Heteropolyacid Ionic Liquid-Based MCF: An Efficient Heterogeneous Catalyst for Oxidative Desulfurization of Fuel
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Catalyst Preparation
2.2.1. Preparation of MCF
2.2.2. Grafting of 1-Butyl-3-methylimidazolium Chloride ([BMIM]Cl) on MCF
2.2.3. Preparation of Heteropolyacid Ionic Liquid-Based MCF
2.2.4. Catalyst Characterization
2.2.5. Preparation of Model Oil and Desulfurization
3. Results and Discussion
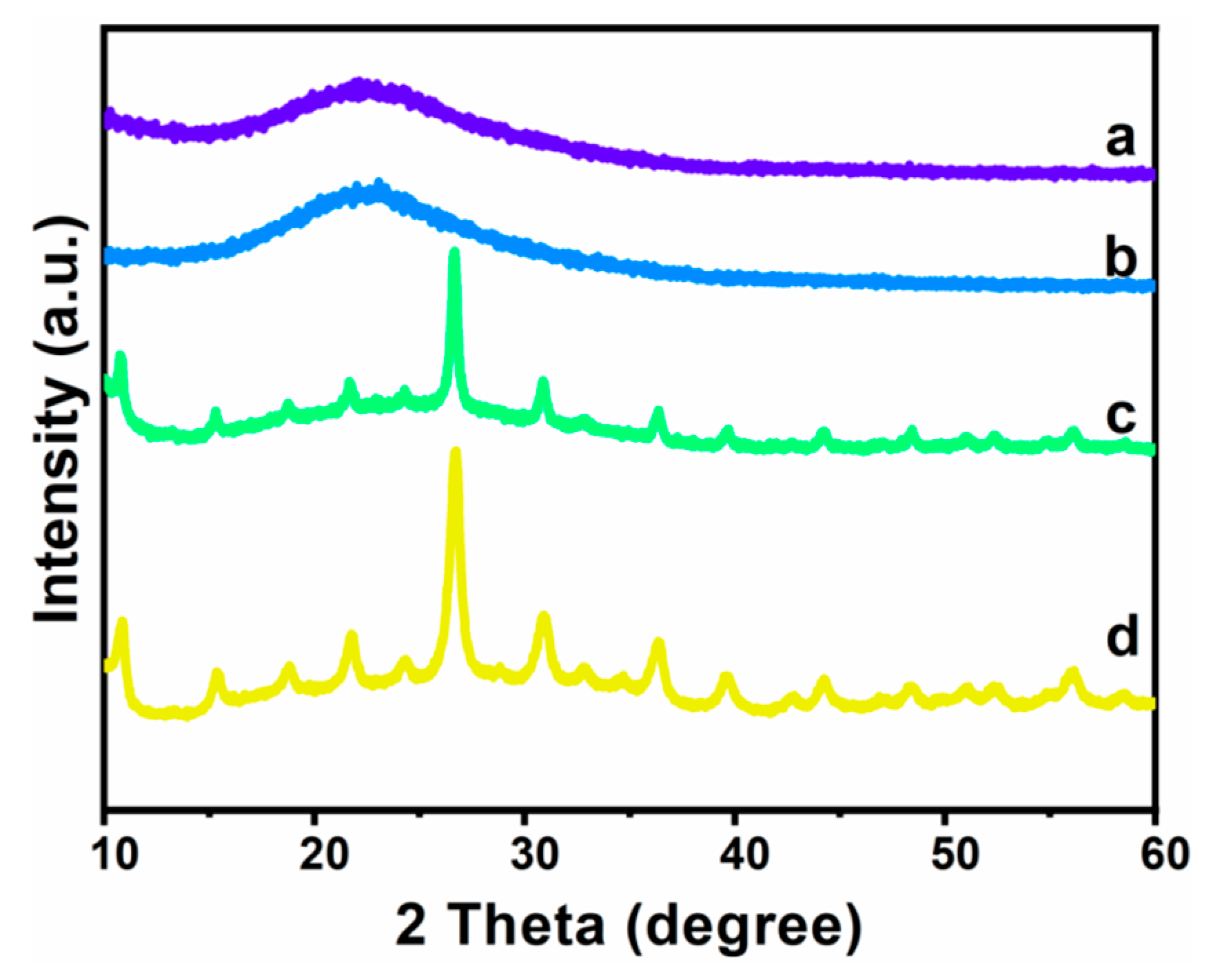
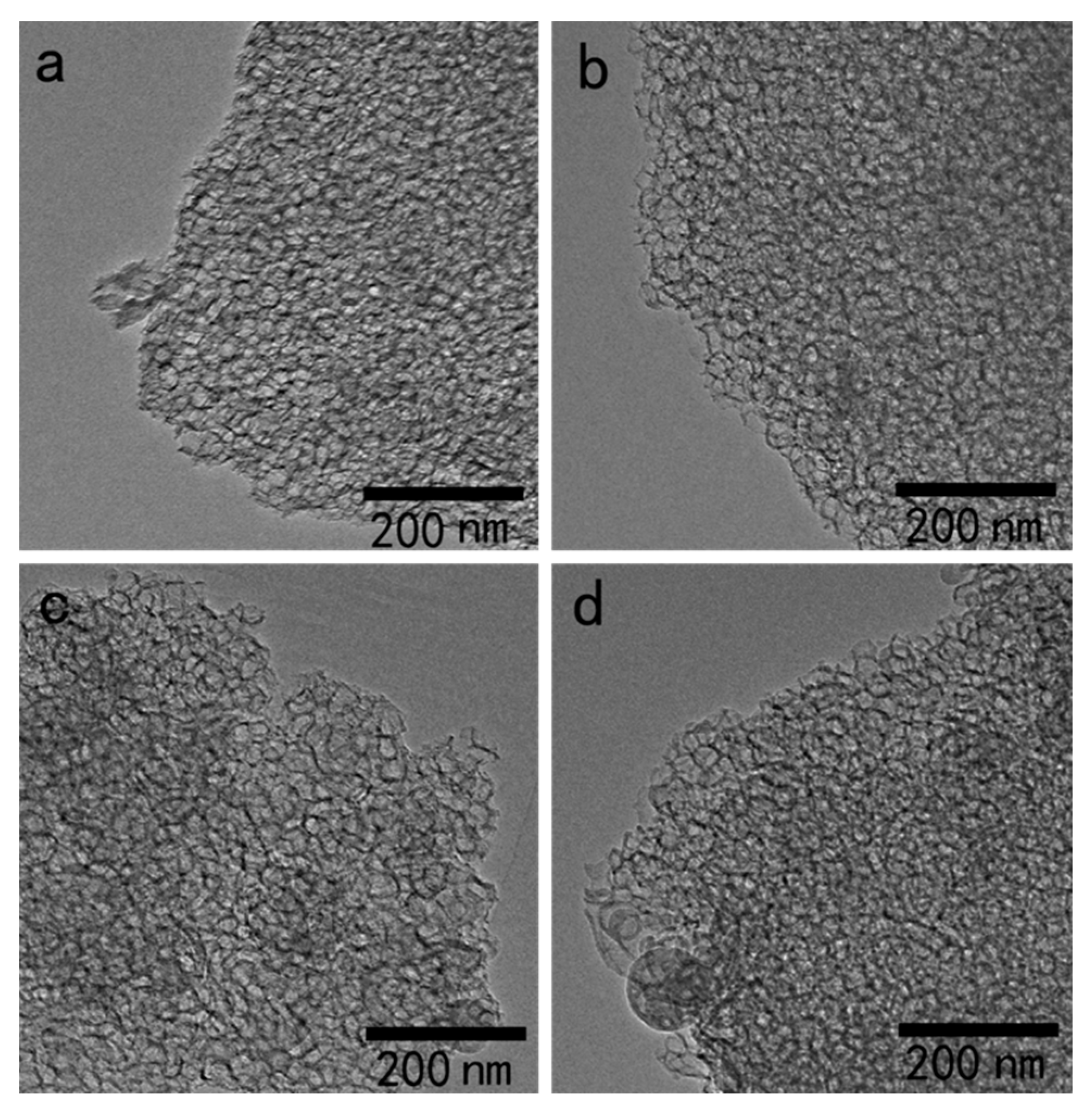
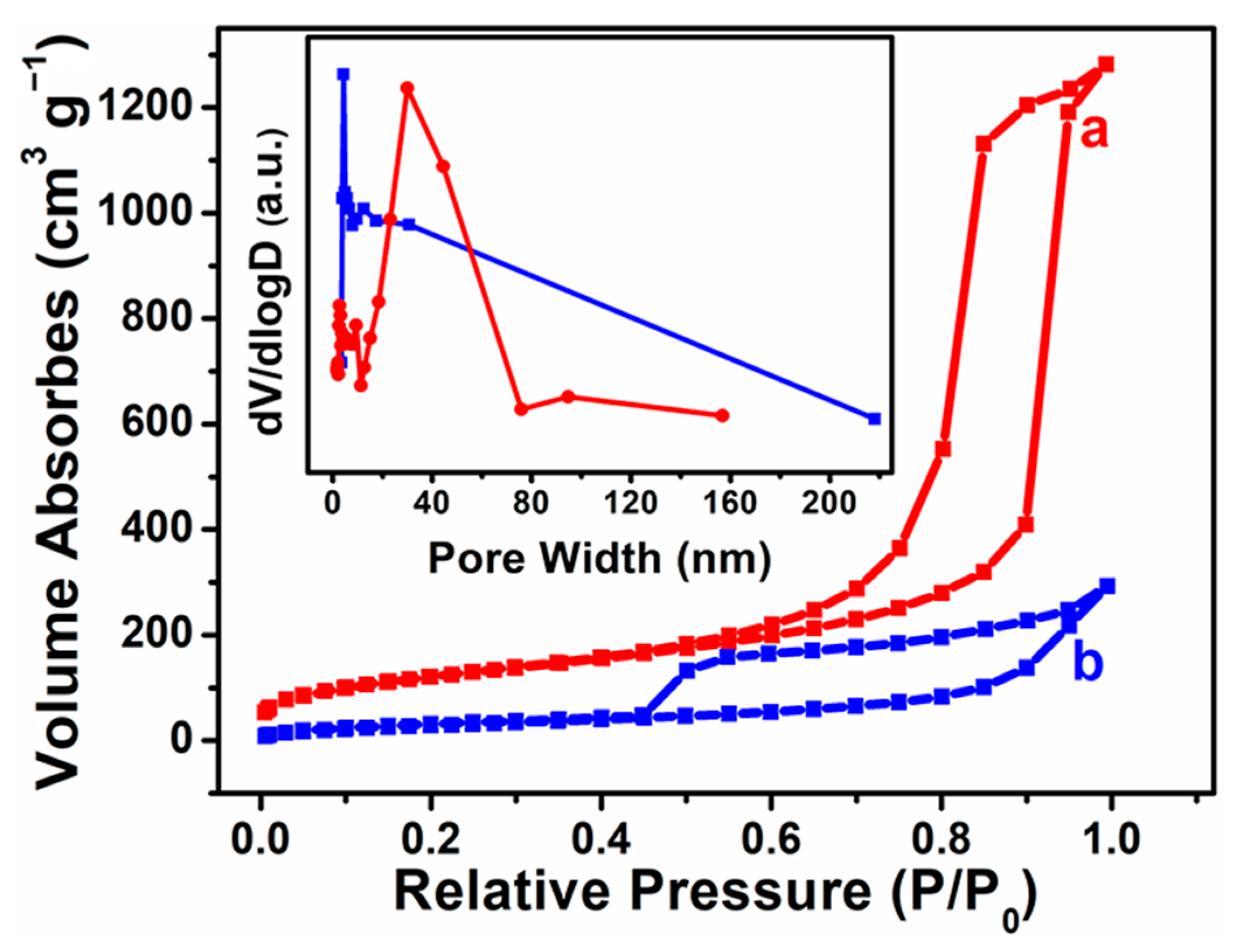
3.1. Morphological Characterization of Heteropolyacid Ionic Liquids-Based MCF
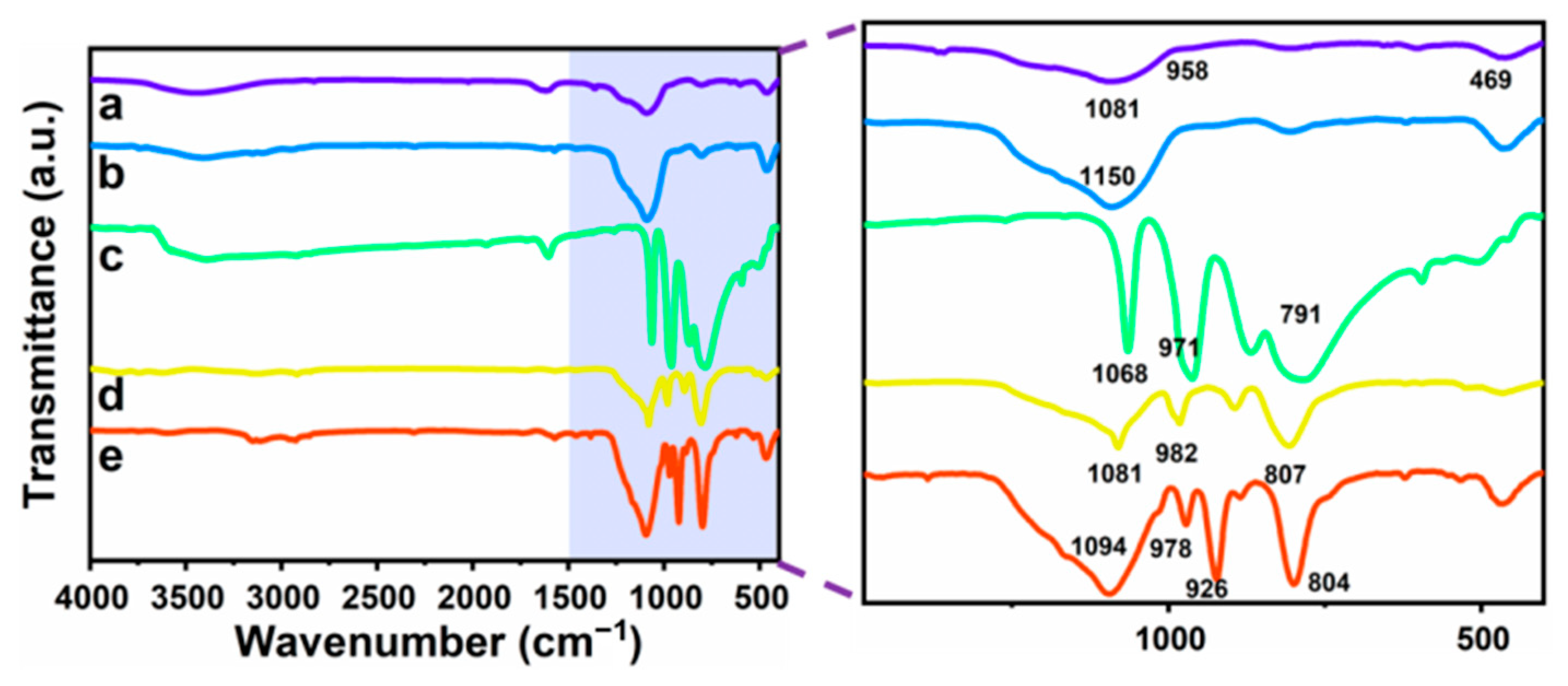
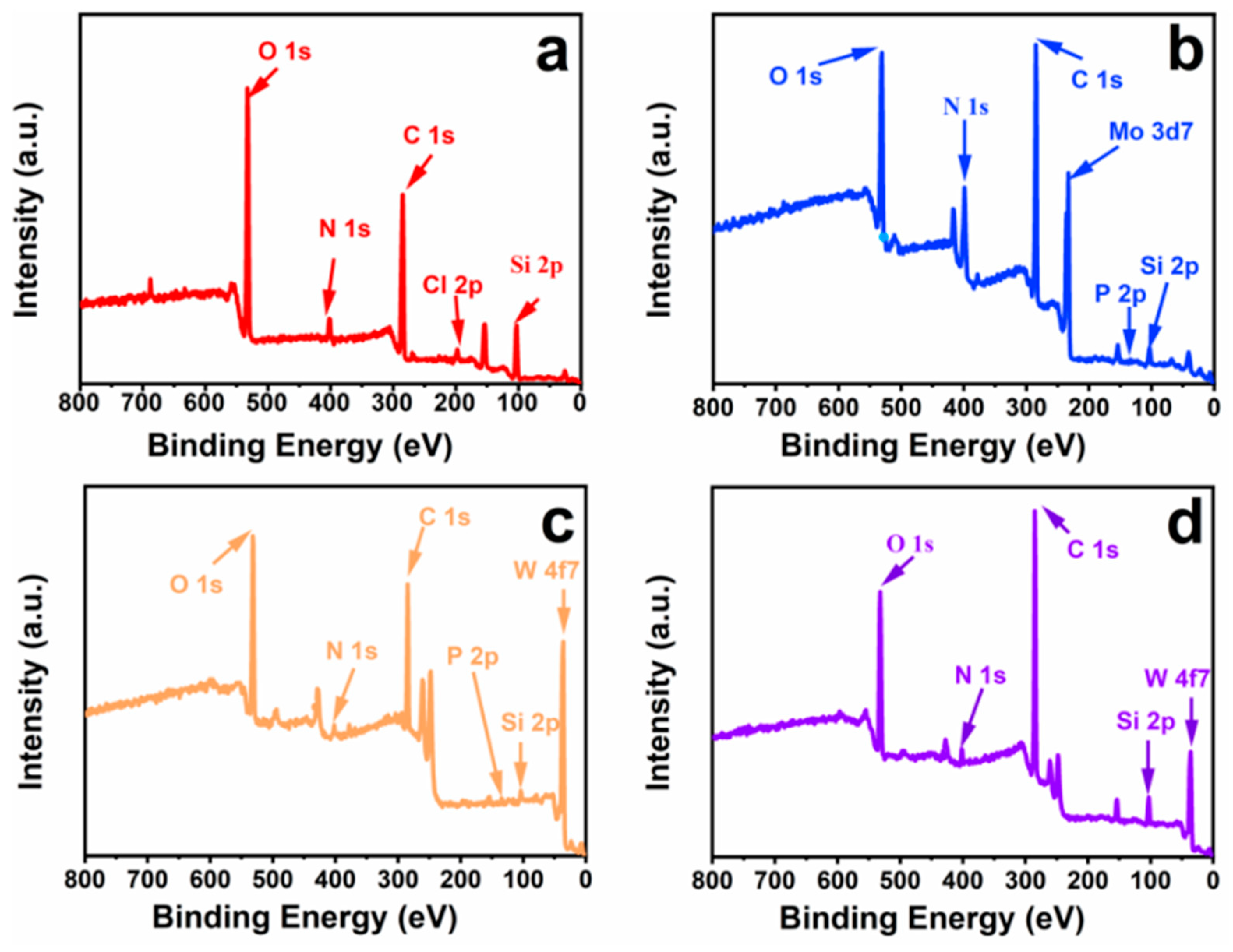
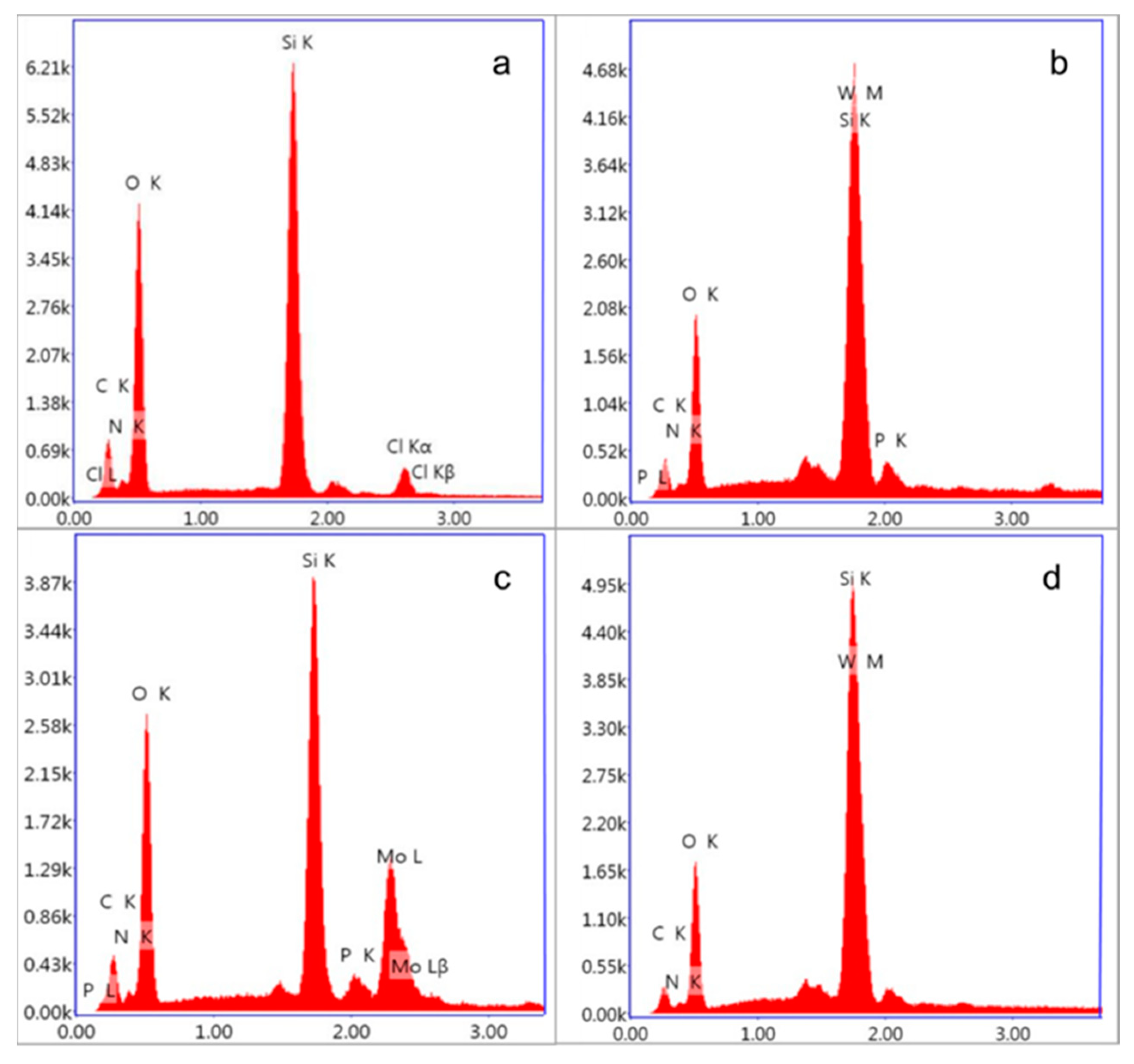
3.2. Composition and Elemental Analysis of Heteropolyacid Ionic Liquid-Based MCF
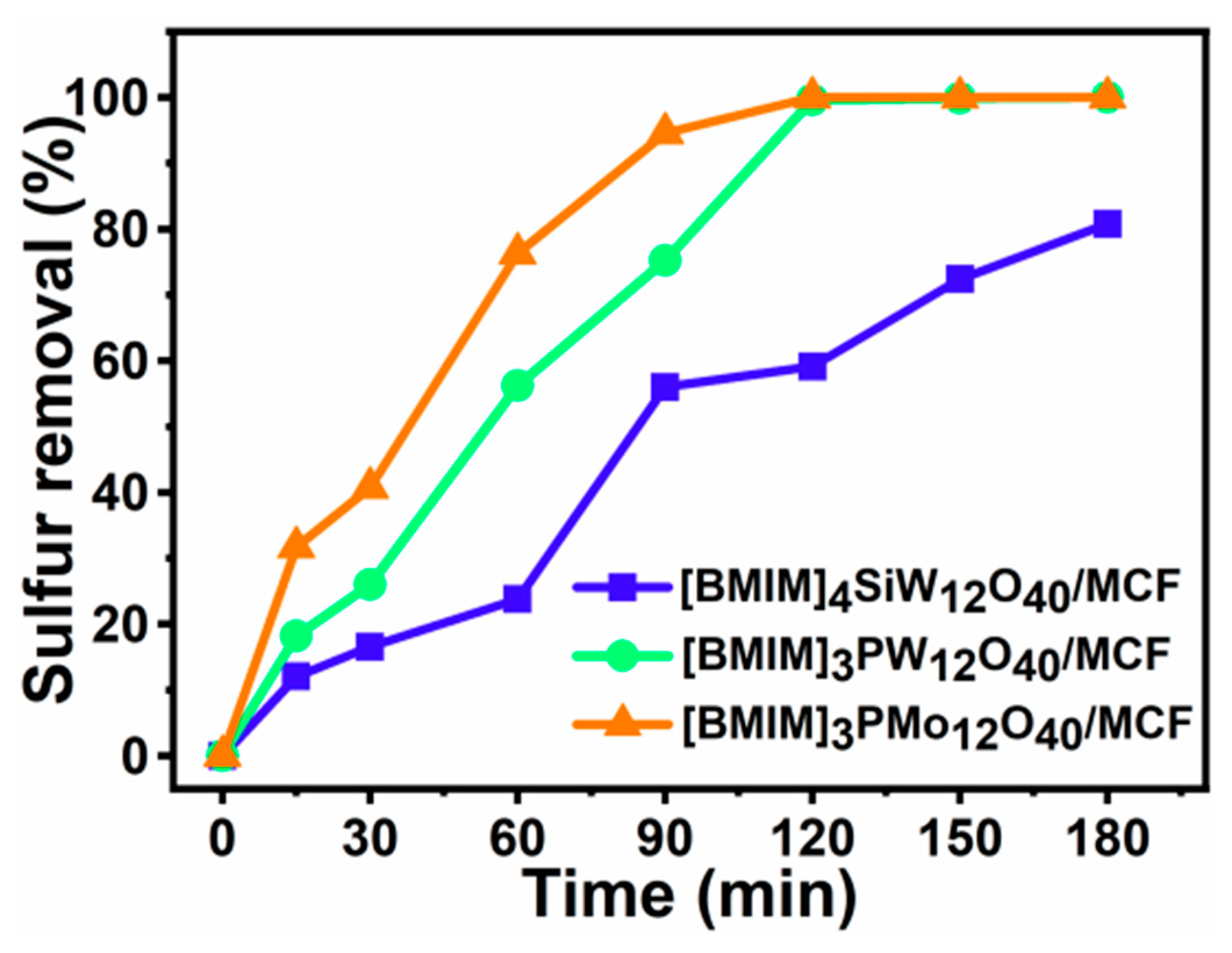
3.3. Influence of Different Catalyst on Sulfur Removal of DBT
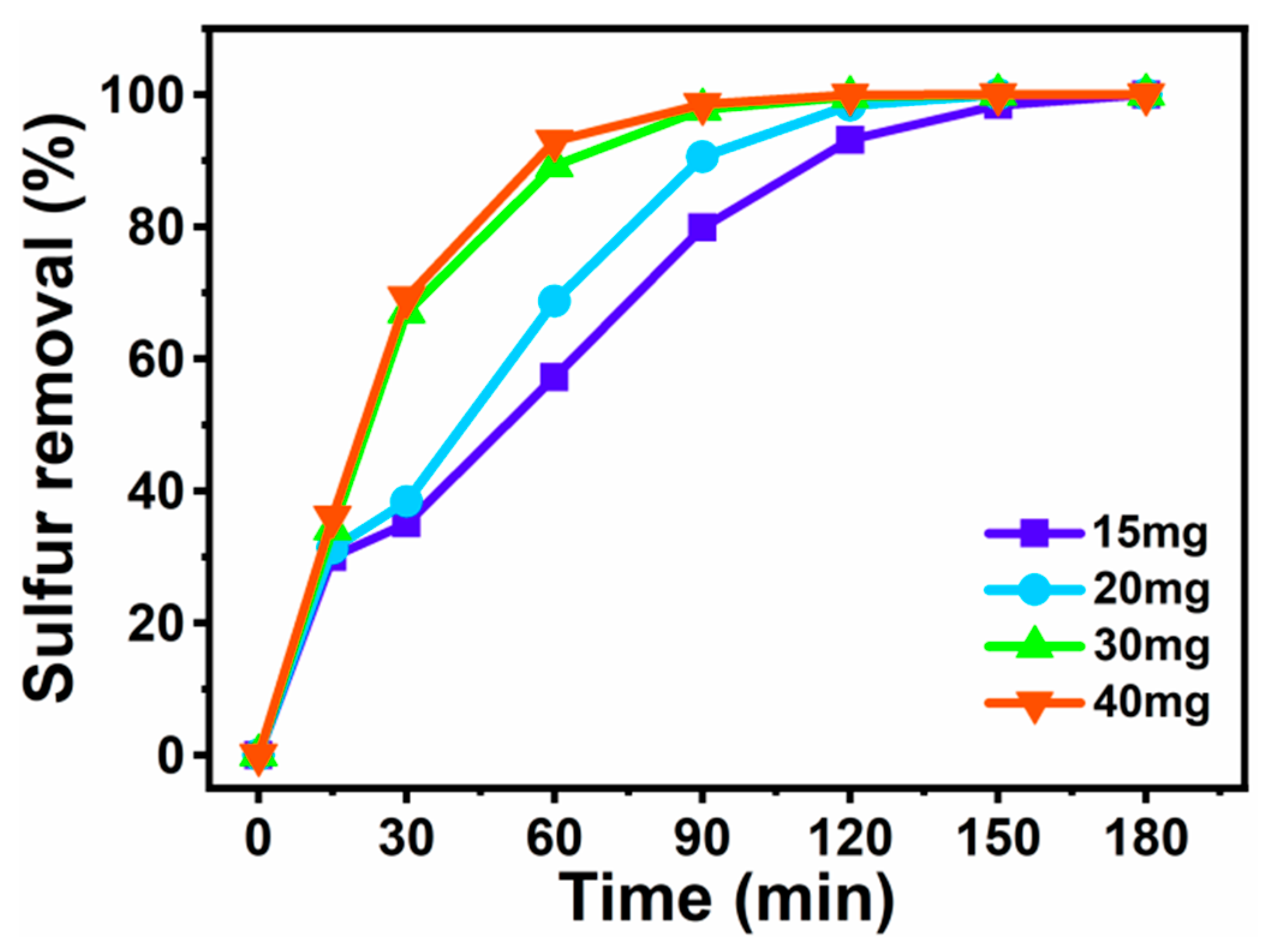
3.4. Effect of the Amount of [BMIM]3PMo12O40-Based MCF on Sulfur Removal of DBT
3.5. Influence of O/S Ratio on Sulfur Removal
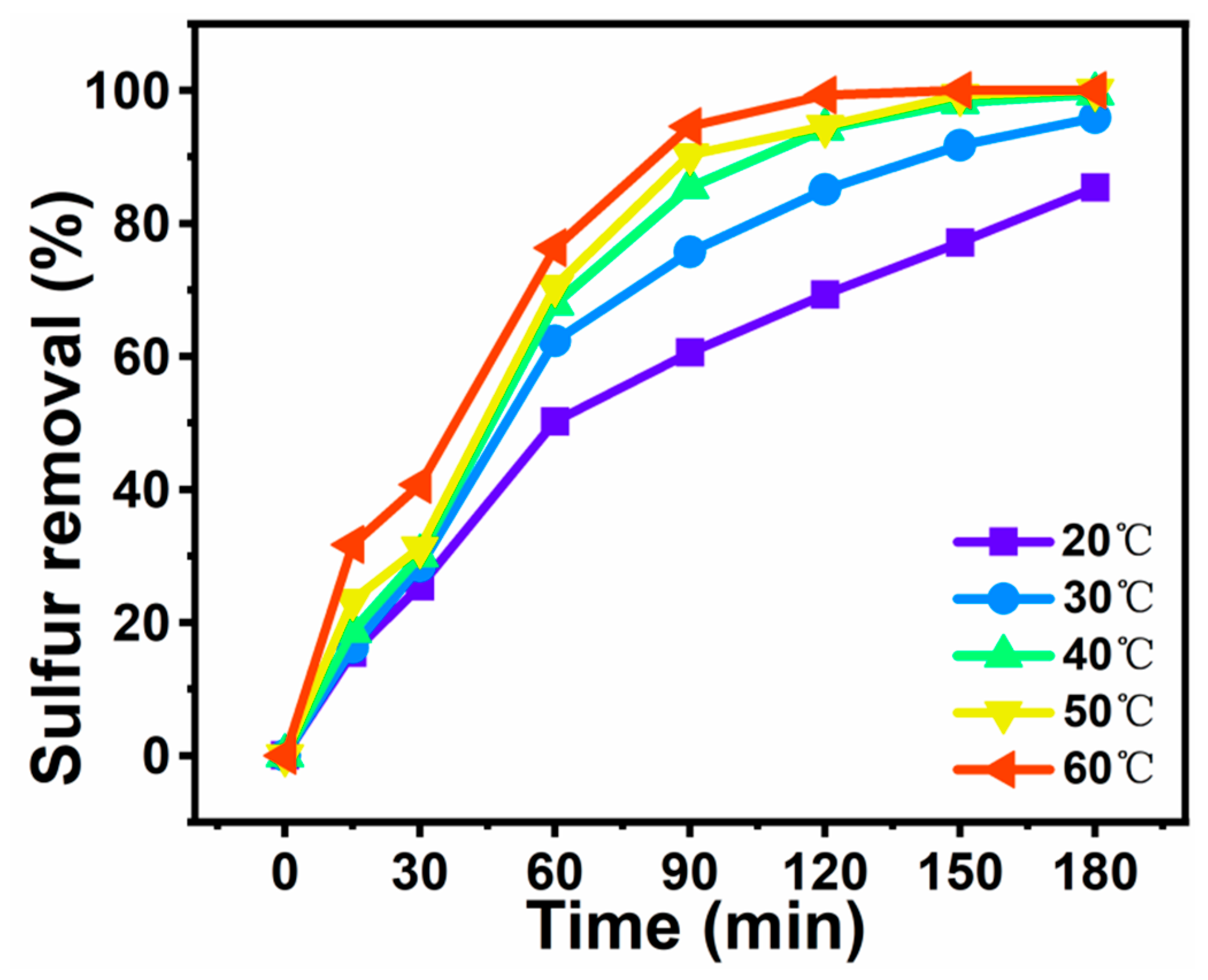
3.6. Sulfur Removal of Different Temperatures
3.7. Sulfur Removal of Different Sulfur-Containing Compounds
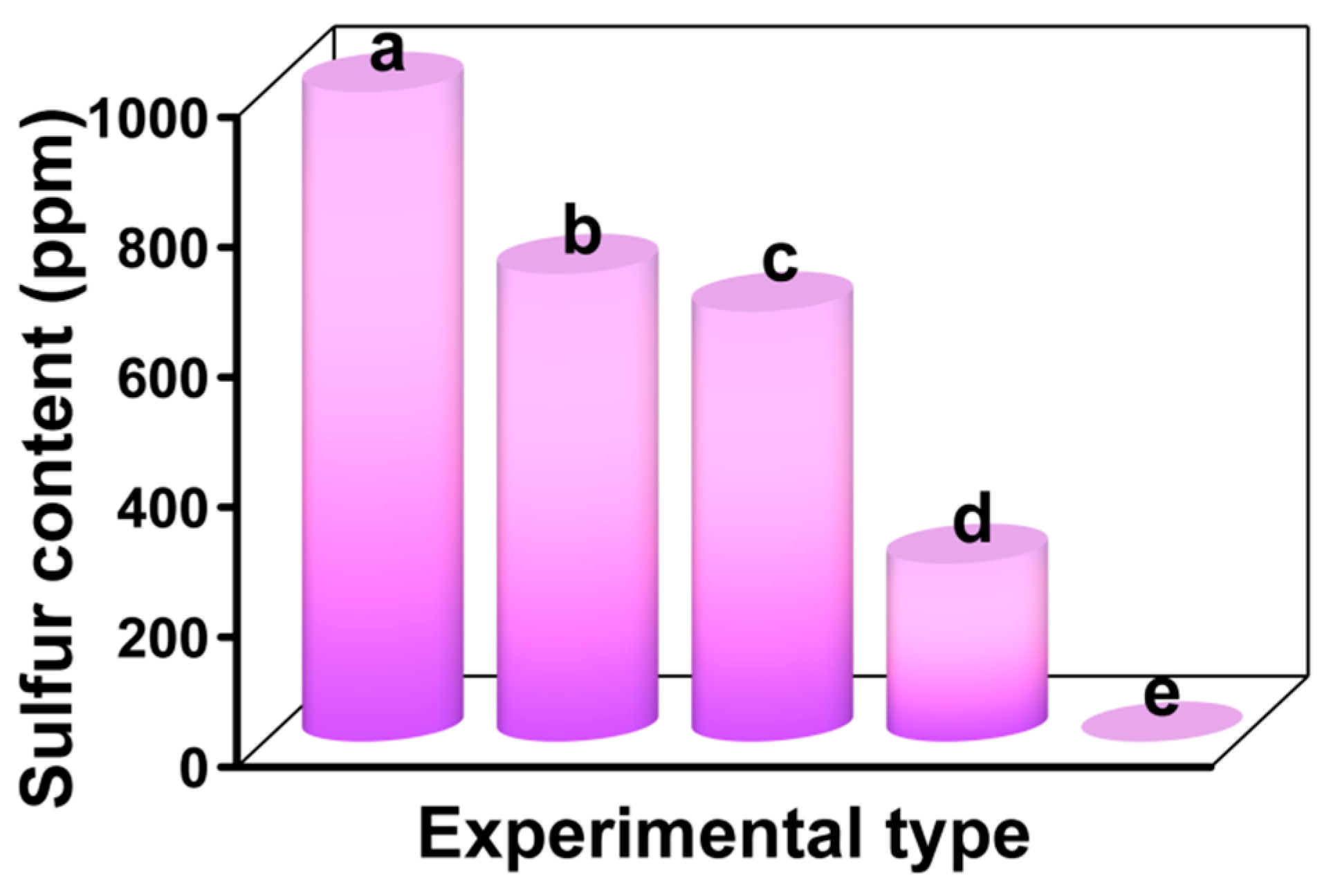
3.8. Sulfur Removal of Different Desulfurization Systems
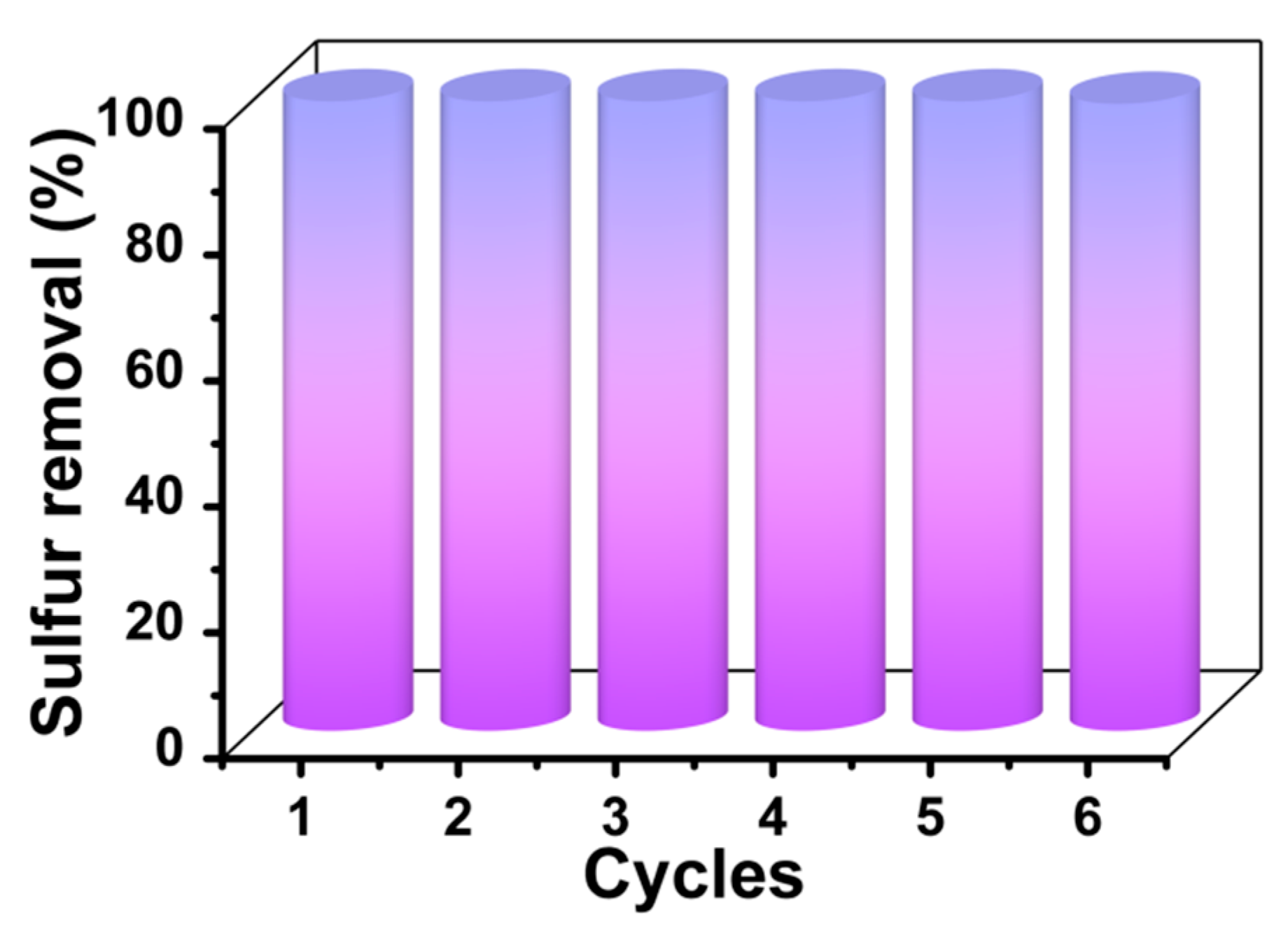
3.9. Reusability of [BMIM]3PMo12O40-Based MCF
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abd Al-Khodor, Y.A.; Albayati, T.M. Employing sodium hydroxide in desulfurization of the actual heavy crude oil: Theoretical optimization and experimental evaluation. Process Saf. Environ. Prot. 2020, 136, 334–342. [Google Scholar] [CrossRef]
- Houda, S.; Lancelot, C.; Blanchard, P.; Poinel, L.; Lamonier, C. Oxidative Desulfurization of Heavy Oils with High Sulfur Content: A Review. Catalysts 2018, 8, 344. [Google Scholar] [CrossRef]
- Ebadi, M.; Asri, M.; Beshkar, F. Novel Mo/Bi2MoO6/Bi3ClO4 heterojunction photocatalyst for ultra-deep desulfurization of thiophene under simulated sunlight irradiation. Adv. Powder Technol. 2021, 32, 2160–2170. [Google Scholar] [CrossRef]
- Qi, Z.; Qiu, T.; Wang, H.; Ye, C. Synthesis of ionic-liquid-functionalized UiO-66 framework by post-synthetic ligand exchange for the ultra-deep desulfurization. Fuel 2020, 268, 117336. [Google Scholar] [CrossRef]
- Wu, L.; Miao, G.; Dai, X.; Dong, L.; Li, Z.; Xiao, J. Ultra-Deep Desulfurization of Real Diesel Using Two-Layer Silica Gels under Mild Conditions. Energy Fuels 2019, 33, 7287–7296. [Google Scholar] [CrossRef]
- Lebeau, B.; Bonne, M.; Comparot, J.D.; Rousseau, J.; Michelin, L.; Blin, J.L.; Brunet, S. HDS of 4,6-dimethyldibenzothiophene over CoMoS supported mesoporous SiO2-TiO2 materials. Catal. Today 2020, 357, 675–683. [Google Scholar] [CrossRef]
- Liu, X.; Wei, Q.; Huang, W.; Zhou, Y.; Zhang, P.; Xu, Z. DFT insights into the stacking effects on HDS of 4,6-DMDBT on Ni-Mo-S corner sites. Fuel 2020, 280, 118669. [Google Scholar] [CrossRef]
- Saleh, T.A.; AL-Hammadi, S.A. A novel catalyst of nickel-loaded graphene decorated on molybdenum-alumina for the HDS of liquid fuels. Chem. Eng. J. 2021, 406, 125167. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, T.; Zhang, S.; Fan, Y.; Chen, B. Extractive desulfurization of fuels using trialkylamine-based protic ionic liquids. Sep. Purif. Technol. 2020, 231, 115923. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, S.; Dai, Y.; Xiong, C.; Li, C.; Yang, W.; Jiang, X. Performance and mechanism for extractive desulfurization of fuel oil using modified polyethylene glycol. Fuel 2018, 233, 704–713. [Google Scholar] [CrossRef]
- Wagle, D.V.; Zhao, H.; Deakyne, C.A.; Baker, G.A. Quantum Chemical Evaluation of Deep Eutectic Solvents for the Extractive Desulfurization of Fuel. ACS Sustain. Chem. Eng. 2018, 6, 7525–7531. [Google Scholar] [CrossRef]
- Huo, Q.; Li, J.; Qi, X.; Liu, G.; Zhang, X.; Zhang, B.; Ning, Y.; Fu, Y.; Liu, J.; Liu, S. Cu, Zn-embedded MOF-derived bimetallic porous carbon for adsorption desulfurization. Chem. Eng. J. 2019, 378, 122106. [Google Scholar] [CrossRef]
- Luo, J.; Chao, Y.; Tang, Z.; Hua, M.; Li, X.; Wei, Y.; Ji, H.; Xiong, J.; Zhu, W.; Li, H. Design of Lewis Acid Centers in Bundlelike Boron Nitride for Boosting Adsorptive Desulfurization Performance. Ind. Eng. Chem. Res. 2019, 58, 13303–13312. [Google Scholar] [CrossRef]
- Ding, R.-D.; Zu, Y.; Zhou, C.-H.; Wang, H.; Mo, Z.-S.; Qin, Y.-C.; Sun, Z.-L.; Song, L.-J. Insight into the correlation between the effective adsorption sites and adsorption desulfurization performance of CuNaY zeolite. J. Fuel Chem. Technol. 2018, 46, 451–458. [Google Scholar] [CrossRef]
- Dasgupta, S.; Divekar, S.; Arya, A.; Gupta, P.; Chauhan, R.; Bhadauria, S.; Hanif, A.; Garg, M.O.; Nanoti, A. A vapor phase adsorptive desulfurization process for producing ultra low sulfur diesel using NiY zeolite as a regenerable adsorbent. RSC Adv. 2015, 5, 56060–56066. [Google Scholar] [CrossRef]
- Ribeiro, S.O.; Duarte, B.; De Castro, B.; Granadeiro, C.M.; Balula, S.S. Improving the Catalytic Performance of Keggin [PW12O40]3− for Oxidative Desulfurization: Ionic Liquids versus SBA-15 Composite. Materials 2018, 11, 1196. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Zhou, F.; Zhang, J.; Yang, H.; Wang, Y.; Zhao, Y.; Yuan, X.; Ju, J.; Hu, S. Construction of novel amphiphilic [Bmin]3PMo12O40/g-C3N4 heterojunction catalyst with outstanding photocatalytic oxidative desulfurization performance under visible light. J. Taiwan Inst. Chem. Eng. 2019, 100, 210–219. [Google Scholar] [CrossRef]
- Rajendran, A.; Fan, H.-X.; Cui, T.-Y.; Feng, J.; Li, W.-Y. Enrichment of polymeric WOx species in WOx@SnO2 catalysts for ultra-deep oxidative desulfurization of liquid fuels. Fuel 2021, 290, 120036. [Google Scholar] [CrossRef]
- Shafiq, I.; Hussain, M.; Rashid, R.; Shafique, S.; Akhter, P.; Yang, W.; Ahmed, A.; Nawaz, Z.; Park, Y.-K. Development of hierarchically porous LaVO4 for efficient visible-light-driven photocatalytic desulfurization of diesel. Chem. Eng. J. 2021, 420, 130529. [Google Scholar] [CrossRef]
- Ribeiro, S.O.; Granadeiro, C.M.; Almeida, P.L.; Pires, J.; Capel-Sanchez, M.C.; Campos-Martin, J.M.; Gago, S.; De Castro, B.; Balula, S.S. Oxidative desulfurization strategies using Keggin-type polyoxometalate catalysts: Biphasic versus solvent-free systems. Catal. Today 2019, 333, 226–236. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Dong, J.; Liu, D.; Liu, C.; Chi, Y.; Hu, C. Polyoxometalates encapsulated into hollow double-shelled nanospheres as amphiphilic nanoreactors for an effective oxidative desulfurization. Nanoscale 2020, 12, 16586–16595. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, M.A.; Khalafi, N. Deep oxidative desulfurization of real fuel and thiophenic model fuels using polyoxometalate-based catalytic nanohybrid material. Mater. Today Commun. 2020, 22, 100730. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, G.; Long, Z.; Wang, J. Recent advances in polyoxometalate-based heterogeneous catalytic materials for liquid-phase organic transformations. RSC Adv. 2014, 4, 42092–42113. [Google Scholar] [CrossRef]
- Zhang, Q.; Lei, D.; Luo, Q.; Wang, J.; Deng, T.; Zhang, Y.; Ma, P. Efficient biodiesel production from oleic acid using metal–organic framework encapsulated Zr-doped polyoxometalate nano-hybrids. RSC Adv. 2020, 10, 8766–8772. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, L.; Sun, Y.; Jiang, B.; Chen, Y.; Gao, X.; Yang, H. Deep catalytic oxidative desulfurization of fuels by novel Lewis acidic ionic liquids. Fuel Process. Technol. 2018, 177, 81–88. [Google Scholar] [CrossRef]
- Li, S.-W.; Gao, R.-M.; Zhao, J.-S. Deep Oxidative Desulfurization of Fuel Catalyzed by Modified Heteropolyacid: The Comparison Performance of Three Kinds of Ionic Liquids. ACS Sustain. Chem. Eng. 2018, 6, 15858–15866. [Google Scholar] [CrossRef]
- Jiang, B.; Yang, H.; Zhang, L.; Zhang, R.; Sun, Y.; Huang, Y. Efficient oxidative desulfurization of diesel fuel using amide-based ionic liquids. Chem. Eng. J. 2016, 283, 89–96. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Z.; Yao, X.; Jiang, W.; Zhang, M.; Li, H.; Liu, H.; Zhu, W.; Li, H. One-pot extraction and aerobic oxidative desulfurization with highly dispersed V2O5/SBA-15 catalyst in ionic liquids. RSC Adv. 2017, 7, 39383–39390. [Google Scholar] [CrossRef]
- Rezvani, M.A.; Aghbolagh, Z.S.; Monfared, H.H. Green and efficient organic–inorganic hybrid nanocatalyst for oxidative desulfurization of gasoline. Appl. Organomet. Chem. 2018, 32, e4592. [Google Scholar] [CrossRef]
- Haghighi, M.; Gooneh-Farahani, S. Insights to the oxidative desulfurization process of fossil fuels over organic and inorganic heterogeneous catalysts: Advantages and issues. Environ. Sci. Pollut. Res. 2020, 27, 39923–39945. [Google Scholar] [CrossRef]
- Huang, P.; Luo, G.; Kang, L.; Zhu, M.; Dai, B. Preparation, characterization and catalytic performance of HPW/aEVM catalyst on oxidative desulfurization. RSC Adv. 2017, 7, 4681–4687. [Google Scholar] [CrossRef]
- Rajendran, A.; Cui, T.-Y.; Fan, H.-X.; Yang, Z.-F.; Feng, J.; Li, W.-Y. A comprehensive review on oxidative desulfurization catalysts targeting clean energy and environment. J. Mater. Chem. A 2020, 8, 2246–2285. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Song, J.Y.; Khan, N.A.; Jhung, S.H. TiO2-Containing Carbon Derived from a Metal–Organic Framework Composite: A Highly Active Catalyst for Oxidative Desulfurization. ACS Appl. Mater. Interfaces 2017, 9, 31192–31202. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, J.; Yu, H.; Han, S.; Wei, Y. Recent Advances of Anderson-Type Polyoxometalates as Catalysts Largely for Oxidative Transformations of Organic Molecules. Molecules 2022, 27, 5212. [Google Scholar] [CrossRef]
- Shah, W.A.; Ibrahim, S.; Abbas, S.; Naureen, L.; Batool, M.; Imran, M.; Nadeem, M.A. Nickel containing polyoxometalates incorporated in two different metal-organic frameworks for hydrogen evolution reaction. J. Environ. Chem. Eng. 2021, 9, 106004. [Google Scholar] [CrossRef]
- Bai, F.; Liu, X.; Sani, S.; Liu, Y.; Guo, W.; Sun, C. Amine functionalized mesocellular silica foam as highly efficient sorbents for CO2 capture. Sep. Purif. Technol. 2022, 299, 121539. [Google Scholar] [CrossRef]
- Balistreri, N.; Gaboriau, D.; Jolivalt, C.; Launay, F. Covalent immobilization of glucose oxidase on mesocellular silica foams: Characterization and stability towards temperature and organic solvents. J. Mol. Catal. B Enzym. 2016, 127, 26–33. [Google Scholar] [CrossRef]
- Bhadra, K.H.; Yadav, G.D. Solventless triarylmethane synthesis via hydroxyalkylation of anisole with benzaldehyde by modified heteropoly acid on mesocellular foam silica (MCF). Mol. Catal. 2018, 455, 150–158. [Google Scholar] [CrossRef]
- Yongquan, D.; Ming, W.; Lin, C.; Mingjun, L. Preparation, characterization of P(VDF-HFP)/[bmim]BF4 ionic liquids hybrid membranes and their pervaporation performance for ethyl acetate recovery from water. Desalination 2012, 295, 53–60. [Google Scholar] [CrossRef]
- Holomb, R.; Martinelli, A.; Albinsson, I.; Lassègues, J.C.; Johansson, P.; Jacobsson, P. Ionic liquid structure: The conformational isomerism in 1-butyl-3-methyl-imidazolium tetrafluoroborate ([bmim] [BF4]). J. Raman Spectrosc. 2008, 39, 793–805. [Google Scholar] [CrossRef]
- Kim, H.; Jung, J.C.; Kim, P.; Yeom, S.H.; Lee, K.-Y.; Song, I.K. Preparation of H3PMo12O40 catalyst immobilized on surface modified mesostructured cellular foam (SM-MCF) silica and its application to the ethanol conversion reaction. J. Mol. Catal. A Chem. 2006, 259, 150–155. [Google Scholar] [CrossRef]
- Rajkumar, T.; Ranga Rao, G. Investigation of hybrid molecular material prepared by ionic liquid and polyoxometalate anion. J. Chem. Sci. 2008, 120, 587–594. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, A.; Li, X.; Ma, X. Oxidative desulfurization of dibenzothiophene and diesel over [Bmim]3PMo12O40. J. Catal. 2011, 279, 269–275. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, H.; Wei, Z.; Jiang, Y.; Zhang, L.; Zhao, J.; Zhang, J.; Dong, L.; Li, E.; Ruhlmann, L. Catalytic Emulsion Based on Janus Nanosheets for Ultra-Deep Desulfurization. Chem.—A Eur. J. 2017, 23, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kang, T.H.; Song, J.H.; Bang, Y.; Song, I.K. Redox behavior and oxidation catalysis of HnXW12O40 (X = Co2+, B3+, Si4+ and P5+) Keggin heteropolyacid catalysts. Catal. Commun. 2014, 43, 155–158. [Google Scholar] [CrossRef]
- Capel-Sanchez, M.C.; Campos-Martin, J.M.; Fierro, J.L. Removal of refractory organosulfur compounds via oxidation with hydrogen peroxide on amorphous Ti/SiO2 catalysts. Energy Environ. Sci. 2010, 3, 328–333. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Xiao, J.; Zhao, Z.; Yu, M.; Li, Z. Carbon nanotube catalysts for oxidative desulfurization of a model diesel fuel using molecular oxygen. Green Chem. 2014, 16, 211–220. [Google Scholar] [CrossRef]
- Kavoosi, G.; Teixeira da Silva, J.A.; Saharkhiz, M.J. Inhibitory effects of Zataria multiflora essential oil and its main components on nitric oxide and hydrogen peroxide production in lipopolysaccharide-stimulated macrophages. J. Pharm. Pharmacol. 2012, 64, 1491–1500. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, T.; Chen, Y.; Wang, H.; Xia, L. Heteropolyacid Ionic Liquid-Based MCF: An Efficient Heterogeneous Catalyst for Oxidative Desulfurization of Fuel. Materials 2023, 16, 3195. https://doi.org/10.3390/ma16083195
Pei T, Chen Y, Wang H, Xia L. Heteropolyacid Ionic Liquid-Based MCF: An Efficient Heterogeneous Catalyst for Oxidative Desulfurization of Fuel. Materials. 2023; 16(8):3195. https://doi.org/10.3390/ma16083195
Chicago/Turabian StylePei, Tingting, Yaxian Chen, Huiting Wang, and Lixin Xia. 2023. "Heteropolyacid Ionic Liquid-Based MCF: An Efficient Heterogeneous Catalyst for Oxidative Desulfurization of Fuel" Materials 16, no. 8: 3195. https://doi.org/10.3390/ma16083195
APA StylePei, T., Chen, Y., Wang, H., & Xia, L. (2023). Heteropolyacid Ionic Liquid-Based MCF: An Efficient Heterogeneous Catalyst for Oxidative Desulfurization of Fuel. Materials, 16(8), 3195. https://doi.org/10.3390/ma16083195



