Engineered Highly Porous Polyvinyl Alcohol Hydrogels with Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Graphene Nanosheets for Musculoskeletal Tissue Engineering: Morphology, Water Sorption, Thermal, Mechanical, Electrical Properties, and Biocompatibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
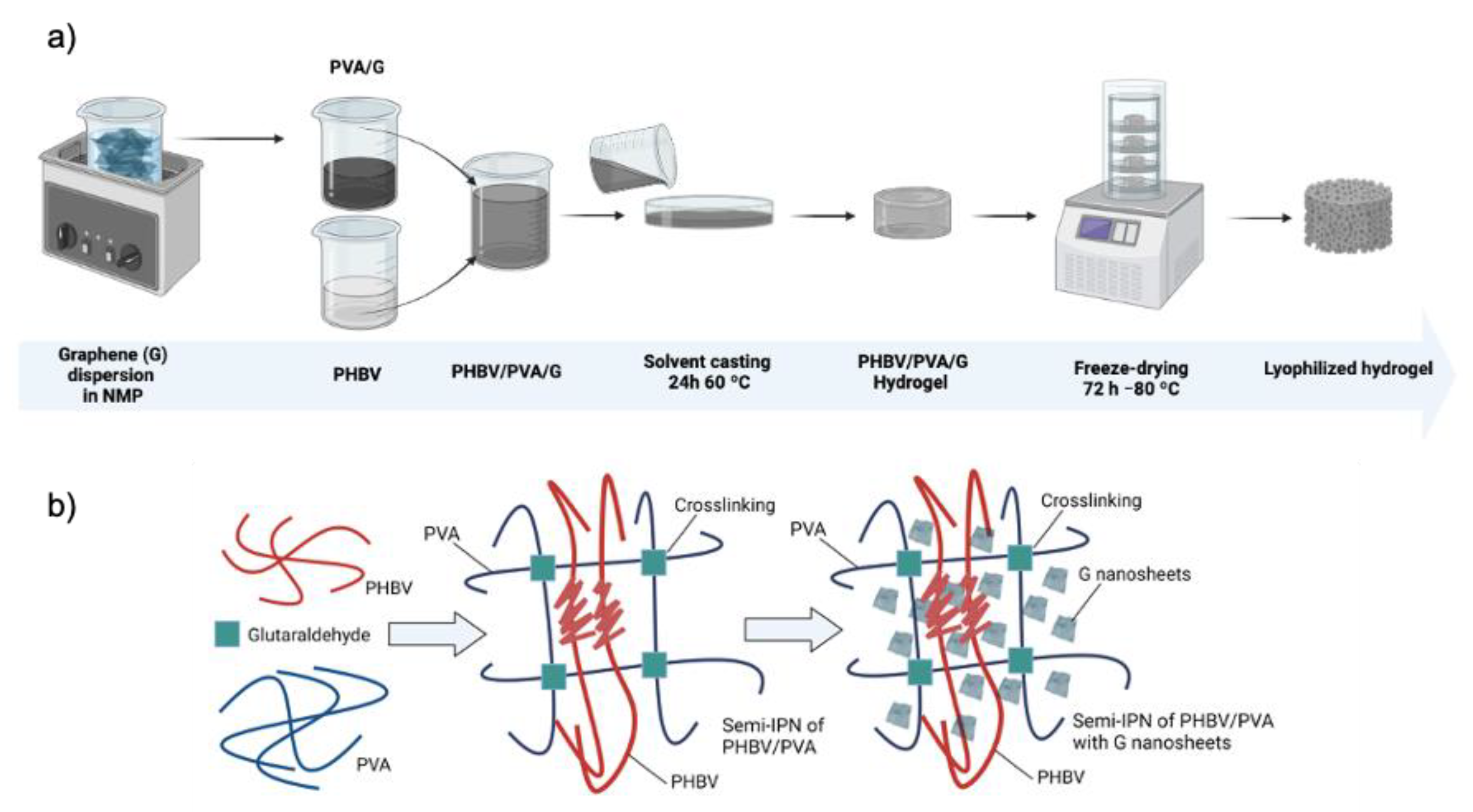
2.2. Preparation of Semi-IPN PHBV/PVA/G Hydrogels
2.3. Morphological and Physicochemical Characterization
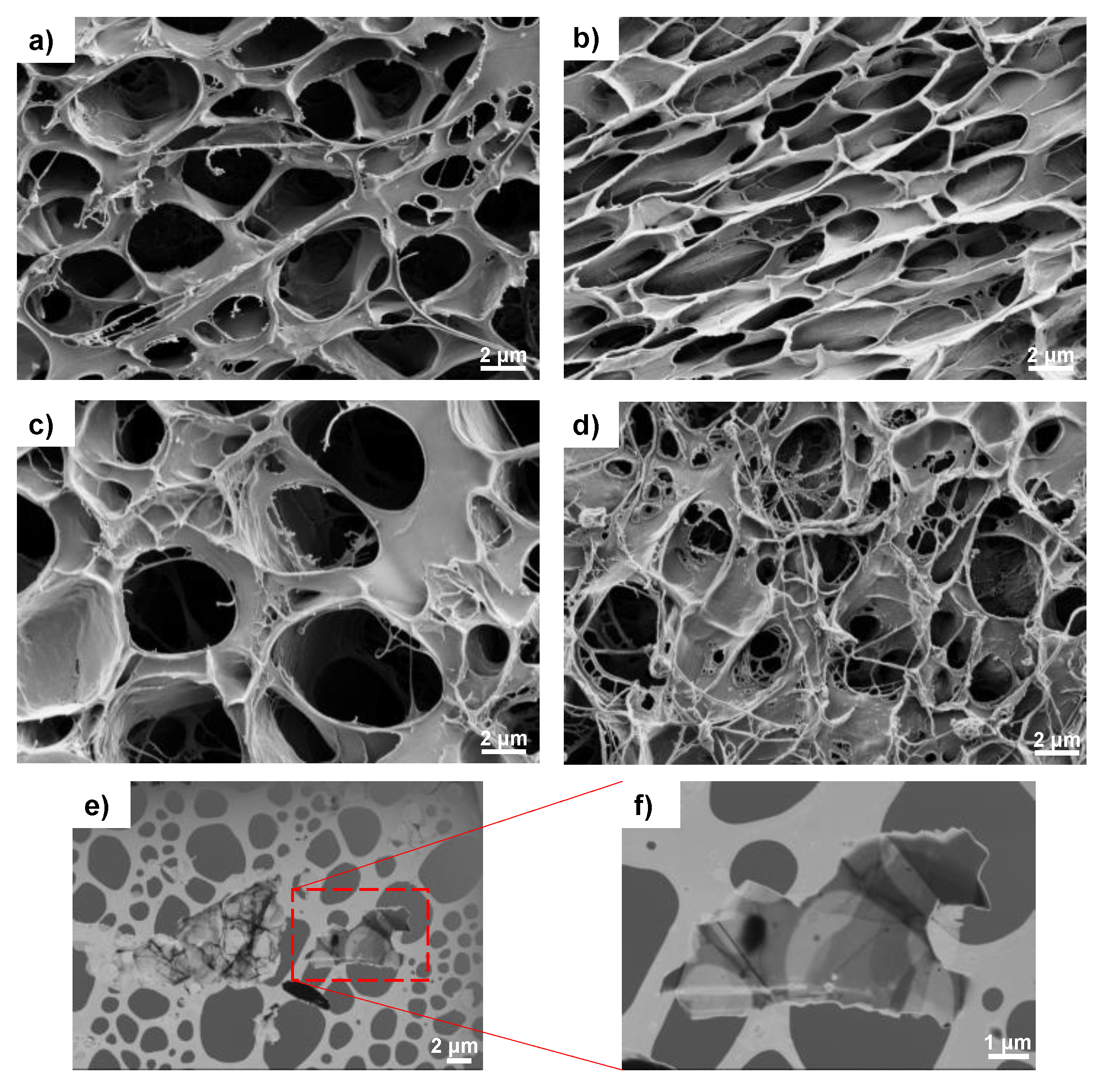
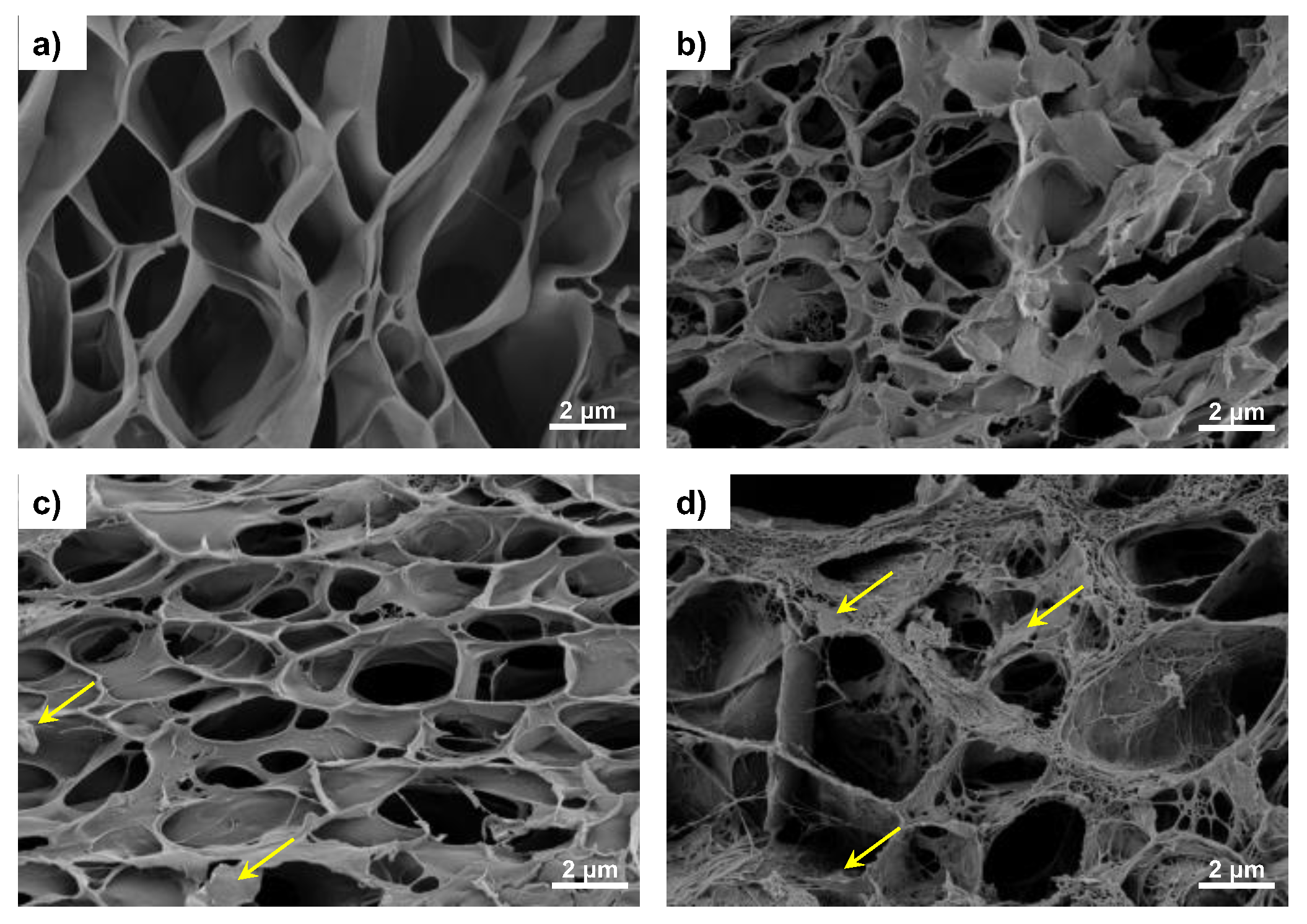
2.3.1. Electron Microscopy
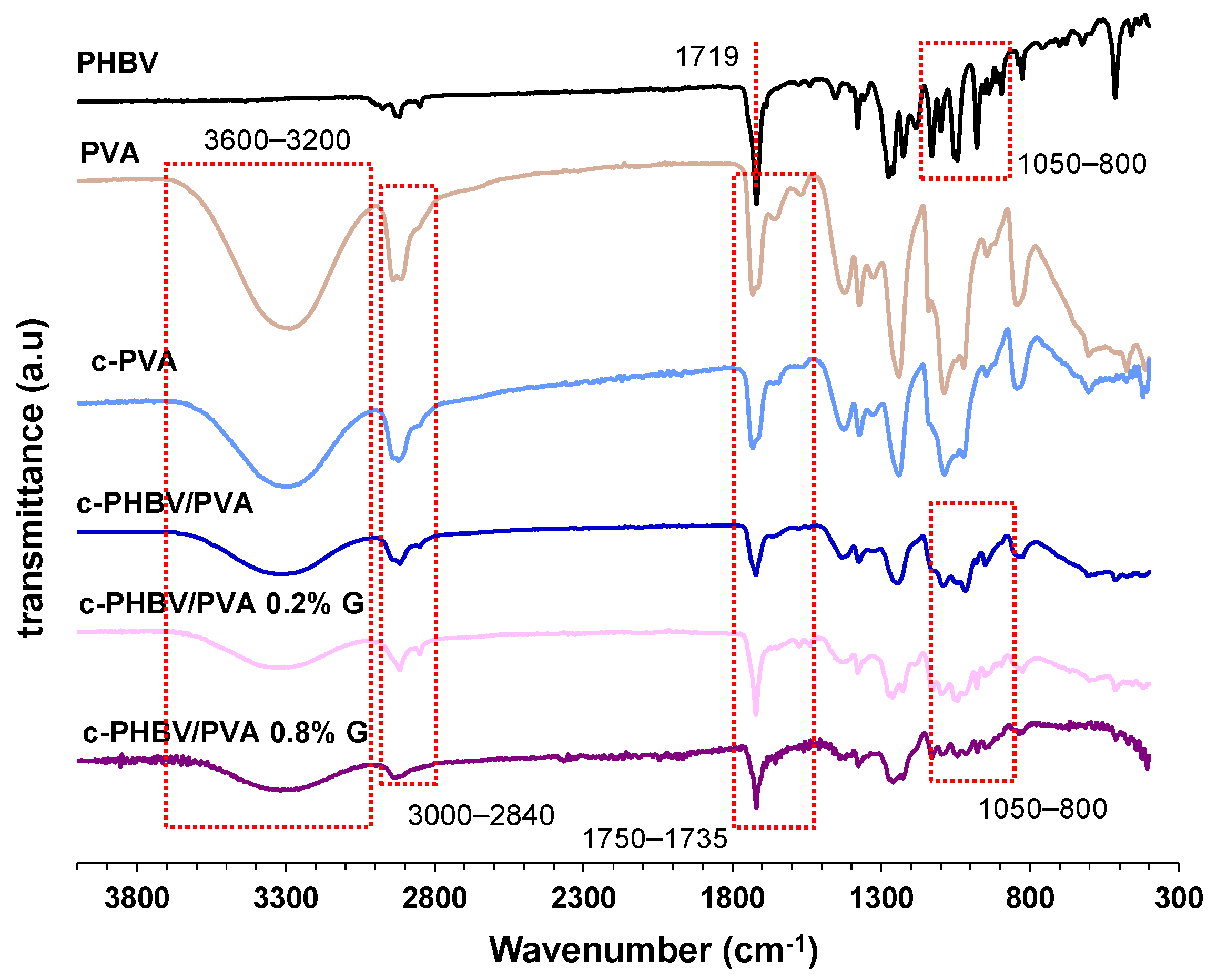
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.3. Swelling Assay
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. Thermogravimetric Analysis (TGA)
2.3.6. Mechanical Properties
2.3.7. Electrical Conductivity
2.4. Cytotoxicity Assessment
2.5. Statistical Analysis
3. Results and Discussion
3.1. Microstructure and FTIR Analysis of the Nanohybrid Hydrogels
3.2. Physical Characterization
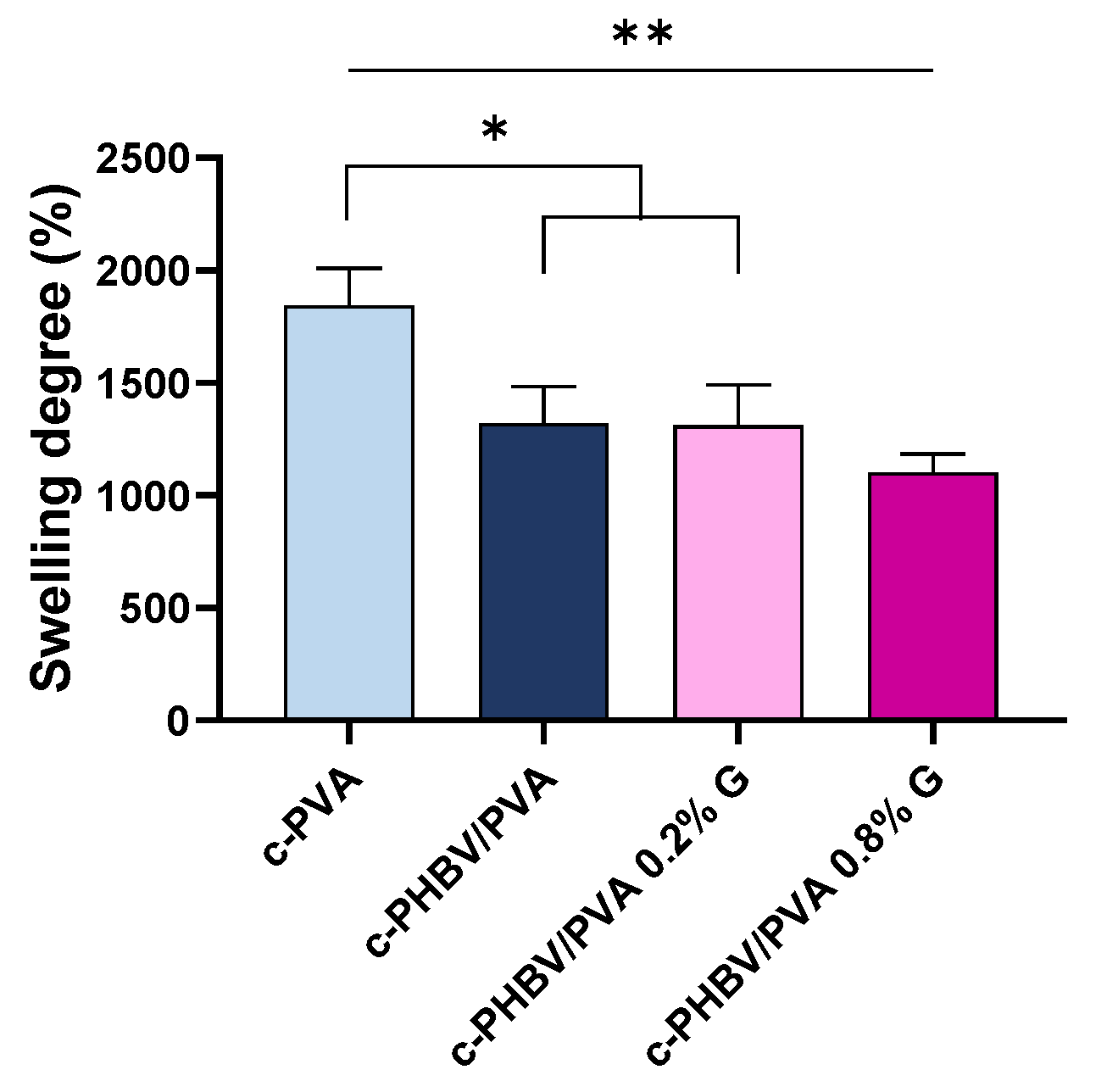
3.2.1. Swelling Properties
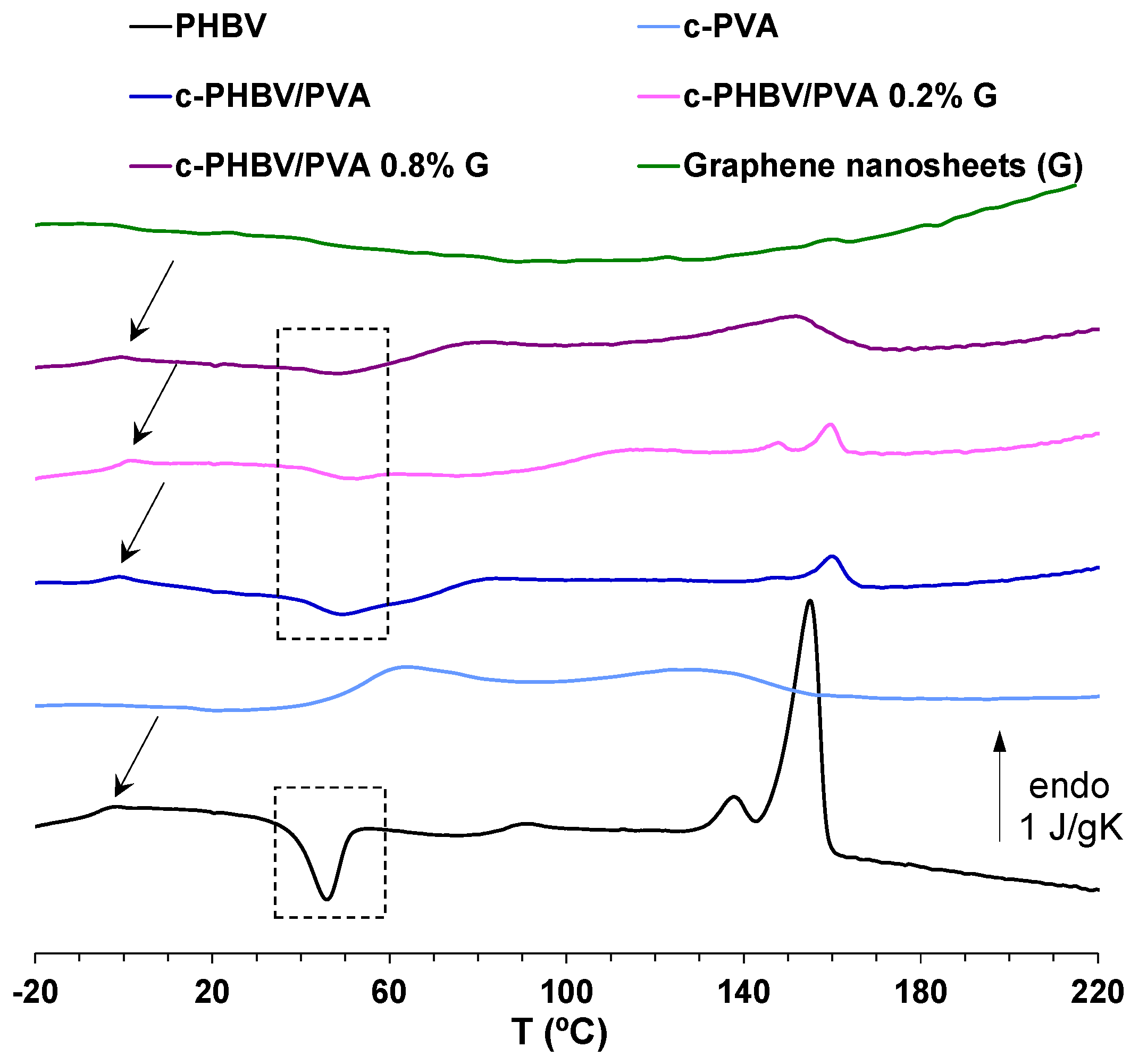
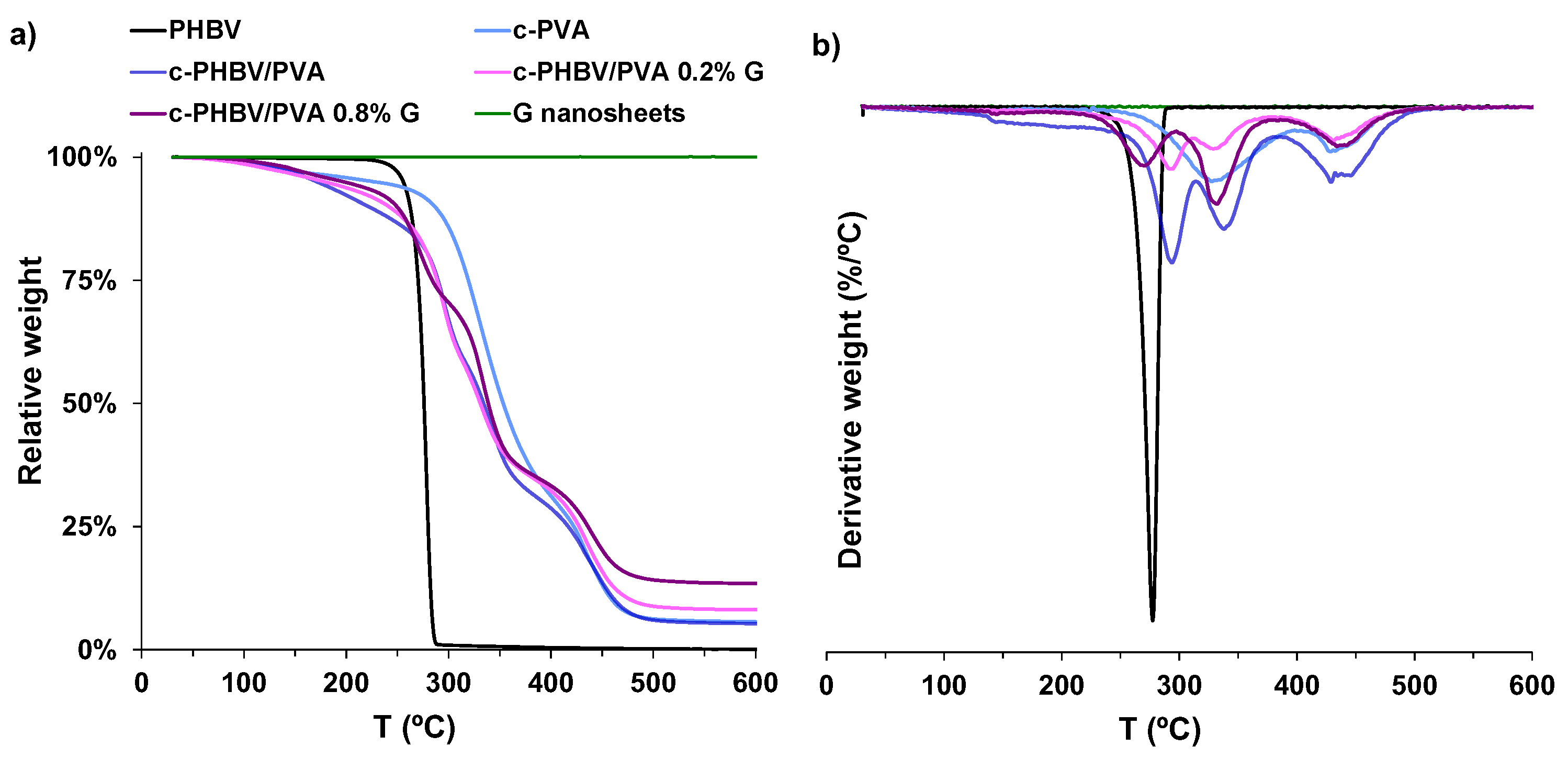
3.2.2. Thermal Behavior and Thermal Degradation
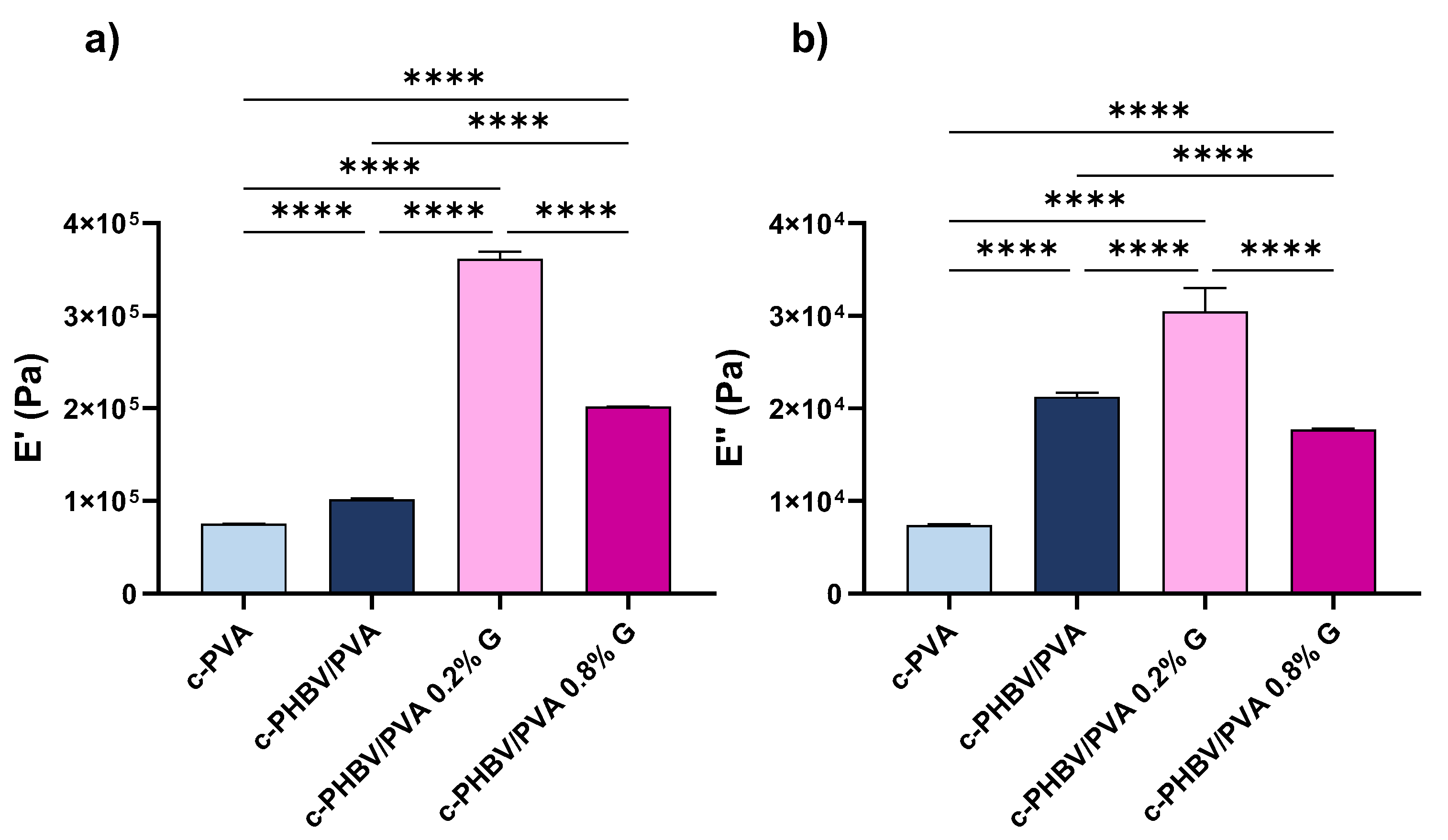
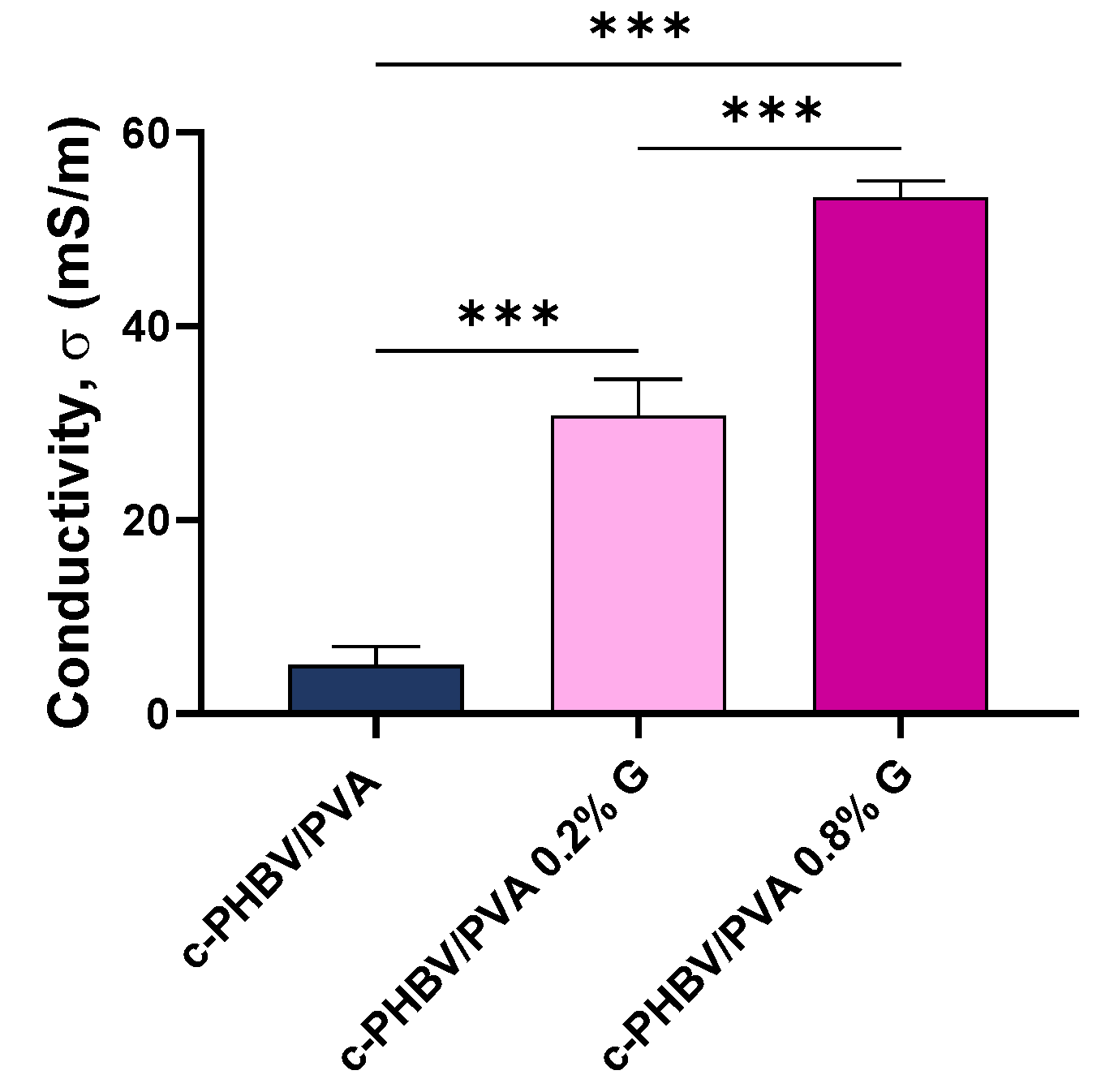
3.2.3. Mechanical and Electrical Properties
3.3. Biocompatibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and Novel Polymeric Biomaterials for Neural Tissue Engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef] [PubMed]
- Di Marzio, N.; Eglin, D.; Serra, T.; Moroni, L. Bio-Fabrication: Convergence of 3D Bioprinting and Nano-Biomaterials in Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Mujawar, M.A.; Kaushik, A. State-of-Art Functional Biomaterials for Tissue Engineering. Front. Mater. 2019, 6. [Google Scholar] [CrossRef]
- Choi, G.; Cha, H.J. Recent Advances in the Development of Nature-Derived Photocrosslinkable Biomaterials for 3D Printing in Tissue Engineering. Biomater. Res. 2019, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, R.; Kumar, A.; Ranjbar-Mohammadi, M.; Ashrafizadeh, M.; Han, S.S.; Khang, G.; Roveimiab, Z. Recent Advances in Natural Gum-Based Biomaterials for Tissue Engineering and Regenerative Medicine: A Review. Polymers 2020, 12, 176. [Google Scholar] [CrossRef]
- Ullah, S.; Chen, X. Fabrication, Applications and Challenges of Natural Biomaterials in Tissue Engineering. Appl. Mater. Today 2020, 20, 100656. [Google Scholar] [CrossRef]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic Polymer Scaffolds for Tissue Engineering. Chem. Soc. Rev. 2009, 38, 1139–1151. [Google Scholar] [CrossRef]
- Yang, D. Recent Advances in Hydrogels. Chem. Mater. 2022, 34, 1987–1989. [Google Scholar] [CrossRef]
- Luis Aparicio-Collado, J.; Novoa, J.J.; Molina-Mateo, J.; Torregrosa-Cabanilles, C.; Serrano-Aroca, Á.; Sabater i Serra, R. Novel Semi-Interpenetrated Polymer Networks of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Poly (Vinyl Alcohol) with Incorporated Conductive Polypyrrole Nanoparticles. Polymers 2021, 13, 57. [Google Scholar] [CrossRef]
- Gajra, B.; Pandya, S.S.; Vidyasagar, G.; Rabari, H.; Dedania, R.R.; Rao, S. Poly Vinyl Alcohol Hydrogel and Its Pharmaceutical and Biomedical Applications: A Review. Int. J. Pharm. Res. 2012, 4, 20–26. [Google Scholar]
- Boso, D.; Maghin, E.; Carraro, E.; Giagante, M.; Pavan, P.; Piccoli, M. Extracellular Matrix-Derived Hydrogels as Biomaterial for Different Skeletal Muscle Tissue Replacements. Materials 2020, 13, 2483. [Google Scholar] [CrossRef] [PubMed]
- Gull, N.; Khan, S.M.; Butt, O.M.; Islam, A.; Shah, A.; Jabeen, S.; Khan, S.U.; Khan, A.; Khan, R.U.; Butt, M.T.Z. Inflammation Targeted Chitosan-Based Hydrogel for Controlled Release of Diclofenac Sodium. Int. J. Biol. Macromol. 2020, 162, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Han, S.S. PVA-Based Hydrogels for Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Sionkowska, A. Current Research on the Blends of Natural and Synthetic Polymers as New Biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Crosby, C.O.; Stern, B.; Kalkunte, N.; Pedahzur, S.; Ramesh, S.; Zoldan, J. Interpenetrating Polymer Network Hydrogels as Bioactive Scaffolds for Tissue Engineering. Rev. Chem. Eng. 2022, 38, 347–361. [Google Scholar] [CrossRef]
- Rivera-Briso, A.L.; Serrano-Aroca, Á. Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate): Enhancement Strategies for Advanced Applications. Polymers 2018, 10, 732. [Google Scholar] [CrossRef]
- Pryadko, A.; Surmeneva, M.A.; Surmenev, R.A. Review of Hybrid Materials Based on Polyhydroxyalkanoates for Tissue Engineering Applications. Polymers 2021, 13, 1738. [Google Scholar] [CrossRef]
- Gheibi, A.; Khoshnevisan, K.; Ketabchi, N.; Derakhshan, M.A.; Babadi, A.A. Application of Electrospun Nanofibrous PHBV Scaffold in Neural Graft and Regeneration: A Mini-Review. Nanomed. Res. J. 2016, 1, 107–111. [Google Scholar] [CrossRef]
- Rivera-Briso, A.L.; Aparicio-Collado, J.L.; Serra, R.S.I.; Serrano-Aroca, Á. Graphene Oxide versus Carbon Nanofibers in Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Films: Degradation in Simulated Intestinal Environments. Polymers 2022, 14, 348. [Google Scholar] [CrossRef]
- Janowski, G.; Frącz, W.; Bąk, Ł. The Mechanical Properties Prediction of Poly [(3-Hydroxybutyrate)-Co-(3-Hydroxyvalerate)] (PHBV) Biocomposites on a Chosen Example. Materials 2022, 15, 7531. [Google Scholar] [CrossRef]
- Hurtado, A.; Cano-Vicent, A.; Tuñón-Molina, A.; Aparicio-Collado, J.L.; Salesa, B.; i Serra, R.S.; Serrano-Aroca, Á. Engineering Alginate Hydrogel Films with Poly(3-Hydroxybutyrate-Co-3-Valerate) and Graphene Nanoplatelets: Enhancement of Antiviral Activity, Cell Adhesion and Electroactive Properties. Int. J. Biol. Macromol. 2022, 219, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, L.; Lan, L.; Gao, Y.; Zhang, Q.; Dawit, H.; Mao, J.; Guo, L.; Shen, L.; Wang, L. Conductive Biomaterials for Cardiac Repair: A Review. Acta Biomater 2022, 139, 157–178. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Ma, P.X.; Guo, B. Conductive Biomaterials for Muscle Tissue Engineering. Biomaterials 2020, 229, 119584. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Zangene, E.; Manouchehri, S.; Amirabad, L.M.; Baheiraei, N.; Hadjighasem, M.R.; Farokhi, M.; Ganjali, M.R.; Walker, B.W.; Saeb, M.R.; et al. Conductive Biomaterials as Nerve Conduits: Recent Advances and Future Challenges. Appl. Mater. Today 2020, 20, 100784. [Google Scholar] [CrossRef]
- Chen, C.; Xi, Y.; Weng, Y. Progress in the Development of Graphene-Based Biomaterials for Tissue Engineering and Regeneration. Materials 2022, 15, 2164. [Google Scholar] [CrossRef]
- Gajendiran, M.; Choi, J.; Kim, S.J.; Kim, K.; Shin, H.; Koo, H.J.; Kim, K. Conductive Biomaterials for Tissue Engineering Applications. J. Ind. Eng. Chem. 2017, 51, 12–26. [Google Scholar] [CrossRef]
- Ma, C.; Jiang, L.; Wang, Y.; Gang, F.; Xu, N.; Li, T.; Liu, Z.; Chi, Y.; Wang, X.; Zhao, L.; et al. 3D Printing of Conductive Tissue Engineering Scaffolds Containing Polypyrrole Nanoparticles with Different Morphologies and Concentrations. Materials 2019, 12, 2491. [Google Scholar] [CrossRef]
- Bellet, P.; Gasparotto, M.; Pressi, S.; Fortunato, A.; Scapin, G.; Mba, M.; Menna, E.; Filippini, F. Graphene-Based Scaffolds for Regenerative Medicine. Nanomaterials 2021, 11, 404. [Google Scholar] [CrossRef]
- Shin, S.R.; Li, Y.C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-Based Materials for Tissue Engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef]
- Hermenean, A.; Codreanu, A.; Herman, H.; Balta, C.; Rosu, M.; Mihali, C.V.; Ivan, A.; Dinescu, S.; Ionita, M.; Costache, M. Chitosan-Graphene Oxide 3D Scaffolds as Promising Tools for Bone Regeneration in Critical-Size Mouse Calvarial Defects. Sci. Rep. 2017, 7, 16641. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Collado, J.L.; García-San-Martín, N.; Molina-Mateo, J.; Torregrosa Cabanilles, C.; Donderis Quiles, V.; Serrano-Aroca, A.; Sabater i Serra, R. Electroactive Calcium-Alginate/Polycaprolactone/Reduced Graphene Oxide Nanohybrid Hydrogels for Skeletal Muscle Tissue Engineering. Colloids Surf. B Biointerfaces 2022, 214, 112455. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhao, X.; Han, Q.; Chen, W.; Li, H.; Yuan, W. An Integrated Multi-Layer 3D-Fabrication of PDA/RGD Coated Graphene Loaded PCL Nanoscaffold for Peripheral Nerve Restoration. Nat. Commun. 2018, 9, 323. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Su, M.; Liu, C.; Shen, C.; Liu, X. Poly (Vinyl Alcohol)/Graphene Nanocomposite Hydrogel Scaffolds for Control of Cell Adhesion. J. Renew. Mater. 2020, 8, 89–99. [Google Scholar] [CrossRef]
- Das, B.; Eswar Prasad, K.; Ramamurty, U.; Rao, C.N.R. Nano-Indentation Studies on Polymer Matrix Composites Reinforced by Few-Layer Graphene. Nanotechnology 2009, 20, 125705. [Google Scholar] [CrossRef]
- Sridhar, V.; Lee, I.; Chun, H.H.; Park, H. Graphene Reinforced Biodegradable Poly(3-Hydroxybutyrate-Co-4-Hydroxybutyrate) Nano-Composites. Express Polym. Lett. 2013, 7, 320–328. [Google Scholar] [CrossRef]
- Aparicio-Collado, J.L.; Molina-Mateo, J.; Cabanilles, C.T.; Vidaurre, A.; Salesa, B.; Serrano-Aroca, Á.; Sabater i Serra, R. Pro-Myogenic Environment Promoted by the Synergistic Effect of Conductive Polymer Nanocomposites Combined with Extracellular Zinc Ions. Biology 2022, 11, 1706. [Google Scholar] [CrossRef]
- Sabater i Serra, R.; Serrano-Aroca, Á.; Molina Mateo, J.; Aparicio Collado, J.L. Biomaterial. Biodegradable. Patent ES 2812048 B2, 5 May 2022. [Google Scholar]
- Sgarminato, V.; Tonda-Turo, C.; Ciardelli, G. Reviewing Recently Developed Technologies to Direct Cell Activity through the Control of Pore Size: From the Macro- to the Nanoscale. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1176–1185. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR Spectroscopy Characterization of Poly (Vinyl Alcohol) Hydrogel with Different Hydrolysis Degree and Chemically Crosslinked with Glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Mansur, H.S.; Oréfice, R.L.; Mansur, A.A.P. Characterization of Poly(Vinyl Alcohol)/Poly(Ethylene Glycol) Hydrogels and PVA-Derived Hybrids by Small-Angle X-Ray Scattering and FTIR Spectroscopy. Polymer 2004, 45, 7193–7202. [Google Scholar] [CrossRef]
- Paşcu, E.I.; Stokes, J.; McGuinness, G.B. Electrospun Composites of PHBV, Silk Fibroin and Nano-Hydroxyapatite for Bone Tissue Engineering. Mater. Sci. Eng. C 2013, 33, 4905–4916. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- Singh, S.; Mohanty, A.K. Wood Fiber Reinforced Bacterial Bioplastic Composites: Fabrication and Performance Evaluation. Compos. Sci. Technol. 2007, 67, 1753–1763. [Google Scholar] [CrossRef]
- Voronova, M.I.; Surov, O.V.; Guseinov, S.S.; Barannikov, V.P.; Zakharov, A.G. Thermal Stability of Polyvinyl Alcohol/Nanocrystalline Cellulose Composites. Carbohydr. Polym. 2015, 130, 440–447. [Google Scholar] [CrossRef]
- Lewandowska, K. Miscibility and Thermal Stability of Poly(Vinyl Alcohol)/Chitosan Mixtures. Thermochim. Acta 2009, 493, 42–48. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Jin, C.; Zhang, T.; Yin, Q. Preparation of Polypyrrole/Graphene Nanosheets Composites with Enhanced Thermoelectric Properties. RSC Adv. 2014, 4, 46187–46193. [Google Scholar] [CrossRef]
- Zeinali, K.; Khorasani, M.T.; Rashidi, A.; Daliri Joupari, M. Preparation and Characterization of Graphene Oxide Aerogel/Gelatin as a Hybrid Scaffold for Application in Nerve Tissue Engineering. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 674–683. [Google Scholar] [CrossRef]
- Jitphuthi, P.; Tangtrakulwanich, B.; Meesane, J. Hierarchical Porous Formation, Collagen and Mineralized Collagen Modification of Polylactic Acid to Design Mimicked Scaffolds for Maxillofacial Bone Surgery. Mater. Today Commun. 2017, 13, 46–52. [Google Scholar] [CrossRef]
- Silva, M.; Pinho, I.; Gonçalves, H.; Vale, A.C.; Paiva, M.C.; Alves, N.M.; Covas, J.A. Engineering Ligament Scaffolds Based on PLA/Graphite Nanoplatelet Composites by 3D Printing or Braiding. J. Compos. Sci. 2023, 7, 104. [Google Scholar] [CrossRef]
- Le, M.T.; Huang, S.C. Thermal and Mechanical Behavior of Hybrid Polymer Nanocomposite Reinforced with Graphene Nanoplatelets. Materials 2015, 8, 5526–5536. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xie, G.; Fang, M.; Ying, Z.; Tong, Y.; Zeng, Y. Mechanical Reinforcement of Graphene/Poly(Vinyl Chloride) Composites Prepared by Combining the in-Situ Suspension Polymerization and Melt-Mixing Methods. Compos. B Eng. 2017, 113, 278–284. [Google Scholar] [CrossRef]
- Kaur, T.; Thirugnanam, A.; Pramanik, K. Effect of Carboxylated Graphene Nanoplatelets on Mechanical and In-Vitro Biological Properties of Polyvinyl Alcohol Nanocomposite Scaffolds for Bone Tissue Engineering. Mater. Today Commun. 2017, 12, 34–42. [Google Scholar] [CrossRef]
- Yang, D.; Yang, S.; Jiang, Z.; Yu, S.; Zhang, J.; Pan, F.; Cao, X.; Wang, B.; Yang, J. Polydimethyl Siloxane-Graphene Nanosheets Hybrid Membranes with Enhanced Pervaporative Desulfurization Performance. J. Memb. Sci. 2015, 487, 152–161. [Google Scholar] [CrossRef]
- Carnes, M.E.; Pins, G.D. Skeletal Muscle Tissue Engineering: Biomaterials-Based Strategies for the Treatment of Volumetric Muscle Loss. Bioengineering 2020, 7, 85. [Google Scholar] [CrossRef]
- Zhou, J.; Vijayavenkataraman, S. 3D-Printable Conductive Materials for Tissue Engineering and Biomedical Applications. Bioprinting 2021, 24, e00166. [Google Scholar] [CrossRef]
- Pien, N.; Krzyslak, H.; Shastry Kallaje, S.; Van Meerssche, J.; Mantovani, D.; De Schauwer, C.; Dubruel, P.; Van Vlierberghe, S.; Pennisi, C.P. Tissue Engineering of Skeletal Muscle, Tendons and Nerves: A Review of Manufacturing Strategies to Meet Structural and Functional Requirements. Appl. Mater. Today 2023, 31, 101737. [Google Scholar] [CrossRef]
- Yu, C.; Yao, F.; Li, J. Rational Design of Injectable Conducting Polymer-Based Hydrogels for Tissue Engineering. Acta Biomater. 2022, 139, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ge, J.; Li, Y.; Ma, P.X.; Lei, B. Biomimetic Elastomeric, Conductive and Biodegradable Polycitrate-Based Nanocomposites for Guiding Myogenic Differentiation and Skeletal Muscle Regeneration. Biomaterials 2018, 157, 40–50. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Mahmoudifard, M.; Mohamadyar-Toupkanlou, F.; Dodel, M.; Hajarizadeh, A.; Adabi, M.; Soleimani, M. The Nanofibrous PAN-PANi Scaffold as an Efficient Substrate for Skeletal Muscle Differentiation Using Satellite Cells. Bioprocess Biosyst. Eng. 2016, 39, 1163–1172. [Google Scholar] [CrossRef]
- Deepthi, S.; Nivedhitha Sundaram, M.; Vijayan, P.; Nair, S.V.; Jayakumar, R. Engineering Poly(Hydroxy Butyrate-Co-Hydroxy Valerate) Based Vascular Scaffolds to Mimic Native Artery. Int. J. Biol. Macromol. 2018, 109, 85–98. [Google Scholar] [CrossRef]
- Tebaldi, M.L.; Maia, A.L.C.; Poletto, F.; de Andrade, F.V.; Soares, D.C.F. Poly(-3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHBV): Current Advances in Synthesis Methodologies, Antitumor Applications and Biocompatibility. J. Drug Deliv. Sci. Technol. 2019, 51, 115–126. [Google Scholar] [CrossRef]
- Sreena, R.; Nathanael, A.J. Biodegradable Biopolymeric Nanoparticles for Biomedical Applications-Challenges and Future Outlook. Materials 2023, 16, 2364. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Song, W.; Feng, M.; Zhou, R. Potential Interference of Graphene Nanosheets in Immune Response: Via Disrupting the Recognition of HLA-Presented KK10 by TCR: A Molecular Dynamics Simulation Study. Nanoscale 2021, 13, 19255–19263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ali, S.F.; Dervishi, E.; Xu, Y.; Li, Z.; Casciano, D.; Biris, A.S. Cytotoxicity Effects of Graphene and Single-Wall Carbon Nanotubes in Neural Phaeochromocytoma-Derived Pc12 Cells. ACS Nano 2010, 4, 3181–3186. [Google Scholar] [CrossRef]
- Gies, V.; Lopinski, G.; Augustine, J.; Cheung, T.; Kodra, O.; Zou, S. The Impact of Processing on the Cytotoxicity of Graphene Oxide. Nanoscale Adv. 2019, 1, 817–826. [Google Scholar] [CrossRef]
- Fan, H.; Wang, L.; Zhao, K.; Li, N.; Shi, Z.; Ge, Z.; Jin, Z. Fabrication, Mechanical Properties, and Biocompatibility of Graphene-Reinforced Chitosan Composites. Biomacromolecules 2010, 11, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Nilawar, S.; BS, M.; Chatterjee, K. Nanoceria-Decorated Graphene Nanosheets Enhance Mechanical Properties and Bioactivity of a Degradable Polyurethane for Biomedical Applications. J. Polym. Environ. 2023. [Google Scholar] [CrossRef]

| Identification | Description |
|---|---|
| PHBV | PHBV 3% wt/wt film (solvent casting) |
| c-PVA | PVA 5% wt/wt film crosslinked with 4% wt/wt GA (solvent casting–freeze-drying) |
| c-PHBV/PVA | 30% PHBV/70% PVA semi-IPN crosslinked with 4% wt/wt GA (solvent casting–freeze-drying) |
| c-PHBV/PVA 0.2% G | 30% PHBV/70% PVA semi-IPN crosslinked with 4% wt/wt GA + 0.2% G nanosheets (solvent casting–freeze-drying) |
| c-PHBV/PVA 0.8% G | 30% PHBV/70% PVA semi-IPN crosslinked with 4% wt/wt GA + 0.8% G nanosheets (solvent casting–freeze-drying) |
| Sample | Tg (°C) | Tc(cold) (°C) | Tm (°C) | ΔHm (J/g) | Xc (%) | Td−50% (°C) |
|---|---|---|---|---|---|---|
| PHBV | −3.6 | 46 | 156 | 21.2 | 16.1 | 276.3 |
| c-PVA | 57 | - | - | - | 352.8 | |
| c-PHBV/PVA | −3.5 | 50 | 162 | 1.0 | 2.6 | 334.5 |
| c-PHBV/PVA 0.2% G | −2 | 50,5 | 160 | 1.8 | 4.7 | 331.1 |
| c-PHBV/PVA 0.8% G | −3.1 | 48 | 156 | 4.8 | 12.3 | 339.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aparicio-Collado, J.L.; Zheng, Q.; Molina-Mateo, J.; Torregrosa Cabanilles, C.; Vidaurre, A.; Serrano-Aroca, Á.; Sabater i Serra, R. Engineered Highly Porous Polyvinyl Alcohol Hydrogels with Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Graphene Nanosheets for Musculoskeletal Tissue Engineering: Morphology, Water Sorption, Thermal, Mechanical, Electrical Properties, and Biocompatibility. Materials 2023, 16, 3114. https://doi.org/10.3390/ma16083114
Aparicio-Collado JL, Zheng Q, Molina-Mateo J, Torregrosa Cabanilles C, Vidaurre A, Serrano-Aroca Á, Sabater i Serra R. Engineered Highly Porous Polyvinyl Alcohol Hydrogels with Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Graphene Nanosheets for Musculoskeletal Tissue Engineering: Morphology, Water Sorption, Thermal, Mechanical, Electrical Properties, and Biocompatibility. Materials. 2023; 16(8):3114. https://doi.org/10.3390/ma16083114
Chicago/Turabian StyleAparicio-Collado, José Luis, Qiqi Zheng, José Molina-Mateo, Constantino Torregrosa Cabanilles, Ana Vidaurre, Ángel Serrano-Aroca, and Roser Sabater i Serra. 2023. "Engineered Highly Porous Polyvinyl Alcohol Hydrogels with Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Graphene Nanosheets for Musculoskeletal Tissue Engineering: Morphology, Water Sorption, Thermal, Mechanical, Electrical Properties, and Biocompatibility" Materials 16, no. 8: 3114. https://doi.org/10.3390/ma16083114
APA StyleAparicio-Collado, J. L., Zheng, Q., Molina-Mateo, J., Torregrosa Cabanilles, C., Vidaurre, A., Serrano-Aroca, Á., & Sabater i Serra, R. (2023). Engineered Highly Porous Polyvinyl Alcohol Hydrogels with Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Graphene Nanosheets for Musculoskeletal Tissue Engineering: Morphology, Water Sorption, Thermal, Mechanical, Electrical Properties, and Biocompatibility. Materials, 16(8), 3114. https://doi.org/10.3390/ma16083114








