Modification Effect of Ca(OH)2 on the Carbonation Resistance of Fly Ash-Metakaolin-Based Geopolymer
Abstract
1. Introduction
2. Experiment Design
2.1. Test Materials and Parameters
2.2. Test Mix Design and Specimen Preparation
2.3. Accelerated Carbonization Design
2.4. Compressive Strength Test
2.5. Carbonization Depth Test
2.6. Material Alkalinity Test
2.7. Microstructure Test
2.8. Phase Composition Test
3. Results and Discussion
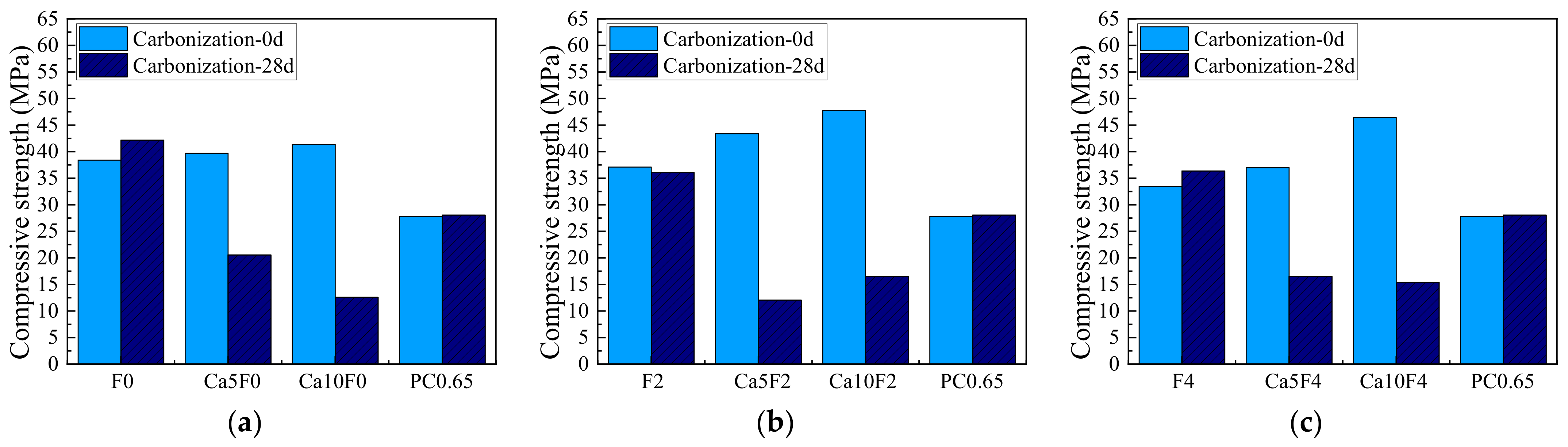
3.1. Compressive Strength
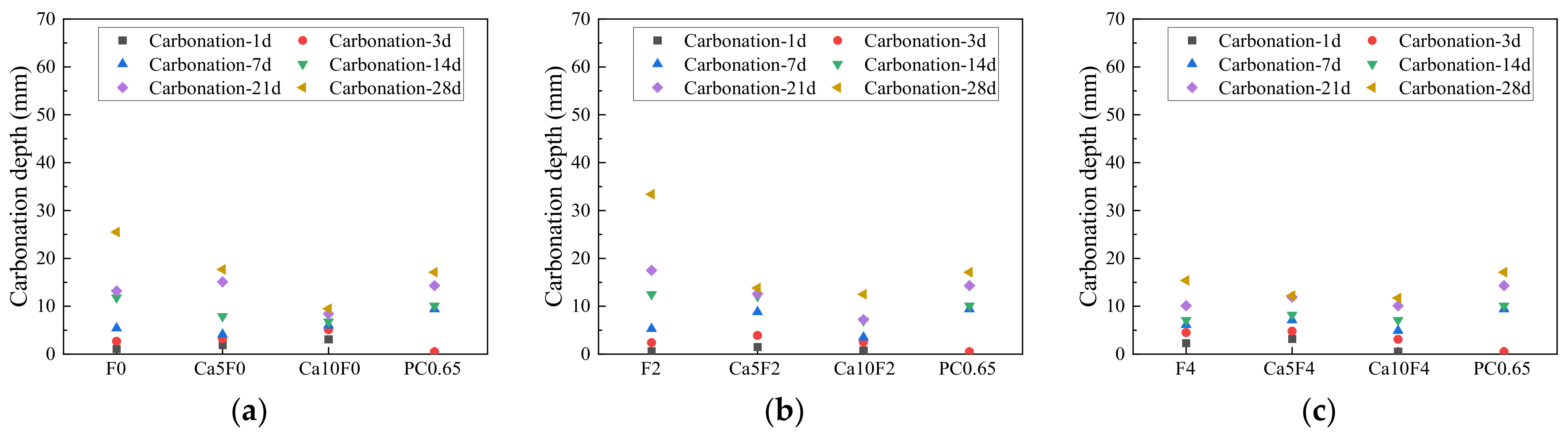
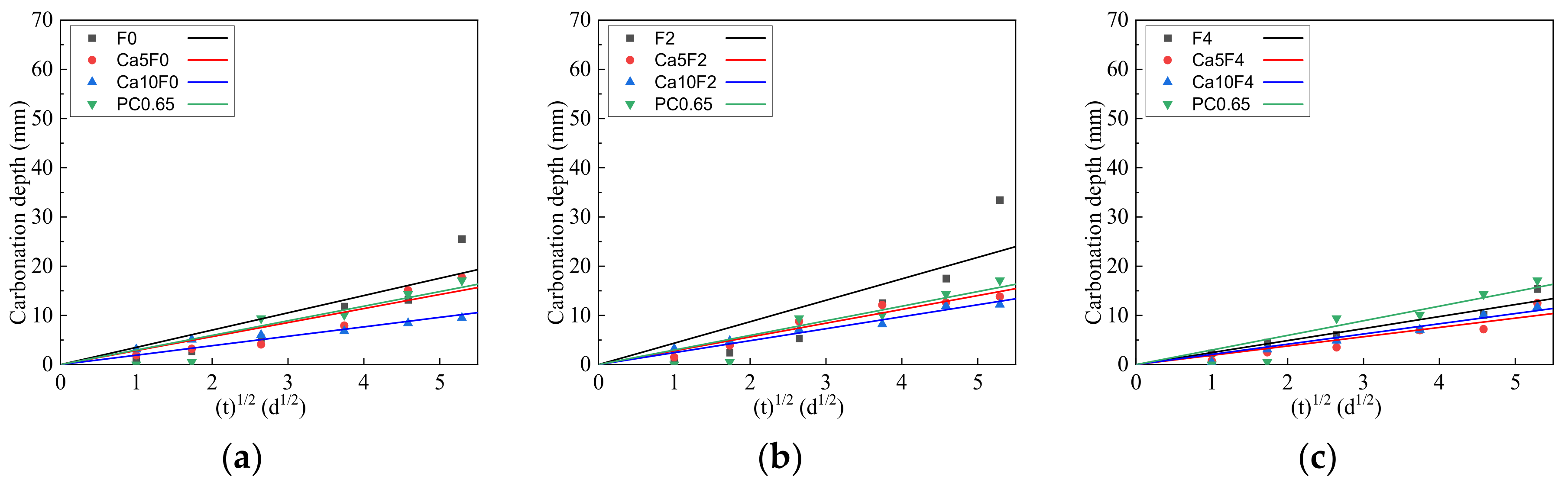
3.2. Carbonization Depth
3.3. Material Alkalinity
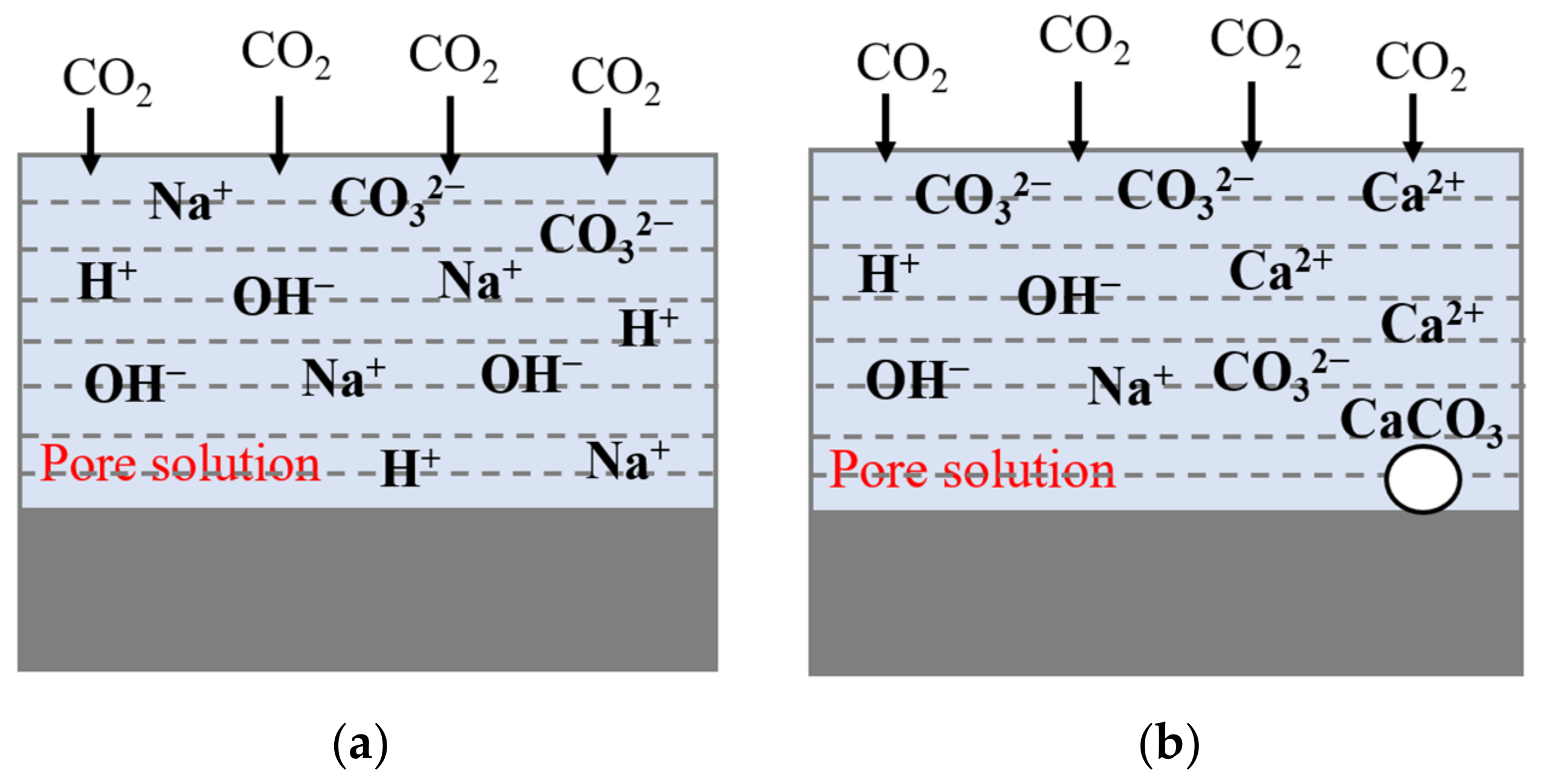
3.4. Microstructure Analysis
3.4.1. Mercury Intrusion Method
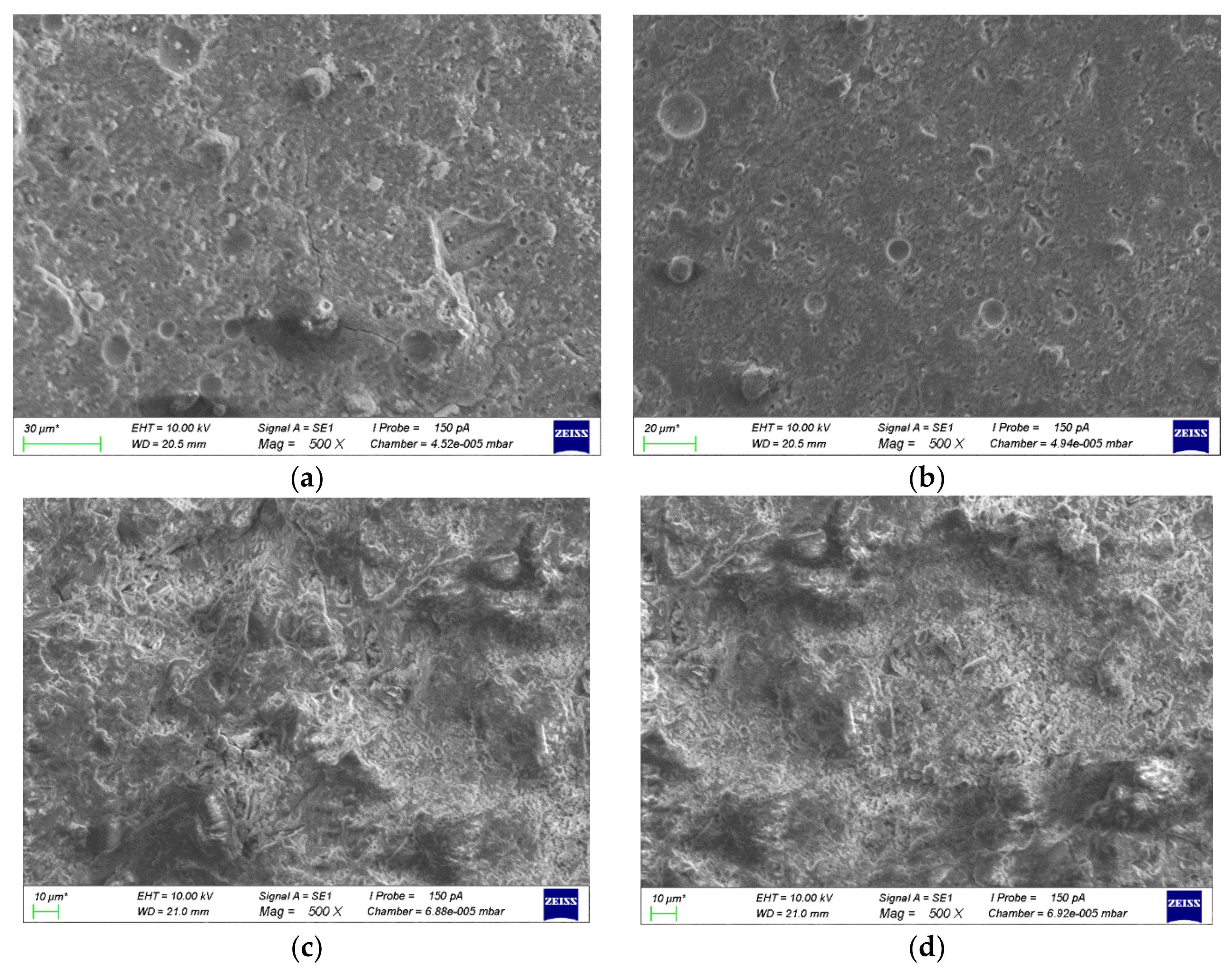
3.4.2. Scanning Electron Microscopy
3.5. Phase Composition Analysis
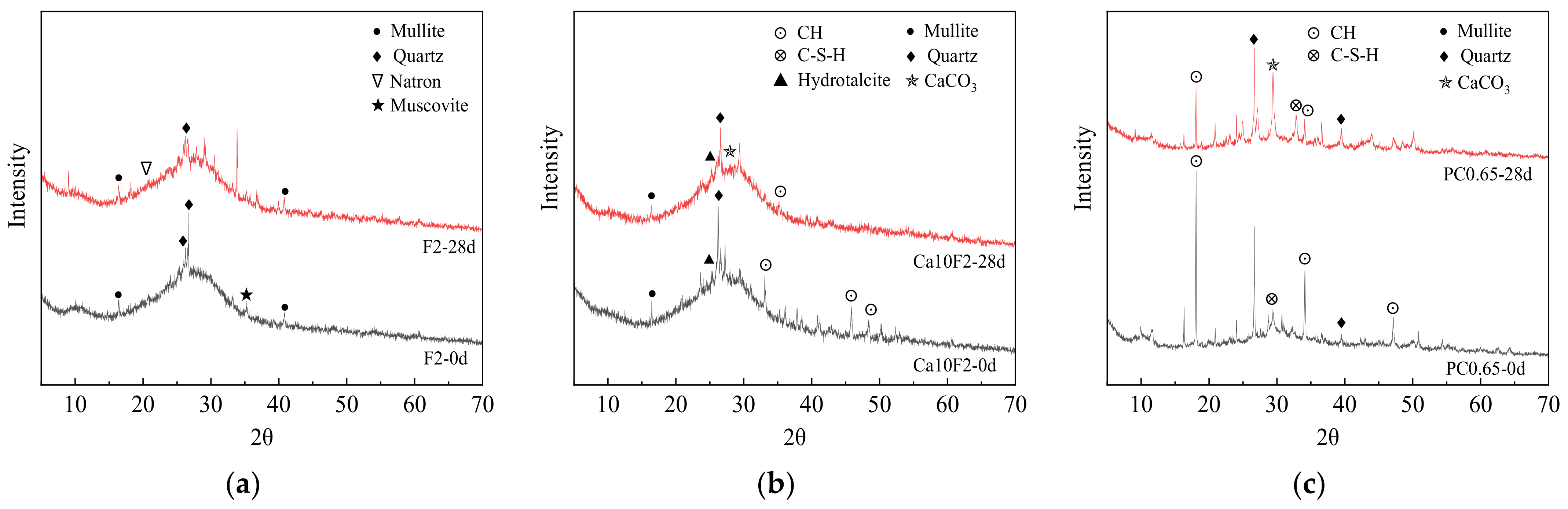
3.5.1. XRD Phase Analysis
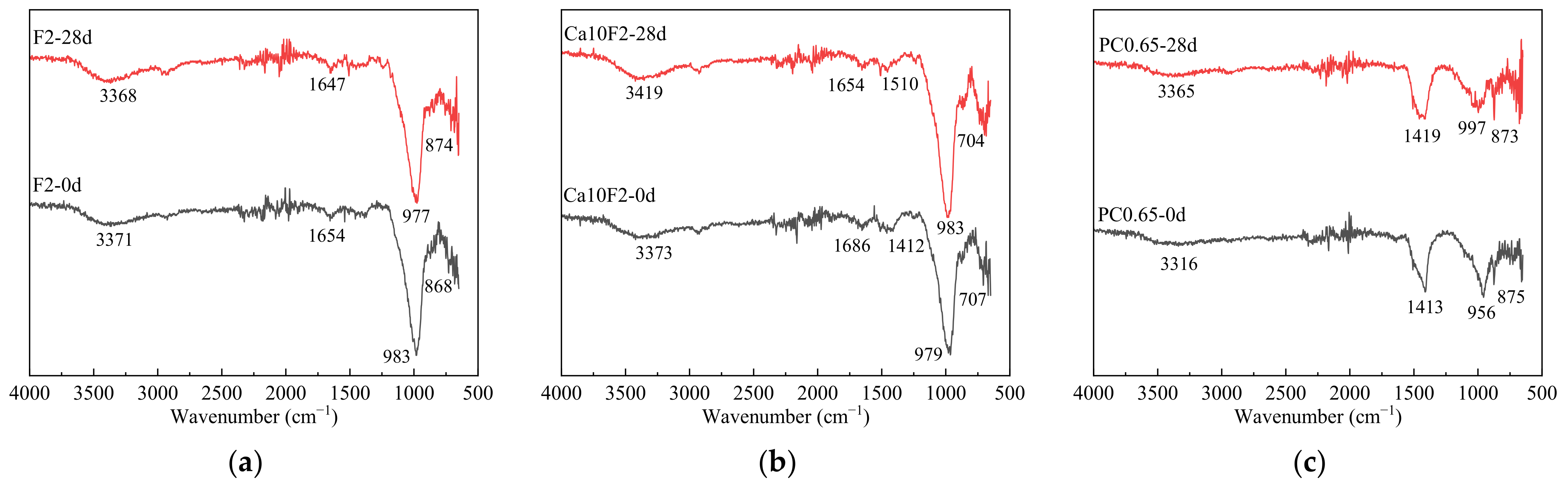
3.5.2. FT-IR Chemical Structure Test and Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Cong, P.; Cheng, Y. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng. Engl. Ed. 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H.; Chen, Y.; Huseien, G.; Shah, K. Shrinkage mechanisms and shrinkage-mitigating strategies of alkali-activated slag composites: A critical review. Constr. Build. Mater. 2022, 318, 125993. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.; Brouwers, H.J.H. Reaction kinetics, gel character and strength of ambient temperature cured alkali activated slag–fly ash blends. Constr. Build. Mater. 2015, 80, 105–115. [Google Scholar] [CrossRef]
- Ding, Y.; Dai, J.G.; Shi, C.J. Mechanical properties of alkali-activated concrete: A state-of-the-art review. Constr. Build. Mater. 2016, 127, 68–79. [Google Scholar] [CrossRef]
- Abdelli, K.; Tahlaiti, M.; Belarbi, R.; Oudjit, M.N. Influence of the origin of metakaolin on pozzolanic reactivity of mortars. Energy Procedia 2017, 139, 230–235. [Google Scholar] [CrossRef]
- Imtiaz, L.; Rehman, S.; Memon, S.A.; Khan, M.K.; Javed, M.F. A review of recent developments and advances in eco-friendly geopolymer concrete. Appl. Sci. 2020, 10, 7838. [Google Scholar] [CrossRef]
- Sitarz, M.; Hager, I.; Choińska, M. Evolution of mechanical properties with time of fly-ash-based geopolymer mortars under the effect of granulated ground blast furnace slag addition. Energies 2020, 13, 1135. [Google Scholar] [CrossRef]
- Wu, X.; Shen, Y.; Hu, L. Performance of geopolymer concrete activated by sodium silicate and silica fume activator. Case Stud. Constr. Mater. 2022, 17, e01513. [Google Scholar] [CrossRef]
- Wang, F.; Sun, X.; Tao, Z.; Zhu, P. Effect of silica fume on compressive strength of ultra-high-performance concrete made of calcium aluminate cement/fly ash based geopolymer. J. Build. Eng. 2022, 62, 105398. [Google Scholar] [CrossRef]
- Yang, J.; Bai, H.; He, X.; Zeng, J.; Su, Y.; Wang, X.; Zhao, H.; Mao, C. Performances and microstructure of one-part fly ash geopolymer activated by calcium carbide slag and sodium metasilicate powder. Constr. Build. Mater. 2023, 367, 130303. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, H.; Zhang, Z. Influence of fly ash on the pore structure and shrinkage characteristics of metakaolin-based geopolymer pastes and mortars. Constr. Build. Mater. 2017, 153, 284–293. [Google Scholar] [CrossRef]
- Elmesalami, N.; Celik, K. A critical review of engineered geopolymer composite: A low-carbon ultra-high-performance concrete. Constr. Build. Mater. 2022, 346, 128491. [Google Scholar] [CrossRef]
- Gharzouni, A.; Joussein, E.; Samet, B.; Baklouti, S.; Rossignol, S. Effect of the reactivity of alkaline solution and metakaolin on geopolymer formation. J. Non-Cryst. Solids 2015, 410, 127–134. [Google Scholar] [CrossRef]
- Hadi MN, S.; Al-Azzawi, M.; Yu, T. Effects of fly ash characteristics and alkaline activator components on compressive strength of fly ash-based geopolymer mortar. Constr. Build. Mater. 2018, 175, 41–54. [Google Scholar] [CrossRef]
- Njimou, J.R.; Pengou, M.; Tchakoute, H.K.; Tamwa, M.S.; Tizaoui, C.; Fannang, U.; Lemougna, P.N.; Nanseu-Njiki, C.P.; Emmanuel Ngameni, E. Removal of lead ions from aqueous solution using phosphate-based geopolymer cement composite. J. Chem. Technol. Biotechnol. 2021, 96, 1358–1369. [Google Scholar] [CrossRef]
- Babu, G.K.; Rao, K.V.; Dey, S.; Veerendra, G.T.N. Performance studies on quaternary blended Geopolymer concrete. Hybrid Adv. 2023, 2, 100019. [Google Scholar] [CrossRef]
- Tang, Z.Q.; Sui, H.; De Souza, F.B.; Sagoe-Crentsil, K.; Duan, W.H. Silane-modified graphene oxide in geopolymer: Reaction kinetics, microstructure, and mechanical performance. Cem. Concr. Compos. 2023, 139, 104997. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W.; Luo, W.; Shen, C. An investigation of the microstructure and durability of a fluidized bed fly ash–metakaolin geopolymer after heat and acid exposure. Mater. Des. 2015, 74, 125–137. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Pławecka, K.; Bazan, P.; Lin, W.T.; Korniejenko, K.; Sitarz, M.; Nykiel, M. Development of Geopolymers Based on Fly Ashes from Different Combustion Processes. Polymers 2022, 14, 1954. [Google Scholar] [CrossRef]
- Bernal, S.A.; San Nicolas, R.; Myers, R.J.; Gutiérrez, R.M.; Puertas, F.; Deventer, J.S.J.; Provis, J.L. MgO content of slag controls phase evolution and structural changes induced by accelerated carbonation in alkali-activated binders. Cem. Concr. Res. 2014, 57, 33–43. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Resistance of alkali-activated slag concrete to carbonation. Cem. Concr. Res. 2001, 31, 1277–1283. [Google Scholar] [CrossRef]
- Godwin, J.; Njimou, J.R.; Abdus-Salam, N.; Panda, P.K.; Tripathy, B.C.; Ghosh, M.K.; Basu, S. Nanoscale ZnO-adsorbent carefully designed for the kinetic and thermodynamic studies of Rhodamine B. Inorg. Chem. Commun. 2022, 138, 109287. [Google Scholar] [CrossRef]
- Lv, Y.; Xiao, B.; Han, W.; Peng, H.; Qiao, J.; Wang, C.; Li, X. Research Progress on carbonization properties of alkali excited cementified materials. Sci. Technol. Rev. 2022, 40, 94–104. [Google Scholar]
- Gao, K.; Lin, K.L.; Wang, D.Y.; Hwang, C.L.; Shiu, H.S.; Chang, Y.M.; Cheng, T.W. Effects SiO2/Na2O molar ratio on mechanical properties and the microstructure of nano-SiO2 metakaolin-based geopolymers. Constr. Build. Mater. 2014, 53, 503–510. [Google Scholar] [CrossRef]
- Park, S.M.; Jang, J.G.; Lee, H.K. Unlocking the role of MgO in the carbonation of alkali-activated slag cement. Inorg. Chem. Front. 2018, 5, 1661–1670. [Google Scholar] [CrossRef]
- He, J.; Gao, Q.; Wu, Y.; He, J.; Pu, X. Study on improvement of carbonation resistance of alkali-activated slag concrete. Constr. Build. Mater. 2018, 176, 60–67. [Google Scholar] [CrossRef]
- Glukhovsky, V.D. Slag-Alkali Concretes Produced from Fine-Grained Aggregate; Vishcha Shkola: Kiev, Ukraine, 1981. (In Russian) [Google Scholar]
- Li, N.; Farzadnia, N.; Shi, C. Microstructural changes in alkali-activated slag mortars induced by accelerated carbonation. Cem. Concr. Res. 2017, 100, 214–226. [Google Scholar] [CrossRef]
- GB/T50082; Standard of Test Methods for Long-Term Performance and Durability of Ordinary Concrete. China Academy of Building Research: Beijing, China, 2009.
- Paudel, S.R.; Yang, M.; Gao, Z. pH level of pore solution in alkali-activated fly-ash geopolymer concrete and its effect on ASR of aggregates with different silicate contents. J. Mater. Civ. Eng. 2020, 32, 04020257. [Google Scholar] [CrossRef]
- Natkunarajah, K.; Masilamani, K.; Maheswaran, S.; Lothenbach, B.; Amarasinghe, D.A.S.; Attygalle, D. Analysis of the trend of pH changes of concrete pore solution during the hydration by various analytical methods. Cem. Concr. Res. 2022, 156, 106780. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. Fundamental modeling and experimental investigation of concrete carbonation. Mater. J. 1991, 88, 363–373. [Google Scholar]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. A reaction engineering approach to the problem of concrete carbonation. AIChE J. 1989, 35, 1639–1650. [Google Scholar] [CrossRef]
- Papadakis, V.; Vayenas, C.; Fardis, M. Physical and chemical characteristics affecting the durability of concrete. ACI Mater. J. 1991, 88, 186–196. [Google Scholar]
- Chang, P.H.; Chang, Y.P.; Chen, S.Y.; Yu, C.T.; Chyou, Y.P. Ca-rich Ca–Al-oxide, high-temperature-stable sorbents prepared from hydrotalcite precursors: Synthesis, characterization, and CO2 capture capacity. ChemSusChem 2011, 4, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- Kljajević, L.; Nenadović, M.; Ivanović, M.; Bučevac, D.; Mirković, M.; Nikolić, N.M.; Nenadović, S. Heat Treatment of Geopolymer Samples Obtained by Varying Concentration of Sodium Hydroxide as Constituent of Alkali Activator. Gels 2022, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Lodeiro, I.G.; Macphee, D.E.; Palomo, A.; Fernández-Jiménez, A. Effect of alkalis on fresh C–S–H gels. FTIR analysis. Cem. Concr. Res. 2009, 39, 147–153. [Google Scholar] [CrossRef]
- Tian, X.; Rao, F.; León-Patiño, C.A.; Song, S. Co-disposal of MSWI fly ash and spent caustic through alkaline-activation consolidation. Cem. Concr. Compos. 2021, 116, 103888. [Google Scholar] [CrossRef]



| Component (%) | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | Na2O | SO3 | K2O | TiO2 | Others |
|---|---|---|---|---|---|---|---|---|---|---|
| MK | - | 54.5 | 43 | 1 | 0.8 | 0.1 | - | 0.4 | - | 0.2 |
| FA | 3.05 | 59.91 | 31.24 | 3.80 | 0.54 | - | 0.61 | 2.06 | 1.34 | 0.45 |
| Serial Number | Incorporation Amount of Ca(OH)2 (%) | FA (%) | MK (%) | The Water–Binder Ratio | Mode of Maintenance |
|---|---|---|---|---|---|
| F0 | - | 0 | 100 | 0.65 | Seal curing |
| Ca5F0 | 5 | ||||
| Ca10F0 | 10 | ||||
| F2 | - | 20 | 80 | 0.65 | Seal curing |
| Ca5F2 | 5 | ||||
| Ca10F2 | 10 | ||||
| F4 | - | 40 | 60 | 0.65 | Seal curing |
| Ca5F4 | 5 | ||||
| Ca10F4 | 10 | ||||
| PC0.65 | - | - | - | 0.65 | Seal curing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Qiao, J.; Han, W.; Pan, B.; Jin, X.; Peng, H. Modification Effect of Ca(OH)2 on the Carbonation Resistance of Fly Ash-Metakaolin-Based Geopolymer. Materials 2023, 16, 2305. https://doi.org/10.3390/ma16062305
Lv Y, Qiao J, Han W, Pan B, Jin X, Peng H. Modification Effect of Ca(OH)2 on the Carbonation Resistance of Fly Ash-Metakaolin-Based Geopolymer. Materials. 2023; 16(6):2305. https://doi.org/10.3390/ma16062305
Chicago/Turabian StyleLv, Yigang, Jie Qiao, Weiwei Han, Bei Pan, Xiafei Jin, and Hui Peng. 2023. "Modification Effect of Ca(OH)2 on the Carbonation Resistance of Fly Ash-Metakaolin-Based Geopolymer" Materials 16, no. 6: 2305. https://doi.org/10.3390/ma16062305
APA StyleLv, Y., Qiao, J., Han, W., Pan, B., Jin, X., & Peng, H. (2023). Modification Effect of Ca(OH)2 on the Carbonation Resistance of Fly Ash-Metakaolin-Based Geopolymer. Materials, 16(6), 2305. https://doi.org/10.3390/ma16062305








