The Effects of the Al and Zr Contents on the Microstructure Evolution of Light-Weight AlxNbTiVZry High Entropy Alloy
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
3.1. Density of AlNbTiVZr HEAs
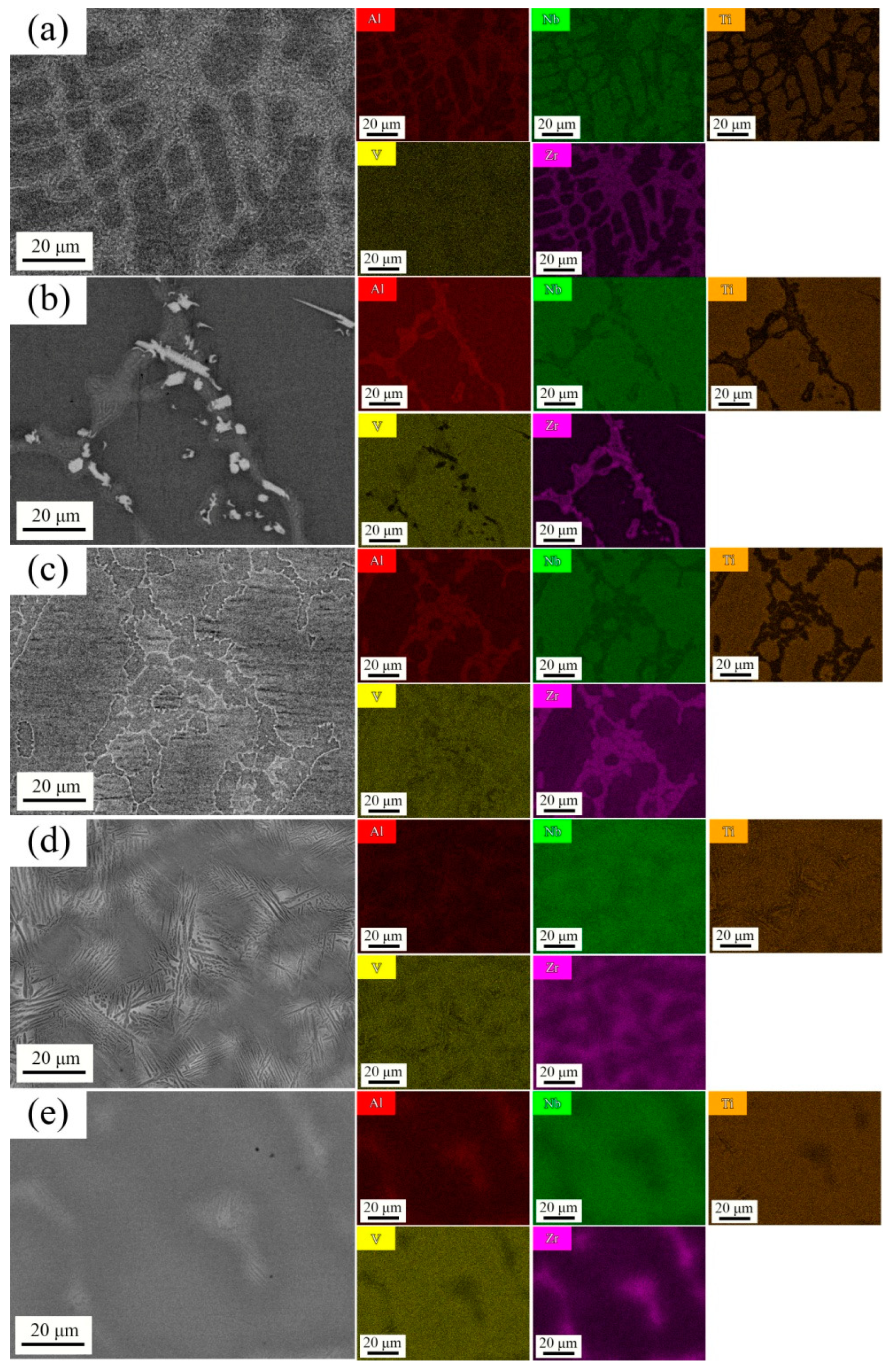
3.2. Structure of AlNbTiVZr HEAs
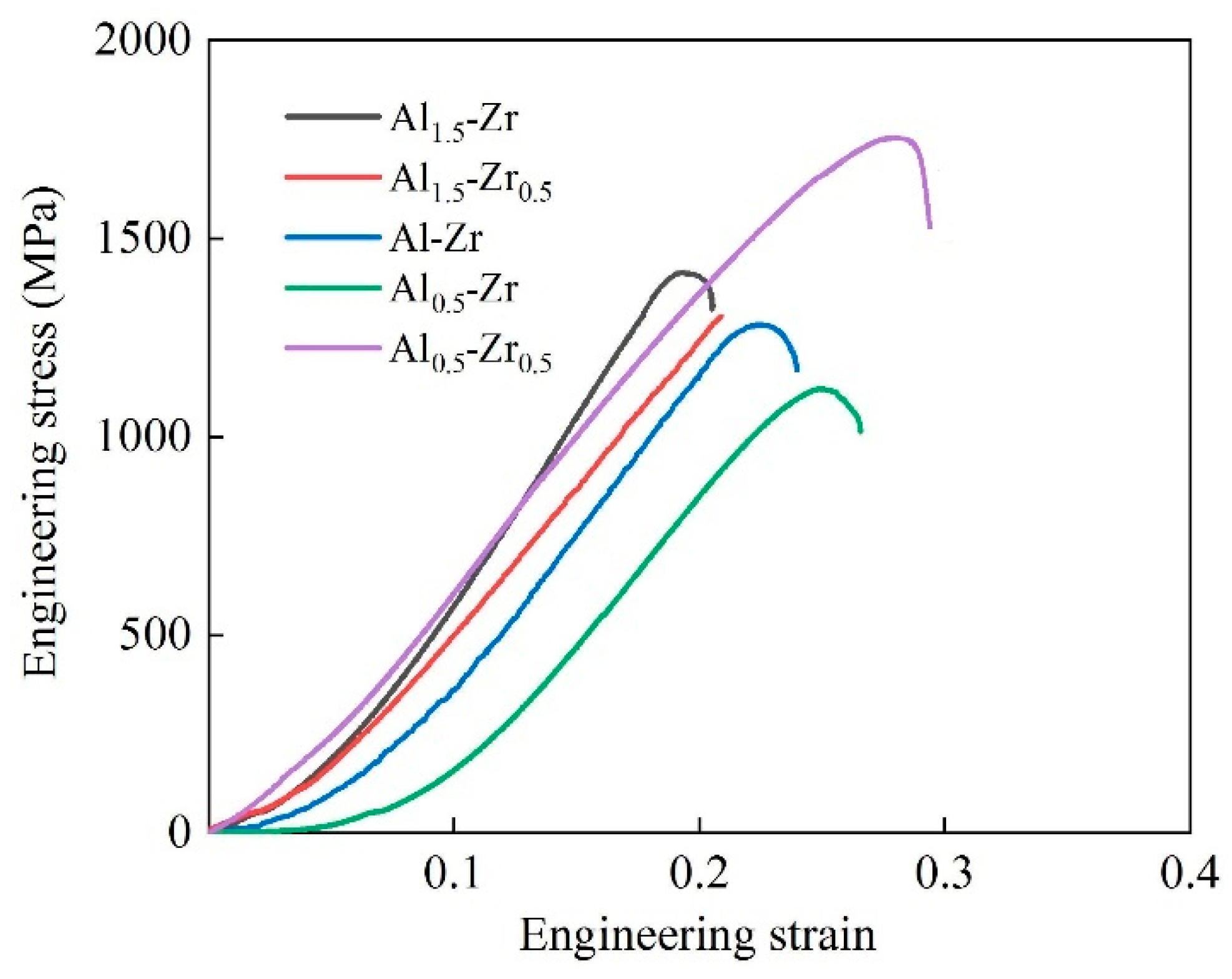
3.3. Compression Property of AlNbTiVZr HEAs
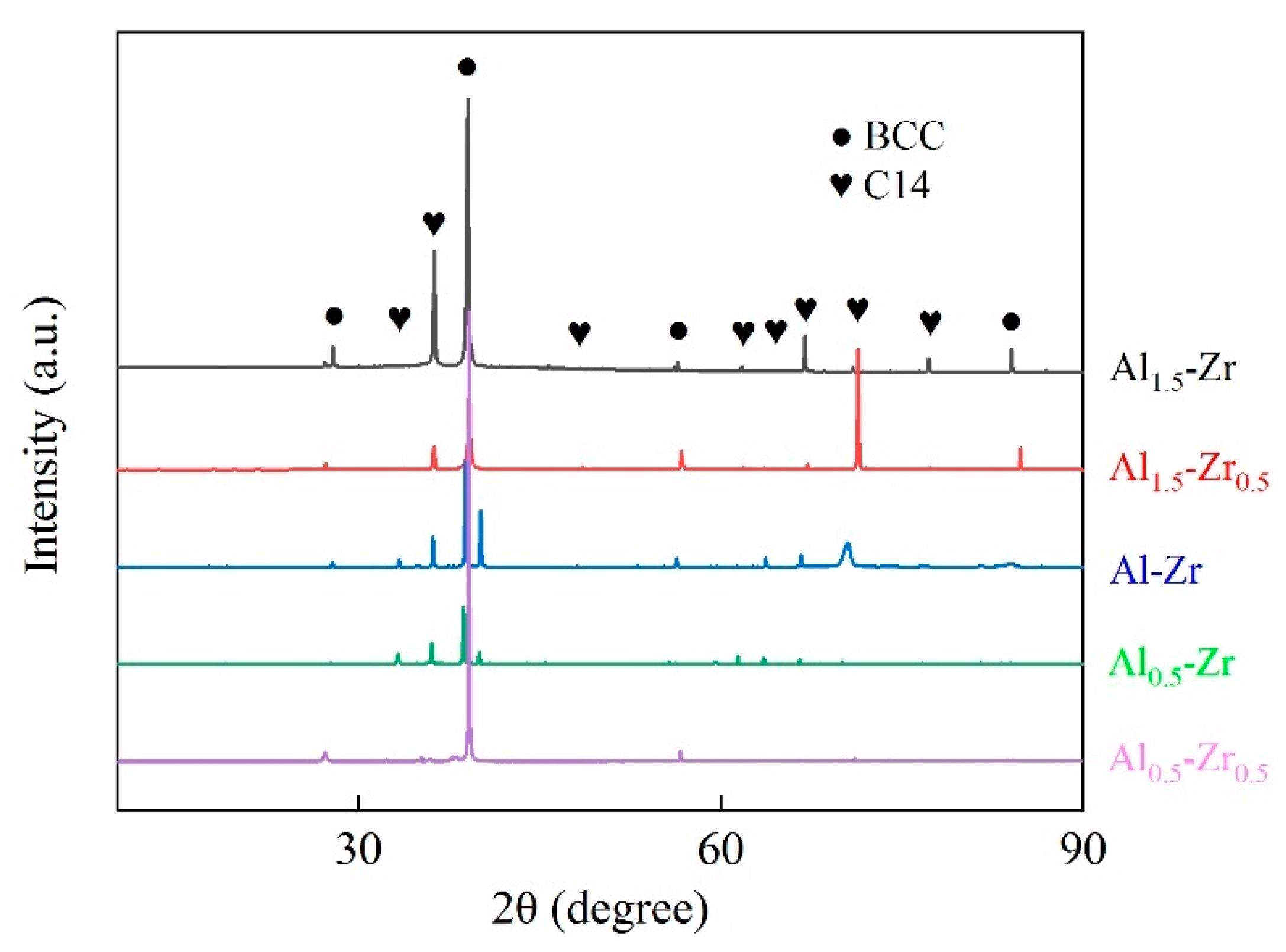
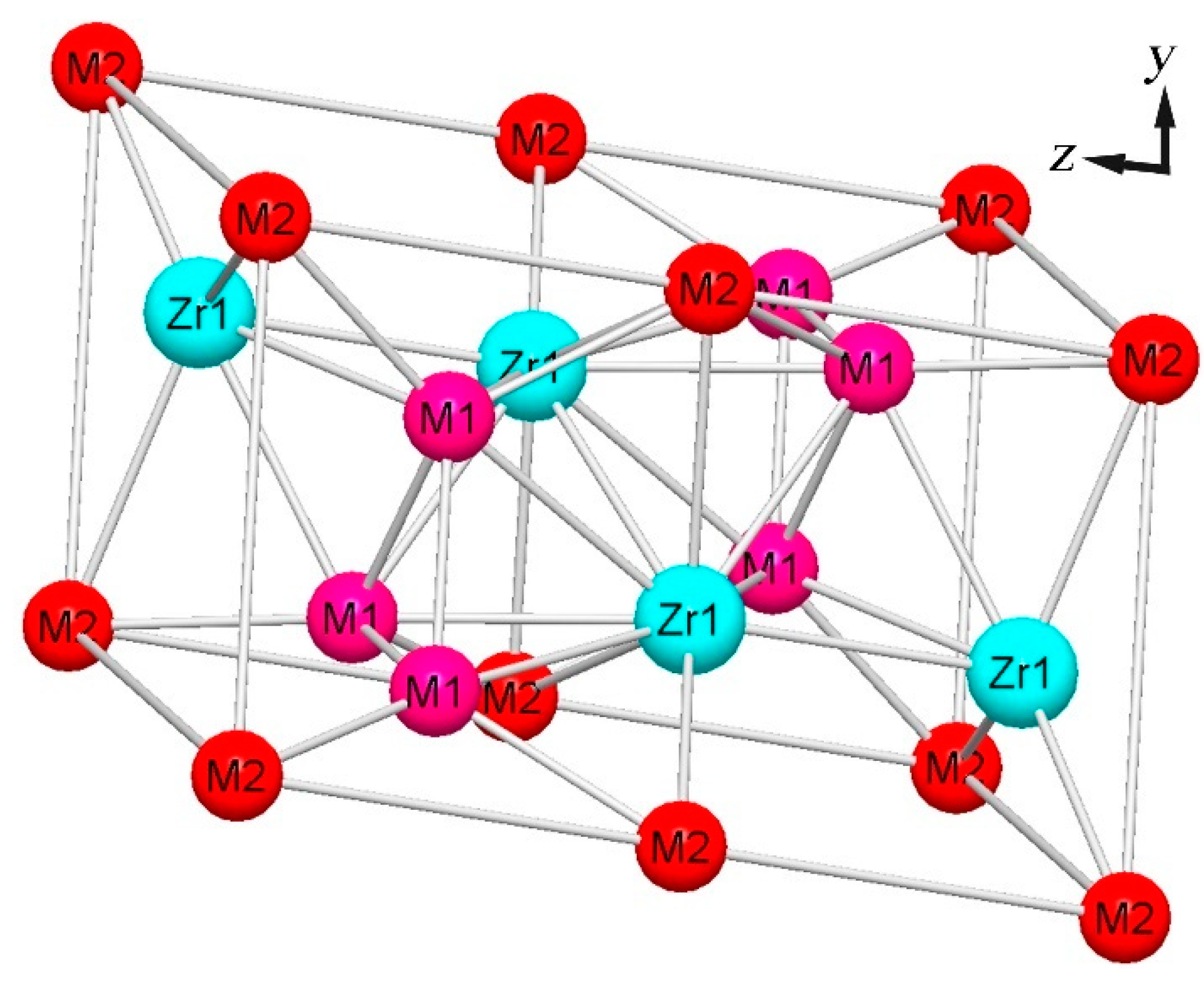
3.4. Phase Composition of AlNbTiVZr HEAs
4. Conclusions
- The actual densities of the studied AlNbTiVZr series HEAs are all below 6 g·cm−3, ranging from 5.291 to 5.826 g·cm−3. And, these alloys can be classified as light-weight HEAs.
- These AlNbTiVZr HEAs predominantly exhibit the BCC structure and C14-Laves phase, which was further confirmed to be the ZrAlV intermetallic. Both the Al and Zr element contents influence the microstructure evolution, and the Zr element has a more dominant influence on the phase composition, as the Zr atoms occupy the central position in the ZrAlV Laves phase. The Laves phase content notably decreases from 29.2% to 23.1% and 1.6% for the Al1.5-Zr sample compared to the Al-Zr and Al0.5-Zr samples which have lower Al element contents. The width of the Laves phase decreases as the Al element content becomes lower.
- The brittle Laves phase is prone to aggregation and separation of the BCC structure, and it significantly influences the compression mechanical properties of the AlNbTiVZr HEAs. Remarkably, the Al0.5-Zr0.5 sample exhibits the best compression mechanical properties with a compression strength (σp) of 1783 MPa and an engineering strain of 28.8%, due to having the lowest ZrAlV Laves phase content (0.7%). In contrast, the Al1.5-Zr0.5 sample fails to reach the yield stage as a consequence of solidification cracks induced by its elevated Al element content.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cantor, B.; Chang, I.T.H.; Knight, P. Microstructural development in equiatomic multicomponent alloys. Mat. Sci. Eng. A-Struct. 2004, 376, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.; Chen, S.; Lin, S.; Gan, J.; Chin, T.; Shun, T.; Tsau, C.; Chang, S. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.P. Development and exploration of refractory high entropy alloys-A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, W.; Tan, Q.; Wu, M.; Qiao, S.; Zhu, G.; Dong, A.; Shu, D.; Sun, B. A review of refractory high-entropy alloys. Trans. Nonferrous Met. Soc. 2022, 32, 3487–3515. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, S.; Yang, S.; Luo, Q.; Jin, Y.; Xie, W.; Zhang, L.; Li, Q. Light-weight refractory high-entropy alloys: A comprehensive review. J. Mater. Sci. Technol. 2023, 151, 41–65. [Google Scholar] [CrossRef]
- Youssef, K.; Zddach, A.; Niu, C.; Irving, D.; Koch, C. A novel low-density, high-hardness, high-entropy alloy with close-packed single-phase nanocrystalline structures. Mater. Res. Lett. 2014, 3, 95–99. [Google Scholar] [CrossRef]
- Tong, Y.; Chen, D.; Han, B.; Wang, J.; Feng, R.; Yang, T.; Zhao, C.; Zhao, Y.; Guo, W.; Shimizu, Y. Outstanding tensile properties of a precipitation-strengthened FeCoNiCrTi0.2 high-entropy alloy at room and cryogenic temperatures. Acta Mater. 2018, 165, 228–240. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Liaw, P.K. Corrosion-resistant high-entropy alloys: A review. Metals 2017, 7, 344. [Google Scholar] [CrossRef]
- Pickering, E.J.; Carruthers, A.W.; Barron, P.J.; Middleburge, S.C.; Armstrong, D.E.J.; Gandy, A.S. High-entropy alloys for advanced nuclear applications. Entropy 2021, 23, 144. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Woodward, C.; Miracle, D.B. Low-density, refractory multi-principal element alloys of the Cr-Nb-Ti-V-Zr system: Microstructure and phase analysis. Acta Mater. 2013, 61, 1545–1557. [Google Scholar] [CrossRef]
- Liao, M.; Liu, Y.; Min, L.; Lai, Z.; Han, T.; Yang, D.; Zhu, J. Alloying effect on phase stability, elastic and thermodynamic properties of Nb-Ti-V-Zr high entropy alloy. Intermetallics 2018, 101, 152–164. [Google Scholar] [CrossRef]
- King, D.J.M.; Cheung, S.T.Y.; Humphry-Baker, S.A.; Parkin, C.; Couet, A.; Cortie, M.B.; Lumpkin, G.R.; Middleburge, S.C.; Knowles, A.J. High temperature, low neutron cross-section high-entropy alloys in the Nb-Ti-V-Zr system. Acta Mater. 2019, 116, 435–446. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Yurchenko, N.Y.; Shaysultanov, D.G.; Salishchev, G.A.; Tikhonovsky, M.A. Effect of Al on structure and mechanical properties of AlxNbTiVZr (x = 0, 0.5, 1, 1.5) high entropy alloys. Mater. Sci. Technol. 2015, 31, 1184–1193. [Google Scholar] [CrossRef]
- Qiu, S.; Chen, S.; Naihua, N.; Zhou, J.; Hu, Q.; Sun, Z. Structural stability and mechanical properties of B2 ordered refractory AlNbTiVZr high entropy alloys. J. Alloys Compd. 2021, 886, 161289. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Yurchrnko, N.Y.; Slkolovsky, V.S.; Tikhonovsky, M.A.; Salishchev, G.A. An AlNbTiVZr0.5 high-entropy alloy combining high specific strength and good ductility. Mater. Lett. 2015, 161, 136–139. [Google Scholar] [CrossRef]
- Yurchenko, N.; Panina, E.; Belyakov, A.; Salishchev, G.; Zherebstov, S.; Stepanov, N. On the yield stress anomaly in a B2-ordered refractory AlNbTiVZr0.25 high-entropy alloy. Mater. Lett. 2021, 311, 131584. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, B.; Liu, S.; Zhang, X.; Liu, R. Microstructure and mechanical properties of novel Zr-Al-V alloys processed by hot rolling. Intermetallics 2020, 116, 106639. [Google Scholar] [CrossRef]
- Junior, J.M.M.; Chaia, N.; Cotton, J.D.; Coelho, G.C.; Nunes, C.A. A multi-principal element alloy combining high specific strength and good ductility. Mater. Lett. 2022, 325, 132905. [Google Scholar]
- Wang, Q.; Chen, F.; Wu, J.; Qiang, J.; Dong, C.; Zhang, Y.; Xu, F.; Sun, L. Hydrogen absorption by Laves phase related BCC solid solution. Rare Matels 2006, 25, 252–255. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, Y.; Che, Y.; Schützendübe, P.; Wan, J.; Huang, Y.; Liu, Y.; Wang, Z. Anomalous formation of micrometer-thick amorphous oxide surficial layers during high-temperature oxidation of ZrAl2. J. Mater. Sci. Technol. 2019, 35, 1479–1484. [Google Scholar] [CrossRef]



| Sample | Alloy (abbr.) | Nominal Density/g·cm−3 | Actual Density/g·cm−3 |
|---|---|---|---|
| 1 | Al1.5NbTiVZr (Al1.5-Zr) | 5.482 | 5.337 |
| 2 | Al1.5NbTiVZr0.5 (Al1.5-Zr0.5) | 5.345 | 5.291 |
| 3 | AlNbTiVZr (Al-Zr) | 5.739 | 5.599 |
| 4 | Al0.5NbTiVZr (Al0.5-Zr) | 6.049 | 5.870 |
| 5 | Al0.5NbTiVZr0.5 (Al0.5-Zr0.5) | 5.975 | 5.826 |
| Alloy | Al | Nb | Ti | V | Zr |
|---|---|---|---|---|---|
| Al1.5-Zr | 22 | 25 | 15 | 17 | 21 |
| Al1.5-Zr0.5 | 25 | 25 | 17 | 19 | 14 |
| Al-Zr | 16 | 27 | 16 | 18 | 23 |
| Al0.5-Zr | 10 | 30 | 17 | 19 | 25 |
| Al0.5-Zr0.5 | 11 | 31 | 30 | 22 | 17 |
| ∆Hmix (kJ·mol−1) | Al | Nb | Ti | V | Zr |
|---|---|---|---|---|---|
| Al | - | −18 | −30 | −16 | −44 |
| Nb | - | - | 2 | −1 | 4 |
| Ti | - | - | - | −2 | 0 |
| V | - | - | - | - | −4 |
| Phase Content (%) | Al1.5-Zr | Al1.5-Zr0.5 | Al-Zr | Al0.5-Zr | Al0.5-Zr0.5 |
|---|---|---|---|---|---|
| BCC | 70.8 | 93.9 | 76.9 | 98.4 | 99.3 |
| Laves | 29.2 | 6.1 | 23.1 | 1.6 | 0.7 |
| BOaverage | Zr-Zr | Al-Al | V-V | Zr-V | Zr-Al | Al-V |
|---|---|---|---|---|---|---|
| ZrAlV Laves phase | 0.227 | 0.285 | 0.602 | 0.228 | 0.217 | 0.360 |
| AlNbTiVZr alloy | 0.354 | 0.236 | 0.331 | 0.273 | 0.243 | 0.255 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Liu, R.; Li, S.; Zhang, Y.; Xiao, W.; Xue, B.; Xiong, B.; Li, X.; Li, Z. The Effects of the Al and Zr Contents on the Microstructure Evolution of Light-Weight AlxNbTiVZry High Entropy Alloy. Materials 2023, 16, 7581. https://doi.org/10.3390/ma16247581
Yan H, Liu R, Li S, Zhang Y, Xiao W, Xue B, Xiong B, Li X, Li Z. The Effects of the Al and Zr Contents on the Microstructure Evolution of Light-Weight AlxNbTiVZry High Entropy Alloy. Materials. 2023; 16(24):7581. https://doi.org/10.3390/ma16247581
Chicago/Turabian StyleYan, Hongwei, Rui Liu, Shenglong Li, Yong’an Zhang, Wei Xiao, Boyu Xue, Baiqing Xiong, Xiwu Li, and Zhihui Li. 2023. "The Effects of the Al and Zr Contents on the Microstructure Evolution of Light-Weight AlxNbTiVZry High Entropy Alloy" Materials 16, no. 24: 7581. https://doi.org/10.3390/ma16247581
APA StyleYan, H., Liu, R., Li, S., Zhang, Y., Xiao, W., Xue, B., Xiong, B., Li, X., & Li, Z. (2023). The Effects of the Al and Zr Contents on the Microstructure Evolution of Light-Weight AlxNbTiVZry High Entropy Alloy. Materials, 16(24), 7581. https://doi.org/10.3390/ma16247581






