Zirconium Surface Treatment via Chemical Etching
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Preliminary Investigations
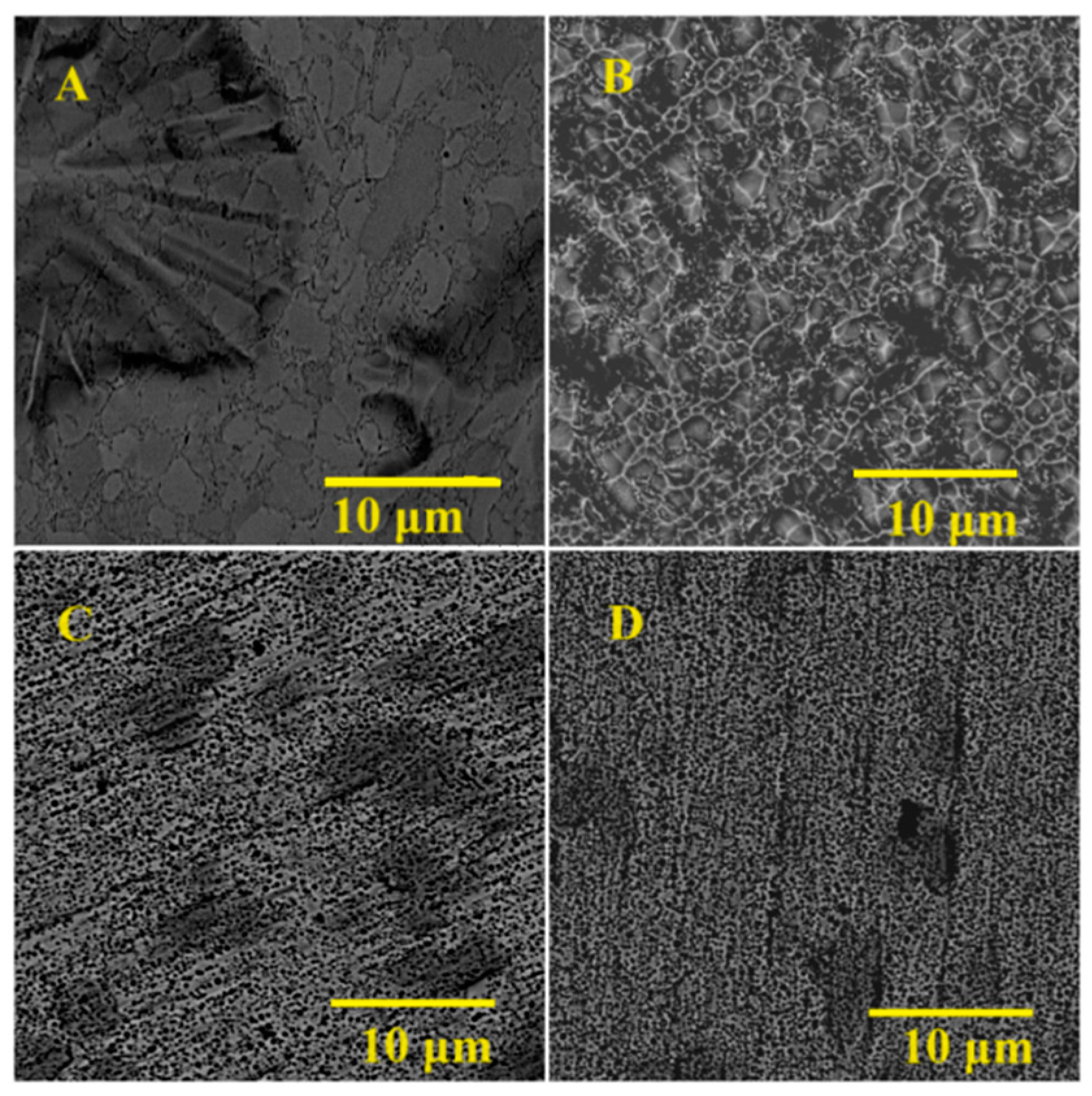
3.2. Main Samples’ Surface Morphology
3.3. Main Samples’ EDX
3.4. Main Samples’ Surface Roughness
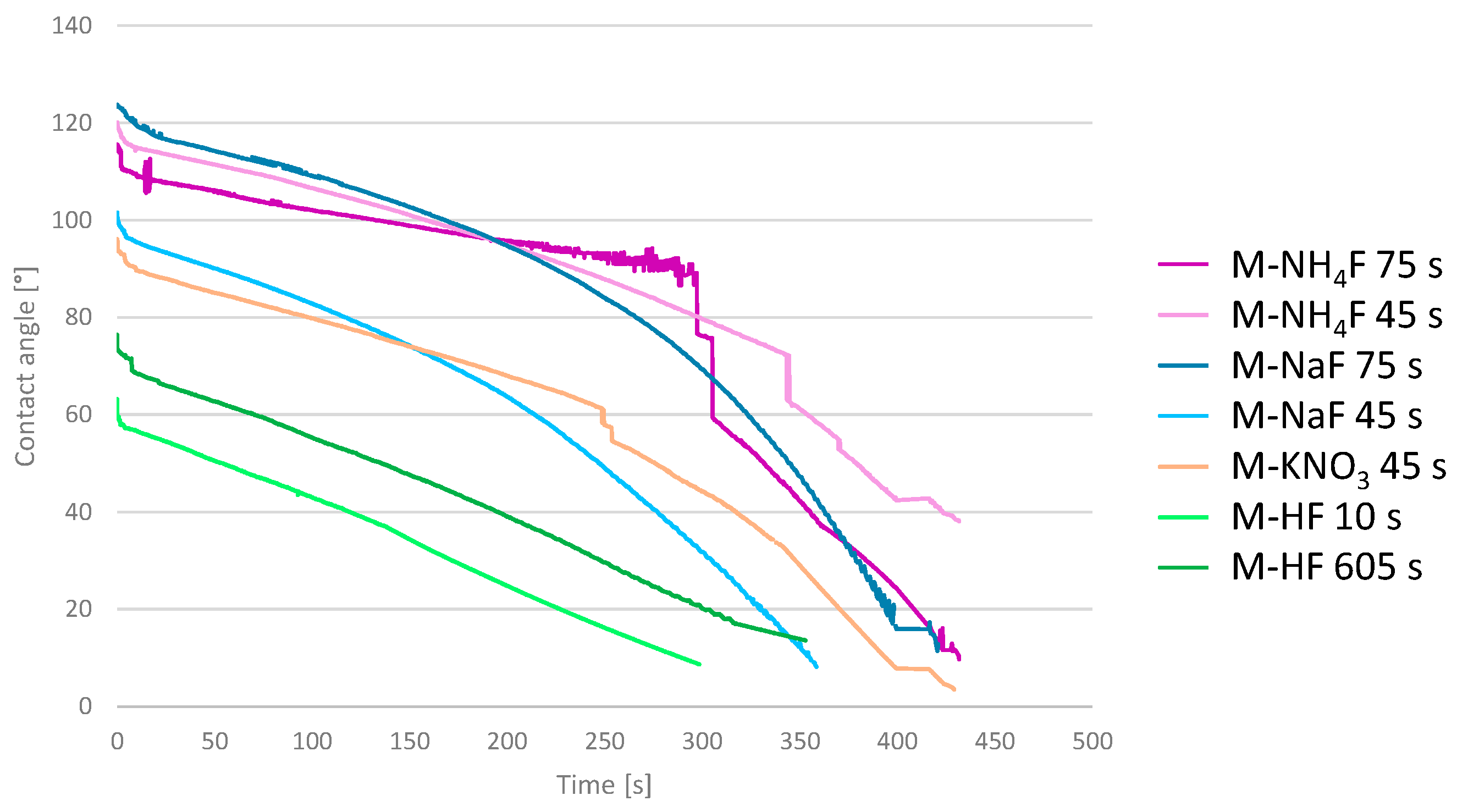
3.5. Main Samples’ Surface Wettability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, D. Implants in Dental and Maxillofacial Surgery. Biomaterials 1981, 2, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Tschernitschek, H.; Borchers, L.; Geurtsen, W. Nonalloyed Titanium as a Bioinert Metal—A Review. J. Prosthet. Dent. 2006, 96, 12. [Google Scholar] [CrossRef]
- Sykaras, N.; Iacopino, A.M.; Marker, V.A.; Triplett, R.G.; Woody, R.D. Implant Materials, Designs, and Surface Topographies: Their Effect on Osseointegration. A Literature Review. Int. J. Oral Maxillofac. Implant. 2000, 15, 675–690. [Google Scholar]
- Ravnholt, G. Corrosion Current and PH Rise around Titanium Coupled to Dental Alloys. Eur. J. Oral Sci. 1988, 96, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Chiapasco, M.; Casentini, P.; Zaniboni, M.; Corsi, E.; Anello, T. Titanium-Zirconium Alloy Narrow-Diameter Implants (Straumann Roxolid®) for the Rehabilitation of Horizontally Deficient Edentulous Ridges: Prospective Study on 18 Consecutive Patients. Clin. Oral Implant. Res. 2011, 23, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Grandin, H.M.; Berner, S.; Dard, M. A Review of Titanium Zirconium (TIZR) Alloys for Use in Endosseous Dental Implants. Materials 2012, 5, 1348–1360. [Google Scholar] [CrossRef]
- Zhao, Q.; Ueno, T.; Wakabayashi, N. A review in titanium-zirconium binary alloy for use in dental implants: Is there an ideal Ti-Zr composing ratio? Jpn. Dent. Sci. Rev. 2023, 59, 28–37. [Google Scholar] [CrossRef]
- Al-Radha, A.S.D. The Influence of Different Acids Etch on Dental Implants Titanium Surface. IOSR J. Dent. Med. Sci. 2016, 15, 87–91. [Google Scholar] [CrossRef]
- Ma, T.; Ge, X.; Zhang, Y.; Lin, Y. Effect of Titanium Surface Modifications of Dental Implants on Rapid Osseointegration; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Sriamporn, T.; Thamrongananskul, N.; Busabok, C.; Poolthong, S.; Uo, M.; Tagami, J. Dental Zirconia Can Be Etched by Hydrofluoric Acid. Dent. Mater. J. 2014, 33, 79–85. [Google Scholar] [CrossRef]
- Ruyter, E.; Vajeeston, N.; Knarvang, T.; Kvam, K. A Novel Etching Technique for Surface Treatment of Zirconia Ceramics to Improve Adhesion of Resin-Based Luting Cements. Acta Biomater. Odontol. Scand. 2017, 3, 36–46. [Google Scholar] [CrossRef]
- Śmielak, B.; Klimek, L. Effect of Hydrofluoric Acid Concentration and Etching Duration on Select Surface Roughness Parameters for Zirconia. J. Prosthet. Dent. 2015, 113, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Chaiyabutr, Y.; McGowan, S.; Phillips, K.; Kois, J.C.; Giordano, R. The Effect of Hydrofluoric Acid Surface Treatment and Bond Strength of a Zirconia Veneering Ceramic. J. Prosthet. Dent. 2008, 100, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Ehrensberger, M.T.; Gibson, M.P.; Kim, H.-I.; Monaco, E.A. Biomaterial Properties of Titanium in Dentistry. J. Oral Biosci. 2015, 57, 192–199. [Google Scholar] [CrossRef]
- Kohn, D.H. Metals in Medical Applications. Curr. Opin. Solid State Mater. Sci. 1998, 3, 309–316. [Google Scholar] [CrossRef]
- Arango, S.C.; Peláez-Vargas, A.; Garćıa, C. Coating and Surface Treatments on Orthodontic Metallic Materials. Coatings 2012, 3, 1–15. [Google Scholar] [CrossRef]
- Canabarro, A.; Paiva, C.G.; Ferreira, H.T.; Tholt-De-Vasconcellos, B.; De-Deus, G.; Prioli, R.; Linhares, A.; Alves, G.G.; Granjeiro, J.M. Short-Term Response of Human Osteoblast-Like Cells on Titanium Surfaces with Micro- and Nano-Sized Features. Scanning 2012, 34, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-I.; Choi, S.; Ryu, J.J.; Koh, S.Y.; Park, J.H.; Lee, I.S. The Biocompatibility of SLA-Treated Titanium Implants. Biomed. Mater. 2008, 3, 025011. [Google Scholar] [CrossRef]
- Wally, Z.J.; Van Grunsven, W.; Claeyssens, F.; Goodall, R.; Reilly, G.C. Porous Titanium for Dental Implant Applications. Metals 2015, 5, 1902–1920. [Google Scholar] [CrossRef]
- Mur, F.J.G.; Manero, J.M.; Rúperez, E.; Ortega, E.V.; Jiménez-Guerra, Á.; Ortiz-García, I.; Monsalve-Guil, L. Mineralization of Titanium Surfaces: Biomimetic Implants. Materials 2021, 14, 2879. [Google Scholar] [CrossRef]
- Anselme, K.; Bigerelle, M.; Noël, B.; Iost, A.; Hardouin, P. Effect of Grooved Titanium Substratum on Human Osteoblastic Cell Growth. J. Biomed. Mater. Res. 2002, 60, 529–540. [Google Scholar] [CrossRef]
- Schenck, J.F. The Role of Magnetic Susceptibility in Magnetic Resonance Imaging: MRI Magnetic Compatibility of the First and Second Kinds. Med. Phys. 1996, 23, 815–850. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Mechanical Properties of Biomedical Titanium Alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Xue, R.; Wang, D.; Tian, Y.; Wang, J.; Liu, L.; Zhang, L. Zr-XNb-4Sn Alloys with Low Young’s Modulus and Magnetic Susceptibility for Biomedical Implants. Prog. Nat. Sci. Mater. Int. 2021, 31, 772–778. [Google Scholar] [CrossRef]
- Poli, P.P.; De Miranda, F.V.; Polo, T.O.B.; Júnior, J.F.S.; De Lima Neto, T.J.; Rios, B.R.; Assunção, W.G.; Ervolino, E.; Maiorana, C.; Faverani, L.P. Titanium Allergy Caused by Dental Implants: A Systematic Literature Review and Case Report. Materials 2021, 14, 5239. [Google Scholar] [CrossRef] [PubMed]
- As, D.S.M. Titanium Allergy: Is Zirconia a Viable Alternative? J. Dent. Probl. Solut. 2017, 4, 031–035. [Google Scholar] [CrossRef]
- Nagano, H.; Kajimura, H.; Yamanaka, K. Corrosion Resistance of Zirconium and Zirconium-Titanium Alloy in Hot Nitric Acid. Mater. Sci. Eng. A 1995, 198, 127–134. [Google Scholar] [CrossRef]
- Malakhova, É.K.; Kuzyukov, A.N.; Meshcheryakov, A.V. Corrosion Resistance of Zirconium Alloys in Acetic Acid Media. Chem. Pet. Eng. 1995, 31, 183–185. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, J.; Yan, G.; Liu, H.; Huang, J.-C.; Wang, L. Thermodynamic Properties of Zirconium-Oxygen Solid Solution and Its Deoxidation in Calcium Chloride Molten Salt. J. Alloys Compd. 2019, 810, 151964. [Google Scholar] [CrossRef]
- Considine, D.M.; Considine, G.D. Van Nostrand’s Scientific Encyclopedia; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Yeniyol, S.; Bölükbaşι, N.; Çakır, A.; Bılır, A.; Özdemir, T. Effects of Surface Modifications with Oxalic Acid Etching and Sandblasting on Surface Topography and Biocompatibility of CpTi Surfaces. Biotechnol. Biotechnol. Equip. 2013, 27, 3995–4001. [Google Scholar] [CrossRef]
- Iwaya, Y.; Machigashira, M.; Kanbara, K.; Miyamoto, M.; Noguchi, K.; Izumi, Y.; Ban, S. Surface Properties and Biocompatibility of Acid-Etched Titanium. Dent. Mater. J. 2008, 27, 415–421. [Google Scholar] [CrossRef]
- Wall, E.M.V.; Whitener, E.M. Concentrated Nitric and Dilute Hydrofluoric Acid Mixtures in Dissolution of Zirconium Metal. Ind. Eng. Chem. 1959, 51, 51–54. [Google Scholar] [CrossRef]
- Klein, R. Dissolution of Zirconium in Nitric-Hydrofluoric Acid Mixtures. Corrosion 1997, 53, 327–332. [Google Scholar] [CrossRef]
- Straumanis, M.E.; James, W.J.; Neiman, A.S. Rates of Dissolution and Passivation of Hafnium-Free Zirconium in Hydrofluoric Acid. Corrosion 1959, 15, 20–24. [Google Scholar] [CrossRef]
- Ban, S.; Iwaya, Y.; Kōno, H.; Sato, H. Surface Modification of Titanium by Etching in Concentrated Sulfuric Acid. Dent. Mater. 2006, 22, 1115–1120. [Google Scholar] [CrossRef]
- Rosa, M.B.; Albrektsson, T.; Francischone, C.E.; Schwartz-Filho, H.O.; Wennerberg, A. The Influence of Surface Treatment on the Implant Roughness Pattern. J. Appl. Oral Sci. 2012, 20, 550–555. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A Review on the Wettability of Dental Implant Surfaces II: Biological and Clinical Aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Baek, B.J.; Park, S.Y. Waterside Corrosion of Zirconium Alloys in Nuclear Power Plants; Nuclear Fuel Cycle and Materials Section International Atomic Energy Agency: Taejon, Republic of Korea, 1999. [Google Scholar]



| Label | Composition [w/w] | Etching Time | |
|---|---|---|---|
| Group A | A-OXA7.5 | 7.5% oxalic acid | 75 min |
| A-OXA10 | 10% oxalic acid | 75 min | |
| A-OXA15 | 15% oxalic acid | 75 min | |
| A-OXA20 | 20% oxalic acid | 45, 60, 75 min | |
| A-CIT30 | 30% citric acid | 45, 60, 75 min | |
| A-CITOXA15-10 | 15% citric acid; 10% oxalic acid | 75 min | |
| A-PHOS10 | 10% Na3PO4; 10% NaOH | 45, 60, 75 min | |
| A-SULF70 | 70% H2SO4 | 45, 60, 75 min | |
| Group B | B-NaF2 | 10.0% (NH4)2S2O8; 2.10% NaF | 45, 60, 75 s |
| B-NaF3 | 10.0% (NH4)2S2O8; 3.15% NaF | 45, 60, 75 s | |
| B-NaF4 | 10.0% (NH4)2S2O8; 4.20% NaF | 45, 60, 75 s | |
| B-HNO3 | 48%HNO3; 10% HF | 20, 30 s |
| Composition [w/w] | Etching Times | |
|---|---|---|
| M-NH4F | 10.0% (NH4)2S2O8; 1.85% NH4F | 45, 75 s |
| M-KNO3 | 10.0% (NH4)2S2O8; 1.85%NH4F; 3% KNO3 | 45 s |
| M-HF | 17.4% H2SO4; 1% HF | 10, 60 s |
| M-NaF | 10.0% (NH4)2S2O8; 2.10% NaF | 45, 75 s |
| Etching Time, s | Zr | C * | N * | O * | F * | |
|---|---|---|---|---|---|---|
| Machined Zr | — | 92 | — | — | 8 | — |
| M-NaF | 45 | 41 | 28 | 18 | 10 | 3 |
| 75 | 38 | 31 | 17 | 10 | 3 | |
| M-NH4F | 45 | 43 | 27 | 20 | 9 | 2 |
| 75 | 39 | 31 | 22 | 7 | 1 | |
| M-KNO3 | 45 | 41 | 33 | 20 | 6 | 1 |
| M-HF | 10 | 50 | 39 | - | 10 | 1 |
| 60 | 47 | 41 | - | 10 | 2 |
| Etching Time, s | Ra, μm | Rz, μm | Sa, μm | Average Contact Angle, ° | |
|---|---|---|---|---|---|
| Machined Zr | — | 0.43 | 3.94 | 0.95 | 93.8 |
| M-NaF | 45 | 0.33 | 2.80 | 0,91 | 112.9 |
| 75 | 0.46 | 3.29 | 1.49 | 116.1 | |
| M-NH4F | 45 | 0.42 | 3.34 | 1.27 | 107.1 |
| 75 | 0.34 | 2.75 | 0.87 | 106.2 | |
| M-KNO3 | 45 | 0.42 | 3.37 | 1.16 | 103.3 |
| M-HF | 10 | 0.83 | 5.94 | 3.05 | 59.0 |
| 60 | 3.28 | 18.32 | 3.21 | 55.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gołasz, P.; Kołkowska, A.; Zieliński, R.; Simka, W. Zirconium Surface Treatment via Chemical Etching. Materials 2023, 16, 7404. https://doi.org/10.3390/ma16237404
Gołasz P, Kołkowska A, Zieliński R, Simka W. Zirconium Surface Treatment via Chemical Etching. Materials. 2023; 16(23):7404. https://doi.org/10.3390/ma16237404
Chicago/Turabian StyleGołasz, Przemysław, Agata Kołkowska, Rafał Zieliński, and Wojciech Simka. 2023. "Zirconium Surface Treatment via Chemical Etching" Materials 16, no. 23: 7404. https://doi.org/10.3390/ma16237404
APA StyleGołasz, P., Kołkowska, A., Zieliński, R., & Simka, W. (2023). Zirconium Surface Treatment via Chemical Etching. Materials, 16(23), 7404. https://doi.org/10.3390/ma16237404







