Contrast Enhancement in MRI Using Combined Double Action Contrast Agents and Image Post-Processing in the Breast Cancer Model
Abstract
1. Introduction
2. Materials and Methods
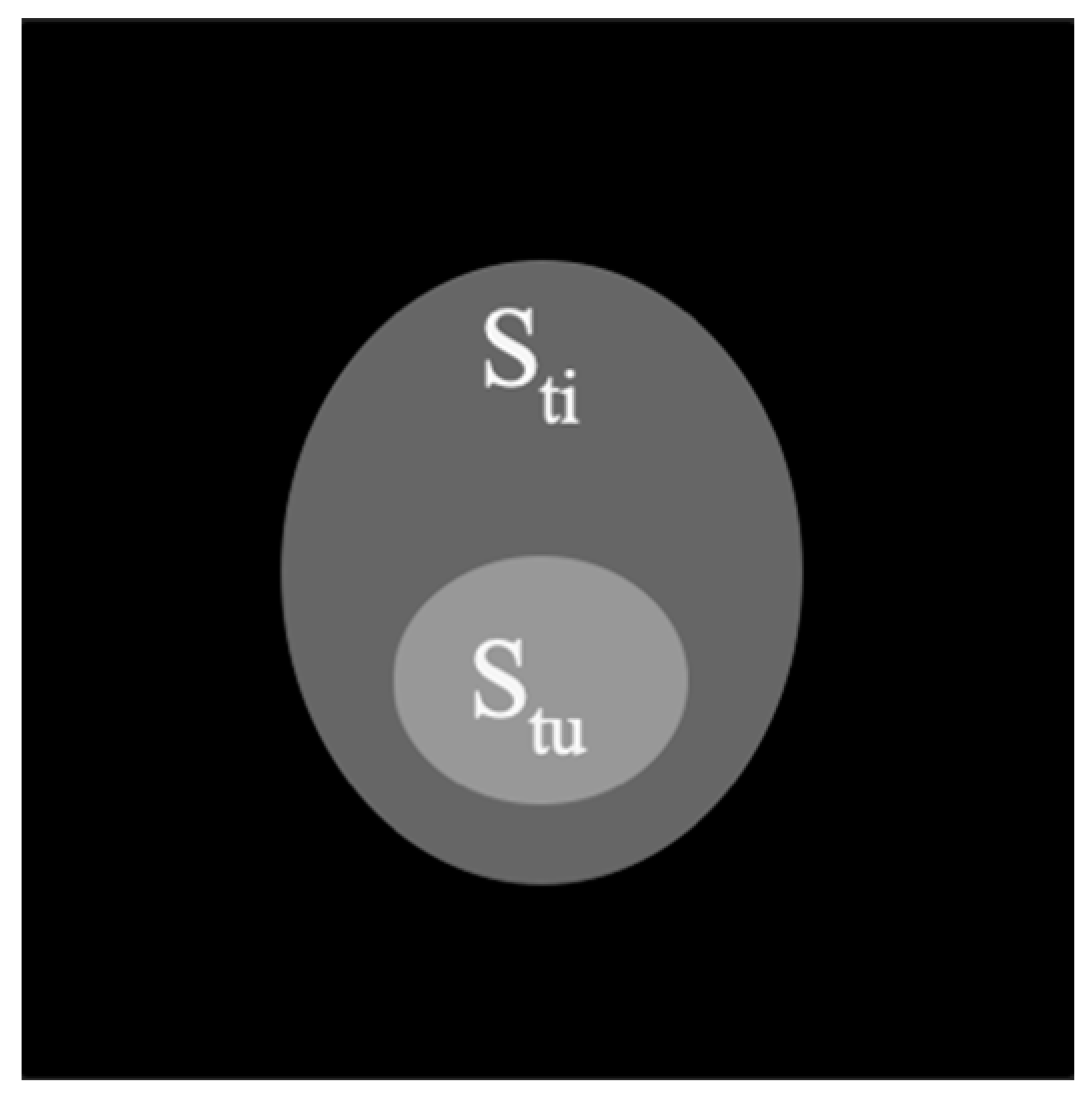
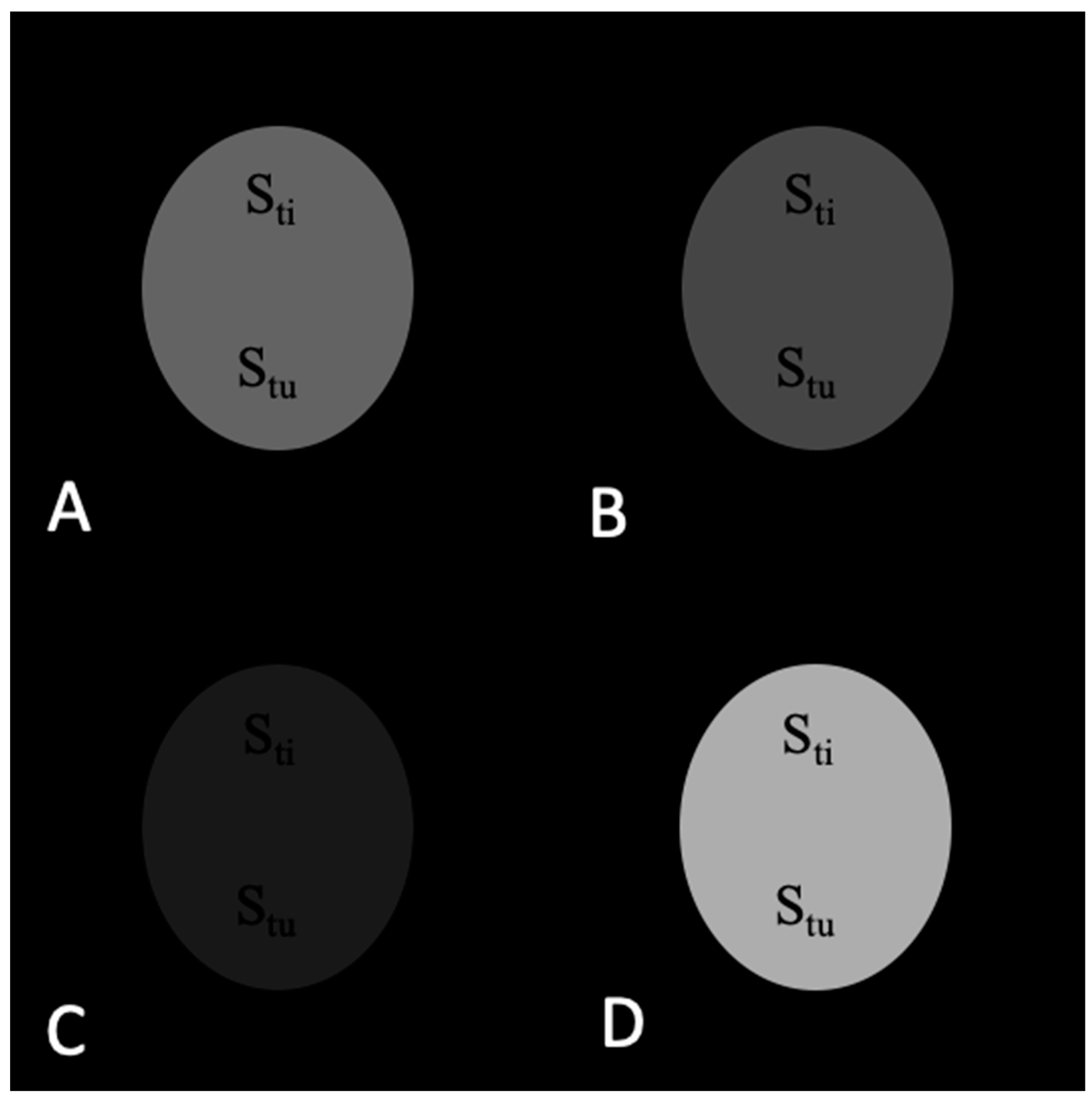
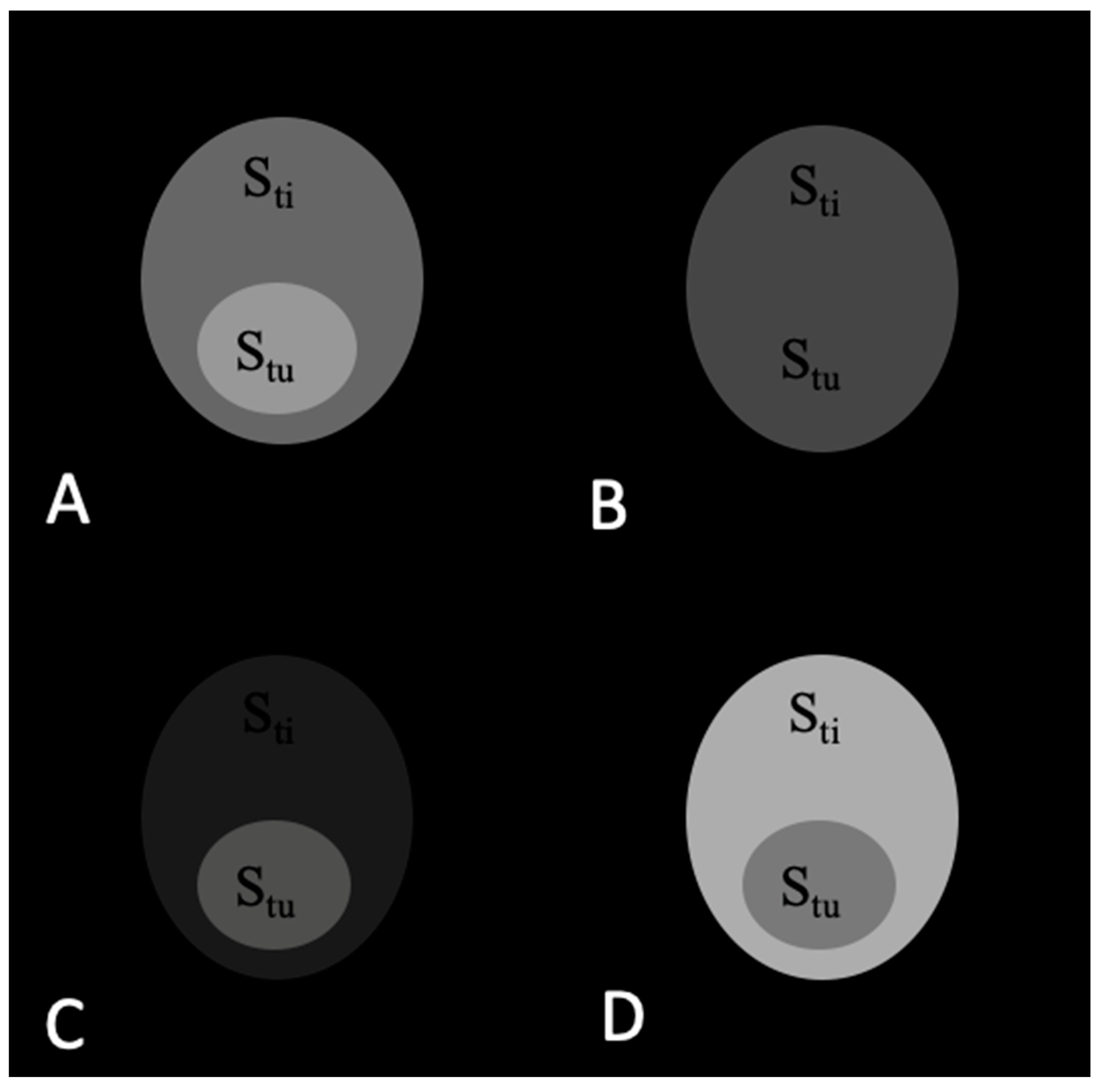
2.1. Theoretical Analysis of a Phantom Tumor Model
2.2. Animal Model of Breast Cancer
2.3. Synthesis and Characterisation of the NaDyF4/NaGdF4 Nanoparticles
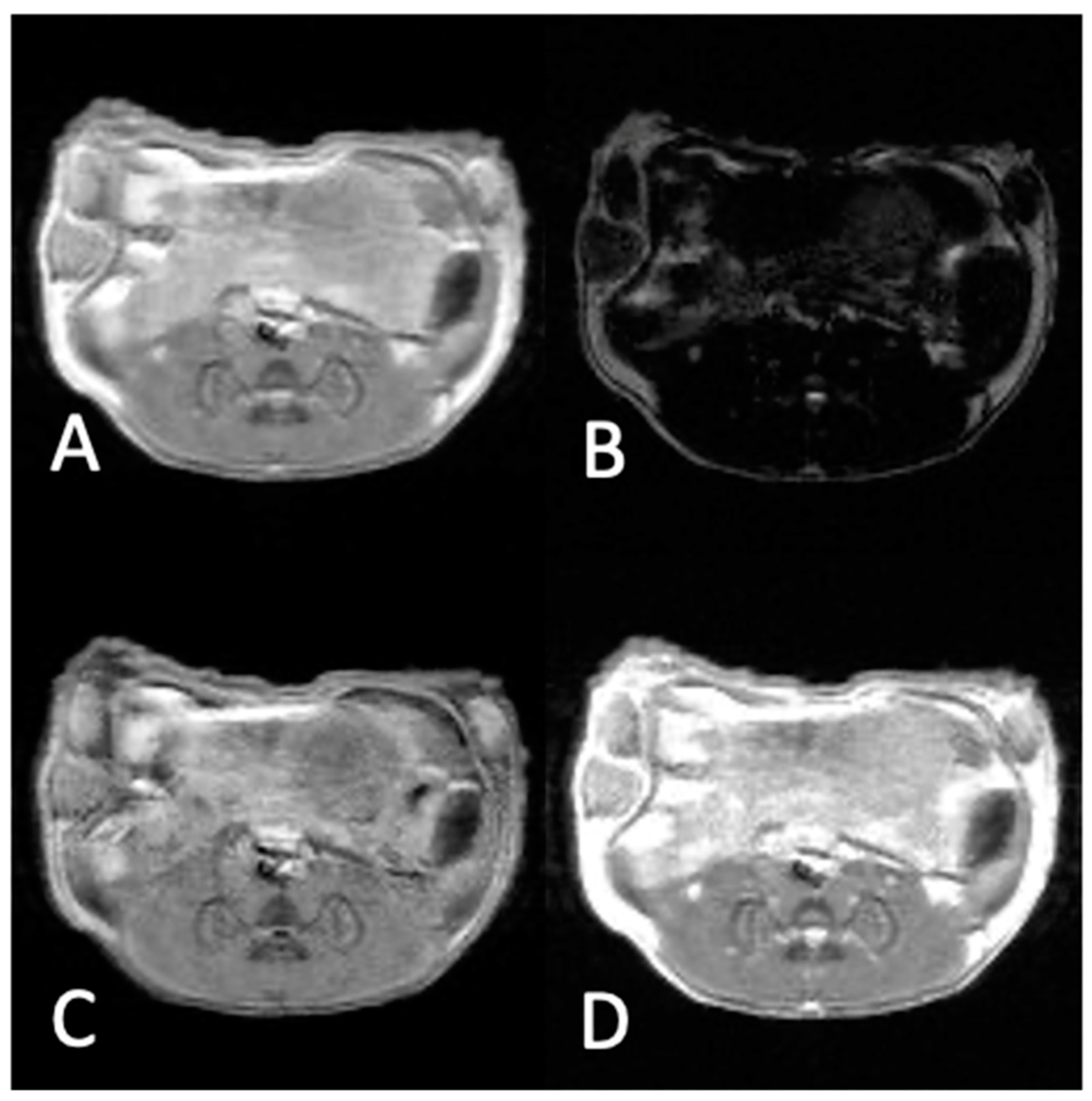
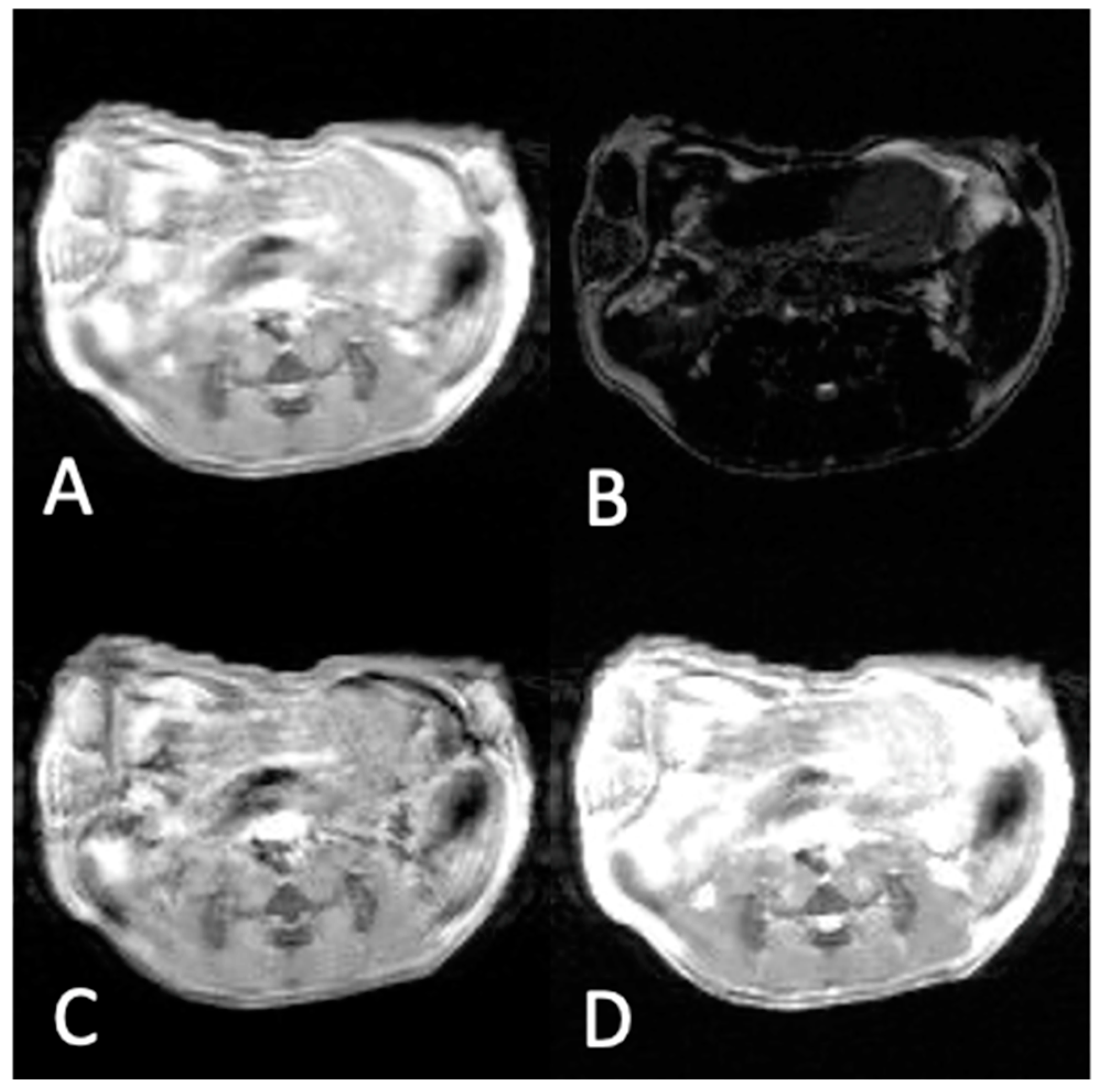
2.4. In Vivo MRI Experiments
2.5. MR Imaging Parameters
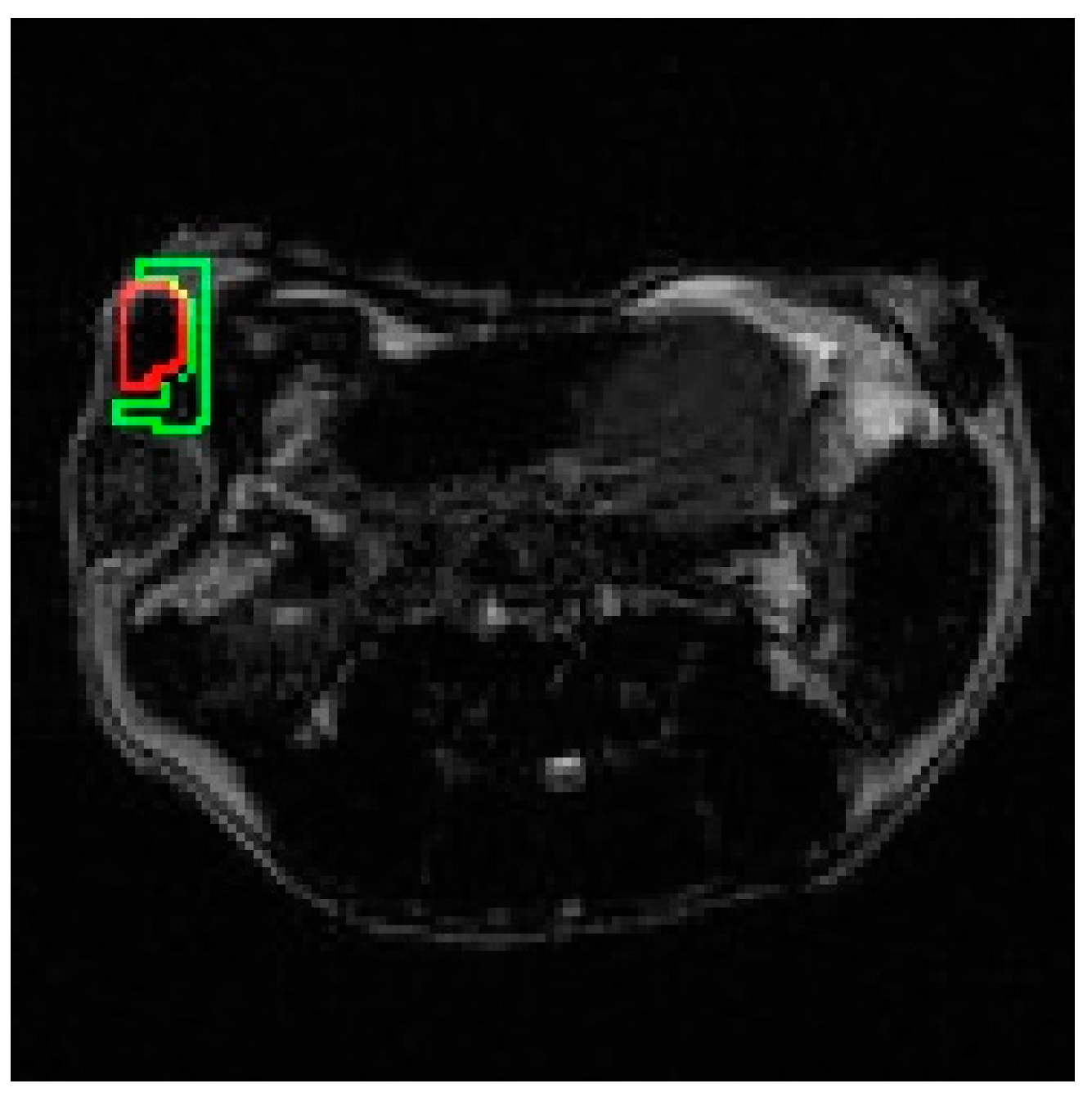
2.6. Selection of ROIs
3. Results
3.1. Calculations of Contrast in a Tumor Phantom Model
3.2. In Vivo Experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demer, J.L.; Dushyanth, A. T2-weighted fast spin-echo magnetic resonance imaging of extraocular muscles. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2011, 15, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.J.; Löffler, R.; Deimling, M.; Peller, M.; Reiser, M. MR lung imaging at 0.2 T with T1-weighted true FISP: Native and oxygen-enhanced. J. Magn. Reson. Imaging 2001, 14, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Busquets, M.A.; Sabaté, R.; Estelrich, J. Potential applications of magnetic particles to detect and treat Alzheimer’s disease. Nanoscale Res. Lett. 2014, 9, 538. [Google Scholar] [CrossRef]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, D.; Bao, J.; Chen, Q.; Liu, G.; Chen, Z.; Chen, X.; Gao, J. A Synergistically Enhanced T1–T2 Dual-Modal Contrast Agent. Adv. Mater. 2012, 24, 6223–6228. [Google Scholar] [CrossRef]
- Nazir, S.; Hussain, T.; Ayub, A.; Rashid, U.; MacRobert, A.J. Nanomaterials in combating cancer: Therapeutic applications and developments. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Barua, S.; Sharma, G.; Dey, S.K.; Rege, K. Inorganic nanoparticles for cancer imaging and therapy. J. Control. Release 2011, 155, 344–357. [Google Scholar] [CrossRef]
- Ravichandran, M.; Oza, G.; Velumani, S.; Ramirez, J.T.; Vera, A.; Leija, L. Design and evaluation of surface functionalized superparamagneto-plasmonic nanoparticles for cancer therapeutics. Int. J. Pharm. 2017, 524, 16–29. [Google Scholar] [CrossRef]
- Kievit, F.M.; Zhang, M. Surface Engineering of Iron Oxide Nanoparticles for Targeted Cancer Therapy. Acc. Chem. Res. 2011, 44, 853–862. [Google Scholar] [CrossRef]
- Blasiak, B.; Van Veggel, F.C.J.M.; Tomanek, B. Applications of Nanoparticles for MRI Cancer Diagnosis and Therapy. J. Nanomater. 2013, 148578. [Google Scholar] [CrossRef]
- Anzai, Y. Superparamagnetic Iron Oxide Nanoparticles Nodal Metastases and Beyond. Top. Mag. Reson. 2004, 15, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.H.; Graif, M.; Niendorf, H.P.; Brown, J.; Steiner, R.E.; Blumgart, L.H.; Young, I.R. Gadolinium-DTPA in the assessment of liver tumours by magnetic resonance imaging. Clin. Radiol. 1986, 37, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.J.; Rocha, A.J.; Mendes, M.F.; Maia, A.C.; Braga, F.T.; Tilbery, C.P. Trigeminal involvement in multiple sclerosis: Magnetic resonance imaging findings with clinical correlation in a series of patients. Mult Scler. 2005, 11, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Dash, A.; Blasiak, B.; Tomanek, B.; Latta, P.; van Veggel, F.C.J.M. Target-Specific Magnetic Resonance Imaging of Human Prostate Adenocarcinoma Using NaDyF4–NaGdF4 Core–Shell Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 24345–24355. [Google Scholar] [CrossRef]
- Harel, N.; Lin, J.; Moeller, S.; Ugurbil, K.; Yacoub, E. Combined imaging–histological study of cortical laminar specificity of fMRI signals. Neuroimage 2006, 29, 879–887. [Google Scholar] [CrossRef]
- Carvalho, A.; Domingues, I.; Gonçalves, M.C. Core–shell superparamagnetic nanoparticles with interesting properties as contrast agents for MRI. Mater. Chem. Phys. 2015, 168, 42–49. [Google Scholar] [CrossRef]
- Sounderya, N.; Zhang, Y. Use of Core/Shell Structured Nanoparticles for Biomedical Applications. Recent Pat. Biomed. Eng. 2008, 1, 34–42. [Google Scholar] [CrossRef]
- Yang, H.; Zhuang, Y.; Sun, Y.; Dai, A.; Shi, X.; Wu, D.; Li, F.; Hu, H.; Yang, S. Targeted dual-contrast T1- and T2-weighted magnetic resonance imaging of tumors using multifunctional gadolinium-labeled superparamagnetic iron oxide nanoparticles. Biomaterials 2011, 32, 4584–4593. [Google Scholar] [CrossRef]
- Norek, M.; Peters, J.A. MRI contrast agents based on dysprosium or holmium. Prog. Nucl. Magn. Reson. Spectrosc. 2011, 59, 64–82. [Google Scholar] [CrossRef]
- Li, F.; Zhi, D.; Luo, Y.; Zhang, J.; Nan, X.; Zhang, Y.; Zhou, W.; Qiu, B.; Wen, L.-P.; Liang, G. Core/shell Fe3O4/Gd2O3 nanocubes as T1–T2 dual modal MRI contrast agents. Nanoscale 2016, 8, 12826–12833. [Google Scholar] [CrossRef]
- Cheng, K.; Yang, M.; Zhang, R.; Qin, C.; Su, X.; Cheng, Z. Hybrid Nanotrimers for Dual T1 and T2-Weighted Magnetic Resonance Imaging. ACS Nano 2014, 8, 9884–9896. [Google Scholar] [CrossRef] [PubMed]
- Girard, O.M.; Du, J.; Agemy, L.; Sugahara, K.N.; Kotamraju, V.R.; Ruoslahti, E.; Bydder, G.M.; Mattrey, R.F. Optimization of iron oxide nanoparticle detection using ultrashort echo time pulse sequences: Comparison of T1, T2*, and synergistic T1−T2* contrast mechanisms. Magn. Reson. Med. 2011, 65, 1649–1660. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, X.; Lu, H.; He, Y.; Lu, Z.; Chen, Z.; Yuan, Y.; Tang, N.; Dreyer, C.A.; Quigley, L.; et al. Two-way magnetic resonance tuning and enhanced subtraction imaging for non-invasive and quantitative biological imaging. Nat. Nanotechnol. 2020, 15, 482–490. [Google Scholar] [CrossRef]
- Hendrick, R.E. High-Quality Breast MRI. Radiol. Clin. N. Am. 2014, 52, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Strijkers, G.J.; Mulder, W.J.M.; van Tilborg, G.A.F.; Nicolay, K. MRI Contrast Agents: Current Status and Future Perspectives. Anti-Cancer Agents Med. Chem. 2007, 7, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.-S.; Zhou, S.-K. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef]
- Johnson, N.J.J.; Korinek, A.; Dong, C.; van Veggel, F.C.J.M. Self-Focusing by Ostwald Ripening: A Strategy for Layer-by-Layer Epitaxial Growth on Upconverting Nanocrystals. J. Am. Chem. Soc. 2012, 134, 11068–11071. [Google Scholar] [CrossRef]
- Tomanek, B.; Iqbal, U.; Blasiak, B.; Abulrob, A.; Albaghdadi, H.; Matyas, J.R.; Ponjevic, D.; Sutherland, G.R. Evaluation of brain tumor vessels specific contrast agents for glioblastoma imaging. Neuro-Oncology 2012, 14, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, H.; Tao, Q.; Ye, C.; Yu, M.; Li, J.; Zhou, H.; Yang, S.; Ding, G.; Xie, X. Enhancing the magnetic relaxivity of MRI contrast agents via the localized superacid microenvironment of graphene quantum dots. Biomaterials 2020, 250, 120056. [Google Scholar] [CrossRef]
- Shang, L.; Li, Y.; Xiao, Y.; Xu, Y.; Chen, L.; Wang, H.; Tao, Q.; Ma, P.; Yang, S.; Ding, G.; et al. Synergistic Effect of Oxygen- and Nitrogen-Containing Groups in Graphene Quantum Dots: Red Emitted Dual-Mode Magnetic Resonance Imaging Contrast Agents with High Relaxivity. ACS Appl. Mater. Interfaces 2022, 14, 39885–39895. [Google Scholar] [CrossRef]
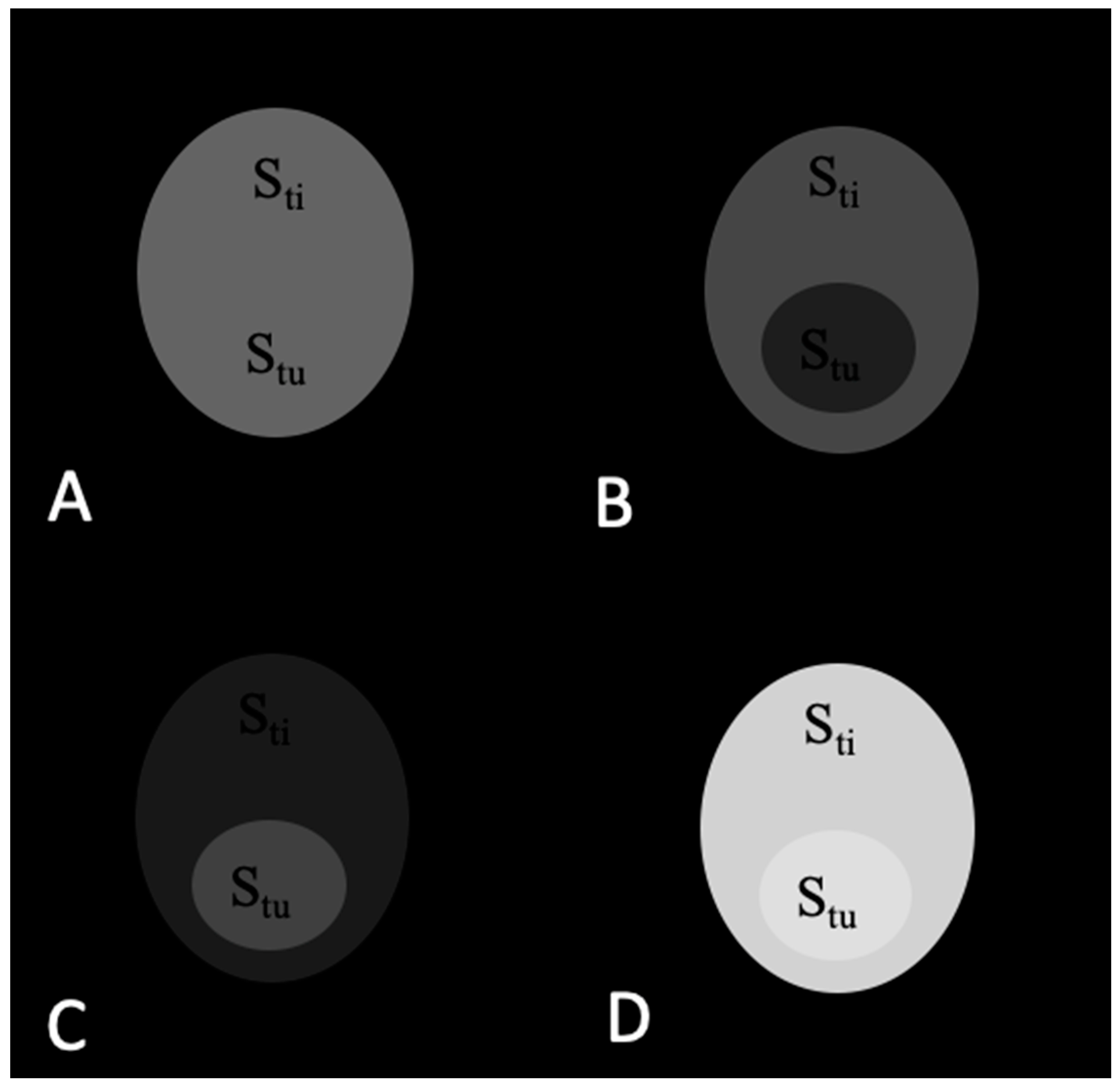
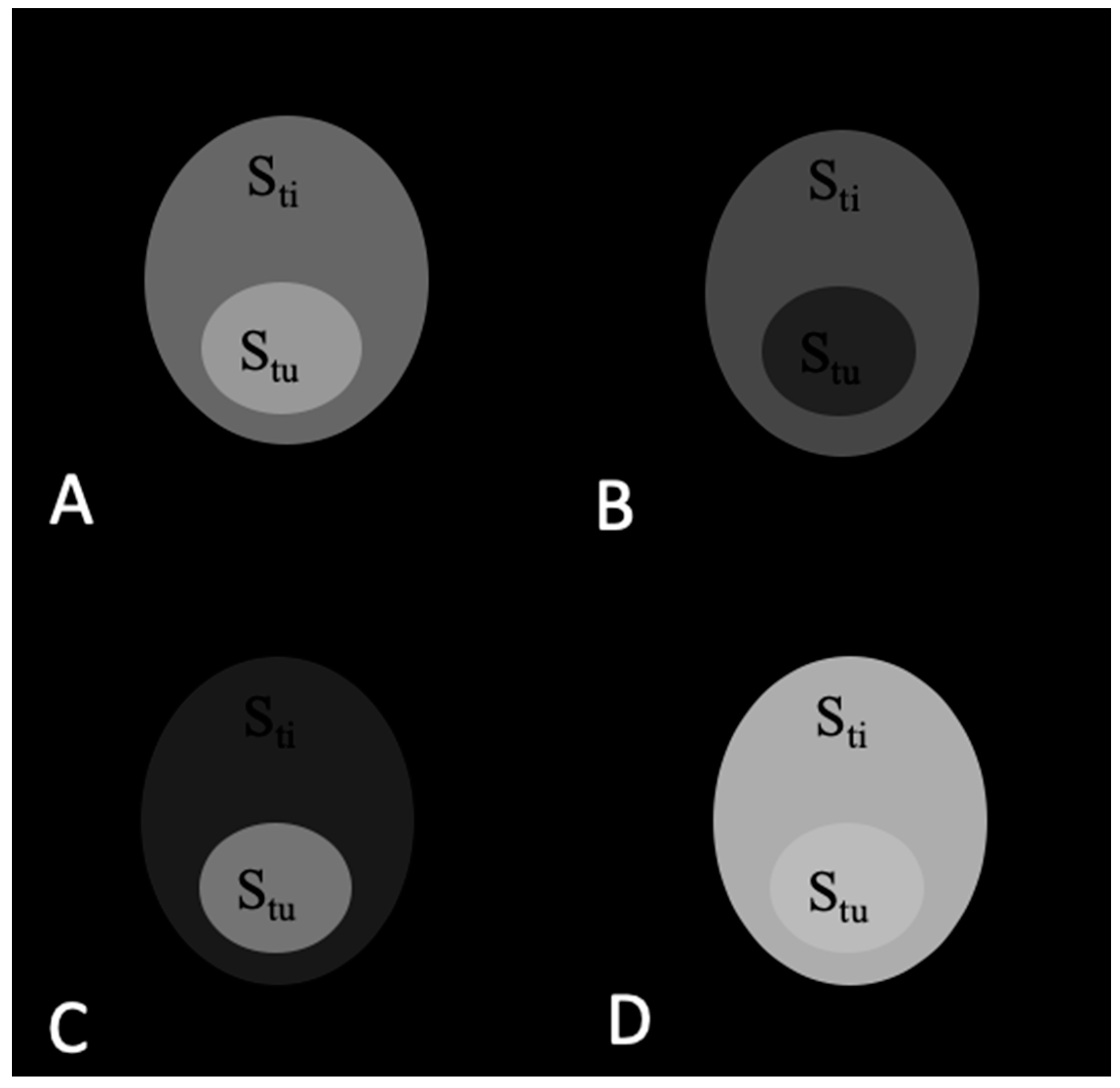
| Stu | Sti | TC = Stu/Sti | ||
|---|---|---|---|---|
| Pre-injection | T1w | 100 | 100 | 1.00 |
| T2w | 70 | 70 | 1.00 | |
| T1w − T2w | 30 | 30 | 1.00 | |
| T1w + T2w | 170 | 170 | 1.00 | |
| Post-T1 contrast injection | T1w | 150 | 100 | 1.50 |
| T2w | 70 | 70 | 1.00 | |
| T1w − T2w | 80 | 30 | 2.67 | |
| T1w + T2w | 120 | 170 | 0.71 | |
| Post-T2 contrast injection | T1w | 100 | 100 | 1.00 |
| T2w | 35 | 70 | 0.47 | |
| T1w − T2w | 65 | 30 | 2.17 | |
| T1w + T2w | 222 | 208 | 1.07 | |
| Post-T1/T2 contrast injection | T1w | 150 | 100 | 1.50 |
| T2w | 35 | 70 | 0.50 | |
| T1w − T2w | 115 | 30 | 3.83 | |
| T1w + T2w | 185 | 170 | 1.09 |
| Stu | Sti | TC = Stu/Sti | ||
|---|---|---|---|---|
| Pre-injection | T1w | 187.0 | 162.0 | 1.15 |
| T2w | 20.0 | 60.9 | 0.33 | |
| T1w − T2w | 167.0 | 110.0 | 1.52 | |
| T1w + T2w | 204.0 | 204.0 | 1.00 | |
| Post T1/T2 contrast injection | T1w | 230.0 | 186.0 | 1.24 |
| T2w | 18.6 | 57.6 | 0.32 | |
| T1w − T2w | 216.0 | 138.0 | 1.56 | |
| T1w + T2w | 239.0 | 219.0 | 1.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacDonald, D.; van Veggel, F.C.J.M.; Tomanek, B.; Blasiak, B. Contrast Enhancement in MRI Using Combined Double Action Contrast Agents and Image Post-Processing in the Breast Cancer Model. Materials 2023, 16, 3096. https://doi.org/10.3390/ma16083096
MacDonald D, van Veggel FCJM, Tomanek B, Blasiak B. Contrast Enhancement in MRI Using Combined Double Action Contrast Agents and Image Post-Processing in the Breast Cancer Model. Materials. 2023; 16(8):3096. https://doi.org/10.3390/ma16083096
Chicago/Turabian StyleMacDonald, David, Frank C. J. M. van Veggel, Boguslaw Tomanek, and Barbara Blasiak. 2023. "Contrast Enhancement in MRI Using Combined Double Action Contrast Agents and Image Post-Processing in the Breast Cancer Model" Materials 16, no. 8: 3096. https://doi.org/10.3390/ma16083096
APA StyleMacDonald, D., van Veggel, F. C. J. M., Tomanek, B., & Blasiak, B. (2023). Contrast Enhancement in MRI Using Combined Double Action Contrast Agents and Image Post-Processing in the Breast Cancer Model. Materials, 16(8), 3096. https://doi.org/10.3390/ma16083096





