High-Grade Ferronickel Concentrates Prepared from Laterite Nickel Ore by a Carbothermal Reduction and Magnetic Separation Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization and Thermodynamic Equilibrium Calculation Methods
2.2. Raw Materials
2.2.1. Laterite Nickel Ore
2.2.2. Reductants and Additives
2.2.3. Thermodynamic Feasibility Analysis of Carbothermal Reduction
2.3. Experimental Methods
2.3.1. Sample Preparation
2.3.2. Carbothermal Reduction and Magnetic Separation
3. Results and Discussion
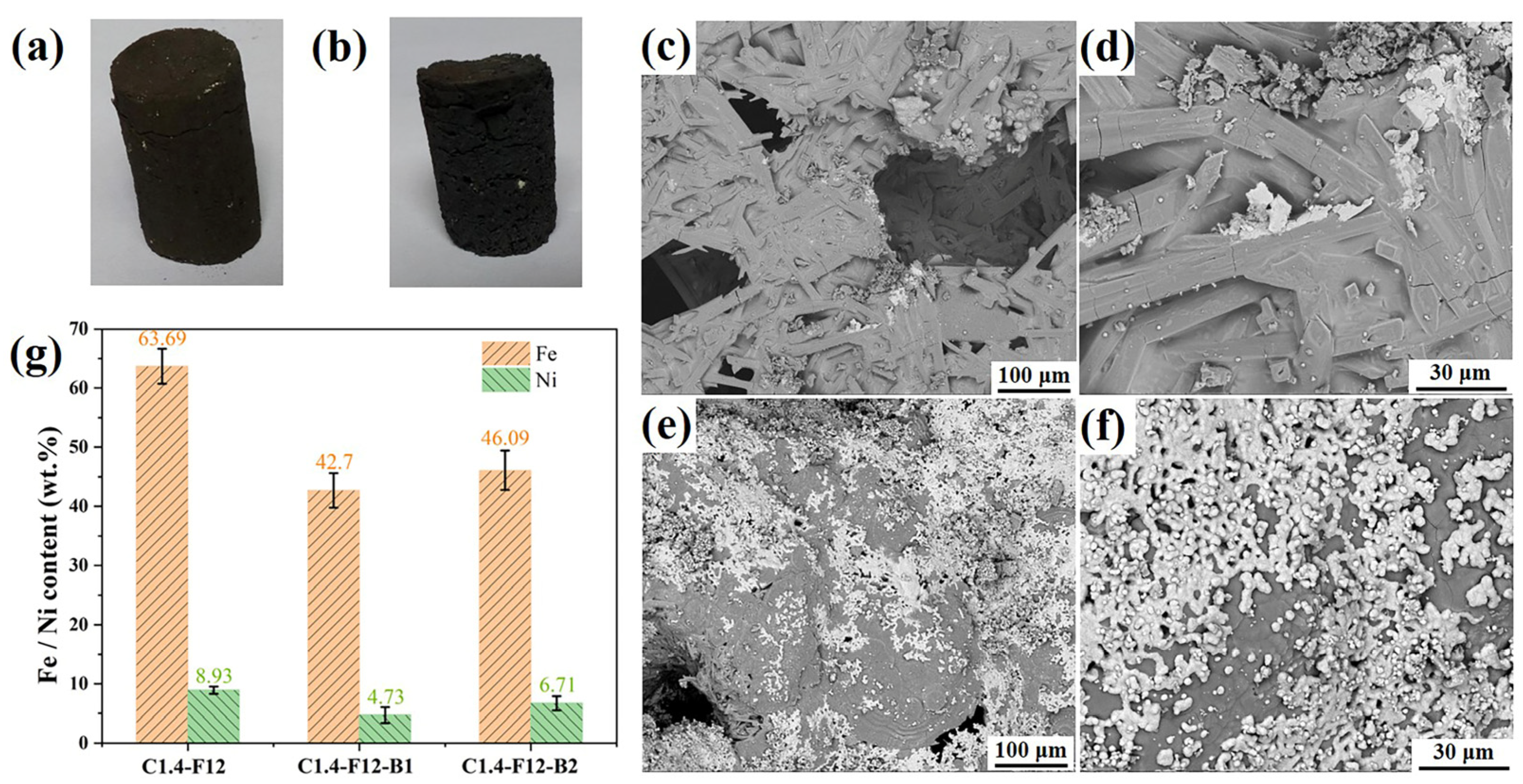
3.1. The Morphology of As-Prepared Ferronickel Concentrates
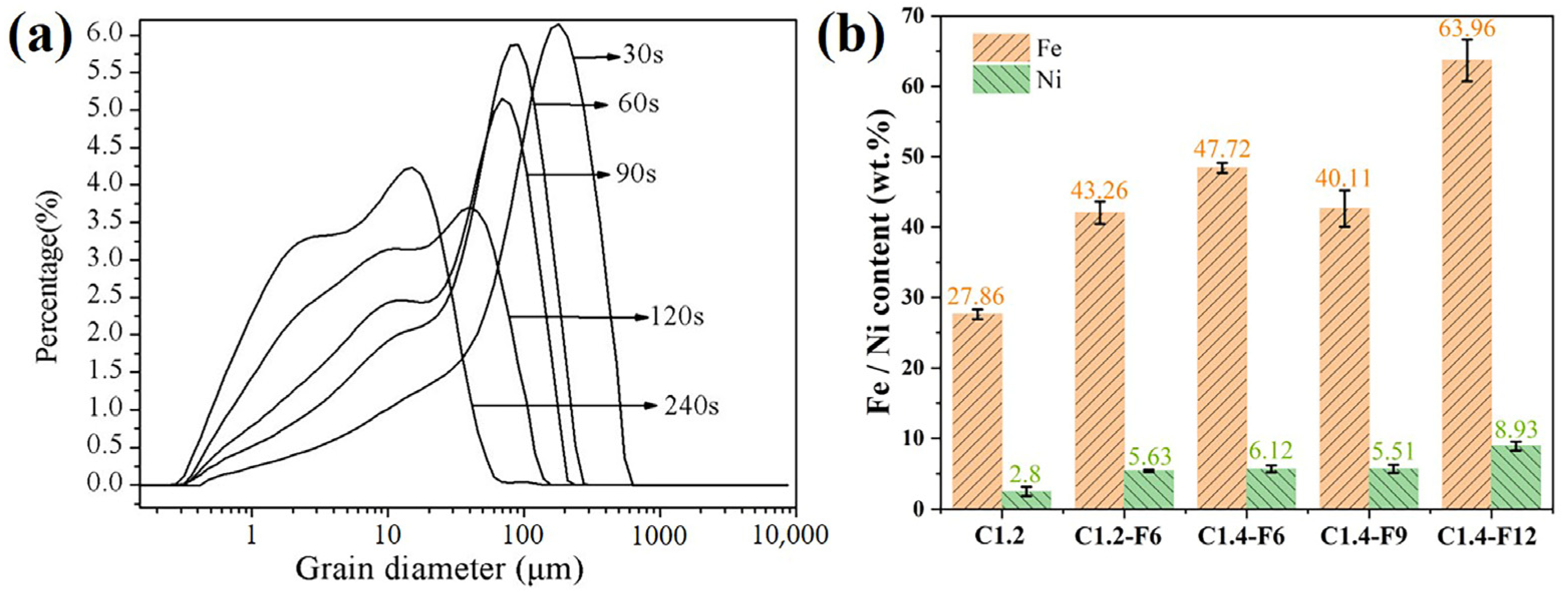
3.2. Iron and Nickel Contents of As-Prepared Ferronickel Concentrates after Fine Grinding and Magnetic Separation
3.3. Effects of the Additive Type on the Iron and Nickel Contents of As-Prepared Ferronickel Concentrates
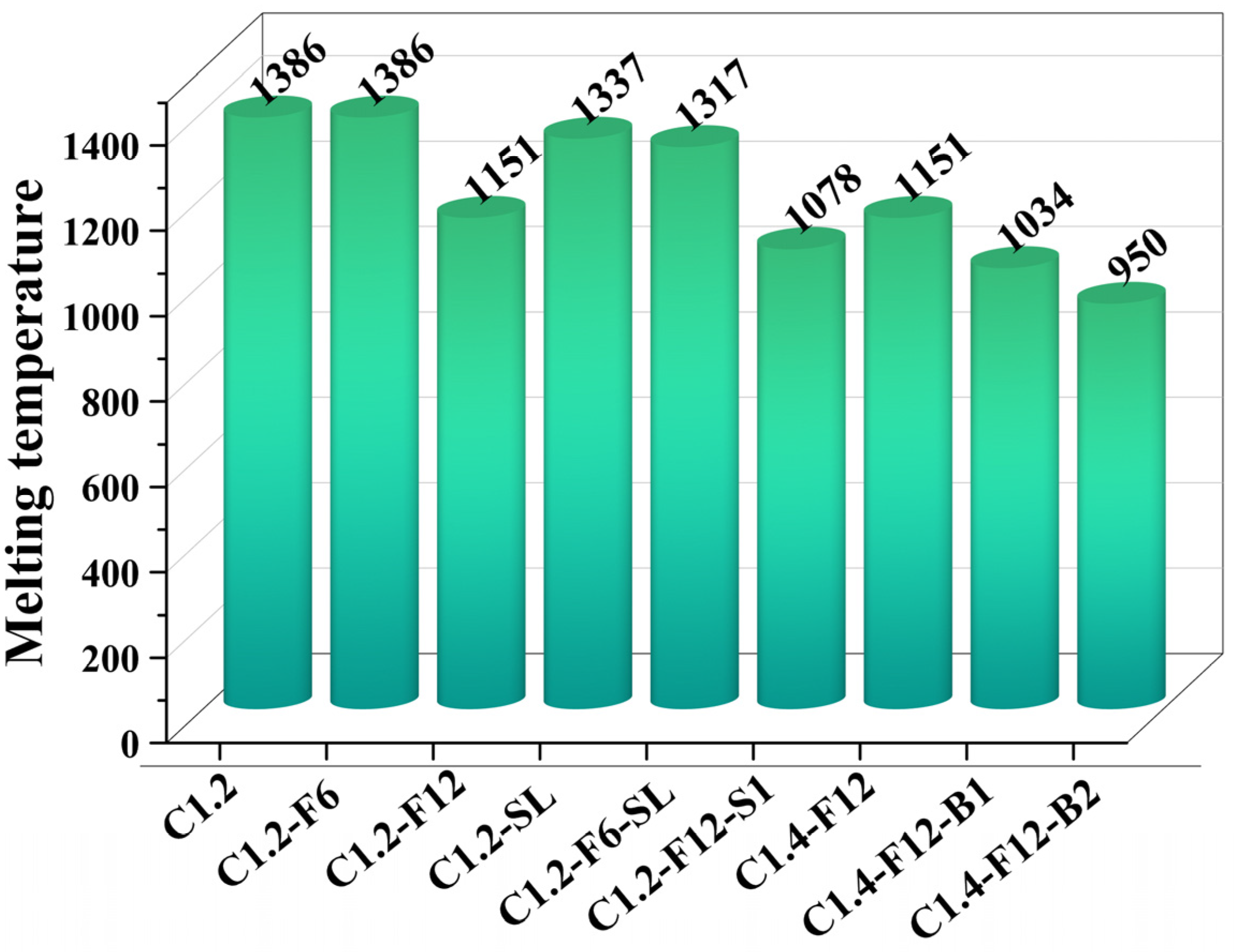
3.4. Thermodynamic Equilibrium and Melting Temperature Calculation
4. Conclusions
- The addition of Ca(OH)2 and Na2SO4 played negative roles in the formation of iron and nickel, while CaF2 and H3BO3 were beneficial to that. The higher the amount of CaF2 and H3BO3 added, the higher the grade of the obtained ferronickel concentrate. Thermodynamic calculation also confirmed that the addition of CaF2 can reduce the melting temperature of the sample, which facilitated the reduction of iron and nickel.
- The thermodynamic calculation revealed that the simultaneous addition of CaF2 and H3BO3 can significantly reduce the formation temperature of the melting phase in the carbothermal reduction process, facilitating the reduction of iron and nickel, which was aligned with experimental results. However, the sizes of reduced ferronickel alloy particles were too small (<10 μm), making it difficult to separate them from the ore even after fine grinding, thus decreasing the recovery of the ferronickel alloy. Conversely, in the cases of only adding CaF2, natural magnetism was sufficient to separate the ferronickel particles from the product after fine grinding, eliminating the need for additional electromagnetic fields and thereby reducing energy consumption.
- Increasing the fine grinding time was beneficial for improving the grade of the ferronickel concentrate. The optimal fine grinding time of 240 s was conductive to the magnetic separation of ferronickel particles.
- High-grade ferronickel concentrates with nickel content of 8.93 wt% and iron content of 63.96 wt% were obtained after reducing laterite nickel ore at 1250 °C for 15 min with a carbon ratio of 1.4 and 9.85 wt% CaF2, which can serve as raw materials in the blast furnace iron smelting, stainless steel, alloy manufacturing, and electroplating industries.
- The metal recoveries of nickel and iron in this work reached 96.28% and 82.14%, respectively, which offers guidance on effectively exploiting and utilizing low-grade laterite nickel ore to maintain the nickel supply. In addition, the design and implementation of a larger-scale carbothermal reduction process for laterite nickel ores are expected to be achieved.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hang, G.; Xue, Z.; Wu, Y.J.; Zhang, B. Effect of CaF2 on the aggregation and growth of ferronickel particles in the self-reduction of Nickel laterite ore. Met. Res. Technol. 2021, 118, 407. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, X.; Wei, Y.; Xia, L.; Pi, C.; Song, H.; Zheng, Y.; Gao, B.; Fu, J.; Chu, P.K. General synthesis of NiCo alloy nanochain arrays with thin oxide coating: A highly efficient bifunctional electrocatalyst for overall water splitting. J. Alloys Compd. 2019, 797, 1216–1223. [Google Scholar] [CrossRef]
- Li, B.; Chen, J.; Liu, D.; Gridnev, I.D.; Zhang, W. Nickel-catalysed asymmetric hydrogenation of oximes. Nat. Chem. 2022, 14, 920–927. [Google Scholar] [CrossRef]
- Wang, Z.; Li, B.; Feng, Z.; Hui, S.; Yang, Y.; Wang, H. Extraction of Ferronickel Concentrate by Reduction Roasting-Magnetic Separation from Low Grade Laterite Nickel Ore under the Action of Compound Additives. Mater. Trans. 2022, 63, 1197–1204. [Google Scholar] [CrossRef]
- Tian, H.; Guo, Z.; Zhan, R.; Pan, J.; Zhu, D.; Yang, C.; Pan, L. Effective and economical treatment of low-grade nickel laterite by a duplex process of direct reduction-magnetic separation & rotary kiln-electric furnace and its industrial application. Powder Technol. 2021, 394, 120–132. [Google Scholar] [CrossRef]
- Yuan, S.; Zhou, W.-T.; Li, Y.-J.; Han, Y.-X. Efficient enrichment of nickel and iron in laterite nickel ore by deep reduction and magnetic separation. Trans. Nonferr. Met. Soc. China 2020, 30, 812–822. [Google Scholar] [CrossRef]
- Shi, R.; Li, X.; Cui, Y.; Zhao, J.; Zou, C.; Qiu, G. Coupled Preparation of Ferronickel and Cementitious Material from Laterite Nickel Ores. Materials 2020, 13, 4992. [Google Scholar] [CrossRef] [PubMed]
- Forster, J.; Pickles, C.; Elliott, R. Microwave carbothermic reduction roasting of a low grade nickeliferous silicate laterite ore. Miner. Eng. 2016, 88, 18–27. [Google Scholar] [CrossRef]
- De Alvarenga Oliveira, V.; dos Santos, C.G.; de Albuquerque Brocchi, E. Assessing the Influence of NaCl on the Reduction of a Siliceous Laterite Nickel Ore Under Caron Process Conditions. Met. Mater. Trans. B 2019, 50, 1309–1321. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Cathelineau, M.; Blancher, S.B.; Laugier, O.; Filippov, L. Characterization of Weda Bay nickel laterite ore from Indonesia. J. Geochem. Explor. 2018, 196, 270–281. [Google Scholar] [CrossRef]
- Pan, C.; Ma, K.; Chen, X.; Feng, S.; Fang, C.; Yang, H.; He, P. Corrosion behavior of Ni-based coating with Ni-coated TiC-reinforced particles by induction cladding in molten brass. Mater. Chem. Phys. 2023, 296, 127257. [Google Scholar] [CrossRef]
- Tian, H.; Pan, J.; Zhu, D.; Yang, C.; Guo, Z.; Xue, Y. Improved beneficiation of nickel and iron from a low-grade saprolite laterite by addition of limonitic laterite ore and CaCO3. J. Mater. Res. Technol. 2020, 9, 2578–2589. [Google Scholar] [CrossRef]
- Chang, L.; Bi, X.; Luo, S.; Yang, W.; Wei, A.; Yang, R.; Liu, J. Insight into the high-efficiency separation of Si element from low-grade laterite nickel ore and the preparation of Li2FeSiO4/C cathode materials for lithium-ion batteries. J. Alloys Compd. 2023, 937, 168357. [Google Scholar] [CrossRef]
- Liu, P.; Liu, Z.; Chu, M.; Yan, R.; Li, F.; Tang, J. A novel process for preparing Fe–Cr-Ni-C alloy: Synergetic reduction of stainless steel dust and laterite nickel ore. Environ. Sci. Pollut. Res. 2022, 29, 65500–65520. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, L.; He, Z.; Hou, X.; Zhan, W.; Pang, Q. Separation and recovery of iron and nickel from low-grade laterite nickel ore by microwave carbothermic reduction roasting. J. Mater. Res. Technol. 2020, 9, 12223–12235. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, C.; Li, B.; Wei, Y. Migration and Aggregation Behavior of Nickel and Iron in Low Grade Laterite Ore with New Additives. Metals 2021, 11, 2033. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, X.; Luo, Y.; Pan, J.; Bai, B. Reduction Smelting Low Ferronickel from Pre-concentrated Nickel-Iron Ore of Nickel Laterite. High Temp. Mater. Process. 2016, 35, 1031–1036. [Google Scholar] [CrossRef]
- Dong, J.; Wei, Y.; Lu, C.; Zhou, S.; Li, B.; Ding, Z.; Wang, C.; Ma, B. Influence of Calcium Chloride Addition on Coal-Based Reduction Roasting of Low-Nickel Garnierite Ore. Mater. Trans. 2017, 58, 1161–1168. [Google Scholar] [CrossRef]
- Ma, B.; Yang, W.; Xing, P.; Wang, C.; Chen, Y.; Lv, D. Pilot-scale plant study on solid-state metalized reduction–magnetic separation for magnesium-rich nickel oxide ores. Int. J. Miner. Process. 2017, 169, 99–105. [Google Scholar] [CrossRef]
- Zhu, D.; Pan, L.; Guo, Z.; Pan, J.; Zhang, F. Utilization of limonitic nickel laterite to produce ferronickel concentrate by the selective reduction-magnetic separation process. Adv. Powder Technol. 2018, 30, 451–460. [Google Scholar] [CrossRef]
- Ma, B.; Xing, P.; Yang, W.; Wang, C.; Chen, Y.; Wang, H. Solid-State Metalized Reduction of Magnesium-Rich Low-Nickel Oxide Ores Using Coal as the Reductant Based on Thermodynamic Analysis. Met. Mater. Trans. B 2017, 48, 2037–2046. [Google Scholar] [CrossRef]
- Hang, G.; Xue, Z.; Wang, J.; Wu, Y. Mechanism of Calcium Sulphate on the Aggregation and Growth of Ferronickel Particles in the self-Reduction of Saprolitic Nickel Laterite Ore. Metals 2020, 10, 423. [Google Scholar] [CrossRef]
- Qu, G.; Zhou, S.; Wang, H.; Li, B.; Wei, Y. Production of Ferronickel Concentrate from Low-Grade Nickel Laterite Ore by Non-Melting Reduction Magnetic Separation Process. Metals 2019, 9, 1340. [Google Scholar] [CrossRef]
- Ma, B.; Li, X.; Yang, W.; Hu, D.; Xing, P.; Liu, B.; Wang, C. Nonmolten state metalized reduction of saprolitic laterite ores: Effective extraction and process optimization of nickel and iron. J. Clean. Prod. 2020, 256, 120415. [Google Scholar] [CrossRef]
- Cao, C.; Xue, Z.; Duan, H. Making Ferronickel from Laterite Nickel Ore by Coal-Based Self-Reduction and High Temperature Melting Process. Int. J. Nonferr. Met. 2016, 5, 9–15. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Liu, J.; Wang, T.; Xu, Y.; Liang, J.; Jiang, F.; Feng, G.; Jiang, W. Partially stabilized tetragonal ZrO2 whiskers with preferred [001] direction derived from CaF2. J. Mater. 2021, 7, 879–885. [Google Scholar] [CrossRef]
- Cao, W.; Yang, Q. Properties of a Carbonated Steel Slag-Slaked Lime Mixture. J. Mater. Civ. Eng. 2015, 27, 04014115. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Filippov, L. Challenges in processing nickel laterite ores by flotation. Int. J. Miner. Process. 2016, 151, 59–67. [Google Scholar] [CrossRef]
- Cao, Z.; Ma, B.; Zhou, J.; Chen, Y.; Wang, C. The study for reduction roasting of laterite residue in the presence of CaF2. Process. Saf. Environ. Prot. 2022, 168, 1–9. [Google Scholar] [CrossRef]
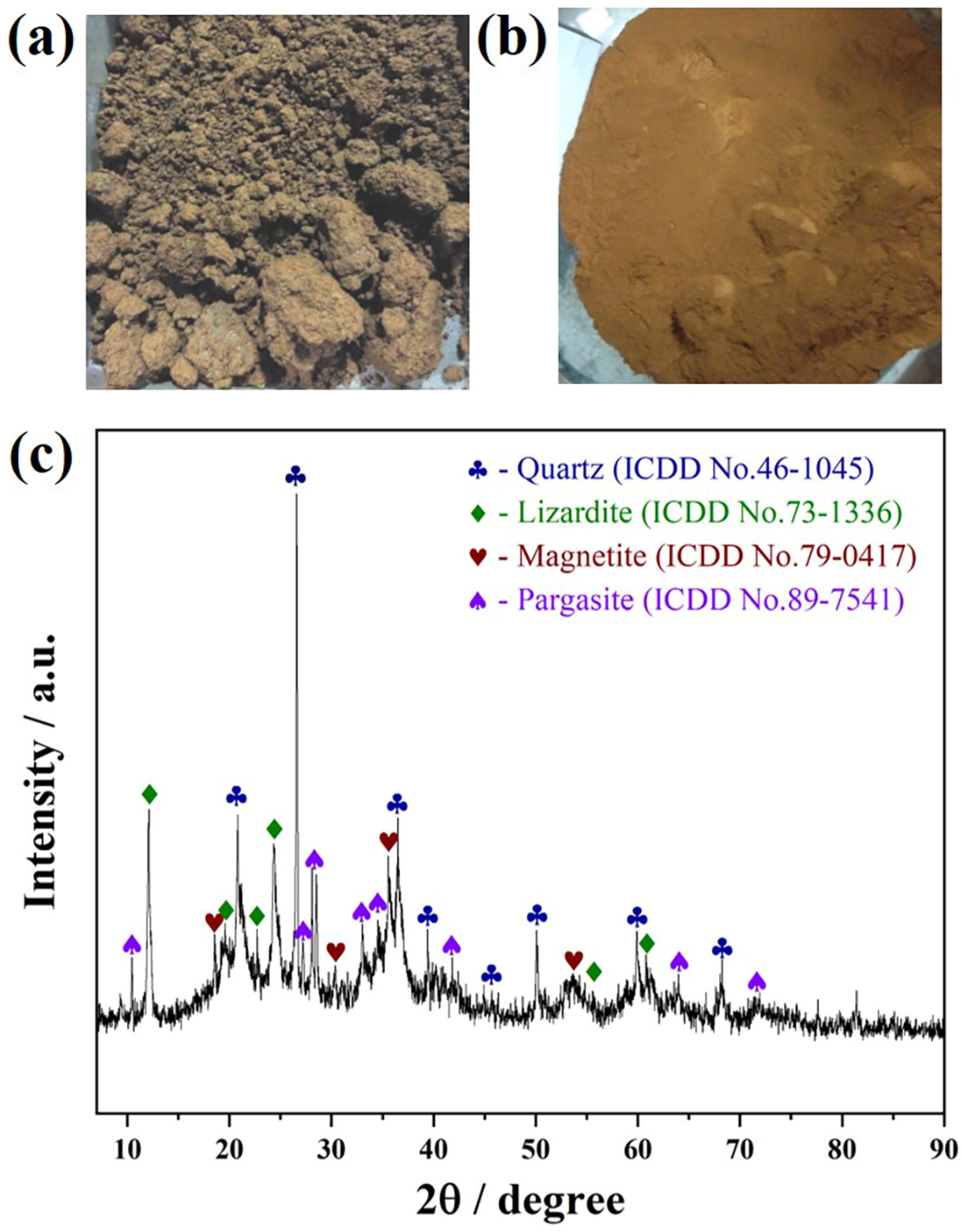
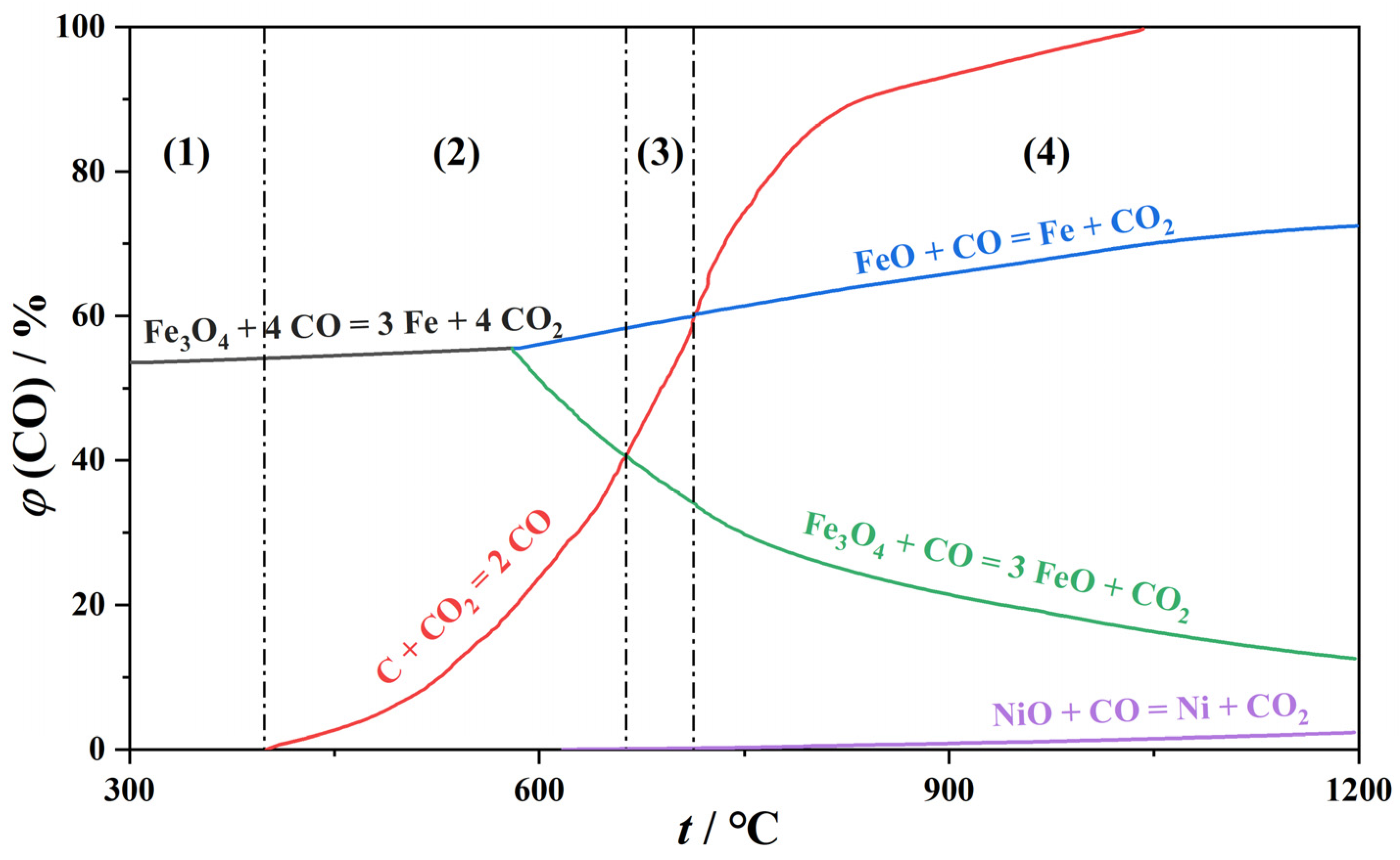
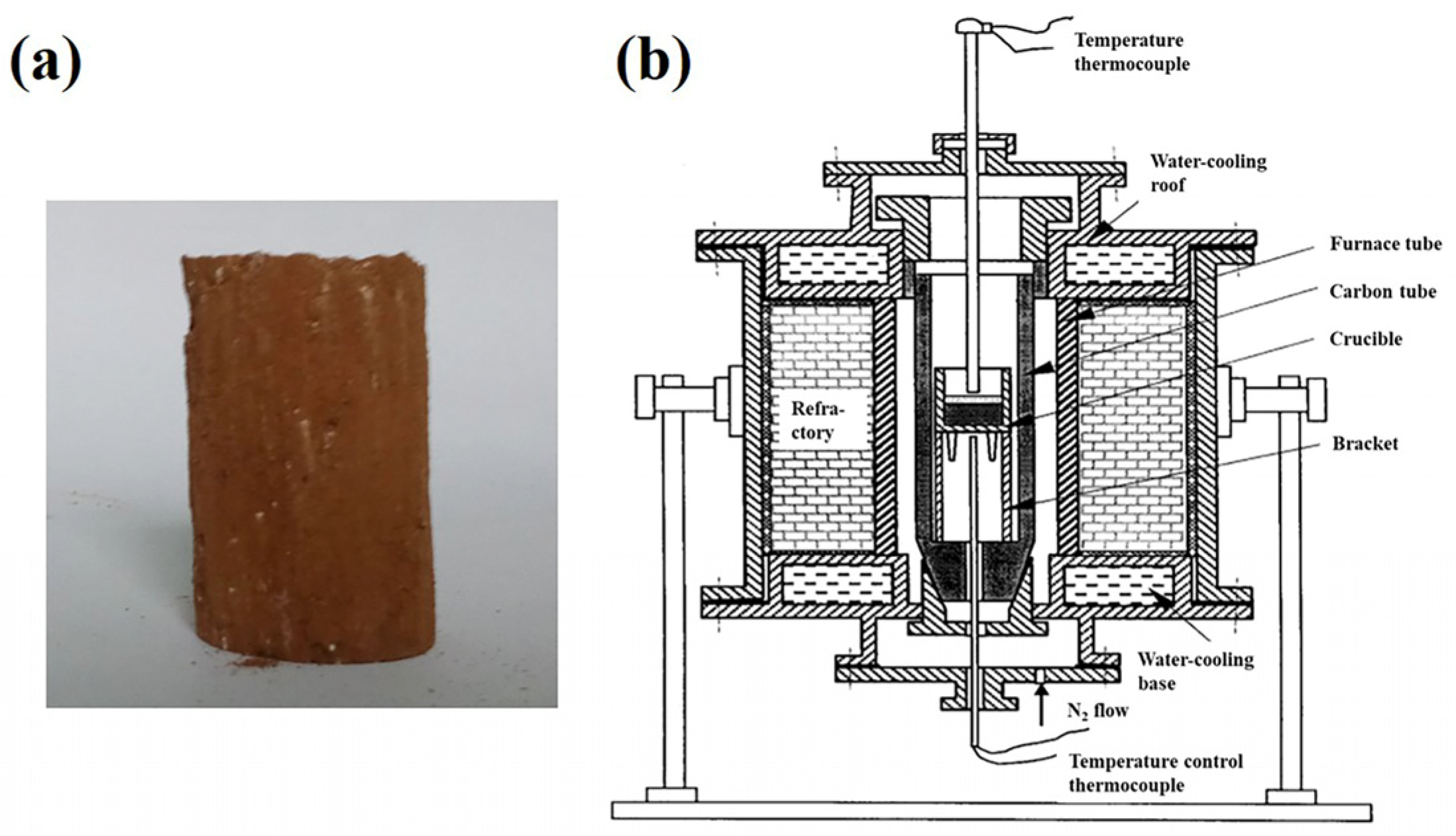
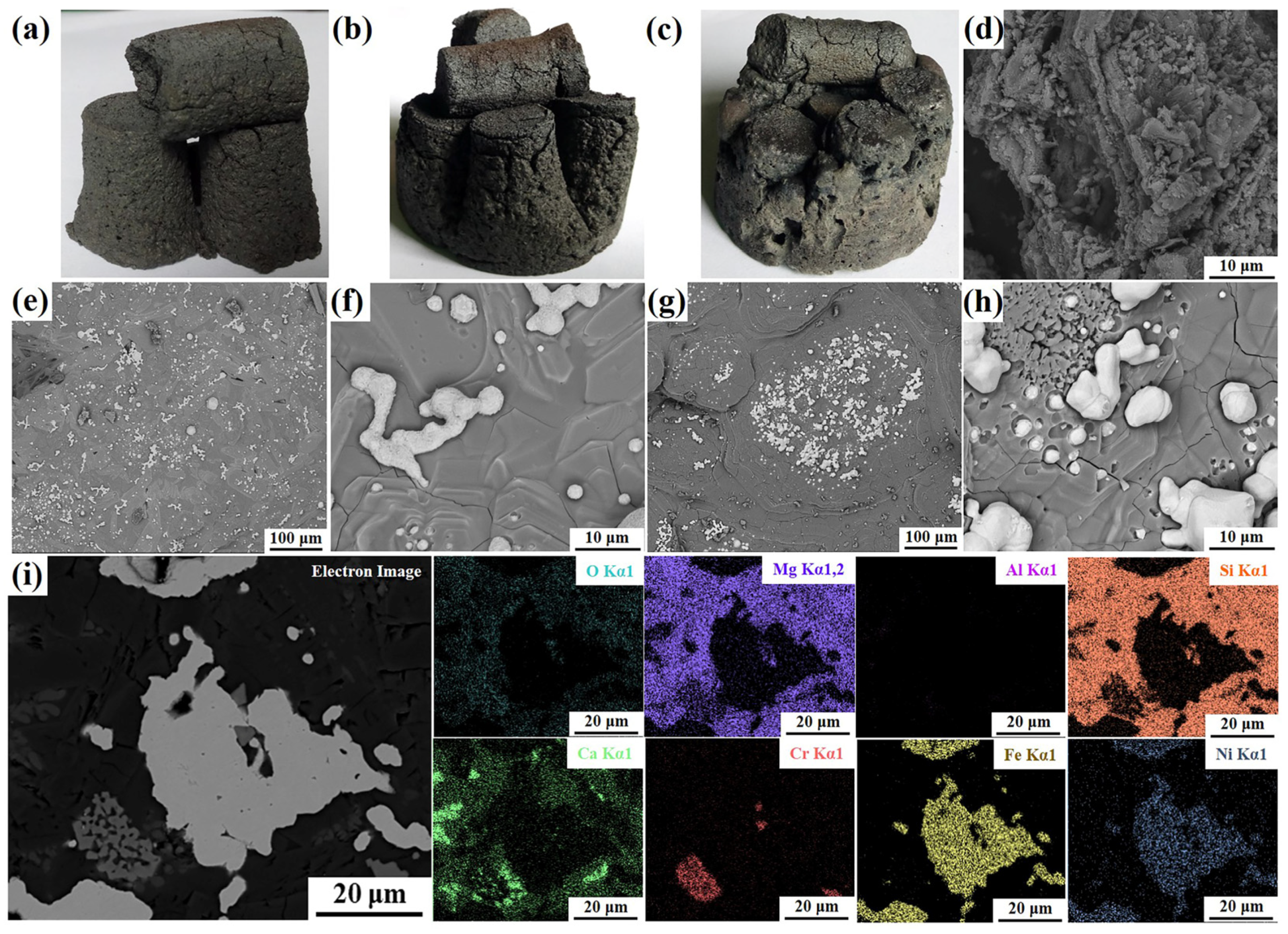
| Component | Fetotal | Nitotal | CaO | MgO | SiO2 | Al2O3 | P2O5 | Na2O | K2O | TiO2 | S | LOI 1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt%) | 15.87 | 1.32 | 0.80 | 15.10 | 40.46 | 2.79 | 0.02 | 0.23 | 0.29 | 0.02 | 0.02 | 15.24 |
| Component (wt%) | CaO | MgO | SiO2 | Al2O3 | C | P2O5 | S | Na2O | K2O | TiO2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | |||||||||||
| Anthracite | 1.28 | 0.35 | 6.09 | 2.68 | 76.56 | 0.23 | 0.54 | 0.02 | 0.41 | 0.15 | |
| Slaked lime | 67.52 | 4.56 | 0.56 | - | 2.11 | 0.01 | - | 0.04 | 0.06 | - | |
| Component (wt%) | Laterite | Carbon Ratio | Anthracite | Slaked Lime | CaF2 | Na2SO4 | H3BO3 | |
|---|---|---|---|---|---|---|---|---|
| Sample | ||||||||
| C1.2 | 92.21 | 1.2 | 7.79 | - | - | - | - | |
| C1.4 | 91.03 | 1.4 | 8.97 | - | - | - | - | |
| C1.2-F6 | 87.37 | 1.2 | 7.38 | - | 5.24 | - | - | |
| C1.2-F12 | 83.02 | 1.2 | 7.02 | - | 9.96 | - | - | |
| C1.4-F6 | 86.32 | 1.4 | 8.50 | - | 5.18 | - | - | |
| C1.4-F9 | 84.14 | 1.4 | 8.29 | - | 7.57 | - | - | |
| C1.4-F12 | 82.07 | 1.4 | 8.08 | - | 9.85 | - | - | |
| C1.2-SL | 50.84 | 1.2 | 4.30 | 44.86 | - | - | - | |
| C1.2-F6-SL | 49.34 | 1.2 | 4.17 | 43.53 | 2.96 | - | - | |
| C1.4-F6-S1 | 85.58 | 1.4 | 8.43 | - | 5.13 | 0.86 | - | |
| C1.4-F12-B1 | 81.40 | 1.4 | 8.02 | - | 9.77 | - | 0.81 | |
| C1.4-F12-B2 | 80.74 | 1.4 | 7.95 | - | 9.69 | - | 1.61 | |
| Grinding Time (s) | 30 | 60 | 90 | 120 | 240 | |
|---|---|---|---|---|---|---|
| Distribution (μm) | ||||||
| D10 | 10 | 4 | 3 | 2 | 1 | |
| D50 | 52 | 52 | 32 | 12 | 6 | |
| D90 | 339 | 147 | 114 | 63 | 26 | |
| Raw Material | Reduction Temperature (°C) | Additive | Metal Grade in Raw Material (wt%) | Metal Content in Reduction Product (wt%) | Metal Recovery (%) | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Ni | Fe | Ni | Fe | Ni | Fe | ||||
| Saprolitic laterite ore | 1300 | CaF2 | 0.8 | 10.9 | 6.1 | 68.2 | 90 | 75.7 | [24] |
| 1200 | CaSO4 | 1.4 | 16.18 | 8.08 | 79.98 | 92.6 | 79.9 | [22] | |
| Laterite nickel ore | 1250 | NaCl | 1.13 | 35.79 | 8.15 | 64.28 | 97.76 | - | [23] |
| 1500 | CaF2 | 1.27 | 46.54 | 1.79 | 89.65 | 71.3 | 59.96 | [25] | |
| 1550 | - | 1.29 | 16.31 | 10.02 | 84.02 | 95.51 | - | [5] | |
| 1275 | - | 2.26 | 14.24 | 6.96 | 34.74 | 94.06 | 80.44 | [6] | |
| 1200 | CaF2 | 1.4 | 16.18 | 7.1 | 68.5 | 84.14 | 70.24 | [1] | |
| 1250 | CaF2 | 1.06 | 11.56 | 8.39 | 67.7 | 98.54 | 71.73 | [4] | |
| 1250 | CaF2 | 1.32 | 15.87 | 8.93 | 63.96 | 96.28 | 82.14 | This work | |
| Sample | Fe Content (g) | Reduction Ratio of Fe * (%) | Ni Content (g) | Reduction Ratio of Ni * (%) |
|---|---|---|---|---|
| C1.2-F6 | 13.26 | 99.98 | 1.38 | 100 |
| C1.2-F12 | 13.26 | 99.98 | 1.38 | 100 |
| C1.4-F6 | 13.26 | 99.98 | 1.38 | 100 |
| C1.4-F9 | 13.26 | 99.98 | 1.38 | 100 |
| C1.4-F12 | 13.26 | 99.98 | 1.38 | 100 |
| C1.2-F12-S1 | 13.25 | 99.90 | 1.38 | 100 |
| C1.4-F12-B1 | 13.26 | 100 | 1.38 | 100 |
| C1.4-F12-B2 | 13.26 | 99.98 | 1.38 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Cao, C.; Xue, Z.; Li, F.; Li, S.; Duan, H.; Zhang, H. High-Grade Ferronickel Concentrates Prepared from Laterite Nickel Ore by a Carbothermal Reduction and Magnetic Separation Method. Materials 2023, 16, 7132. https://doi.org/10.3390/ma16227132
Zhang J, Cao C, Xue Z, Li F, Li S, Duan H, Zhang H. High-Grade Ferronickel Concentrates Prepared from Laterite Nickel Ore by a Carbothermal Reduction and Magnetic Separation Method. Materials. 2023; 16(22):7132. https://doi.org/10.3390/ma16227132
Chicago/Turabian StyleZhang, Jingzhe, Chang Cao, Zhengliang Xue, Faliang Li, Shaoping Li, Hongjuan Duan, and Haijun Zhang. 2023. "High-Grade Ferronickel Concentrates Prepared from Laterite Nickel Ore by a Carbothermal Reduction and Magnetic Separation Method" Materials 16, no. 22: 7132. https://doi.org/10.3390/ma16227132
APA StyleZhang, J., Cao, C., Xue, Z., Li, F., Li, S., Duan, H., & Zhang, H. (2023). High-Grade Ferronickel Concentrates Prepared from Laterite Nickel Ore by a Carbothermal Reduction and Magnetic Separation Method. Materials, 16(22), 7132. https://doi.org/10.3390/ma16227132






