Application and Research Progress of Covalent Organic Frameworks for Solid-State Electrolytes in Lithium Metal Batteries
Abstract
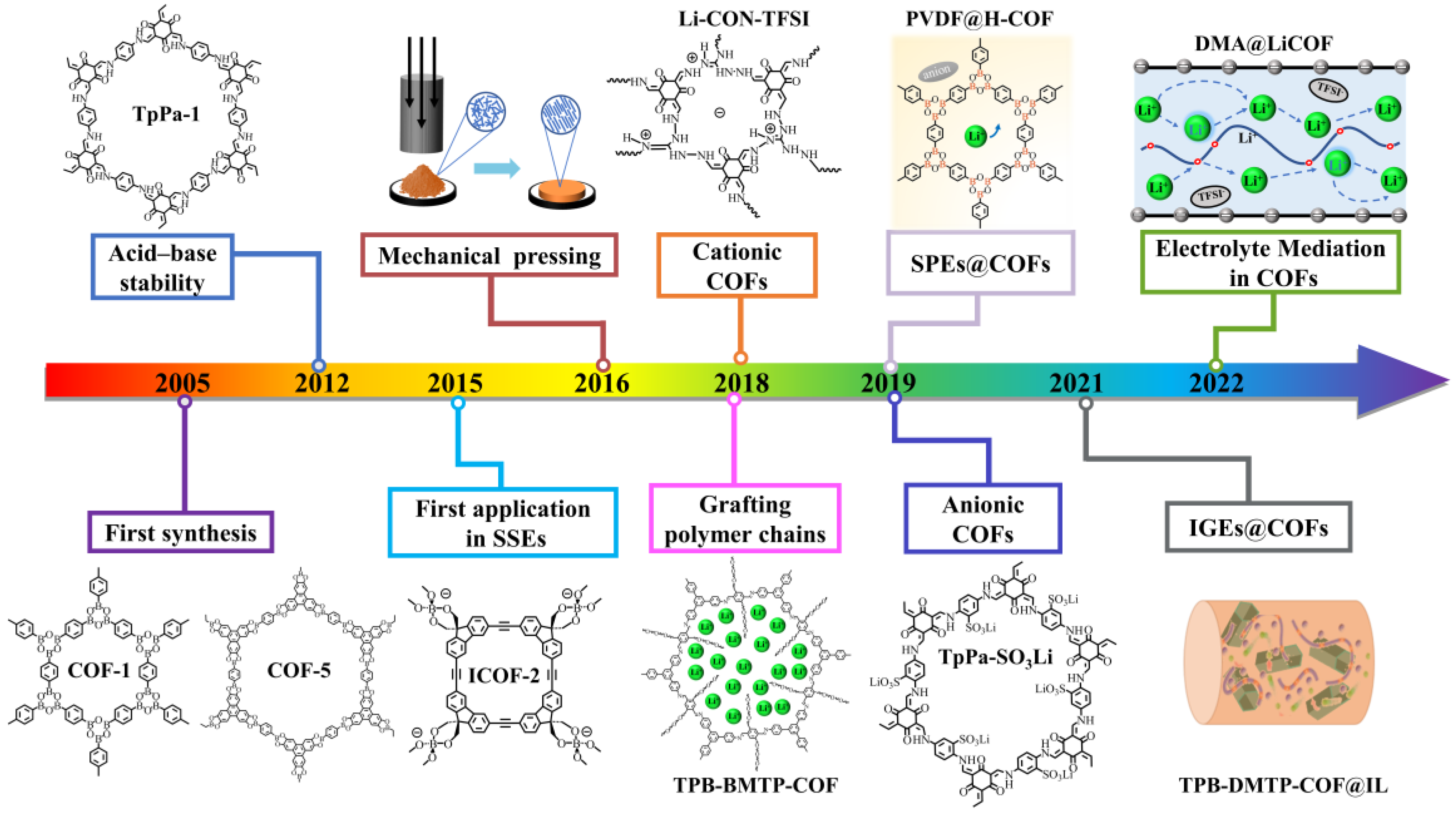
1. Introduction
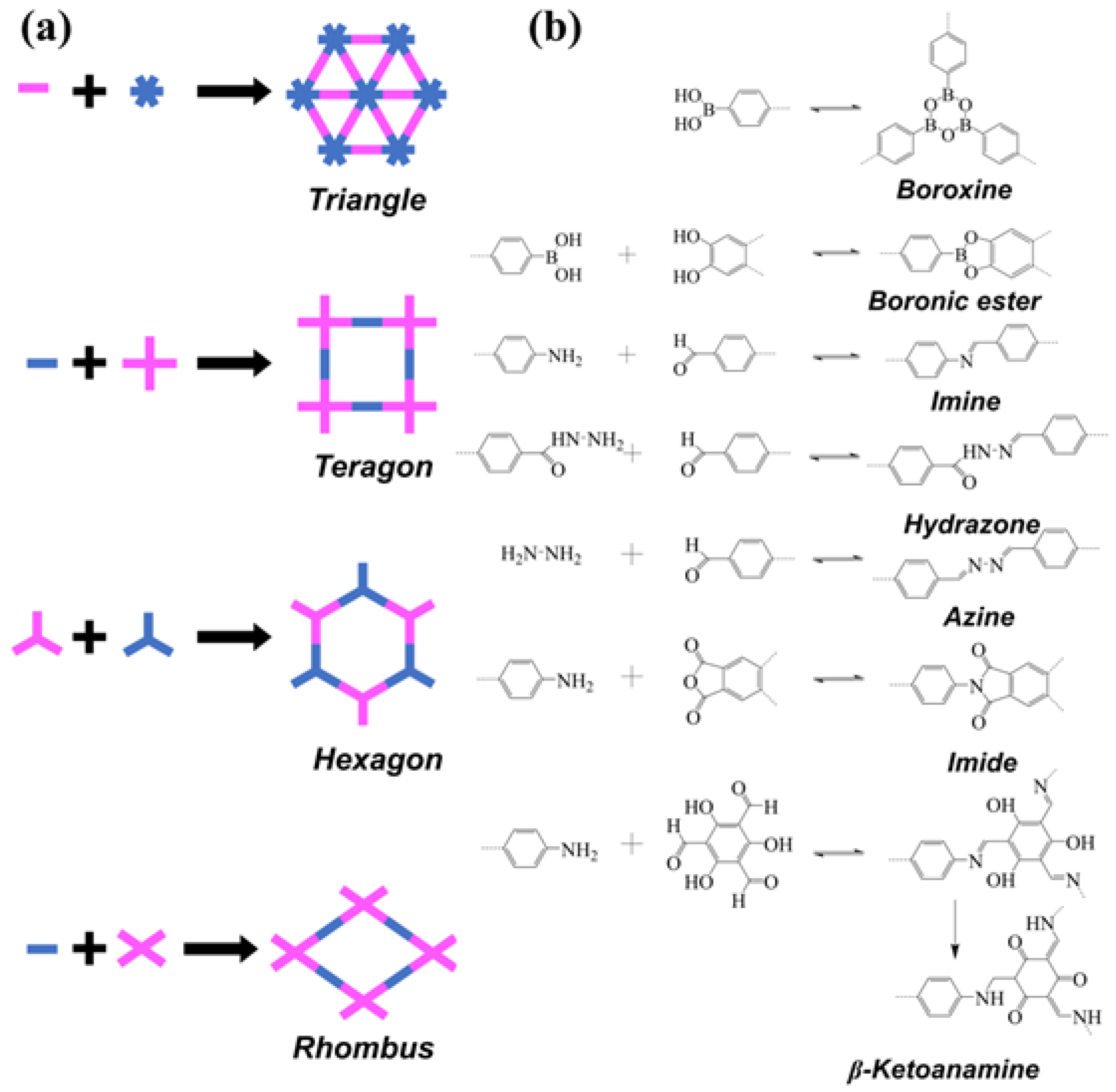
2. Design Principles and Structural Features of COFs
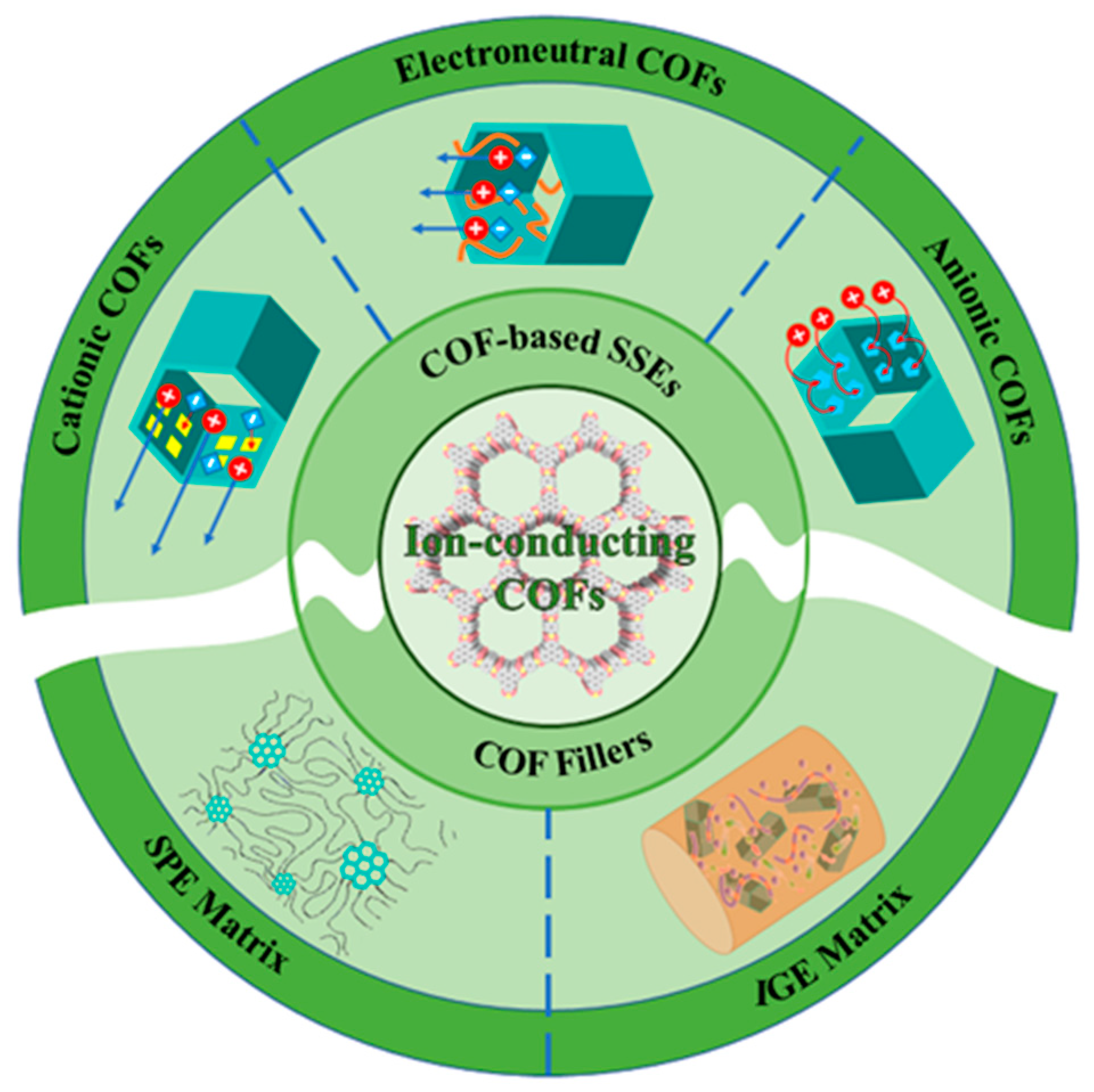
3. COF-Based Electrolytes
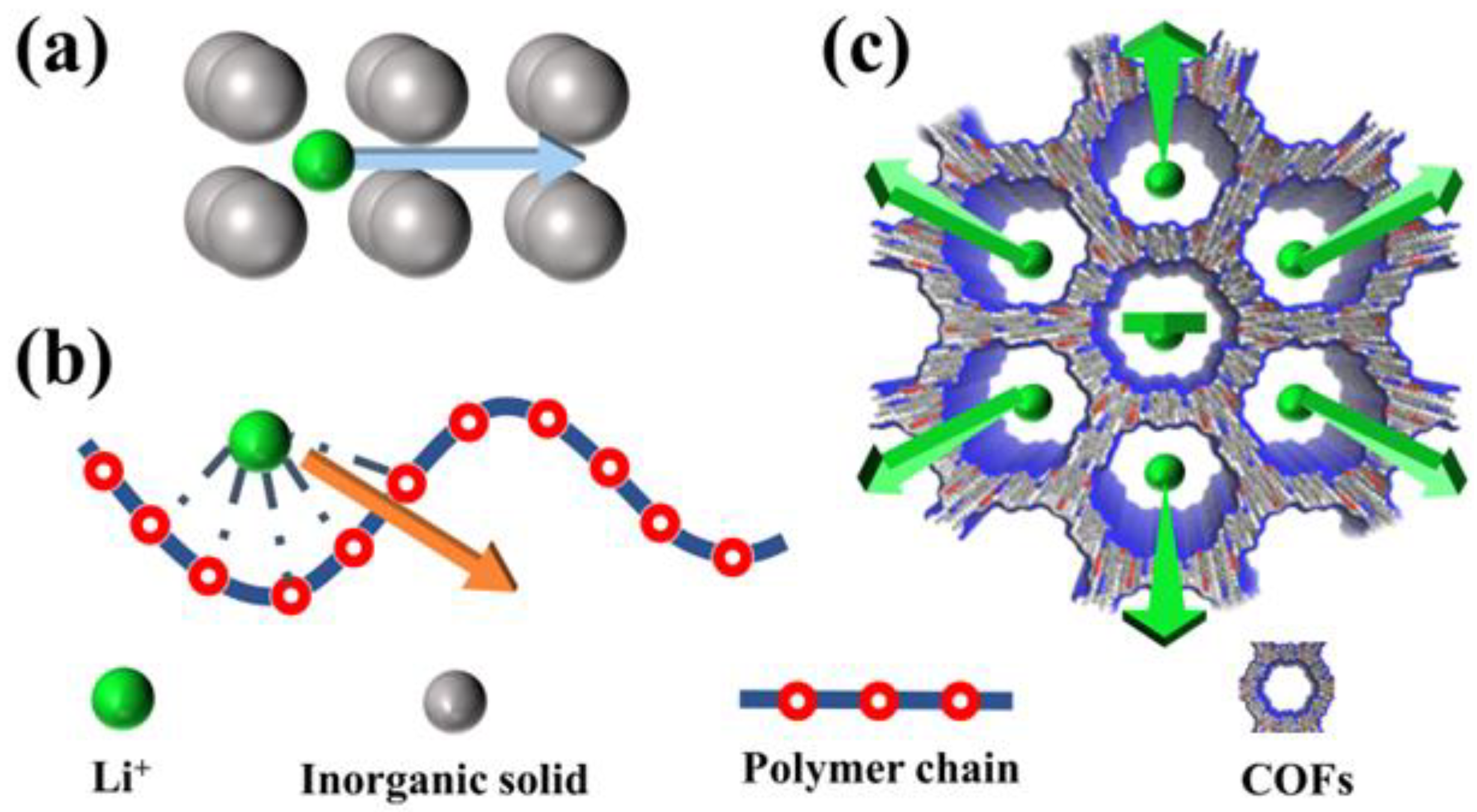
3.1. Performance Optimizing Strategies for Electrolytes
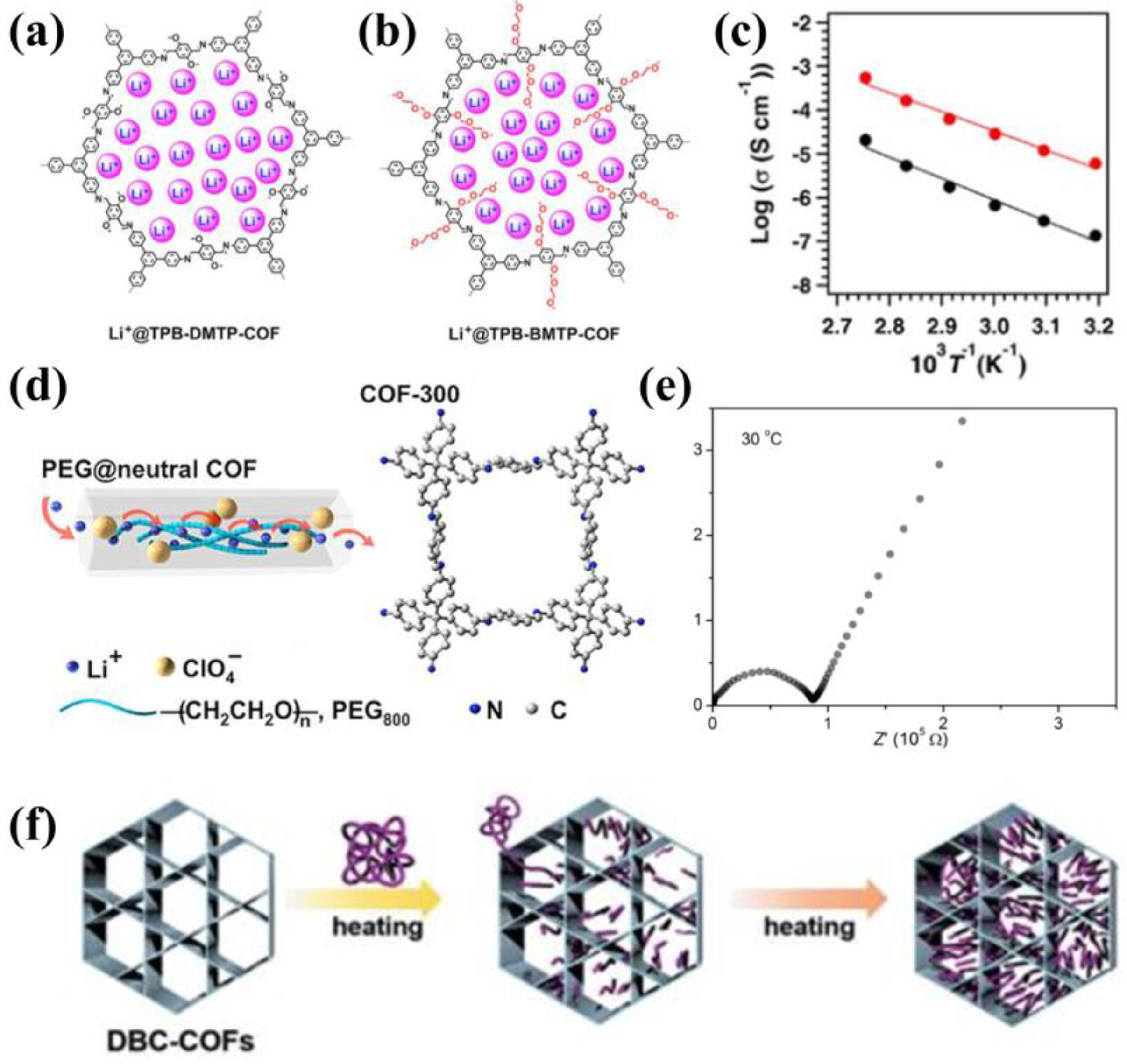
3.2. Electroneutral COFs
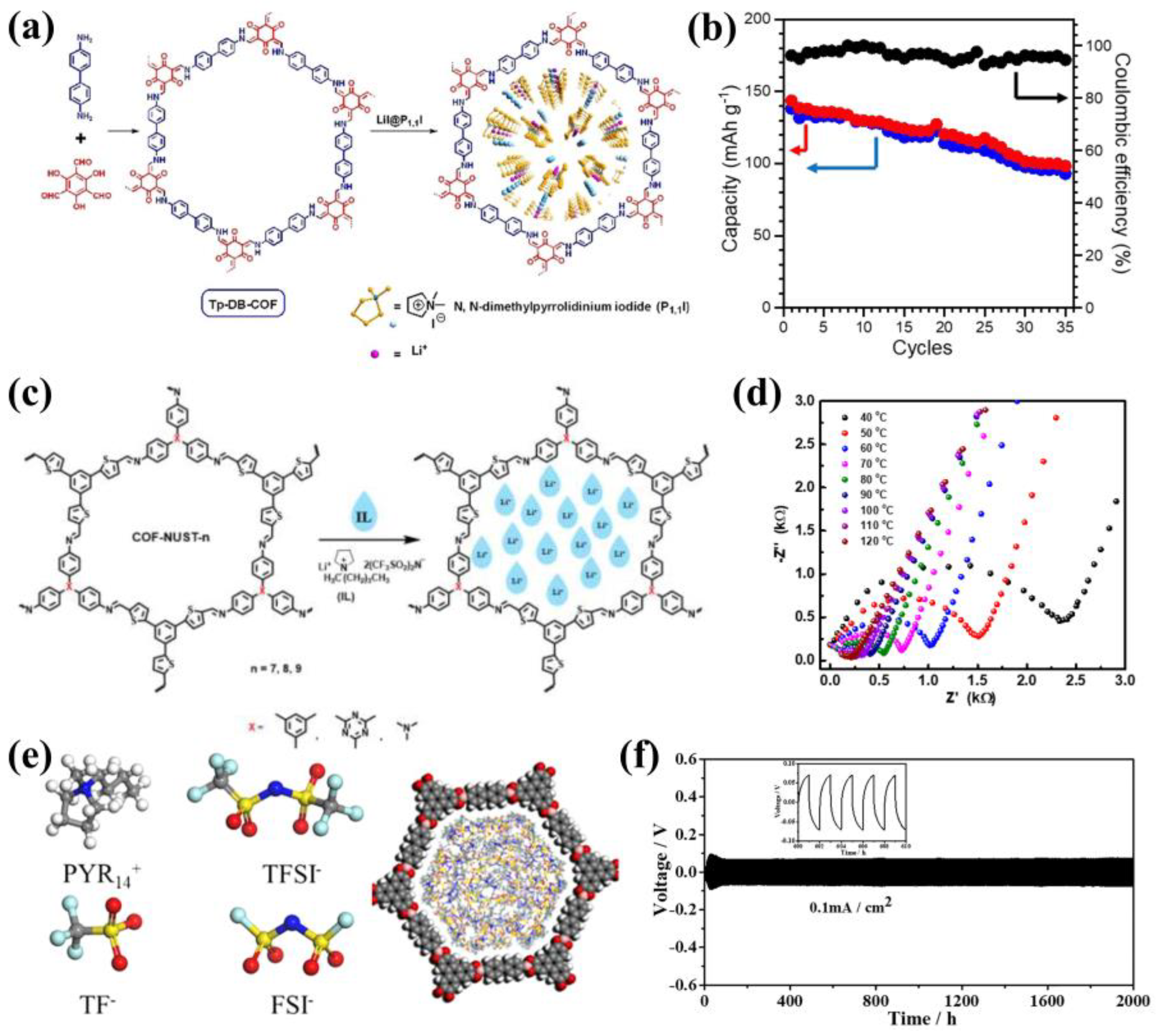
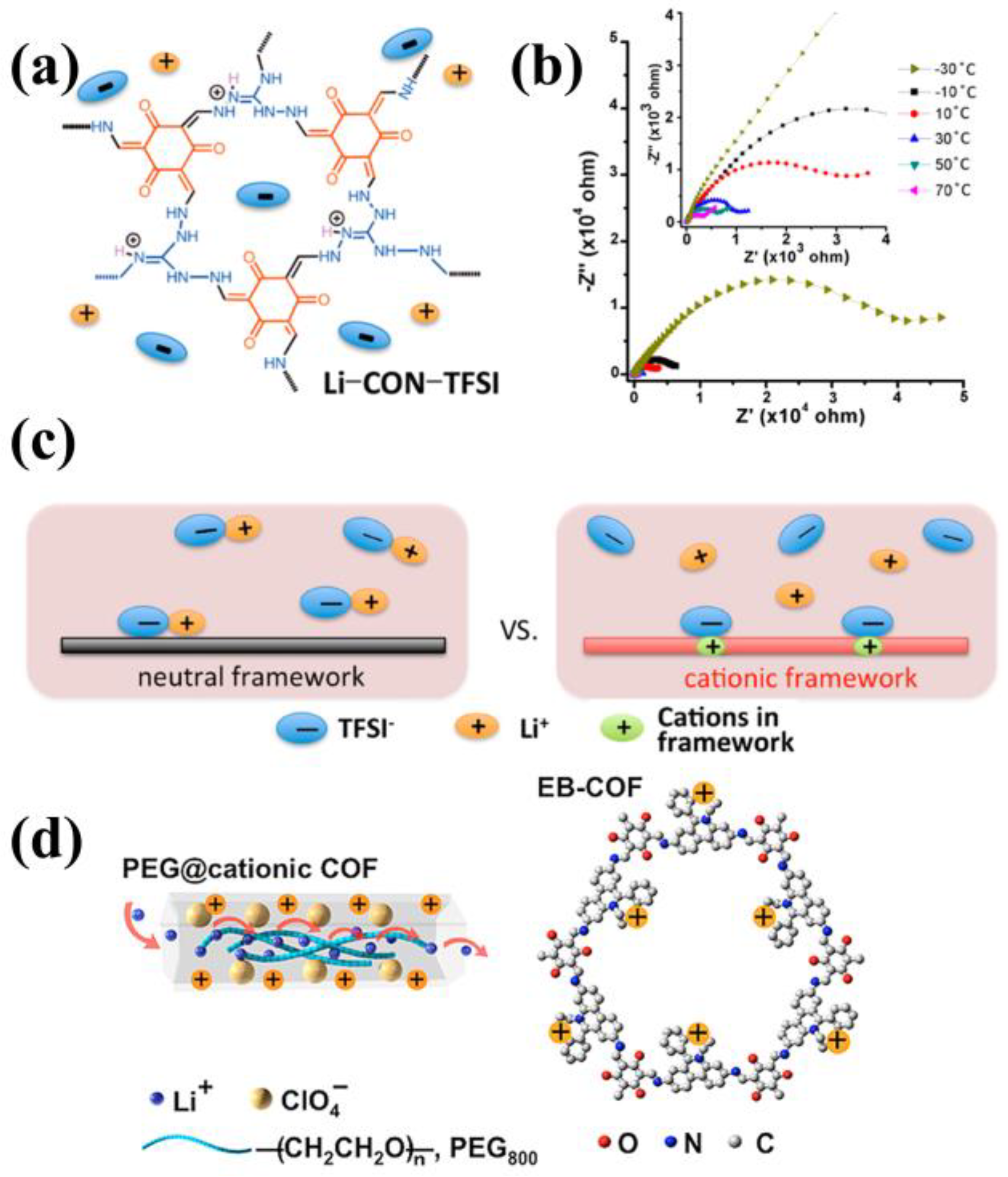
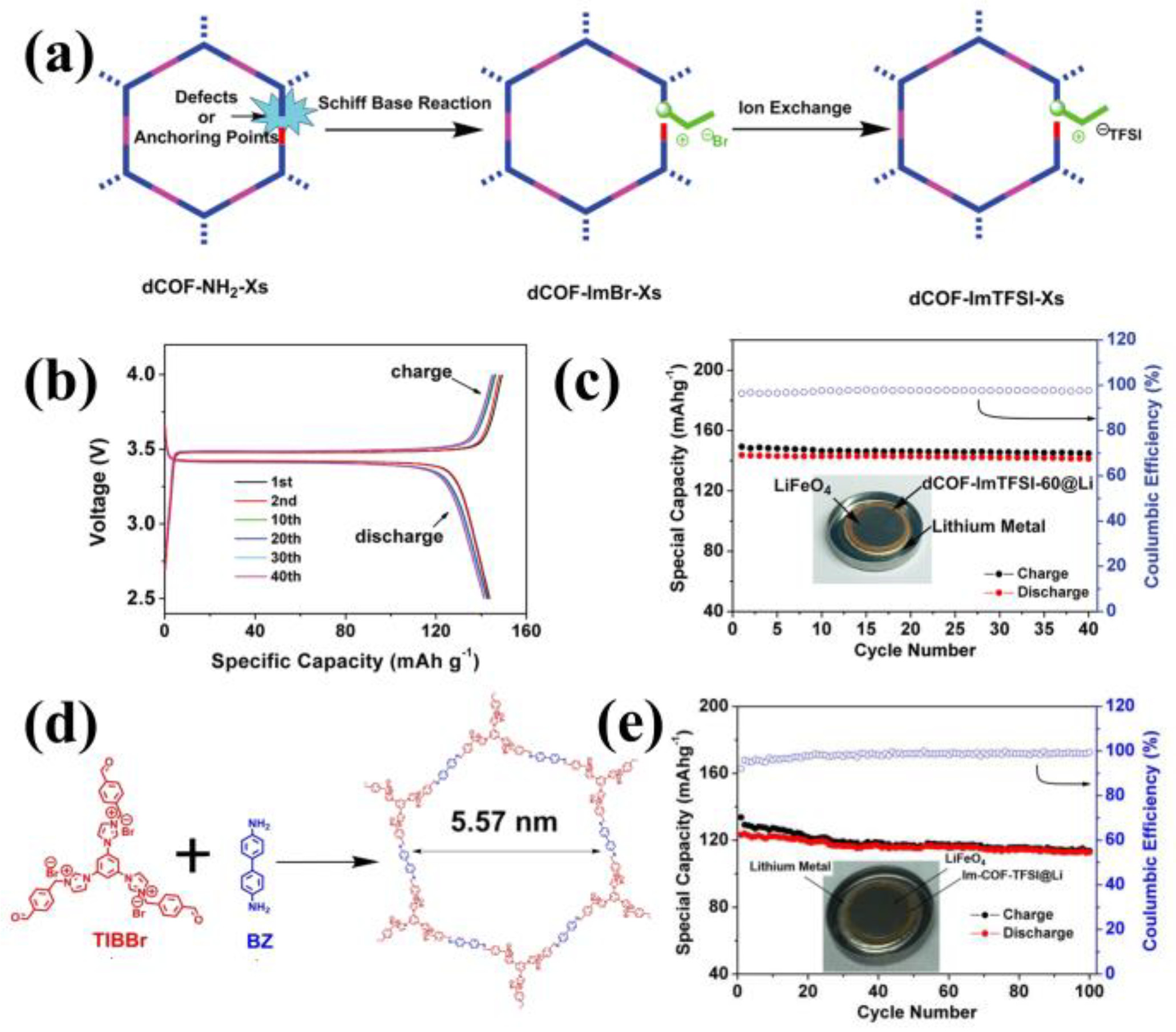
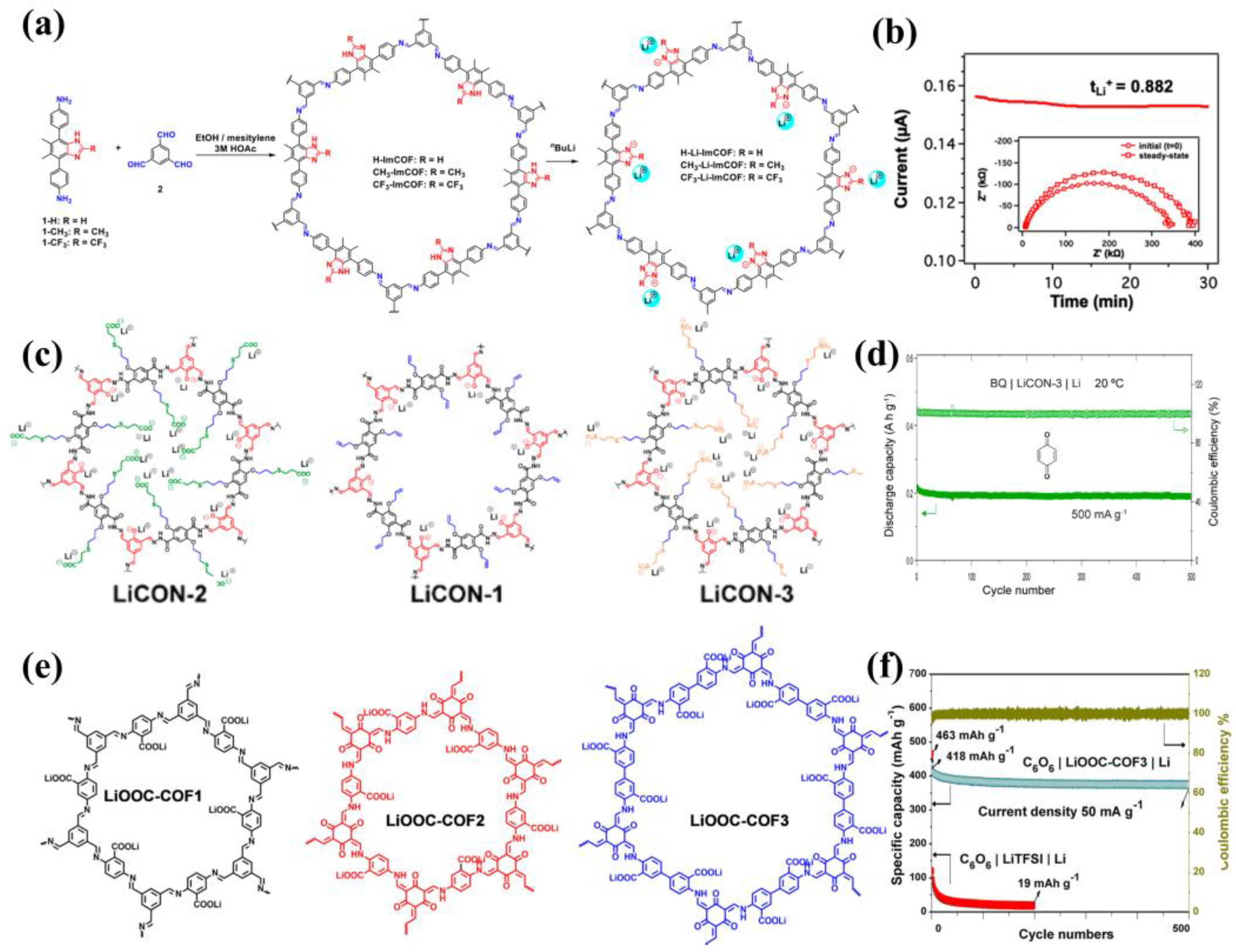
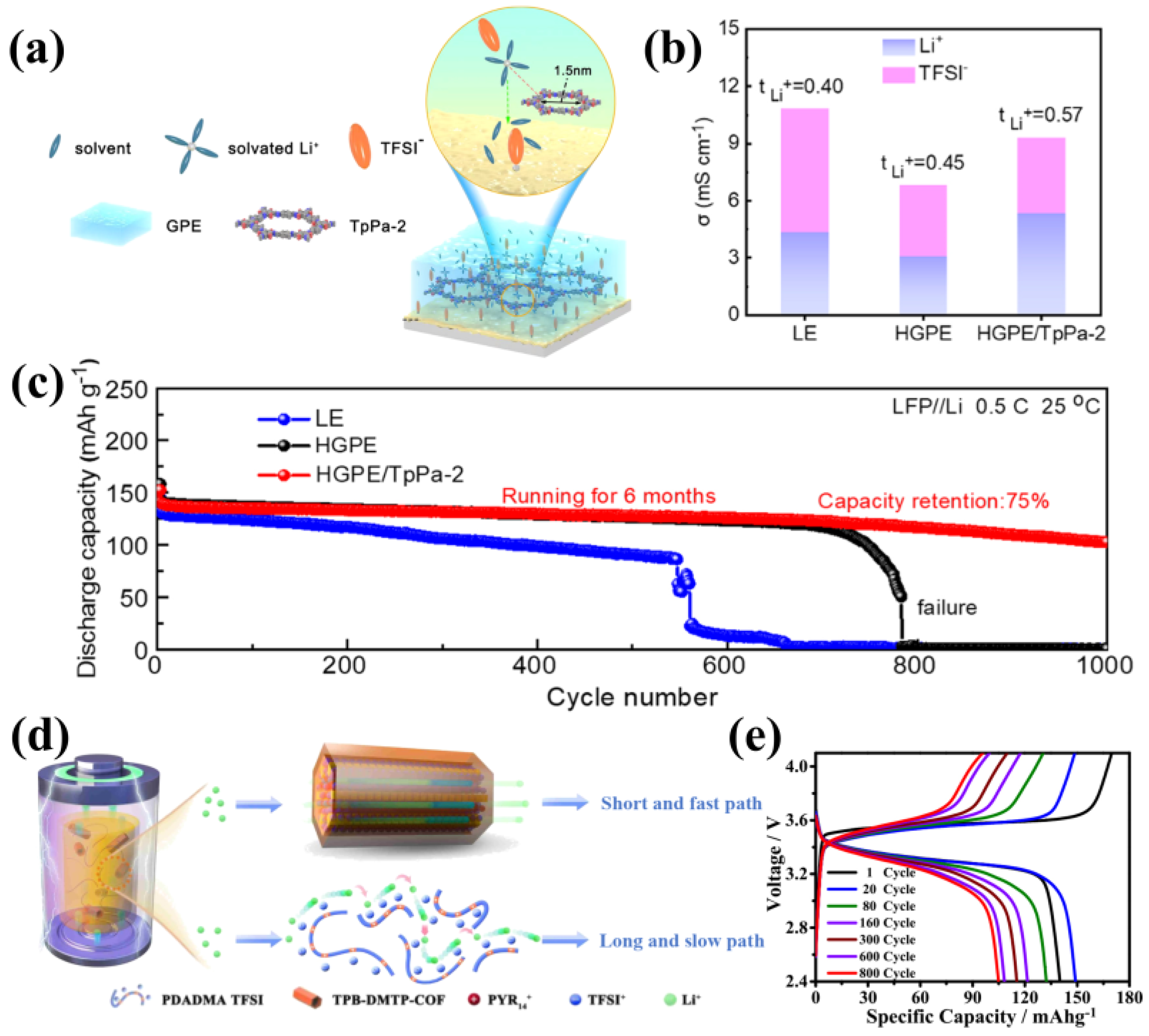
3.3. Cationic COFs
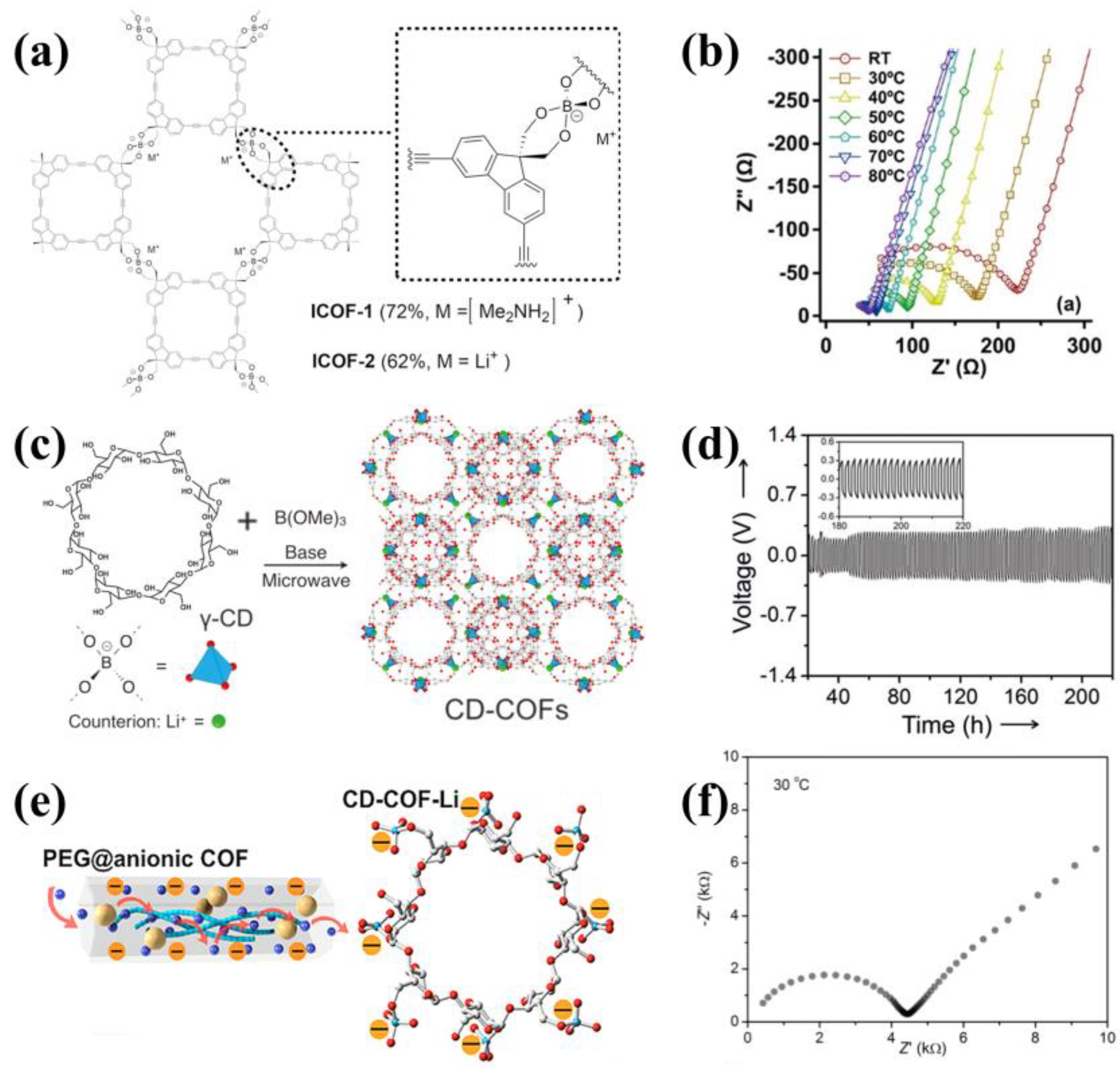
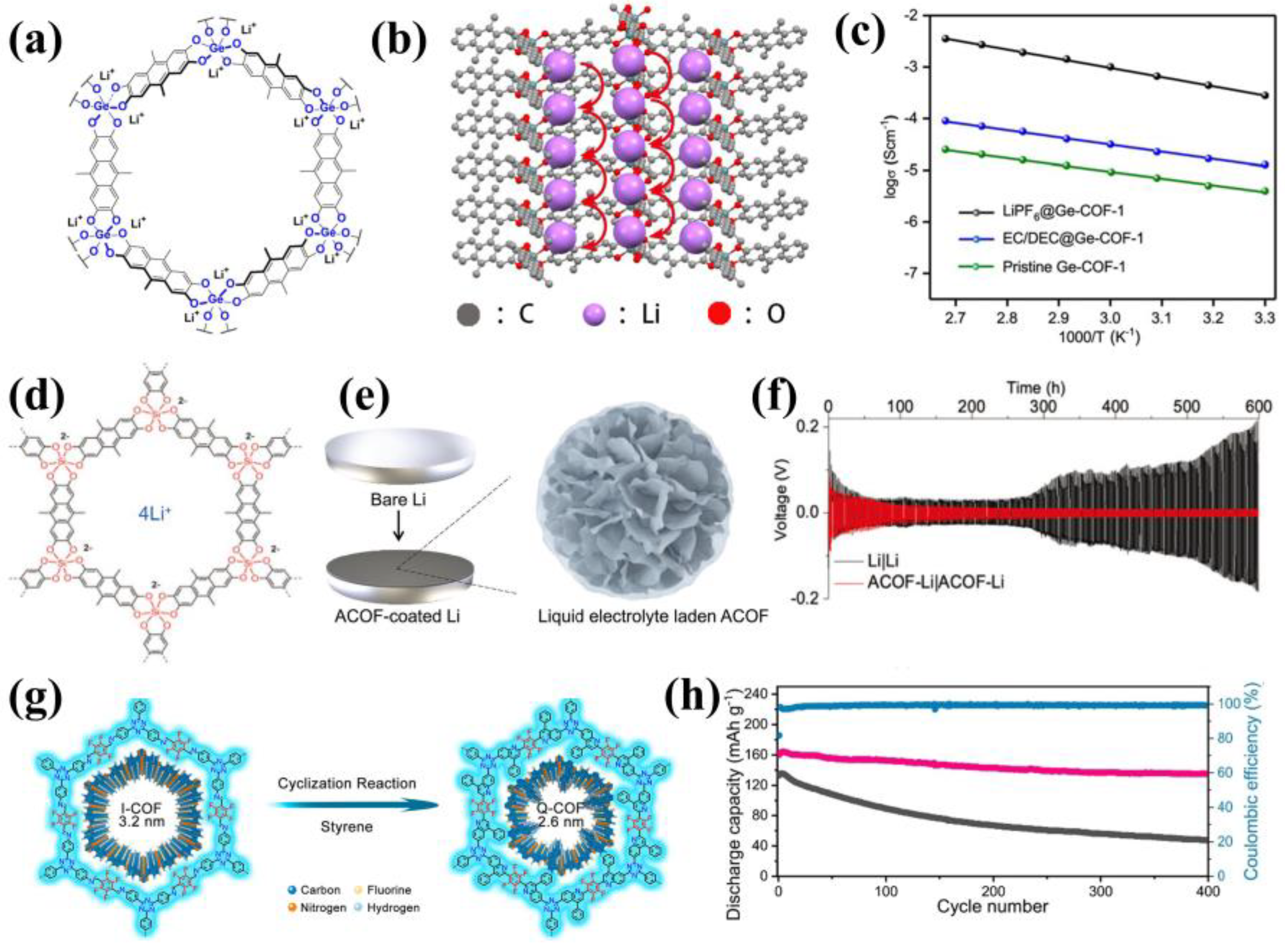
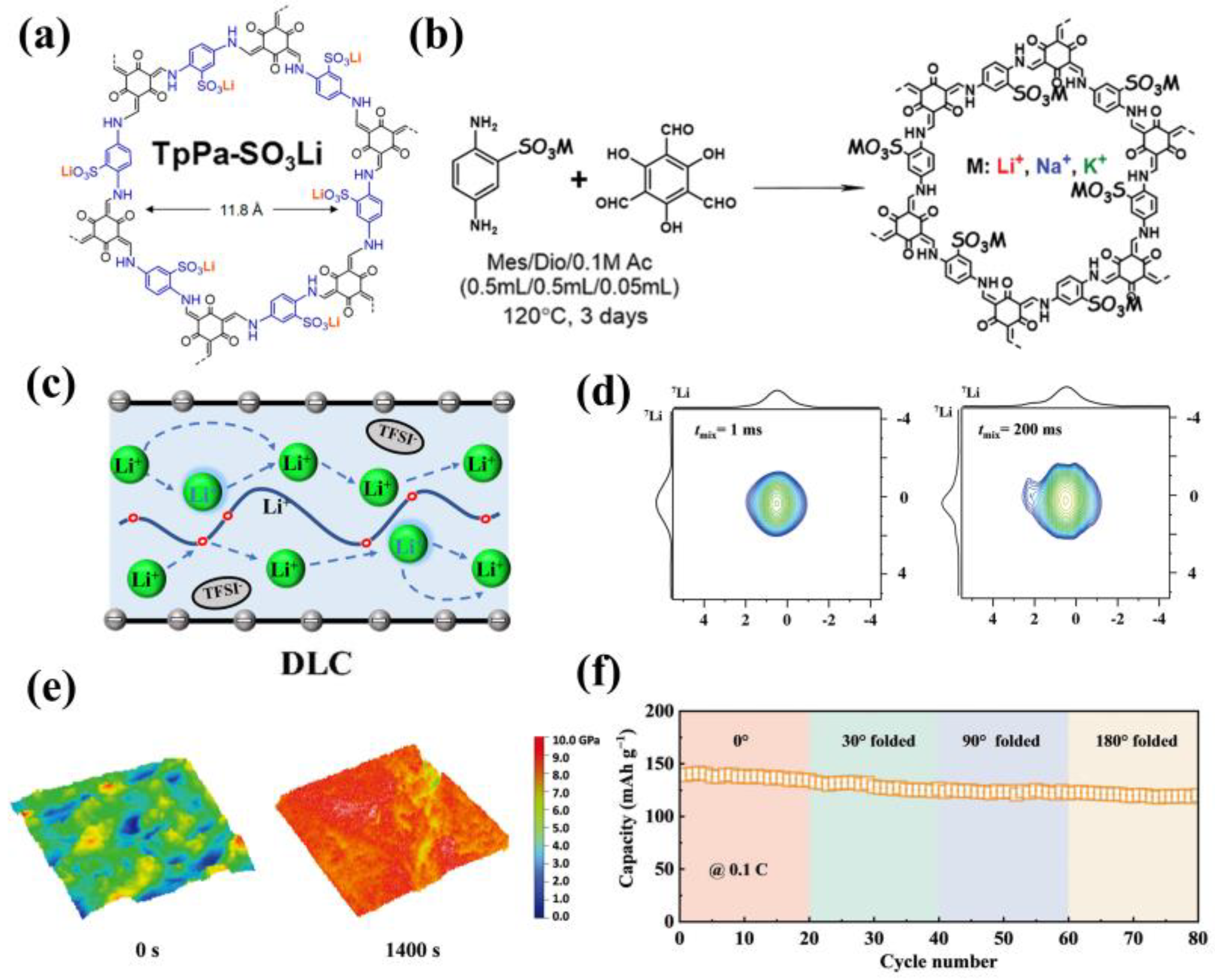
3.4. Anionic COFs
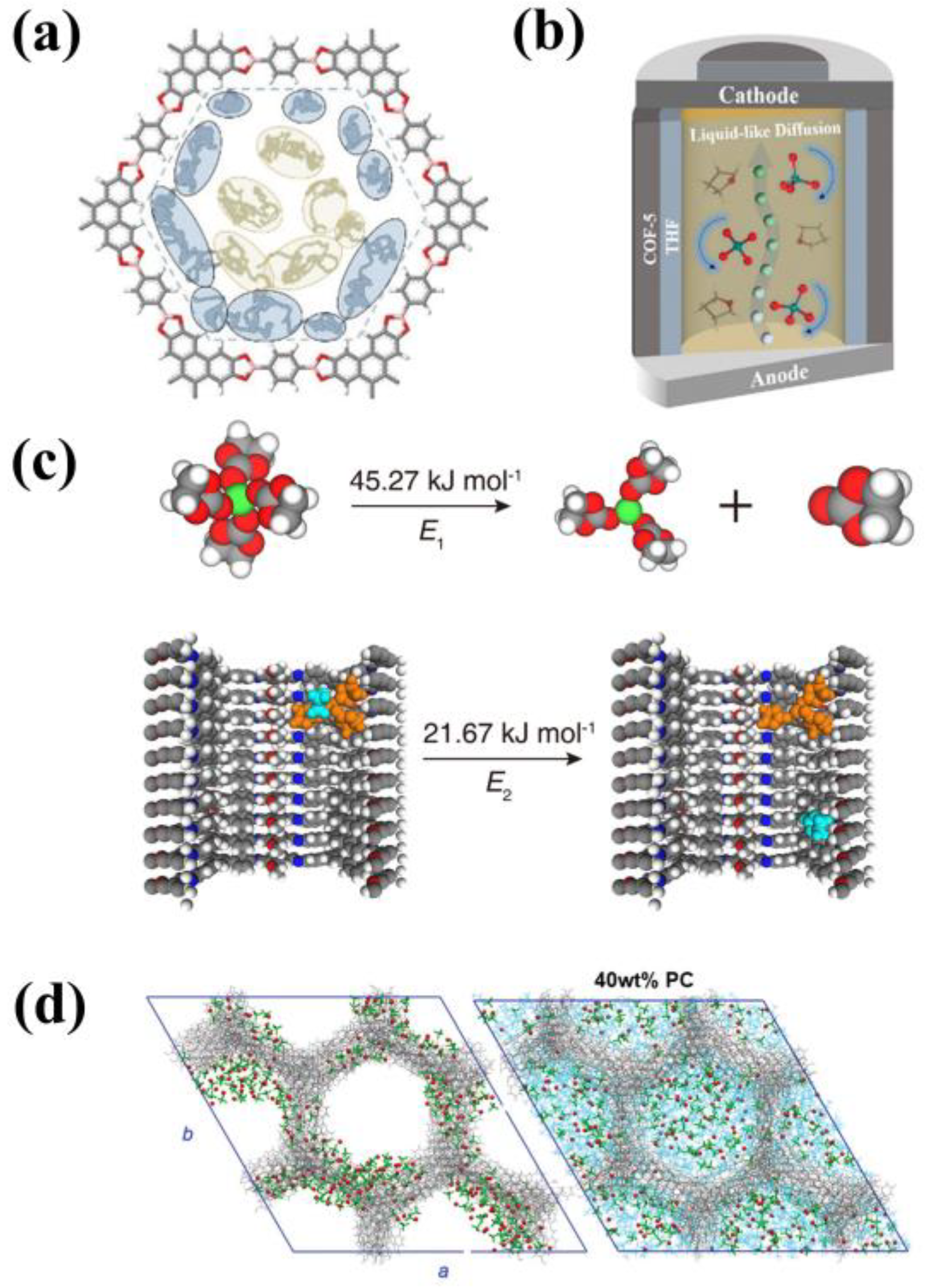
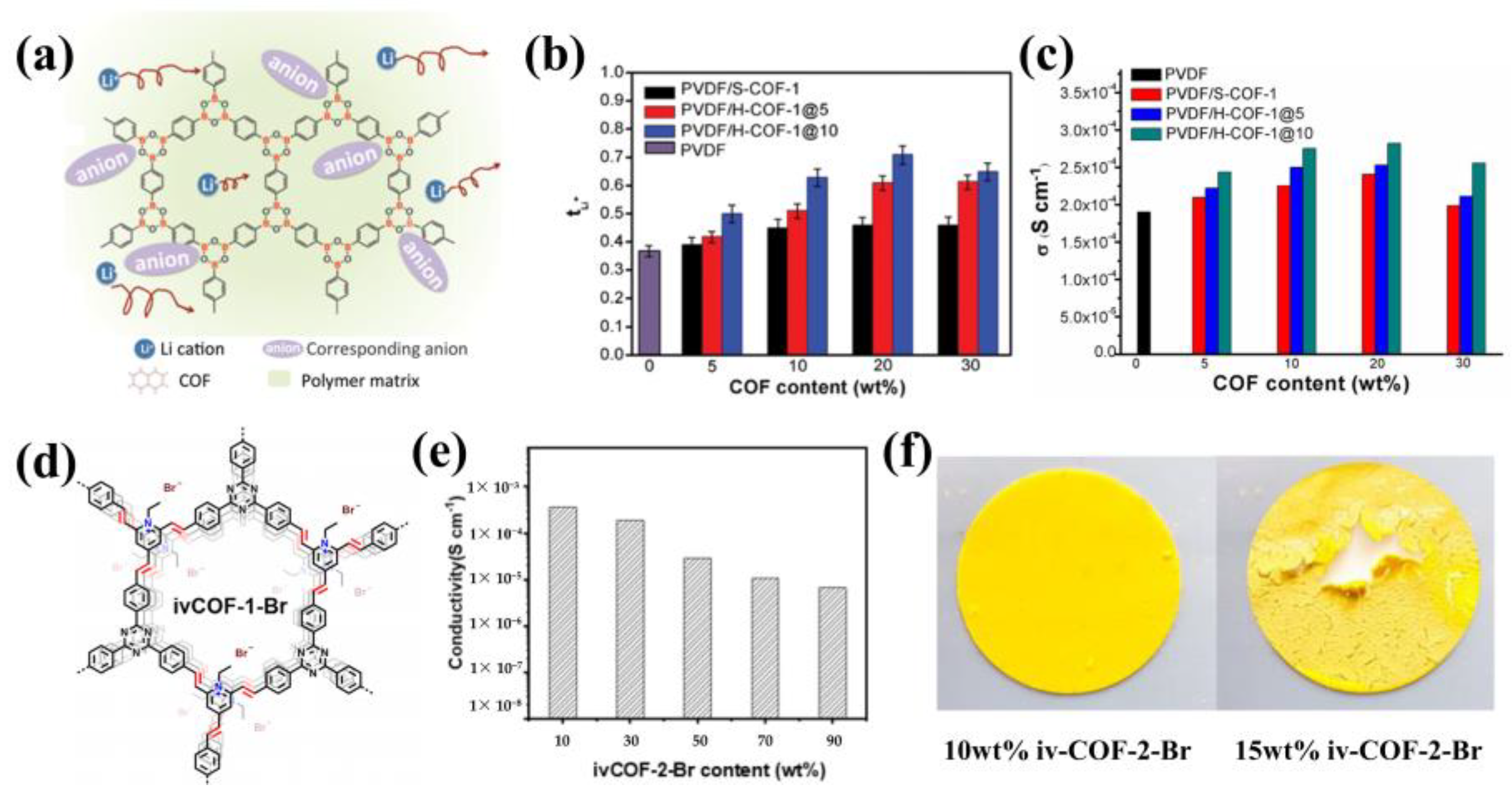
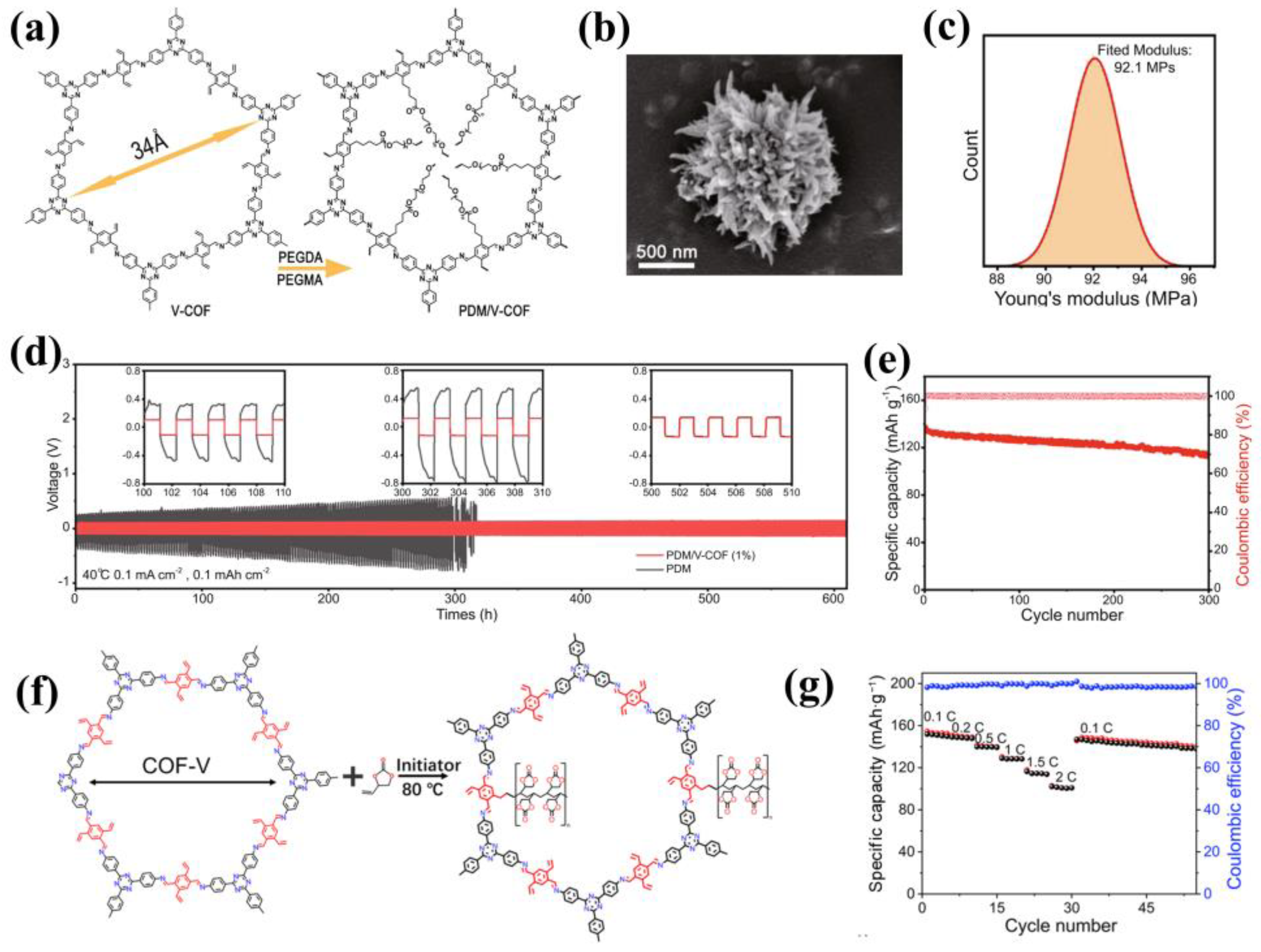
4. Composite Electrolytes with COFs as Fillers
5. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar]
- Yang, X.; Liu, T.; Wang, C. Thermally modulated lithium iron phosphate batteries for mass-market electric vehicles. Nat. Energy 2021, 6, 176–185. [Google Scholar] [CrossRef]
- Albertus, P.; Babinec, S.; Litzelman, S.; Newman, A. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat. Energy 2017, 3, 16–21. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, B.; Shen, Y.; Wu, J.; Zhao, Z.; Zhong, C.; Hu, W. Confronting the Challenges in Lithium Anodes for Lithium Metal Batteries. Adv. Sci. 2021, 8, 2101111. [Google Scholar] [CrossRef]
- Wan, J.; Xie, J.; Mackanic, D.G.; Burke, W.; Bao, Z.; Cui, Y. Status, promises, and challenges of nanocomposite solid-state electrolytes for safe and high performance lithium batteries. Mater. Today Nano 2018, 4, 1–16. [Google Scholar] [CrossRef]
- Kim, K.J.; Balaish, M.; Wadaguchi, M.; Kong, L.; Rupp, J.L.M. Solid-State Li-Metal Batteries: Challenges and Horizons of Oxide and Sulfide Solid Electrolytes and Their Interfaces. Adv. Energy Mater. 2020, 11, 2002689. [Google Scholar] [CrossRef]
- Yue, L.; Ma, J.; Zhang, J.; Zhao, J.; Dong, S.; Liu, Z.; Cui, G.; Chen, L. All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater. 2016, 5, 139–164. [Google Scholar] [CrossRef]
- An, Y.; Han, X.; Liu, Y.; Azhar, A.; Na, J.; Nanjundan, A.K.; Wang, S.; Yu, J.; Yamauchi, Y. Progress in Solid Polymer Electrolytes for Lithium-Ion Batteries and Beyond. Small 2022, 18, 2103617. [Google Scholar] [CrossRef]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem. Soc. Rev. 2014, 43, 4714–4727. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, J.; Dong, S.; Li, X.; Cui, G. Intermolecular Chemistry in Solid Polymer Electrolytes for High-Energy-Density Lithium Batteries. Adv. Mater. 2019, 31, 1902029. [Google Scholar] [CrossRef] [PubMed]
- Dirican, M.; Yan, C.; Zhu, P.; Zhang, X. Composite solid electrolytes for all-solid-state lithium batteries. Mater. Sci. Eng. R Rep. 2019, 136, 27–46. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Guo, W.; Fu, Y. Advances in Composite Polymer Electrolytes for Lithium Batteries and Beyond. Adv. Energy Mater. 2020, 11, 2000802. [Google Scholar] [CrossRef]
- Bonnick, P.; Muldoon, J. The quest for the holy grail of solid-state lithium batteries. Energy Environ. Sci. 2022, 15, 1840–1860. [Google Scholar] [CrossRef]
- Feng, X.; Ding, X.; Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022. [Google Scholar] [CrossRef]
- Lohse, M.S.; Bein, T. Covalent Organic Frameworks: Structures, Synthesis, and Applications. Adv. Funct. Mater. 2018, 28, 1705553. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Karak, S.; Dey, K.; Banerjee, R. Maneuvering Applications of Covalent Organic Frameworks via Framework-Morphology Modulation. Adv. Mater. 2022, 34, 2202751. [Google Scholar] [CrossRef] [PubMed]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, S.; Chen, Y.; Zhang, Z.; Ma, S. Covalent organic frameworks for separation applications. Chem. Soc. Rev. 2020, 49, 708–735. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, A.; Wagner, A.; Inamdar, S.; Acharya, A.P. Covalent Organic Frameworks for Biomedical Applications. Adv. Healthc. Mater. 2021, 10, 2002090. [Google Scholar] [CrossRef]
- Cui, X.; Lei, S.; Wang, A.C.; Gao, L.; Zhang, Q.; Yang, Y.; Lin, Z. Emerging covalent organic frameworks tailored materials for electrocatalysis. Nano Energy 2020, 70, 104525. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Yu, G. Covalent organic frameworks: Design, synthesis, and performance for photocatalytic applications. Nano Today 2021, 40, 101247. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, M.; Wang, H.; Han, C.; Pan, F.; Sun, J. Covalent Organic Framework for Rechargeable Batteries: Mechanisms and Properties of Ionic Conduction. Adv. Energy Mater. 2022, 12, 2200057. [Google Scholar] [CrossRef]
- Sahoo, R.; Mondal, S.; Pal, S.C.; Mukherjee, D.; Das, M.C. Covalent-Organic Frameworks (COFs) as Proton Conductors. Adv. Energy Mater. 2021, 11, 2102300. [Google Scholar] [CrossRef]
- Lanni, L.M.; Tilford, R.W.; Bharathy, M.; Lavigne, J.J. Enhanced hydrolytic stability of self-assembling alkylated two-dimensional covalent organic frameworks. J. Am. Chem. Soc. 2011, 133, 13975–13983. [Google Scholar] [CrossRef]
- Spitler, E.L.; Giovino, M.R.; White, S.L.; Dichtel, W.R. A mechanistic study of Lewis acid-catalyzed covalent organic framework formation. Chem. Sci. 2011, 2, 1588–1593. [Google Scholar] [CrossRef]
- Kandambeth, S.; Mallick, A.; Lukose, B.; Mane, M.V.; Heine, T.; Banerjee, R. Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. J. Am. Chem. Soc. 2012, 134, 19524–19527. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Pachfule, P.; Thomas, A. Covalent organic frameworks (COFs) for electrochemical applications. Chem. Soc. Rev. 2021, 50, 6871–6913. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Loh, K.P. Recent Progress in Covalent Organic Frameworks as Solid-State Ion Conductors. ACS Mater. Lett. 2019, 1, 327–335. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, Q.; Zhao, G.; Sun, Y.; Guo, H. Covalent organic frameworks for solid-state electrolytes of lithium metal batteries. J. Mater. Chem. A 2022, 10, 7497–7516. [Google Scholar] [CrossRef]
- Zhu, D.; Xu, G.; Barnes, M.; Li, Y.; Tseng, C.; Zhang, Z.; Zhang, J.; Zhu, Y.; Khalil, S.; Rahman, M.M.; et al. Covalent Organic Frameworks for Batteries. Adv. Funct. Mater. 2021, 31, 2100505. [Google Scholar] [CrossRef]
- Yang, H.; Wu, N. Ionic conductivity and ion transport mechanisms of solid-state lithium-ion battery electrolytes: A review. Energy Sci. Eng. 2022, 10, 1643–1671. [Google Scholar] [CrossRef]
- Yang, L.; Huang, N. Covalent organic frameworks for applications in lithium batteries. J. Polym. Sci. 2022, 60, 2225–2238. [Google Scholar] [CrossRef]
- Wu, M.; Shan, Z.; Xu, B.; Zhang, G. Anion anchored conjugated microporous polymers as solid electrolytes. Chem. Eng. J. 2022, 427, 131728. [Google Scholar] [CrossRef]
- Lee, J.M.; Cooper, A.I. Advances in Conjugated Microporous Polymers. Chem. Rev. 2020, 120, 2171–2214. [Google Scholar] [CrossRef]
- Uthappa, U.T.; Jung, H.; Kurkuri, M.D. Recent advances in conjugated polymers for lithium-ion and supercapacitor applications. In Polymer-Based Advanced Functional Composites for Optoelectronic and Energy Applications; Subramani, N.K., Siddaramaiah, H., Lee, J.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 12, pp. 265–289. [Google Scholar]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Vazquez-Molina, D.A.; Mohammad-Pour, G.S.; Lee, C.; Logan, M.W.; Duan, X.; Harper, J.K.; Uribe-Romo, F.J. Mechanically Shaped Two-Dimensional Covalent Organic Frameworks Reveal Crystallographic Alignment and Fast Li-Ion Conductivity. J. Am. Chem. Soc. 2016, 138, 9767–9770. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, B.; Weng, M.; Zheng, J.; Li, S.; Pan, F. Lithium ion diffusion mechanism in covalent organic framework based solid state electrolyte. Phys. Chem. Chem. Phys. 2019, 21, 9883–9888. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Wang, L.; Wang, J.; Xu, H.; He, X. Accelerated lithium-ion conduction in covalent organic frameworks. Chem. Commun. 2020, 56, 10465–10468. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H. Lithium-ion distribution and motion in two-dimensional covalent organic frameworks: The example of TAPB-PDA COF. J. Mater. Chem. C 2022, 10, 13834–13843. [Google Scholar] [CrossRef]
- Xu, Q.; Tao, S.; Jiang, Q.; Jiang, D. Ion Conduction in Polyelectrolyte Covalent Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 7429–7432. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Y.; Dong, Y.; Li, J.; Li, S.; Shao, P.; Feng, X.; Wang, B. Fast Ion Transport Pathway Provided by Polyethylene Glycol Confined in Covalent Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 1923–1927. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, B.; Yang, Z.; Yang, X.; Yu, X.; Xing, G.; Zhang, Y.; Chen, L. STable 2D Heteroporous Covalent Organic Frameworks for Efficient Ionic Conduction. Angew. Chem. Int. Ed. 2019, 58, 15742–15746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hong, Y.L.; Nishiyama, Y.; Bai, S.; Kitagawa, S.; Horike, S. Accumulation of Glassy Poly(ethylene oxide) Anchored in a Covalent Organic Framework as a Solid-State Li(+) Electrolyte. J. Am. Chem. Soc. 2019, 141, 1227–1234. [Google Scholar] [CrossRef]
- Xu, Q.; Tao, S.; Jiang, Q.; Jiang, D. Designing Covalent Organic Frameworks with a Tailored Ionic Interface for Ion Transport across One-Dimensional Channels. Angew. Chem. Int. Ed. 2020, 59, 4557–4563. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, K.; Huang, G.; Xu, B.; Hong, Y.L.; Wu, X.; Nishiyama, Y.; Horike, S.; Zhang, G.; Kitagawa, S. Highly Processable Covalent Organic Framework Gel Electrolyte Enabled by Side-Chain Engineering for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2022, 61, 202110695. [Google Scholar]
- Wu, Z.; Xu, Q.; Li, J.; Zhang, X.M. Liquid-like Phase of N,N-Dimethylpyrrolidinium Iodide Impregnated into COFs Endows Fast Lithium Ion Conduction in the Solid State. Chem. Eur. J. 2021, 27, 4583–4587. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Wu, M.; Du, Y.; Xu, B.; He, B.; Wu, X.; Zhang, G. Covalent Organic Framework-Based Electrolytes for Fast Li+ Conduction and High-Temperature Solid-State Lithium-Ion Batteries. Chem. Mater. 2021, 33, 5058–5066. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, W.; Li, B.; Sun, W.; Zhao, L.; Yuan, W. Confined ionic liquids in covalent organic frameworks toward the rational design of high-safety lithium metal battery. Chem. Eng. J. 2022, 433, 133749. [Google Scholar] [CrossRef]
- Forsyth, M.; Porcarelli, L.; Wang, X.; Goujon, N.; Mecerreyes, D. Innovative Electrolytes Based on Ionic Liquids and Polymers for Next-Generation Solid-State Batteries. Acc. Chem. Res. 2019, 52, 686–694. [Google Scholar] [CrossRef]
- Park, M.; Zhang, X.; Chung, M.; Less, G.B.; Sastry, A.M. A review of conduction phenomena in Li-ion batteries. J. Power Sources 2010, 195, 7904–7929. [Google Scholar] [CrossRef]
- Horike, S.; Umeyama, D.; Kitagawa, S. Ion Conductivity and Transport by Porous Coordination Polymers and Metal Organic Frameworks. Acc. Chem. Res. 2013, 46, 2376. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Piszcz, M.; Coya, E.; Rojo, T.; Rodriguez-Martinez, L.M.; Armand, M.; Zhou, Z. Single lithium-ion conducting solid polymer electrolytes: Advances and perspectives. Chem. Soc. Rev. 2017, 46, 797–815. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tu, H.; Hu, C.; Liu, Y.; Dong, D.; Sun, Y.; Dai, Y.; Wang, S.; Qian, H.; Lin, Z.; et al. Cationic Covalent Organic Framework Nanosheets for Fast Li-Ion Conduction. J. Am. Chem. Soc. 2018, 140, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Ogihara, W.; Washiro, S.; Nakajima, H.; Ohno, H. Effect of cation structure on the electrochemical and thermal properties of ion conductive polymers obtained from polymerizable ionic liquids. Electrochim. Acta 2006, 51, 2614–2619. [Google Scholar] [CrossRef]
- Lewandowski, A.; Świderska-Mocek, A. Ionic liquids as electrolytes for Li-ion batteries—An overview of electrochemical studies. J. Power Sources 2009, 194, 601–609. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Li, Z.; Wang, T.; Zhao, F.; Ding, X.; Feng, W.; Han, B. Defective 2D Covalent Organic Frameworks for Postfunctionalization. Adv. Funct. Mater. 2020, 30, 1909267. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Mu, Z.; Cao, C.; Li, Z.; Wang, T.; Li, Y.; Ding, X.; Han, B.; Feng, W. Cationic covalent organic framework based all-solid-state electrolytes. Mater. Chem. Front. 2020, 4, 1164–1173. [Google Scholar] [CrossRef]
- Du, Y.; Yang, H.; Whiteley, J.M.; Wan, S.; Jin, Y.; Lee, S.H.; Zhang, W. Ionic Covalent Organic Frameworks with Spiroborate Linkage. Angew. Chem. Int. Ed. 2016, 55, 1737–1741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, J.; Ma, D.; Li, P.; Li, S.; Li, H.; Zhou, J.; Ma, X.; Feng, X.; Wang, B. Three-Dimensional Anionic Cyclodextrin-Based Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2017, 56, 16313–16317. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, H.; Strangmüller, S.; Klein, W.; Kirchhain, H.; Dietrich, C.; Zeier, W.G.; van Wüllen, L.; Fässler, T.F. Lithium Phosphidogermanates α- and β-Li8GeP4—A Novel Compound Class with Mixed Li+ Ionic and Electronic Conductivity. Chem. Mater. 2018, 30, 6440–6448. [Google Scholar] [CrossRef]
- Ashraf, S.; Zuo, Y.; Li, S.; Liu, C.; Wang, H.; Feng, X.; Li, P.; Wang, B. Crystalline Anionic Germanate Covalent Organic Framework for High CO2 Selectivity and Fast Li Ion Conduction. Chem. Eur. J. 2019, 25, 13479–13483. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, Y.; Shen, L.; Qu, Z.; Ma, T.; Sun, F.; Liu, X.; Zhang, C.; Shen, J.; Li, X.; et al. Electrolyte Interphase Built from Anionic Covalent Organic Frameworks for Lithium Dendrite Suppression. Adv. Funct. Mater. 2021, 31, 2009718. [Google Scholar] [CrossRef]
- Niu, C.; Luo, W.; Dai, C.; Yu, C.; Xu, Y. High-Voltage-Tolerant Covalent Organic Framework Electrolyte with Holistically Oriented Channels for Solid-State Lithium Metal Batteries with Nickel-Rich Cathodes. Angew. Chem. Int. Ed. 2021, 60, 24915–24923. [Google Scholar] [CrossRef]
- Hu, Y.; Dunlap, N.; Wan, S.; Lu, S.; Huang, S.; Sellinger, I.; Ortiz, M.; Jin, Y.; Lee, S.; Zhang, W. Crystalline Lithium Imidazolate Covalent Organic Frameworks with High Li-Ion Conductivity. J. Am. Chem. Soc. 2019, 141, 7518–7525. [Google Scholar] [CrossRef]
- Li, X.; Hou, Q.; Huang, W.; Xu, H.; Wang, X.; Yu, W.; Li, R.; Zhang, K.; Wang, L.; Chen, Z.; et al. Solution-Processable Covalent Organic Framework Electrolytes for All-Solid-State Li–Organic Batteries. ACS Energy Lett. 2020, 5, 3498–3506. [Google Scholar] [CrossRef]
- Zhao, G.; Mei, Z.; Duan, L.; An, Q.; Yang, Y.; Zhang, C.; Tan, X.; Guo, H. COF-based single Li+ solid electrolyte accelerates the ion diffusion and restrains dendrite growth in quasi-solid-state organic batteries. Carbon Energy 2022, 5, 248. [Google Scholar] [CrossRef]
- Jeong, K.; Park, S.; Jung, G.Y.; Kim, S.H.; Lee, Y.H.; Kwak, S.K.; Lee, S.Y. Solvent-Free, Single Lithium-Ion Conducting Covalent Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 5880–5885. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, F.Q.; Li, F.; Wu, Z.; Ma, C.; Xu, Q.; Wang, P.; Zhang, X.M. A pre-synthetic strategy to construct single ion conductive covalent organic frameworks. Chem. Commun. 2020, 56, 2747–2750. [Google Scholar] [CrossRef]
- Guo, D.; Shinde, D.B.; Shin, W.; Abou-Hamad, E.; Emwas, A.H.; Lai, Z.; Manthiram, A. Foldable Solid-State Batteries Enabled by Electrolyte Mediation in Covalent Organic Frameworks. Adv. Mater. 2022, 34, 2201410. [Google Scholar] [CrossRef]
- Dong, D.; Zhang, H.; Zhou, B.; Sun, Y.; Zhang, H.; Cao, M.; Li, J.; Zhou, H.; Qian, H.; Lin, Z.; et al. Porous covalent organic frameworks for high transference number polymer-based electrolytes. Chem. Commun. 2019, 55, 1458–1461. [Google Scholar] [CrossRef]
- Kandambeth, S.; Dey, K.; Banerjee, R. Covalent Organic Frameworks: Chemistry beyond the Structure. J. Am. Chem. Soc. 2019, 141, 1807–1822. [Google Scholar] [CrossRef]
- Meng, F.; Bi, S.; Sun, Z.; Jiang, B.; Wu, D.; Chen, J.S.; Zhang, F. Synthesis of Ionic Vinylene-Linked Covalent Organic Frameworks through Quaternization-Activated Knoevenagel Condensation. Angew. Chem. Int. Ed. 2021, 60, 13614–13620. [Google Scholar] [CrossRef]
- Hou, Z.; Xia, S.; Niu, C.; Pang, Y.; Sun, H.; Li, Z.; Xu, Y.; Zheng, S. Tailoring the interaction of covalent organic framework with the polyether matrix toward high-performance solid-state lithium metal batteries. Carbon Energy 2022, 4, 506–516. [Google Scholar] [CrossRef]
- Zhang, K.; Niu, C.; Yu, C.; Zhang, L.; Xu, Y. Highly crystalline vinylene-linked covalent organic frameworks enhanced solid polycarbonate electrolyte for dendrite-free solid lithium metal batteries. Nano Res. 2022, 15, 8083–8090. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, Y.; Liu, Y.; Zhong, S.; Xie, M.; Yan, F.; Zhang, Z.; Peng, J.; Li, J.; Wang, A.; et al. Regulating solvation structure in gel polymer electrolytes with covalent organic frameworks for lithium metal batteries. Energy Storage Mater. 2022, 53, 917–926. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, W.; Sun, W.; Zhao, L.; Yuan, W. Covalent Organic Frameworks-Enhanced Ionic Conductivity of Polymeric Ionic Liquid-Based Ionic Gel Electrolyte for Lithium Metal Battery. ACS Appl. Energy Mater. 2021, 4, 2808–2819. [Google Scholar] [CrossRef]
- Thote, J.; Barike Aiyappa, H.; Rahul Kumar, R.; Kandambeth, S.; Biswal, B.P.; Balaji Shinde, D.; Chaki Roy, N.; Banerjee, R. Constructing covalent organic frameworks in water via dynamic covalent bonding. IUCrJ 2016, 3, 402–407. [Google Scholar] [CrossRef]
- Karak, S.; Kandambeth, S.; Biswal, B.P.; Sasmal, H.S.; Kumar, S.; Pachfule, P.; Banerjee, R. Constructing Ultraporous Covalent Organic Frameworks in Seconds via an Organic Terracotta Process. J. Am. Chem. Soc. 2017, 139, 1856–1862. [Google Scholar] [CrossRef]
- Wei, H.; Chai, S.; Hu, N.; Yang, Z.; Wei, L.; Wang, L. The microwave-assisted solvothermal synthesis of a crystalline two-dimensional covalent organic framework with high CO2 capacity. Chem. Commun. 2015, 51, 12178–12181. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Q.; Huang, Y.; An, H.; Zhao, Y.; Feng, Y.; Li, X.; Shi, X.; Liang, J.; Pan, F.; et al. PolyCOFs: A New Class of Freestanding Responsive Covalent Organic Framework Membranes with High Mechanical Performance. ACS Cent. Sci. 2019, 5, 1352–1359. [Google Scholar] [CrossRef] [PubMed]

| Electrolyte | Li Metal Battery | Ref. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COFs | Li Salt | Solvent | σ (S cm−1) | tLi+ | Ea (eV) | EW (V) | Cathode | Cycling Conditions | Qdis1 (mAh/g) | Capacity Retention | |||
| Cycle | % | ||||||||||||
| ICOF-2 | PC | 3.05 × 10−5 (RT) | 0.80 | 0.24 | 4.5 | [65] | |||||||
| COF-5 | LiClO4 | 2.6 × 10−4 (RT) | 0.37 | [43] | |||||||||
| TpPa-1 | LiClO4 | 1.5 × 10−4 (RT) | [43] | ||||||||||
| CD-COF | LiPF6 | EC/DMC | 2.7 × 10−3 (30 °C) | 0.26 | [66] | ||||||||
| Li-CON-TFSI | LiTFSI | 5.74 × 10−5 (30 °C) | 0.61 | 3.8 | [60] | ||||||||
| TPB-DMTP-COF | LiClO4 | 1.36 × 10−7 (40 °C) | 0.96 | [47] | |||||||||
| TPB-BMTP-COF | LiClO4 | 6.04×10−6 (40 °C) | 0.87 | [47] | |||||||||
| PEG-Li+@COF-5 | LiClO4 | 3.60 × 10−8 (30 °C) | 0.40 | 0.35 | [48] | ||||||||
| PEG-Li+@COF-300 | LiClO4 | 1.40 × 10−6 (30 °C) | 0.44 | 0.20 | [48] | ||||||||
| PEG-Li+@EB-COF-ClO4 | LiClO4 | 1.93 × 10−5 (30 °C) | 0.60 | 0.21 | N/A | [48] | |||||||
| PEG--Li+@CD-COF-Li | LiClO4 | 2.60 × 10−5 (30 °C) | 0.20 | 0.17 | [48] | ||||||||
| TpPa-SO3Li | 2.7 × 10−5 (RT) | 0.90 | 0.18 | 4.0 | [74] | ||||||||
| H-Li-ImCOF | PC | 5.3 × 10−3 (RT) | 0.88 | 0.12 | 4.5 | [71] | |||||||
| CH3-Li-ImCOF | PC | 8.0 × 10−3 (RT) | 0.93 | 0.27 | 4.5 | [71] | |||||||
| CF3-Li-ImCOF | PC | 7.2 × 10−3 (RT) | 0.81 | 0.10 | 4.5 | [71] | |||||||
| DBC-2P@PEG | LiBF4 | 2.1 × 10−3 (70 °C) | 0.09 | [49] | |||||||||
| Ge-COF-1 | 1.1 × 10−6 (30 °C) | 0.26 | [68] | ||||||||||
| Ge-COF-1 | LiPF6 | EC/DEC | 2.5 × 10−4 (30 °C) | 0.67 | 0.29 | [68] | |||||||
| COF-PEO-3-Li | LiTFSI | 9.73 × 10−5 (200 °C) | [50] | ||||||||||
| COF-PEO-6-Li | LiTFSI | 3.71 × 10−4 (200 °C) | [50] | ||||||||||
| COF-PEO-9-Li | LiTFSI | 1.33 × 10−3 (200 °C) | 5.2 | LiFePO4 | 2.5–4.2 V, 0.02 C (100 °C) | 120 | 10 | 91.7 | [50] | ||||
| [TEO]0.33TPB-DMTP-COF | LiClO4 | 7.77 × 10−6 (40 °C) | 0.78 | [51] | |||||||||
| [TEO]0.5TPB-DMTP-COF | LiClO4 | 1.31 × 10−5 (40 °C) | 0.68 | [51] | |||||||||
| [TEO]0.33TPB-BMTP-COF | LiClO4 | 8.43 × 10−6 (40 °C) | 0.87 | [51] | |||||||||
| [TEO]0.5TPB-BMTP-COF | LiClO4 | 5.51 × 10−6 (40 °C) | 0.82 | [51] | |||||||||
| [TEO]1TPB-BPTA-COF | LiClO4 | 9.04 × 10−7 (40 °C) | [51] | ||||||||||
| dCOF-ImTFSI-60@Li | LiTFSI | 9.74 × 10−5 (30 °C) | 0.72 | 0.28 | 5.3 | LiFePO4 | 2.5–4.0 V, 0.1 C (80 °C) | 143.7 | 40 | 98.3 | [63] | ||
| Tp-PaSO3Li-COFs | EC/DMC | 1.6 × 10−3 (20 °C) | 0.94 | 0.13 | LiFePO4 | 2.5–3.6 V, 0.2 C | 152 | N/A | N/A | [75] | |||
| Im-COF-TFSI@Li | LiTFSI | 2.92 × 10−5 (30 °C) | 0.62 | 0.32 | 4.2 | LiFePO4 | 2.5–4.0 V, 0.1 C (80 °C) | 123.3 | 100 | 91.6 | [64] | ||
| Li-CON-1 | 2.13 × 10−7 (20 °C) | 0.86 | 0.25 | 4.2 | [72] | ||||||||
| Li-CON-2 | 4.36 × 10−6 (20 °C) | 0.83 | 0.22 | 3.8 | [72] | ||||||||
| Li-CON-3 | 3.21 × 10−5 (20 °C) | 0.92 | 0.13 | 4.3 | BQ | 1.7–4.0 V, 1 C (20 °C) | 214.8 | 500 | 87.7 | [72] | |||
| LPC-2 | LiI | P1,1I | 8.9 × 10−4 (40 °C) | 0.58 | 0.23 | LiFePO4 | 2.5–3.6 V, 0.2 C | 144.6 | 35 | 68.6 | [53] | ||
| ACOF | LiPF6 | EC/DEC | 3.7 × 10−3 (20 °C) | 0.82 | 0.15 | LiCoO2 | 3.0–4.5 V, 0.2 C (25 °C) | 180 | 500 | 70 | [69] | ||
| COF-NUST-7 | LiTFSI | PyR14TFSI | 9.66 × 10−4 (120 °C) | 0.11 | 0.317 | 4.2 | LiFePO4 | 2.5–3.8 V, 0.05 C (100 °C) | 140.8 | 60 | 93.3 | [54] | |
| COF-NUST-8 | LiTFSI | PyR14TFSI | 1.4 × 10−3 (120 °C) | 0.301 | [54] | ||||||||
| COF-NUST-9 | LiTFSI | PyR14TFSI | 2.3 × 10−3 (120 °C) | 0.323 | [54] | ||||||||
| TAPB-TPA | LiTFSI | PyR14TFSI | 8.7 × 10−4 (30 °C) | 0.29 | LiFePO4 | 2.5–4 V, 0.1 C (60 °C) | 163.1 | 50 | 92.2 | [55] | |||
| Q-COF | LiTFSI | 7.5 × 10−5 (30 °C) | 0.72 | 0.15 | 5.6 | NMC811 | 3.0–4.4 V (60 °C) | 160 | 400 | 82 | [70] | ||
| PEG-Li+@COF-M | LiTFSI | 2.2 × 10−5 (20 °C) | 0.46 | 5.0 | [48] | ||||||||
| DMA@COF-SO3Li | LiTFSI | 1.65 × 10−4 (23 °C) | 0.85 | 4.5 | LiFePO4 | 2.8–3.8 V, 0.5 C (65 °C) | 122 | 130 | 90.2 | [76] | |||
| LiOOC-COF1 | 7.23 × 10−7 (30 °C) | 0.82 | 0.21 | 3.5 | [73] | ||||||||
| LiOOC-COF2 | 2.66 × 10−6 (30 °C) | 0.86 | 0.2 | 4.06 | [73] | ||||||||
| LiOOC-COF3 | LiPF6 | EC/DEC | 1.36 × 10−5 (30 °C) | 0.91 | 0.17 | 4.2 | C6O6 | 1.5–4 V, 50 mA g−1 (25 °C) | 418 | 500 | 89.7 | [73] | |
| Electrolyte | Li Metal Battery | Refs. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| The Composition of Electrolyte | Filler Content (wt%) | σ (S cm−1) | tLi+ | EW (V) | Cathode | Cycling Conditions | Qdis1 (mAh/g) | Capacity Retention | |||||
| Matrix | COFs | Li Salt | Solvent | Cycle | % | ||||||||
| PVDF | H-COF-1@10 | LiClO4 | 20 | 2.74 × 10−4 (RT) | 0.71 | 4.3 | LiFePO4 | 1 C (RT) | 128 | 80 | 94 | [78] | |
| PEO | ivCOF-2-Br | LiTFSI | 10 | 3.84 × 10−4 (20 °C) | [79] | ||||||||
| PDADMA TFSI | TPB-DMTP-COF | LiTFSI | PYR14TFSI | 3.2 | 2.8 × 10−4 (30 °C) | 0.16 | 5 V | LiFePO4 | 1 C (60 °C) | 140 | 800 | 76.4 | [83] |
| PDM (PEGDA/ PEGMA) | V-COF | LiTFSI | 1 | 1.1 × 10−4 (40 °C) | 0.45 | 5.0 | LiFePO4 | 1 C (40 °C) | 136 | 300 | 83.8 | [80] | |
| PVEC | V-COF | LiTFSI | 2 | 1.1 × 10−4 (40 °C) | LiFePO4 | 1 C (40 °C) | 125 | 400 | 82.25 | [81] | |||
| AN+PEGDMA | TpPa-2 | LiTFSI | EC/DEC | 0.375 | 9.3 × 10−3 (RT) | 0.57 | 4.8 | LiFePO4 | 0.5 C (RT) | ~140 | 850 | 85 | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Y.; Zeng, X.; Wang, H.; Long, J.; Tian, Y.; Lan, J.; Yu, Y.; Yang, X. Application and Research Progress of Covalent Organic Frameworks for Solid-State Electrolytes in Lithium Metal Batteries. Materials 2023, 16, 2240. https://doi.org/10.3390/ma16062240
Qiao Y, Zeng X, Wang H, Long J, Tian Y, Lan J, Yu Y, Yang X. Application and Research Progress of Covalent Organic Frameworks for Solid-State Electrolytes in Lithium Metal Batteries. Materials. 2023; 16(6):2240. https://doi.org/10.3390/ma16062240
Chicago/Turabian StyleQiao, Yufeng, Xiaoyue Zeng, Haihong Wang, Jianlin Long, Yanhong Tian, Jinle Lan, Yunhua Yu, and Xiaoping Yang. 2023. "Application and Research Progress of Covalent Organic Frameworks for Solid-State Electrolytes in Lithium Metal Batteries" Materials 16, no. 6: 2240. https://doi.org/10.3390/ma16062240
APA StyleQiao, Y., Zeng, X., Wang, H., Long, J., Tian, Y., Lan, J., Yu, Y., & Yang, X. (2023). Application and Research Progress of Covalent Organic Frameworks for Solid-State Electrolytes in Lithium Metal Batteries. Materials, 16(6), 2240. https://doi.org/10.3390/ma16062240







