Enhancement of the Solubility of BS Class II Drugs with MOF and MOF/GO Composite Materials: Case Studies of Felodipine, Ketoprofen and Ibuprofen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.1.1. A HKUST-1 Synthesis
2.1.2. GO Synthesis
2.2. Synthesis of MOFs-Composite
2.3. Characterization of the Samples -FTIR, SEM XRD BET
2.4. Drug Loading Tests
2.5. Drug Release
3. Results and Discussion
3.1. Sample Characterisation
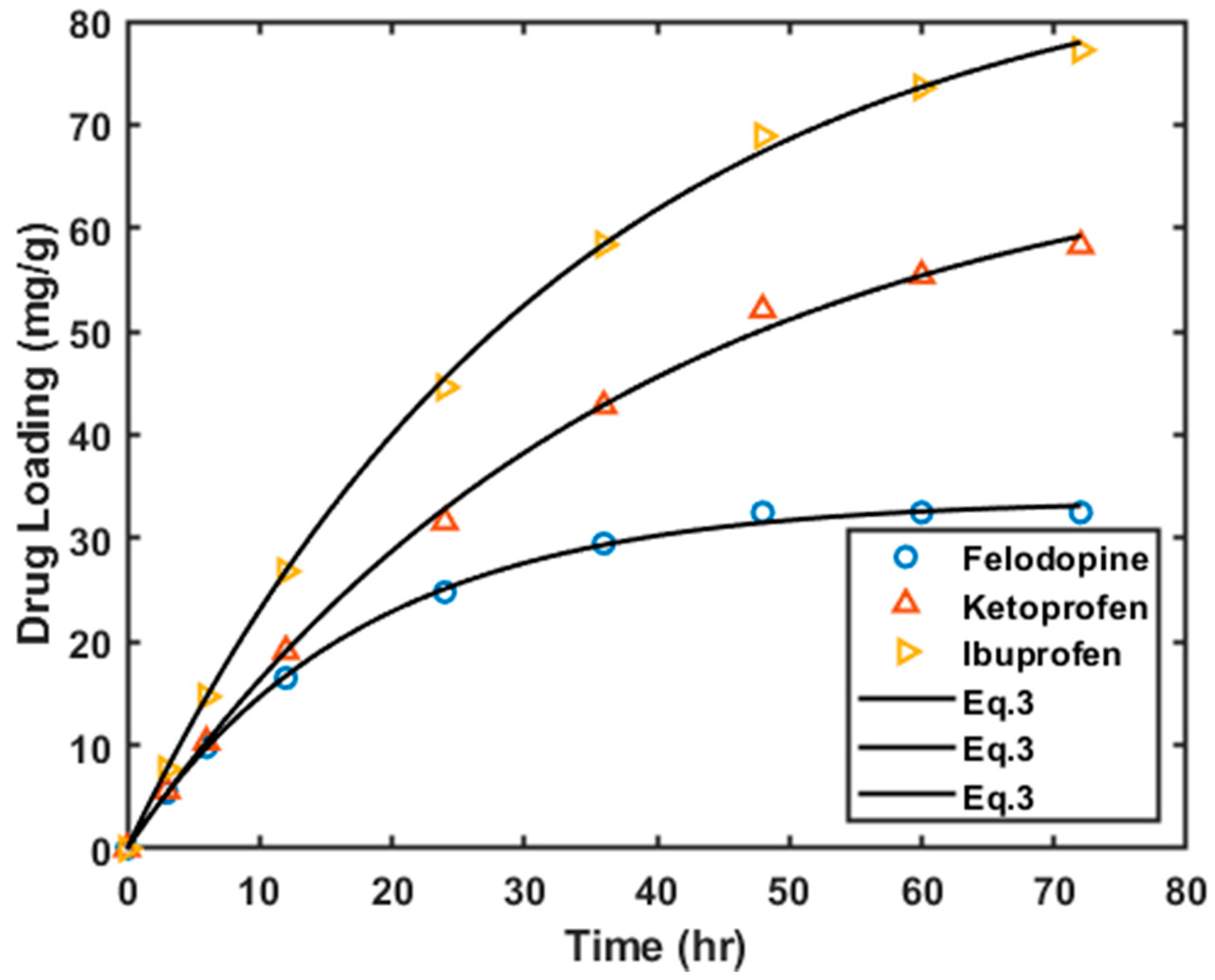
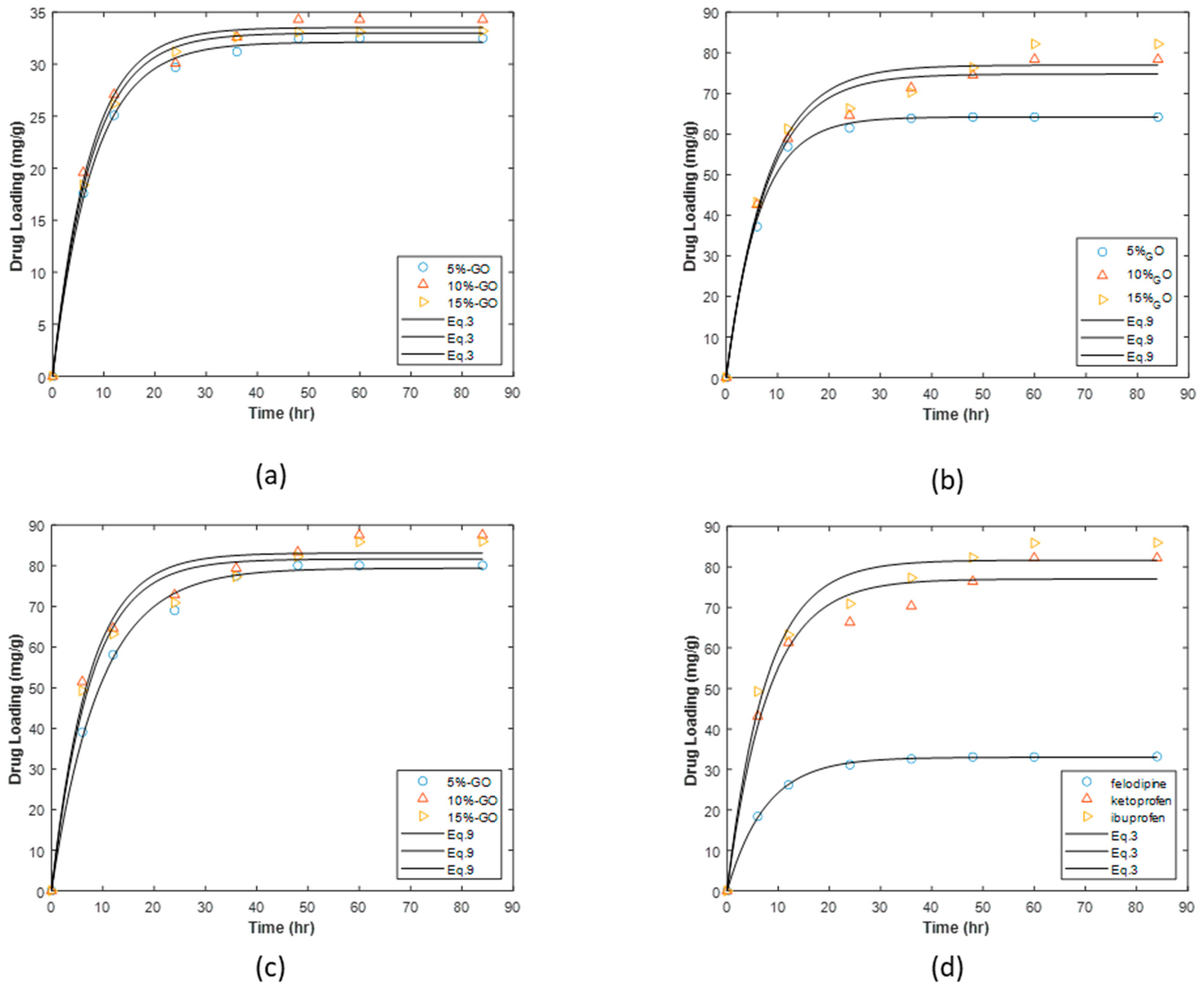
3.2. Drug Loading
3.2.1. Loading on Graphite Oxide and HKUST-1 Matrices
3.2.2. Loading on HKUST-1/GO Composites
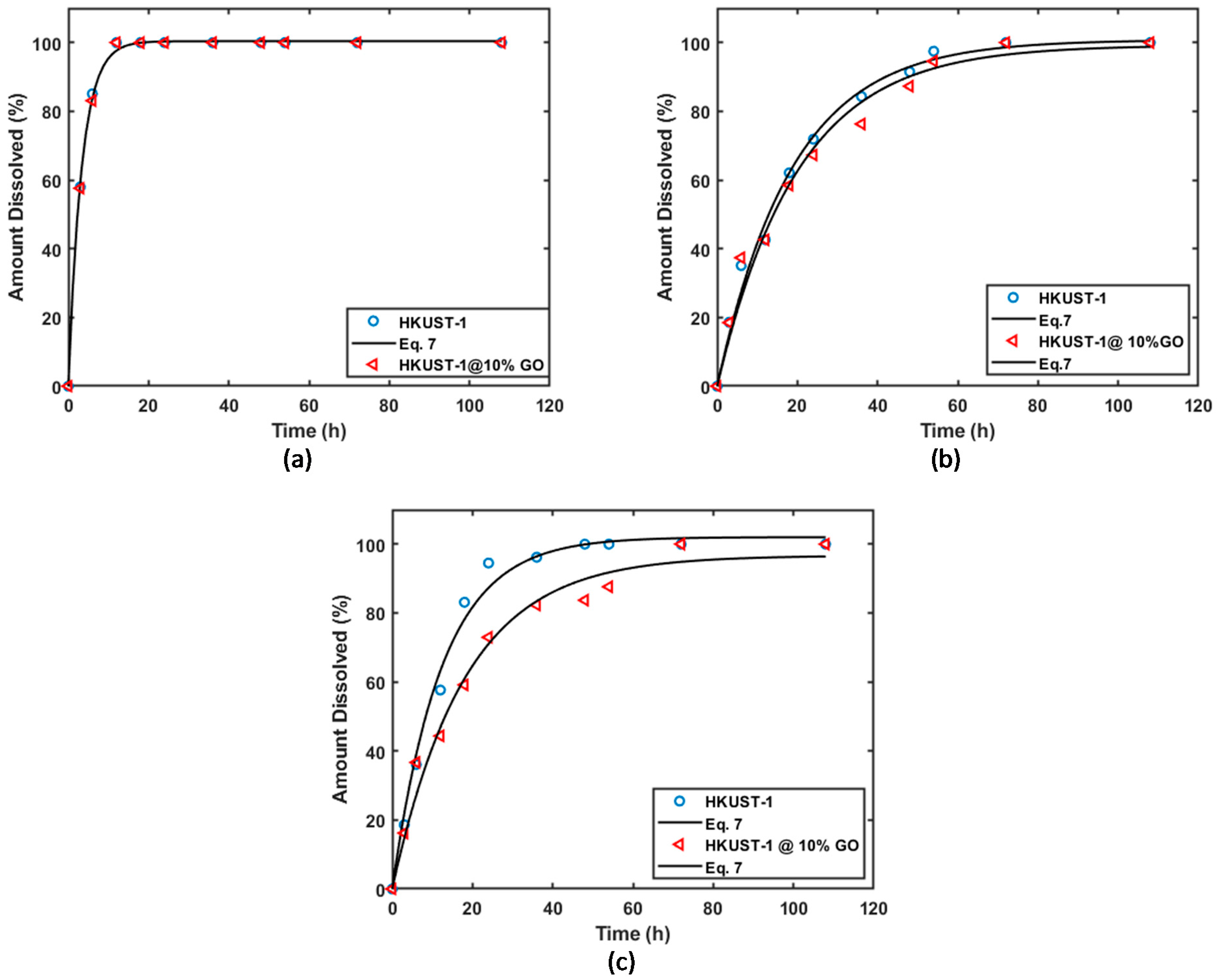
3.3. Drug Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef]
- Wang, C.; Song, P.; Zhang, Q.; Du, R.; Zhong, T.; Zhang, W.; Song, W.; Zhang, X.; Zhao, Y. The preparation and characteristics of febuxostat SiO2 solid dispersions. J. Chin. Pharm. Sci. 2014, 23, 463–470. [Google Scholar] [CrossRef]
- Chiou, W.L.; Riegelman, S. Pharmaceutical applications of solid dispersion systems. J. Pharm. Sci. 1971, 60, 1281–1302. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.K.; Li, L.; He, Y.Q.; Fan, L.; Wu, Y.Q.; Liu, L. Preparation and Drug-Delivery Properties of HKUST-1/GO Hybrid. J. Nanosci. Nanotechnol. 2016, 16, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Servatan, M.; Zarrintaj, P.; Mahmodi, G.; Kim, S.J.; Ganjali, M.R.; Saeb, M.R.; Mozafari, M. Zeolites in drug delivery: Progress, challenges and opportunities. Drug Discov. Today 2020, 25, 642–656. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Qin, C.; Wang, X.-L.; Yang, G.-S.; Shao, K.-Z.; Lan, Y.-Q.; Su, Z.-M.; Huang, P.; Wang, C.-G.; Wang, E.-B. Zeolitic imidazolate framework-8 as efficient pH-sensitive drug delivery vehicle. Dalton Trans. 2012, 41, 6906–6909. [Google Scholar] [CrossRef]
- Seo, J.S.; Whang, D.; Lee, H.; Jun, S.I.; Oh, J.; Jeon, Y.J.; Kim, K. A homochiral metal-organic porous material for enantioselective separation and catalysis. Nature 2000, 404, 982–986. [Google Scholar] [CrossRef]
- Schlogl, R. Surface Composition and Structure of Active Carbons. In Handbook of Porous Solids; Schüth, F., Sing, K., Weitkamp, J., Eds.; Wiley: Weinheim, Germany, 2002. [Google Scholar]
- Férey, G.; Latroche, M.; Serre, C.; Millange, F.; Loiseau, T.; Percheron-Guégan, A. Hydrogen adsorption in the nanoporous metal-benzenedicarboxylate M(OH)(O2C-C6H4-CO2) (M = Al3+, Cr3+), MIL-53. Chem. Commun. 2003, 24, 2976–2977. [Google Scholar] [CrossRef]
- Ma, D.-Y.; Li, Z.; Xiao, J.-X.; Deng, R.; Lin, P.-F.; Chen, R.-Q.; Liang, Y.-Q.; Guo, H.-F.; Liu, B.; Liu, J.-Q. Hydrostable and Nitryl/Methyl-Functionalized Metal–Organic Framework for Drug Delivery and Highly Selective CO2 Adsorption. Inorg. Chem. 2015, 54, 6719–6726. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal-organic frameworks as efficient materials for drug delivery. Angew. Chem. 2006, 45, 5974–5978. [Google Scholar] [CrossRef]
- Huxford, R.C.; Della Rocca, J.; Lin, W. Metal–organic frameworks as potential drug carriers. Curr. Opin. Chem. Biol. 2010, 14, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Shen, J. Cyclodextrins Modified/Coated Metal–Organic Frameworks. Materials 2020, 13, 1273. [Google Scholar] [CrossRef] [PubMed]
- Preiß, T.; Zimpel, A.; Wuttke, S.; Rädler, J.O. Kinetic Analysis of the Uptake and Release of Fluorescein by Metal-Organic Framework Nanoparticles. Materials 2017, 10, 216. [Google Scholar] [CrossRef]
- Liu, X.-M.; Xie, L.-H.; Wu, Y. Efficient Propylene/Ethylene Separation in Highly Porous Metal–Organic Frameworks. Materials 2023, 16, 154. [Google Scholar]
- Yan, Y.; Chu, Y.; Khan, M.A.; Xia, M.; Shi, M.; Zhu, S.; Lei, W.; Wang, F. Facile immobilization of ethylenediamine tetramethylene-phosphonic acid into UiO-66 for toxic divalent heavy metal ions removal: An experimental and theoretical exploration. Sci. Total Environ. 2022, 806, 150652. [Google Scholar] [CrossRef]
- Li, H.; Xu, C.; Li, N.; Rao, T.; Zhou, Z.; Zhou, Q.; Wang, C.; Xu, S.; Tang, J. Synthesis of Bimetallic FeCu-MOF and Its Performance as Catalyst of Peroxymonosulfate for Degradation of Methylene Blue. Materials 2022, 15, 7252. [Google Scholar] [CrossRef]
- Xie, Y.; Lyu, S.; Zhang, Y.; Cai, C. Adsorption and Degradation of Volatile Organic Compounds by Metal–Organic Frameworks (MOFs): A Review. Materials 2022, 15, 7727. [Google Scholar]
- Pavlovskii, A.A.; Pushnitsa, K.; Kosenko, A.; Novikov, P.; Popovich, A.A. Organic Anode Materials for Lithium-Ion Batteries: Recent Progress and Challenges. Materials 2023, 16, 177. [Google Scholar] [CrossRef]
- Song, D.; Hu, C.; Gao, Z.; Yang, B.; Li, Q.; Zhan, X.; Tong, X.; Tian, J. Metal Organic Frameworks (MOFs) Derived Materials Used in Zn-Air Battery. Materials 2022, 15, 5837. [Google Scholar] [CrossRef]
- Chen, F.; Xu, H.; Cai, Y.; Zhang, W.; Shen, P.; Zhang, W.; Xie, H.; Bai, G.; Xu, S.; Gao, J. Multi-Responsive Sensor Based on Porous Hydrogen-Bonded Organic Frameworks for Selective Sensing of Ions and Dopamine Molecules. Molecules 2022, 27, 8750. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Adhikari, S.; Riju, H.; Maji, T.K. Terbium(III), Europium(III), and Mixed Terbium(III)–Europium(III) Mucicate Frameworks: Hydrophilicity and Stoichiometry-Dependent Color Tunability. Inorg. Chem. 2012, 51, 4891–4893. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Liang, L.; Song, J.; Wei, Z.; Yang, S.; Ma, Y.; Chen, W.R.; Lu, C.; Wen, L. Doxorubicin-Loaded Metal-Organic Framework Nanoparticles as Acid-Activatable Hydroxyl Radical Nanogenerators for Enhanced Chemo/Chemodynamic Synergistic Therapy. Materials 2022, 15, 1096. [Google Scholar] [CrossRef] [PubMed]
- Saeb, M.R.; Rabiee, N.; Mozafari, M.; Verpoort, F.; Voskressensky, L.G.; Luque, R. Metal–Organic Frameworks (MOFs) for Cancer Therapy. Materials 2021, 14, 7277. [Google Scholar]
- Roh, D.K.; Jae, H.; Mun, H.; Jo, J.H.; Chi, W.S. Precise tuning of morphology and pore size of amine-functionalized MIL metal–organic frameworks using a directing agent. Mater. Sci. Eng. B 2021, 263, 114833. [Google Scholar] [CrossRef]
- An, J.; Geib, S.J.; Rosi, N.L. Cation-Triggered Drug Release from a Porous Zinc−Adeninate Metal−Organic Framework. J. Am. Chem. Soc. 2009, 131, 8376–8377. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Petit, C. MOF/graphite oxide hybrid materials: Exploring the new concept of adsorbents and catalysts. Adsorption 2011, 17, 5–16. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.L.; Geng, H.Y.; Hu, B.; Song, G.W.; Xu, Z.S. A MOF/graphite oxide hybrid (MOF: HKUST-1) material for the adsorption of methylene blue from aqueous solution. J. Mater. Chem. A 2013, 1, 10292–10299. [Google Scholar] [CrossRef]
- Sule, R.; Mishra, A.K. Synthesis of Mesoporous MWCNT/HKUST-1 Composite for Wastewater Treatment. Appl. Sci. 2019, 9, 4407. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Xu, Q. Metal–organic framework composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef]
- Ke, F.; Yuan, Y.-P.; Qiu, L.-G.; Shen, Y.-H.; Xie, A.-J.; Zhu, J.-F.; Tian, X.-Y.; Zhang, L.-D. Facile fabrication of magnetic metal–organic framework nanocomposites for potential targeted drug delivery. J. Mater. Chem. 2011, 21, 3843–3848. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Liu, Z.; Ma, Y.; Huang, Y.; Chen, Y. High-Efficiency Loading and Controlled Release of Doxorubicin Hydrochloride on Graphene Oxide. J. Phys. Chem. C 2008, 112, 17554–17558. [Google Scholar] [CrossRef]
- Chui, S.S.; Lo, S.M.; Charmant, J.P.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Q.; Qiu, L.-G.; Xu, T.; Wu, Y.; Wang, W.; Wu, Z.-Y.; Jiang, X. Ultrasonic synthesis of the microporous metal–organic framework Cu3(BTC)2 at ambient temperature and pressure: An efficient and environmentally friendly method. Mater. Lett. 2009, 63, 78–80. [Google Scholar] [CrossRef]
- Li, Y.; Miao, J.; Sun, X.; Xiao, J.; Li, Y.; Wang, H.; Xia, Q.; Li, Z. Mechanochemical synthesis of Cu-BTC@GO with enhanced water stability and toluene adsorption capacity. Chem. Eng. J. 2016, 298, 191–197. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, X.; Lester, E.; Wu, T. High efficiency synthesis of HKUST-1 under mild conditions with high BET surface area and CO2 uptake capacity. Prog. Nat. Sci. Mater. Int. 2018, 28, 584–589. [Google Scholar] [CrossRef]
- Hartmann, M.; Kunz, S.; Himsl, D.; Tangermann, O.; Ernst, S.; Wagener, A. Adsorptive Separation of Isobutene and Isobutane on Cu3(BTC)2. Langmuir 2008, 24, 8634–8642. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Lin, K.-S.; Adhikari, A.K.; Ku, C.-N.; Chiang, C.-L.; Kuo, H. Synthesis and characterization of porous HKUST-1 metal organic frameworks for hydrogen storage. Int. J. Hydrog. Energy 2012, 37, 13865–13871. [Google Scholar] [CrossRef]
- Zarei Mohammadabad, M.; Moeinaddini, M.; Nowrouzi, M.; Rafiee, R.; Abbasi, A. Facile and cost-efficient synthesis of highly efficient CO2 adsorbents: A pathway towards a green environment. J. Porous Mater. 2020, 27, 1659–1668. [Google Scholar] [CrossRef]
- Bartoszynski, R.; Powers, J.D.; Herderick, E.E.; Pultz, J.A. Statistical Comparison of Dissolution Curves. Pharmacol. Res. 2001, 43, 369–387. [Google Scholar] [CrossRef] [PubMed]
- Noce, L.; Gwaza, L.; Mangas-Sanjuan, V.; Garcia-Arieta, A. Comparison of free software platforms for the calculation of the 90% confidence interval of f2 similarity factor by bootstrap analysis. Eur. J. Pharm. Sci. 2020, 146, 105259. [Google Scholar] [CrossRef] [PubMed]
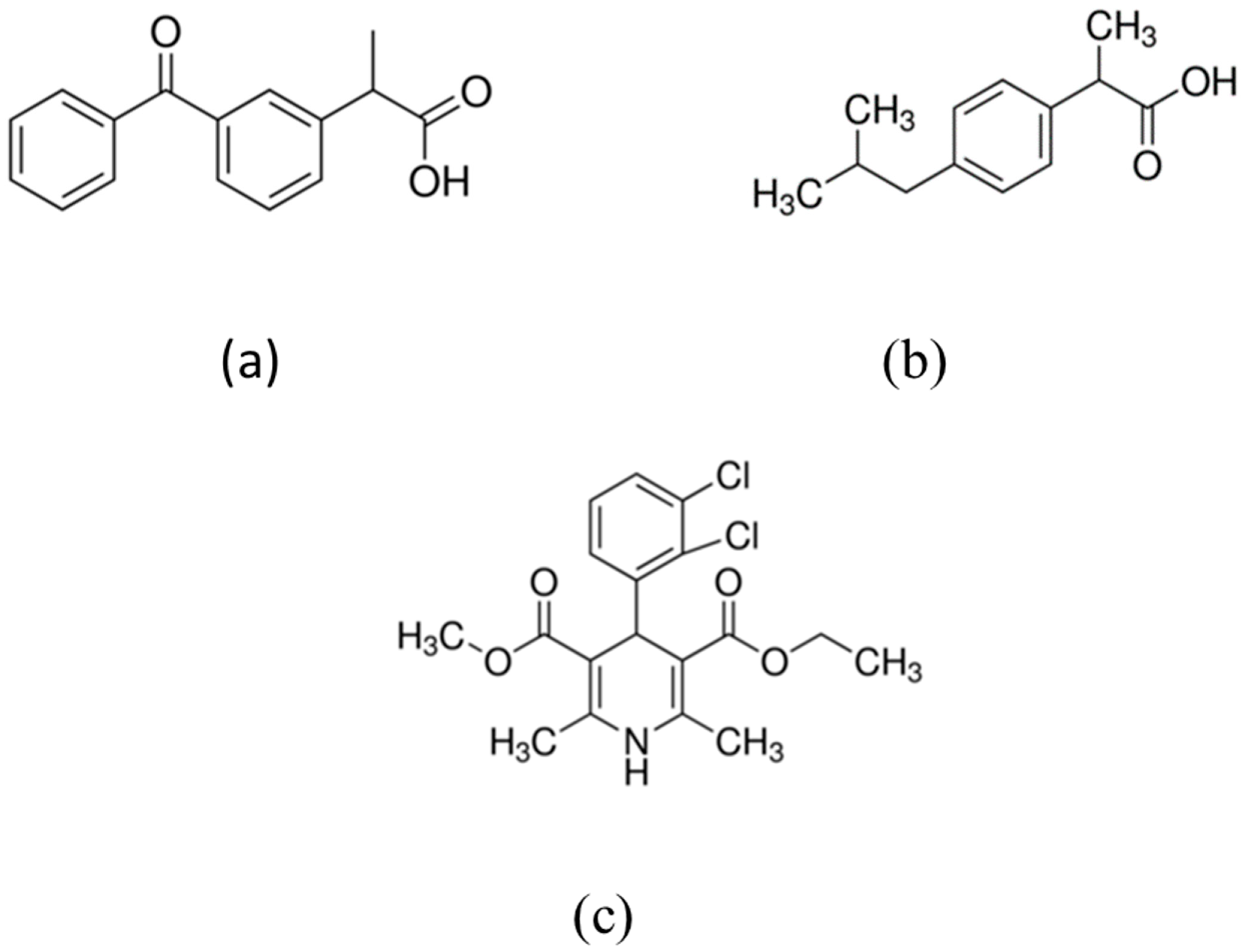

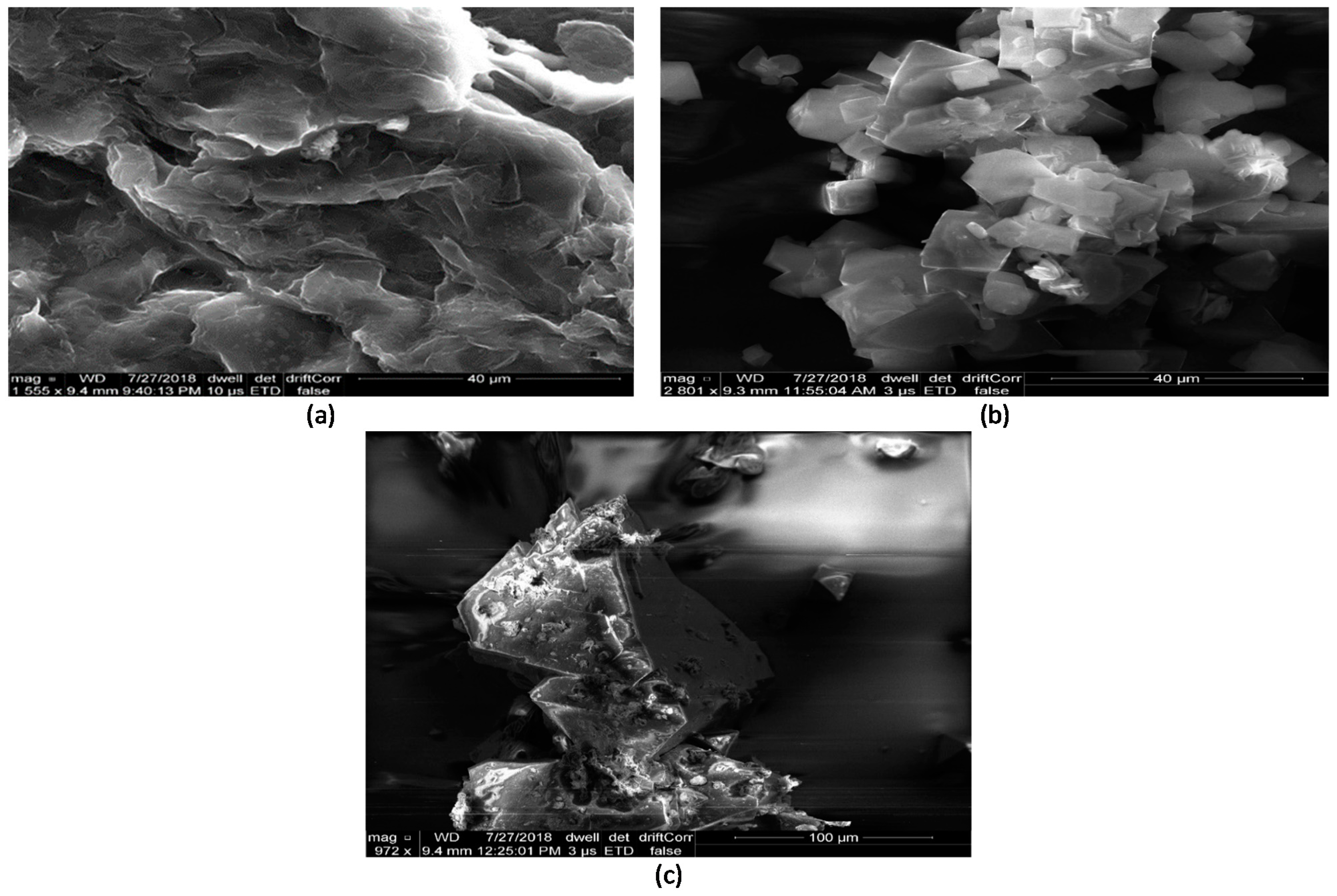
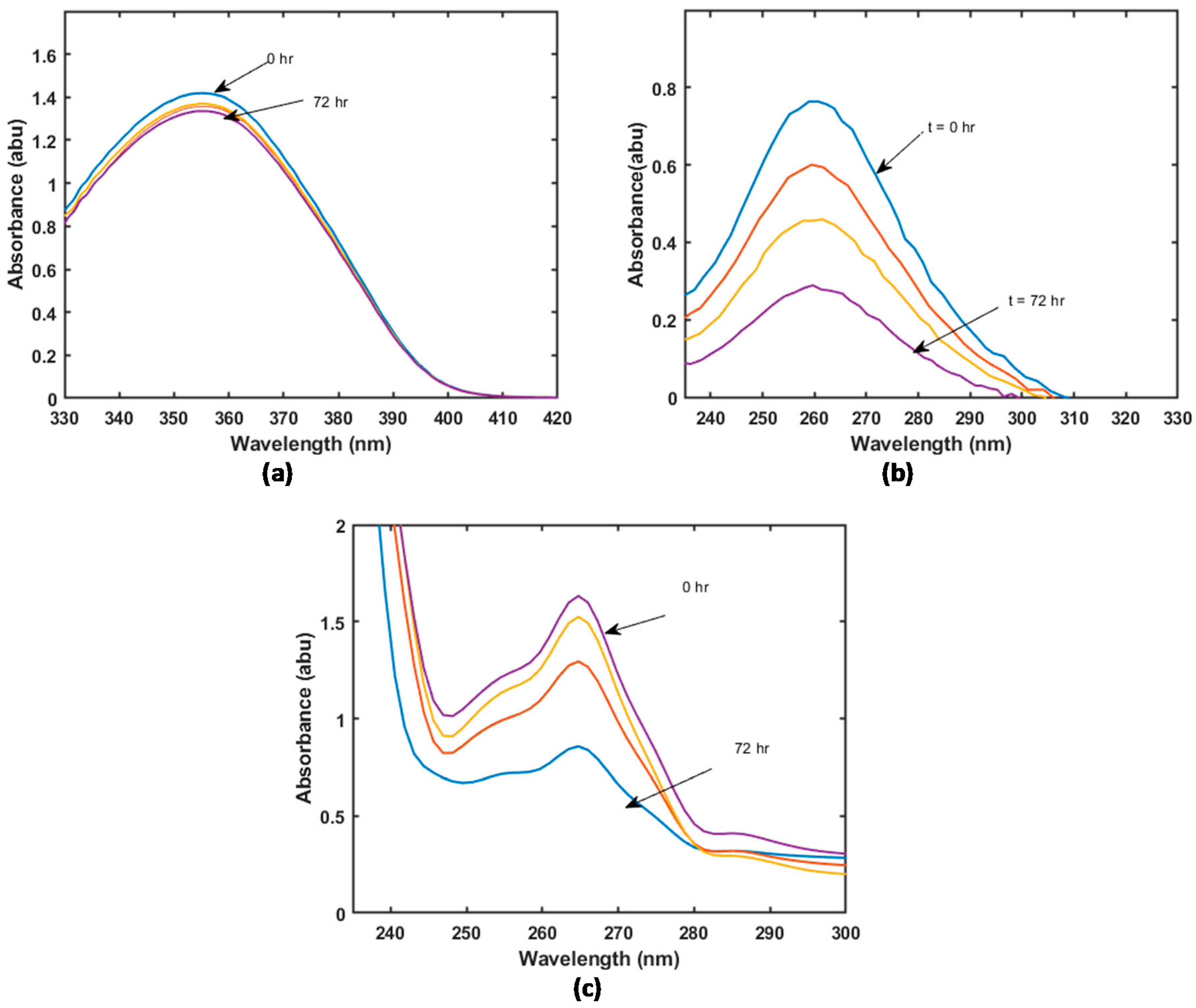
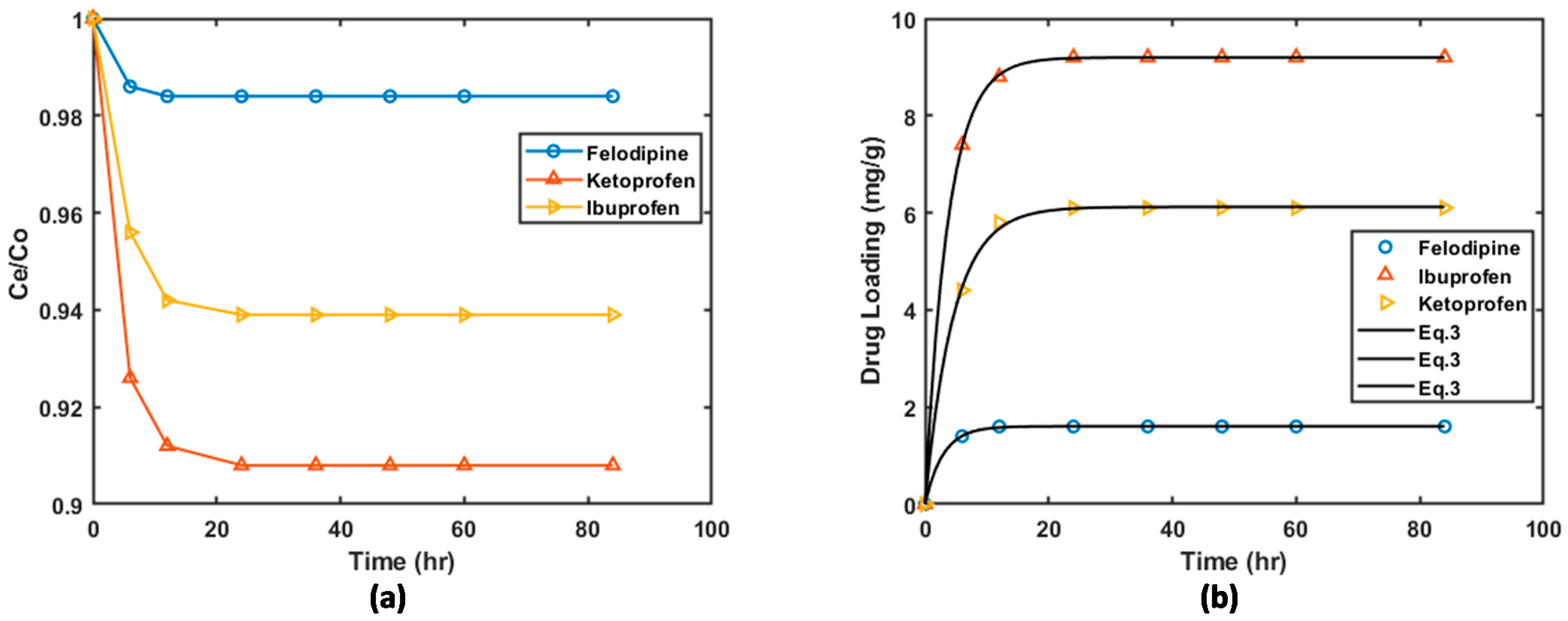
| Material | BET Surface Area (m2/g) |
|---|---|
| GO | 216 |
| HKUST-1 | 1523 |
| HKUST-1-5%GO | 1612 |
| HKUST-1-10%GO | 1625 |
| HKUST-1-15%GO | 1608 |
| Drug | Loading Vehicle | Loading Rate, kL (h−1) | qm (mg/g) | R2 |
|---|---|---|---|---|
| Felodipine | HKUST-1 | 0.057 ± 0.013 | 33.7 ± 0.67 | 0.998 |
| HKUST-1@ 5% GO | 0.127 ± 0.013 | 32.12 ± 0.67 | 0.998 | |
| HKUST-1@ 10% GO | 0.137 ± 0.027 | 33.53 ± 1.38 | 0.992 | |
| HKUST-1@ 15% GO | 0.133 ± 0.057 | 33.00 ± 0.30 | 0.996 | |
| GO | 0.349 ± 0.021 | 1.60 ± 0.01 | 0.999 | |
| Ketoprofen | HKUST-1 | 0.0346 ± 0.0033 | 58.30 ± 0.19 | 0.990 |
| HKUST-1@ 5% GO | 0.156 ± 0.019 | 64.19 ± 1.57 | 0.997 | |
| HKUST-1@ 10% GO | 0.127 ± 0.036 | 74.76 ± 0.013 | 0.983 | |
| HKUST-1@ 15% GO | 0.126 ± 0.046 | 76.98 ± 5.92 | 0.976 | |
| GO | 0.271 ± 0.004 | 9.198 ± 0.021 | 0.999 | |
| Ibuprofen | HKUST-1 | 0.0220 ± 0.0012 | 79.10 ± 0.15 | 0.998 |
| HKUST-1@ 5% GO | 0.107 ± 0.006 | 79.38 ± 2.53 | 0.996 | |
| HKUST-1@ 10% GO | 0.137 ± 0.044 | 83.14 ± 5.55 | 0.978 | |
| HKUST-1@ 15% GO | 0.132 ± 0.013 | 81.64 ± 5.52 | 0.978 | |
| GO | 0.218 ± 0.014 | 6.12 ± 0.07 | 0.9993 |
| Drug | Loading Vehicle | Release Constant, | R2 |
|---|---|---|---|
| Felodipine | HKUST-1 | 0.2914 | 0.999 |
| HKUST-1@ 10% GO | 0.2915 | 0.999 | |
| Ketoprofen | HKUST-1 | 0.0542 | 0.991 |
| HKUST-1@ 10% GO | 0.0498 | 0.976 | |
| Ibuprofen | HKUST-1 | 0.081 | 0.999 |
| HKUST-1@ 10% GO | 0.055 | 0.983 |
| Drug | Similarity Factor, |
|---|---|
| Felodipine | 95.6 |
| Ketoprofen | 72.3 |
| Ibuprofen | 44.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Xiao, B.; Le, P.K.; Mangwandi, C. Enhancement of the Solubility of BS Class II Drugs with MOF and MOF/GO Composite Materials: Case Studies of Felodipine, Ketoprofen and Ibuprofen. Materials 2023, 16, 1554. https://doi.org/10.3390/ma16041554
Han J, Xiao B, Le PK, Mangwandi C. Enhancement of the Solubility of BS Class II Drugs with MOF and MOF/GO Composite Materials: Case Studies of Felodipine, Ketoprofen and Ibuprofen. Materials. 2023; 16(4):1554. https://doi.org/10.3390/ma16041554
Chicago/Turabian StyleHan, Jinyang, Bo Xiao, Phung Kim Le, and Chirangano Mangwandi. 2023. "Enhancement of the Solubility of BS Class II Drugs with MOF and MOF/GO Composite Materials: Case Studies of Felodipine, Ketoprofen and Ibuprofen" Materials 16, no. 4: 1554. https://doi.org/10.3390/ma16041554
APA StyleHan, J., Xiao, B., Le, P. K., & Mangwandi, C. (2023). Enhancement of the Solubility of BS Class II Drugs with MOF and MOF/GO Composite Materials: Case Studies of Felodipine, Ketoprofen and Ibuprofen. Materials, 16(4), 1554. https://doi.org/10.3390/ma16041554









