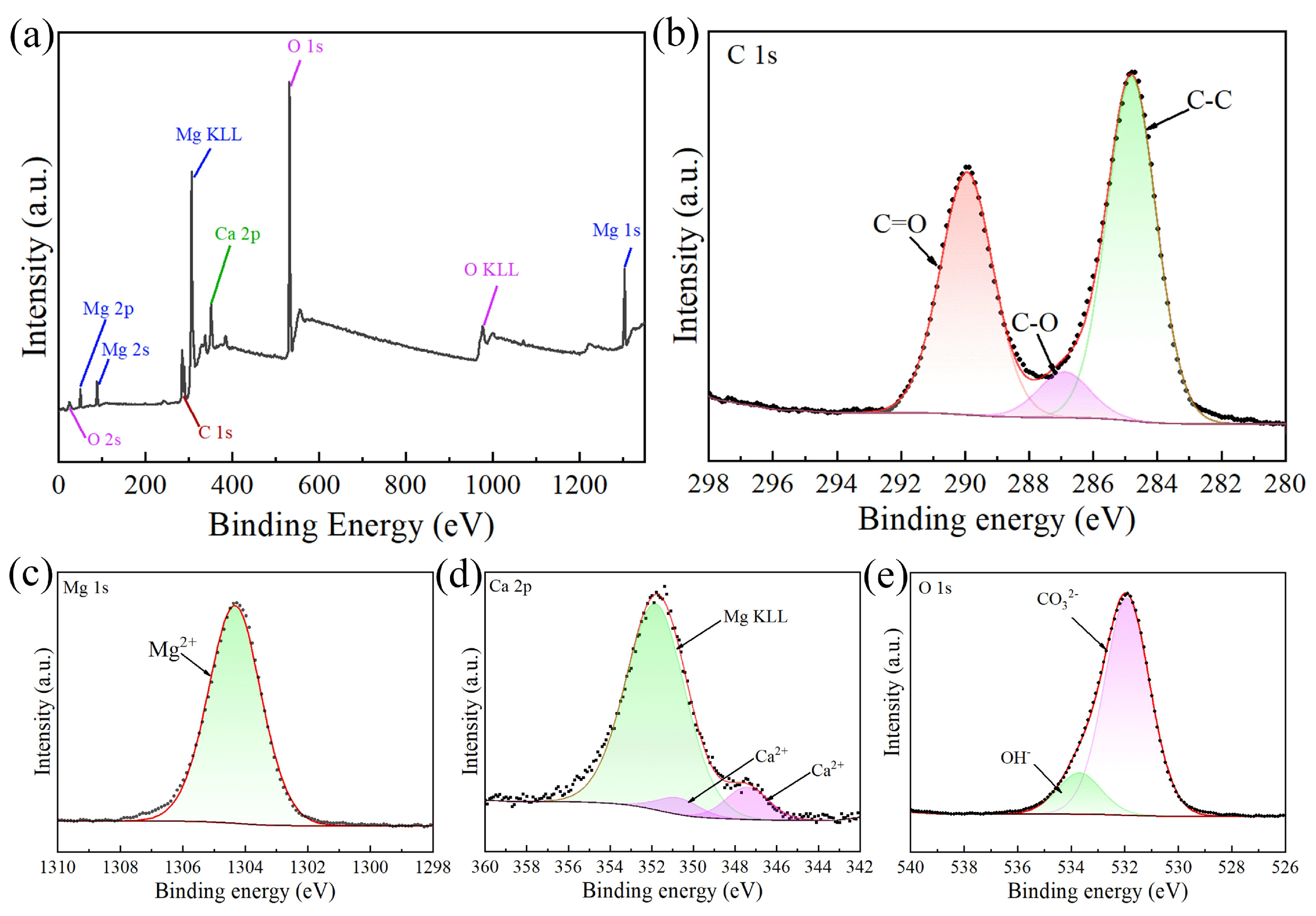
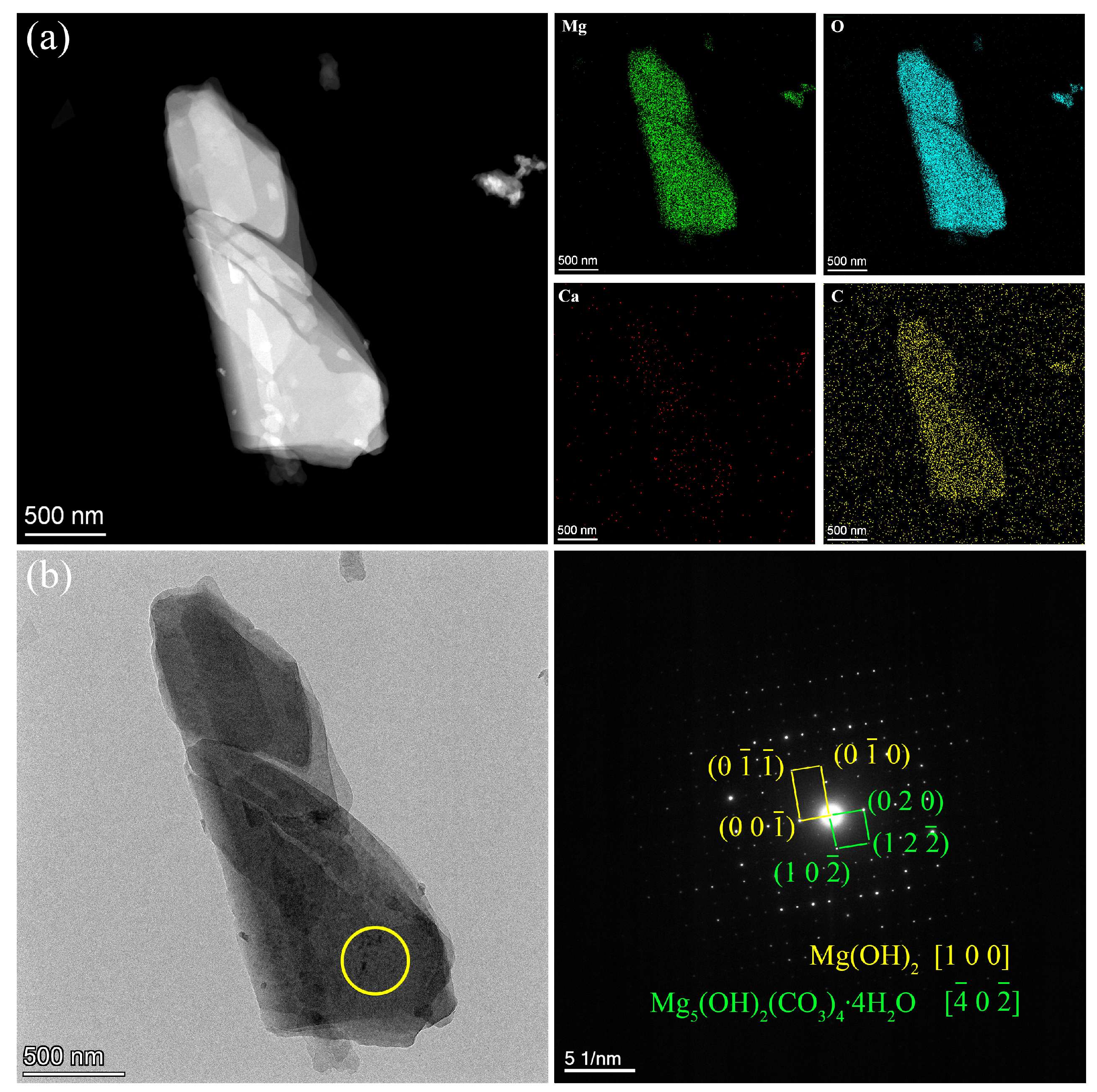
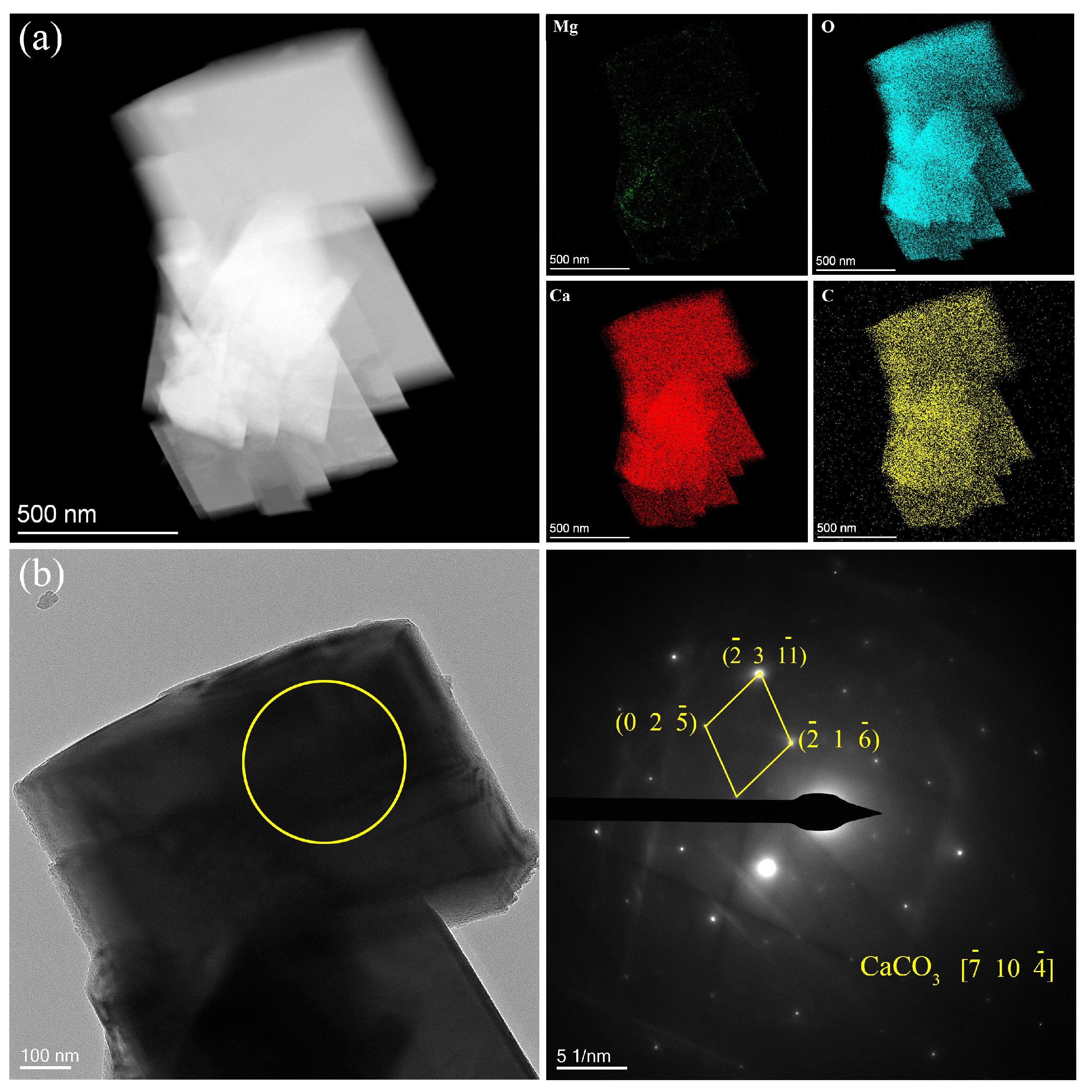
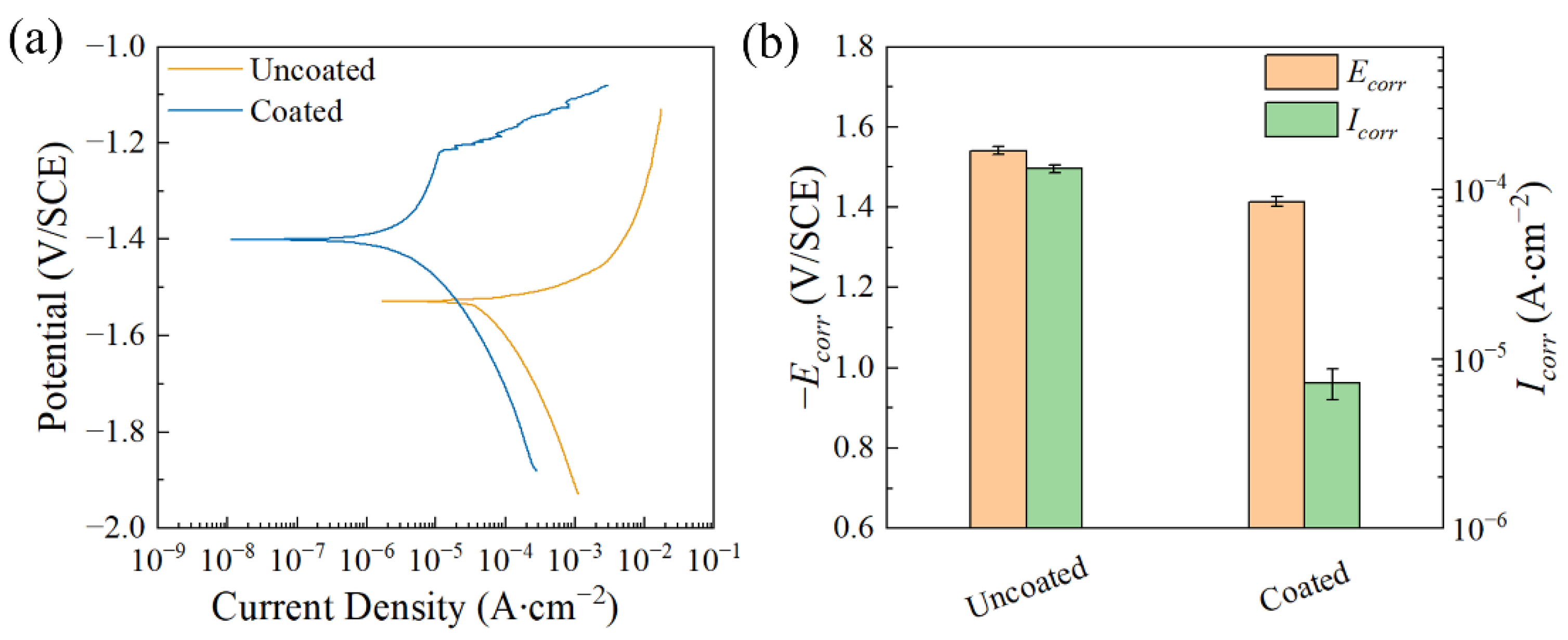
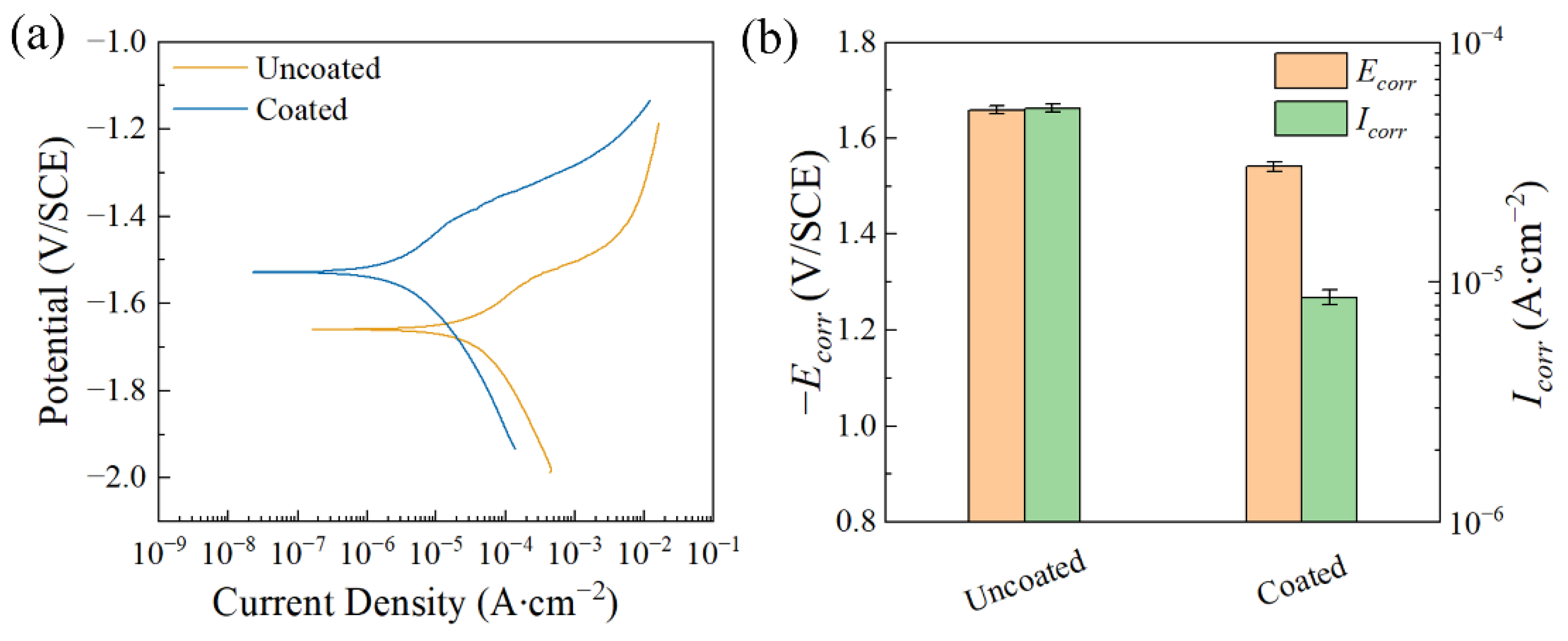
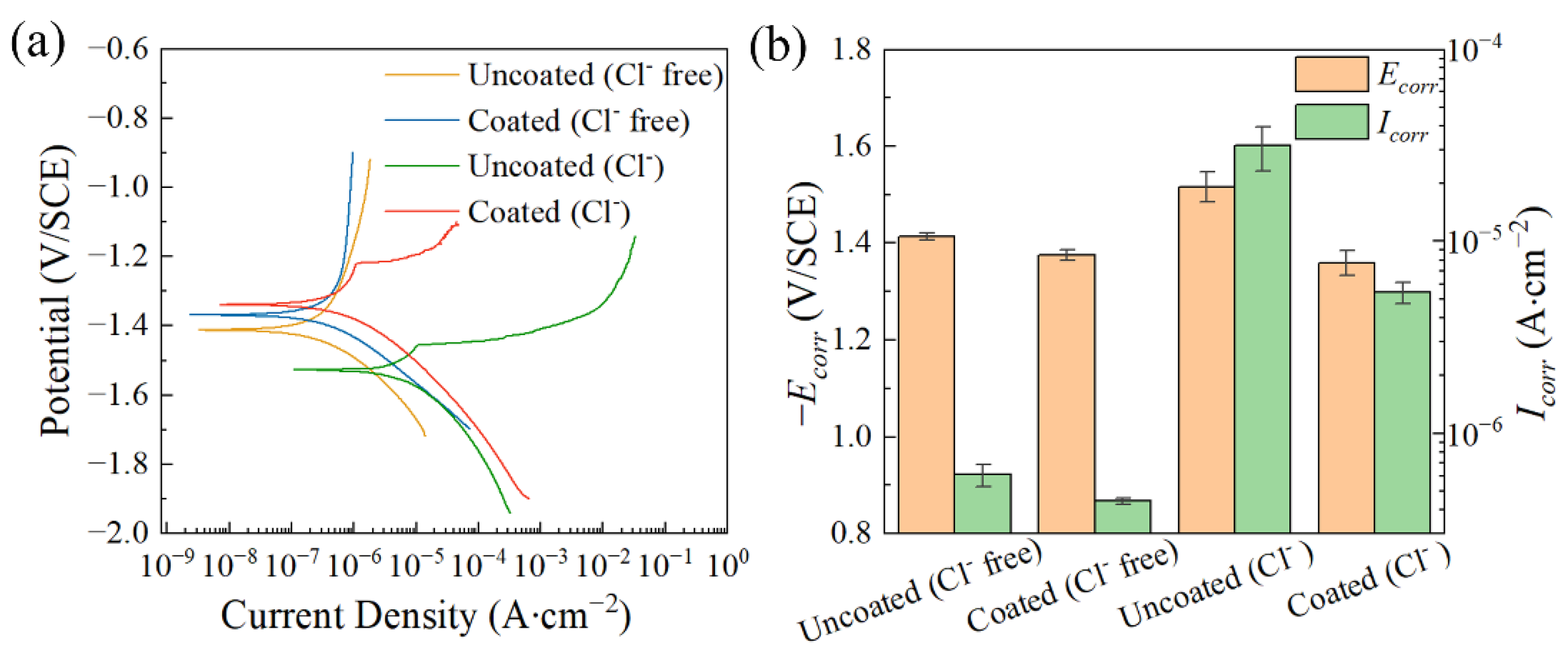
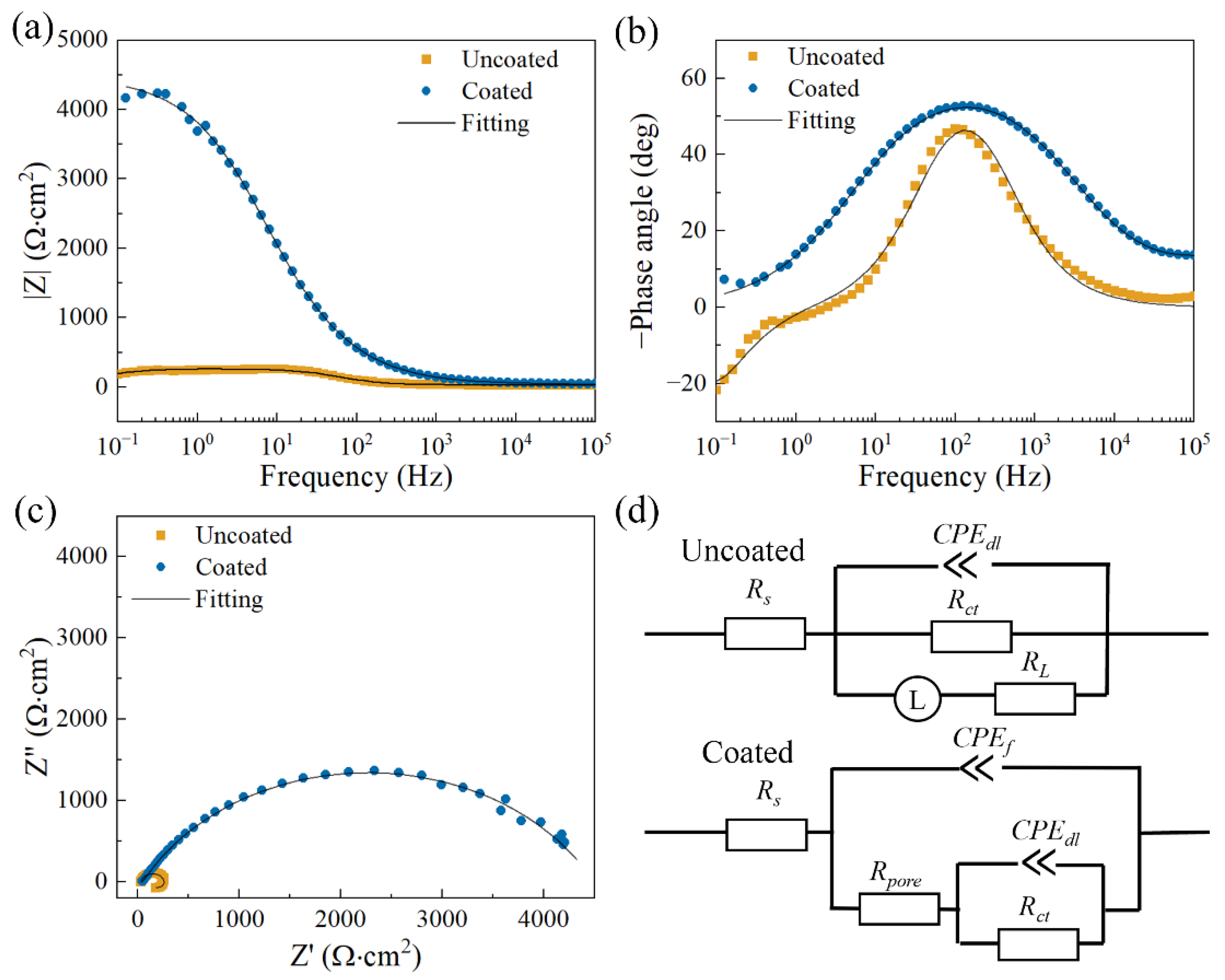

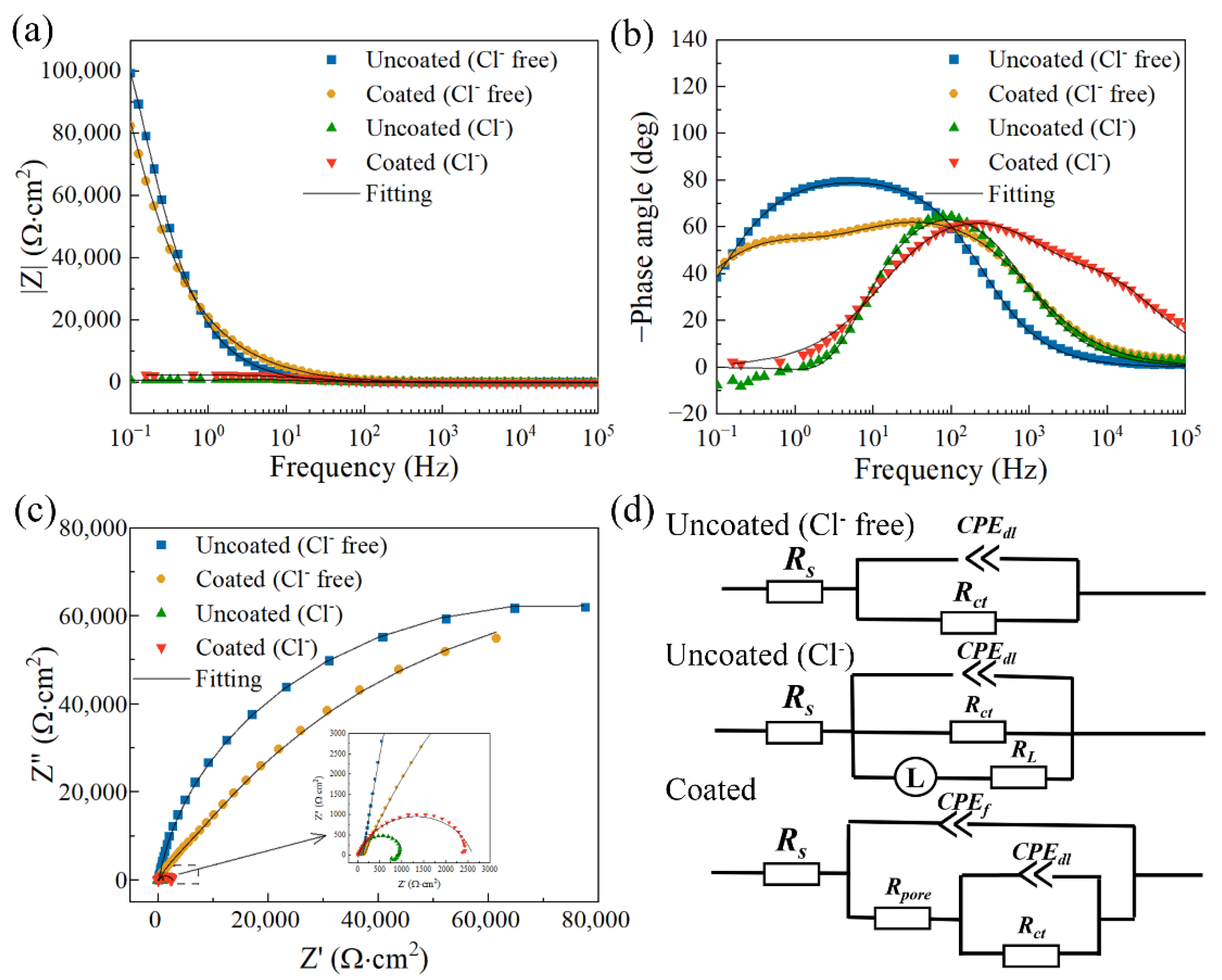
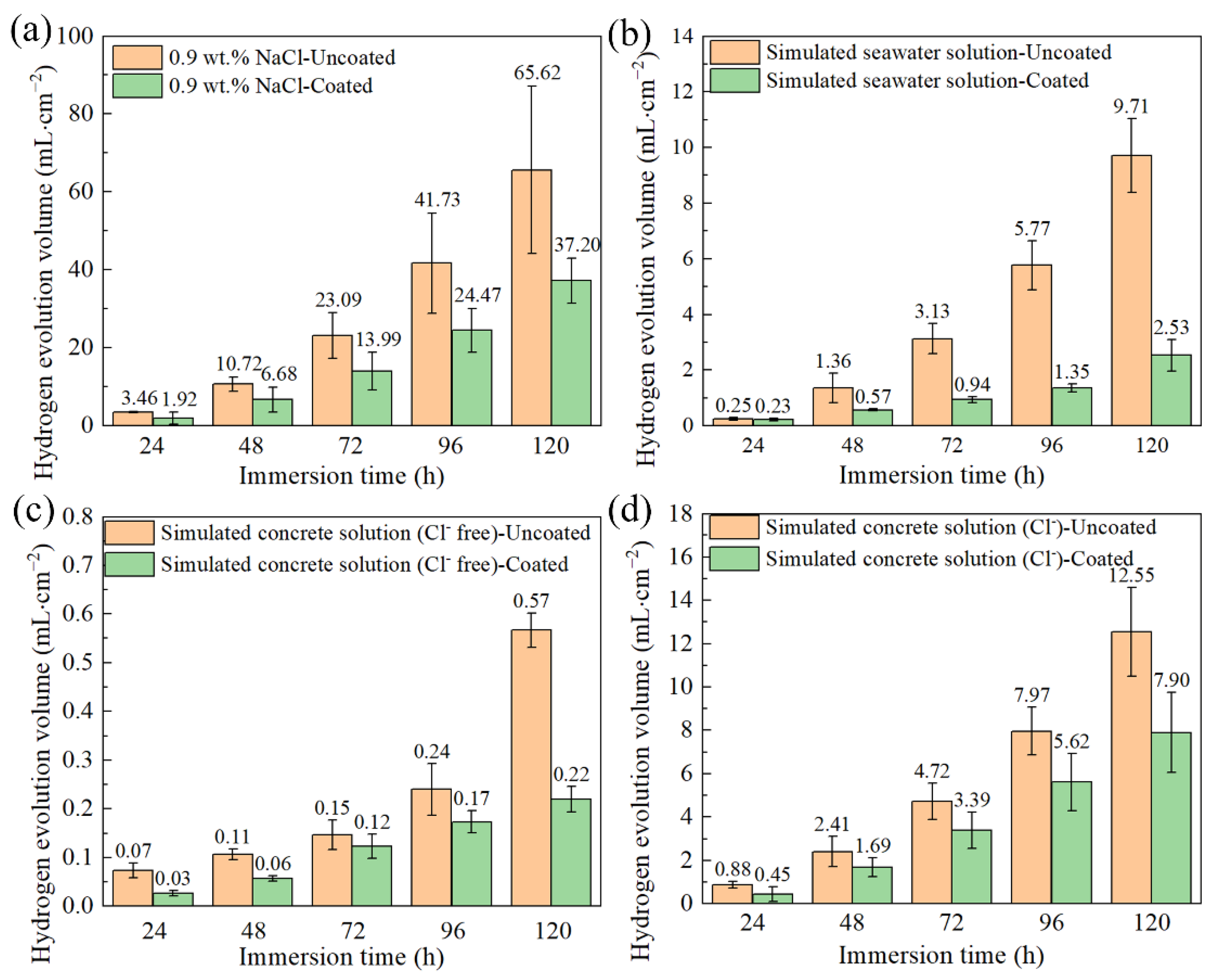

| Ecorr (V/SCE) | Icorr (A∙cm−2) | |
|---|---|---|
| Uncoated | −1.541 ± 0.011 | (1.330 ± 0.072) × 10−4 |
| Coated | −1.414 ± 0.013 | (7.548 ± 1.490) × 10−6 |
| Ecorr (V/SCE) | Icorr (A∙cm−2) | |
|---|---|---|
| Uncoated | −1.659 ± 0.003 | (5.310 ± 0.019) × 10−4 |
| Coated | −1.541 ± 0.010 | (8.650 ± 0.615) × 10−6 |
| Ecorr (V/SCE) | Icorr (A∙cm−2) | |
|---|---|---|
| Uncoated (Cl− free) | −1.413 ± 0.008 | (6.092 ± 0.801) × 10−7 |
| Coated (Cl− free) | −1.375 ± 0.001 | (4.444 ± 0.152) × 10−7 |
| Uncoated (Cl−) | −1.516 ± 0.031 | (3.147 ± 0.816) × 10−5 |
| Coated (Cl−) | −1.359 ± 0.026 | (5.414 ± 0.664) × 10−6 |
| Uncoated | Coated | |
|---|---|---|
| Rs (Ω∙cm2) | 33.127 ± 3.614 | 35.897 ± 0.808 |
| Y0,f (Ω−1∙cm−2∙sn) | - | (5.434 ± 1.239) × 10−6 |
| nf | - | 0.700 ± 0.007 |
| Rpore (Ω∙cm2) | - | 57.270 ± 6.850 |
| Y0,dl (Ω−1∙cm−2∙sn) | (2.754 ± 0.491) × 10−5 | (1.668 ± 0.193) × 10−5 |
| ndl | 0.922 ± 0.010 | 0.691 ± 0.009 |
| Rct (Ω∙cm2) | 232.800 ± 24.447 | (4.436 ± 0.495) × 103 |
| L (H∙cm2) | 550.533 ± 101.946 | - |
| RL (Ω∙cm2) | 151.633 ± 24.908 | - |
| Uncoated | Coated | |
|---|---|---|
| Rs (Ω∙cm2) | 19.493 ± 5.007 | 20.067 ± 8.183 |
| Y0,f (Ω−1∙cm−2∙sn) | - | (7.825 ± 6.382) × 10−6 |
| nf | - | 0.679 ± 0.071 |
| Rpore (Ω∙cm2) | - | 64.747 ± 20.530 |
| Y0,dl (Ω−1∙cm−2∙sn) | (1.014 ± 0.215) × 10−3 | (4.262 ± 2.370) × 10−6 |
| ndl | 0.721 ± 0.042 | 0.867 ± 0.124 |
| Rct (Ω∙cm2) | 574.900 ± 54.277 | (3.326 ± 0.411) × 103 |
| Y0,diff (Ω−1∙cm−2∙sn) | (1.425 ± 0.063) × 10−5 | (4.591 ± 0.842) × 10−5 |
| ndiff | 0.923 ± 0.003 | 0.719 ± 0.082 |
| Rdiff (Ω∙cm2) | 859.100 ± 76.464 | (6.403 ± 0.423) × 103 |
| Uncoated (Cl− Free) | Coated (Cl− Free) | Uncoated (Cl−) | Coated (Cl−) | |
|---|---|---|---|---|
| Rs (Ω∙cm2) | 113.833 ± 8.014 | 157.733 ± 9.880 | 17.730 ± 6.760 | 12.330 ± 0.771 |
| Y0,f (Ω−1∙cm−2∙sn) | - | (7.792 ± 1.517) × 10−6 | - | (6.801 ± 0.897) × 10−6 |
| nf | - | 0.770 ± 0.044 | - | 0.821 ± 0.018 |
| Rpore (Ω∙cm2) | - | (1.960 ± 0.549) × 104 | - | 62.923 ± 17.766 |
| Y0,dl (Ω−1∙cm−2∙sn) | (9.776 ± 0.440) × 10−6 | (7.236 ± 1.286) × 10−6 | (2.018 ± 0.208) × 10−5 | (4.893 ± 0.095) × 10−6 |
| ndl | 0.913 ± 0.001 | 0.709 ± 0.026 | 0.882 ± 0.007 | 0.815 ± 0.006 |
| Rct (Ω∙cm2) | (1.431 ± 0.187) × 105 | (1.440 ± 0.215) × 105 | (1.540 ± 0.304) × 103 | (2.436 ± 0.242) × 103 |
| L (H∙cm2) | - | - | 177.700 ± 58.613 | - |
| RL (Ω∙cm2) | - | - | (3.995 ± 0.131) × 103 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Kuang, Y.; Xu, B.; Liu, C.; Wu, G. Simultaneously Regulating Electrochemical Corrosion Behavior and Wettability of Magnesium–Neodymium Alloy by Self-Layered Chemical Conversion Coating. Materials 2024, 17, 2815. https://doi.org/10.3390/ma17122815
Yang K, Kuang Y, Xu B, Liu C, Wu G. Simultaneously Regulating Electrochemical Corrosion Behavior and Wettability of Magnesium–Neodymium Alloy by Self-Layered Chemical Conversion Coating. Materials. 2024; 17(12):2815. https://doi.org/10.3390/ma17122815
Chicago/Turabian StyleYang, Keke, Yulian Kuang, Bingqian Xu, Changyang Liu, and Guosong Wu. 2024. "Simultaneously Regulating Electrochemical Corrosion Behavior and Wettability of Magnesium–Neodymium Alloy by Self-Layered Chemical Conversion Coating" Materials 17, no. 12: 2815. https://doi.org/10.3390/ma17122815
APA StyleYang, K., Kuang, Y., Xu, B., Liu, C., & Wu, G. (2024). Simultaneously Regulating Electrochemical Corrosion Behavior and Wettability of Magnesium–Neodymium Alloy by Self-Layered Chemical Conversion Coating. Materials, 17(12), 2815. https://doi.org/10.3390/ma17122815







