Technological Advances and Market Developments of Solid-State Batteries: A Review
Abstract
1. Introduction
2. Solid-State Electrolyte Materials
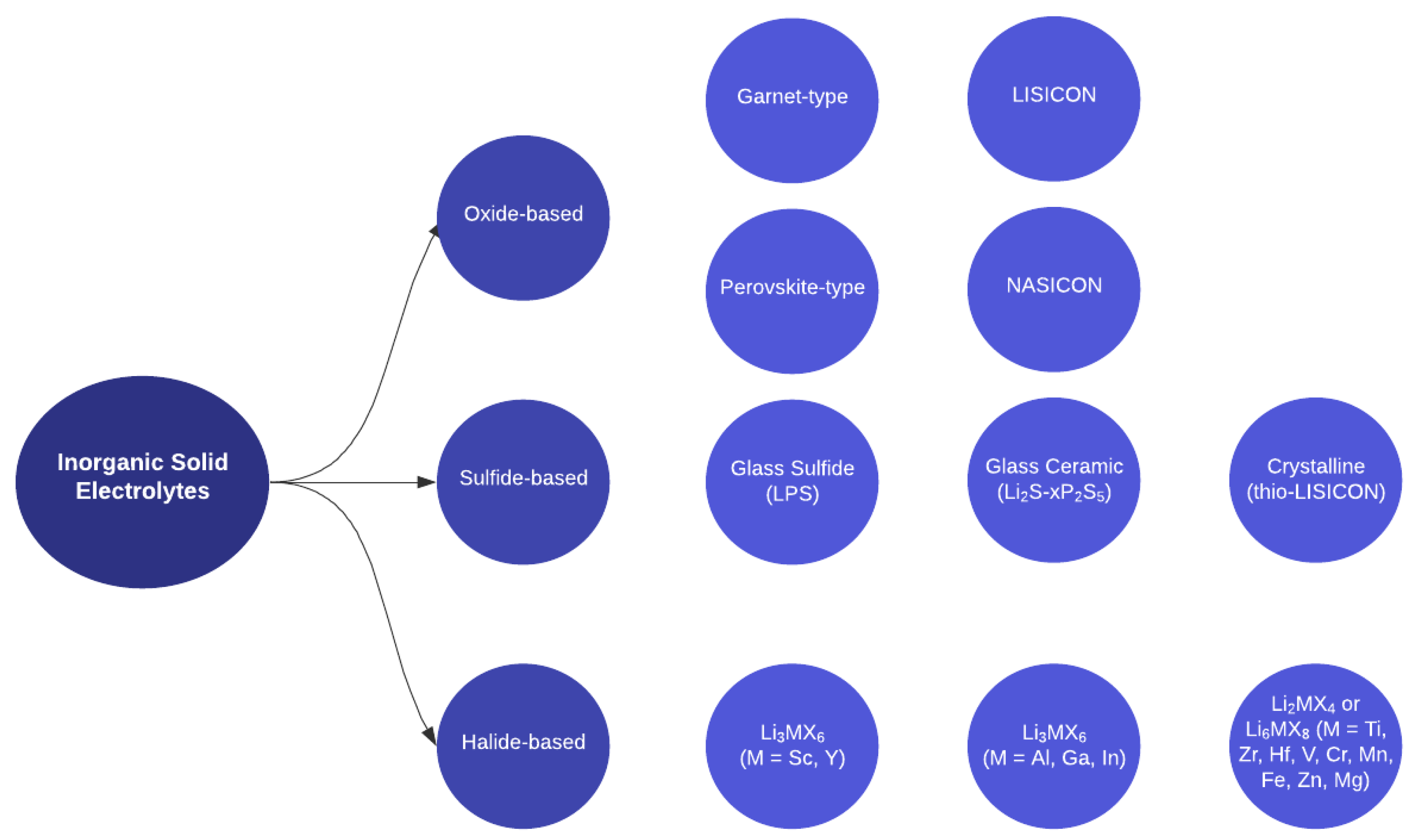
2.1. Inorganic Solid Electrolytes
2.1.1. Oxide-Based ISEs
2.1.2. Sulfide-Based ISEs
2.1.3. Halide-Based ISEs
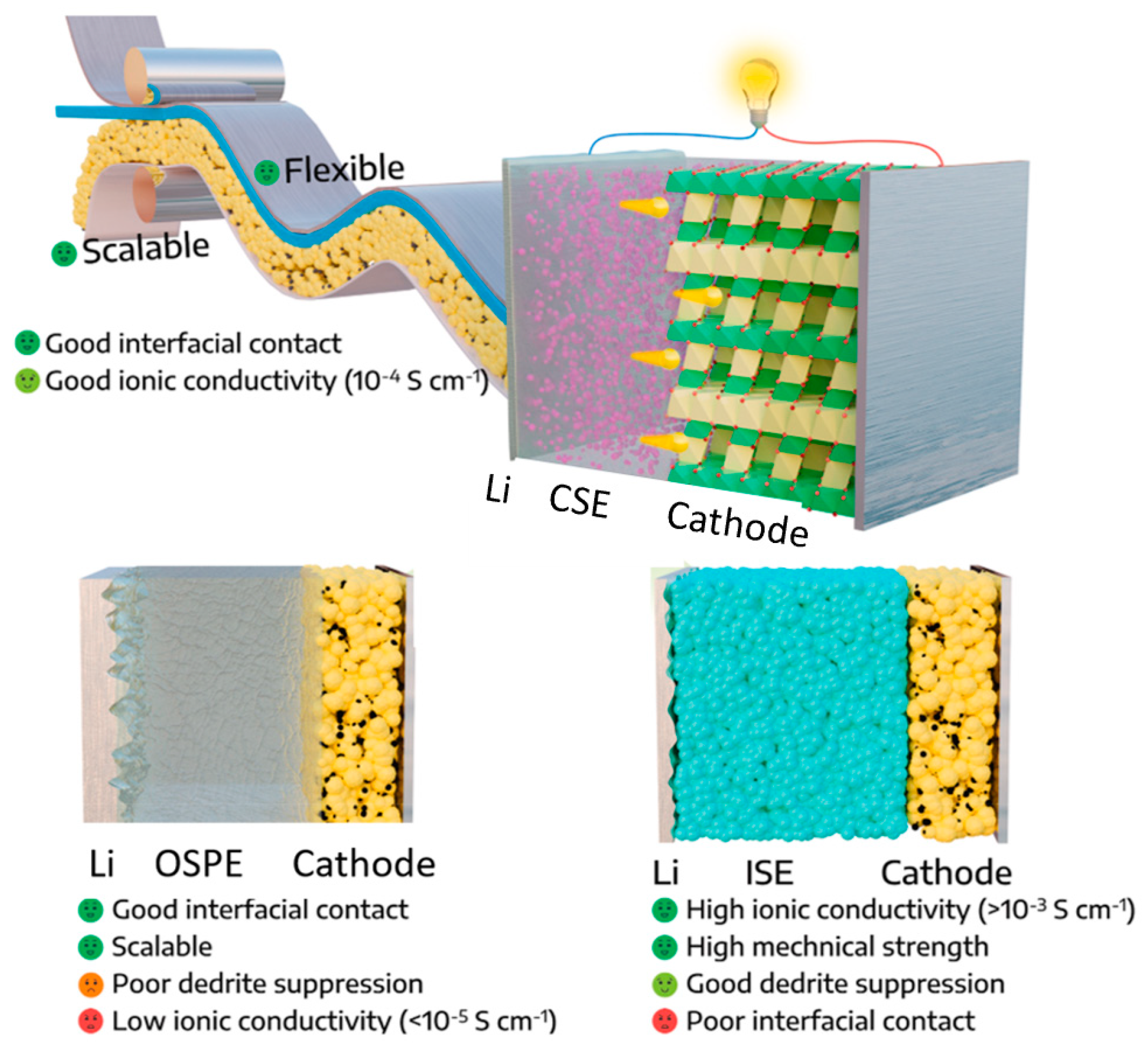
2.2. Organic Solid Polymer Electrolytes (OSPEs)
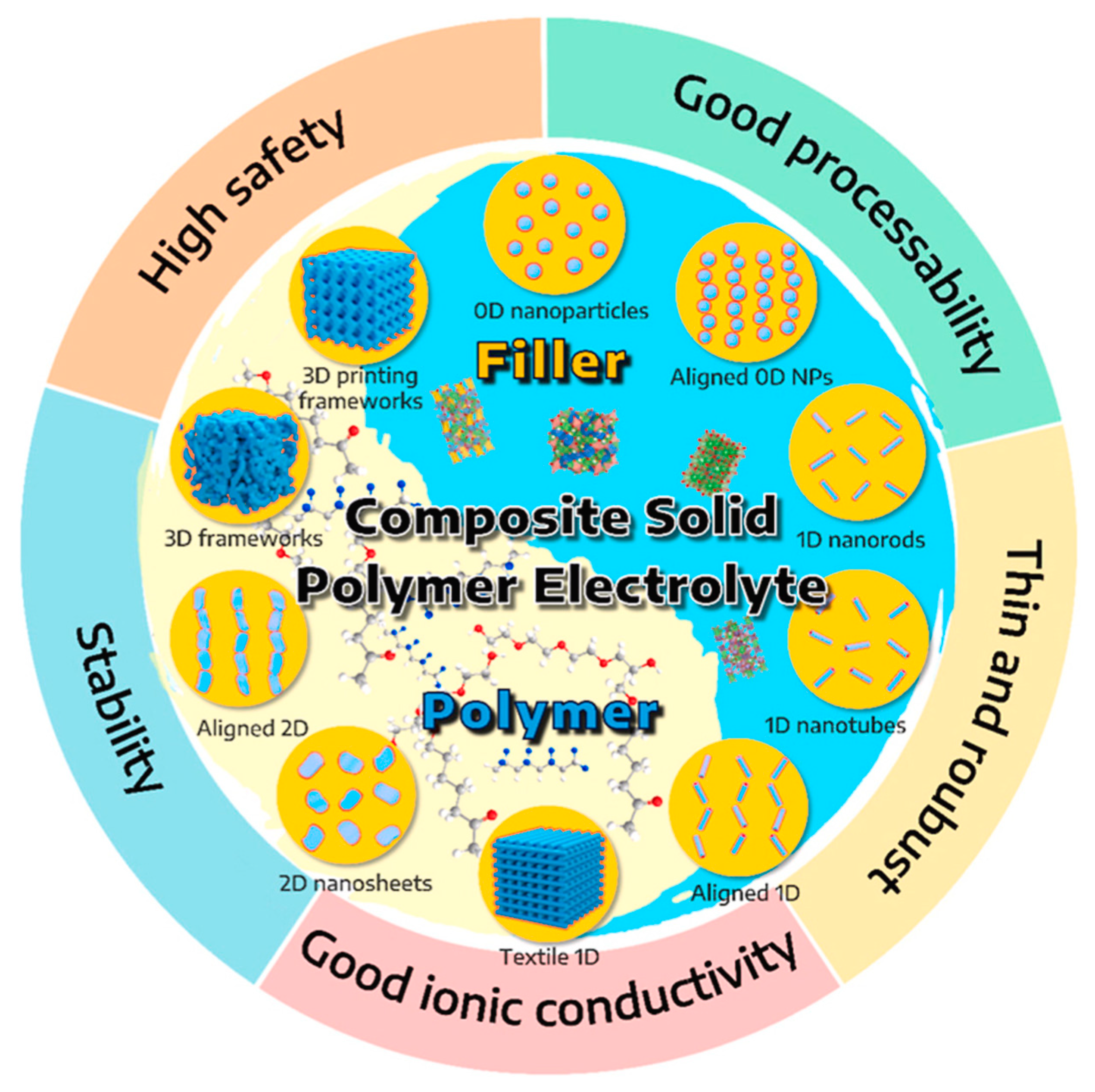
2.3. Composite Solid Electrolytes (CSEs)
3. Electrode Materials for SSBs
3.1. Anode
3.2. Cathode
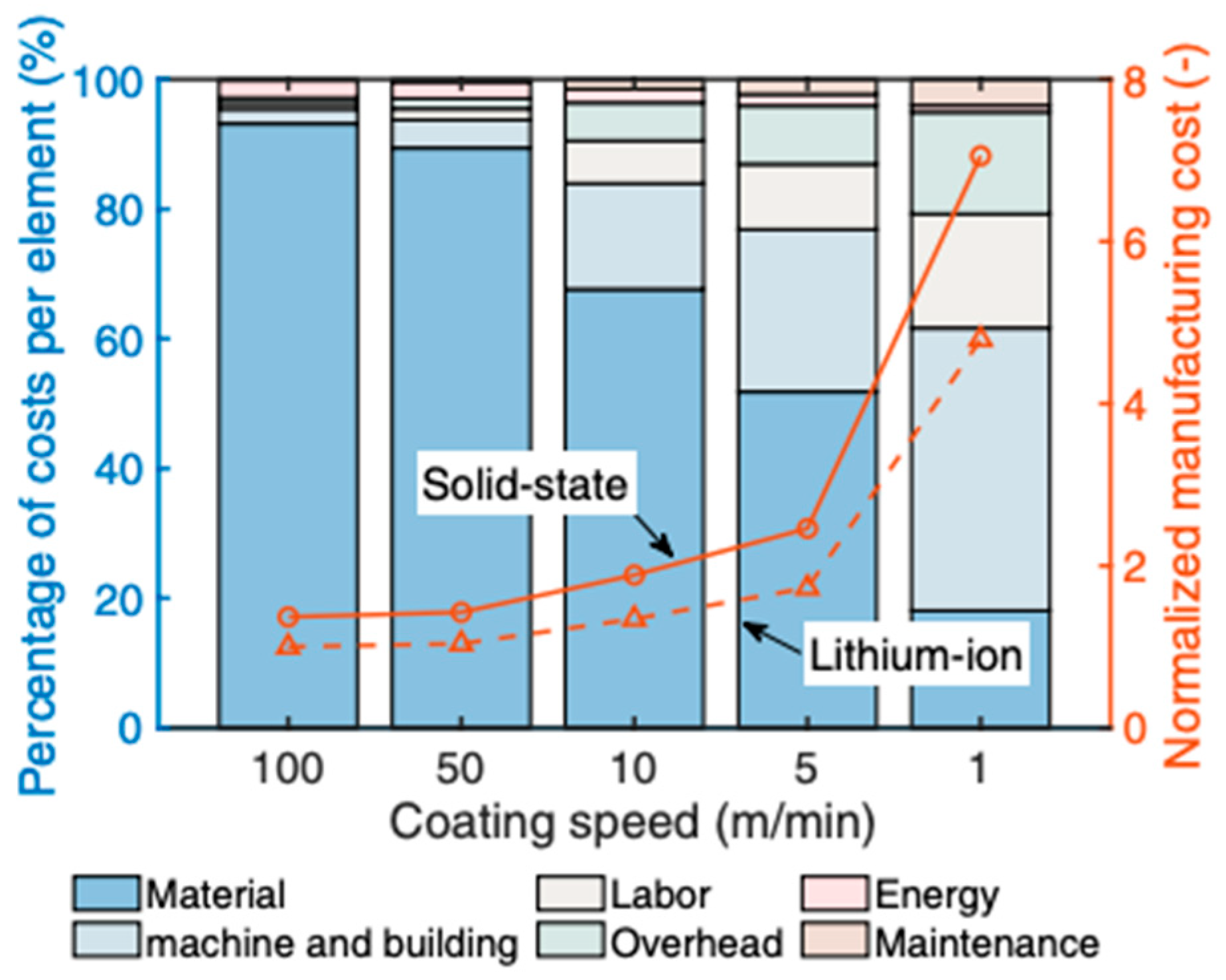
4. Additive Manufacturing of SSBs
5. Solid-State Battery Market
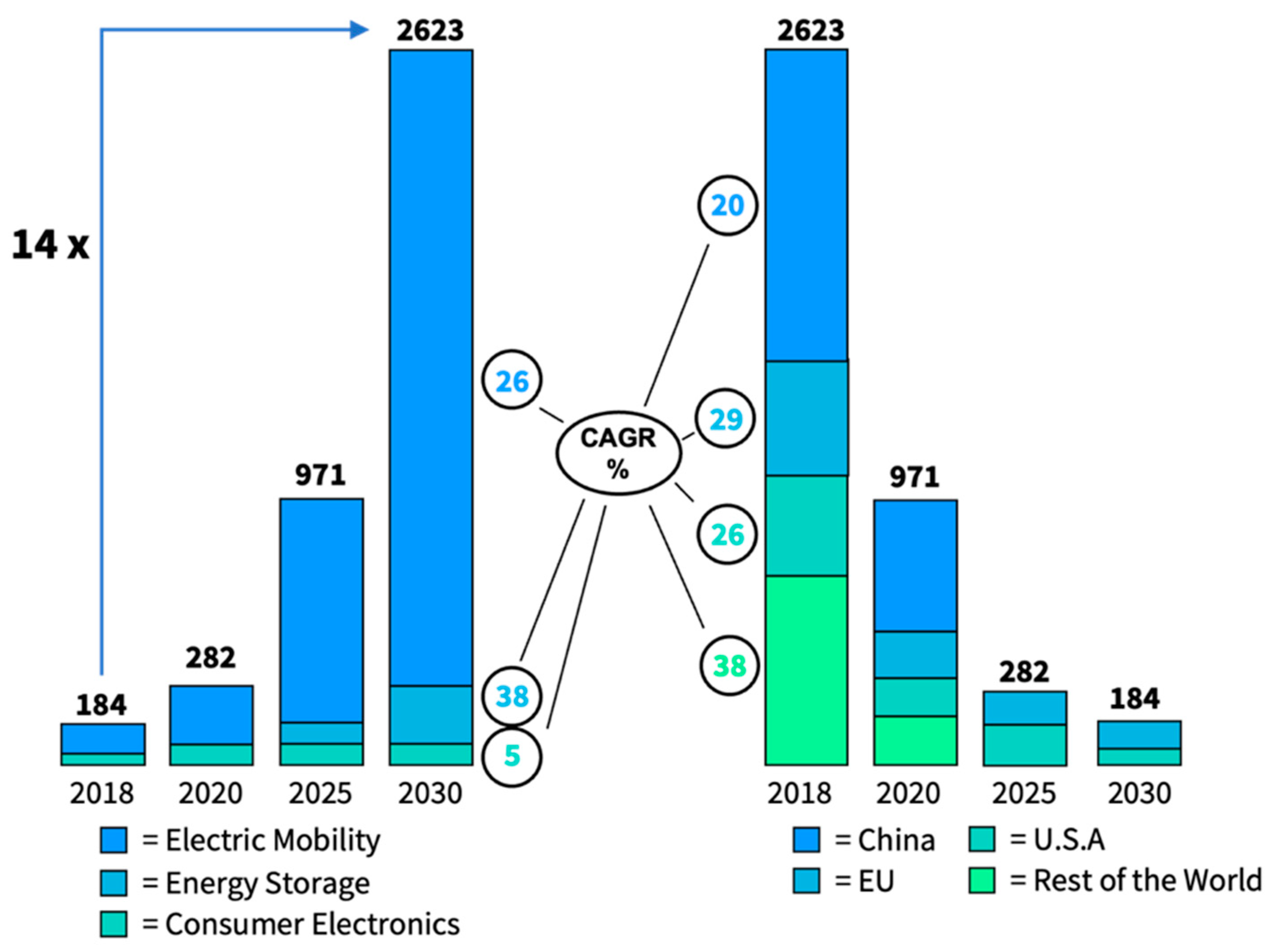
5.1. Market Overview
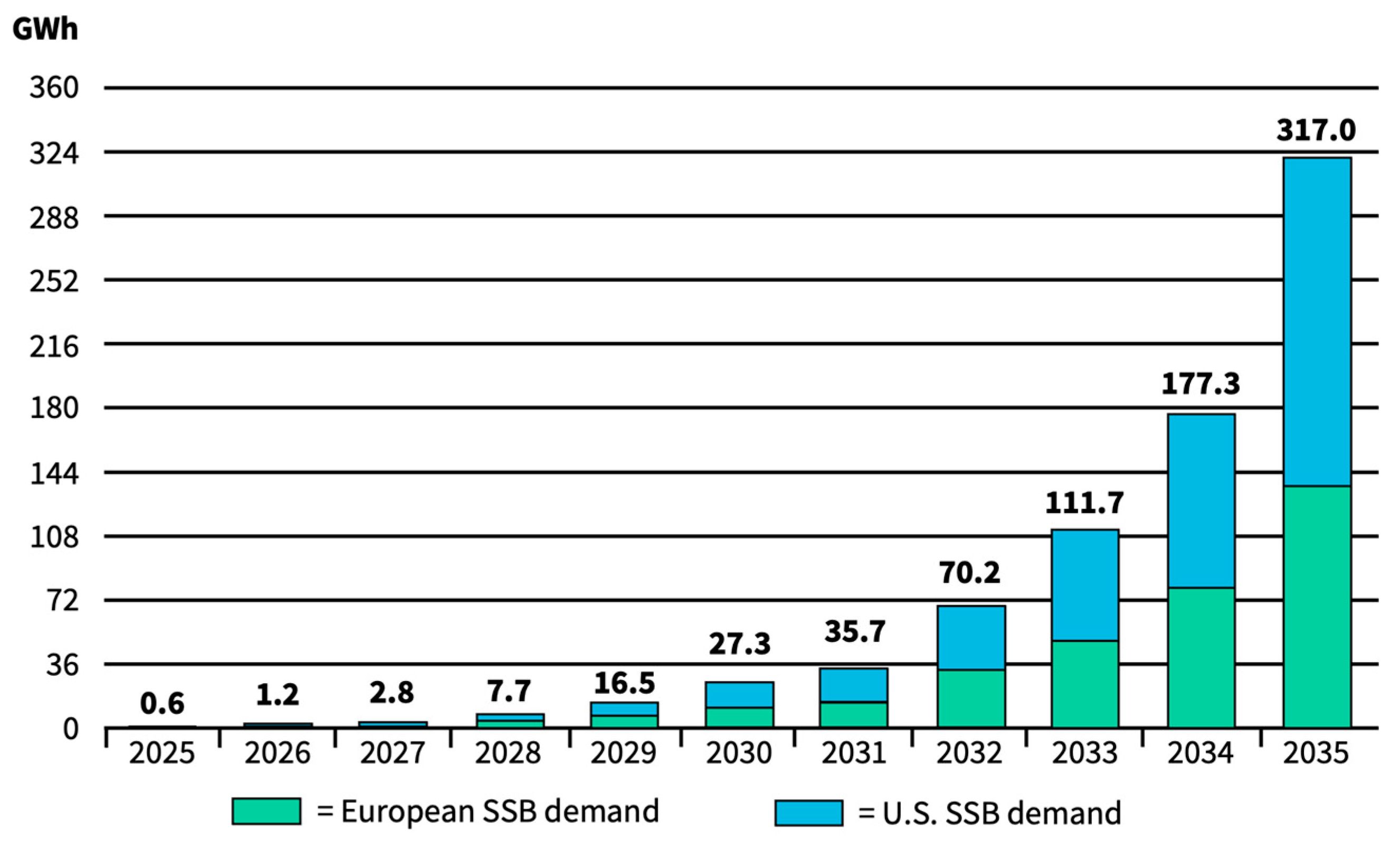
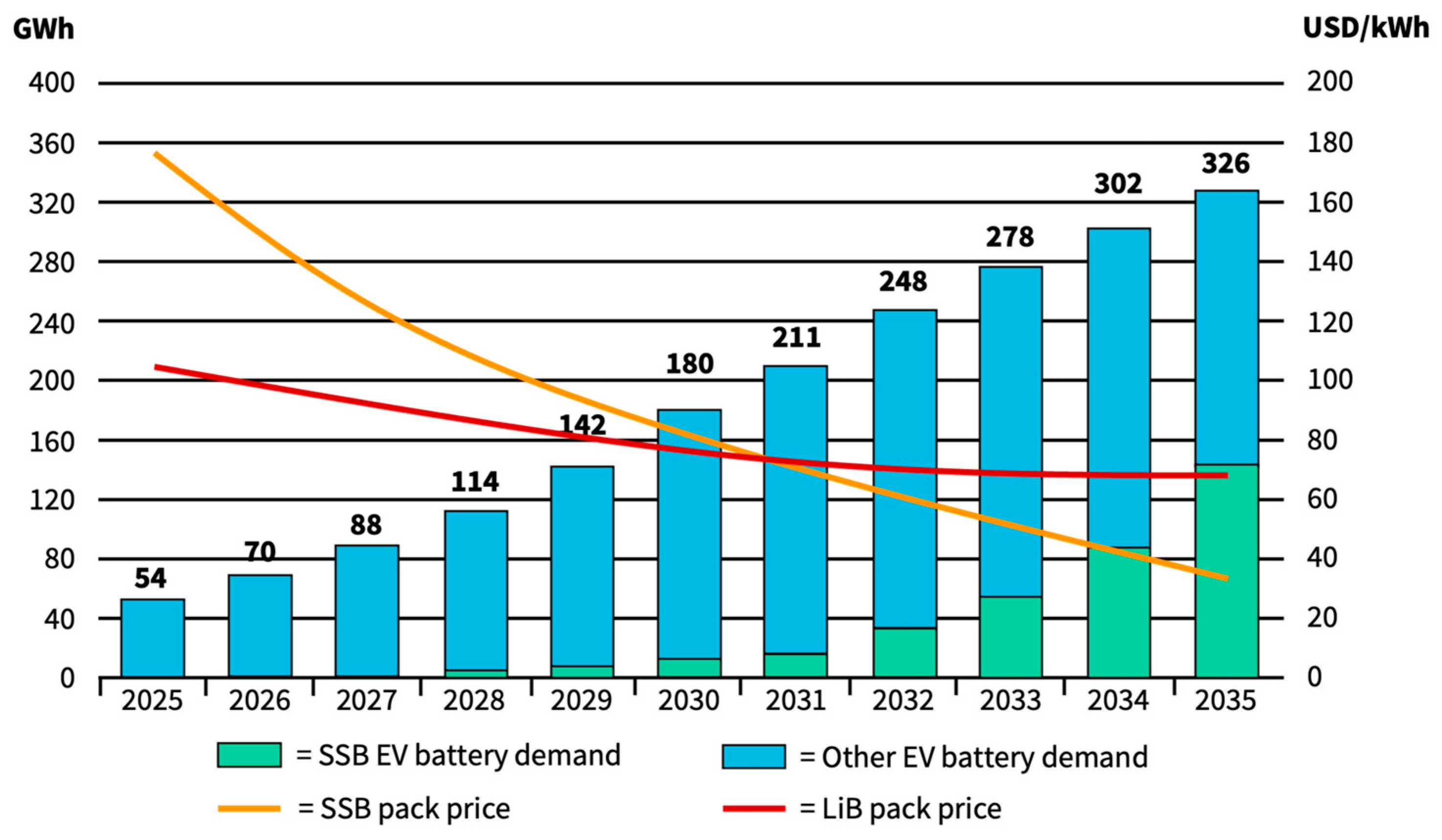
5.2. SSB Market Size
5.3. Economics of SSBs
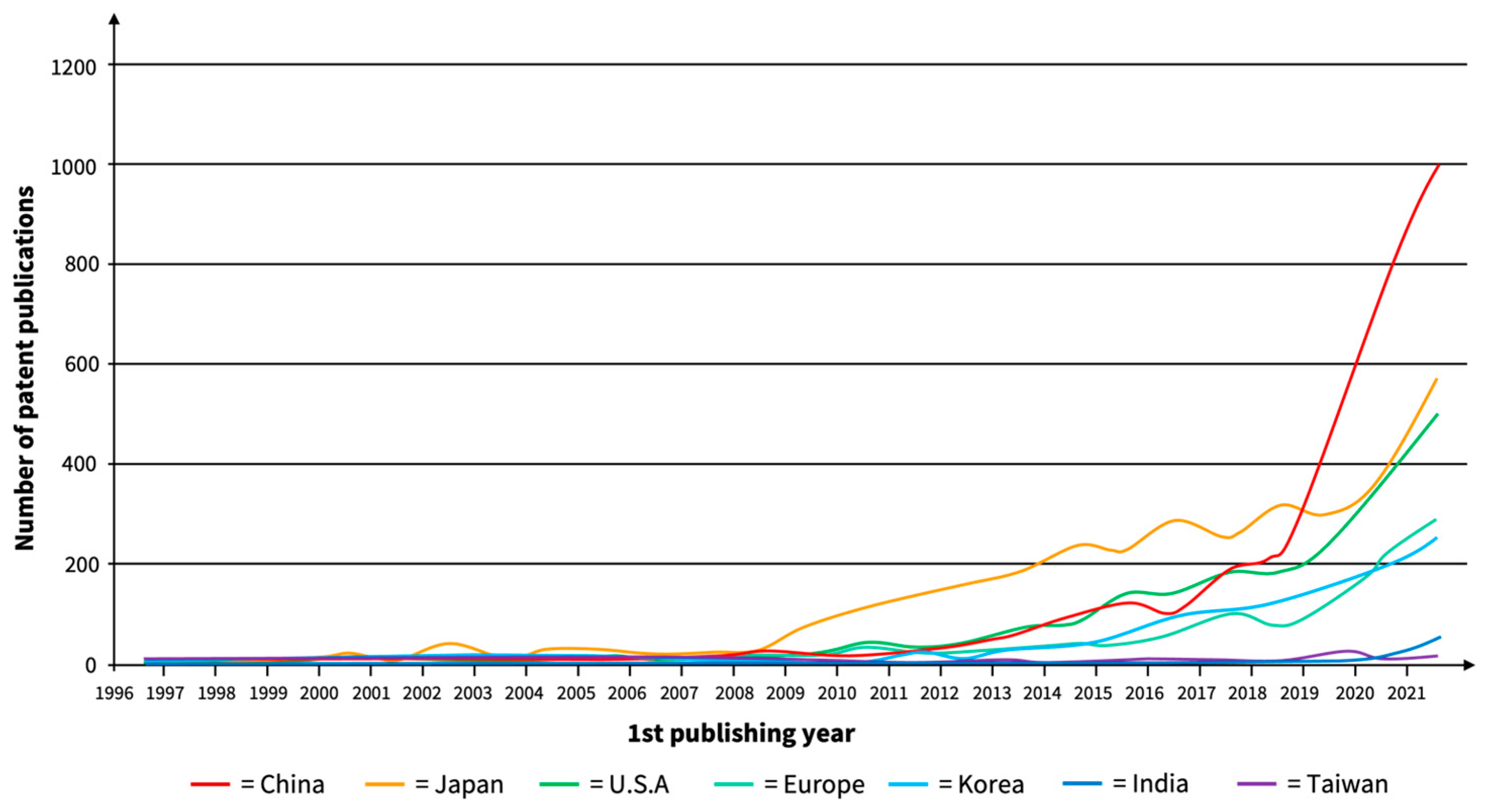
5.4. Key SSB Players and Collaborations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0 < x < −1): A New Cathode Material for Batteries of High Energy Density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- New Energy Outlook 2022|BloombergNEF|Bloomberg Finance LP; BloombergNEF: New York, NY, USA, 2022; Available online: https://about.bnef.com/new-energy-outlook/ (accessed on 23 November 2023).
- Nykvist, B.; Nilsson, M. Rapidly Falling Costs of Battery Packs for Electric Vehicles. Nat. Clim. Change 2015, 5, 329–332. [Google Scholar] [CrossRef]
- Deng, D. Li-ion batteries: Basics, progress, and challenges. Energy Sci. Eng. 2015, 3, 385–418. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; He, Z.; Li, Y.; Mao, J.; Dai, K.; Yan, C.; Zheng, J. An Advance Review of Solid-State Battery: Challenges, Progress and Prospects. Sustain. Mater. Technol. 2021, 29, e00297. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A Review of Composite Solid-State Electrolytes for Lithium Batteries: Fundamentals, Key Materials and Advanced Structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef]
- Wu, F.; Liu, L.; Wang, S.; Xu, J.; Lu, P.; Yan, W.; Peng, J.; Wu, D.; Li, H. Solid State Ionics—Selected Topics and New Directions. Progress. Mater. Sci. 2022, 126, 100921. [Google Scholar] [CrossRef]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of Inorganic Solid-State Electrolytes for Batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef]
- Raju, M.M.; Altayran, F.; Johnson, M.; Wang, D.; Zhang, Q. Crystal Structure and Preparation of Li7La3Zr2O12 (LLZO) Solid-State Electrolyte and Doping Impacts on the Conductivity: An Overview. Electrochem 2021, 2, 390–414. [Google Scholar] [CrossRef]
- Song, H.; Wang, S.; Song, X.; Wang, J.; Jiang, K.; Huang, S.; Han, M.; Xu, J.; He, P.; Chen, K.; et al. Solar-Driven All-Solid-State Lithium–Air Batteries Operating at Extreme Low Temperatures. Energy Environ. Sci. 2020, 13, 1205–1211. [Google Scholar] [CrossRef]
- Kitaura, H.; Zhou, H. All-Solid-State Lithium-Oxygen Battery with High Safety in Wide Ambient Temperature Range. Sci. Rep. 2015, 5, 13271. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Zhong, D.; Li, H.; Wang, G.; Chang, H. Halide Solid-State Electrolytes for All-Solid-State Batteries: Structural Design, Synthesis, Environmental Stability, Interface Optimization and Challenges. Chem. Sci. 2023, 14, 8693–8722. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, C.; Zhang, W.; Xia, Y.; Huang, H.; Gan, Y.; He, X.; Xia, X.; Tao, X.; Zhang, J. A Critical Review on Composite Solid Electrolytes for Lithium Batteries: Design Strategies and Interface Engineering. J. Energy Chem. 2023, 84, 189–209. [Google Scholar] [CrossRef]
- Nguyen, A.-G.; Park, C.-J. Insights into Tailoring Composite Solid Polymer Electrolytes for Solid-State Lithium Batteries. J. Membr. Sci. 2023, 675, 121552. [Google Scholar] [CrossRef]
- Yan, J.; Zhu, D.; Ye, H.; Sun, H.; Zhang, X.; Yao, J.; Chen, J.; Geng, L.; Su, Y.; Zhang, P.; et al. Atomic-Scale Cryo-TEM Studies of the Thermal Runaway Mechanism of Li1.3Al0.3Ti1.7P3O12 Solid Electrolyte. ACS Energy Lett. 2022, 7, 3855–3863. [Google Scholar] [CrossRef]
- Lu, P.; Xia, Y.; Huang, Y.; Li, Z.; Wu, Y.; Wang, X.; Sun, G.; Shi, S.; Sha, Z.; Chen, L.; et al. Wide-Temperature, Long-Cycling, and High-Loading Pyrite All-Solid-State Batteries Enabled by Argyrodite Thioarsenate Superionic Conductor. Adv. Funct. Mater. 2023, 33, 2211211. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Z.; Chen, L.; Li, H.; Wu, F. Doping Strategy and Mechanism for Oxide and Sulfide Solid Electrolytes with High Ionic Conductivity. J. Mater. Chem. A 2022, 10, 4517–4532. [Google Scholar] [CrossRef]
- Suci, W.G.; Aliwarga, H.K.; Azinuddin, Y.R.; Setyawati, R.B.; Stulasti, K.N.R.; Purwanto, A. Review of Various Sulfide Electrolyte Types for Solid-State Lithium-Ion Batteries. Open Eng. 2022, 12, 409–423. [Google Scholar] [CrossRef]
- Xiao, Y.; Jun, K.; Wang, Y.; Miara, L.J.; Tu, Q.; Ceder, G. Lithium Oxide Superionic Conductors Inspired by Garnet and NASICON Structures. Adv. Energy Mater. 2021, 11, 2101437. [Google Scholar] [CrossRef]
- Thangadurai, V.; Kaack, H.; Weppner, W.J.F. Novel Fast Lithium Ion Conduction in Garnet-Type Li5La3M2O12 (M = Nb, Ta). J. Am. Ceram. Soc. 2003, 86, 437–440. [Google Scholar] [CrossRef]
- Afyon, S.; Kravchyk, K.V.; Wang, S.; Broek, J.V.D.; Hänsel, C.; Kovalenko, M.V.; Rupp, J.L.M. Building Better All-Solid-State Batteries with Li-Garnet Solid Electrolytes and Metalloid Anodes. J. Mater. Chem. A 2019, 7, 21299–21308. [Google Scholar] [CrossRef]
- Taylor, N.J.; Stangeland-Molo, S.; Haslam, C.G.; Sharafi, A.; Thompson, T.; Wang, M.; Garcia-Mendez, R.; Sakamoto, J. Demonstration of High Current Densities and Extended Cycling in the Garnet Li7La3Zr2O12 Solid Electrolyte. J. Power Source 2018, 396, 314–318. [Google Scholar] [CrossRef]
- Rangasamy, E.; Wolfenstine, J.; Sakamoto, J. The Role of Al and Li Concentration on the Formation of Cubic Garnet Solid Electrolyte of Nominal Composition Li7La3Zr2O12. Solid. State Ion. 2012, 206, 28–32. [Google Scholar] [CrossRef]
- Huang, J.; Liang, F.; Hou, M.; Zhang, Y.; Chen, K.; Xue, D. Garnet-Type Solid-State Electrolytes and Interfaces in All-Solid-State Lithium Batteries: Progress and Perspective. Appl. Mater. Today 2020, 20, 100750. [Google Scholar] [CrossRef]
- Chen, R.; Nolan, A.M.; Lu, J.; Wang, J.; Yu, X.; Mo, Y.; Chen, L.; Huang, X.; Li, H. The Thermal Stability of Lithium Solid Electrolytes with Metallic Lithium. Joule 2020, 4, 812–821. [Google Scholar] [CrossRef]
- Samson, A.J.; Hofstetter, K.; Bag, S.; Thangadurai, V. A Bird’s-Eye View of Li-Stuffed Garnet-Type Li7La3Zr2O12 Ceramic Electrolytes for Advanced All-Solid-State Li Batteries. Energy Environ. Sci. 2019, 12, 2957–2975. [Google Scholar] [CrossRef]
- Zhao, W.; Yi, J.; He, P.; Zhou, H. Solid-State Electrolytes for Lithium-Ion Batteries: Fundamentals, Challenges and Perspectives. Electrochem. Energ. Rev. 2019, 2, 574–605. [Google Scholar] [CrossRef]
- Yu, T.; Yang, X.; Yang, R.; Bai, X.; Xu, G.; Zhao, S.; Duan, Y.; Wu, Y.; Wang, J. Progress and Perspectives on Typical Inorganic Solid-State Electrolytes. J. Alloys Compd. 2021, 885, 161013. [Google Scholar] [CrossRef]
- Liu, J.; Wang, T.; Yu, J.; Li, S.; Ma, H.; Liu, X. Review of the Developments and Difficulties in Inorganic Solid-State Electrolytes. Materials 2023, 16, 2510. [Google Scholar] [CrossRef]
- Parameswaran, A.K.; Pazhaniswamy, S.; Dekanovsky, L.; Balakrishnan, N.; Paneerselvam, C.S.; Reddy, M.V.; Adams, S.; Sofer, Z. An Integrated Study on the Ionic Migration across the Nano Lithium Lanthanum Titanate (LLTO) and Lithium Iron Phosphate-Carbon (LFP-C) Interface in All-Solid-State Li-Ion Batteries. J. Power Sources 2023, 565, 232907. [Google Scholar] [CrossRef]
- Inaguma, Y.; Liquan, C.; Itoh, M.; Nakamura, T.; Uchida, T.; Ikuta, H.; Wakihara, M. High Ionic Conductivity in Lithium Lanthanum Titanate. Solid. State Commun. 1993, 86, 689–693. [Google Scholar] [CrossRef]
- Yu, K.; Gu, R.; Wu, L.; Sun, H.; Ma, R.; Jin, L.; Xu, Y.; Xu, Z.; Wei, X. Ionic and Electronic Conductivity of Solid Electrolyte Li0.5La0.5TiO3 Doped with LiO2-SiO2-B2O3 Glass. J. Alloys Compd. 2018, 739, 892–896. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y. Perovskite-type Li-ion Solid Electrolytes: A Review. J. Mater. Sci. Mater. Electron. 2021, 32, 9736–9754. [Google Scholar] [CrossRef]
- Chen, C. Ionic Conductivity, Lithium Insertion and Extraction of Lanthanum Lithium Titanate. Solid. State Ion. 2001, 144, 51–57. [Google Scholar] [CrossRef]
- Ban, C. The Effect of Sintering on the Grain Boundary Conductivity of Lithium Lanthanum Titanates. Solid. State Ion. 2001, 140, 285–292. [Google Scholar] [CrossRef]
- Chen, K.; Huang, M.; Shen, Y.; Lin, Y.; Nan, C.W. Improving Ionic Conductivity of Li0.35La0.55TiO3 Ceramics by Introducing Li7La3Zr2O12 Sol into the Precursor Powder. Solid. State Ion. 2013, 235, 8–13. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hong, H.Y.-P.; Kafalas, J.A. Fast Na+-Ion Transport in Skeleton Structures. Mater. Res. Bull. 1976, 11, 203–220. [Google Scholar] [CrossRef]
- Zhang, L.; Malys, M.; Jamroz, J.; Krok, F.; Wrobel, W.; Hull, S.; Yan, H.; Abrahams, I. Structure and Conductivity in LISICON Analogues within the Li4GeO4–Li2MoO4 System. Inorg. Chem. 2023, 62, 11876–11886. [Google Scholar] [CrossRef]
- Boaretto, N.; Garbayo, I.; Valiyaveettil-SobhanRaj, S.; Quintela, A.; Li, C.; Casas-Cabanas, M.; Aguesse, F. Lithium Solid-State Batteries: State-of-the-Art and Challenges for Materials, Interfaces and Processing. J. Power Source 2021, 502, 229919. [Google Scholar] [CrossRef]
- Lau, J.; DeBlock, R.H.; Butts, D.M.; Ashby, D.S.; Choi, C.S.; Dunn, B.S. Sulfide Solid Electrolytes for Lithium Battery Applications. Adv. Energy Mater. 2018, 8, 1800933. [Google Scholar] [CrossRef]
- Chen, S.; Xie, D.; Liu, G.; Mwizerwa, J.P.; Zhang, Q.; Zhao, Y.; Xu, X.; Yao, X. Sulfide Solid Electrolytes for All-Solid-State Lithium Batteries: Structure, Conductivity, Stability and Application. Energy Storage Mater. 2018, 14, 58–74. [Google Scholar] [CrossRef]
- Chang, D.; Oh, K.; Kim, S.J.; Kang, K. Super-Ionic Conduction in Solid-State Li7P3S11-Type Sulfide Electrolytes. Chem. Mater. 2018, 30, 8764–8770. [Google Scholar] [CrossRef]
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A Lithium Superionic Conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Richards, W.D.; Ong, S.P.; Miara, L.J.; Kim, J.C.; Mo, Y.; Ceder, G. Design Principles for Solid-State Lithium Superionic Conductors. Nat. Mater. 2015, 14, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Kudu, Ö.U.; Famprikis, T.; Fleutot, B.; Braida, M.-D.; Le Mercier, T.; Islam, M.S.; Masquelier, C. A Review of Structural Properties and Synthesis Methods of Solid Electrolyte Materials in the Li2S−P2S5 Binary System. J. Power Source 2018, 407, 31–43. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, D.; Ma, Y.; Natan, A.; Aurora, P.; Zhu, H. Sulfide-Based Solid-State Electrolytes: Synthesis, Stability, and Potential for All-Solid-State Batteries. Adv. Mater. 2019, 31, 1901131. [Google Scholar] [CrossRef] [PubMed]
- Deiseroth, H.-J.; Kong, S.-T.; Eckert, H.; Vannahme, J.; Reiner, C.; Zaiß, T.; Schlosser, M. Li6PS5X: A Class of Crystalline Li-Rich Solids With an Unusually High Li+ Mobility. Angew. Chem. Int. Ed. 2008, 47, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Lian, P.-J.; Zhao, B.-S.; Zhang, L.-Q.; Xu, N.; Wu, M.-T.; Gao, X.-P. Inorganic Sulfide Solid Electrolytes for All-Solid-State Lithium Secondary Batteries. J. Mater. Chem. A 2019, 7, 20540–20557. [Google Scholar] [CrossRef]
- Xu, R.; Wu, Z.; Zhang, S.; Wang, X.; Xia, Y.; Xia, X.; Huang, X.; Tu, J. Construction of All-Solid-State Batteries Based on a Sulfur-Graphene Composite and Li9.54Si1.74P1.44S11.7Cl0.3 Solid Electrolyte. Chem. A Eur. J. 2017, 23, 13950–13956. [Google Scholar] [CrossRef]
- Liang, F.; Sun, Y.; Yuan, Y.; Huang, J.; Hou, M.; Lu, J. Designing Inorganic Electrolytes for Solid-State Li-Ion Batteries: A Perspective of LGPS and Garnet. Mater. Today 2021, 50, 418–441. [Google Scholar] [CrossRef]
- Hori, S.; Suzuki, K.; Hirayama, M.; Kato, Y.; Kanno, R. Lithium Superionic Conductor Li9.42Si1.02P2.1S9.96O2.04 with Li10GeP2S12-Type Structure in the Li2S–P2S5–SiO2 Pseudoternary System: Synthesis, Electrochemical Properties, and Structure–Composition Relationships. Front. Energy Res. 2016, 4, 38. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Zheng, C.; Xia, Y.; Gan, Y.; Huang, H.; Liang, C.; He, X.; Tao, X.; Zhang, W. Silicon-Doped Argyrodite Solid Electrolyte Li6PS5 I with Improved Ionic Conductivity and Interfacial Compatibility for High-Performance All-Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2020, 12, 41538–41545. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Yamashita, H.; Tatsumisago, M.; Minami, T. Characterization of Li2S–SiS2–LiMO (M = Si, P, Ge) Amorphous Solid Electrolytes Prepared by Melt-Quenching and Mechanical Milling. Solid. State Ion. 2002, 148, 381–389. [Google Scholar] [CrossRef]
- Hu, Y.; Li, W.; Zhu, J.; Hao, S.-M.; Qin, X.; Fan, L.-Z.; Zhang, L.; Zhou, W. Multi-Layered Electrolytes for Solid-State Lithium Batteries. Next Energy 2023, 1, 100042. [Google Scholar] [CrossRef]
- Alexander, G.V.; Patra, S.; Raj, S.V.S.; Sugumar, M.K.; Din, M.M.U.; Murugan, R. Electrodes-Electrolyte Interfacial Engineering for Realizing Room Temperature Lithium Metal Battery Based on Garnet Structured Solid Fast Li+ Conductors. J. Power Source 2018, 396, 764–773. [Google Scholar] [CrossRef]
- Luo, W.; Gong, Y.; Zhu, Y.; Li, Y.; Yao, Y.; Zhang, Y.; Fu, K.; Pastel, G.; Lin, C.; Mo, Y.; et al. Reducing Interfacial Resistance between Garnet-Structured Solid-State Electrolyte and Li-Metal Anode by a Germanium Layer. Adv. Mater. 2017, 29, 1606042. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Cui, Z.; Chen, C.; Li, Y.; Guo, X. Formation of Self-Limited, Stable and Conductive Interfaces between Garnet Electrolytes and Lithium Anodes for Reversible Lithium Cycling in Solid-State Batteries. J. Mater. Chem. A 2018, 6, 11463–11470. [Google Scholar] [CrossRef]
- Ogawa, M.; Kanda, R.; Yoshida, K.; Uemura, T.; Harada, K. High-Capacity Thin Film Lithium Batteries with Sulfide Solid Electrolytes. J. Power Source 2012, 205, 487–490. [Google Scholar] [CrossRef]
- Kim, H.; Watanabe, K.; Matsui, N.; Suzuki, K.; Kanno, R.; Hirayama, M. Crack Suppression by Downsizing Sulfide-Electrolyte Particles for High-Current-Density Operation of Metal/Alloy Anodes. Batter. Supercaps 2023, 6, e202300306. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-Power All-Solid-State Batteries Using Sulfide Superionic Conductors. Nat. Energy 2016, 1, 16030. [Google Scholar] [CrossRef]
- Wu, F.; Fitzhugh, W.; Ye, L.; Ning, J.; Li, X. Advanced Sulfide Solid Electrolyte by Core-Shell Structural Design. Nat. Commun. 2018, 9, 4037. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhao, Y.; Li, W.; Meng, L.; Bai, Y.; Chen, G. New Insight for Solid Sulfide Electrolytes LSiPSI by Using Si/P/S as the Raw Materials and I Doping. ACS Sustain. Chem. Eng. 2019, 7, 12930–12937. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, M.-X.; Zhang, W.; Yan, J.-L.; Shao, G.-Q. Synthesis and Purification of SiS2 and Li2S for Li9.54Si1.74P1.44S11.7Cl0.3 Solid Electrolyte in Lithium-Ion Batteries. Mater. Lett. 2020, 266, 127508. [Google Scholar] [CrossRef]
- Ye, L.; Gil-González, E.; Li, X. Li9.54Si1.74(P1−xSbx)1.44S11.7Cl0.3: A Functionally Stable Sulfide Solid Electrolyte in Air for Solid-State Batteries. Electrochem. Commun. 2021, 128, 107058. [Google Scholar] [CrossRef]
- Asano, T.; Sakai, A.; Ouchi, S.; Sakaida, M.; Miyazaki, A.; Hasegawa, S. Solid Halide Electrolytes with High Lithium-Ion Conductivity for Application in 4 V Class Bulk-Type All-Solid-State Batteries. Adv. Mater. 2018, 30, 1803075. [Google Scholar] [CrossRef] [PubMed]
- Tuo, K.; Sun, C.; Liu, S. Recent Progress in and Perspectives on Emerging Halide Superionic Conductors for All-Solid-State Batteries. Electrochem. Energy Rev. 2023, 6, 17. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Pei, N.; Chen, Z.; Li, R.; Fu, L.; Zhang, P.; Zhao, J. The Critical Role of Fillers in Composite Polymer Electrolytes for Lithium Battery. Nano-Micro Lett. 2023, 15, 74. [Google Scholar] [CrossRef]
- Sheng, O.; Zheng, J.; Ju, Z.; Jin, C.; Wang, Y.; Chen, M.; Nai, J.; Liu, T.; Zhang, W.; Liu, Y.; et al. In Situ Construction of a LiF-Enriched Interface for Stable All-Solid-State Batteries and Its Origin Revealed by Cryo-TEM. Adv. Mater. 2020, 32, 2000223. [Google Scholar] [CrossRef]
- Marzantowicz, M.; Struzik, M. Overview of Polymer and Solid Electrolytes: Towards All Solid-State Batteries. In Designing Electrolytes for Lithium-Ion and Post-Lithium Batteries; Wieczorek, W., Płocharski, J., Eds.; Jenny Stanford Publishing: Singapore, 2021; pp. 71–138. ISBN 978-1-00-305093-3. [Google Scholar]
- Agrawal, R.C.; Pandey, G.P. Solid Polymer Electrolytes: Materials Designing and All-Solid-State Battery Applications: An Overview. J. Phys. D Appl. Phys. 2008, 41, 223001. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Wang, Y.; Liu, Q.; Chen, Q.; Chen, M. Advances and Prospects of PVDF Based Polymer Electrolytes. J. Energy Chem. 2022, 64, 62–84. [Google Scholar] [CrossRef]
- Wang, H.; Sheng, L.; Yasin, G.; Wang, L.; Xu, H.; He, X. Reviewing the Current Status and Development of Polymer Electrolytes for Solid-State Lithium Batteries. Energy Storage Mater. 2020, 33, 188–215. [Google Scholar] [CrossRef]
- Dirican, M.; Yan, C.; Zhu, P.; Zhang, X. Composite Solid Electrolytes for All-Solid-State Lithium Batteries. Mater. Sci. Eng. R. Rep. 2019, 136, 27–46. [Google Scholar] [CrossRef]
- Puente, P.M.G.; Song, S.; Cao, S.; Rannalter, L.Z.; Pan, Z.; Xiang, X.; Shen, Q.; Chen, F. Garnet-Type Solid Electrolyte: Advances of Ionic Transport Performance and Its Application in All-Solid-State Batteries. J. Adv. Ceram. 2021, 10, 933–972. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Chen, Y.; Wang, P.; Zhang, H.; He, X. PEO Based Polymer-Ceramic Hybrid Solid Electrolytes: A Review. Nano Converg. 2021, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, W.; Zhu, X.; Liu, S.; Wang, Y.; Xu, Y.; Zhou, S.; He, X.; Xue, Z. Composite Polymer Electrolytes Reinforced by Hollow Silica Nanotubes for Lithium Metal Batteries. J. Membr. Sci. 2021, 618, 118697. [Google Scholar] [CrossRef]
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Wu, J.; Liu, X. Review on Polymer-Based Composite Electrolytes for Lithium Batteries. Front. Chem. 2019, 7, 522. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, Y.; Liao, C.; Gong, W.; Yang, M.; Li, R.K.Y.; Tjong, S.C.; Lu, Z. Enhanced Electrochemical Performance of Solid PEO/LiClO4 Electrolytes with a 3D Porous Li6.28La3Zr2Al0.24O12 Network. Compos. Sci. Technol. 2019, 184, 107863. [Google Scholar] [CrossRef]
- Wang, G.; Liu, H.; Liang, Y.; Wang, C.; Fan, L.-Z. Composite Polymer Electrolyte with Three-Dimensional Ion Transport Channels Constructed by NaCl Template for Solid-State Lithium Metal Batteries. Energy Storage Mater. 2022, 45, 1212–1219. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. A Review of Composite Polymer-Ceramic Electrolytes for Lithium Batteries. Energy Storage Mater. 2021, 34, 282–300. [Google Scholar] [CrossRef]
- Didwal, P.N.; Singhbabu, Y.N.; Verma, R.; Sung, B.-J.; Lee, G.-H.; Lee, J.-S.; Chang, D.R.; Park, C.-J. An Advanced Solid Polymer Electrolyte Composed of Poly(Propylene Carbonate) and Mesoporous Silica Nanoparticles for Use in All-Solid-State Lithium-Ion Batteries. Energy Storage Mater. 2021, 37, 476–490. [Google Scholar] [CrossRef]
- Liu, S.; Shan, H.; Xia, S.; Yan, J.; Yu, J.; Ding, B. Polymer Template Synthesis of Flexible SiO2 Nanofibers to Upgrade Composite Electrolytes. ACS Appl. Mater. Interfaces 2020, 12, 31439–31447. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.H.; Didwal, P.N.; Kim, J.M.; Chang, D.R.; Park, C.-J. Poly(Ethylene Oxide)-Based Composite Solid Polymer Electrolyte Containing Li7La3Zr2O12 and Poly(Ethylene Glycol) Dimethyl Ether. J. Membr. Sci. 2020, 595, 117538. [Google Scholar] [CrossRef]
- Wan, Z.; Lei, D.; Yang, W.; Liu, C.; Shi, K.; Hao, X.; Shen, L.; Lv, W.; Li, B.; Yang, Q.; et al. Low Resistance–Integrated All-Solid-State Battery Achieved by Li7La3Zr2O12 Nanowire Upgrading Polyethylene Oxide (PEO) Composite Electrolyte and PEO Cathode Binder. Adv. Funct. Mater. 2019, 29, 1805301. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, P.; Fang, Q.; Jing, M.; Shen, X.; Yang, L. A Novel Solid PEO/LLTO-Nanowires Polymer Composite Electrolyte for Solid-State Lithium-Ion Battery. Electrochim. Acta 2018, 292, 718–726. [Google Scholar] [CrossRef]
- Sivaraj, P.; Abhilash, K.P.; Nalini, B.; Perumal, P.; Selvin, P.C. Free-Standing, High Li-Ion Conducting Hybrid PAN/PVdF/LiClO4/Li0.5La0.5TiO3 Nanocomposite Solid Polymer Electrolytes for All-Solid-State Batteries. J. Solid. State Electrochem. 2021, 25, 905–917. [Google Scholar] [CrossRef]
- Kammampata, S.P.; Garakani, M.A.; Zhang, Z.; Thangadurai, V. 4.19—Solid-State Electrolytes for Lithium-Ion Batteries. In Comprehensive Inorganic Chemistry III, 3rd ed.; Reedijk, J., Poeppelmeier, K.R., Eds.; Elsevier: Oxford, UK, 2023; pp. 658–680. ISBN 978-0-12-823153-1. [Google Scholar]
- Xiao, J. How Lithium Dendrites Form in Liquid Batteries. Science 2019, 366, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Oh, P.; Lee, H.; Park, S.; Cha, H.; Kim, J.; Cho, J. Improvements to the Overpotential of All-Solid-State Lithium-Ion Batteries during the Past Ten Years. Adv. Energy Mater. 2020, 10, 2000904. [Google Scholar] [CrossRef]
- Jetybayeva, A.; Aaron, D.S.; Belharouak, I.; Mench, M.M. Critical Review on Recently Developed Lithium and Non-Lithium Anode-Based Solid-State Lithium-Ion Batteries. J. Power Source 2023, 566, 232914. [Google Scholar] [CrossRef]
- Sonomura, H. Lithium-Compound-Coated Graphite as an Anode Material for All-Solid-State Lithium Batteries. Solid. State Ion. 2022, 386, 116044. [Google Scholar] [CrossRef]
- Sreejith, O.V.; Murugan, R. An Enhanced Interface between Garnet Solid Electrolyte and Lithium through Multifunctional Lithium Titanate Anode-Additive for Solid-State Lithium Batteries. J. Alloys Compd. 2023, 939, 168774. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Q.; Zhang, H.; Cheng, J.; Li, Y.; Zeng, Z.; Zhang, S.; Xu, X.; Ji, F.; Li, D.; et al. The Application Road of Silicon-Based Anode in Lithium-Ion Batteries: From Liquid Electrolyte to Solid-State Electrolyte. Energy Storage Mater. 2023, 55, 244–263. [Google Scholar] [CrossRef]
- Grandjean, M.; Pichardo, M.; Biecher, Y.; Haon, C.; Chenevier, P. Matching Silicon-Based Anodes with Sulfide-Based Solid-State Electrolytes for Li-Ion Batteries. J. Power Sources 2023, 580, 233386. [Google Scholar] [CrossRef]
- Numan-Al-Mobin, A.M.; Smirnova, A. 5—Silicon-Based Lithium-Ion Battery Anodes and Their Application in Solid-State Batteries. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Smirnova, A., Numan-Al-Mobin, A., Inamuddin, Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 129–169. ISBN 978-0-323-90635-7. [Google Scholar]
- Xia, X.; Qian, X.; Chen, C.; Li, W.; He, D.; He, G.; Chen, H. Recent Progress of Si-Based Anodes in the Application of Lithium-Ion Batteries. J. Energy Storage 2023, 72, 108715. [Google Scholar] [CrossRef]
- Xie, J.; Zhuang, R.; Du, Y.; Pei, Y.; Tan, D.; Xu, F. Advances in Sulfur-Doped Carbon Materials for Use as Anodes in Sodium-Ion Batteries. New Carbon. Mater. 2023, 38, 305–316. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, X.; Yan, R.; Zhang, J. Recent Research Progress of Alloy-Containing Lithium Anodes in Lithium-Metal Batteries. Curr. Opin. Solid. State Mater. Sci. 2023, 27, 101079. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Yang, Y.; Xie, J.; Deng, Q.; Zou, W.; Zhou, A.; Li, J. Polyacrylonitrile Fibers Network Reinforced Polymer Electrolyte with Li-Sn Alloy Layer Protected Li Anode toward Ultra-Long Cycle Lifespan for Room-Temperature Solid-State Batteries. Chem. Eng. J. 2023, 461, 141993. [Google Scholar] [CrossRef]
- Lewis, J.A.; Cavallaro, K.A.; Liu, Y.; McDowell, M.T. The promise of alloy anodes for solid-state batteries. Joule 2022, 6, 1418–1430. [Google Scholar] [CrossRef]
- Spencer-Jolly, D.; Agarwal, V.; Doerrer, C.; Hu, B.; Zhang, S.; Melvin, D.L.R.; Gao, H.; Gao, X.; Adamson, P.; Magdysyuk, O.V.; et al. Structural Changes in the Silver-Carbon Composite Anode Interlayer of Solid-State Batteries. Joule 2023, 7, 503–514. [Google Scholar] [CrossRef]
- Ahmad, H.; Kubra, K.T.; Butt, A.; Nisar, U.; Iftikhar, F.J.; Ali, G. Recent Progress, Challenges, and Perspectives in the Development of Solid-State Electrolytes for Sodium Batteries. J. Power Source 2023, 581, 233518. [Google Scholar] [CrossRef]
- Li, M.; Lian, Y.; Li, Z.; Zhu, M.; Qiao, Q.; Liu, J.; Xiao, L. An Integrated Architecture of Mutually Infiltrated Nanoarray Cathode and Polymer Electrolyte Improves the Performances of Solid-State Lithium−oxygen Batteries. Electrochim. Acta 2023, 453, 142360. [Google Scholar] [CrossRef]
- Zhao, C.; Pan, Y.; Li, R.; Hu, A.; Zhou, B.; He, M.; Chen, J.; Yan, Z.; Fan, Y.; Chen, N.; et al. A Safe Anode-Free Lithium Metal Pouch Cell Enabled by Integrating Stable Quasi-Solid Electrolytes with Oxygen-Free Cathodes. Chem. Eng. J. 2023, 463, 142386. [Google Scholar] [CrossRef]
- Jung, J.Y.; Kim, K.; Suh, J.H.; Kim, H.; You, M.J.; Park, K.-H.; Song, J.H.; Yu, J.-S.; Lee, J.-W.; Cho, W.; et al. Customizing the Morphology and Microstructure of Single-Crystalline Ni-Rich Layered Cathode Materials for All-Solid-State Batteries. Chem. Eng. J. 2023, 470, 144381. [Google Scholar] [CrossRef]
- de Klerk, N.J.J.; Wagemaker, M. Space-Charge Layers in All-Solid-State Batteries; Important or Negligible? ACS Appl. Energy Mater. 2018, 1, 5609–5618. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, M.; Wu, N.; Cui, L.; Huang, X.; Liu, X.; Zhao, Y.; Chen, H.; Yuan, W.; Bai, Y. Single-Crystalline Particles: An Effective Way to Ameliorate the Intragranular Cracking, Thermal Stability, and Capacity Fading of the LiNi0.6Co0.2Mn0.2O2 Electrodes. J. Electrochem. Soc. 2018, 165, A3040. [Google Scholar] [CrossRef]
- Li, X.; Jin, L.; Song, D.; Zhang, H.; Shi, X.; Wang, Z.; Zhang, L.; Zhu, L. LiNbO3-Coated LiNi0.8Co0.1Mn0.1O2 Cathode with High Discharge Capacity and Rate Performance for All-Solid-State Lithium Battery. J. Energy Chem. 2020, 40, 39–45. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, L.; Hu, J.; Liu, Y.; Liu, Y.; Feng, Q.; Zhu, G.; Fan, L.-Z. Solid-State Lithium Metal Batteries Enabled with High Loading Composite Cathode Materials and Ceramic-Based Composite Electrolytes. J. Power Sources 2019, 442, 227230. [Google Scholar] [CrossRef]
- Yu, J.; Chen, L.; Wu, Q.; Wang, J.; Cheng, L.; Wang, H.-G. Stable Quasi-Solid-State Lithium-Organic Battery Based on Composite Gel Polymer Electrolyte and Compatible Organic Cathode Material. J. Colloid. Interface Sci. 2023, 649, 159–165. [Google Scholar] [CrossRef]
- Ponnada, S.; Gorle, D.B.; Bose, R.S.C.; Kiai, M.S.; Devi, M.; Raju, C.V.; Baydogan, N.; Nanda, K.K.; Marken, F.; Sharma, R.K. Current insight into 3D printing in solid-state lithium-ion batteries: A perspective. Batter. Supercaps 2022, 5, e202200223. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, M.; Yang, X.; Sun, R.; Zhang, J.; Sun, X. Emerging application of 3D-printing techniques in lithium batteries: From liquid to solid. Mater. Today 2022, 59, 161–181. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, Q.; Liu, L.; Xu, J.; Yan, M.; Jiang, Z. A novel and facile route of ink-jet printing to thin film SnO2 anode for rechargeable lithium ion batteries. Electrochim. Acta 2006, 51, 2639–2645. [Google Scholar] [CrossRef]
- Li, J.; Leu, M.C.; Panat, R.; Park, J. A hybrid three-dimensionally structured electrode for lithium-ion batteries via 3D printing. Mater. Des. 2017, 119, 417–424. [Google Scholar] [CrossRef]
- Delannoy, P.-E.; Riou, B.; Lestriez, B.; Guyomard, D.; Brousse, T.; Le Bideau, J. Toward fast and cost-effective ink-jet printing of solid electrolyte for lithium microbatteries. J. Power Sources 2015, 274, 1085–1090. [Google Scholar] [CrossRef]
- Cheng, M.; Jiang, Y.; Yao, W.; Yuan, Y.; Deivanayagam, R.; Foroozan, T.; Huang, Z.; Song, B.; Rojaee, R.; Shokuhfar, T.; et al. Elevated-temperature 3D printing of hybrid solid-state electrolyte for Li-ion batteries. Adv. Mater. 2018, 30, 1800615. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, S.; Nie, L.; Sun, Z.; Wu, X.; Liu, W. Stereolithography Three-Dimensional Printing Solid Polymer Electrolytes for All-Solid-State Lithium Metal Batteries. Nano Lett. 2020, 20, 7136–7143. [Google Scholar] [CrossRef]
- Executive Agency for Small and Medium Sized Enterprises; Fraunhofer ISI. Advanced Technologies for Industry: Product Watch: Solid State Lithium Ion Batteries for Electric Vehicles, November 2020. Available online: https://op.europa.eu/en/publication-detail/-/publication/0d9b6e6e-3e83-11eb-b27b-01aa75ed71a1/language-en/format-PDF/source-193964815 (accessed on 23 November 2023).
- Schmaltz, T.; Wicke, T.; Weymann, L.; Voß, P.; Neef, C.; Thielmann, A. Solid-State Battery Roadmap 2035+; Fraunhofer ISI: Karlsruhe, Germany, 2022; Available online: https://www.isi.fraunhofer.de/content/dam/isi/dokumente/cct/2022/SSB_Roadmap.pdf (accessed on 23 November 2023).
- A Vision for a Sustainable Battery Value Chain in 2030. Available online: https://www.weforum.org/reports/a-vision-for-a-sustainable-battery-value-chain-in-2030/ (accessed on 25 September 2023).
- Battery Pack Prices Fall to an Average of $132/kWh, But Rising Commodity Prices Start to Bite. Available online: https://about.bnef.com/blog/battery-pack-prices-fall-to-an-average-of-132-kwh-but-rising-commodity-prices-start-to-bite/ (accessed on 25 September 2023).
- Thielmann, A.; Neef, C.; Hettesheimer, T.; Döscher, H.; Wietschel, M.; Tübke, J. Energiespeicher-Roadmap (Update 2017); Fraunhofer ISI: Karlsruhe, Germany, 2017. [Google Scholar] [CrossRef]
- Grylls, B. Online and on Point. New Electron. 2018, 51, 8. [Google Scholar] [CrossRef]
- STMicroelectronics EnFilmTM—Rechargeable Solid State Lithium Thin Film Battery. Available online: https://www.st.com/resource/en/datasheet/efl700a39.pdf (accessed on 25 September 2023).
- Thielmann, A.; Wietschel, M.; Funke, S.Á.; Grimm, A.; Hettesheimer, T.; Langkau, S.; Loibl, A.; Moll, C.; Neef, C.; Plötz, P.; et al. Batterien Für Elektroautos: Faktencheck Und Handlungsbedarf; Fraunhofer ISI: Karlsruhe, Germany, 2020. [Google Scholar] [CrossRef]
- Solid State Battery Installations Forecast in Europe and USA. Available online: https://www.grepow.com/industry-news/solid-state-battery-in-stallations-forecast-in-europe-and-usa.html (accessed on 25 September 2023).
- Solid-State and Polymer Batteries 2019–2029: Technology, Patents, Forecasts. Available online: https://www.idtechex.com/de/research-report/solid-state-and-polymer-batteries-2019-2029-technology-patents-forecasts/641 (accessed on 25 September 2023).
- Next-Generation Batteries Beyond Li-Ion Will Be Worth $10 Billion in 2030. Available online: http://finance.yahoo.com/news/next-generation-batteries-beyond-li-123000445.html (accessed on 25 September 2023).
- Will Japanese Companies Win the Race on Solid-State Batteries? Here’s What the Patents Tell Us. Available online: https://www.knowmade.com/technology-news/energy-technology-news/batteries-news/will-japanese-companies-win-the-race-on-solid-state-batteries-heres-what-the-patents-tell-us/ (accessed on 25 September 2023).
- Toyota and Panasonic Lead in Solid-State Battery Patents. Available online: https://www.greencarreports.com/news/1136415_toyota-and-panasonic-lead-in-solid-state-battery-patents (accessed on 25 September 2023).
- Electric Vehicle Solid State Battery Market. Available online: https://www.alliedmarketresearch.com/electric-vehicle-solid-state-battery-market-A31607#:~:text=Solid%2Dstate%20battery%20prices%20are,become%20an%20economically%20viable%20alternative (accessed on 25 September 2023).
- Ampcera Announces Scaling Production of Its Engineered Solid-State Electrolytes. Available online: https://www.prnewswire.com/news-releases/ampcera-announces-scaling-production-of-its-engineered-solid-state-electrolytes-301293895.html (accessed on 25 September 2023).
- Davison, M.; Cranney, J.; Summers, T.; Townsend, C.D. Decentralised Energy Market for Implementation into the Intergrid Concept—Part 2: Integrated System. In Proceedings of the 2018 7th International Conference on Renewable Energy Research and Applications (ICRERA), Paris, France, 14–17 October 2018; pp. 287–293. [Google Scholar] [CrossRef]
- Hatzell, K.B.; Zheng, Y. Prospects on Large-Scale Manufacturing of Solid State Batteries. MRS Energy Sustain. 2021, 8, 33–39. [Google Scholar] [CrossRef]
- Hsieh, I.-Y.L.; Pan, M.S.; Chiang, Y.-M.; Green, W.H. Learning Only Buys You so Much: Practical Limits on Battery Price Reduction. Appl. Energy 2019, 239, 218–224. [Google Scholar] [CrossRef]
- Ganfeng Lithium Plans to Build China’s Largest Solid-State Battery Production Base. Available online: https://newsletter.cnevpost.com/embed (accessed on 23 November 2023).
- Flaherty, N. Ilika, Nexeon Team for UK Silicon Solid State Batteries with BMW. eeNews Europe: Lasne, Belgium, 2023; Available online: https://www.eenewseurope.com/en/ilika-nexeon-team-for-uk-silicon-solid-state-batteries-with-bmw/ (accessed on 23 November 2023).
| Material | Ionic Conductivity */S cm−1 at RT | Source |
|---|---|---|
| LATP | 10−3 | [75] |
| LLZO | 10−6–10−3 | [51] |
| LTTO | 10−3 | [32] |
| LISICON | 10−6–10−4 | [51] |
| LPS | 10−2 | [47] |
| LGPS | 10−2 | [52] |
| PVDF | 10−8–10−6 | [68] |
| PEO | 10−8–10−6 | [68] |
| PAN | 10−8–10−6 | [68] |
| Compound | Ionic Conductivity/S cm−1 at T/°C | Source |
|---|---|---|
| SiO2/PPC/LiTFSI | 8.5 × 10−4/60 °C | [82] |
| SiO2 NTs/PEO/LiTFSI | 4.35 × 10−4/30 °C | [77] |
| SiO2 NFs/PEO-LiTFSI-SN | 1.3 × 10−4/30 °C | [83] |
| LLZO/PEO/LiTFSI/PEGDME | 4.7 × 10−4/60 °C | [84] |
| LLZO NWs/PEO/LiTFSI | 2.39 × 10−4/RT | [85] |
| Li/LATP-3D/LiFePO4 | 7.47 × 10−4/60 °C | [80] |
| LLZAO-PEO/LiClO4 | 2.25 × 10−5/30 °C | [79] |
| LLTO/PEO | 3.31 × 10−4/RT | [86] |
| LLTO/PAN-PVDF | 1.43 × 10−3/RT | [87] |
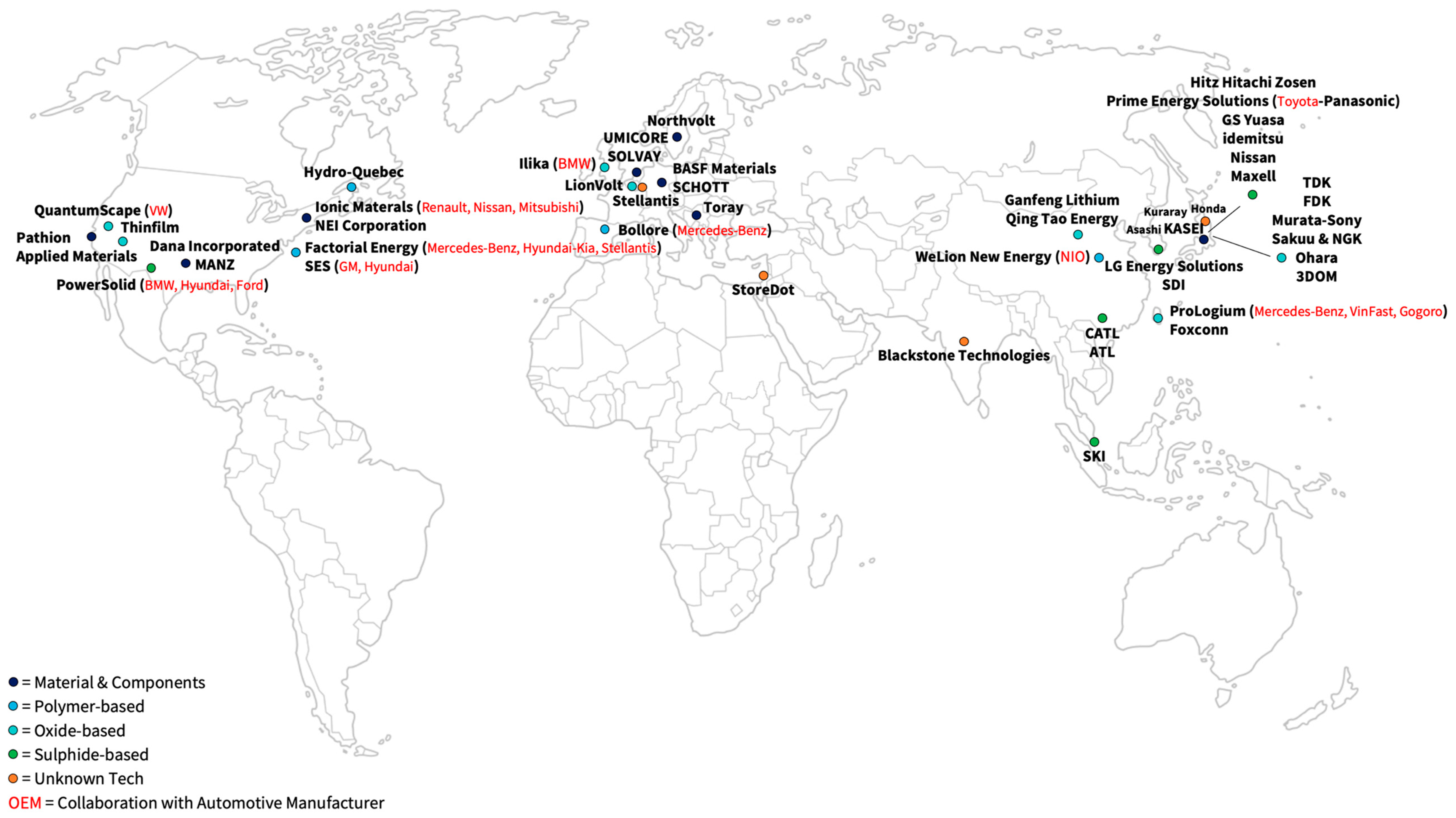
| Player | Description | OEM Collaboration | Year of Market Entry | Source |
|---|---|---|---|---|
| Bollore (BlueSolutions) | Bollore developed a passenger car with an SSB (BlueCar) in 2011. In addition, buses equipped with SSBs were launched in 2020 together with Mercedes. The Bollore-owned company “BlueSolutions” has been selling LMP technology (all-solid-state Li-metal polymer) since 2011. It is now selling its SSBs to Daimler for the eCitaro-bus. | Mercedes-Benz | 2020 | [119,120] |
| WeLion New Energy Technology | Car manufacturer NIO, together with WeLion New Energy Technology, launched a polymer battery with a Li metal anode and an NMC cathode in 2022. It also announced the start of construction of a production plant that will initially produce 20 GWh of hybrid SSBs with liquid electrolyte, as well as ASSB. An expansion to 100 GWh is targeted. | NIO | 2022 | [119,120] |
| Factorial Energy | Factorial Energy presented a cell with a solid separator, liquid electrolyte, and a Li metal anode that achieved 40 Ah capacity in 2021. The OEM Hyundai-Kia, Mercedes-Benz, and Stellantis have already invested in Factorial Energy. Mercedes-Benz and Factorial Energy scheduled a small series to enter the market for automotive applications by the end of 2026. | Hyundai-Kia, Mercedes-Benz, Stellantis | 2026 | [119,120] |
| Solid Energy Systems | Solid Energy Systems plans to develop a prototype car together with GM and Hyundai by 2023 and reach market maturity by 2030. SES relies on a hybrid cell concept with an LCO or an NCA cathode active material and a Li metal anode. | GM, Hyundai | 2030 | [119,120] |
| Hydro-Quebec | Hydro Quebec plans to start production between 2025 and 2027, initially launching polymer electrolytes with a Li metal anode and an LFP cathode. Later, the LFP cathode will be replaced with NMC, and the polymer electrolyte will be replaced by a composite electrolyte with ceramic components. | Mercedes-Benz | 2025–2027 | [119,120] |
| Ionic Materials | Furthermore, the company Ionic Materials is developing a polymer battery with Renault-Nissan-Mitsubishi. Ionic Materials is known to only use a Li metal anode. A123 Systems LLC will invest in the project. | Renault, Nissan, Mitsubishi | - | [119,120] |
| Player * | Description | OEM Collaboration | Year of Market Entry | Source |
|---|---|---|---|---|
| QuantumScape | In another cooperation between carmaker VW and cell manufacturer Quantum Scape, market-ready batteries for the automotive sector will be developed by 2025. In 2024, Quantum Scape will build up a production capacity of 1 GWh, which will be expanded to 20 GWh by 2026. QuantumScape describes the electrolyte material as ceramic and has already demonstrated prototype cells with Li anodes. Due to the potential proximity to oxide materials, the announcements are classified as oxides. | VW | 2024 | [119,120] |
| ProLogium | Cell maker ProLogium and car manufacturers are teaming up to put an SSB in a commercial vehicle (VinFast) or Prototypes (Mercedes-Benz) by 2023. For this goal, production capacities of 1 to 2 GWh were planned to be built up in 2022. A battery with a ceramic separator and a capacity of 2.5 kWh was demonstrated together with scooter manufacturer Gogoro in 2022. | VinFast, Mercedes-Benz, Gogoro | 2023 | [119,120] |
| Ganfeng Lithium | Ganfeng Lithium is one of China’s largest battery producers. The Li and battery manufacturer started to build a 10 GWh SSB factory in 2022 with a second 10 GWh factory planned to produce SSBs with 360 Wh/kg. | - | - | [137] |
| Qing Tao Energy | Qing Tao Energy Development and Ampcera are also working on solid oxide electrolytes. Quin Tao announced a production capacity of 1 GWh in 2020 and a second production facility with an optional capacity of 10 GWh in 2022. | SAIC Motor | - | [119,120] |
| Ilika | Founded in 2004, Ilika started designing the Stereax family of mm-scale SSBs for medical implants and industrial IoT devices in 2014. Financed by three rounds of venture capital, the company was publicly listed in 2010. The company currently plans to start its MWh scale-up of EV batteries. | BMW | - | [119,120,138] |
| Player * | Description | OEM Collaboration | Year of Market Entry | Source |
|---|---|---|---|---|
| Samsung SDI | In 2020, SDI introduced a prototype cell with an in situ Li-metal anode and started the construction of a pilot production plant in 2022. | - | 2027 | [119,120] |
| CATL | According to the company‘s own roadmap, CATL plans to be the first SSB cell manufacturer by developing a sulfide SSB ready for market introduction by 2025. | - | 2025 | [119,120] |
| LGES | SKI has announced their SSB will be ready for market penetration by 2030. | - | 2030+ | [119,120] |
| PowerSolid | In addition, PowerSolid plans to develop a prototype car with an SSB before 2025 and a series-produced SSB for passenger cars by the end of the decade in collaboration with BMW and Ford. Since 2018, Hyundai has also taken a financial stake in PowerSolid. PowerSolid plans to develop a 100 Ah cell with a Si anode by 2026 and a 100 Ah cell with a Li metal anode by 2028, with investments from A123 Systems LLC. | BMW, Ford, Hyundai | 2025 | [119,120] |
| Prime Planet Energy | The cooperation between Toyota and Panasonic presented a prototype of a car equipped with an SSB in 2021. Although no technical data were published on this battery, it can be assumed that a sulfide electrolyte was used. They plan to bring the SSB to the market by 2025. | Toyota, Panasonic | 2025 | [119,120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, F.; Mahdi, L.; Lemaire, J.; Santos, D.M.F. Technological Advances and Market Developments of Solid-State Batteries: A Review. Materials 2024, 17, 239. https://doi.org/10.3390/ma17010239
Thomas F, Mahdi L, Lemaire J, Santos DMF. Technological Advances and Market Developments of Solid-State Batteries: A Review. Materials. 2024; 17(1):239. https://doi.org/10.3390/ma17010239
Chicago/Turabian StyleThomas, Felix, Lauren Mahdi, Julien Lemaire, and Diogo M. F. Santos. 2024. "Technological Advances and Market Developments of Solid-State Batteries: A Review" Materials 17, no. 1: 239. https://doi.org/10.3390/ma17010239
APA StyleThomas, F., Mahdi, L., Lemaire, J., & Santos, D. M. F. (2024). Technological Advances and Market Developments of Solid-State Batteries: A Review. Materials, 17(1), 239. https://doi.org/10.3390/ma17010239












