Synthesis and Properties of Biodegradable Hydrogel Based on Polysaccharide Wound Dressing
Abstract
:1. Introduction
2. Materials and Methods
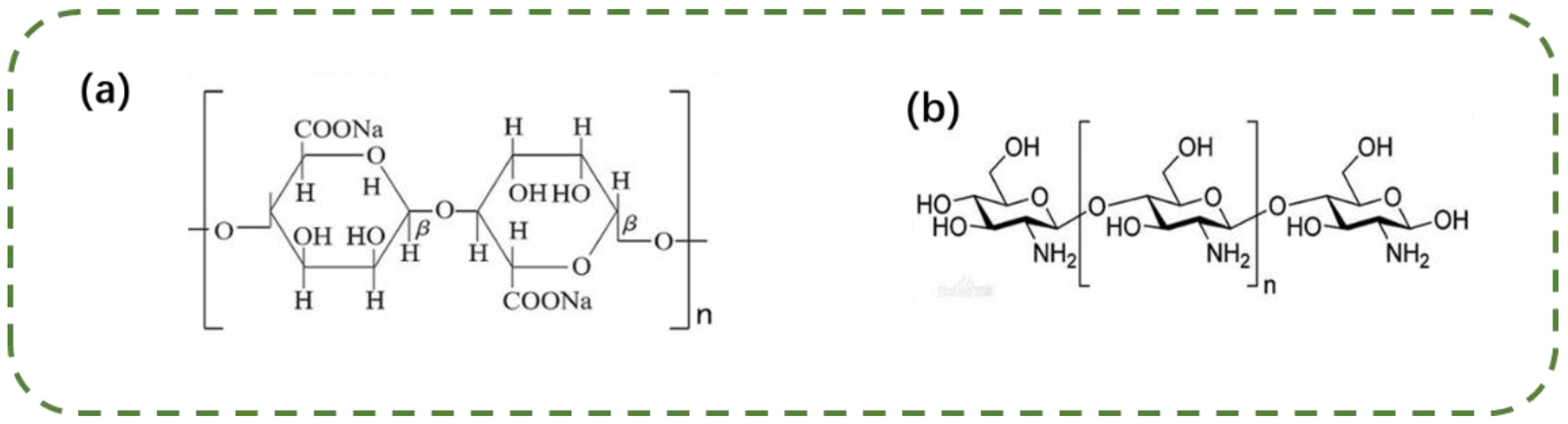
2.1. Materials and Reagents
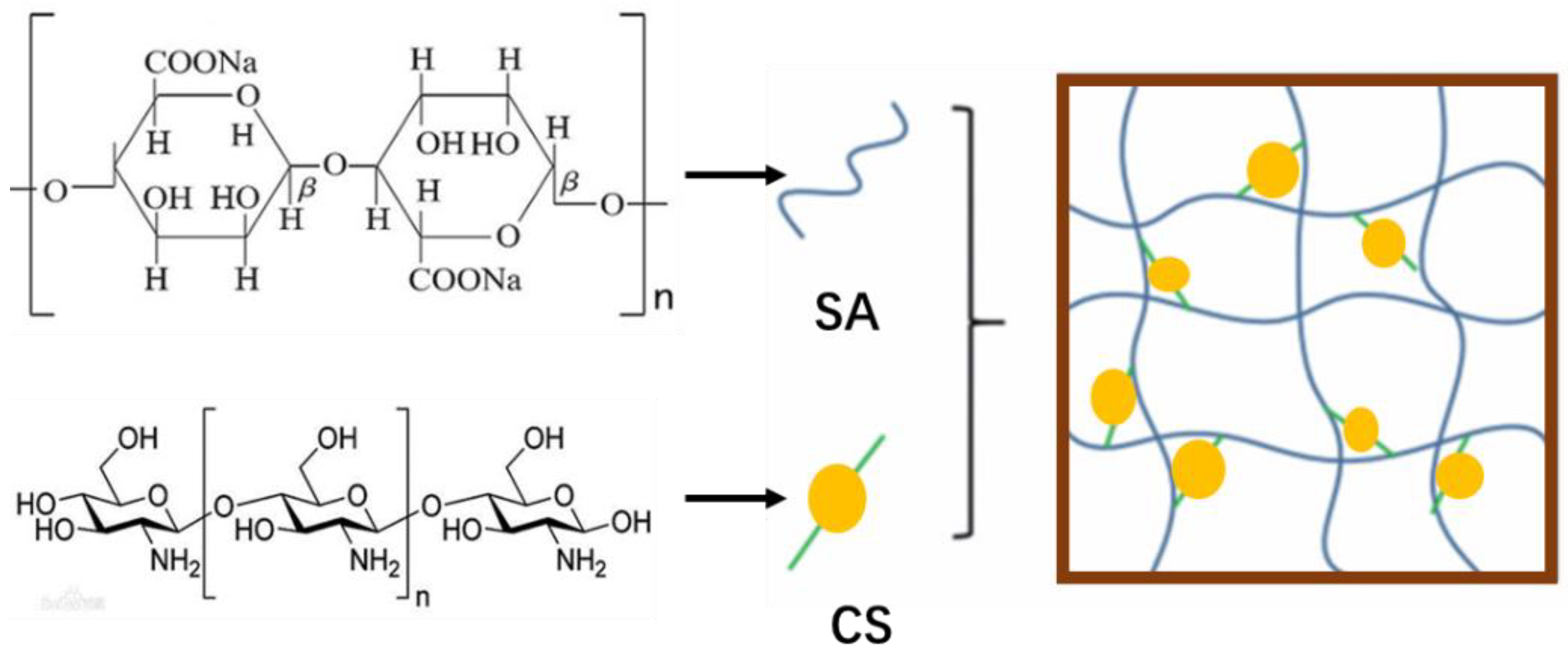
2.2. Methods
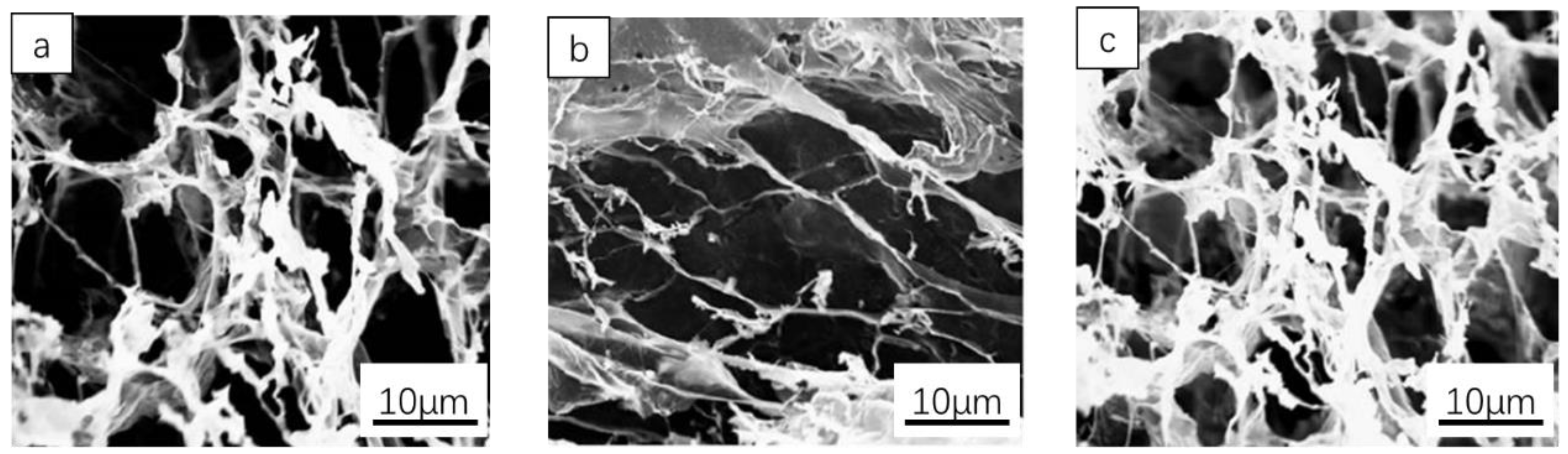
3. Results and Discussion
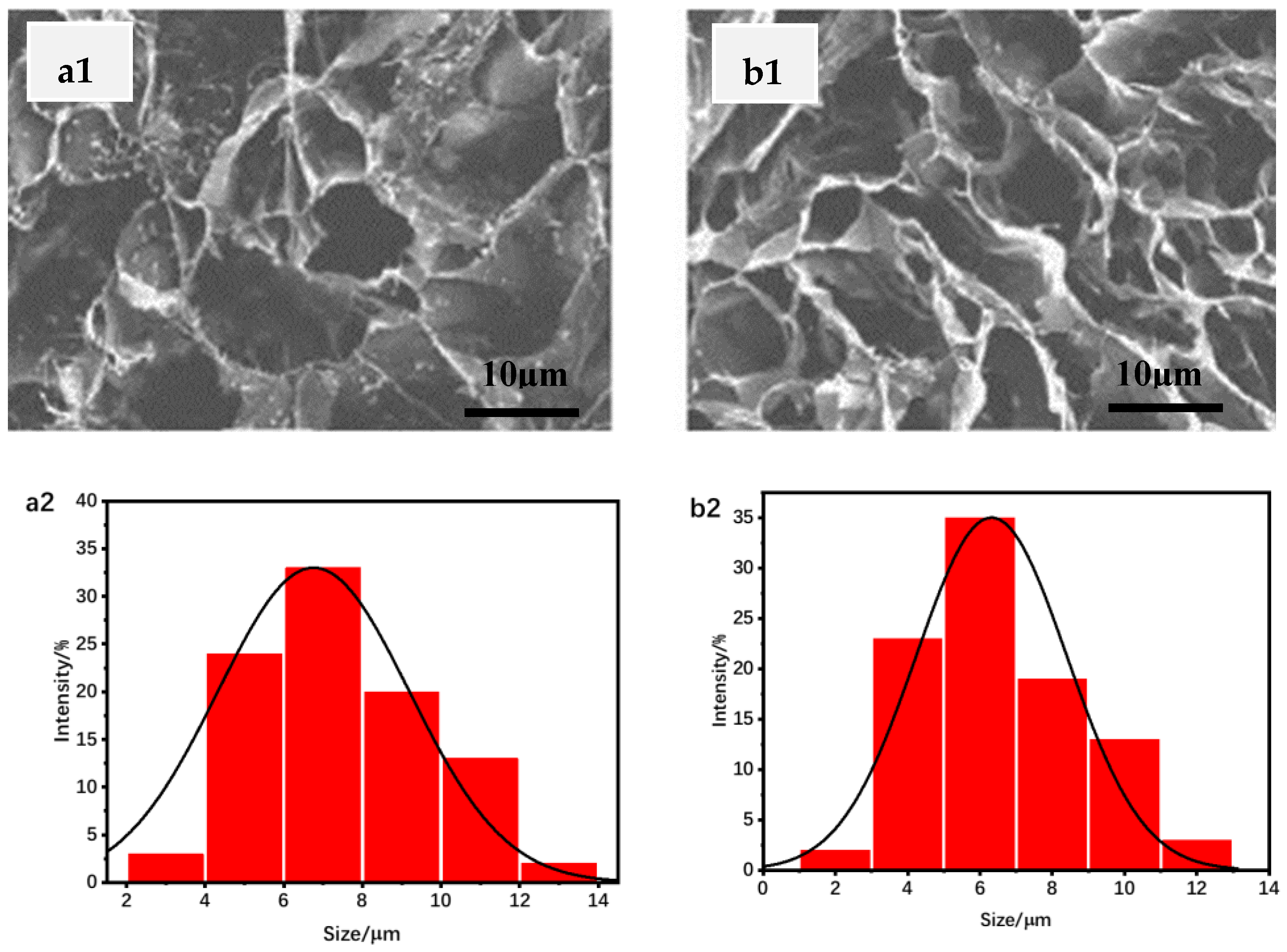
3.1. Swelling Degree Analysis
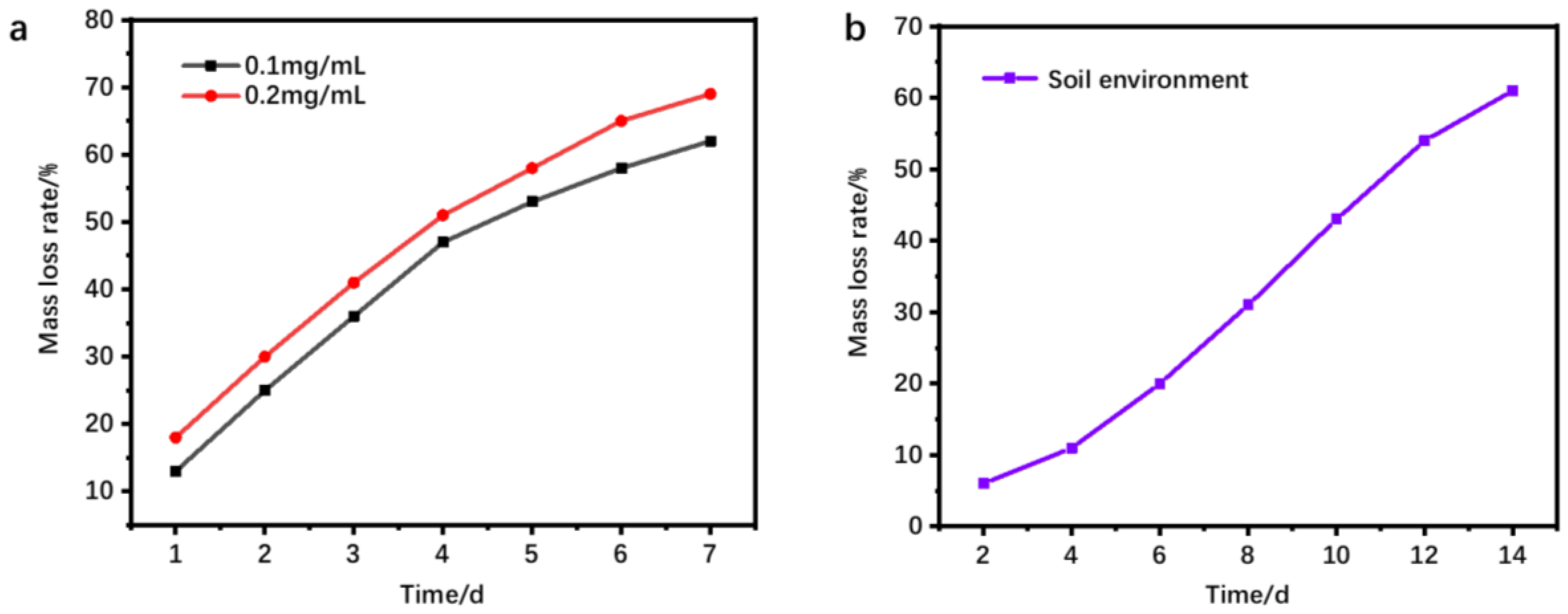
3.2. Hydrogel Degradability
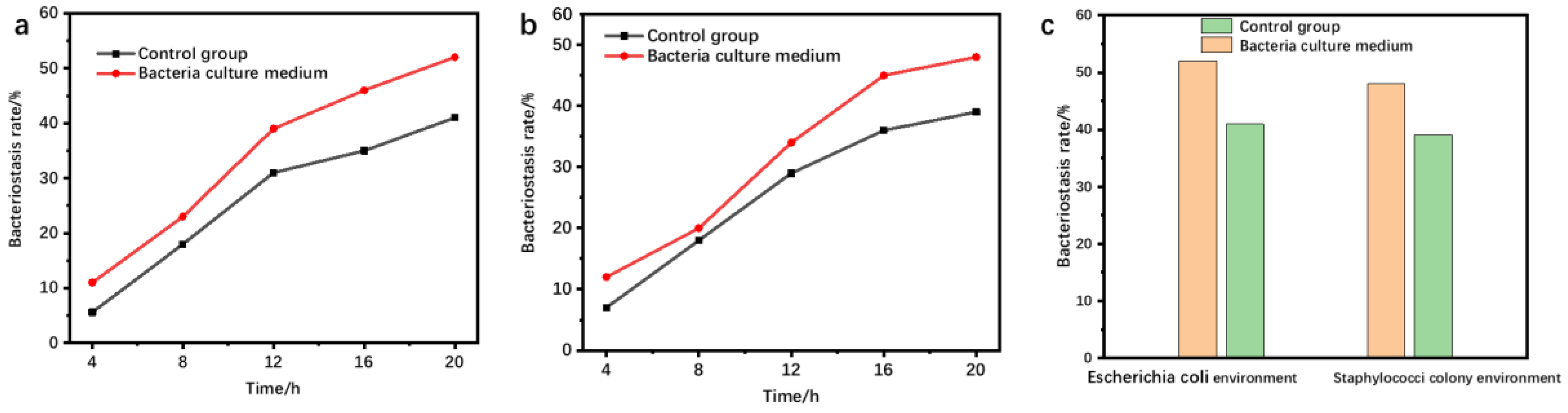
3.3. Bacteriostatic Performance
3.4. Tissue Collagen Deposition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, N.; Youssef, G. Dynamic properties of hydrogels and fiber-reinforced hydrogels. J. Mech. Behav. Biomed. Mater. 2018, 85, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Gunes, O.C.; Ziylan Albayrak, A. Antibacterial Polypeptide nisin containing cotton modified hydrogel composite wound dressings. Polym. Bull. 2021, 78, 6409–6428. [Google Scholar] [CrossRef]
- Huang, J.; Lei, X.; Huang, Z.; Rong, Z.; Li, H.; Xie, Y.; Duan, L.; Xiong, J.; Wang, D.; Zhu, S.; et al. Bioprinted Gelatin-Recombinant Type III Collagen Hydrogel Promotes Wound Healing. Int. J. Bioprint. 2022, 8, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Pardo, A.; Gómez-Florit, M.; Barbosa, S.; Taboada, P.; Domingues, R.M.A.; Gomes, M.E. Magnetic Nanocomposite Hydrogels for Tissue Engineering: Design Concepts and Remote Actuation Strategies to Control Cell Fate. ACS Nano 2021, 15, 175–209. [Google Scholar] [CrossRef]
- Jin, J.; Chen, Z.-L.; Xiang, Y.; Tang, T.; Zhou, H.; Hong, X.-D.; Fan, H.; Zhang, X.-D.; Luo, P.-F.; Ma, B.; et al. Development of aPHMBhydrogel-modified wound scaffold dressing with antibacterial activity. Wound Repair Regen. 2020, 28, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Konieczynska, M.D.; Villa-Camacho, J.C.; Ghobril, C.; Perez-Viloria, M.; Tevis, K.M.; Blessing, W.A.; Nazarian, A.; Rodriguez, E.K.; Grinstaff, M.W. On-Demand Dissolution of a Dendritic Hydrogel-based Dressing for Second-Degree Burn Wounds through Thiol-Thioester Exchange Reaction. Angew. Chem. Int. Ed. 2016, 55, 9984–9987. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, B.; Tian, Y.; Pang, L.; Xu, T.; Cong, H.; Shen, Y. Diversified antibacterial modification and latest applications of polysaccharide-based hydrogels for wound healthcare. Appl. Mater. Today 2022, 26, 101396. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Lin, Z.; Huang, T.; Li, W.; Gong, W.; Guo, Y.; Su, J.; Wang, J.; Tu, Q. A green method of preparing a natural and degradable wound dressing containing aloe vera as an active ingredient. Compos. Part B Eng. 2021, 222, 109047. [Google Scholar] [CrossRef]
- Shang, K.; Tao, L.; Jiang, S.; Yan, J.; Hu, S.; Yang, G.; Ma, C.; Cheng, S.; Wang, X.; Yin, J. Highly flexible hydrogel dressing with efficient antibacterial, antioxidative, and wound healing performances. Biomater. Sci. 2022, 10, 1373–1383. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, T.; Guo, R.; Li, C.; Lu, C.; Yang, G.; Nie, J.; Wang, F.; Yang, X.; Chen, Z. Injectable and Degradable PEG Hydrogel with Antibacterial Performance for Promoting Wound Healing. ACS Appl. Bio Mater. 2021, 4, 2769–2780. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zhu, C.; Fan, D. Optimization of human-like collagen composite polysaccharide hydrogel dressing preparation using response surface for burn repair. Carbohydr. Polym. 2020, 239, 116249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ding, F.; Liu, Y.; Ren, X. Glucose-responsive biomimetic nanoreactor in bacterial cellulose hydrogel for antibacterial and hemostatic therapies. Carbohydr. Polym. 2022, 292, 119615. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Peng, S.; Yao, Y.; Xie, J.; Li, S.; Tu, C.; Gao, C. A nanofibrous membrane loaded with doxycycline and printed with conductive hydrogel strips promotes diabetic wound healing in vivo. Acta Biomater. 2022, 152, 60–73. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, R.; Chen, B.; Liu, X.; Jia, Q.; Wang, X.; Yang, Z.; Ning, P.; Wang, Z.; Yang, Y. Injectable Reactive Oxygen Species-Responsive Hydrogel Dressing with Sustained Nitric Oxide Release for Bacterial Ablation and Wound Healing. Adv. Funct. Mater. 2022, 32, 2202857. [Google Scholar] [CrossRef]
- Chen, H.; Li, B.; Feng, B.; Wang, H.; Yuan, H.; Xu, Z. Tetracycline hydrochloride loaded citric acid functionalized chitosan hydrogel for wound healing. RSC Adv. 2019, 9, 19523–19530. [Google Scholar] [CrossRef]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Ghorbani, S.; Ai, J.; Sahrapeyma, H. Chitosan/alginate hydrogels containing Alpha-tocopherol for wound healing in rat model. J. Drug Deliv. Sci. Technol. 2019, 51, 204–213. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Raza, M.A.; Razak, S.I.A.; Abdul Kadir, M.R.; Haider, A.; Shah, S.A.; Mohd Yusof, A.H.; Haider, S.; Shakir, I.; Aftab, S. Novel functional antimicrobial and biocompatible arabinoxylan/guar gum hydrogel for skin wound dressing applications. J. Tissue Eng. Regen. Med. 2020, 14, 1488–1501. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Adeli, H.; Mansoori-Moghadam, Z.; Moghaddam, A. Design and optimization of process parameters of polyvinyl (alcohol)/chitosan/nano zinc oxide hydrogels as wound healing materials. Carbohydr. Polym. 2019, 207, 542–554. [Google Scholar] [CrossRef]
- Rezaei, N.; Hamidabadi, H.G.; Khosravimelal, S.; Zahiri, M.; Ahovan, Z.A.; Bojnordi, M.N.; Eftekhari, B.S.; Hashemi, A.; Ganji, F.; Darabi, S.; et al. Antimicrobial peptides-loaded smart chitosan hydrogel: Release behavior and antibacterial potential against antibiotic resistant clinical isolates. Int. J. Biol. Macromol. 2020, 164, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Kong, Y.; Shao, C.; Cheng, Y.; Lu, J.; Tao, Y.; Du, J.; Wang, H. Chitosan-based multifunctional flexible hemostatic bio-hydrogel. Acta Biomater. 2021, 136, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kaur, P.; Bernela, M.; Rani, R.; Thakur, R. Ketoconazole encapsulated in chitosan-gellan gum nanocomplexes exhibits prolonged antifungal activity. Int. J. Biol. Macromol. 2016, 93, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; He, H.; Ma, G.; Li, Y.; Jiang, S.; Xuan, X.; Song, Y.; Zhang, C.; Xiao, J.; Xu, Y.; et al. Biodegradable copolypeptide hydrogel prodrug accelerates dermal wound regeneration by enhanced angiogenesis and epithelialization. RSC Adv. 2018, 8, 10620–10626. [Google Scholar] [CrossRef]
- Suzuki, Y.; Tanihara, M.; Nishimura, Y.; Suzuki, K.; Kakimaru, Y.; Shimizu, Y. A novel wound dressing with an antibiotic delivery system stimulated by microbial infection. Asaio J. 1997, 43, M854–M857. [Google Scholar] [CrossRef] [PubMed]

| Temperature (°C) | 30 | 35 | 40 | 45 | 50 | 60 | 70 |
|---|---|---|---|---|---|---|---|
| Swelling rate (%) | 26.8 | 31.2 | 34.2 | 38.9 | 42.5 | 47.6 | 53.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, M.; Shi, Z.; Zhang, X.; Zhai, B.; Sun, J. Synthesis and Properties of Biodegradable Hydrogel Based on Polysaccharide Wound Dressing. Materials 2023, 16, 1358. https://doi.org/10.3390/ma16041358
Shao M, Shi Z, Zhang X, Zhai B, Sun J. Synthesis and Properties of Biodegradable Hydrogel Based on Polysaccharide Wound Dressing. Materials. 2023; 16(4):1358. https://doi.org/10.3390/ma16041358
Chicago/Turabian StyleShao, Meiling, Zhan Shi, Xiangfei Zhang, Bin Zhai, and Jiashu Sun. 2023. "Synthesis and Properties of Biodegradable Hydrogel Based on Polysaccharide Wound Dressing" Materials 16, no. 4: 1358. https://doi.org/10.3390/ma16041358
APA StyleShao, M., Shi, Z., Zhang, X., Zhai, B., & Sun, J. (2023). Synthesis and Properties of Biodegradable Hydrogel Based on Polysaccharide Wound Dressing. Materials, 16(4), 1358. https://doi.org/10.3390/ma16041358




