Effects of Space Dimensionality within Scaffold for Bone Regeneration with Large and Oriented Blood Vessels
Abstract
:1. Introduction
2. Materials and Methods
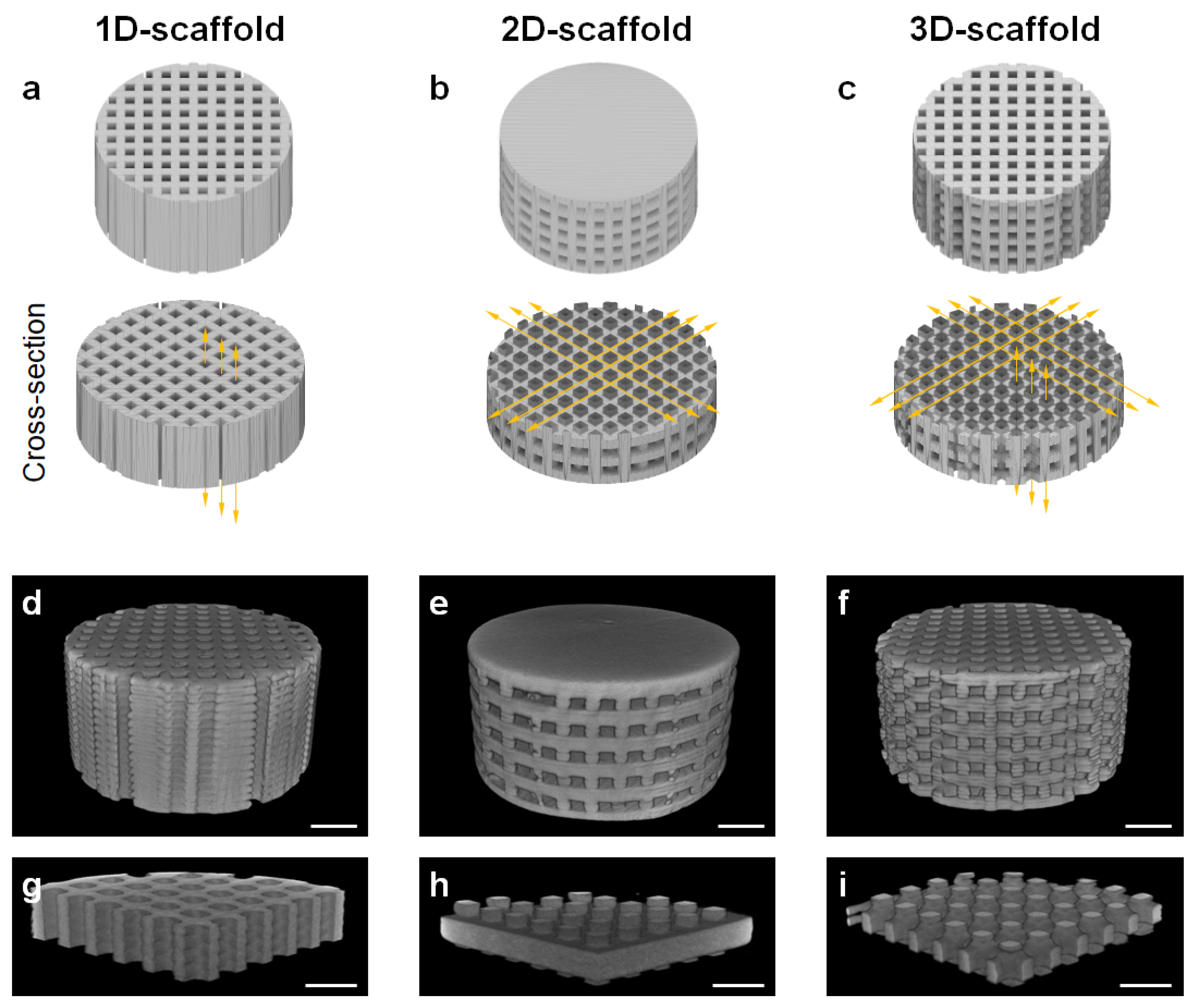
2.1. Fabrication of 1D-, 2D-, and 3D-Scaffolds
2.2. Characterization of 1D-, 2D-, and 3D-Scaffolds
2.3. Ethics Statement
2.4. Sample Size Calculations
2.5. Animals
2.6. Surgical Procedure
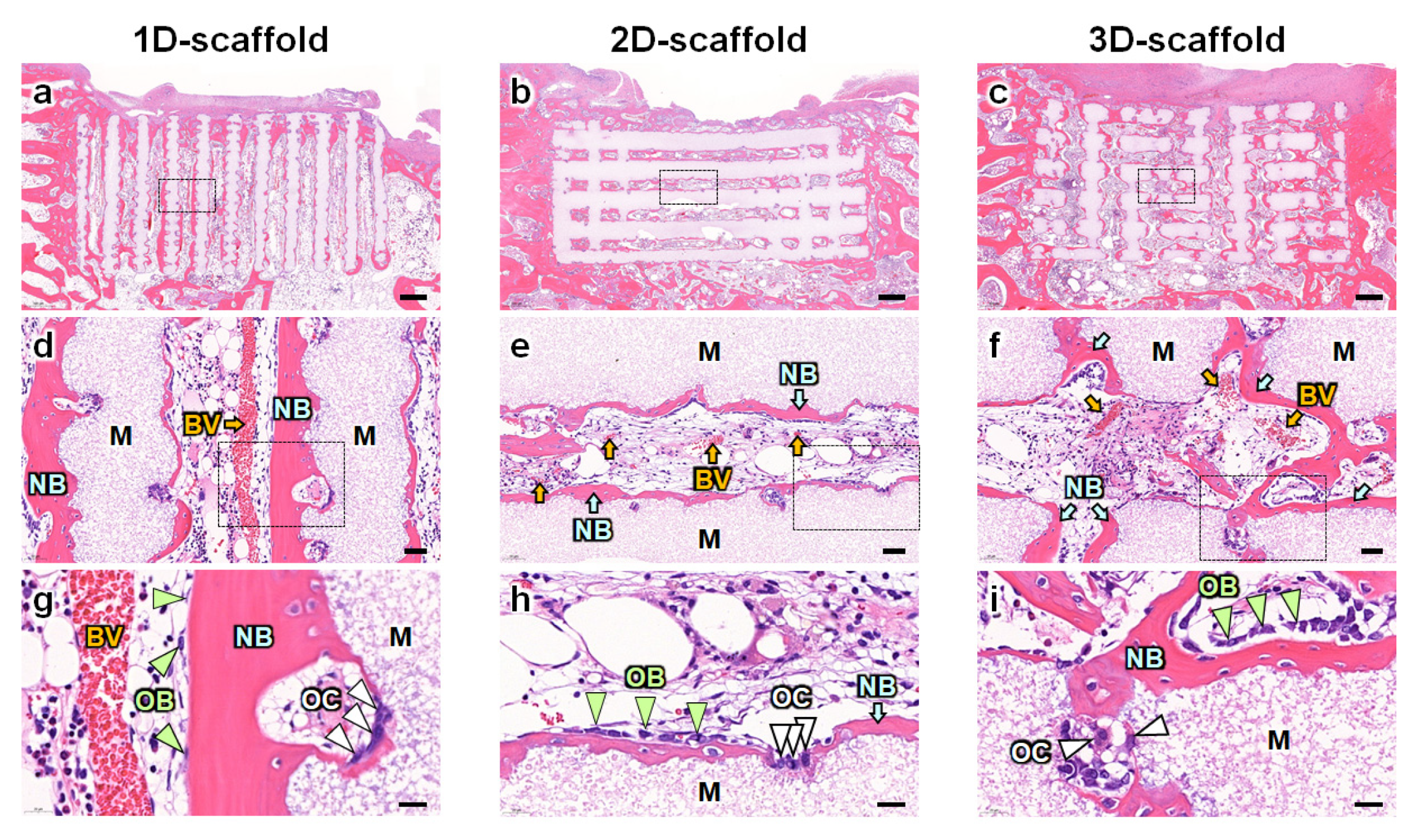
2.7. Histological Analyses
2.8. Statistical Analysis
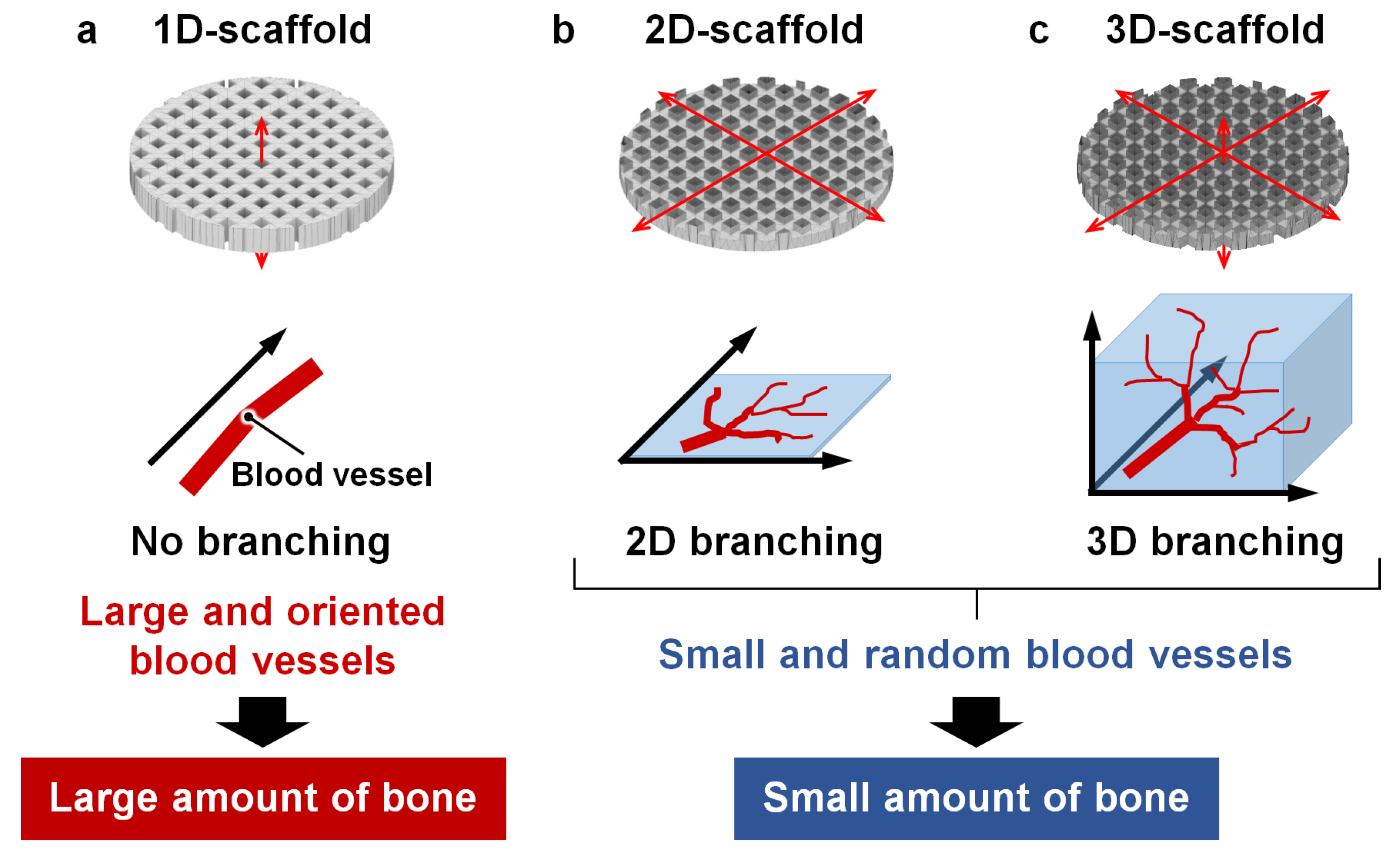
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial Inflammation in Osteoarthritis Progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Bei, T.; Yang, L.; Huang, Q.; Wu, J.; Liu, J. Effectiveness of Bone Substitute Materials in Opening Wedge High Tibial Osteotomy: A Systematic Review and Meta-Analysis. Ann. Med. 2022, 54, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Motta, F.; Barone, E.; Sica, A.; Selmi, C. Inflammaging and Osteoarthritis. Clinic. Rev. Allerg. Immunol. 2022, 64, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Cui, J.; Lin, K.; Xie, J.; Wang, X. Recent Advances in Smart Stimuli-Responsive Biomaterials for Bone Therapeutics and Regeneration. Bone Res. 2022, 10, 17. [Google Scholar] [CrossRef]
- Dixon, D.T.; Gomillion, C.T. Conductive Scaffolds for Bone Tissue Engineering: Current State and Future Outlook. J. Funct. Biomater. 2021, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Mirkhalaf, M.; Men, Y.; Wang, R.; No, Y.; Zreiqat, H. Personalized 3D Printed Bone Scaffolds: A Review. Acta Biomater. 2023, 156, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yu, H.; Chen, C. Biological Properties of Calcium Phosphate Biomaterials for Bone Repair: A Review. RSC Adv. 2018, 8, 2015–2033. [Google Scholar] [CrossRef]
- Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Honeycomb Blocks Composed of Carbonate Apatite, β-Tricalcium Phosphate, and Hydroxyapatite for Bone Regeneration: Effects of Composition on Biological Responses. Mater. Today Bio 2019, 4, 100031. [Google Scholar] [CrossRef]
- Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Granular Honeycombs Composed of Carbonate Apatite, Hydroxyapatite, and β-Tricalcium Phosphate as Bone Graft Substitutes: Effects of Composition on Bone Formation and Maturation. ACS Appl. Bio Mater. 2020, 3, 1787–1795. [Google Scholar] [CrossRef]
- Ribas, R.G.; Schatkoski, V.M.; do Amaral Montanheiro, T.L.; de Menezes, B.R.C.; Stegemann, C.; Leite, D.M.G.; Thim, G.P. Current Advances in Bone Tissue Engineering Concerning Ceramic and Bioglass Scaffolds: A Review. Ceram. Int. 2019, 45, 21051–21061. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, V.; Baino, F.; Mauro, J.C.; Pickrell, G.; Evans, I.; Bretcanu, O. Mechanical Properties of Bioactive Glasses, Ceramics, Glass-Ceramics and Composites: State-of-the-Art Review and Future Challenges. Mater. Sci. Eng. C 2019, 104, 109895. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-Tricalcium Phosphate for Bone Substitution: Synthesis and Properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Flores-Jiménez, M.S.; Garcia-Gonzalez, A.; Fuentes-Aguilar, R.Q. Review on Porous Scaffolds Generation Process: A Tissue Engineering Approach. ACS Appl. Bio Mater. 2023, 6, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Tadic, D.; Beckmann, F.; Schwarz, K.; Epple, M. A Novel Method to Produce Hydroxyapatite Objects with Interconnecting Porosity That Avoids Sintering. Biomaterials 2004, 25, 3335–3340. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.C.T.; Camilli, J.A.; Kawachi, E.Y.; Bertran, C.A. Behavior of Dense and Porous Hydroxyapatite Implants and Tissue Response in Rat Femoral Defects. J. Biomed. Mater. Res. 2002, 62, 30–36. [Google Scholar] [CrossRef]
- Zhu, W.; Ma, Q.; Borg, S.; Öhman Mägi, C.; Weng, X.; Engqvist, H.; Xia, W. Cemented Injectable Multi-Phased Porous Bone Grafts for the Treatment of Femoral Head Necrosis. J. Mater. Chem. B 2019, 7, 2997–3006. [Google Scholar] [CrossRef]
- Lee, D.S.H.; Pai, Y.; Chang, S.; Kim, D.H. Microstructure, Physical Properties, and Bone Regeneration Effect of the Nano-Sized β-Tricalcium Phosphate Granules. Mater. Sci. Eng. C 2016, 58, 971–976. [Google Scholar] [CrossRef]
- Almirall, A.; Larrecq, G.; Delgado, J.A.; Martínez, S.; Planell, J.A.; Ginebra, M.P. Fabrication of Low Temperature Macroporous Hydroxyapatite Scaffolds by Foaming and Hydrolysis of an α-TCP Paste. Biomaterials 2004, 25, 3671–3680. [Google Scholar] [CrossRef]
- Li, S.H.; De Wijn, J.R.; Layrolle, P.; De Groot, K. Synthesis of Macroporous Hydroxyapatite Scaffolds for Bone Tissue Engineering. J. Biomed. Mater. Res. 2002, 61, 109–120. [Google Scholar] [CrossRef]
- Fiume, E.; Ciavattini, S.; Verné, E.; Baino, F. Foam Replica Method in the Manufacturing of Bioactive Glass Scaffolds: Out-of-Date Technology or Still Underexploited Potential? Materials 2021, 14, 2795. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass®-Derived Glass–Ceramic Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.M.; Silva, S.S.; Malafaya, P.B.; Rodrigues, M.T.; Kotobuki, N.; Hirose, M.; Gomes, M.E.; Mano, J.F.; Ohgushi, H.; Reis, R.L. Macroporous Hydroxyapatite Scaffolds for Bone Tissue Engineering Applications: Physicochemical Characterization and Assessment of Rat Bone Marrow Stromal Cell Viability. J. Biomed. Mater. Res. 2009, 91A, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Gervaso, F.; Scalera, F.; Kunjalukkal Padmanabhan, S.; Sannino, A.; Licciulli, A. High-Performance Hydroxyapatite Scaffolds for Bone Tissue Engineering Applications: High-Performance Hydroxyapatite Scaffolds. Int. J. Appl. Ceram. Technol. 2012, 9, 507–516. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D Bioactive Composite Scaffolds for Bone Tissue Engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Tsuchiya, A.; Shimabukuro, M.; Ishikawa, K. Multiscale Porous Scaffolds Constructed of Carbonate Apatite Honeycomb Granules for Bone Regeneration. Mater. Des. 2022, 215, 110468. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Zhang, C.; Wu, Z.; Liu, J. Recent Developments of Biomaterials for Additive Manufacturing of Bone Scaffolds. Adv. Healthc. Mater. 2020, 9, 2000724. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Wang, C.; Zhou, X.; Libanori, A.; Jiang, X.; Xu, W.; Zhu, S.; Chen, Q.; Sun, W.; Khademhosseini, A. Multi-Dimensional Printing for Bone Tissue Engineering. Adv. Healthc. Mater. 2021, 10, 2001986. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, S.; Yu, J.; Gu, Z.; Zhang, Y. Advances in Translational 3D Printing for Cartilage, Bone, and Osteochondral Tissue Engineering. Small 2022, 18, 2201869. [Google Scholar] [CrossRef]
- Guzzi, E.A.; Tibbitt, M.W. Additive Manufacturing of Precision Biomaterials. Adv. Mater. 2020, 32, 1901994. [Google Scholar] [CrossRef]
- Cámara-Torres, M.; Fucile, P.; Sinha, R.; Mota, C.; Moroni, L. Boosting Bone Regeneration Using Augmented Melt-Extruded Additive-Manufactured Scaffolds. Int. Mater. Rev. 2022, 68, 755–785. [Google Scholar] [CrossRef]
- Sun, J.; Ye, D.; Zou, J.; Chen, X.; Wang, Y.; Yuan, J.; Liang, H.; Qu, H.; Binner, J.; Bai, J. A Review on Additive Manufacturing of Ceramic Matrix Composites. J. Mater. Sci. Technol. 2023, 138, 1–16. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Liu, J.; Wang, H.; Li, X.; Cao, J.; Cao, H.; Tang, B.; Yuan, H. Recent Advances on Bioactive Baghdadite Ceramic for Bone Tissue Engineering Applications: 20 Years of Research and Innovation (a Review). Mater. Today Bio 2022, 17, 100473. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Superiority of Triply Periodic Minimal Surface Gyroid Structure to Strut-Based Grid Structure in Both Strength and Bone Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 34570–34577. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, Z.; Liu, L.; Shi, Z.; Liu, J.; Li, Z.; Hao, Y. Design and Study of in Vivo Bone Formation Characteristics of Biodegradable Bioceramic. Mater. Des. 2021, 212, 110242. [Google Scholar] [CrossRef]
- Raja, N.; Han, S.H.; Cho, M.; Choi, Y.-J.; Jin, Y.-Z.; Park, H.; Lee, J.H.; Yun, H. Effect of Porosity and Phase Composition in 3D Printed Calcium Phosphate Scaffolds on Bone Tissue Regeneration in Vivo. Mater. Des. 2022, 219, 110819. [Google Scholar] [CrossRef]
- Li, Y.; Wu, R.; Yu, L.; Shen, M.; Ding, X.; Lu, F.; Liu, M.; Yang, X.; Gou, Z.; Xu, S. Rational Design of Nonstoichiometric Bioceramic Scaffolds via Digital Light Processing: Tuning Chemical Composition and Pore Geometry Evaluation. J. Biol. Eng. 2021, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Wu, R.; Shen, M.; Xie, L.; Liu, M.; Li, Y.; Xu, S.; Wan, L.; Yang, X.; Gao, C.; et al. Rational Design of Bioceramic Scaffolds with Tuning Pore Geometry by Stereolithography: Microstructure Evaluation and Mechanical Evolution. J. Eur. Ceram. Soc. 2021, 41, 1672–1682. [Google Scholar] [CrossRef]
- Mirkhalaf, M.; Wang, X.; Entezari, A.; Dunstan, C.R.; Jiang, X.; Zreiqat, H. Redefining Architectural Effects in 3D Printed Scaffolds through Rational Design for Optimal Bone Tissue Regeneration. Appl. Mater. Today 2021, 25, 101168. [Google Scholar] [CrossRef]
- Entezari, A.; Roohani, I.; Li, G.; Dunstan, C.R.; Rognon, P.; Li, Q.; Jiang, X.; Zreiqat, H. Architectural Design of 3D Printed Scaffolds Controls the Volume and Functionality of Newly Formed Bone. Adv. Healthc. Mater. 2019, 8, 1801353. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Z.; Xu, M.; Wang, S.; Li, N.; Yang, J. A Review of 3D Printed Porous Ceramics. J. Eur. Ceram. Soc. 2022, 42, 3351–3373. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Song, P.; Pei, X.; Sun, H.; Wu, L.; Zhou, C.; Wang, K.; Fan, Y.; Zhang, X. 3D Printed Bone Tissue Regenerative PLA/HA Scaffolds with Comprehensive Performance Optimizations. Mater. Des. 2021, 201, 109490. [Google Scholar] [CrossRef]
- Hayashi, K.; Yanagisawa, T.; Kishida, R.; Ishikawa, K. Effects of Scaffold Shape on Bone Regeneration: Tiny Shape Differences Affect the Entire System. ACS Nano 2022, 16, 11755–11768. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Carbonate Apatite Micro-Honeycombed Blocks Generate Bone Marrow-Like Tissues as Well as Bone. Adv. Biosyst. 2019, 3, 1900140. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ishikawa, K. Honeycomb Scaffolds Fabricated Using Extrusion Molding and the Sphere-Packing Theory for Bone Regeneration. ACS Appl. Bio Mater. 2021, 4, 721–730. [Google Scholar] [CrossRef]
- Hayashi, K.; Ishikawa, K. Effects of Nanopores on the Mechanical Strength, Osteoclastogenesis, and Osteogenesis in Honeycomb Scaffolds. J. Mater. Chem. B 2020, 8, 8536–8545. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Munar, M.L.; Ishikawa, K. Effects of Macropore Size in Carbonate Apatite Honeycomb Scaffolds on Bone Regeneration. Mater. Sci. Eng. C 2020, 111, 110848. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Shimabukuro, M.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Honeycomb Scaffolds Capable of Achieving Barrier Membrane-Free Guided Bone Regeneration. Mater. Adv. 2021, 2, 7638–7649. [Google Scholar] [CrossRef]
- Hayashi, K.; Shimabukuro, M.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Structurally Optimized Honeycomb Scaffolds with Outstanding Ability for Vertical Bone Augmentation. J. Adv. Res. 2022, 41, 101–112. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, J.; Jing, W. Motivating Role of Type H Vessels in Bone Regeneration. Cell Prolif. 2020, 53, e12874. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, S.; Li, Y.; Crane, J.L. Type H Blood Vessels in Bone Modeling and Remodeling. Theranostics 2020, 10, 426–436. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Huang, X.; Gu, Y.; Li, S.; Yin, P.; Zhang, L.; Tang, P. Skeleton-Vasculature Chain Reaction: A Novel Insight into the Mystery of Homeostasis. Bone Res. 2021, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Sadek, A.A.; Abd-Elkareem, M.; Abdelhamid, H.N.; Moustafa, S.; Hussein, K. Repair of Critical-Sized Bone Defects in Rabbit Femurs Using Graphitic Carbon Nitride (g-C3N4) and Graphene Oxide (GO) Nanomaterials. Sci. Rep. 2023, 13, 5404. [Google Scholar] [CrossRef] [PubMed]
- Sadek, A.A.; Abd-Elkareem, M.; Abdelhamid, H.N.; Moustafa, S.; Hussein, K. Enhancement of Critical-Sized Bone Defect Regeneration Using UiO-66 Nanomaterial in Rabbit Femurs. BMC Vet. Res. 2022, 18, 260. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.K.; Li, L.; Qin, L.; Wang, X.L.; Lai, Y.X. Bone Defect Animal Models for Testing Efficacy of Bone Substitute Biomaterials. J. Orthop. Translat. 2015, 3, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Yang, Z.Y.; Tan, L.L.; Li, H.; Zhang, Y.Z. An Animal Experimental Study of Porous Magnesium Scaffold Degradation and Osteogenesis. Braz. J. Med. Biol. Res. 2014, 47, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Jia, J.; Wu, F.; Wei, S.; Zhou, H.; Zhang, H.; Shin, J.W.; Liu, C. Hierarchically Microporous/Macroporous Scaffold of Magnesium–Calcium Phosphate for Bone Tissue Regeneration. Biomaterials 2010, 31, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Boudaoud, A.; Burian, A.; Borowska-Wykręt, D.; Uyttewaal, M.; Wrzalik, R.; Kwiatkowska, D.; Hamant, O. FibrilTool, an ImageJ Plug-in to Quantify Fibrillar Structures in Raw Microscopy Images. Nat. Protoc. 2014, 9, 457–463. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software ‘EZR’ for Medical Statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Ghayor, C.; Bhattacharya, I.; Weber, F.E. The Optimal Microarchitecture of 3D-Printed β-TCP Bone Substitutes for Vertical Bone Augmentation Differs from That for Osteoconduction. Mater. Des. 2021, 204, 109650. [Google Scholar] [CrossRef]
- Guerrero, J.; Ghayor, C.; Bhattacharya, I.; Weber, F.E. Osteoconductivity of Bone Substitutes with Filament-Based Microarchitectures: Influence of Directionality, Filament Dimension, and Distance. IJB 2022, 9, 626. [Google Scholar] [CrossRef]
- Ha, Y.; Ma, X.; Li, S.; Li, T.; Li, Z.; Qian, Y.; Shafiq, M.; Wang, J.; Zhou, X.; He, C. Bone Microenvironment-Mimetic Scaffolds with Hierarchical Microstructure for Enhanced Vascularization and Bone Regeneration. Adv. Funct. Mater. 2022, 32, 2200011. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, T.; Lin, X. 3D Printed Hydrogel Scaffolds with Macro Pores and Interconnected Microchannel Networks for Tissue Engineering Vascularization. Chem. Eng. J. 2022, 430, 132926. [Google Scholar] [CrossRef]
- Liang, Q.; Gao, F.; Zeng, Z.; Yang, J.; Wu, M.; Gao, C.; Cheng, D.; Pan, H.; Liu, W.; Ruan, C. Coaxial Scale-Up Printing of Diameter-Tunable Biohybrid Hydrogel Microtubes with High Strength, Perfusability, and Endothelialization. Adv. Funct. Mater. 2020, 30, 2001485. [Google Scholar] [CrossRef]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular Networks and Functional Intravascular Topologies within Biocompatible Hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef]
- Kennedy, S.M.; Amudhan, K.; Jeen Robert, R.B.; Vasanthanathan, A.; Vignesh Moorthi Pandian, A. Experimental and Finite Element Analysis on the Effect of Pores on Bio-Printed Polycaprolactone Bone Scaffolds. Bioprinting 2023, 34, e00301. [Google Scholar] [CrossRef]
- Reyes, R.L.; Ghim, M.S.; Kang, N.U.; Park, J.W.; Gwak, S.J.; Cho, Y.S. Development and Assessment of Modified-Honeycomb-Structure Scaffold for Bone Tissue Engineering. Addit. Manuf. 2022, 54, 102740. [Google Scholar] [CrossRef]
- He, F.; Lu, T.; Fang, X.; Tian, Y.; Li, Y.; Zuo, F.; Ye, J. Modification of Honeycomb Bioceramic Scaffolds for Bone Regeneration under the Condition of Excessive Bone Resorption. J. Biomed. Mater. Res. 2019, 107, 1314–1323. [Google Scholar] [CrossRef]
- Naudot, M.; Garcia Garcia, A.; Jankovsky, N.; Barre, A.; Zabijak, L.; Azdad, S.Z.; Collet, L.; Bedoui, F.; Hébraud, A.; Schlatter, G.; et al. The Combination of a Poly-caprolactone/Nano-hydroxyapatite Honeycomb Scaffold and Mesenchymal Stem Cells Promotes Bone Regeneration in Rat Calvarial Defects. J. Tissue Eng. Regen. Med. 2020, 14, 1570–1580. [Google Scholar] [CrossRef]
- Rumpler, M.; Woesz, A.; Dunlop, J.W.C.; van Dongen, J.T.; Fratzl, P. The Effect of Geometry on Three-Dimensional Tissue Growth. J. R. Soc. Interface 2008, 5, 1173–1180. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, M.; Wang, Z.; Gao, H.; Chen, S.; Cong, Y.; Yang, L.; Wen, S.; Cheng, D.; He, J.; et al. Radially Porous Nanocomposite Scaffolds with Enhanced Capability for Guiding Bone Regeneration In Vivo. Adv. Funct. Mater. 2022, 32, 2110931. [Google Scholar] [CrossRef]
- Magnaudeix, A. Calcium Phosphate Bioceramics: From Cell Behavior to Chemical-Physical Properties. Front. Biomater. Sci. 2022, 1, 942104. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Bone Tissue Regeneration: The Role of Scaffold Geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar] [CrossRef]
- Pieuchot, L.; Marteau, J.; Guignandon, A.; Dos Santos, T.; Brigaud, I.; Chauvy, P.-F.; Cloatre, T.; Ponche, A.; Petithory, T.; Rougerie, P.; et al. Curvotaxis Directs Cell Migration through Cell-Scale Curvature Landscapes. Nat. Commun. 2018, 9, 3995. [Google Scholar] [CrossRef]
- Werner, M.; Blanquer, S.B.G.; Haimi, S.P.; Korus, G.; Dunlop, J.W.C.; Duda, G.N.; Grijpma, D.W.; Petersen, A. Surface Curvature Differentially Regulates Stem Cell Migration and Differentiation via Altered Attachment Morphology and Nuclear Deformation. Adv. Sci. 2017, 4, 1600347. [Google Scholar] [CrossRef]
- Werner, M.; Petersen, A.; Kurniawan, N.A.; Bouten, C.V.C. Cell-Perceived Substrate Curvature Dynamically Coordinates the Direction, Speed, and Persistence of Stromal Cell Migration. Adv. Biosys. 2019, 3, 1900080. [Google Scholar] [CrossRef]
- Callens, S.J.P.; Uyttendaele, R.J.C.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Substrate Curvature as a Cue to Guide Spatiotemporal Cell and Tissue Organization. Biomaterial 2020, 232, 119739. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Effects of Space Dimensionality within Scaffold for Bone Regeneration with Large and Oriented Blood Vessels. Materials 2023, 16, 7518. https://doi.org/10.3390/ma16247518
Hayashi K, Kishida R, Tsuchiya A, Ishikawa K. Effects of Space Dimensionality within Scaffold for Bone Regeneration with Large and Oriented Blood Vessels. Materials. 2023; 16(24):7518. https://doi.org/10.3390/ma16247518
Chicago/Turabian StyleHayashi, Koichiro, Ryo Kishida, Akira Tsuchiya, and Kunio Ishikawa. 2023. "Effects of Space Dimensionality within Scaffold for Bone Regeneration with Large and Oriented Blood Vessels" Materials 16, no. 24: 7518. https://doi.org/10.3390/ma16247518
APA StyleHayashi, K., Kishida, R., Tsuchiya, A., & Ishikawa, K. (2023). Effects of Space Dimensionality within Scaffold for Bone Regeneration with Large and Oriented Blood Vessels. Materials, 16(24), 7518. https://doi.org/10.3390/ma16247518






