XRD and Spectroscopic Investigations of ZIF—Microchannel Glass Plates Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of ZIF-8 and ZIF-8@MCG Composite
2.2. Synthesis of ZIF-67 and ZIF-67@MCG Composite
2.2.1. Synthesis of ZIF-67-1 and ZIF-67-1@MCG Composite (Route A Method)
2.2.2. Synthesis of ZIF-67-2 and ZIF-67-2@MCG Composite (Route B Method)
2.3. Characterizations of ZIF-8, ZIF-67, and Their Composites
2.3.1. XRD Measurements
2.3.2. Micro-Raman Spectroscopy
2.3.3. FTIR Spectroscopy
3. Results and Discussion
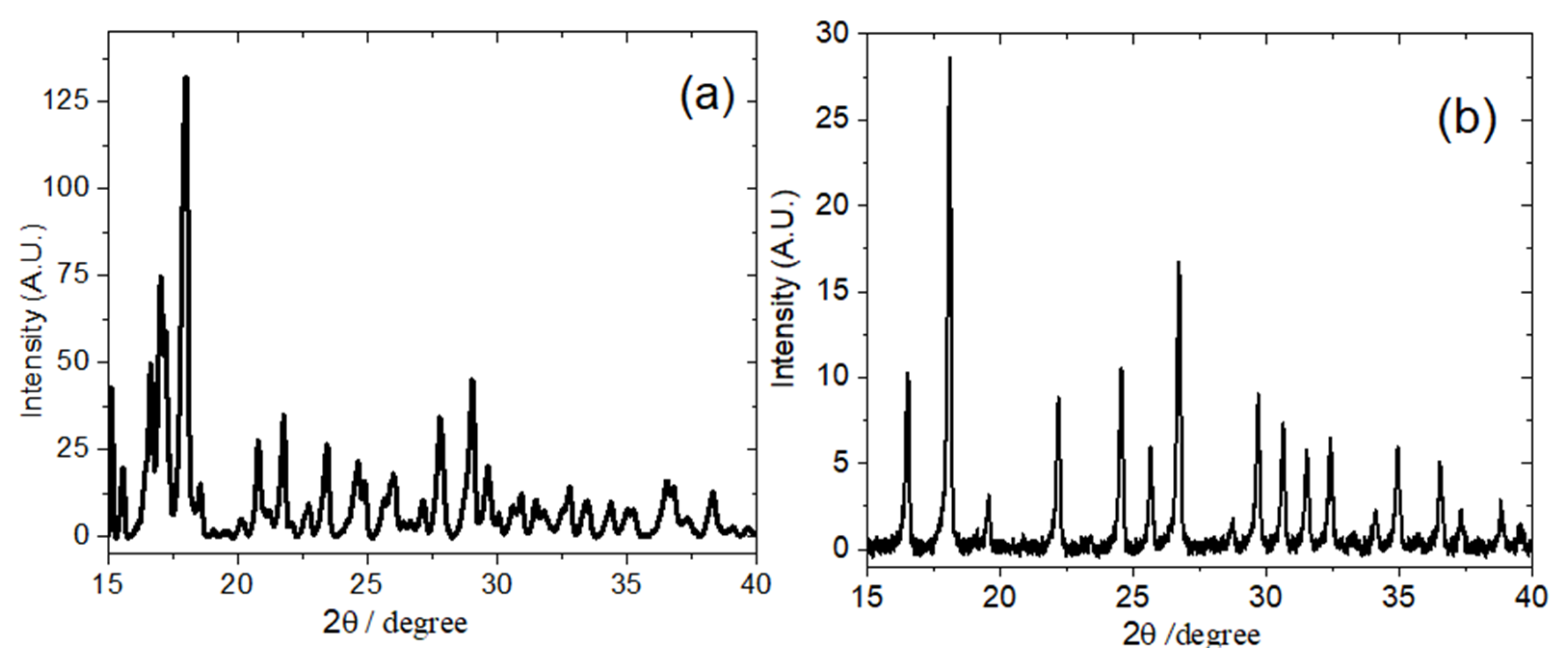
3.1. XRD Analysis
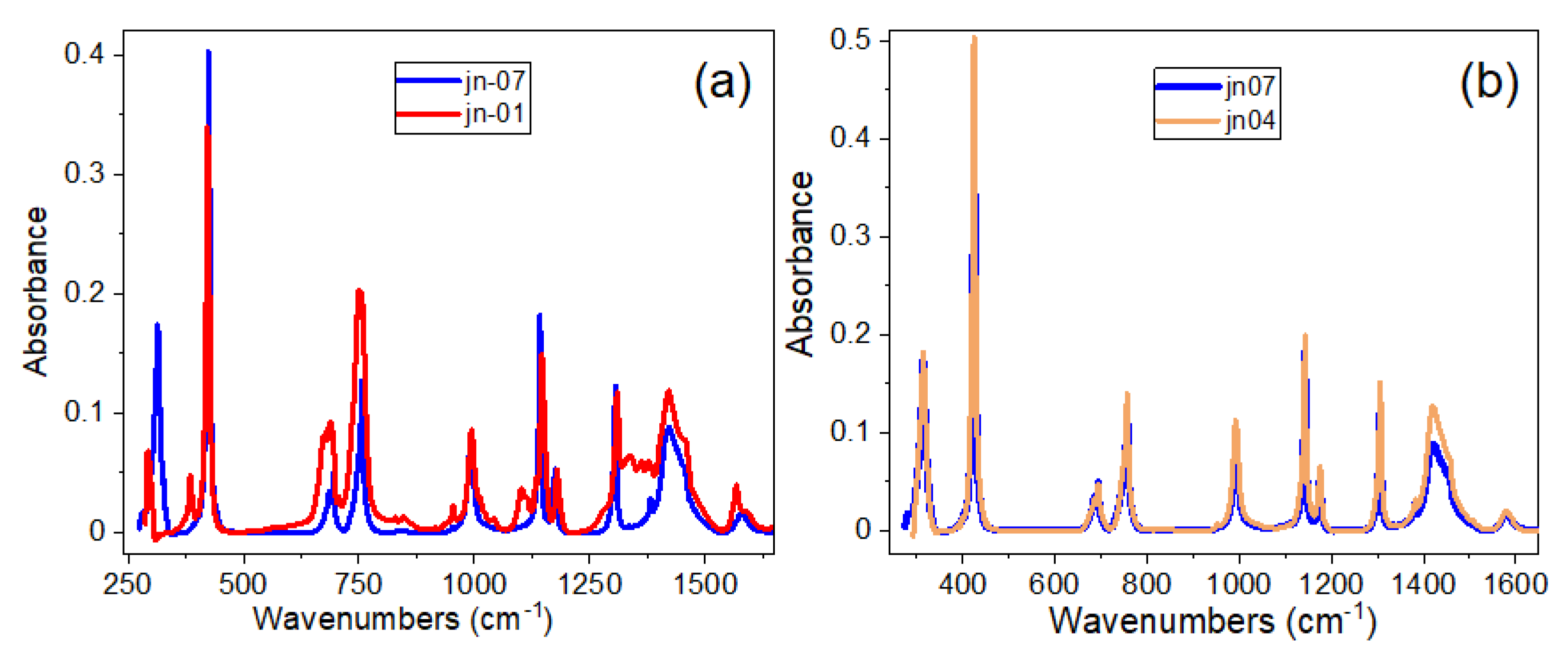
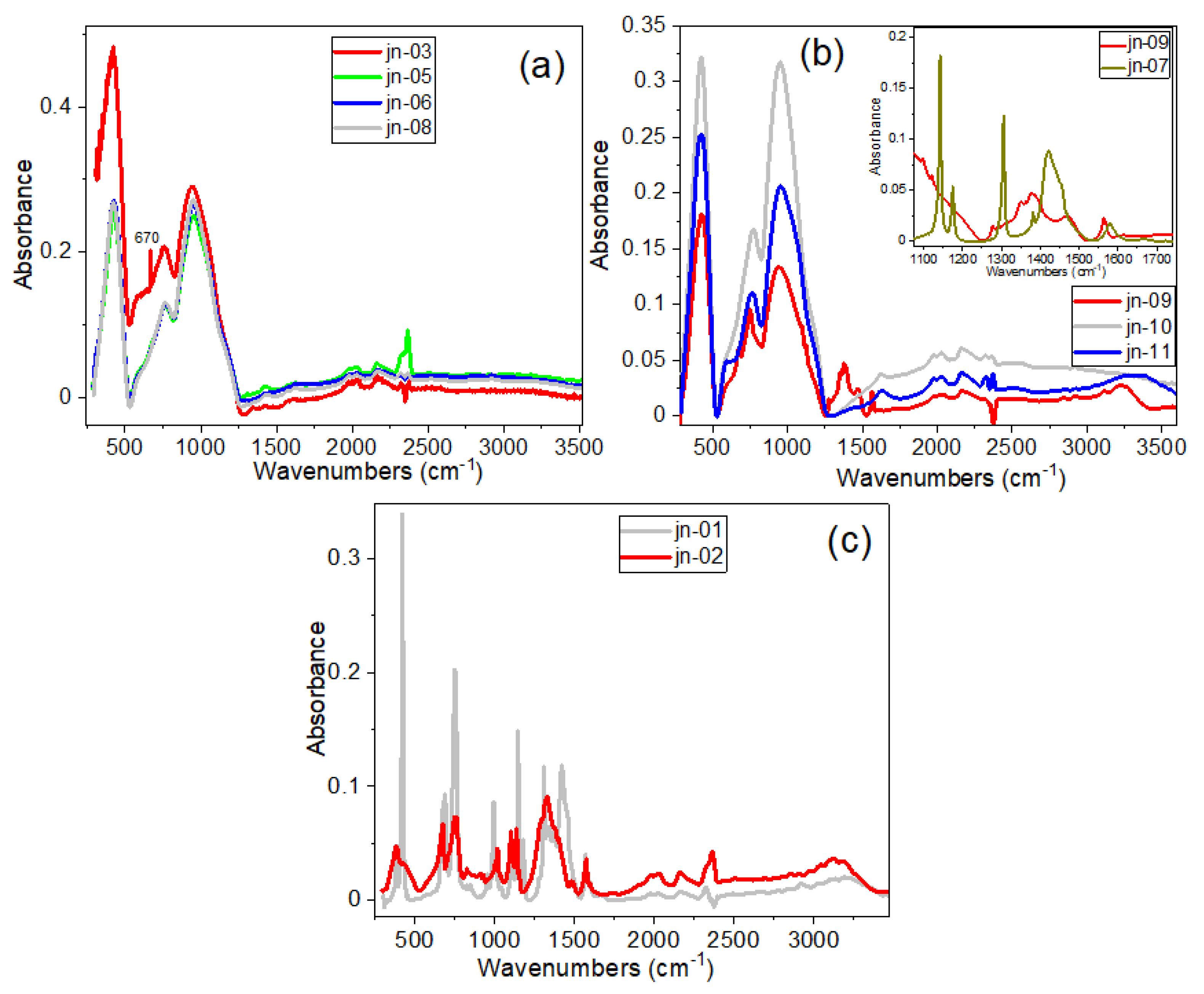
3.2. FTIR Spectra
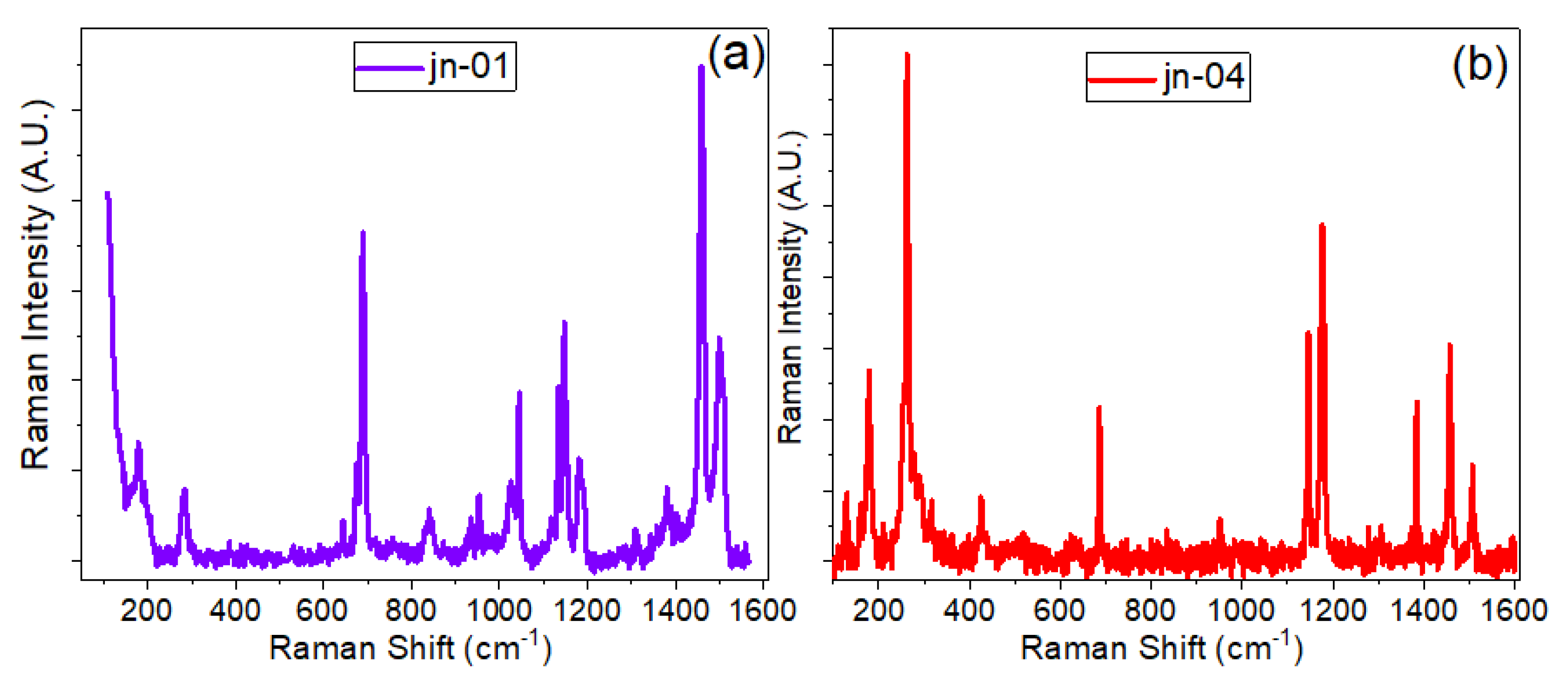
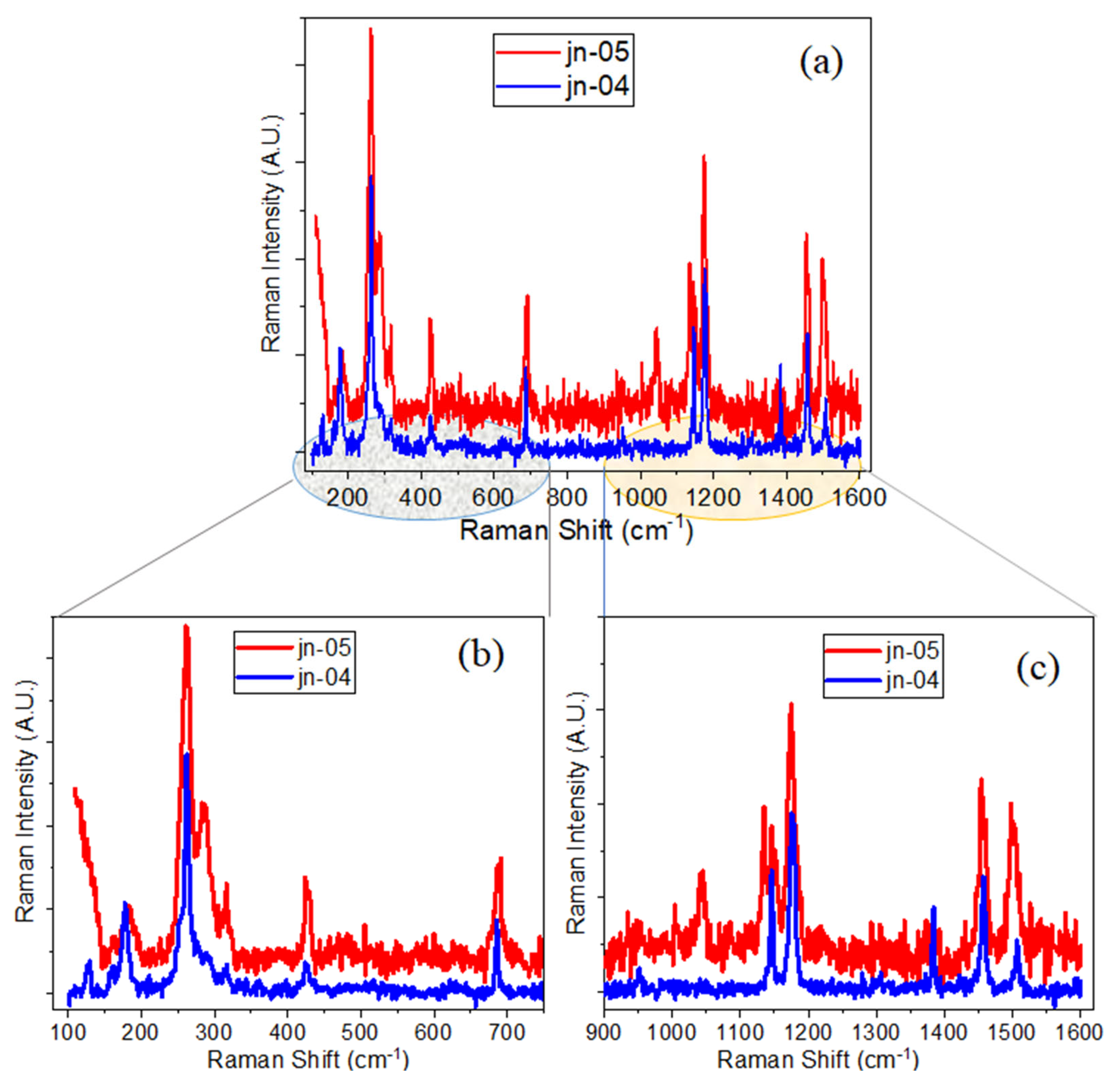
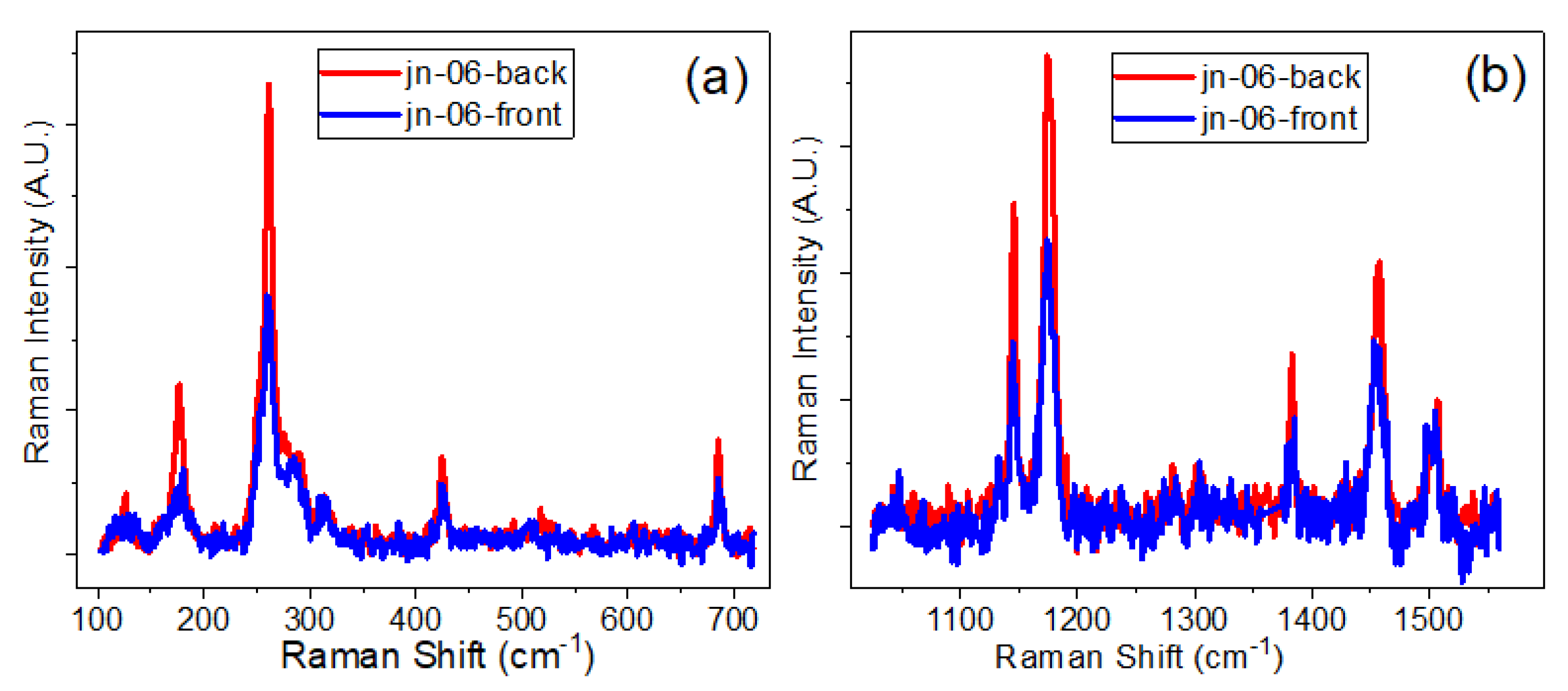
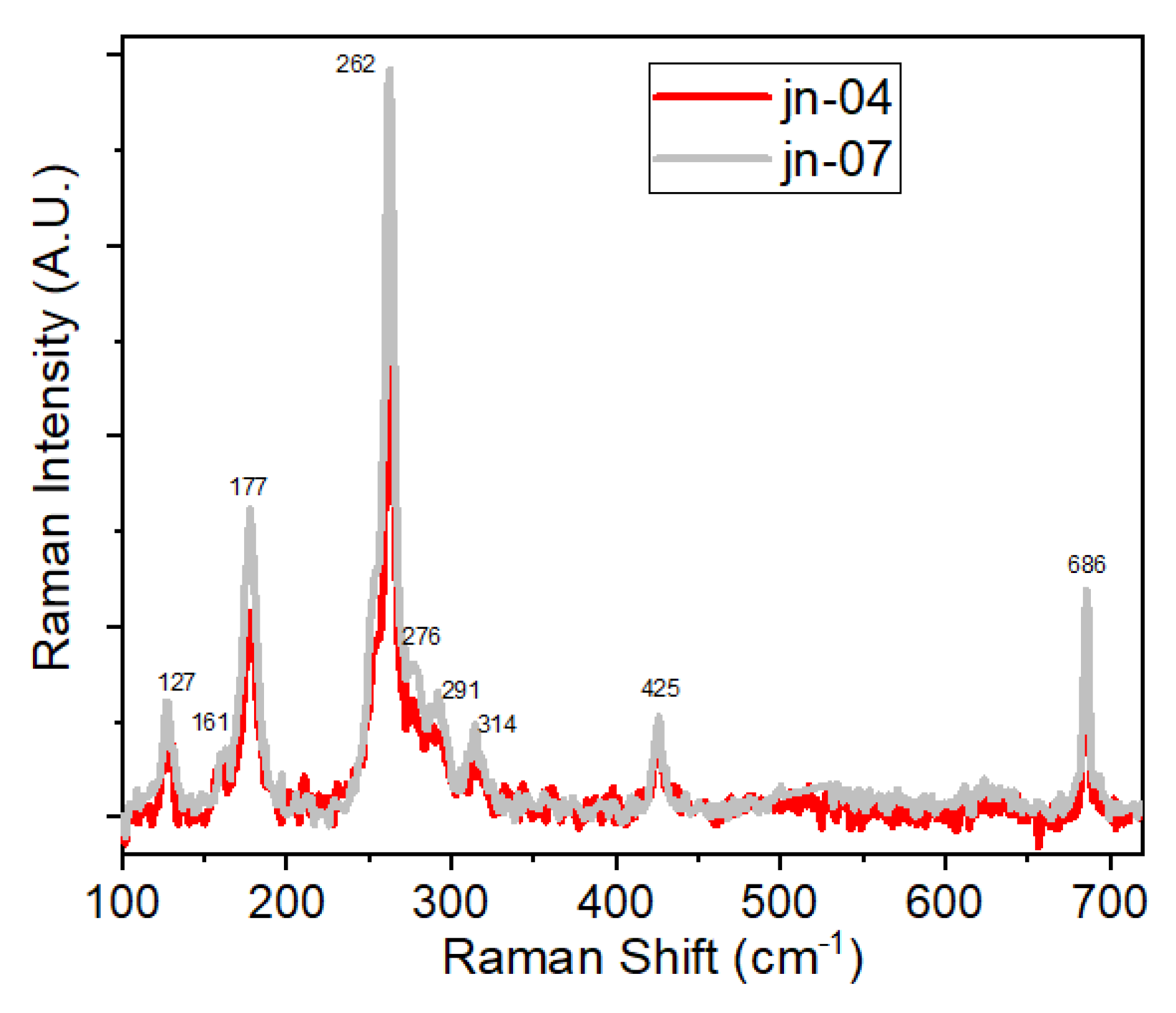
3.3. Raman Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, F.; Cerro, A.M.; Mosier, A.M.; Wayment-Steele, H.K.; Shine, R.S.; Park, A.; Benz, L. Surface and Stability Characterization of a Nanoporous ZIF-8 Thin Film. J. Phys. Chem. C 2014, 118, 14449–14456. [Google Scholar] [CrossRef]
- Wen, L.; Li, F.; Cheng, H.-M. Carbon nanotubes and graphene for flexible electrochemical energy storage: From materials to devices. Adv. Mater. 2016, 28, 4306–4337. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-organic framework functionalization and design strategies for advanced electrochemical energy storage devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef]
- Ricco, R.; Malfatti, L.; Takahashi, M.; Hill, A.J.; Falcaro, P. Applications of magnetic metal-organic framework composites. J. Mater. Chem. A 2013, 1, 13033–13045. [Google Scholar] [CrossRef]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal–organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Gangua, K.K.; Maddila, S.; Mukkamalab, S.B.; Jonnalagadda, S.B. A review on contemporary Metal–Organic Framework materials. Inorg. Chim. Acta 2016, 446, 61–74. [Google Scholar] [CrossRef]
- Han, Y.; Qi, P.; Zhou, J.; Feng, X.; Li, S.; Fu, X.; Zhao, J.; Yu, D.; Wang, B. Metal-organic frameworks (MOFs) as sandwich coating cushion for silicon anode in lithium ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 26608–26613. [Google Scholar] [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. TRAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Rasheed, T.; Rizwan, K.; Bilal, M.; Iqbal, H.M.N. Metal-Organic Framework-Based Engineered Materials—Fundamentals and Applications. Molecules 2020, 25, 1598. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.W. Metal–Organic Frameworks for Biomedical Applications. Small 2020, 16, 1–24. [Google Scholar] [CrossRef]
- Raptopoulou, C.P. Metal-Organic Frameworks: Synthetic Methods and Potential Applications. Materials 2021, 14, 310. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-R.; Jang, M.-S.; Cho, H.-Y.; Kwon, H.-J.; Kim, S.; Ahn, W.-S. ZIF-8: A comparison of synthesis methods. Chem. Eng. J. 2015, 271, 276–280. [Google Scholar] [CrossRef]
- Pang, F.; He, M.; Ge, J. Controlled Synthesis of Fe3O4-ZIF-8 Nanoparticles for Magnetically Separable Nanocatalysts. Chem. Eur. J. 2015, 21, 6879–6887. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yang, Z.; Zhu, Y.; Xia, Y. Zeolitic imidazolate framework materials: Recent progress in synthesis and applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Wang, X. Metal–organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef]
- Li, Y.; Yan, X.; Hu, X.; Feng, R.; Zhou, M.; Han, D. In situ growth of ZIF-8 onto porous carbons as an efficient adsorbent for malachite green removal. J. Porous Mater. 2020, 27, 1109–1117. [Google Scholar] [CrossRef]
- Peng, J.; Sun, X.; Li, Y.; Huang, C.; Jin, J.; Dhanjai; Wang, J.; Chen, J. Controllable growth of ZIF-8 layers with nanometer-level precision on SiO2 nano-powders via liquid phase epitaxy stepwise growth approach. Microporous Mesoporous Mater. 2018, 268, 268–275. [Google Scholar] [CrossRef]
- Shayegan, H.; Ali, G.A.M.; Safarifard, V. Recent Progress in the Removal of Heavy Metal Ions from Water Using Metal-Organic Frameworks. ChemistrySelect 2020, 5, 124–146. [Google Scholar] [CrossRef]
- Shen, B.; Wang, B.; Zhu, L.; Jiang, L. Properties of Cobalt- and Nickel-Doped Zif-8 Framework Materials and Their Application in Heavy-Metal Removal from Wastewater. Nanomaterials 2020, 10, 1636. [Google Scholar] [CrossRef]
- Liu, Y.; Ng, Z.; Khan, E.A.; Jeong, H.-K.; Ching, C.-B.; Lai, Z. Synthesis of continuous MOF-5 membranes on porous a-alumina substrates. Microporous Mesoporous Mater. 2009, 118, 296–301. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Downard, A.J.; Telfer, S.G. HKUST-1 growth on glassy carbon. J. Mater. Chem. 2011, 21, 19207–19209. [Google Scholar] [CrossRef]
- Li, W.; Tu, M.; Cao, R.; Fischer, R.A. Metal–organic framework thin films: Electrochemical fabrication techniques and corresponding applications & perspectives. J. Mater. Chem. A 2016, 4, 12356–12369. [Google Scholar] [CrossRef]
- Keppler, N.C.; Deli, K.; Hindricks, J.; Behrens, P. Large refractive index changes in ZIF-8 thin films of optical quality. RSC Adv. 2022, 12, 5807–5815. [Google Scholar] [CrossRef] [PubMed]
- Van Cleuvenbergen, S.; Stassen, I.; Gobechiya, E.; Zhang, Y.; Markey, K.; De Vos, D.E.; Kirschhock, C.; Champagne, B.; Verbiest, T.; van der Veen, M.A. ZIF-8 as nonlinear optical material: Influence of structure and synthesis. Chem. Mater. 2016, 28, 3203–3209. [Google Scholar] [CrossRef]
- Dong, L.; Chu, H.; Li, Y.; Zhao, S.; Li, D. Broadband optical nonlinearity of zeolitic imidazolate framework-8 (ZIF-8) for ultrafast photonics. J. Mater. Chem. C 2021, 9, 8912–8919. [Google Scholar] [CrossRef]
- Pan, H.; Wang, X.; Chu, H.; Li, Y.; Zhao, S.; Li, G.; Li, D. Optical modulation characteristics of zeolitic imidazolate framework-67 (ZIF-67) in the near infrared regime. Opt. Lett. 2019, 44, 5892–5895. [Google Scholar] [CrossRef]
- Pan, H.; Chu, H.; Wang, X.; Li, Y.; Zhao, S.; Li, G.; Li, D. Nonlinear Optical Features of Zeolitic Imidazolate Framework-67 Nanocrystals for Mid-Infrared Pulse Generation. Cryst. Growth Des. 2020, 20, 6683–6690. [Google Scholar] [CrossRef]
- Aboraia, A.M.; Darwish, A.A.A.; Polyakov, V.; Erofeeva, E.; Butova, V.; Zahran, H.Y.; Abd El-Rehim, A.F.; Algarni, H.; Yahia, I.S.; Soldatov, A.V. Structural characterization and optical properties of zeolitic imidazolate frameworks (ZIF-8) for solid-state electronics applications. Opt. Mater. 2020, 100, 109648. [Google Scholar] [CrossRef]
- Sutrisna, P.D.; Prasetya, N.; Himma, N.F.; Wenten, I.G. A mini-review and recent outlooks on the synthesis and applications of zeolite imidazolate framework-8 (ZIF-8) membranes on polymeric substrate. J. Chem. Technol. Biotechnol. 2020, 95, 2767–2774. [Google Scholar] [CrossRef]
- Barankova, E.; Pradeep, N.; Peinemann, K. Zeolite-imidazolate framework (ZIF-8) membrane synthesis on mixed-matrix substrate. Chem. Commun. 2013, 49, 9419–9421. [Google Scholar] [CrossRef]
- Tu, M.; Wannapaiboon, S.; Khaletskaya, K.; Fischer, R.A. Engineering Zeolitic-Imidazolate Framework (ZIF) Thin Film Devices for Selective Detection of Volatile Organic Compounds. Adv. Funct. Mater. 2015, 25, 4470–4479. [Google Scholar] [CrossRef]
- Dangwal, S.; Ronte, A.; Lin, H.; Liu, R.; Zhu, J.; Lee, J.S.; Gappa-Fahlenkamp, H.; Kim, S.-K. ZIF-8 membranes supported on silicalite-seeded substrates for propylene/propane separation. J. Membr. Sci. 2021, 626, 119165. [Google Scholar] [CrossRef]
- Huang, K.; Dong, Z.; Li, Q.; Jin, W. Growth of a ZIF-8 membrane on the inner-surface of a ceramic hollow fiber via cycling precursors. Chem. Commun. 2013, 49, 10326–10328. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhang, X.; Liu, Y.; Li, S.; Liu, H.; Qiu, J.; Yeung, K.L. In situ fabrication of high-permeance ZIF-8 tubular membranes in a continuous flow system. Mater. Chem. Phys. 2014, 148, 10–16. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Jang, M.S.; Kwon, H.J.; Ahn, W.S. Zeolitic Imidazolate Frameworks: Synthesis, Functionalization, and Catalytic/Adsorption Applications. Catal. Surv. Asia 2014, 18, 101–127. [Google Scholar] [CrossRef]
- Popecki, M.A.; Adams, B.; Craven, C.A.; Cremer, T.; Foley, M.R.; Lyashenko, A.; O’Mahony, A.; Minot, M.J.; Aviles, M.; Bond, J.L.; et al. Microchannel plate fabrication using glass capillary arrays with Atomic Layer Deposition films for resistance and gain. J. Geophys. Res. Space Phys. 2016, 121, 7449–7460. [Google Scholar] [CrossRef]
- Kouser, S.; Hezam, A.; Khadri, M.N.; Khanum, S.A. A review on zeolite imidazole frameworks: Synthesis, properties, and applications. J. Porous Mater. 2022, 29, 663–681. [Google Scholar] [CrossRef]
- Ghoshal, S.; Zaccarine, S.; Anderson, G.C.; Martinez, M.B.; Hurst, K.E.; Pylypenko, S.; Pivovar, B.S.; Alia, S.M. ZIF 67 Based Highly Active Electrocatalysts as Oxygen Electrodes in Water Electrolyzer. ACS Appl. Energy Mater. 2019, 2, 5568–5576. [Google Scholar] [CrossRef]
- Song, X.-Z.; Zhao, Y.-H.; Yang, W.-B.; Meng, Y.-L.; Chen, X.; Niu, Z.-Y.; Wang, X.-F.; Tan, Z. Hollow CoP Encapsulated in an N-Doped Carbon Nanocage as an Efficient Bifunctional Electrocatalyst for Overall Water Splitting. ACS Appl. Nano Mater. 2021, 4, 13450–13458. [Google Scholar] [CrossRef]
- Shahsavari, M.; Mortazavi, M.; Tajik, S.; Sheikhshoaie, I.; Beitollahi, H. Synthesis and Characterization of GO/ZIF-67 Nanocomposite: Investigation of Catalytic Activity for the Determination of Epinine in the Presence of Dobutamine. Micromachines 2022, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Balog, E.; Varga, G.; Kukovecz, Á.; Tóth, Á.; Horváth, D.; Lagzi, I.; Schuszter, G. Polymorph Selection of Zeolitic Imidazolate Frameworks via Kinetic and Thermodynamic Control. Cryst. Growth Des. 2022, 22, 4268–4276. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Yu, D.; Xiao, K.; Hong, Y. Transition from ZIF-L-Co to ZIF-67: A new insight into the structural evolution of zeolitic imidazolate frameworks (ZIFs) in aqueous systems. CrystEngComm 2015, 17, 8212–8215. [Google Scholar] [CrossRef]
- Low, Z.-X.; Yao, J.; Liu, Q.; He, M.; Wang, Z.; Suresh, A.K.; Bellare, J.; Wang, H. Crystal Transformation in Zeolitic-Imidazolate Framework. Cryst. Growth Des. 2014, 14, 6589–6598. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, M.; Lin, Y.S. Stability of ZIF-8 in water under ambient conditions. Microporous Mesoporous Mater. 2019, 279, 201–210. [Google Scholar] [CrossRef]
- Hu, Y.; Kazemian, H.; Rohani, S.; Huang, Y.; Song, Y. In situ high pressure study of ZIF-8 by FTIR spectroscopy. Chem. Commun. 2011, 47, 12694–12696. [Google Scholar] [CrossRef]
- Zhou, K.; Mousavi, B.; Luo, Z.; Phatanasri, S.; Chaemchuen, S.; Verpoort, F. Characterization and properties of Zn/Co zeolitic imidazolate frameworks vs. ZIF-8 and ZIF-67. J. Mater. Chem. A 2017, 5, 952–957. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Y.; Hou, L. Synthesis of zeolitic imidazolate framework-8 on polyester fiber for PM2.5 removal. RSC Adv. 2018, 8, 31471–31477. [Google Scholar] [CrossRef]
- Singh, S.; Bajwaa, B.S.; Kaur, I. (Zn/Co)-zeolitic imidazolate frameworks: Room temperature synthesis and application as promising U(VI) scavengers—A comparative study. J. Ind. Eng. Chem. 2021, 93, 351–360. [Google Scholar] [CrossRef]
- Westendorff, K.S.; Paolucci, C.; Giri, G. Polymer-induced polymorphism in a Zn-based metal organic framework. Chem. Commun. 2021, 57, 887–890. [Google Scholar] [CrossRef]
- Deacon, A.; Briquet, L.; Malankowska, M.; Massingberd-Mundy, F.; Rudić, S.; Hyde, T.I.; Cavaye, H.; Coronas, J.; Poulston, S.; Johnson, T. Understanding the ZIF-L to ZIF-8 transformation from fundamentals to fully costed kilogram-scale production. Commun. Chem. 2022, 5, 18. [Google Scholar] [CrossRef]
- Kumari, G.; Jayaramulu, K.; Maji, T.K.; Narayana, C. Temperature Induced Structural Transformations and Gas Adsorption in the Zeolitic Imidazolate Framework ZIF-8: A Raman Study. J. Phys. Chem. A 2013, 117, 11006–11012. [Google Scholar] [CrossRef]
- Tanaka, S.; Fujita, K.; Miyake, Y.; Miyamoto, M.; Hasegawa, Y.; Makino, T.; Van der Perre, S.; Remi, J.C.S.; Assche, T.V.; Baron, G.; et al. Adsorption and Diffusion Phenomena in Crystal Size Engineered ZIF-8 MOF. J. Phys. Chem. C 2015, 119, 28430–28439. [Google Scholar] [CrossRef]
- Ebrahim, A.M.; Plonka, A.M.; Tian, Y.; Senanayake, S.D.; Gordon, W.O.; Balboa, A.; Wang, H.; Collins-Wildman, D.L.; Hill, C.L.; Musaev, D.G.; et al. Capture and Decomposition of the Nerve Agent Simulant, DMCP, Using the Zeolitic Imidazolate Framework (ZIF-8). ACS Appl. Mater. Interfaces 2020, 12, 58326–58338. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chang, H.; Li, Y.; Li, Q.; Shen, K.; Yi, H.; Zhang, J. Synthesis and adsorption performance of La@ZIF-8 composite metal–organic frameworks. RSC Adv. 2020, 10, 3380–3390. [Google Scholar] [CrossRef] [PubMed]
- Olaniyan, B.; Saha, B. Comparison of Catalytic Activity of ZIF-8 and Zr/ZIF-8 for Greener Synthesis of Chloromethyl Ethylene Carbonate by CO2 Utilization. Energies 2020, 13, 521. [Google Scholar] [CrossRef]
- Yang, X.; Gu, J.; Liu, C.; Bai, Z.; Yang, L. Partial deligandation activated ZIF-67 for efficient electrocatalytic oxygen reduction reaction. Front. Chem. 2022, 10, 983549. [Google Scholar] [CrossRef]
- Lu, Y.; Pan, H.; Lai, J.; Xia, Y.; Chen, L.; Liang, R.; Yan, G.; Huang, R. Bimetallic CoCu-ZIF material for efficient visible light photocatalytic fuel denitrification. RSC Adv. 2022, 12, 12702–12709. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Ishikawa, H.; Chervy, T.; Hutchison, J.A.; Uji-I, H. Selective crystallization via vibrational strong coupling. Chem. Sci. 2021, 12, 11986–11994. [Google Scholar] [CrossRef]
- Markham, L.M.; Mayne, L.C.; Hudson, B.S. Resonance Raman Studies of Imidazole, Imidazolium, and Their Derivatives: The Effect of Deuterium Substitution. J. Phys. Chem. 1993, 97, 10319–10325. [Google Scholar] [CrossRef]
- Carter, D.A.; Pemberton, J.E. Raman Spectroscopy and Vibrational Assignments of 1- and 2-Methylimidazole. J. Raman Spectrosc. 1997, 28, 939–946. [Google Scholar] [CrossRef]
- Mao, C.-J.; Hu, X.-W.; Song, J.-M.; Niu, H.-L.; Zhang, S.-Y. Synthesis of zinc 1-(2-pyridylazo)-2-naphthol (Zn(PAN)2) nanobelts with nonlinear optical property. CrystEngComm 2012, 14, 6823–6826. [Google Scholar] [CrossRef]
- Mazuritskiy, M.I.; Lerer, A.M. Spectral and diffraction properties of microchannel plates in the long-wavelength X-Ray range. JETP Lett. 2017, 105, 572–576. [Google Scholar] [CrossRef]
- Zhang, W.; Maul, J.; Vulpe, D.; Moghadam, P.Z.; Fairen-Jimenez, D.; Mittleman, D.M.; Zeitler, J.A.; Erba, A.; Ruggiero, M.T. Probing the Mechanochemistry of Metal-Organic Frameworks with Low-Frequency Vibrational Spectroscopy. J. Phys. Chem. C 2018, 122, 27442–27450. [Google Scholar] [CrossRef]
- Ryder, M.R.; Civalleri, B.; Bennett, T.D.; Henke, S.; Rudic, S.; Cinque, G.; Fernandez-Alonso, F.; Tan, J.-C. Identifying the Role of Terahertz Vibrations in Metal-Organic Frameworks: From Gate Opening Phenomenon to Shear-Driven Structural Destabilization. Phys. Rev. Lett. 2014, 113, 215502. [Google Scholar] [CrossRef]
- Tan, N.Y.; Ruggiero, M.T.; Orellana-Tavra, C.; Tian, T.; Bond, A.D.; Korter, T.M.; Fairen-Jimenez, D.; Zeitler, J.A. Investigation of the Terahertz Vibrational Modes of ZIF-8 and ZIF-90 with Terahertz Time-Domain Spectroscopy. Chem. Commun. 2015, 51, 16037–16040. [Google Scholar] [CrossRef]
- Tanno, T.; Watanabe, Y.; Umeno, K.; Matsuoka, A.; Matsumura, H.; Odaka, M.; Ogawa, N. In Situ Observation of Gas Adsorption onto ZIF-8 Using Terahertz Waves. J. Phys. Chem. C 2017, 121, 17921–17924. [Google Scholar] [CrossRef]
- Maul, J.; Ryder, M.R.; Ruggiero, M.T.; Erba, A. Pressure-driven mechanical anisotropy and destabilization in zeolitic imidazolate frameworks. Phys. Rev. B 2019, 99, 014102. [Google Scholar] [CrossRef]
- Li, Q.; Zaczek, A.J.; Korter, T.M.; Zeitler, J.A.; Ruggiero, M.T. Methyl-rotation dynamics in metal–organic frameworks probed with terahertz spectroscopy. Chem. Commun. 2018, 54, 5776–5779. [Google Scholar] [CrossRef] [PubMed]
- Formalik, F.; Fischer, M.; Rogacka, J.; Firlej, L.; Kuchta, B. Effect of low frequency phonons on structural properties of ZIFs with SOD topology. Microporous Mesoporous Mater. 2020, 304, 109132. [Google Scholar] [CrossRef]
- Sun, D.; Yang, D.; Wei, P.; Liu, B.; Chen, Z.; Zhang, L.; Lu, J. One-Step Electrodeposition of Silver Nanostructures on 2D/3D Metal−Organic Framework ZIF-67: Comparison and Application in Electrochemical Detection of Hydrogen Peroxide. ACS Appl. Mater. Interfaces 2020, 12, 41960–41968. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Deng, Y.; Zhu, L.; Liu, J.; Zhang, B.; Zhang, Y.; Sun, W.; Li, G. Au-Co nanoparticles-embedded N-doped carbon nanotube hollow polyhedron modified electrode for electrochemical determination of quercetin. Microchim. Acta 2020, 187, 546. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narimbi, J.; Balakrishnan, S.; Perova, T.S.; Dee, G.; Swiegers, G.F.; Gun’ko, Y.K. XRD and Spectroscopic Investigations of ZIF—Microchannel Glass Plates Composites. Materials 2023, 16, 2410. https://doi.org/10.3390/ma16062410
Narimbi J, Balakrishnan S, Perova TS, Dee G, Swiegers GF, Gun’ko YK. XRD and Spectroscopic Investigations of ZIF—Microchannel Glass Plates Composites. Materials. 2023; 16(6):2410. https://doi.org/10.3390/ma16062410
Chicago/Turabian StyleNarimbi, Justin, Sivakumar Balakrishnan, Tatiana S. Perova, Garret Dee, Gerhard F. Swiegers, and Yurii K. Gun’ko. 2023. "XRD and Spectroscopic Investigations of ZIF—Microchannel Glass Plates Composites" Materials 16, no. 6: 2410. https://doi.org/10.3390/ma16062410
APA StyleNarimbi, J., Balakrishnan, S., Perova, T. S., Dee, G., Swiegers, G. F., & Gun’ko, Y. K. (2023). XRD and Spectroscopic Investigations of ZIF—Microchannel Glass Plates Composites. Materials, 16(6), 2410. https://doi.org/10.3390/ma16062410








