Investigation of Alkali-Activated Slag-Based Composite Incorporating Dehydrated Cement Powder and Red Mud
Abstract
:1. Introduction
2. Experimental Program
2.1. Materials
2.1.1. Granulated Blast Furnace Slag (GBFS)
2.1.2. Dehydrated Cement Powder (DCP)
2.1.3. Red Mud Powder (RM)
2.1.4. Alkali Solutions
2.2. Mix Proportion and Preparation of Specimens
2.3. Test Methods
3. Results and Discussion
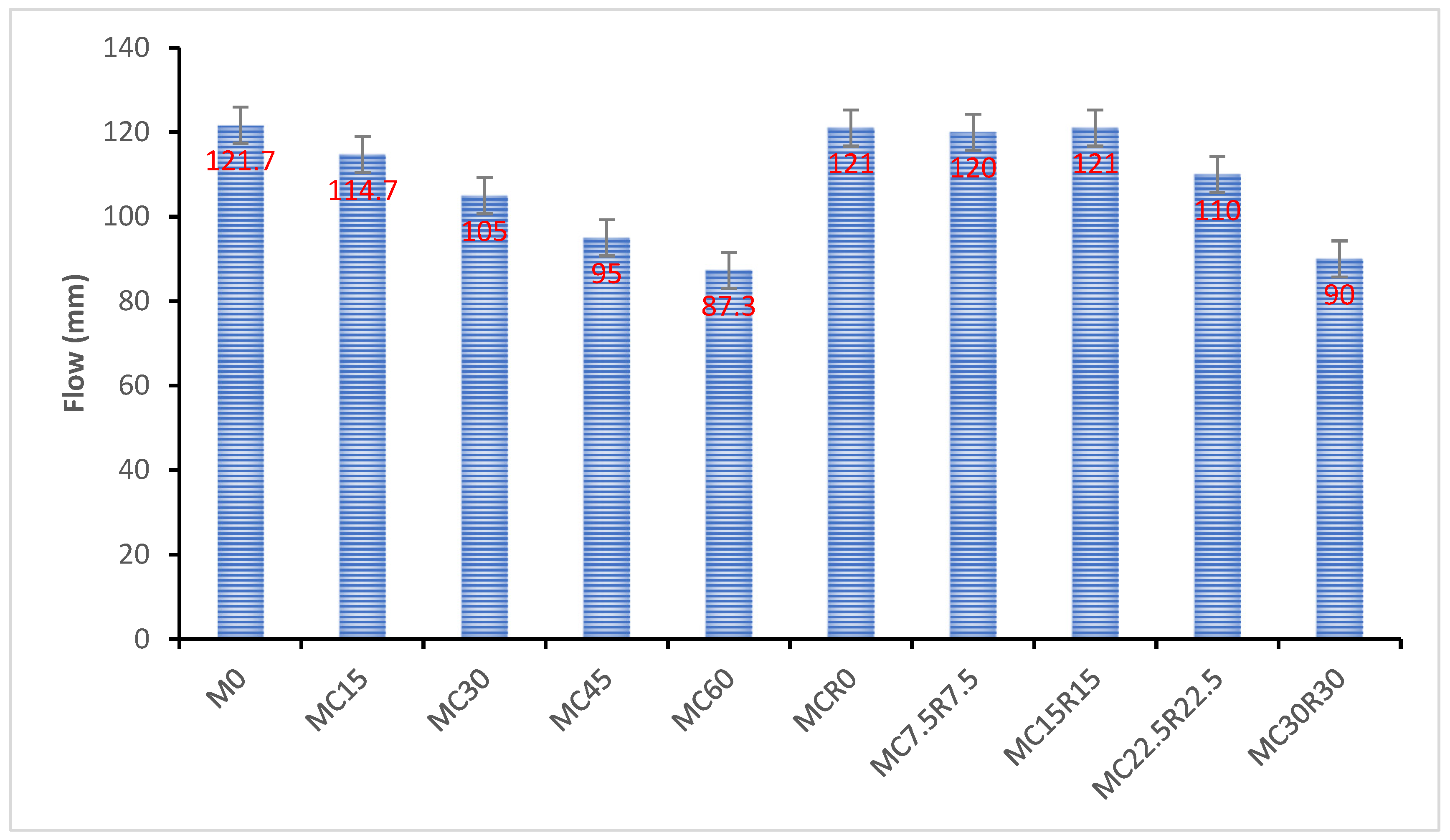
3.1. Flowability
3.2. Compressive Strength
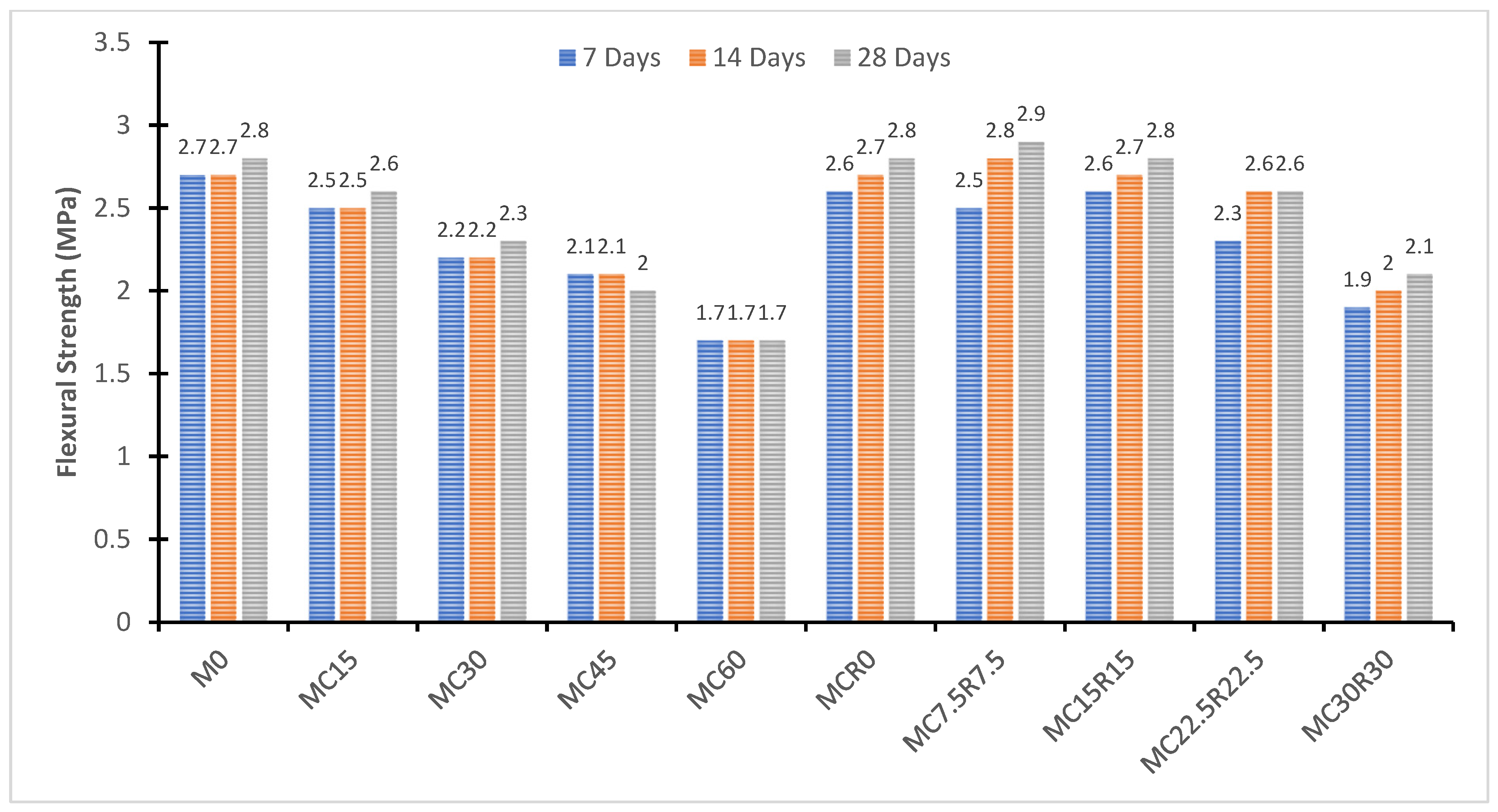
3.3. Flexure Strength
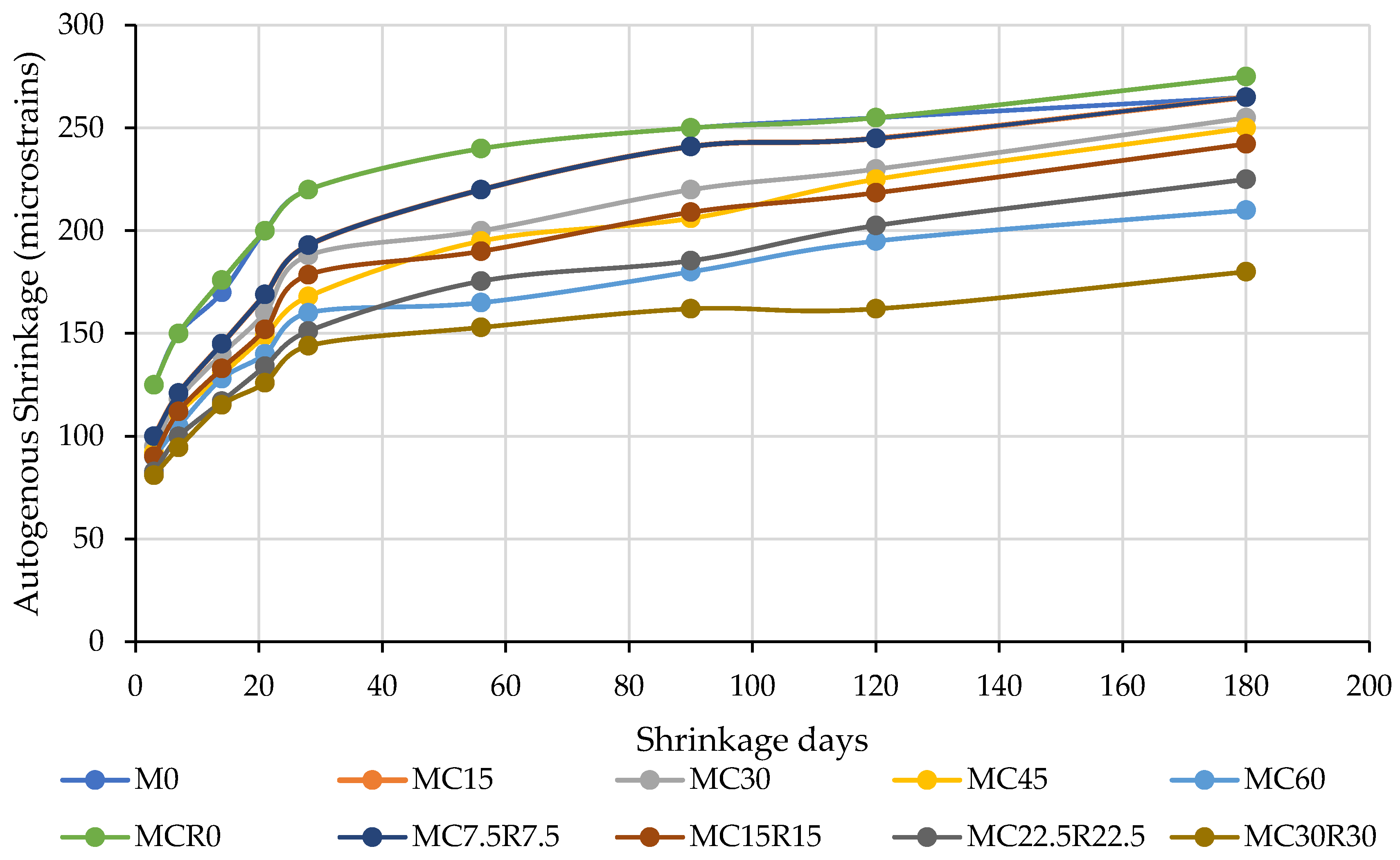
3.4. Drying Shrinkage
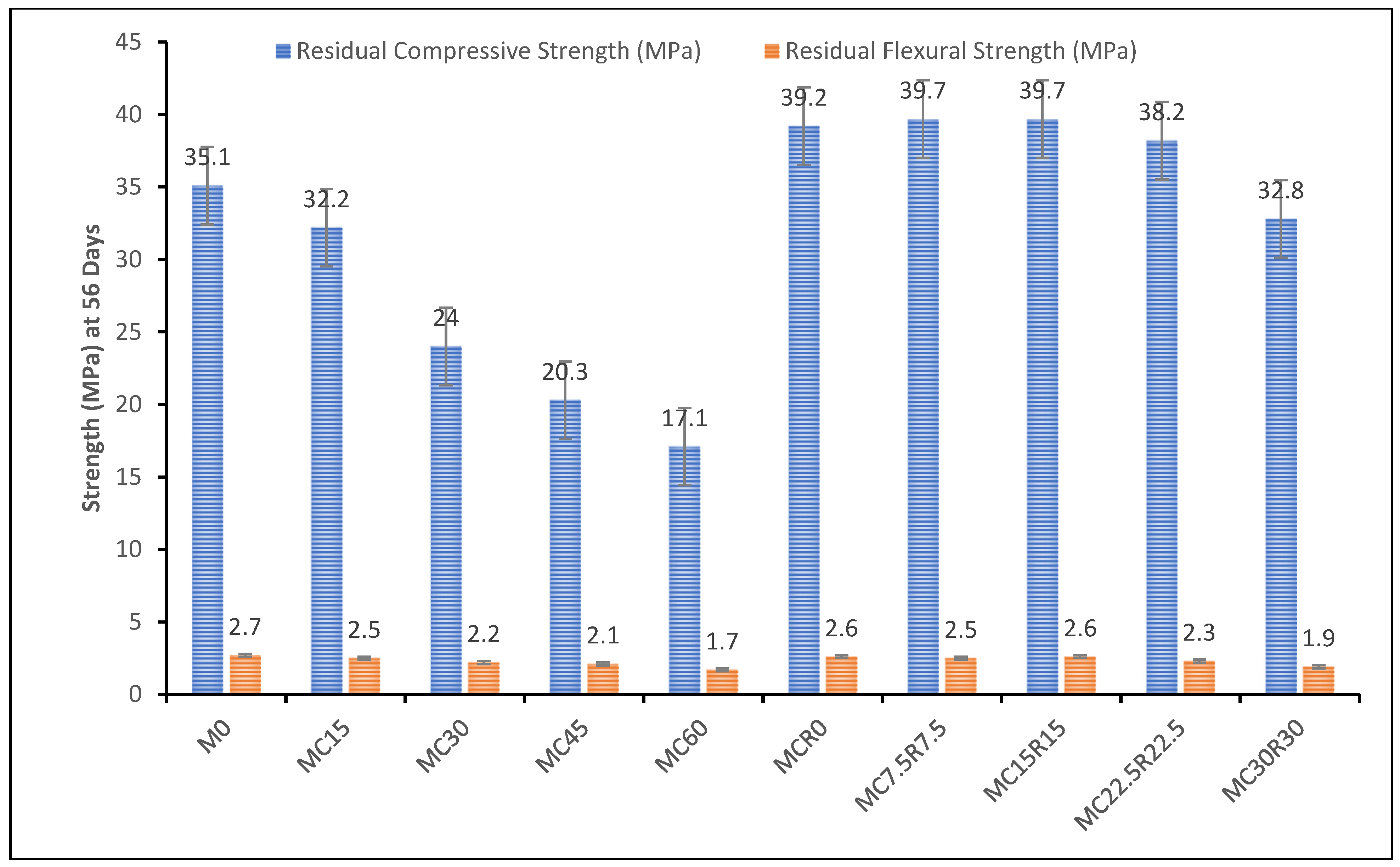
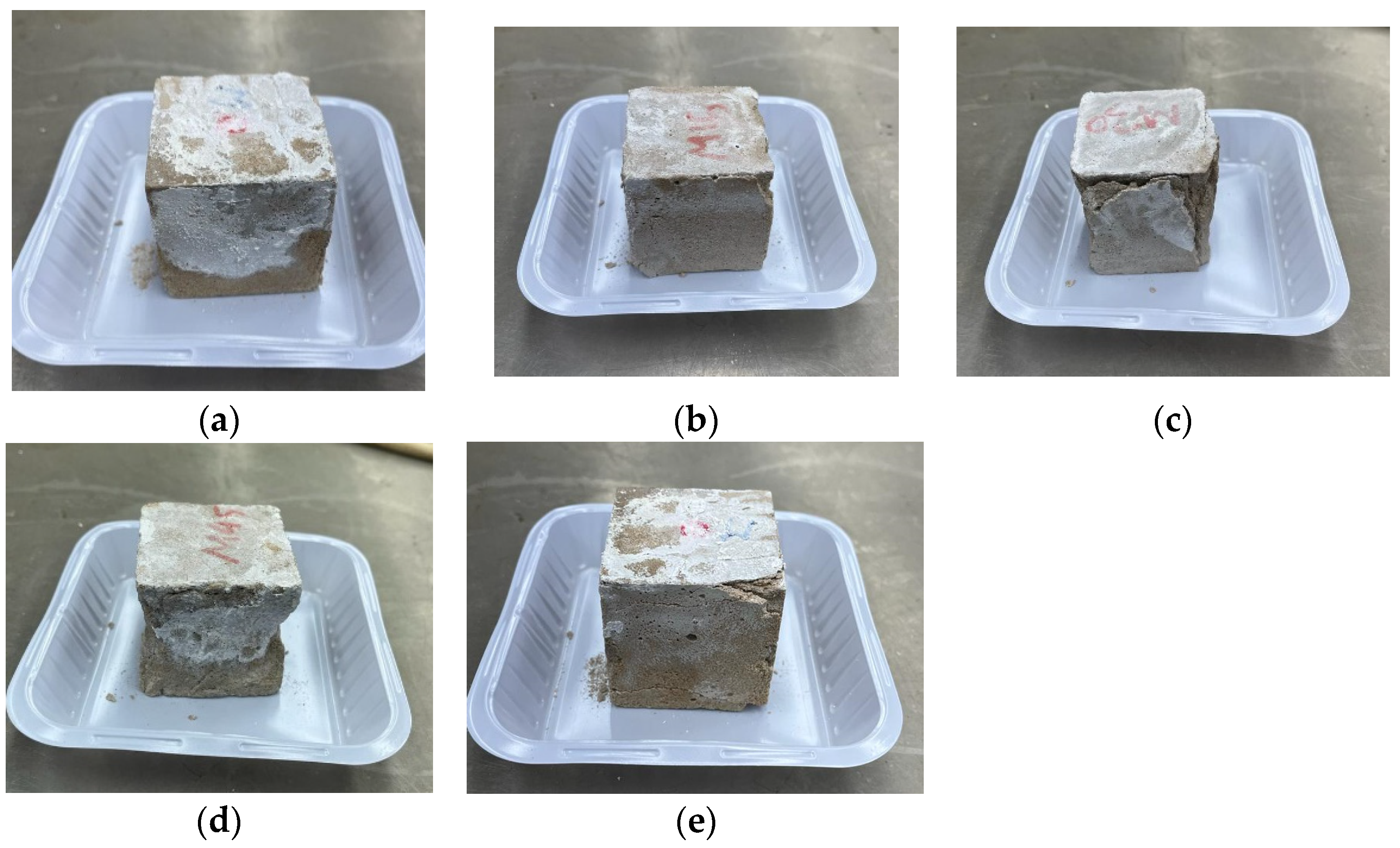
3.5. Resistance against Sulphuric Acid
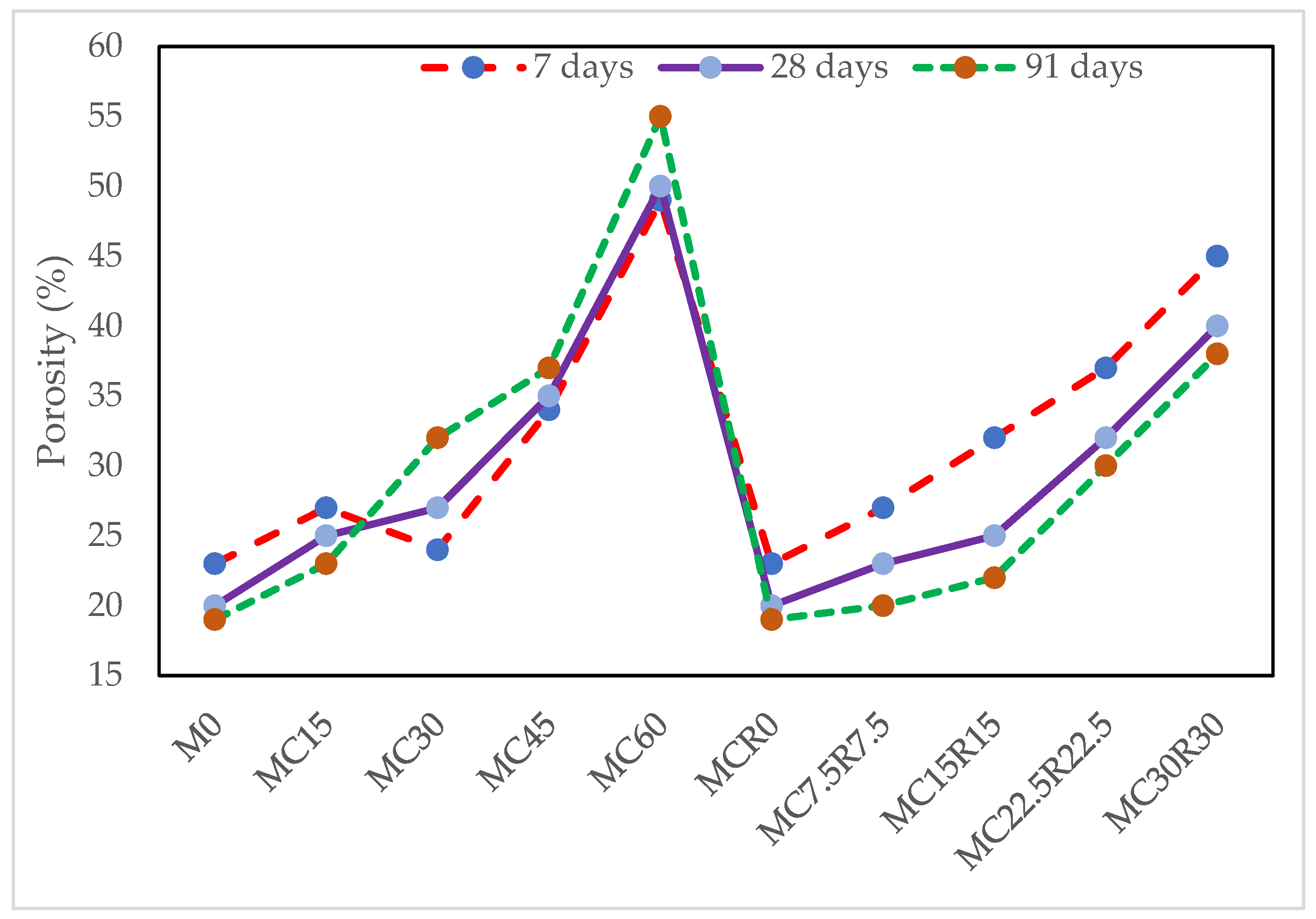
3.6. Porosity Test
3.7. Efflorescence
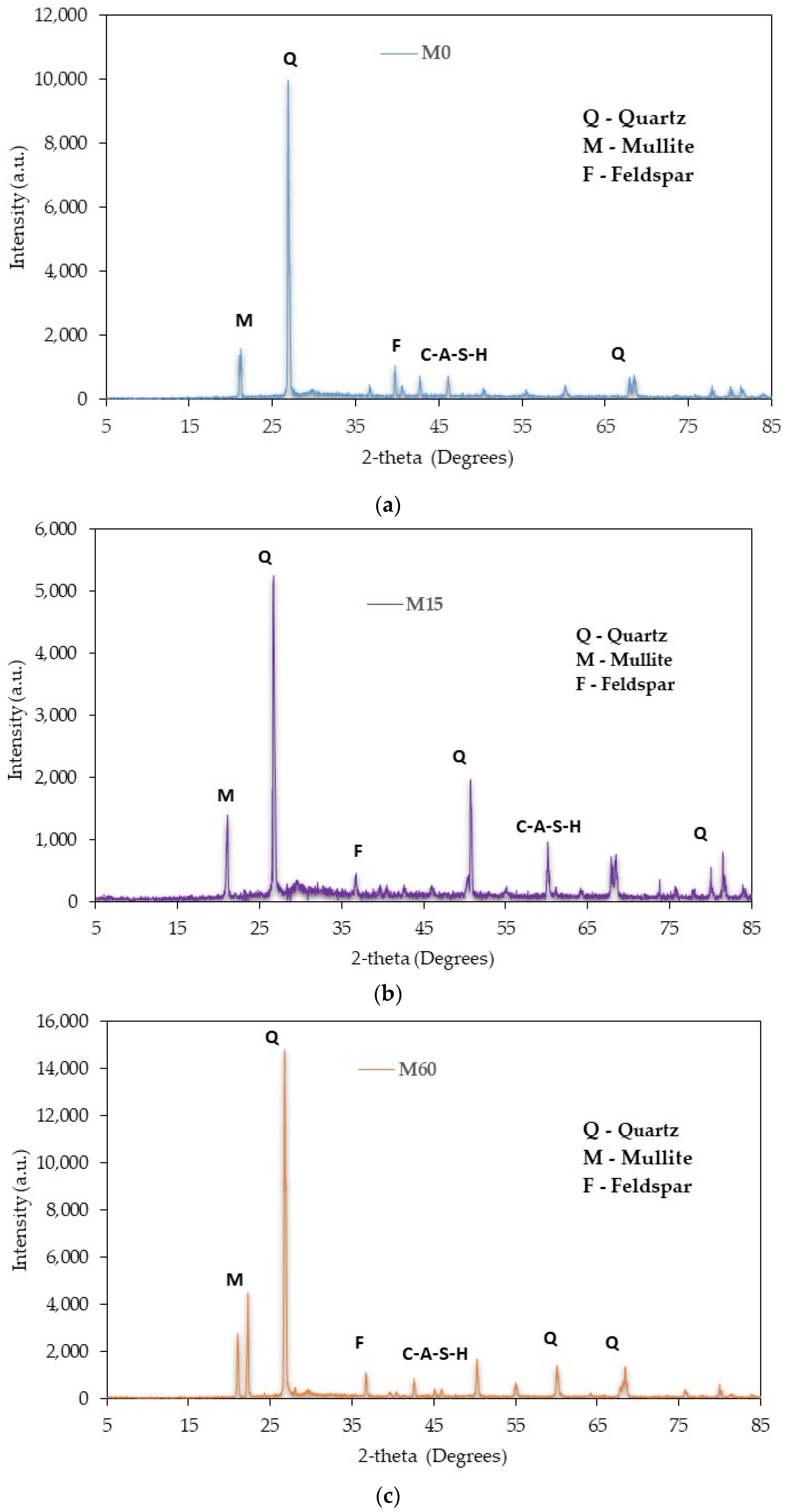
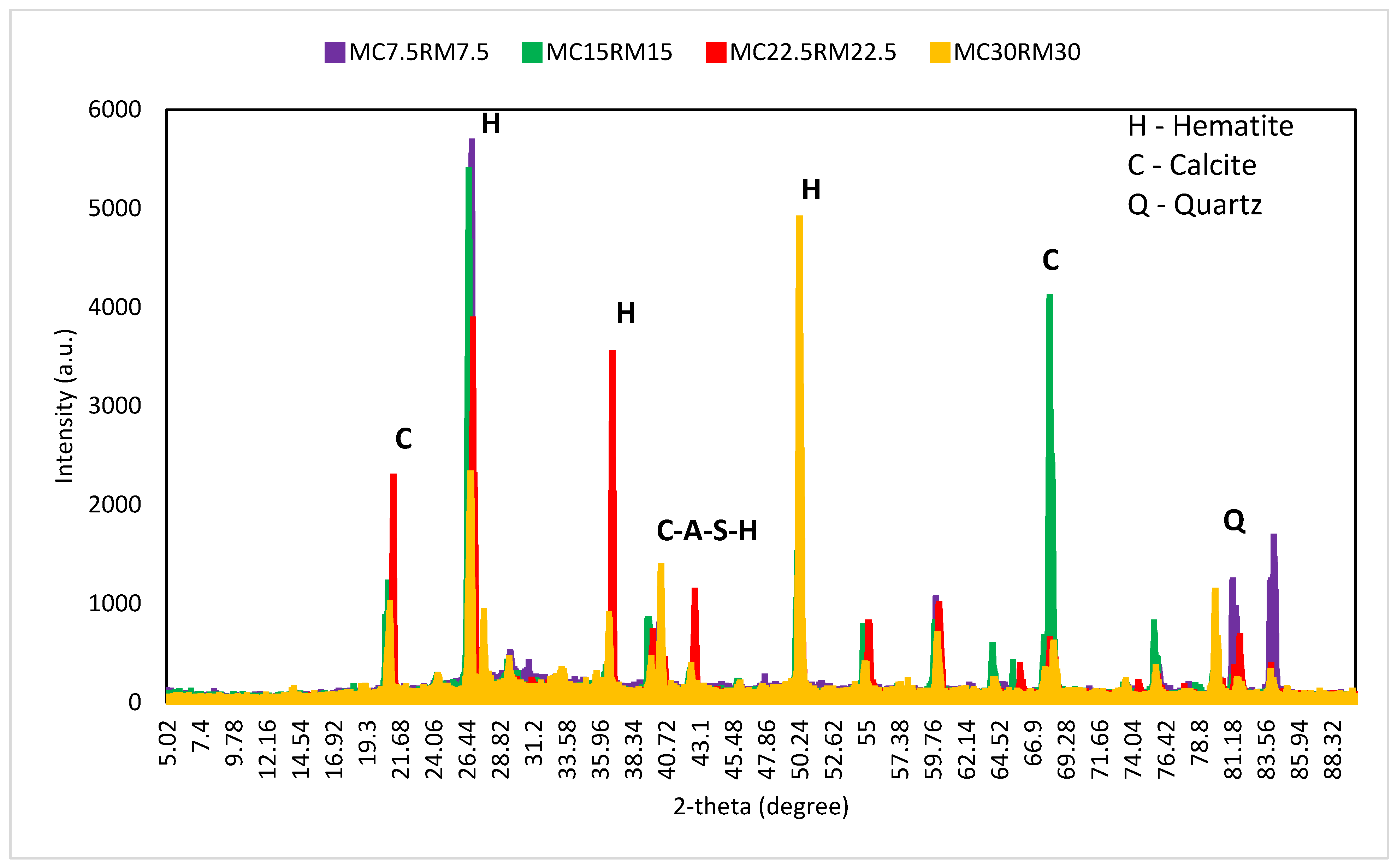
4. X-ray Dispersive (XRD) Analysis
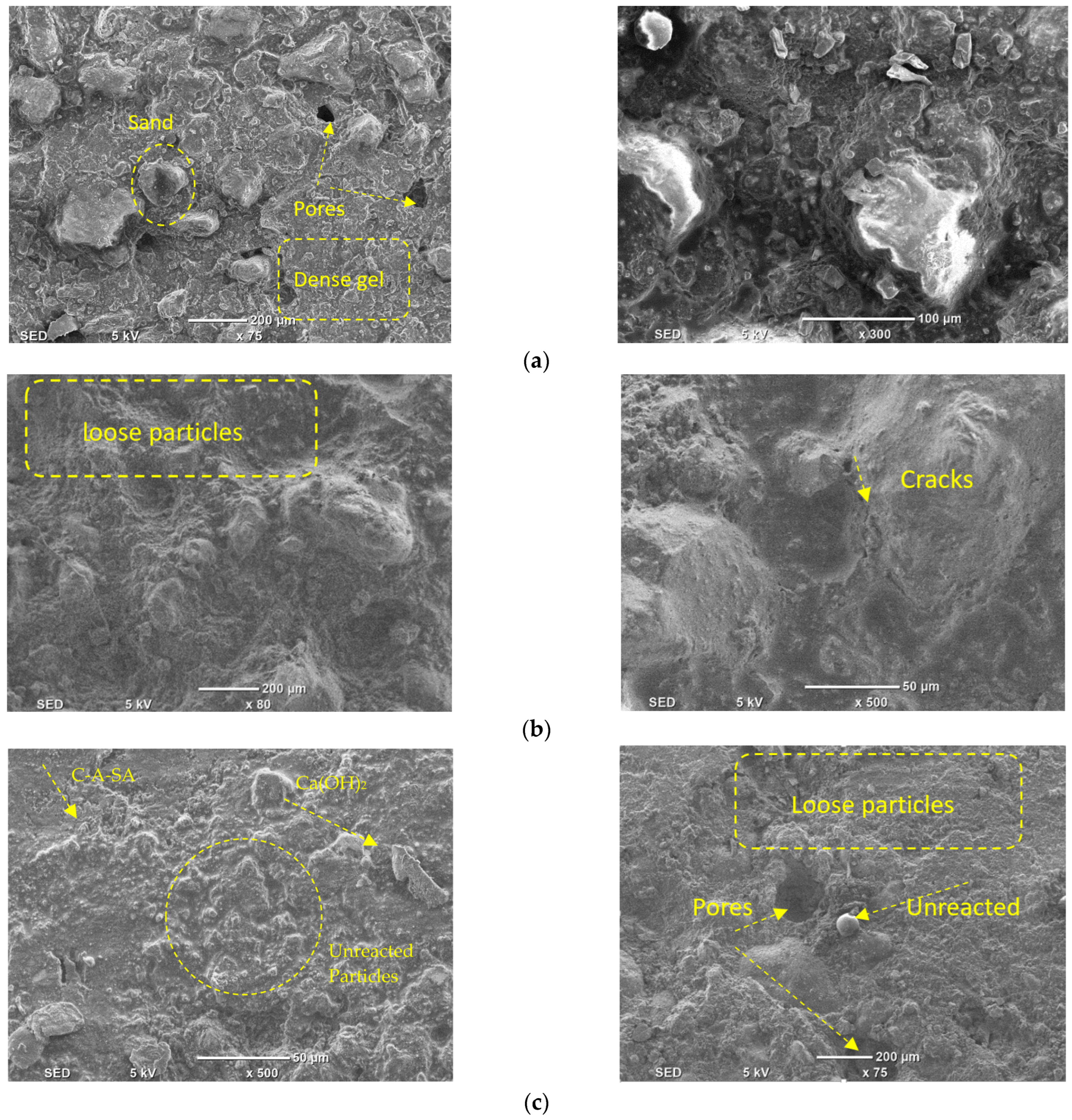
5. Scanning Electron Microscopy (SEM) Analysis
6. Sustainability Perspective of DCP
7. Conclusions
- As the amount of DCP increased, the mixes’ flowability decreased. With both DCP and RM, the flowability rose to a specific level and then decreased. This is due to the different chemical and physical reactions of raw ingredients.
- The mechanical strength of alkali-activated samples was deceased with a rise in the proportion of DCP utilized as a substitute for GBFS from 0% to 60%. The compressive strength was enhanced by 42.2% at 28 days when RM was added to DCP.
- At each level of DCP, the substitution of GBFS with DCP induced a loss in strength due to the detrimentally impacted formulation of calcium-aluminate-silicate-hydrate gel, which ultimately caused a drop in mechanical strength.
- The residual compressive and flexural strength displayed an improvement in the alkali-activated sample’s resistance against acid attack with an increase in DCP. This was prevented significantly when the sample had DCP and RM, with samples almost retaining their original strength values.
- The outcome of the XRD analysis showed that the formation of ettringites and gypsum was constrained by the rise in the amount of DCP as a partial substitute for GBFS in the mixture. Additionally, the development of crystalline phases was observed due to the addition of RM to DCP, which helped in the process of geo-polymerization.
- The study showed that DCP could be used up to a certain level to improve the durability of alkali-activated samples, as observed in the drying shrinkage test. The shrinkage was observed to decrease continuously with adding RM along with DCP. As the testing age increased, shrinkage decreased up to 180 days.
- It is recommended to utilize DCP at a low dosage because, as observed in the study, both the level of porosity and efflorescence increased with the rising level of DCP. Due to the addition of RM, the degree of efflorescence development was better controlled.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abadel, A.A.; Albidah, A.S.; Altheeb, A.H.; Alrshoudi, F.A.; Abbas, H.; Al-Salloum, Y.A. Effect of molar ratios on strength, microstructure & embodied energy of metakaolin geopolymer. Adv. Concr. Constr. 2021, 11, 127–140. [Google Scholar]
- Zaid, O.; Mukhtar, F.M.; M-García, R.; El Sherbiny, M.G.; Mohamed, A.M. Characteristics of high-performance steel fiber reinforced recycled aggregate concrete utilizing mineral filler. Case Stud. Constr. Mater. 2022, 16, e00939. [Google Scholar] [CrossRef]
- Zaid, O.; Aslam, F.; Alabduljabbar, H. To evaluate the performance of waste marble powder and wheat straw ash in steel fiber reinforced concrete. Struct. Concr. 2021, 23, 19–38. [Google Scholar] [CrossRef]
- Ibrahim, Y.E.; Adamu, M.; Marouf, M.L.; Ahmed, O.S.; Drmosh, Q.A.; Malik, M.A. Mechanical Performance of Date-Palm-Fiber-Reinforced Concrete Containing Silica Fume. Buildings 2022, 12, 1642. [Google Scholar] [CrossRef]
- Berndt, M.L. Properties of sustainable concrete containing fly ash, slag and recycled concrete aggregate. Constr. Build. Mater. 2009, 23, 2606–2613. [Google Scholar] [CrossRef]
- Munir, M.J.; Kazmi, S.M.S.; Wu, Y.-F. Efficiency of waste marble powder in controlling alkali–silica reaction of concrete: A sustainable approach. Constr. Build. Mater. 2017, 154, 590–599. [Google Scholar] [CrossRef]
- Sadek, D.M.; El-Attar, M.M.; Ali, H.A. Reusing of marble and granite powders in self-compacting concrete for sustainable development. J. Clean. Prod. 2016, 121, 19–32. [Google Scholar] [CrossRef]
- Khan, K.; Ahmad, W.; Amin, M.N.; Nazar, S. Nano-Silica-Modified Concrete: A Bibliographic Analysis and Comprehensive Review of Material Properties. Nanomaterials 2022, 12, 1989. [Google Scholar] [CrossRef]
- Huseien, G.F.; Shah, K.W. Durability and life cycle evaluation of self-compacting concrete containing fly ash as GBFS replacement with alkali activation. Constr. Build. Mater. 2020, 235, 117458. [Google Scholar] [CrossRef]
- Ahsan, M.B.; Hossain, Z. Supplemental use of rice husk ash (RHA) as a cementitious material in concrete industry. Constr. Build. Mater. 2018, 178, 1–9. [Google Scholar] [CrossRef]
- Punurai, W.; Kroehong, W.; Saptamongkol, A.; Chindaprasirt, P. Mechanical properties, microstructure and drying shrinkage of hybrid fly ash-basalt fiber geopolymer paste. Constr. Build. Mater. 2018, 186, 62–70. [Google Scholar] [CrossRef]
- Huseien, G.F.; Asaad, M.A.; Abadel, A.A.; Ghoshal, S.K.; Hamzah, H.K.; Benjeddou, O.; Mirza, J. Drying Shrinkage, Sulphuric Acid and Sulphate Resistance of High-Volume Palm Oil Fuel Ash-Included Alkali-Activated Mortars. Sustainability 2022, 14, 498. [Google Scholar] [CrossRef]
- Zeyad, A.M.; Megat Johari, M.A.; Abadel, A.; Abutaleb, A.; Mijarsh, M.J.A.; Almalki, A. Transport properties of palm oil fuel ash-based high-performance green concrete subjected to steam curing regimes. Case Stud. Constr. Mater. 2022, 16, e01077. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Zhao, P.; Lu, L.; Cheng, X. Effect of the optimized triple mixing method on the ITZ microstructure and performance of recycled aggregate concrete. Constr. Build. Mater. 2019, 203, 601–607. [Google Scholar] [CrossRef]
- Alharbi, Y.R.; Abadel, A.A. Engineering Properties of High-Volume Fly Ash Modified Cement Incorporated with Bottle Glass Waste Nanoparticles. Sustainability 2022, 14, 12459. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, Z.; Wang, J.; Guo, J.; Hu, S.; Ling, Y. Properties of fresh and hardened fly ash/slag based geopolymer concrete: A review. J. Clean. Prod. 2020, 270, 122389. [Google Scholar] [CrossRef]
- Lee, W.-H.; Wang, J.-H.; Ding, Y.-C.; Cheng, T.-W. A study on the characteristics and microstructures of GGBS/FA based geopolymer paste and concrete. Constr. Build. Mater. 2019, 211, 807–813. [Google Scholar] [CrossRef]
- Temuujin, J.; Minjigmaa, A.; Lee, M.; Chen-Tan, N.; van Riessen, A. Characterisation of class F fly ash geopolymer pastes immersed in acid and alkaline solutions. Cem. Concr. Compos.-Cem. Concr. Compos. 2011, 33, 1086–1091. [Google Scholar] [CrossRef]
- Zhang, P.; Han, S.; Golewski, G.L.; Wang, X. Nanoparticle-reinforced building materials with applications in civil engineering. Adv. Mech. Eng. 2014, 12, 1–4. [Google Scholar] [CrossRef]
- Kotop, M.A.; El-Feky, M.S.; Alharbi, Y.R.; Abadel, A.A.; Binyahya, A.S. Engineering properties of geopolymer concrete incorporating hybrid nano-materials. Ain Shams Eng. J. 2021, 12, 3641–3647. [Google Scholar] [CrossRef]
- Alharbi, Y.R.; Abadel, A.A.; Mayhoub, O.A.; Kohail, M. Effect of using available metakaoline and nano materials on the behavior of reactive powder concrete. Constr. Build. Mater. 2021, 269, 121344. [Google Scholar] [CrossRef]
- Ahmad, J.; Tufail, R.F.; Aslam, F.; Mosavi, A.; Alyousef, R.; Faisal Javed, M.; Zaid, O.; Khan Niazi, M.S. A Step towards Sustainable Self-Compacting Concrete by Using Partial Substitution of Wheat Straw Ash and Bentonite Clay Instead of Cement. Sustainability 2021, 13, 824. [Google Scholar] [CrossRef]
- Wongsa, A.; Zaetang, Y.; Sata, V.; Chindaprasirt, P. Properties of lightweight fly ash geopolymer concrete containing bottom ash as aggregates. Constr. Build. Mater. 2016, 111, 637–643. [Google Scholar] [CrossRef]
- Ariffin, M.A.M.; Bhutta, M.A.R.; Hussin, M.W.; Mohd Tahir, M.; Aziah, N. Sulfuric acid resistance of blended ash geopolymer concrete. Constr. Build. Mater. 2013, 43, 80–86. [Google Scholar] [CrossRef]
- Ramyar, K. Comparison of fly ash, silica fume and metakaolin from mechanical properties and durability performance of mortar mixtures view point. Constr. Build. Mater. 2014, 70, 17–25. [Google Scholar]
- Ribeiro, M.C.S.; Fiúza, A.; Castro, A.C.M.; Silva, F.G.; Dinis, M.L.; Meixedo, J.P.; Alvim, M.R. Mix design process of polyester polymer mortars modified with recycled GFRP waste materials. Compos. Struct. 2013, 105, 300–310. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Composition and microstructure of alkali activated fly ash binder: Effect of the activator. Cem. Concr. Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- Velandia, D.F.; Lynsdale, C.J.; Provis, J.L.; Ramirez, F. Effect of mix design inputs, curing and compressive strength on the durability of Na2SO4-activated high volume fly ash concretes. Cem. Concr. Compos. 2018, 91, 11–20. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Z.; Liu, B.; Zhao, F.; Tang, S.; Jin, M. Effects of Fly Ash Dosage on Shrinkage, Crack Resistance and Fractal Characteristics of Face Slab Concrete. Fractal Fract. 2022, 6, 335. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, S.; Shi, Y.; Huang, Y.; Zhao, F.; Huo, T.; Tang, S. The Influence of Fly Ash Dosages on the Permeability, Pore Structure and Fractal Features of Face Slab Concrete. Fractal Fract. 2022, 6, 476. [Google Scholar] [CrossRef]
- Ali, B.; Raza, S.; Kurda, R.; Alyousef, R. Synergistic effects of fly ash and hooked steel fibers on strength and durability properties of high strength recycled aggregate concrete. Resour. Conserv. Recycl. 2021, 168, 105444. [Google Scholar] [CrossRef]
- Kumar, A.; Muthukannan, M.; Babu, A.; Hariharan, A.; Muthuramalingam, T. Effect on addition of Polypropylene fibers in wood ash-fly ash based geopolymer concrete. IOP Conf. Ser. Mater. Sci. Eng. 2020, 872, 12162. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Abo-El-Enein, S.A. A novel method to produce dry geopolymer cement powder. HBRC J. 2016, 12, 13–24. [Google Scholar] [CrossRef]
- Garau, G.; Silvetti, M.; Deiana, S.; Deiana, P.; Castaldi, P. Long-term influence of red mud on As mobility and soil physico-chemical and microbial parameters in a polluted sub-acidic soil. J. Hazard. Mater. 2011, 185, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liang, S.; Yang, J.; Chen, Y.; Ye, N.; Ke, Y.; Tao, S.; Xiao, K.; Hu, J.; Hou, H.; et al. Role of Fe species in geopolymer synthesized from alkali-thermal pretreated Fe-rich Bayer red mud. Constr. Build. Mater. 2019, 200, 398–407. [Google Scholar] [CrossRef]
- He, X.; Yuhua, Z.; Qaidi, S.; Isleem, H.F.; Zaid, O.; Althoey, F.; Ahmad, J. Mine tailings-based geopolymers: A comprehensive review. Ceram. Int. 2022, 48, 24192–24212. [Google Scholar] [CrossRef]
- Qaidi, S.M.A.; Mohammed, A.S.; Ahmed, H.U.; Faraj, R.H.; Emad, W.; Tayeh, B.A.; Althoey, F.; Zaid, O.; Sor, N.H. Rubberized geopolymer composites: A comprehensive review. Ceram. Int. 2022, 48, 24234–24259. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Lu, G.; Liu, Y.; Zhang, W.; Zhao, Q. Comprehensive Utilization of Red Mud: Current Research Status and a Possible Way Forward for Non-hazardous Treatment. In Proceedings of the Light Metals 2018; Martin, O., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 135–141. [Google Scholar]
- Maglad, A.M.; Zaid, O.; Arbili, M.M.; Ascensão, G.; Șerbănoiu, A.A.; Grădinaru, C.M.; García, R.M.; Qaidi, S.M.A.; Althoey, F.; de Prado-Gil, J. A Study on the Properties of Geopolymer Concrete Modified with Nano Graphene Oxide. Buildings 2022, 12, 1066. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, N. Utilization of red mud in cement production: A review. Waste Manag. Res. 2011, 29, 1053–1063. [Google Scholar] [CrossRef]
- Gomes, H.I.; Mayes, W.M.; Rogerson, M.; Stewart, D.I.; Burked, I.T. Alkaline residues and the environment: A review of impacts, management practices and opportunities. J. Clean. Prod. 2016, 112, 3571–3582. [Google Scholar] [CrossRef]
- Zaid, O.; Hashmi, S.R.Z.; Aslam, F.; Abedin, Z.U.; Ullah, A. Experimental study on the properties improvement of hybrid graphene oxide fiber-reinforced composite concrete. Diam. Relat. Mater. 2022, 124, 108883. [Google Scholar] [CrossRef]
- Chu, S.H.; Poon, C.S.; Lam, C.S.; Li, L. Effect of natural and recycled aggregate packing on properties of concrete blocks. Constr. Build. Mater. 2021, 278, 122247. [Google Scholar] [CrossRef]
- Wang, B.; Yan, L.; Fu, Q.; Kasal, B. A Comprehensive Review on Recycled Aggregate and Recycled Aggregate Concrete. Resour. Conserv. Recycl. 2021, 171, 105565. [Google Scholar] [CrossRef]
- Li, Z.; Ding, Z.; Zhang, Y. Development of sustainable cementitious materials. In Proceedings of the International Workshop on Sustainable Development and Concrete Technology, Beijing, China, 20 May 2004. [Google Scholar]
- Chidiac, S.E.; Panesar, D. Evolution of mechanical properties of concrete containing ground granulated blast furnace slag and effects on the scaling resistance test at 28days. Cem. Concr. Compos. 2008, 30, 63–71. [Google Scholar] [CrossRef]
- Qian, D.; Yu, R.; Shui, Z.; Sun, Y.; Jiang, C.; Zhou, F.; Ding, M.; Tong, X.; He, Y. A novel development of green ultra-high performance concrete (UHPC) based on appropriate application of recycled cementitious material. J. Clean. Prod. 2020, 261, 121231. [Google Scholar] [CrossRef]
- ASTM C 109; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). ASTM Int.: West Conshohocken, PA, USA, 2007.
- ASTM C 579; Standard Test Methods for Compressive Strength of Chemical-Resistant Mortars, Grouts, Monolithic Surfacings, and Polymer Concretes. ASTM Int.: West Conshohocken, PA, USA, 2018.
- ASTM C78-10; Test Method Flexural Strength Concr. (Using Simple Beam with Third-Point Load. ASTM Int.: West Conshohocken, PA, USA, 2010.
- ASTM C 1437; Standard Test Method For Flow Of Hydraulic Cement Mortar. ASTM Int.: West Conshohocken, PA, USA, 2020.
- ASTM C 267; Standard Test Methods for Chemical Resistance of Mortars, Grouts, and Monolithic Surfacing and Polymer Concretes. ASTM Int.: West Conshohocken, PA, USA, 2012.
- ASTM C 157/C 157M-08; Standard Test Method for Length Change of Hardened Hydraulic-Cement Mortar and Concrete. ASTM Int.: West Conshohocken, PA, USA, 2014.
- ASTM C 830; Standard Test Methods for Apparent Porosity. Liquid Absorption: ASTM Int.: West Conshohocken, PA, USA, 2016.
- ASTM C 642; Standard Test Method for Specific Gravity, Absorption and Voids in Hardened Concrete. Annual Book of ASTM Standards: West Conshohocken, PA, USA, 1997.
- Huseien, G.; Sam, A.R.M.; Kwok Wei, S.; Mirza, J. Effects of ceramic tile powder waste on properties of self-compacted alkali-activated concrete. Constr. Build. Mater. 2020, 236, 117574. [Google Scholar] [CrossRef]
- Al-Majidi, M.H.; Lampropoulos, A.; Cundy, A.; Meikle, S. Development of geopolymer mortar under ambient temperature for in situ applications. Constr. Build. Mater. 2016, 120, 198–211. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Criado, M.; Fernández-Jiménez, A.; Palomo, A. Alkali activation of fly ash: Effect of the SiO2/Na2O ratioPart I: FTIR study. Microporous Mesoporous Mater. 2007, 106, 180–191. [Google Scholar] [CrossRef]
- Tang, W.C.; Wang, Z.; Liu, Y.; Cui, H.Z. Influence of red mud on fresh and hardened properties of self-compacting concrete. Constr. Build. Mater. 2018, 178, 288–300. [Google Scholar] [CrossRef]
- Hou, D.; Ma, H.; Li, Z. Morphology of calcium silicate hydrate (C-S-H) gel: A molecular dynamic study. Adv. Cem. Res. 2015, 27, 135–146. [Google Scholar] [CrossRef]
- Alves, L.; Leklou, N.; de Barros, S. A comparative study on the effect of different activating solutions and formulations on the early stage geopolymerization process. MATEC Web Conf. 2020, 322, 1039. [Google Scholar] [CrossRef]
- Hojati, M.; Radlińska, A. Shrinkage and strength development of alkali-activated fly ash-slag binary cements. Constr. Build. Mater. 2017, 150, 808–816. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, H.; Zhang, Z. Influence of fly ash on the pore structure and shrinkage characteristics of metakaolin-based geopolymer pastes and mortars. Constr. Build. Mater. 2017, 153, 284–293. [Google Scholar] [CrossRef]
- Hooton, D.; Stanish, K.; Prusinski, J. The Effect of Ground, Granulated Blast Furnace Slag (Slag Cement) on the Drying Shrinkage of Concrete-A Critical Review of the Literature. Am. Concr. Inst. ACI Spec. Publ. 2004, 263, 79–94. [Google Scholar]
- Anastasiou, E.; Georgiadis Filikas, K.; Stefanidou, M. Utilization of fine recycled aggregates in concrete with fly ash and steel slag. Constr. Build. Mater. 2014, 50, 154–161. [Google Scholar] [CrossRef]
- Ge, X.; Hu, X.; Shi, C. Mechanical properties and microstructure of circulating fluidized bed fly ash and red mud-based geopolymer. Constr. Build. Mater. 2022, 340, 127599. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Fly ash-based geopolymers: The relationship between composition, pore structure and efflorescence. Cem. Concr. Res. 2014, 64, 30–41. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Hyeok-Jung, K.; Kang, S.-P.; Choe, G.-C. Effect of Red Mud Content on Strength and Efflorescence in Pavement using Alkali-Activated Slag Cement. Int. J. Concr. Struct. Mater. 2018, 12, 18. [Google Scholar] [CrossRef]
- Kang, S.-P.; Kwon, S.-J. Effects of red mud and Alkali-Activated Slag Cement on efflorescence in cement mortar. Constr. Build. Mater. 2017, 133, 459–467. [Google Scholar] [CrossRef]
- Brough, A.R.; Atkinson, A. Sodium silicate-based, alkali-activated slag mortars: Part I. Strength, hydration and microstructure. Cem. Concr. Res. 2002, 32, 865–879. [Google Scholar] [CrossRef]
- He, J.; Zhang, J.; Yu, Y.; Zhang, G. The strength and microstructure of two geopolymers derived from metakaolin and red mud-fly ash admixture: A comparative study. Constr. Build. Mater. 2012, 30, 80–91. [Google Scholar] [CrossRef]
- Chi, M.; Huang, R. Binding mechanism and properties of alkali-activated fly ash/slag mortars. Constr. Build. Mater. 2013, 40, 291–298. [Google Scholar] [CrossRef]
- Mohseni, E. Assessment of Na2SiO3 to NaOH ratio impact on the performance of polypropylene fiber-reinforced geopolymer composites. Constr. Build. Mater. 2018, 186, 904–911. [Google Scholar] [CrossRef]
- Zaid, O.; Ahmad, J.; Siddique, M.S.; Aslam, F.; Alabduljabbar, H.; Khedher, K.M. A step towards sustainable glass fiber reinforced concrete utilizing silica fume and waste coconut shell aggregate. Sci. Rep. 2021, 11, 12822. [Google Scholar] [CrossRef] [PubMed]



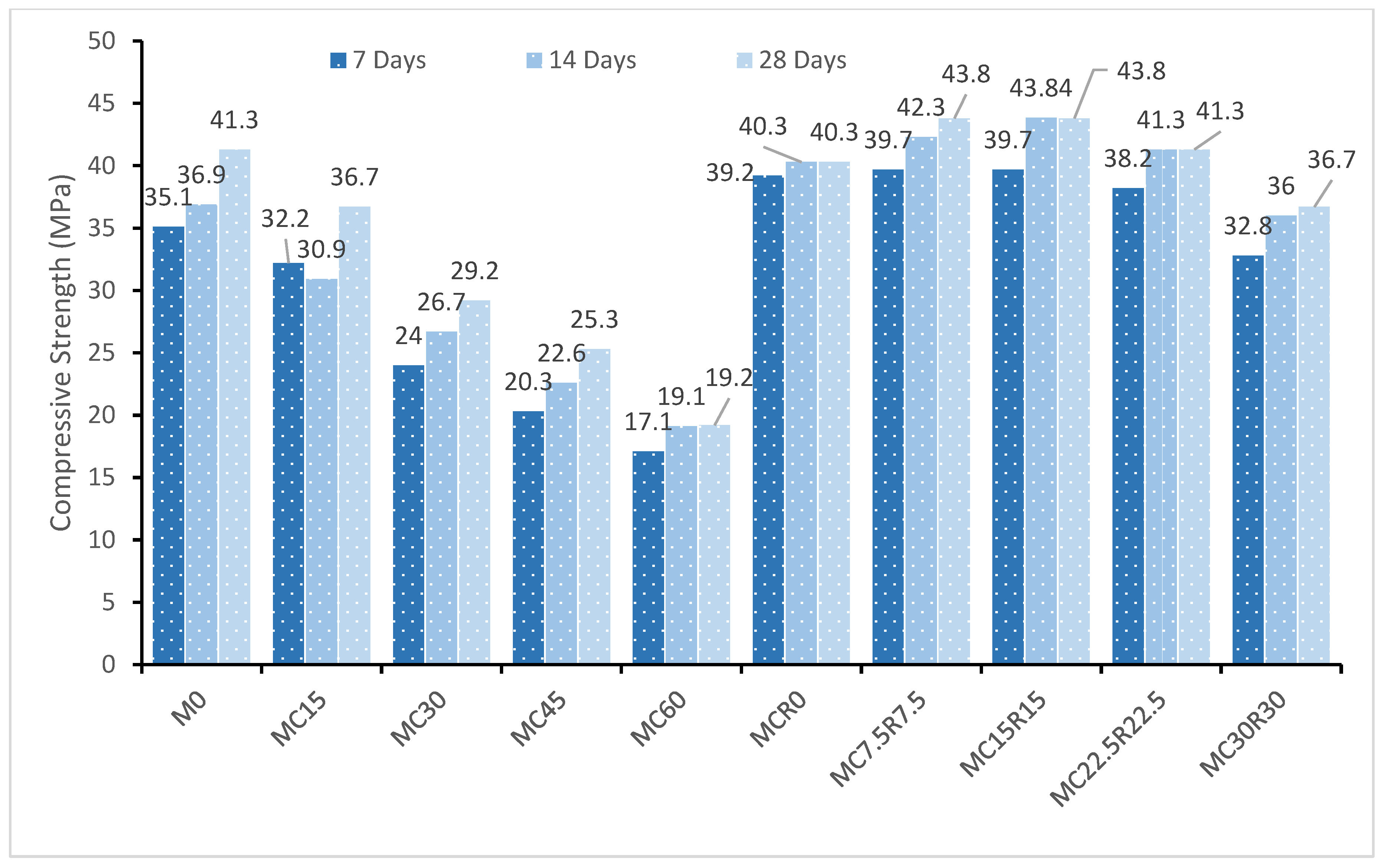


| Oxide Composition | RM (%) | GBFS (%) | DCP (%) |
|---|---|---|---|
| Na2O | 3.87 | 0.23 | 0.14 |
| CaO | 11.5 | 46.1 | 52.2 |
| SiO2 | 19.6 | 30.6 | 19.9 |
| MgO | 0.28 | 5.08 | 2.91 |
| Al2O2 | 14.5 | 13.67 | 5.0 |
| Fe2O3 | 29.5 | 0.33 | 4.125 |
| SO3 | 2.27 | 1.35 | 2.93 |
| K2O | - | 0.36 | 0.78 |
| TiO2 | 12.3 | 0.4 | 0.36 |
| MnO | - | 1.69 | - |
| Total | 93.82 | 99.71 | 88.34 |
| Ignition Loss | 6.18 | 0.29 | 11.66 |
| Mix ID | GBFS (g) | CW Substitution (g) | RM Substitution (g) | Water | NaOH | Sodium Silicate | Sand (g) |
|---|---|---|---|---|---|---|---|
| M0 | 1000 | 0 | 0 | 395 | 36.01 | 253.0 | 2256 |
| MC15 | 850 | 150 | - | 395 | 36.01 | 253.0 | 2280 |
| MC30 | 700 | 300 | - | 395 | 36.01 | 253.0 | 2303 |
| MC45 | 550 | 450 | - | 395 | 36.01 | 253.0 | 2326 |
| MC60 | 400 | 600 | - | 395 | 36.01 | 253.0 | 2326 |
| MCR0 | 1000 | 0 | 0 | 395 | 36.01 | 253.0 | 2256 |
| MC7.5R7.5 | 850 | 75 | 75 | 395 | 36.01 | 253.0 | 2280 |
| MC15R15 | 700 | 150 | 150 | 395 | 36.01 | 253.0 | 2303 |
| MC22.5R22.5 | 550 | 225 | 225 | 395 | 36.01 | 253.0 | 2326 |
| MC30R30 | 400 | 300 | 300 | 395 | 36.01 | 253.0 | 2326 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abadel, A.A.; Alghamdi, H.; Alharbi, Y.R.; Alamri, M.; Khawaji, M.; Abdulaziz, M.A.M.; Nehdi, M.L. Investigation of Alkali-Activated Slag-Based Composite Incorporating Dehydrated Cement Powder and Red Mud. Materials 2023, 16, 1551. https://doi.org/10.3390/ma16041551
Abadel AA, Alghamdi H, Alharbi YR, Alamri M, Khawaji M, Abdulaziz MAM, Nehdi ML. Investigation of Alkali-Activated Slag-Based Composite Incorporating Dehydrated Cement Powder and Red Mud. Materials. 2023; 16(4):1551. https://doi.org/10.3390/ma16041551
Chicago/Turabian StyleAbadel, Aref A., Hussam Alghamdi, Yousef R. Alharbi, Mohammed Alamri, Mohammad Khawaji, Mohammed A. M. Abdulaziz, and Moncef L. Nehdi. 2023. "Investigation of Alkali-Activated Slag-Based Composite Incorporating Dehydrated Cement Powder and Red Mud" Materials 16, no. 4: 1551. https://doi.org/10.3390/ma16041551
APA StyleAbadel, A. A., Alghamdi, H., Alharbi, Y. R., Alamri, M., Khawaji, M., Abdulaziz, M. A. M., & Nehdi, M. L. (2023). Investigation of Alkali-Activated Slag-Based Composite Incorporating Dehydrated Cement Powder and Red Mud. Materials, 16(4), 1551. https://doi.org/10.3390/ma16041551







