The Microstructural Evolution and Corrosion Behavior of Zn-Mg Alloys and Hybrids Processed Using High-Pressure Torsion
Abstract
1. Introduction
2. Materials and Methods
2.1. Processing
2.2. Microstructural Characterization
2.3. Corrosion Behavior Evaluation
3. Results
3.1. Microstructure of the Zn-3Mg Alloy after the Homogenization Heat Treatment
3.2. Microstructural of the Alloy and Hybrid as a Function of the Number of Turns and the Mg Content
3.3. Effect of Mg Content on the Corrosion Behavior of HPT Hybrid Samples
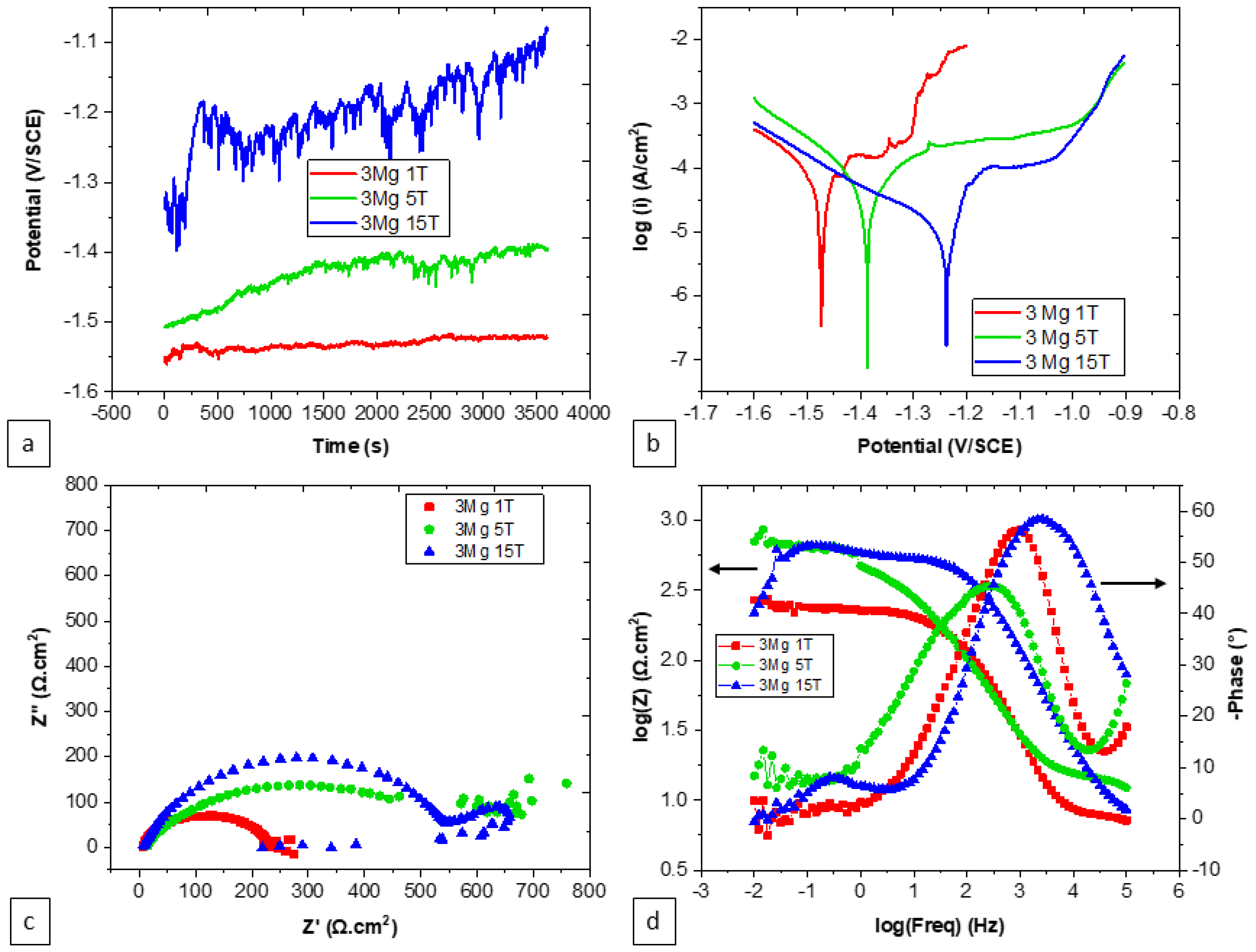
3.4. Effect of Number of Turns on the Corrosion Behavior
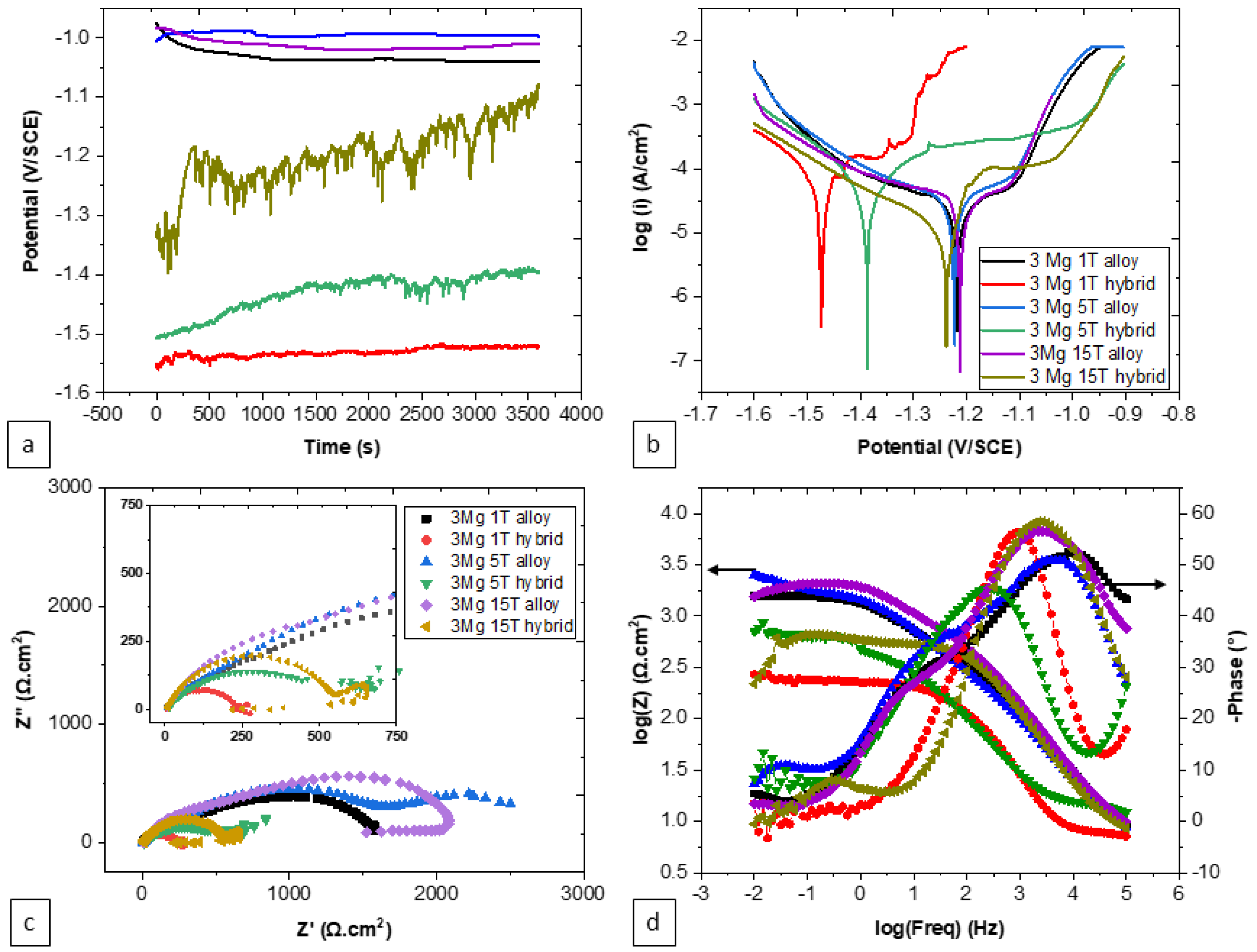
3.5. Effect of Synthesis Approach on the Corrosion Behavior of Zn-3Mg
4. Discussion
4.1. Effect of Homogenization Treatment on the Zn-3Mg Alloy
4.2. Microstructure of the Zn-3Mg HPT Alloys and Hybrids and Effect of Subsequent PDA on the Alloy (All after 30 Turns)
4.3. Processing and Microstructure Effects on the Corrosion Behavior
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grainger, D.W. The Williams dictionary of biomaterials. Mater. Today 1999, 2, 29. [Google Scholar] [CrossRef]
- Katarivas Levy, G.; Goldman, J.; Aghion, E. The prospects of zinc as a structural material for biodegradable implants—A review paper. Metals 2017, 7, 402. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Vedani, M. Zinc-based alloys for degradable vascular stent applications. Acta Biomater. 2018, 71, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, M. Titanium-based biomaterials for preventing stress shielding between implant devices and bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [PubMed]
- Piao, C.; Wu, D.; Luo, M.; Ma, H. Stress shielding effects of two prosthetic groups after total hip joint simulation replacement. J. Orthop. Surg. Res. 2014, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, Y.; Chen, X.H.; Yang, J.A.; Pan, H.; Chen, D.; Wang, L.; Zhang, J.; Zhu, D.; Wu, S.; et al. Fundamental theory of biodegradable metals—Definition, criteria, and design. Adv. Funct. Mater. 2019, 29, 1805402. [Google Scholar] [CrossRef]
- Purnama, A.; Hermawan, H.; Mantovani, D. Biodegradable metal stents: A focused review on materials and clinical studies. J. Biomater. Tissue Eng. 2014, 4, 868–874. [Google Scholar] [CrossRef]
- Francis, A.; Yang, Y.; Virtanen, S.; Boccaccini, A.R. Iron and iron-based alloys for temporary cardiovascular applications. J. Mater. Sci. Mater. Med. 2015, 26, 138. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2015, 23, S28–S40. [Google Scholar] [CrossRef]
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P.; von Schnakenburg, C. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials 2006, 27, 4955–4962. [Google Scholar] [CrossRef]
- Pierson, D.; Edick, J.; Tauscher, A.; Pokorney, E.; Bowen, P.; Gelbaugh, J.; Stinson, J.; Getty, H.; Lee, C.H.; Drelich, J.; et al. A simplified in vivo approach for evaluating the bioabsorbable behavior of candidate stent materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Vojtěch, D.; Kubásek, J.; Šerák, J.; Novák, P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.M.; Rink, L.; Haase, H. The Essential Toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed]
- Champagne, S.; Mostaed, E.; Safizadeh, F.; Ghali, E.; Vedani, M.; Hermawan, H. In vitro degradation of absorbable zinc alloys in artificial urine. Materials 2019, 12, 295. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Tang, Z.; Huang, H.; Pei, J.; Zhang, H.; Yuan, G.; Ding, W. Research on a Zn-Cu alloy as a biodegradable material for potential vascular stents application. Mater. Sci. Eng. C 2016, 69, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Guillory, R.J.; Shearier, E.R.; Seitz, J.-M.; Drelich, J.; Bocks, M.; Zhao, F.; Goldman, J. Metallic Zinc Exhibits Optimal Biocompatibility for Bioabsorbable Endovascular Stents. Mater. Sci. Eng. C 2015, 56, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Paramitha, D.; Chabaud, S.; Bolduc, S.; Hermawan, H. Biological assessment of Zn-based absorbable metals for ureteral stent applications. Materials 2019, 12, 3325. [Google Scholar] [CrossRef]
- Gong, H.; Wang, K.; Strich, R.; Zhou, J.G. In vitro biodegradation behavior, mechanical properties, and cytotoxicity of biodegradable Zn-Mg alloy. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103B, 1632–1640. [Google Scholar] [CrossRef]
- Prosek, T.; Nazarov, A.; Bexell, U.; Thierry, D.; Serak, J. Corrosion mechanism of model zinc-magnesium alloys in atmospheric conditions. Corros. Sci. 2008, 50, 2216–2231. [Google Scholar] [CrossRef]
- Hall, E.O. Variation of hardness of metals with grain size. Nature 1954, 173, 948–949. [Google Scholar] [CrossRef]
- Estrin, Y.; Vinogradov, A. Extreme grain refinement by severe plastic deformation: A wealth of challenging science. Acta Mater. 2013, 61, 782–817. [Google Scholar] [CrossRef]
- Iwahashi, Y.; Wang, J.; Horita, Z.; Nemoto, M.; Langdon, T.G. Principle of equal-channel angular pressing for the processing of ultra-fine grained materials. Scr. Mater. 1996, 35, 143–146. [Google Scholar] [CrossRef]
- Iwahashi, Y.; Horita, Z.; Nemoto, M.; Langdon, T.G. The process of grain refinement in equal-channel angular pressing. Acta Mater. 1998, 46, 3317–3331. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Langdon, T.G. Principles of equal-channel angular pressing as a processing tool for grain refinement. Prog. Mater. Sci. 2006, 51, 881–981. [Google Scholar] [CrossRef]
- Zhilyaev, A.P.; Langdon, T.G. Using high-pressure torsion for metal processing: Fundamentals and applications. Prog. Mater. Sci. 2008, 53, 893–979. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Islamgaliev, R.K.; Alexandrov, I.V. Bulk nanostructured materials from severe plastic deformation. Prog. Mater. Sci. 2000, 45, 103–189. [Google Scholar] [CrossRef]
- Han, J.-K.; Jang, J.; Langdon, T.G.; Kawasaki, M. Bulk-state reactions and improving the mechanical properties of metals through high-pressure torsion. Mater. Trans. 2019, 60, 1131–1138. [Google Scholar] [CrossRef]
- Edalati, K.; Horita, Z. High-pressure torsion of pure metals: Influence of atomic bond parameters and stacking fault energy on grain size and correlation with hardness. Acta Mater. 2011, 59, 6831–6836. [Google Scholar] [CrossRef]
- Edalati, K.; Horita, Z. Significance of homologous temperature in softening behavior and grain size of pure metals processed by high-pressure torsion. Mater. Sci. Eng. A 2011, 528, 7514–7523. [Google Scholar] [CrossRef]
- Maury, N.; Zhang, N.X.; Huang, Y.; Zhilyaev, A.P.; Langdon, T.G. A critical examination of pure tantalum processed by high-pressure torsion. Mater. Sci. Eng. A 2015, 638, 174–182. [Google Scholar] [CrossRef]
- Ito, Y.; Horita, Z. Microstructural evolution in pure aluminum processed by high-pressure torsion. Mater. Sci. Eng. A 2009, 503, 32–36. [Google Scholar] [CrossRef]
- Zhilyaev, A.P.; Lee, S.; Nurislamova, G.V.; Valiev, R.Z.; Langdon, T.G. Microhardness and microstructural evolution in pure nickel during high-pressure torsion. Scr. Mater. 2001, 44, 2753–2758. [Google Scholar] [CrossRef]
- Edalati, K.; Yamamoto, A.; Horita, Z.; Ishihara, T. High-pressure torsion of pure magnesium: Evolution of mechanical properties, microstructures and hydrogen storage capacity with equivalent strain. Scr. Mater. 2011, 64, 880–883. [Google Scholar] [CrossRef]
- Edalati, K.; Matsubara, E.; Horita, Z. Processing pure Ti by high-pressure torsion in wide ranges of pressures and strain. Metall. Mater. Trans. A 2009, 40, 2079–2086. [Google Scholar] [CrossRef]
- Srinivasarao, B.; Zhilyaev, A.P.; Langdon, T.G.; Pérez-Prado, M.T. On the relation between the microstructure and the mechanical behavior of pure Zn processed by high pressure torsion. Mater. Sci. Eng. A 2013, 562, 196–202. [Google Scholar] [CrossRef]
- Wang, Y.C.; Langdon, T.G. Effect of heat treatment on microstructure and microhardness evolution in a Ti-6Al-4V alloy processed by high-pressure torsion. J. Mater. Sci. 2013, 48, 4646–4652. [Google Scholar] [CrossRef]
- Bryła, K.; Morgiel, J.; Faryna, M.; Edalati, K.; Horita, Z. Effect of high-pressure torsion on grain refinement, strength enhancement and uniform ductility of EZ magnesium alloy. Mater. Lett. 2018, 212, 323–326. [Google Scholar] [CrossRef]
- Torbati-Sarraf, S.A.; Sabbaghianrad, S.; Figueiredo, R.B.; Langdon, T.G. Orientation imaging microscopy and microhardness in a ZK60 magnesium alloy processed by high-pressure torsion. J. Alloys Compd. 2017, 712, 185–193. [Google Scholar] [CrossRef]
- Loucif, A.; Figueiredo, R.B.; Kawasaki, M.; Baudin, T.; Brisset, F.; Chemam, R.; Langdon, T.G. Effect of aging on microstructural development in an Al-Mg-Si alloy processed by high-pressure torsion. J. Mater. Sci. 2012, 47, 7815–7820. [Google Scholar] [CrossRef]
- Oh-Ishi, K.; Edalati, K.; Kim, H.S.; Hono, K.; Horita, Z. High-pressure torsion for enhanced atomic diffusion and promoting solid-state reactions in the aluminum-copper system. Acta Mater. 2013, 61, 3482–3489. [Google Scholar] [CrossRef]
- Han, J.-K.; Han, D.K.; Liang, G.Y.; Jang, J.-I.; Langdon, T.G.; Kawasaki, M. Direct bonding of aluminum-copper metals through high-pressure torsion processing. Adv. Eng. Mater. 2018, 20, 1800642. [Google Scholar] [CrossRef]
- Danilenko, V.N.; Sergeev, S.N.; Baimova, J.A.; Korznikova, G.F.; Nazarov, K.S.; Khisamov, R.K.; Glezer, A.M.; Mulyukov, R.R. An approach for fabrication of Al-Cu composite by high pressure torsion. Mater. Lett. 2019, 236, 51–55. [Google Scholar] [CrossRef]
- Kawasaki, M.; Ahn, B.; Lee, H.; Zhilyaev, A.P.; Langdon, T.G. Using high-pressure torsion to process an aluminum-magnesium nanocomposite through diffusion bonding. J. Mater. Res. 2016, 31, 88–99. [Google Scholar] [CrossRef]
- Han, J.-K.; Lee, H.-J.; Jang, J.-I.; Kawasaki, M.; Langdon, T.G. Micro-mechanical and tribological properties of aluminum-magnesium nanocomposites processed by high-pressure torsion. Mater. Sci. Eng. A 2017, 684, 318–327. [Google Scholar] [CrossRef]
- Kawasaki, M.; Han, J.-K.; Lee, D.-H.; Jang, J.; Langdon, T.G. Fabrication of nanocomposites through diffusion bonding under high-pressure torsion. J. Mater. Res. 2018, 33, 2700–2710. [Google Scholar] [CrossRef]
- Hernández-Escobar, D.; Rahman, Z.U.; Yilmazer, H.; Kawasaki, M.; Boehlert, C.J. Microstructural evolution and intermetallic formation in Zn Mg hybrids processed by High Pressure Torsion. Philos. Mag. 2019, 99, 557–584. [Google Scholar] [CrossRef]
- Hernández-Escobar, D.; Marcus, J.; Han, J.-K.; Unocic, R.R.; Kawasaki, M.; Boehlert, C.J. Effect of post-deformation annealing on the microstructure and micro-mechanical behavior of Zn-Mg hybrids processed by High-Pressure Torsion. Mater. Sci. Eng. A 2019, 771, 138578. [Google Scholar] [CrossRef]
- Hernández-Escobar, D. Composition-Processing-Microstructure-Property Relationships of the Zinc-Magnesium System for Absorbable Biomedical Implant Applications. Ph.D. Thesis, Michigan State University, East Lansing, MI, USA, 2021. [Google Scholar]
- Ibrahim, N.; Peterlechner, M.; Emeis, F.; Wegner, M.; Divinski, S.V.; Wilde, G. Mechanical alloying via high-pressure torsion of the immiscible Cu50Ta50 system. Mater. Sci. Eng. A 2017, 685, 19–30. [Google Scholar] [CrossRef]
- Qi, Y.; Kosinova, A.; Kilmametov, A.R.; Straumal, B.B.; Rabkin, E. Plastic flow and microstructural instabilities during high-pressure torsion of Cu/ZnO composites. Mater. Charact. 2018, 145, 389–401. [Google Scholar] [CrossRef]
- Rahman, T.; Yilmazer, H.; Dikici, B.; Edalati, K.; Poplawsky, J.D.; Boehlert, C.J. Microstructural Evolution and Intermetallic Formation in Zn-3Mg (wt.%) Powder Mixture Processed by High-Pressure Torsion. J. Alloys Compd. 2023, 968, 172101. [Google Scholar] [CrossRef]
- Ralston, K.; Birbilis, N. Effect of grain size on corrosion: A review. Corrosion 2010, 66, 075005-13. [Google Scholar] [CrossRef]
- Youssef, K.M.S.; Koch, C.C.; Fedkiw, P.S. Improved corrosion behavior of nanocrystalline zinc produced by pulse-current electrodeposition. Corros. Sci. 2004, 46, 51–64. [Google Scholar] [CrossRef]
- Osório, W.R.; Freire, C.M.; Garcia, A. The role of macrostructural morphology and grain size on the corrosion resistance of Zn and Al castings. Mater. Sci. Eng. A 2005, 402, 22–32. [Google Scholar] [CrossRef]
- Figueiredo, R.B.; Cetlin, P.R.; Langdon, T.G. Using finite element modeling to examine the flow processes in quasi-constrained high-pressure torsion. Mater. Sci. Eng. A 2011, 528, 8198–8204. [Google Scholar] [CrossRef]
- Figueiredo, R.B.; Pereira, P.H.R.; Aguilar, M.T.P.; Cetlin, P.R.; Langdon, T.G. Using finite element modeling to examine the temperature distribution in quasi-constrained high-pressure torsion. Acta Mater. 2012, 60, 3190–3198. [Google Scholar] [CrossRef]
- Edalati, K.; Hashiguchi, Y.; Pereira, P.H.R.; Horita, Z.; Langdon, T.G. Effect of temperature rise on microstructural evolution during high-pressure torsion. Mater. Sci. Eng. A 2018, 714, 167–171. [Google Scholar] [CrossRef]
- Hernández-Escobar, D.; Unocic, R.R.; Kawasaki, M.; Boehlert, C.J. High-pressure torsion processing of Zn-3Mg alloy and its hybrid counterpart: A comparative study. J. Alloys Compd. 2020, 831, 154891. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Mostaed, A.; Loffredo, S.; Demir, A.G.; Previtali, B.; Vedani, M. Novel Zn-based alloys for biodegradable stent applications: Design, development and in vitro degradation. J. Mech. Behav. Biomed. Mater. 2016, 60, 581–602. [Google Scholar] [CrossRef]
- Yao, C.; Tay, S.L.; Zhu, T.; Shang, H.; Gao, W. Effects of Mg content on microstructure and electrochemical properties of Zn-Al-Mg alloys. J. Alloys Compd. 2015, 645, 131–136. [Google Scholar] [CrossRef]


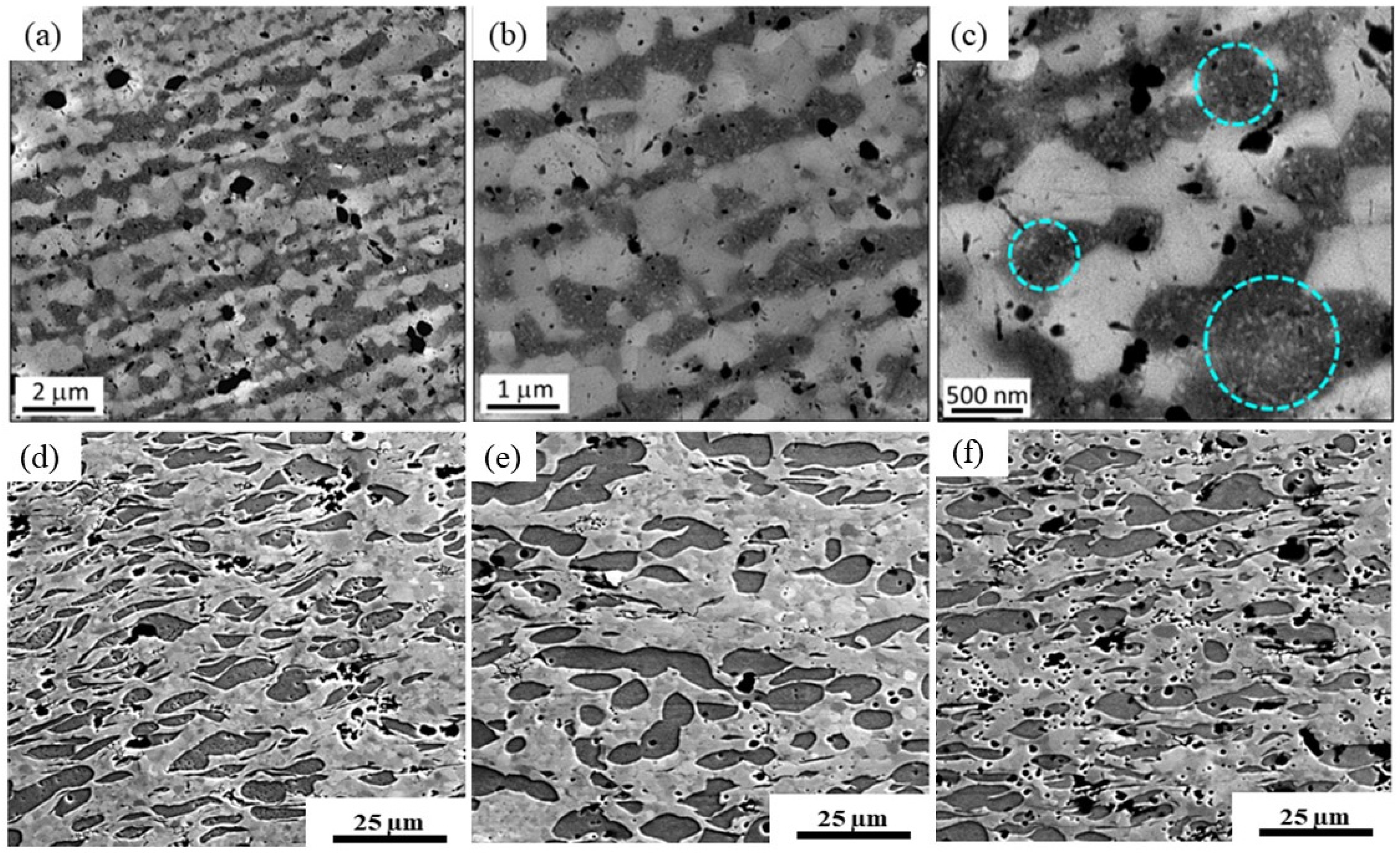

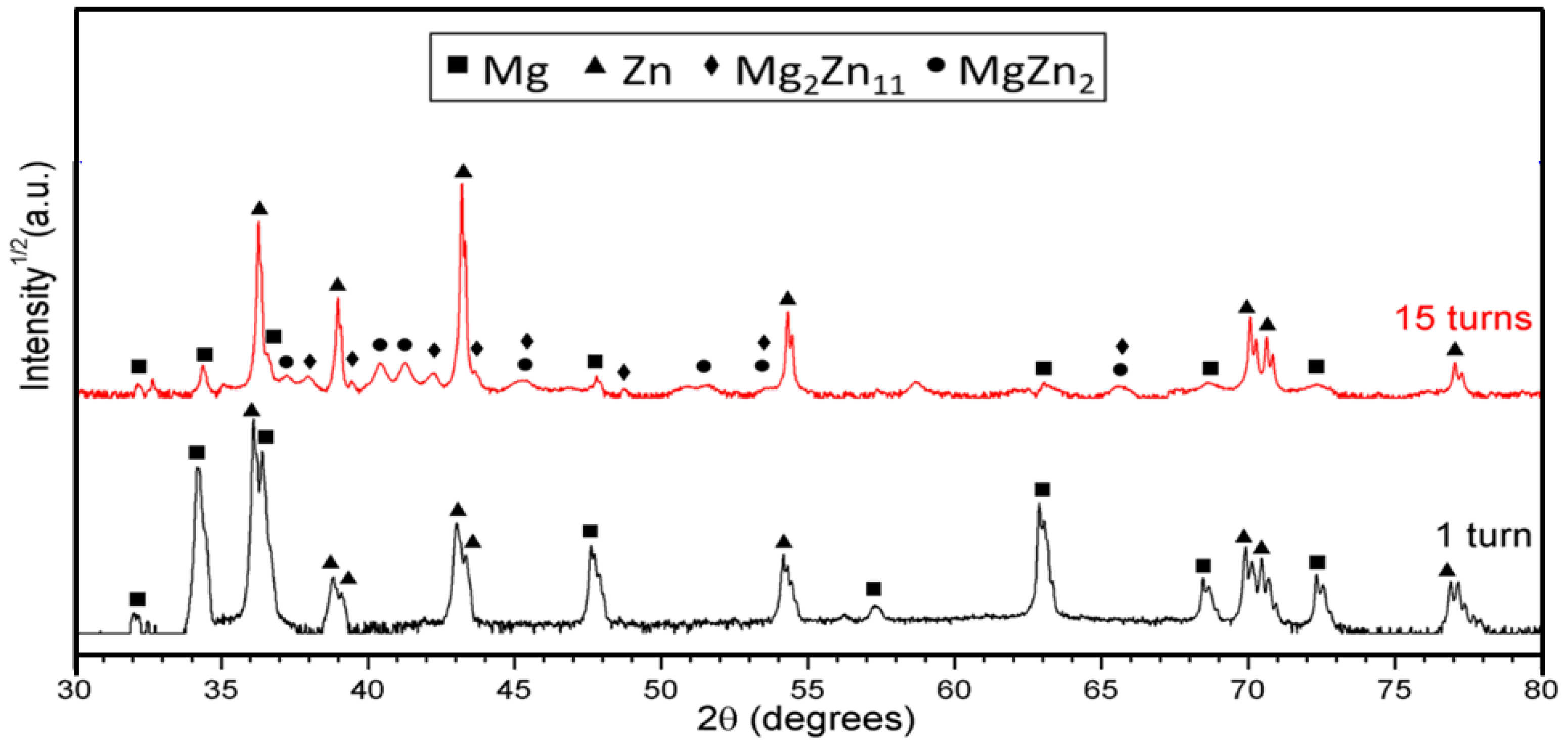
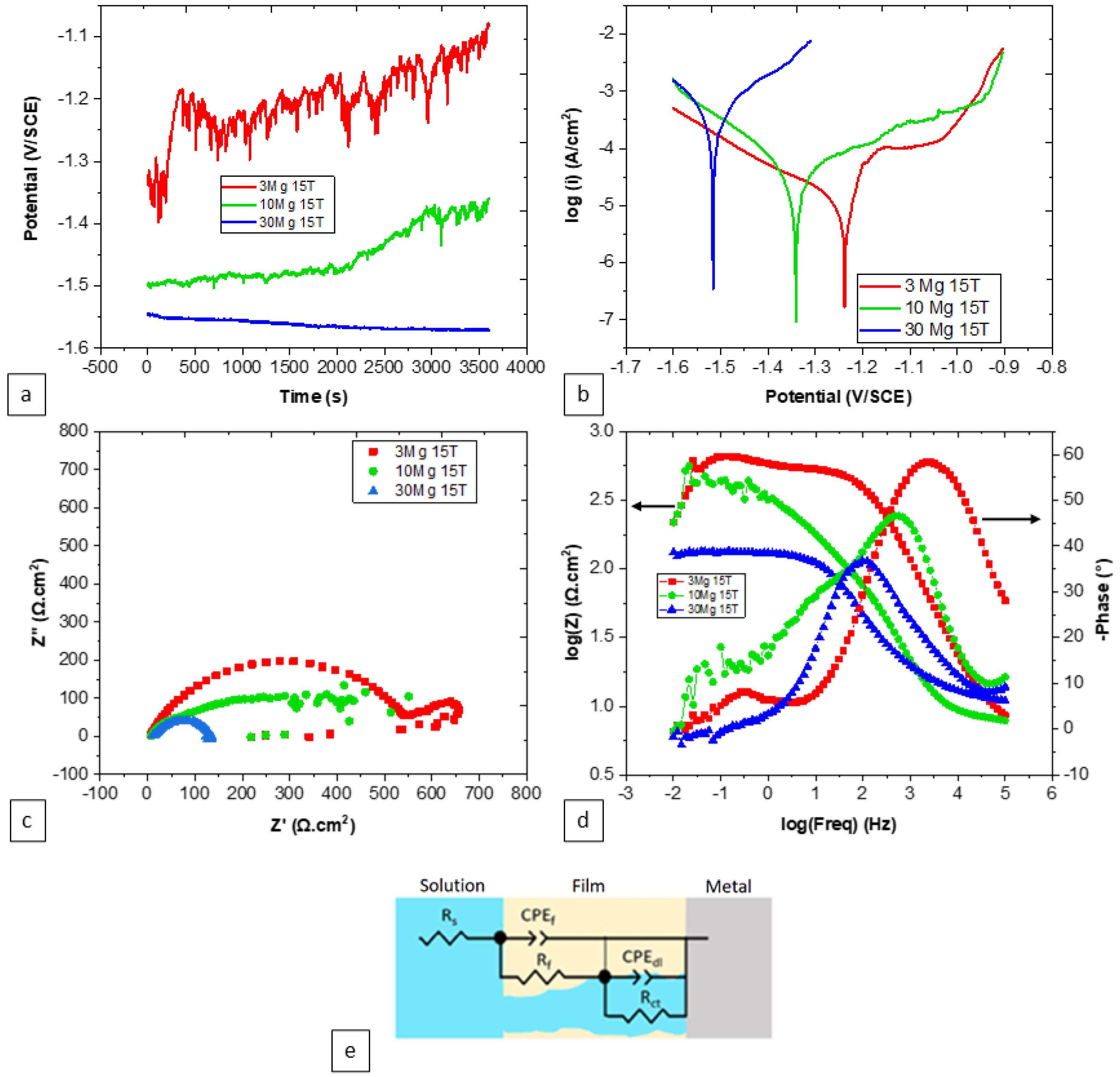
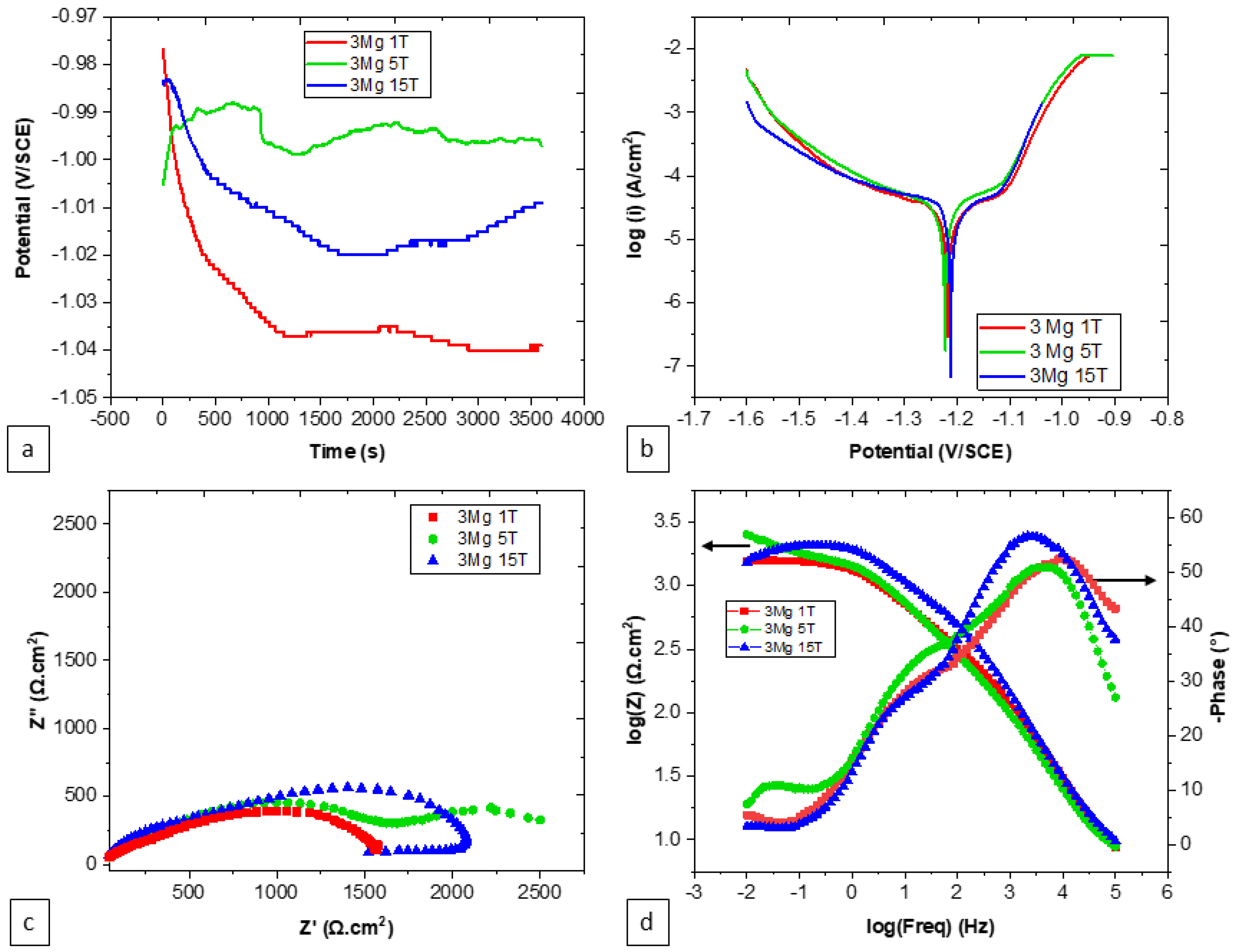
| Number of Turns | Zn | Mg | Mg2Zn11 | MgZn2 |
|---|---|---|---|---|
| (wt. %) | (at. %) | (wt. %) | (at. %) | |
| 1 turn | 18.6 ± 0.5 | 81.4 ± 0.5 | 0 | 0 |
| 15 turns | 57.6 ± 0.4 | 28.0 ± 0.3 | 1.6 ± 0.2 | 12.8 ± 0.4 |
| Composi-tion | E (V vs. SCE) | i (μA·cm−2) | Rs (Ω·cm2) | Qf (F·cm−2·s(n−1)) | nf | Rf (Ω·cm2) | Qdl (F·cm−2·s(n−1)) | ndl | Rct (Ω·cm2) |
|---|---|---|---|---|---|---|---|---|---|
| Zn-3Mg | −1.19 ± 48 × 10−3 | 13.4 ± 5.5 | 6.89 | 80.57 × 10−6 | 0.18 | 3.9 | 2.94 × 10−6 | 1 | 936 |
| Zn-10Mg | −1.35 ± 8 × 10−3 | 40 ± 3.2 | 7.8 | 8.08 × 10−6 | 0.77 | 116 | 71.02 × 10−6 | 0.62 | 314 |
| Zn-30Mg | −1.51 ± 4 × 10−3 | 358.3 ± 30.2 | 5.59 | 0.24 × 10−6 | 0.8 | 5.46 | 4.32 × 10−6 | 0.76 | 127 |
| Number of Turns | E (V vs. SCE) | i (μA·cm−2) | Rs (Ω·cm2) | Qf (F·cm−2·s(n−1)) | nf | Rf (Ω·cm2) | Qdl (F·cm−2·s(n−1)) | ndl | Rct (Ω·cm2) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | −1.21 ± 6 × 10−3 | 20.4 ± 2.6 | 10.66 | 8.38 × 10−9 | 0.9 | 391 | 44.12 × 10−6 | 0.6 | 1300 |
| 5 | −1.20 ± 4 × 10−3 | 17.6 ± 4.0 | 7.8 | 90.17 × 10−9 | 0.7 | 1430 | 16.18 × 10−6 | 0.55 | 1170 |
| 15 | −1.19 ± 4 × 10−3 | 24.7 ± 9.3 | 6.5 | 4.08 × 10−9 | 0.97 | 325 | 80.5 × 10−9 | 0.83 | 1638 |
| Number of Turns | E (V vs. SCE) | i (μA·cm−2) | Rs (Ω·cm2) | Qf (F·cm−2·s(n−1)) | nf | Rf (Ω·cm2) | Qdl (F·cm−2·s(n−1)) | ndl | Rct (Ω·cm2) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | −1.48 ± 6 × 10−3 | 94.6 ± 16.4 | 6.63 | 8 × 10−6 | 0.73 | 3.25 | 55.4 × 10−9 | 1 | 213 |
| 5 | −1.37 ± 11 × 10−3 | 34.6 ± 2.9 | 6 | 26.17 × 10−9 | 0.9 | 14.36 | 14.58 × 10−6 | 0.69 | 457 |
| 15 | −1.19 ± 48 × 10−3 | 13.4 ± 5.5 | 6.89 | 80.57 × 10−6 | 0.18 | 3.9 | 2.94 × 10−6 | 1 | 936 |
| Material, Number of Turns | E (V vs. SCE) | i (μA·cm−2) | Rs (Ω·cm2) | Qf (F·cm−2·s(n−1)) | nf | Rf (Ω·cm2) | Qdl (F·cm−2·s(n−1)) | ndl | Rct (Ω·cm2) |
|---|---|---|---|---|---|---|---|---|---|
| Alloy, 1 | −1.21 ± 6 × 10−3 | 20.4 ± 2.6 | 10.66 | 8.38 × 10−9 | 0.9 | 391 | 44.12 × 10−6 | 0.6 | 1300 |
| Hybrid, 1 | −1.48 ± 6 × 10−3 | 94.6 ± 16.4 | 6.63 | 8 × 10−6 | 0.73 | 3.25 | 55.4 × 10−9 | 1 | 213 |
| Alloy, 5 | −1.20 ± 4 × 10−3 | 17.6 ± 4.0 | 7.8 | 90.17 × 10−9 | 0.7 | 1430 | 16.18 × 10−6 | 0.55 | 1170 |
| Hybrid, 5 | −1.37 ± 11 × 10−3 | 34.6 ± 2.9 | 6 | 26.17 × 10−9 | 0.9 | 14.36 | 14.58 × 10−6 | 0.69 | 457 |
| Alloy, 15 | −1.19 ± 4 × 10−3 | 24.7 ± 9.3 | 6.5 | 4.08 × 10−9 | 0.97 | 325 | 80.5 × 10−9 | 0.83 | 1638 |
| Hybrid, 15 | −1.19 ± 48 × 10−3 | 13.4 ± 5.5 | 6.89 | 80.57 × 10−6 | 0.18 | 3.9 | 2.94 × 10−6 | 1 | 936 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanji, A.; Hermawan, H.; Boehlert, C.J. The Microstructural Evolution and Corrosion Behavior of Zn-Mg Alloys and Hybrids Processed Using High-Pressure Torsion. Materials 2024, 17, 270. https://doi.org/10.3390/ma17010270
Tanji A, Hermawan H, Boehlert CJ. The Microstructural Evolution and Corrosion Behavior of Zn-Mg Alloys and Hybrids Processed Using High-Pressure Torsion. Materials. 2024; 17(1):270. https://doi.org/10.3390/ma17010270
Chicago/Turabian StyleTanji, Ayoub, Hendra Hermawan, and Carl J. Boehlert. 2024. "The Microstructural Evolution and Corrosion Behavior of Zn-Mg Alloys and Hybrids Processed Using High-Pressure Torsion" Materials 17, no. 1: 270. https://doi.org/10.3390/ma17010270
APA StyleTanji, A., Hermawan, H., & Boehlert, C. J. (2024). The Microstructural Evolution and Corrosion Behavior of Zn-Mg Alloys and Hybrids Processed Using High-Pressure Torsion. Materials, 17(1), 270. https://doi.org/10.3390/ma17010270







