Achieving Large-Capability Adsorption of Hg0 in Wet Scrubbing by Defect-Rich Colloidal Copper Sulfides under High-SO2 Atmosphere
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Synthesis of TMSs
2.3. Sample Characterization
2.4. Hg0 Removal Test
2.5. DFT Calculation
3. Results and Discussion
3.1. Selection and Optimization of Transition Metal Sulfides for Hg0 Removal
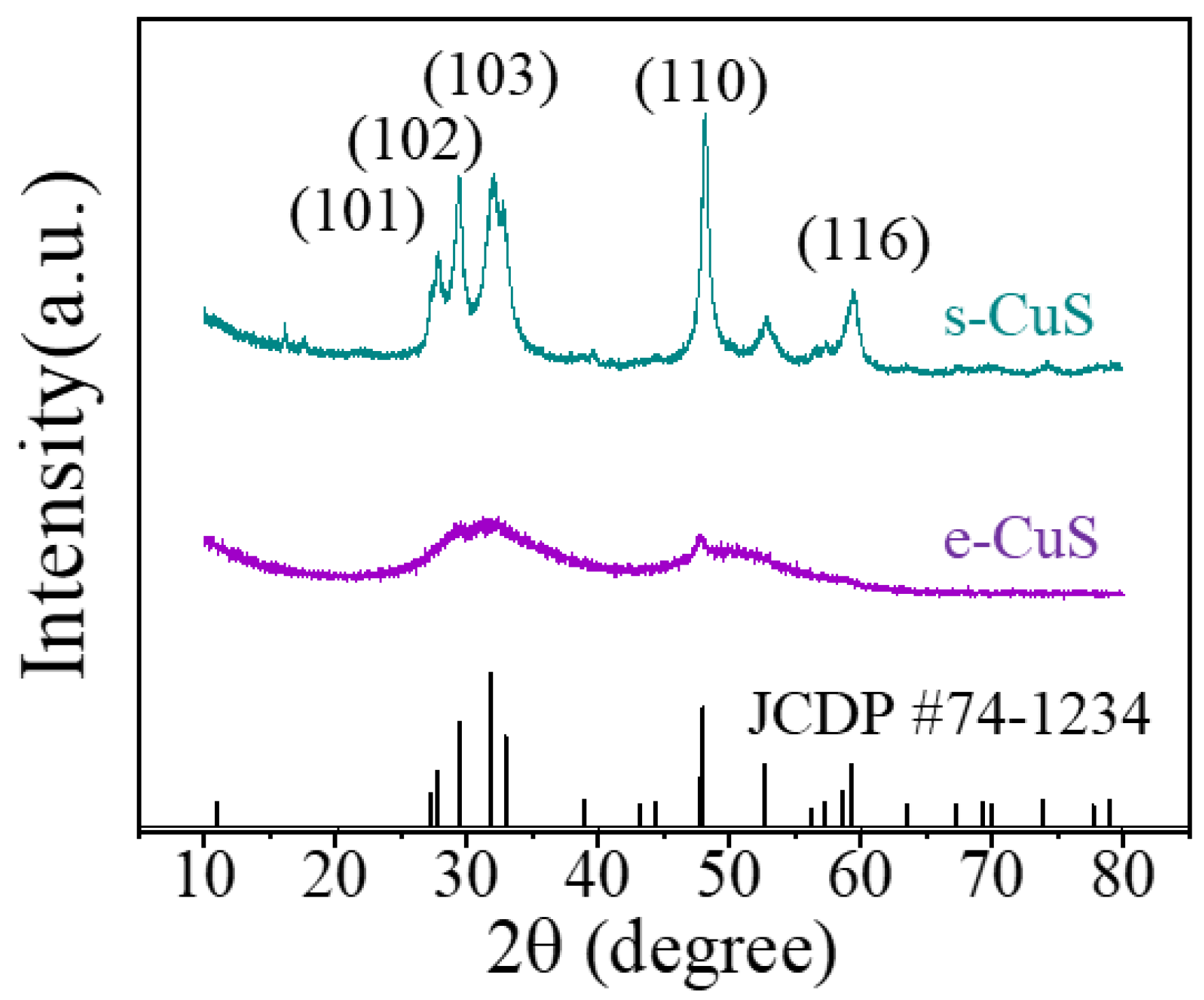
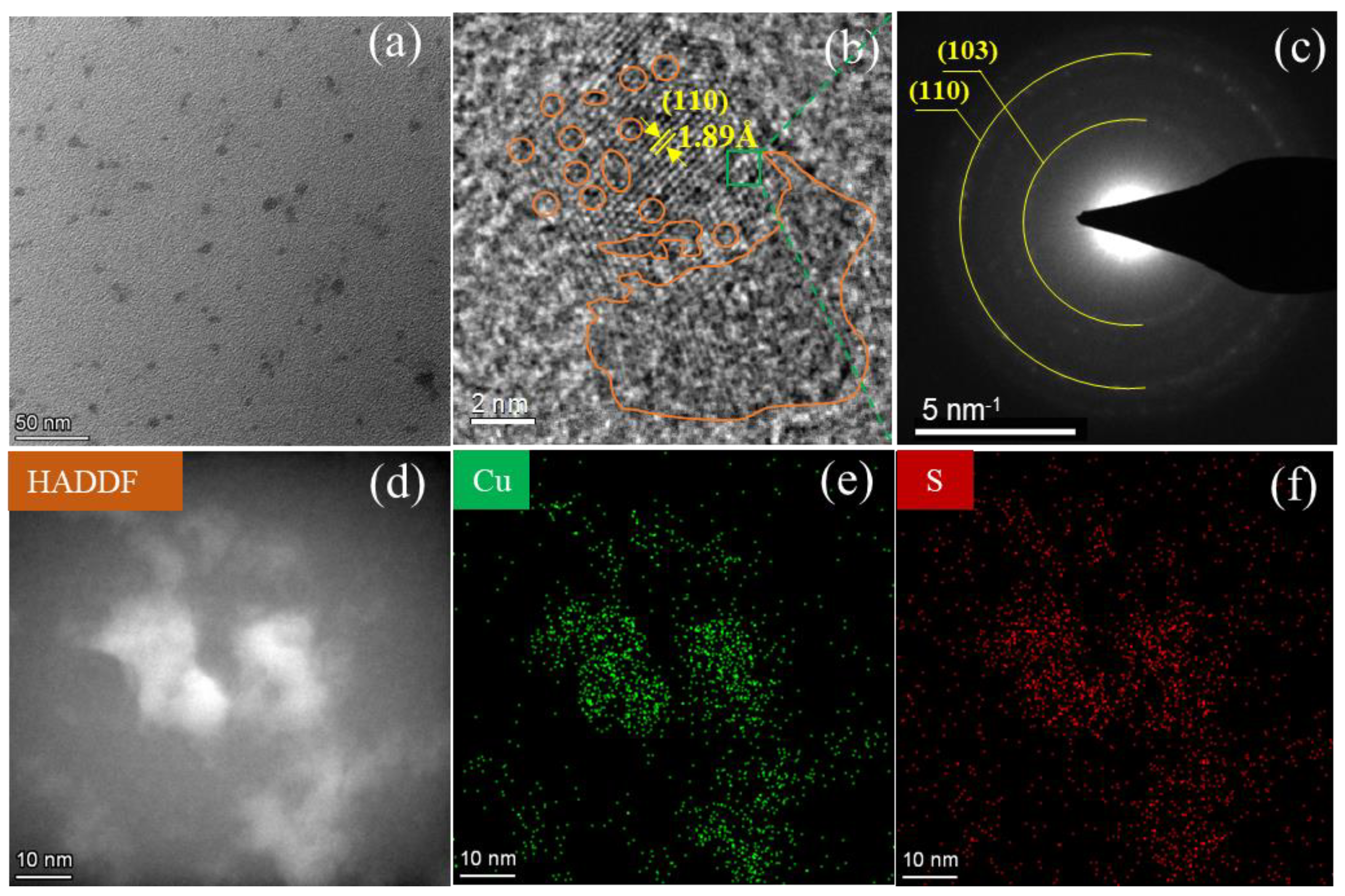
3.2. Structural Characterizations of c-CuS
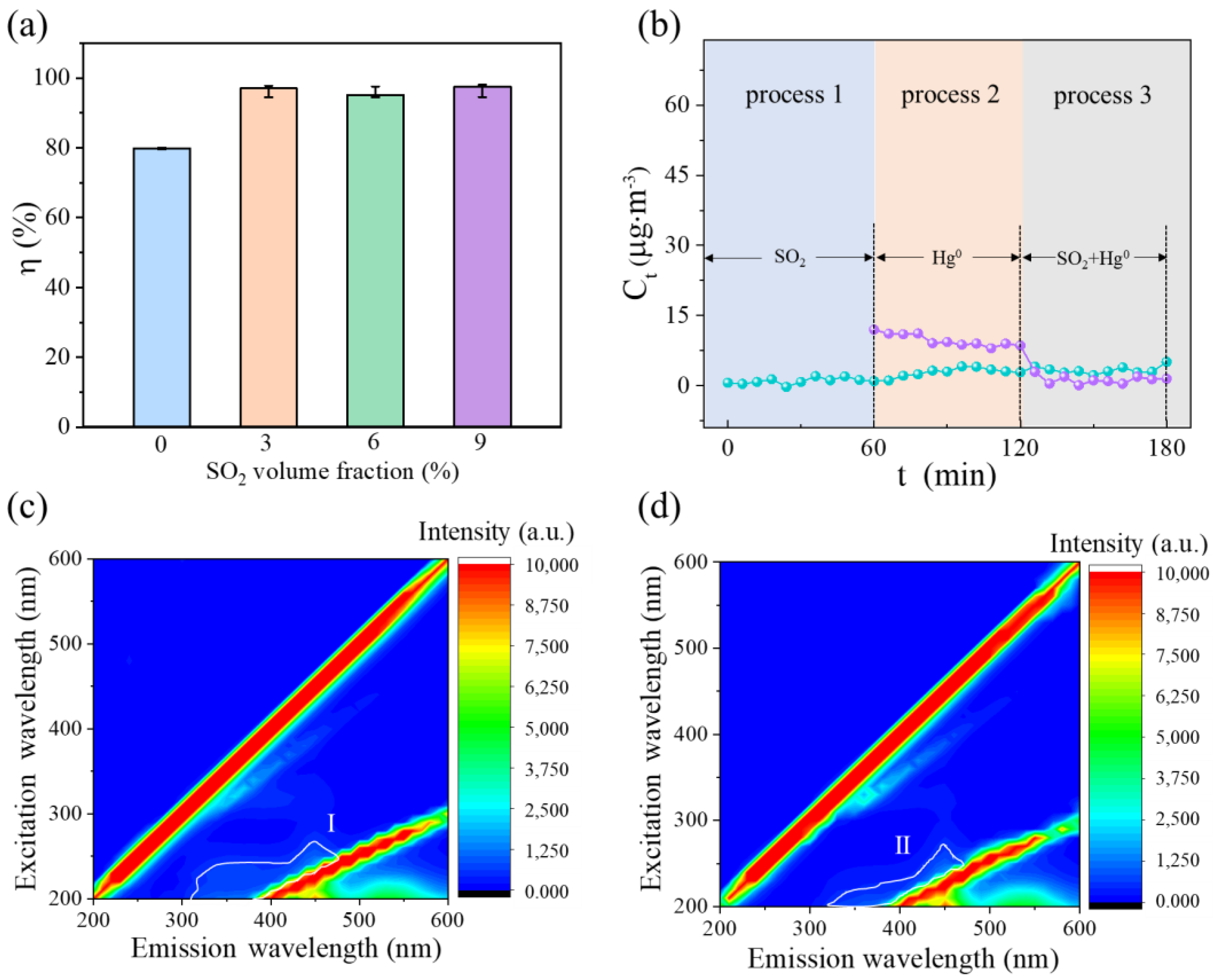
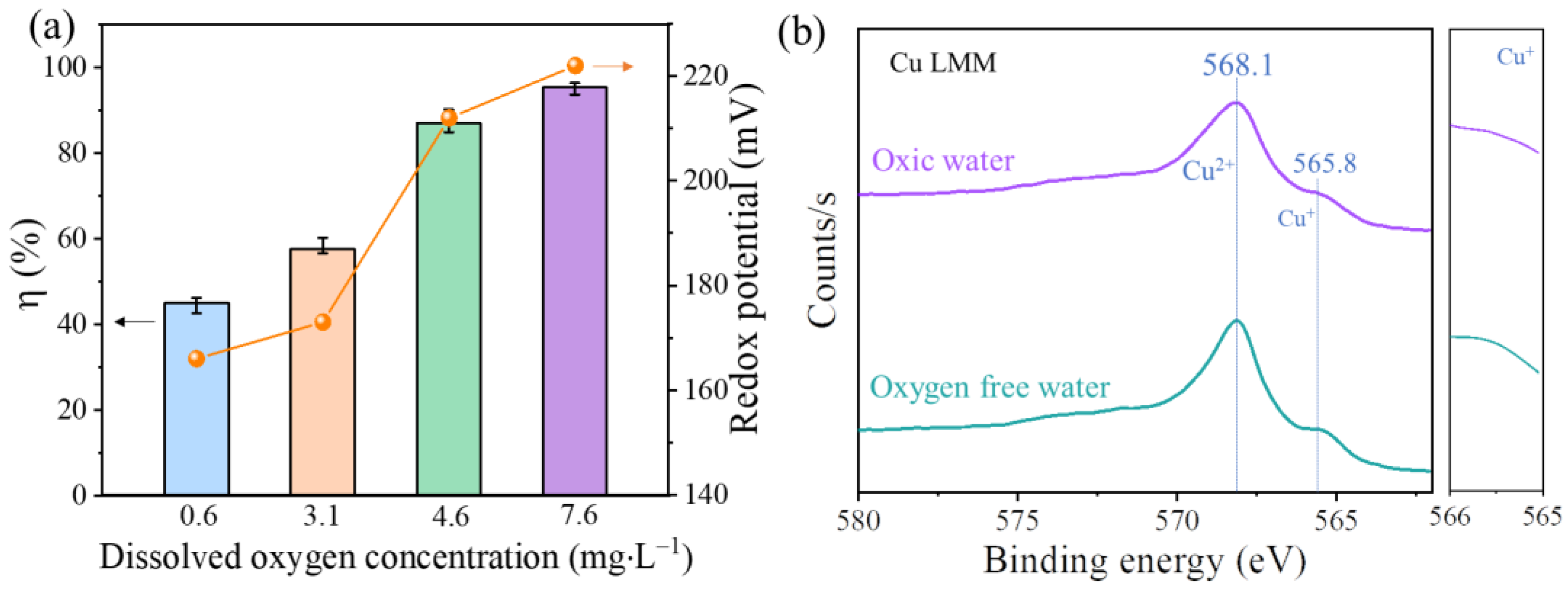
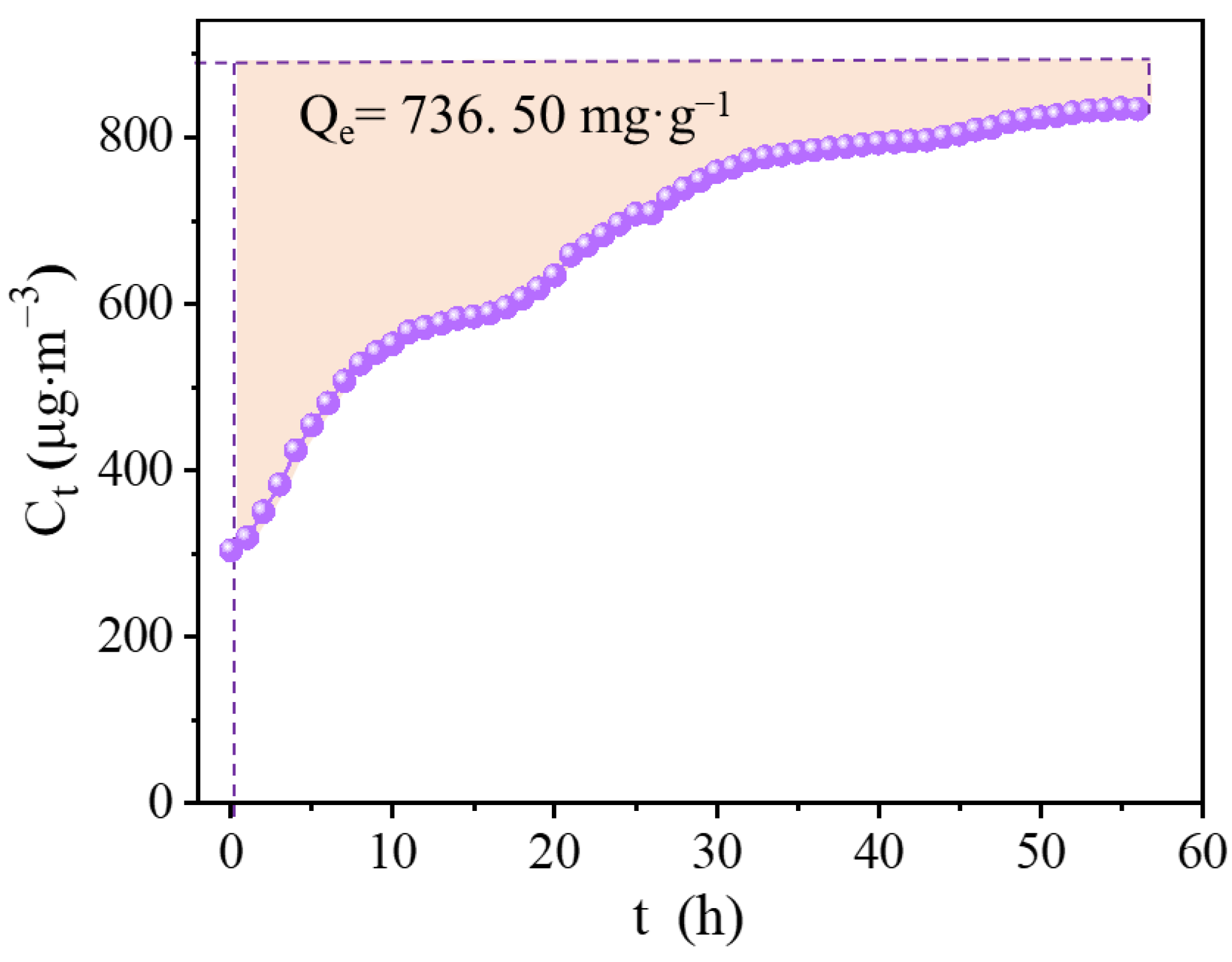
3.3. Hg0 Removal Performance of c-CuS under SO2 and O2 Atmospheres
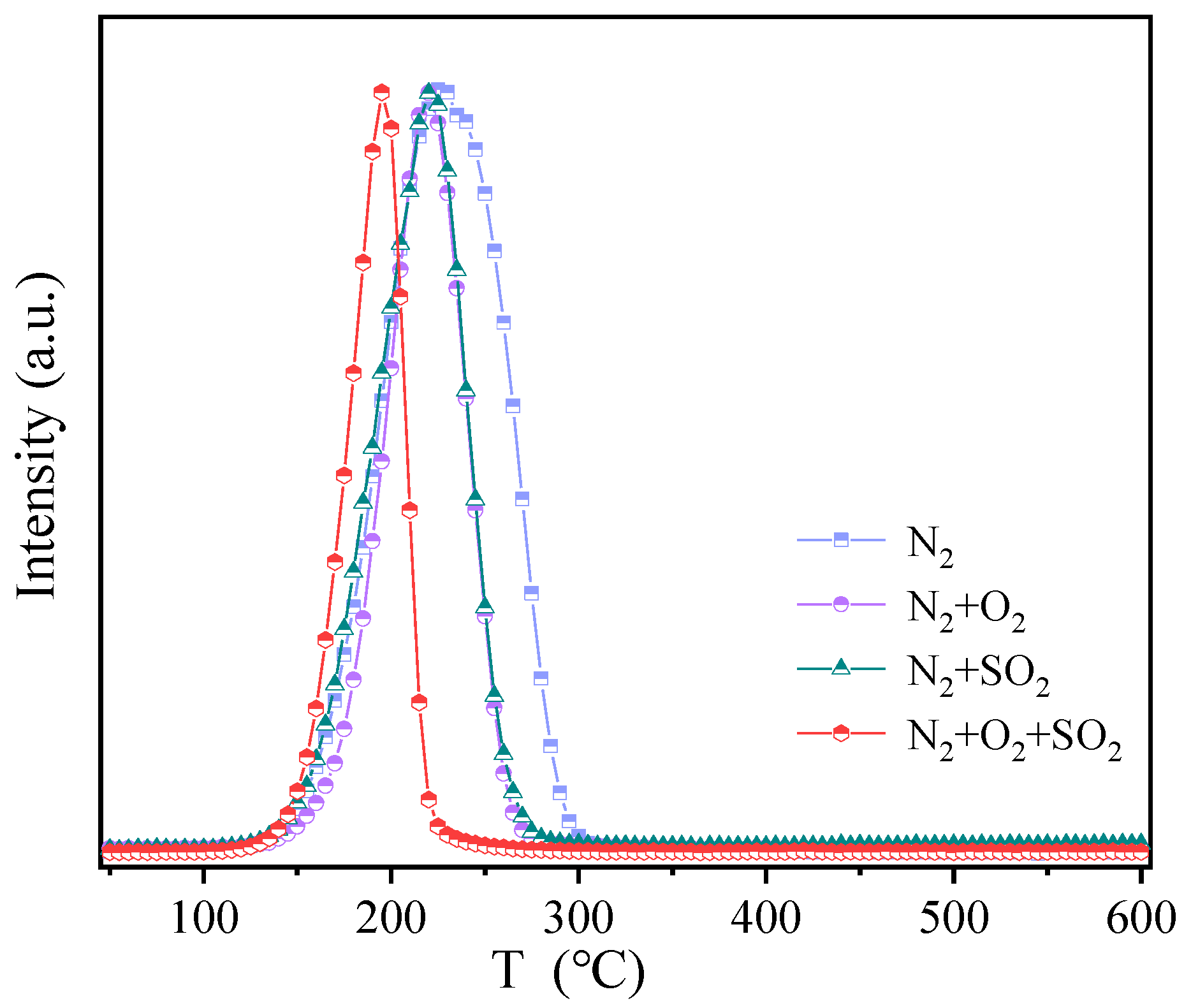
3.4. Mechanism for Hg0 Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selin, H. Global environmental law and treaty-making on hazardous substances: The Minamata Convention and mercury abatement. Glob. Environ. Politics 2014, 14, 1–19. [Google Scholar] [CrossRef]
- Outridge, P.M.; Mason, R.; Wang, F.; Guerrero, S.; Heimburger-Boavida, L. Updated global and oceanic mercury budgets for the United Nations Global Mercury Assessment 2018. Environ. Sci. Technol. 2018, 52, 11466–11477. [Google Scholar] [CrossRef]
- Liu, W.; Xu, H.; Guo, Y.; Yuan, Y.; Liao, Y.; Qu, Z.; Yan, N. Immobilization of elemental mercury in non-ferrous metal smelting gas using ZnSe1−xSx nanoparticles. Fuel 2019, 254, 115641. [Google Scholar] [CrossRef]
- Sun, X.; Huang, W.; Ji, L.; Xu, H.; Qu, Z.; Yan, N. Establishing a Self-supporting System of H2S Production from SO2 with Induced Catalytic Reduction Process for Mercury Capture with Super-large Enrichment. Chem. Eng. J. 2023, 459, 141493. [Google Scholar] [CrossRef]
- Liu, H.; Shen, F.; Li, Q.; Wen, M.; Zhang, H.; Jiang, L.; Zheng, C.; Liu, Y.; Liu, T.; Chai, L. Systematic control technologies for gaseous pollutants from non-ferrous metallurgy. J. Environ. Sci. 2023, 123, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhao, S.; Ma, A.; Sun, K.; Zhu, Y.; Sun, Z. Influence of flue gas components on the mercury adsorption/oxidation by mechanochemical S2Cl2-modified sawdust coke. Fuel 2023, 336, 127154. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, Z.; Chen, Z.; Wu, J.; Li, Z.; Zhong, S.; Liu, H.; Xu, Z.; Zhilou, L. In-situ preparation of zinc sulfide adsorbent using local materials for elemental mercury immobilization and recovery from zinc smelting flue gas. Chem. Eng. J. 2022, 429, 132115. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, P.; Meng, F.; Guo, Q.; He, T.; Yang, Z.; Qu, W.; Li, H. Charge distribution modulation and morphology controlling of copper selenide for an enhanced elemental mercury adsorption activity in flue gas. Chem. Eng. J. 2022, 442, 136145. [Google Scholar] [CrossRef]
- Wu, S.; Ma, Y.; Yu, W.; Wang, H.; Yang, W.; Che, L.; Han, Z. SO2 resistance of CeO2-and Co3O4-supported activated carbon during removal of mercury from flue gas: A comparative study. Fuel 2023, 334, 126636. [Google Scholar] [CrossRef]
- Ishag, A.; Yue, Y.; Xiao, J.; Huang, X.; Sun, Y. Recent advances on the adsorption and oxidation of mercury from coal-fired flue gas: A review. J. Clean. Prod. 2022, 367, 133111. [Google Scholar] [CrossRef]
- Peng, J.; Yin, R.; Yang, X.; Shang, C. A novel UVA/ClO2 advanced oxidation process for the degradation of micropollutants in water. Environ. Sci. Technol. 2022, 56, 1257–1266. [Google Scholar] [CrossRef]
- Huang, T.; Geng, X.; Liu, X.; Liu, J.; Duan, Y.; Zhao, S.; Gupta, R. Reduction of HgCl2 to Hg0 in flue gas at high temperature. Part I: Influences of oxidative species. Fuel 2022, 324, 124417. [Google Scholar] [CrossRef]
- Ye, D.; Wang, X.-X.; Wang, R.-X.; Liu, X.; Liu, H.; Wang, H.-N. Review of elemental mercury (Hg0) removal by CuO-based materials. J. Zhejiang Univ.-Sci. A 2022, 23, 505–526. [Google Scholar] [CrossRef]
- Kumar, A.S.; Kalidhasan, S.; Rajesh, V.; Rajesh, N. A meticulous study on the adsorption of mercury as tetrachloromercurate (II) anion with trioctylamine modified sodium montmorillonite and its application to a coal fly ash sample. Ind. Eng. Chem. Res. 2012, 51, 11312–11327. [Google Scholar] [CrossRef]
- Isinkaralar, K. High-efficiency removal of benzene vapor using activated carbon from Althaea officinalis L. biomass as a lignocellulosic precursor. Environ. Sci. Pollut. Res. 2022, 29, 66728–66740. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Wang, Z.; Yi, H.; Fu, Y.; Wu, L.; Shen, F.; Wang, P.; Liu, M.; Lin, Z.; et al. A gas permeable membrane electrode for selective and durable H2S production from SO2. Chem. Eng. J. 2023, 454, 140052. [Google Scholar] [CrossRef]
- Liu, T.; Xiong, Z.; Ni, P.; Ma, Z.; Tan, Y.; Li, Z.; Deng, S.; Li, Y.; Yang, Q.; Zhang, H. Review on adsorbents in elemental mercury removal in coal combustion flue gas, smelting flue gas and natural gas. Chem. Eng. J. 2023, 454, 140095. [Google Scholar] [CrossRef]
- He, Z.; Zhou, Z.; Wei, P.; Xu, T.; Han, J.; Huang, K.; Guo, K.; Huang, W.; Akasaka, T.; Lu, X. Fullerene-Derived Porous and Defective N-Doped Carbon Nanosheets as Advanced Trifunctional Metal-Free Electrocatalysts. Chem. Asian J. 2023, 18, e202200994. [Google Scholar] [CrossRef]
- Yan, W.; Wang, D.; Botte, G.G. Electrochemical decomposition of urea with Ni-based catalysts. Appl. Catal. B Environ. 2012, 127, 221–226. [Google Scholar] [CrossRef]
- Martellaro, P.; Moore, G.; Peterson, E.; Abbott, E.; Gorenbain, A. Environmental application of mineral sulfides for removal of gas-phase Hg (0) and aqueous Hg2+. Sep. Sci. Technol. 2001, 36, 1183–1196. [Google Scholar] [CrossRef]
- Liu, H.; Ruan, W.; Zhang, Z.; Zhou, Y.; Shen, F.; Liu, J.; Yang, H. Performance and mechanism of CuS-modified MWCNTs on mercury removal: Experimental and density functional theory study. Fuel 2022, 309, 122238. [Google Scholar] [CrossRef]
- Zheng, W.; Li, H.; Yang, Z.; Yang, J.; Qu, W.; Meng, F.; Feng, Y.; Xu, Z.; Guo, X. Advances in flue gas mercury abatement by mineral chalcogenides. Chem. Eng. J. 2021, 411, 128608. [Google Scholar] [CrossRef]
- Li, H.; Zu, H.; Deng, Y.; He, W.; Yang, Z.; Yang, J.; Zhao, S.; Qu, W. Mechanisms of gas-phase mercury immobilized by metal sulfides from combustion flue gas: A mini review. Energy Fuels 2022, 36, 6027–6037. [Google Scholar] [CrossRef]
- Li, Y.; Dong, H.; Li, L.; Tang, L.; Tian, R.; Li, R.; Chen, J.; Xie, Q.; Jin, Z.; Xiao, J.; et al. Recent advances in waste water treatment through transition metal sulfides-based advanced oxidation processes. Water Res. 2021, 192, 116850. [Google Scholar] [CrossRef] [PubMed]
- Kühne, T.D.; Iannuzzi, M.; Del Ben, M.; Rybkin, V.V.; Seewald, P.; Stein, F.; Laino, T.; Khaliullin, R.Z.; Schütt, O.; Schiffmann, F.; et al. CP2K: An electronic structure and molecular dynamics software package-Quickstep: Efficient and accurate electronic structure calculations. J. Chem. Phys. 2020, 152, 194103. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Goedecker, S.; Teter, M.; Hutter, J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B 1996, 54, 1703. [Google Scholar] [CrossRef] [PubMed]
- Hartwigsen, C.; Gœdecker, S.; Hutter, J. Relativistic separable dual-space Gaussian pseudopotentials from H to Rn. Phys. Rev. B 1998, 58, 3641. [Google Scholar] [CrossRef]
- Sathiyaraj, E.; Thirumaran, S. Structural, morphological and optical properties of iron sulfide, cobalt sulfide, copper sulfide, zinc sulfide and copper-iron sulfide nanoparticles synthesized from single source precursors. Chem. Phys. Lett. 2020, 739, 136972. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Z.; Yao, S.; Sun, S.; Ma, J.; Sun, R. CuInS2 quantum dots anchored onto the three-dimensional flexible self-supporting graphene oxide array with regulatable crystallinity and defect density for efficient photocatalytic synthesis of xylonic acid. Appl. Catal. B Environ. 2022, 316, 121573. [Google Scholar] [CrossRef]
- Xiao, J.; Xie, Y.; Tang, R.; Chen, M.; Tian, X. Novel ultrasonically assisted templated synthesis of palladium and silver dendritic nanostructures. Adv. Mater. 2001, 13, 1887–1891. [Google Scholar] [CrossRef]
- Liu, X.; Wu, L.; Wang, Z.; Fu, Y.; Xie, X.; Chen, H.; Long, J.; Xiang, K.; Liu, H. Influence of the Interface Wettability of Membranes on Electrolysis Reduction of SO2 to H2S. Ind. Eng. Chem. Res. 2023, 62, 1550–1557. [Google Scholar] [CrossRef]
- Li, Z.; Ma, H.; Zang, L.; Li, D.; Guo, S.; Shi, L. Construction of nano-flower MIL-125 (Mo)-In2Se3 Z-scheme heterojunctions by one-step solvothermal method for removal of tetracycline from wastewater in the synergy of adsorption and photocatalysis way. Sep. Purif. Technol. 2021, 276, 119355. [Google Scholar] [CrossRef]
- Lesnyak, V.; Brescia, R.; Messina, G.C.; Manna, L. Cu vacancies boost cation exchange reactions in copper selenide nanocrystals. J. Am. Chem. Soc. 2015, 137, 9315–9323. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chao, D.; Wang, Z.; Ni, J.; Li, L. An energetic CuS–Cu battery system based on CuS nanosheet arrays. ACS Nano 2021, 15, 5420–5427. [Google Scholar] [CrossRef]
- Li, H.; Yang, Q.; Qu, W.; Feng, Y.; Leng, L.; Zhao, J.; Meng, F.; Yang, Z.; Yang, J. Facile preparation of nanosized copper sulfide functionalized macroporous skeleton for efficient vapor-phase mercury sequestration. Chem. Eng. J. 2021, 419, 129561. [Google Scholar] [CrossRef]
- Ma, S.; Shim, Y.; Islam, S.M.; Subrahmanyam, K.S.; Wang, P.; Li, H.; Wang, S.; Yang, X.; Kanatzidis, M.G. Efficient Hg vapor capture with polysulfide intercalated layered double hydroxides. Chem. Mater. 2014, 26, 5004–5011. [Google Scholar] [CrossRef]
- Liu, W.; Xu, H.; Liao, Y.; Quan, Z.; Li, S.; Zhao, S.; Qu, Z.; Yan, N. Recyclable CuS sorbent with large mercury adsorption capacity in the presence of SO2 from non-ferrous metal smelting flue gas. Fuel 2019, 235, 847–854. [Google Scholar] [CrossRef]
- Li, H.; Zhu, W.; Yang, J.; Zhang, M.; Zhao, J.; Qu, W. Sulfur abundant S/FeS2 for efficient removal of mercury from coal-fired power plants. Fuel 2018, 232, 476–484. [Google Scholar] [CrossRef]
- Isinkaralar, K.; Gullu, G.; Turkyilmaz, A.; Dogan, M.; Turhan, O. Activated carbon production from horse chestnut shells for hydrogen storage. Int. J. Glob. Warm. 2022, 26, 361–373. [Google Scholar] [CrossRef]
- Waltrovitz, R.R.; Qian, G.; de Groot, F.; Quinton, J.S.; Harmer, S.L. Influence of Co: Fe: Ni ratio on cobalt Pentlandite’s electronic structure and surface speciation. Miner. Eng. 2022, 190, 107935. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Liao, C.; Zhao, J.; Feng, S.; Li, P.; Liu, X.; Yang, J.; Shih, K. Magnetic rattle-type Fe3O4@ CuS nanoparticles as recyclable sorbents for mercury capture from coal combustion flue gas. ACS Appl. Nano Mater. 2018, 1, 4726–4736. [Google Scholar] [CrossRef]
- Hong, Q.; Xu, H.; Li, J.; Huang, W.; Liu, P.; Qu, Z.; Yan, N. Shell-thickness-induced spontaneous inward migration of mercury in porous ZnO@ CuS for gaseous mercury immobilization. Chem. Eng. J. 2021, 420, 127592. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Feng, S.; Li, P.; Liao, C.; Liu, X.; Zhao, J.; Yang, J.; Lee, P.-H.; Shih, K. Multiform sulfur adsorption centers and copper-terminated active sites of nano-CuS for efficient elemental mercury capture from coal combustion flue gas. Langmuir 2018, 34, 8739–8749. [Google Scholar] [CrossRef]
- Quan, Z.; Huang, W.; Liao, Y.; Liu, W.; Xu, H.; Yan, N.; Qu, Z. Study on the regenerable sulfur-resistant sorbent for mercury removal from nonferrous metal smelting flue gas. Fuel 2019, 241, 451–458. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Xie, X.; Yang, S.; Fei, J.; Li, Y.; Xu, Z.; Liu, H. Development of recyclable iron sulfide/selenide microparticles with high performance for elemental mercury capture from smelting flue gas over a wide temperature range. Environ. Sci. Technol. 2019, 54, 604–612. [Google Scholar] [CrossRef]
- Yang, S.; Wang, D.; Liu, H.; Liu, C.; Xie, X.; Xu, Z.; Liu, Z. Highly stable activated carbon composite material to selectively capture gas-phase elemental mercury from smelting flue gas: Copper polysulfide modification. Chem. Eng. J. 2019, 358, 1235–1242. [Google Scholar] [CrossRef]
- Li, H.; Zhu, L.; Wang, J.; Li, L.; Shih, K. Development of nano-sulfide sorbent for efficient removal of elemental mercury from coal combustion fuel gas. Environ. Sci. Technol. 2016, 50, 9551–9557. [Google Scholar] [CrossRef]
- Xia, Y.; Wei, G.; Liang, X.; Zhu, J.; Xian, H.; Su, X.; He, H.; Zhu, R. Sequestration of Gaseous Hg0 by Sphalerite with Fe Substitution: Performance, Mechanism, and Structure–Activity Relationship. J. Phys. Chem. C 2019, 123, 2828–2836. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Chen, H.; Liu, X.; Xiang, K.; Liu, H. Achieving Large-Capability Adsorption of Hg0 in Wet Scrubbing by Defect-Rich Colloidal Copper Sulfides under High-SO2 Atmosphere. Materials 2023, 16, 3157. https://doi.org/10.3390/ma16083157
Xie X, Chen H, Liu X, Xiang K, Liu H. Achieving Large-Capability Adsorption of Hg0 in Wet Scrubbing by Defect-Rich Colloidal Copper Sulfides under High-SO2 Atmosphere. Materials. 2023; 16(8):3157. https://doi.org/10.3390/ma16083157
Chicago/Turabian StyleXie, Xiaofeng, Hao Chen, Xudong Liu, Kaisong Xiang, and Hui Liu. 2023. "Achieving Large-Capability Adsorption of Hg0 in Wet Scrubbing by Defect-Rich Colloidal Copper Sulfides under High-SO2 Atmosphere" Materials 16, no. 8: 3157. https://doi.org/10.3390/ma16083157
APA StyleXie, X., Chen, H., Liu, X., Xiang, K., & Liu, H. (2023). Achieving Large-Capability Adsorption of Hg0 in Wet Scrubbing by Defect-Rich Colloidal Copper Sulfides under High-SO2 Atmosphere. Materials, 16(8), 3157. https://doi.org/10.3390/ma16083157





