A Review: Synthesis and Applications of Titanium Sub-Oxides
Abstract
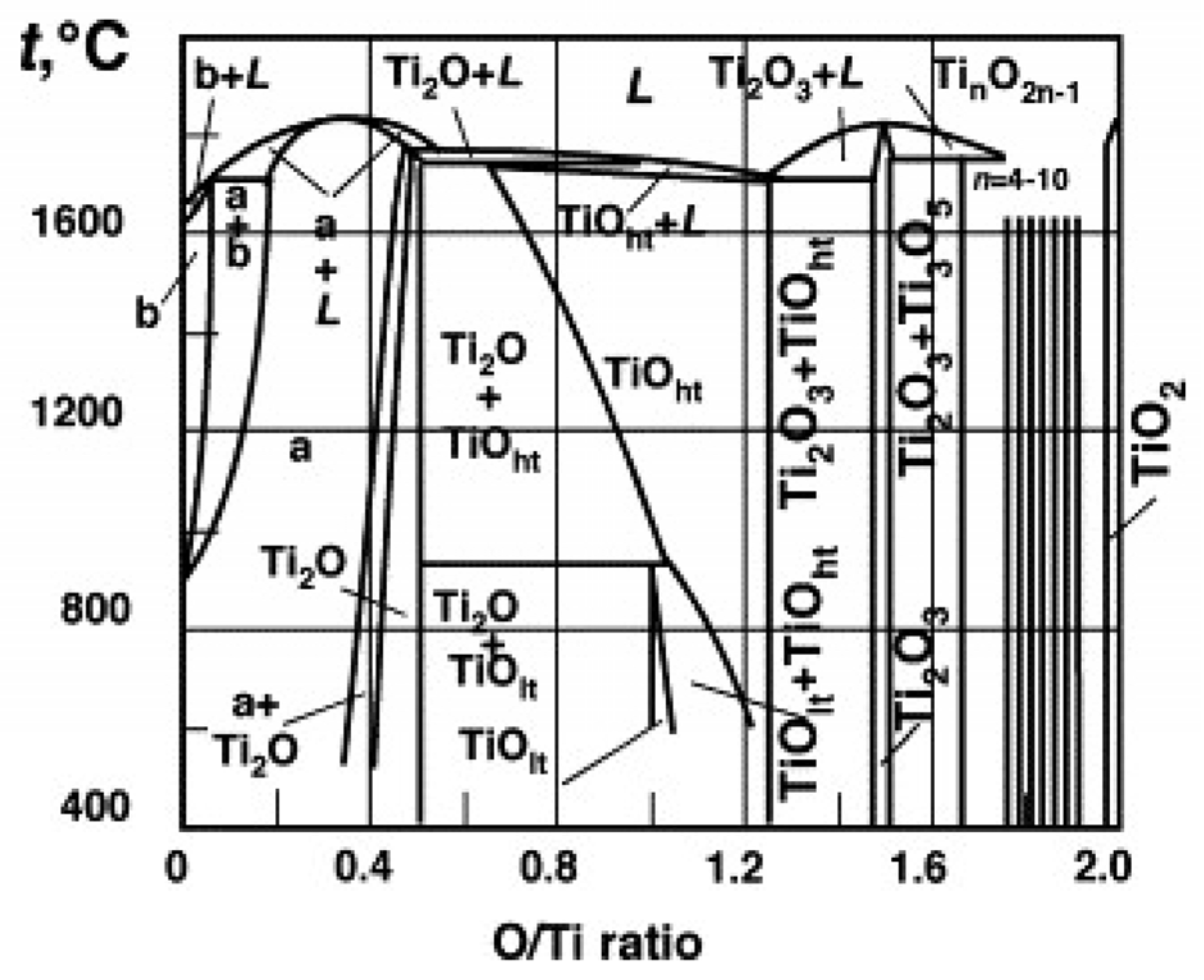
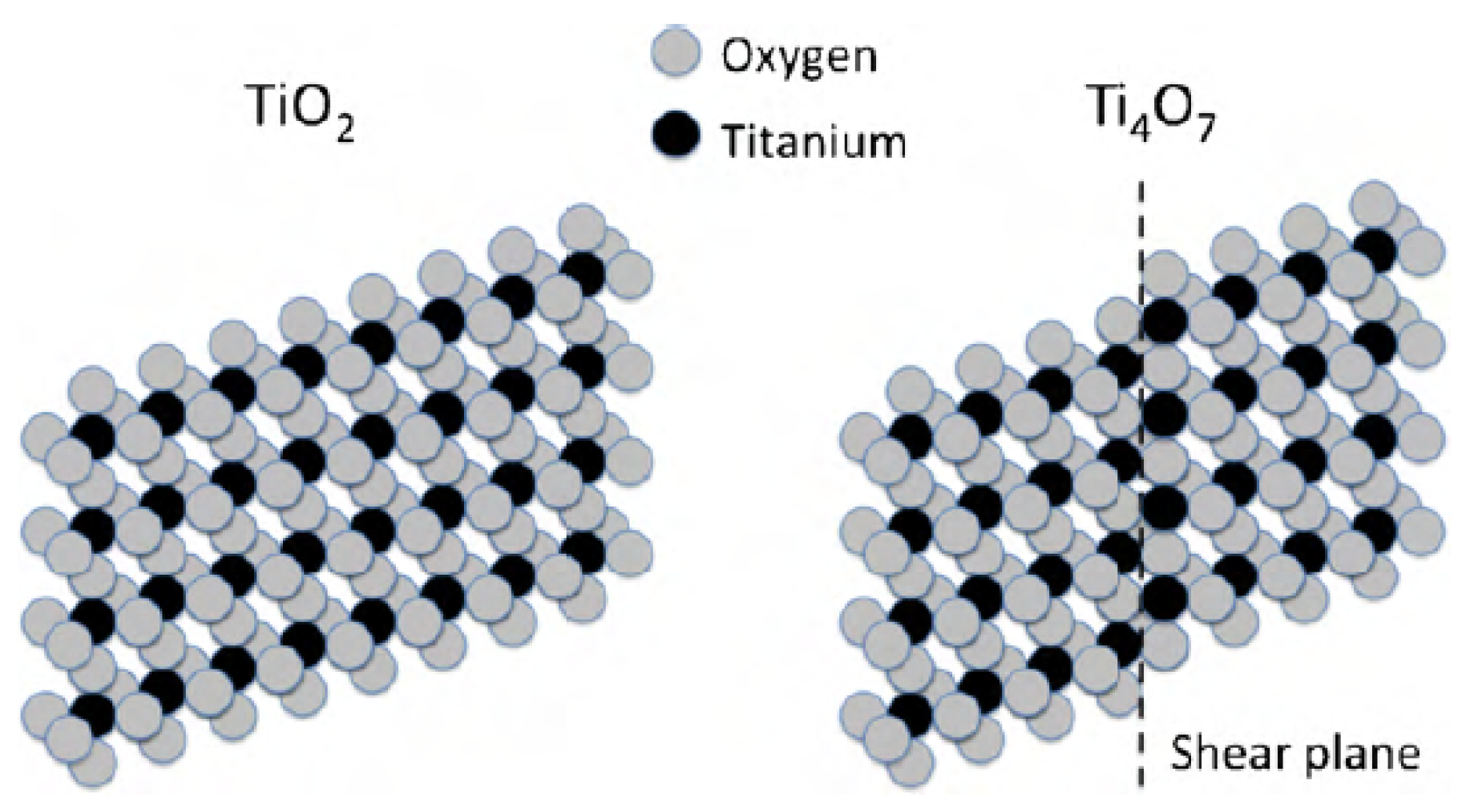
:1. Introduction
2. Synthesis Methods
2.1. Reduction of TiO2 by Hydrogen
2.2. Reduction by Carbon
2.3. Reduction by Metals
2.4. Reduction by Hydride
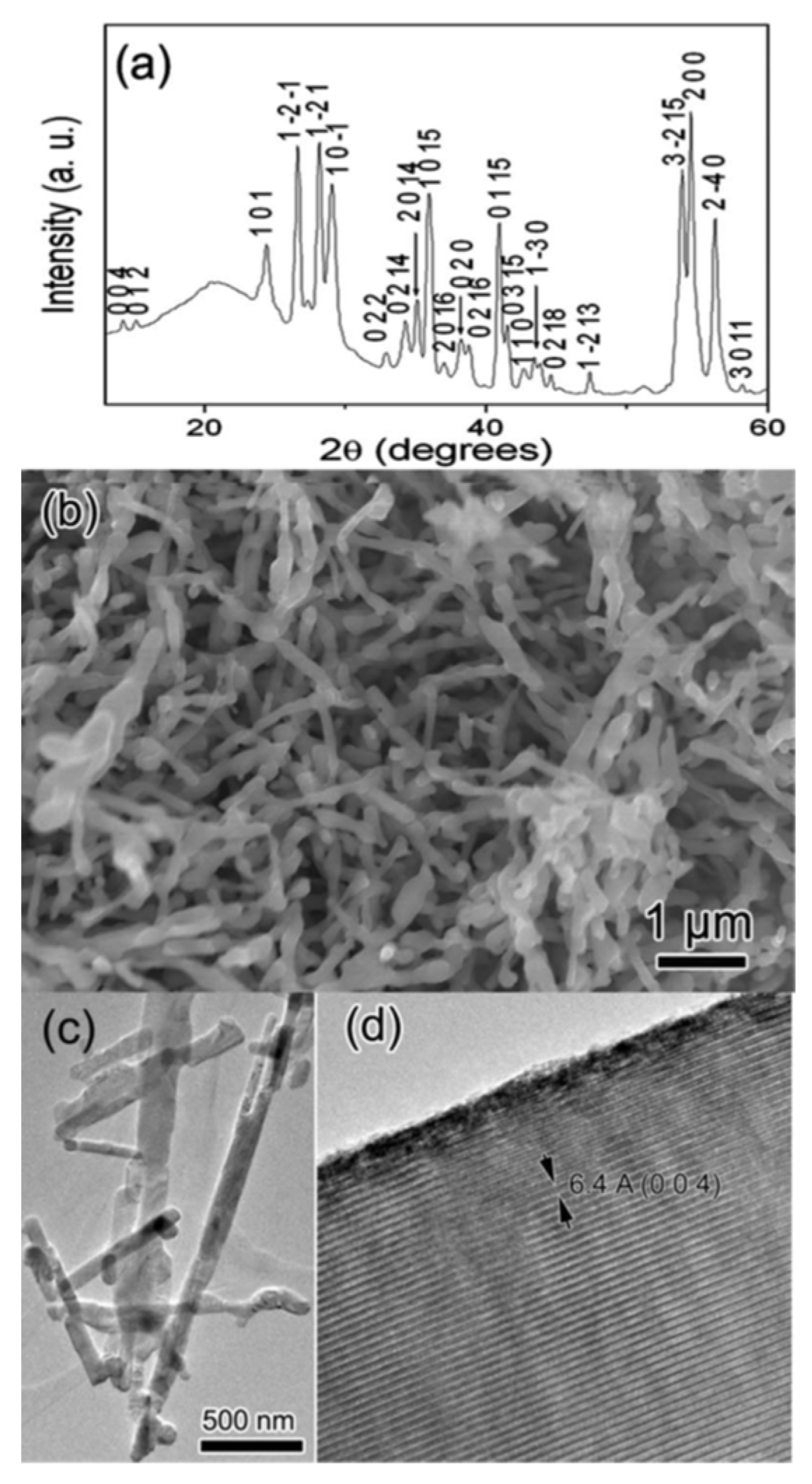
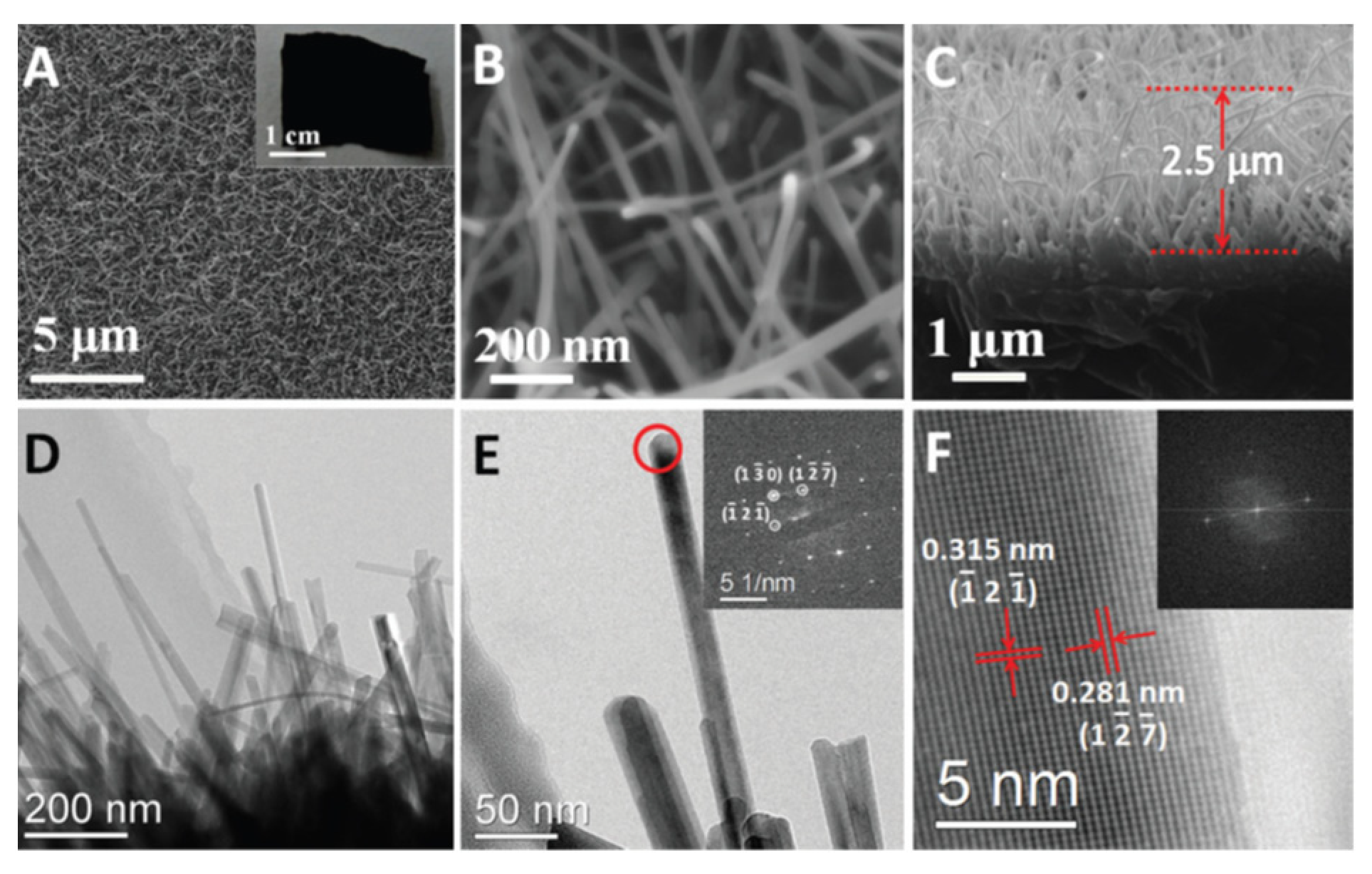
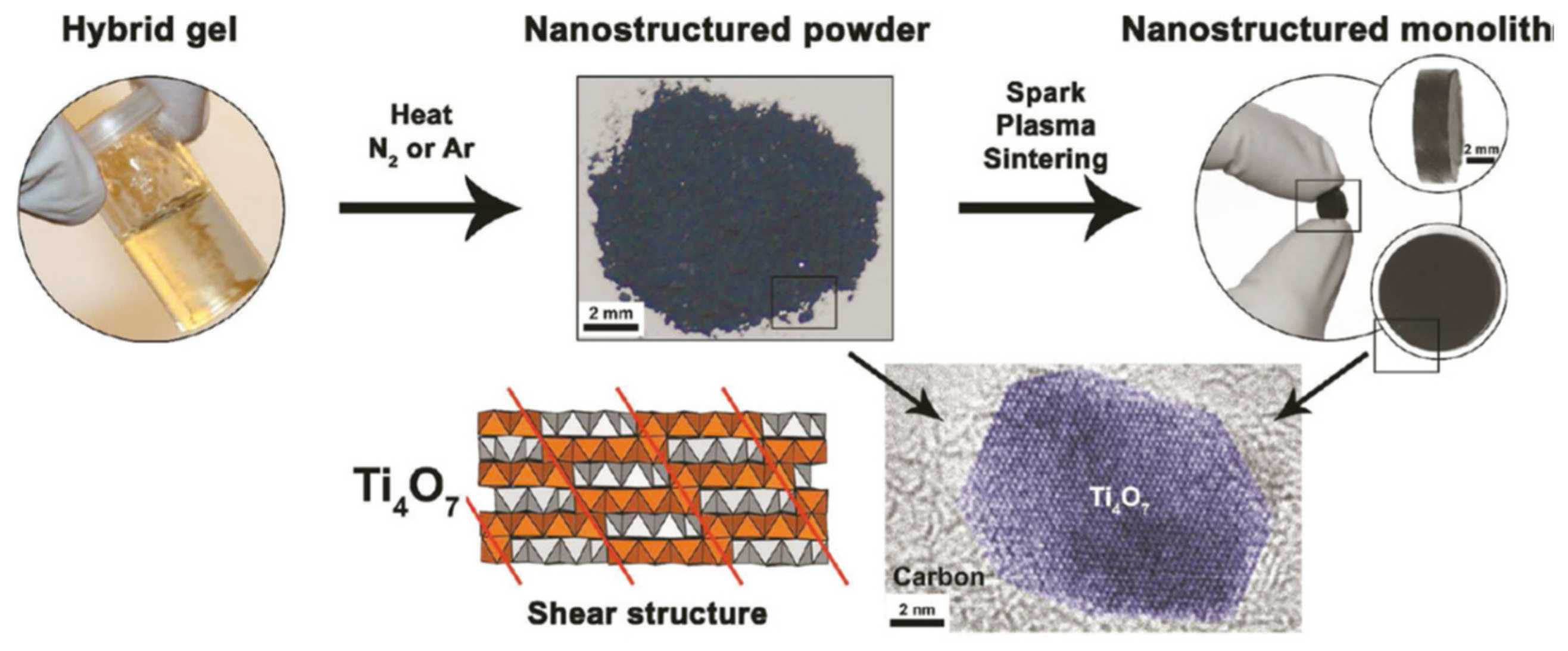
2.5. Synthesis of Nanostructured Titanium Sub-Oxides
3. Applications of Titanium Sub-Oxides
3.1. Catalysis Support in Fuel Cells
3.2. Electrocatalytic Degradation for Wastewater Treatment
3.3. Reactive Electrochemical Membrane
3.4. Batteries
3.5. Other Applications
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, J.R.; Walsh, F.C.; Clarke, R.L. Electrodes based on Magnéli phase titanium oxides: The properties and applications of Ebonex® materials. J. Appl. Electrochem. 1998, 28, 1021–1033. [Google Scholar] [CrossRef]
- Li, X.; Zhu, A.L.; Qu, W.; Wang, H.; Hui, R.; Zhang, L.; Zhang, J. Magnéli phase Ti4O7 electrode for oxygen reduction reaction and its implication for zinc-air rechargeable batteries. Electrochim. Acta 2010, 55, 5891–5898. [Google Scholar] [CrossRef]
- Fan, Y.; Feng, X.; Zhou, W.; Murakami, S.; Kikuchi, K.; Normura, N.; Wang, L.; Jiang, W.; Kawasaki, A. Preparation of monophasic titanium sub-oxides of Magnéli phase with enhanced thermoelectric performance. J. Eur. Ceram. Soc. 2018, 38, 507–513. [Google Scholar] [CrossRef]
- Kolbrecka, K.; Przyluski, J. Sub-stoichiometric titanium oxides as ceramic electrodes for oxygen evolution-structural aspects of the voltammetric behaviour of TinO2n−1. Electrochim. Acta 1994, 39, 1591–1595. [Google Scholar] [CrossRef]
- Inglis, A.D.; Page, Y.L.; Strobel, P.; Hurd, C.M. Electrical conductance of crystalline TinO2n−1 for n = 4–9. J. Phys. C Solid. State Phys. 1983, 16, 317. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodr-guez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; Coss, R.D.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef]
- Harada, S.; Tanaka, K.; Inui, H. Thermoelectric properties and crystallographic shear structures in titanium oxides of the Magnéli phases. J. Appl. Phys. 2010, 108, 083703. [Google Scholar] [CrossRef]
- Ioroi, T.; Senoh, H.; Yamazaki, S.; Siroma, Z.; Fujiwara, N.; Yasuda, K. Stability of corrosion-resistant Magnéli-phase Ti4O7-supported PEMFC catalysts at high potentials. J. Electrochem. Soc. 2008, 155, B321–B326. [Google Scholar] [CrossRef]
- Ehrlich, P. Phase Ratios and Magnetic Properties in the System Titanium-Oxygen. Z. Elektrochem. 1939, 45, 362. [Google Scholar]
- Ehrlich, P. Lösungen von sauerstoff in metallishem titan. Z. Anorg. Chem. 1941, 247, 53–63. [Google Scholar] [CrossRef]
- DeVries, R.C.; Roy, R. Phase diagram for the system Ti-TiO2 constructed from data in the literature. Am. Ceram. Soc. Bull. 1954, 33, 370–372. [Google Scholar]
- Andersson, S.; Collen, B.; Kuylenstierna, U.; Magnéli, A. Phase analysis studies on the titanium-oxygen system. Acta Chem. Scand. 1957, 11, 1641–1652. [Google Scholar] [CrossRef]
- Bartholomew, R.F.; Frankl, D.R. Electrical properties of some titanium oxides. Phys. Rev. 1969, 187, 828. [Google Scholar] [CrossRef]
- Jayashree, S.; Ashokkumar, M. Switchable intrinsic defect chemistry of titania for catalytic applications. Catalysts 2018, 8, 601. [Google Scholar] [CrossRef]
- He, C.; Chang, S.; Huang, X.; Wang, Q.; Mei, A.; Shen, P. Direct synthesis of pure single-crystalline Magnéli phase Ti8O15 nanowires as conductive carbon-free materials for Electrocatalysis. Nanoscale 2015, 7, 2856–2861. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; Kim, K.M.; Jang, J.H.; Jeon, J.M.; Lee, M.H.; Kim, G.H.; Li, X.S.; Park, G.S.; Lee, B.; Han, S.; et al. Atomic structure of conducting nanofilaments in TiO2 resistive switching memory. Nat. Nanotechnol. 2010, 5, 148–153. [Google Scholar] [CrossRef]
- Tsujimoto, Y. Low-temperature solid-state reduction approach to highly reduced titanium oxide nanocrystals. J. Ceram. Soc. Jpn. 2018, 126, 609–613. [Google Scholar] [CrossRef]
- Yao, C.; Li, F.; Lia, X.; Xia, D. Fiber-like nanostructured Ti4O7 used as durable fuel cell catalyst support in oxygen reduction catalysis. J. Mater. Chem. 2012, 22, 16560–16565. [Google Scholar] [CrossRef]
- Geng, P.; Su, J.; Miles, C.; Comninellis, C.; Chen, G. Highly-ordered Magnéli Ti4O7 nanotube arrays as effective anodic material for electro-oxidation. Electrochim. Acta 2015, 153, 316–324. [Google Scholar] [CrossRef]
- Clarke, R.L. Conductive Titanium Suboxide Particulates. US5173215A, 22 December 1992. [Google Scholar]
- Tang, C.; Zhou, D.; Zhang, Q. Synthesis and characterization of Magnéli phases: Reduction of TiO2 in a decomposed NH3 atmosphere. Mater. Lett. 2012, 79, 42–44. [Google Scholar] [CrossRef]
- Yao, S.; Xue, S.; Zhang, Y.; Shen, X.; Qian, Y.; Li, T.; Xiao, K.; Qin, S.; Xiang, J. Synthesis, characterization, and electrochemical performance of spherical nanostructure of Magnéli phase Ti4O7. J. Mater. Sci. Mater. Electron. 2017, 28, 7264–7270. [Google Scholar] [CrossRef]
- Xu, B.; Zhao, D.; Sohn, H.; Mohassab, Y.; Yang, B.; Lan, Y.; Yang, J. Flash synthesis of Magnéli phase (TinO2n−1) nanoparticles by thermal plasma treatment of H2TiO3. Ceram. Int. 2018, 44, 3929–3936. [Google Scholar] [CrossRef]
- Kim, M.; Cho, N.; Kang, T.; Manh, N.; Lee, Y.; Park, K. Synthesis of highly conductive titanium suboxide support materials with superior electrochemical durability for proton exchange membrane fuel cells. Mol. Cryst. Liq. Cryst. 2020, 707, 110–117. [Google Scholar] [CrossRef]
- Afir, A.; Achour, M.; Saoula, N. X-ray diffraction study of Ti–O–C system at high temperature and in a continuous vacuum. J. Alloy. Compd. 1999, 288, 124–140. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Ye, J. Investigation of fabrication of Ti4O7 by carbothermal reduction in argon atmosphere and vacuum. J. Mater. Sci: Mater. Electron. 2015, 27, 3683–3692. [Google Scholar] [CrossRef]
- Toyoda, M.; Yano, T.; Tryba, B.; Mozia, S.; Tsumura, T.; Inagaki, M. Preparation of carbon-coated Magnéli phases TinO2n−1 and their photocatalytic activity under visible light. Appl. Catal. B Environ. 2009, 88, 160–164. [Google Scholar] [CrossRef]
- Fukushima, J.; Takizawa, H. Size Control of Ti4O7 nanoparticles by carbothermal reduction using a multimode microwave furnace. Crystals 2018, 8, 444. [Google Scholar] [CrossRef]
- Adamaki, V.; Clemens, F.; Ragulis, P.; Pennock, S.R.; Taylor, J.; Bowen, C.R. Manufacturing and characterization of Magnéli phase conductive fibres. J. Mater. Chem. A 2014, 2, 8328–8333. [Google Scholar] [CrossRef]
- Acha, C.; Monteverde, M.; Nunez-Regueiro, M.; Kuhn, A.; Franc, M. Electrical resistivity of the Ti4O7 Magnéli phase under high pressure. Eur. Phys. J. 2003, 34, 421–428. [Google Scholar] [CrossRef]
- Gusev, A.; Avvakumov, E.; Vinokurova, O. Synthesis of Ti4O7 Magneli phase using mechanical activation. Sci. Sinter. 2003, 35, 141–145. [Google Scholar] [CrossRef]
- Hauf, C.; Kniep, R.; Pfaff, G. Preparation of various titanium suboxide powders by reduction of TiO2 with silicon. J. Mater. Sci. 1999, 34, 1287–1292. [Google Scholar] [CrossRef]
- Kitada, A.; Hasegawa, G.; Kobayashi, Y.; Kanamori, K.; Nakanishi, K.; Kageyama, H. Selective preparation of macroporous monoliths of conductive titanium oxides TinO2n−1 (n = 2, 3, 4, 6). J. Am. Chem. Soc. 2012, 134, 10894–10898. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Misu, S.; Hirayama, J.; Otomo, R.; Kamiya, Y. Magnéli-phase titanium suboxide nanocrystals as highly active catalysts for selective acetalization of furfural. ACS Appl. Mater. Interfaces 2020, 12, 2539–2547. [Google Scholar] [CrossRef] [PubMed]
- Tominaka, S.; Tsujimoto, Y.; Matsushita, Y.; Yamaura, K. Synthesis of nanostructured reduced titanium oxide: Crystal structure transformation maintaining nanomorphology. Angew. Chem. Int. Ed. Engl. 2011, 50, 7418–7421. [Google Scholar] [CrossRef] [PubMed]
- Kitada, A.; Kasahara, S.; Terashima, T.; Yoshimura, K.; Kobayashi, Y.; Kageyama, H. Highly reduced anatase TiO2−δ thin films obtained via low-temperature reduction. Appl. Phys. Express 2011, 4, 035801. [Google Scholar] [CrossRef]
- Berger, L.M.; Gruner, W.; Langholf, E.; Stolle, S. On the mechanism of carbothermal reduction processes of TiO2 and ZrO2. Int. J. Refract. Met. Hard Mater. 1999, 17, 235–243. [Google Scholar] [CrossRef]
- Lekanova, T.L.; Ryabkov, Y.I.; Sevbo, O.A. Particle-size effect on the rate of carbon reduction of TiO2. Inorg. Mater. 2003, 39, 715–719. [Google Scholar] [CrossRef]
- Maitre, A.; Tetard, D.; Lefort, P. Role of some technological parameters during carburizing titanium dioxide. J. Eur. Ceram. Soc. 2000, 20, 15–22. [Google Scholar] [CrossRef]
- Dewan, M.A.; Zhang, G.; Ostrovski, O. Carbothermal reduction of titania in different gas atmospheres. Metall. Mater. Trans. 2009, 40, 62–69. [Google Scholar] [CrossRef]
- Portehault, D.; Maneeratana, V.; Candolfi, C.; Deschler, N.; Veremchuk, I.; Grin, Y.; Sanchez, C.; Antonietti, M. Facile general route toward tunable Magnéli nanostructures and their use as thermoelectric metal oxide/carbon nanocomposites. ACS Nano. 2011, 5, 9052–9061. [Google Scholar] [CrossRef]
- Mei, S.L.; Jafta, C.J.; Lauermann, I.; Ran, Q.; Kargell, M.; Ballauff, M.; Lu, Y. Porous Ti4O7 particles with interconnected-pore structure as a high-efficiency polysulfide mediator for lithium-sulfur batteries. Adv. Funct. Mater. 2017, 27, 1701176. [Google Scholar] [CrossRef]
- Wei, H.; Rodriguez, E.F.; Best, A.S.; Hollenkamp, A.F.; Chen, D.H.; Caruso, R.A. Chemical bonding and physical trapping of sulfur in mesoporous Magnéli Ti4O7 microspheres for high- performance Li-S battery. Adv. Energy Mater. 2017, 7, 1601616. [Google Scholar] [CrossRef]
- Huang, S.S.; Lin, Y.H.; Chuang, W.; Shao, P.S.; Chuang, C.H.; Lee, J.F.; Lu, M.L.; Weng, Y.T.; Wu, N.L. Synthesis of high-performance titanium sub-oxides for electrochemical applications using combination of sol-gel and vacuum-carbothermic processes. ACS Sustain. Chem. Eng. 2018, 6, 3162–3168. [Google Scholar] [CrossRef]
- Strobel, P.; Le Page, Y. Crystal growth of TinO2n−1 oxides (n = 2 to 9). J. Mater. Sci. 1982, 17, 2424–2430. [Google Scholar] [CrossRef]
- Gusev, A.; Avvakumov, E.; Medvedev, A.; Masliy, A. Ceramic electrodes based on Magnéli phases of titanium oxides. Sci. Sinter. 2007, 39, 51–57. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Smestad, G.; Bignozzi, C.; Argazzi, R. Testing of dye sensitized TiO2 solar cells I: Experimental photocurrent output and conversion efficiencies. Sol. Energy Mater. Sol. Cells 1994, 32, 259–272. [Google Scholar] [CrossRef]
- Kay, A.; Grätzel, M. Low cost photovoltaic modules based on dye sensitized nanocrystalline titanium dioxide and carbon powder. Sol. Energy Mater. Sol. Cells 1996, 44, 99–117. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid. State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Han, W.Q.; Zhang, Y. Magnéli phases TinO2n−1 nanowires: Formation, optical, and transport properties. Appl. Phys. Lett. 2008, 92, 203117. [Google Scholar] [CrossRef]
- Han, W.; Wu, L.; Klie, R.; Zhu, Y. Enhanced optical absorption induced by dense nanocavities inside titania nanorods. Adv. Mater. 2007, 19, 2525–2529. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Titania nanotubes prepared by chemical processing. Adv. Mater. 1999, 15, 1307–1311. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, W.; Du, G.; Peng, L. Trititanate nanotubes made via a single alkali treatment. Adv. Mater. 2002, 14, 1208–1211. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Ye, J.; Zhu, R. Fabrication and characterisation of Magneli phase Ti4O7 nanoparticles. Micro Nano Lett. 2013, 8, 251–253. [Google Scholar] [CrossRef]
- Pang, Q.; Kundu, D.; Cuisinier, M.; Nazar, L. Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium-sulphur batteries. Nat. Commun. 2014, 5, 4759. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Fu, Y.; Su, Y. Challenges and prospects of lithium–sulfur batteries. Acc. Chem. Res. 2012, 46, 1125–1134. [Google Scholar] [CrossRef]
- Evers, S.; Nazar, L.F. New approaches for high energy density lithium–sulfur battery cathodes. Acc. Chem. Res. 2013, 46, 1135–1143. [Google Scholar] [CrossRef]
- Bresser, D.; Passerini, S.; Scrosati, B. Recent progress and remaining challenges in sulfur-based lithium secondary batteries—A review. Chem. Commun. 2013, 49, 10545–10562. [Google Scholar] [CrossRef]
- Davydov, D.A. Preparation of nanostructured Ti4O7. Inorg. Mater. 2014, 50, 682–685. [Google Scholar] [CrossRef]
- Davydov, D.A. Preparation of nanostructured TiCxOy. Inorg. Mater. 2013, 49, 62–65. [Google Scholar] [CrossRef]
- Ioroi, T.; Kageyama, H.; Akita, T.; Yasuda, K. Formation of electro-conductive titanium oxide fine particles by pulsed UV laser irradiation. Phys. Chem. Chem. Phys. 2010, 12, 7529–7535. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Fukushima, J.; Hayashi, Y.; Takizawa, H. Synthesis of Ti4O7 nanoparticles by carbothermal reduction using microwave rapid heating. Catalysts 2017, 7, 65. [Google Scholar] [CrossRef]
- Arif, F.; Balgis, R.; Ogi, T.; Iskandar, F.; Kinoshita, A.; Nakamura, K.; Okuyama, K. Highly conductive nano-sized Magnéli phases titanium oxide (TiOx). Sci. Rep. 2017, 7, 3646. [Google Scholar] [CrossRef] [PubMed]
- Hayfield, P.C.S. Development of a New Materials-Monolithic Ti4O7 Ebonex® Ceramic; Royal Society of Chemistry: Cambridge, UK, 2002. [Google Scholar]
- Walsh, F.C.; Wills, R.G.A. The continuing development of Magnéli phase titanium sub-oxides and Ebonex (R) electrodes. Electrochim. Acta 2010, 55, 6342–6351. [Google Scholar] [CrossRef]
- Pei, S.; Teng, J.; Ren, N.; You, S. Low-temperature removal of refractory organic pollutants by electrochemical oxidation: Role of interfacial joule heating effect. Environ. Sci. Technol. 2020, 54, 4573–4582. [Google Scholar] [CrossRef]
- Nayak, S.; Chaplin, B.P. Fabrication and characterization of porous, conductive, monolithic Ti4O7 electrodes. Electrochim. Acta 2018, 263, 299–310. [Google Scholar] [CrossRef]
- Bunce, N.J.; Bejan, D. Pollutants in Water—Electrochemical remediation using Ebonex electrodes. In Encyclopedia of Applied Electrochemistry; Kreysa, G., Ota, K., Savinell, R.F., Eds.; Springer: New York, NY, USA, 2014; pp. 1629–1633. [Google Scholar] [CrossRef]
- Liang, J.; You, S.; Yuan, Y.; Yuan, Y. A tubular electrode assembly reactor for enhanced electrochemical wastewater treatment with a Magnéli -phase titanium suboxide (M-TiSO) anode and in situ utilization. RSC Adv. 2021, 11, 24976–24984. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Jacobson, M.; Colella, W.; Golden, D. Cleaning the air and improving health with hydrogen fuel-cell vehicles. Science 2005, 308, 1901–1905. [Google Scholar] [CrossRef] [PubMed]
- Service, R.F. Shrinking fuel cells promise power in your pocket. Science 2002, 296, 1222–1224. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, J.; Gu, J.; Su, L.; Cheng, L. An overview of metal oxide materials as electrocatalysts and supports for polymer electrolyte fuel cells. Energy Environ. Sci. 2014, 7, 2535–2558. [Google Scholar] [CrossRef]
- Tian, Z.Q.; Lim, S.H.; Poh, C.K.; Tang, Z.; Xia, Z.; Luo, Z.; Shen, P.K.; Chua, D.; Feng, Y.P.; Shen, Z.; et al. A highly order-structured membrane electrode assembly with vertically aligned carbon nanotubes for ultra-low Pt loading PEM fuel cells. Adv. Energy Mater. 2011, 1, 1205–1214. [Google Scholar] [CrossRef]
- Riese, A.; Banham, D.; Ye, S.; Sun, X. Accelerated stress testing by rotating disk electrode for carbon corrosion in fuel cell catalyst supports. J. Electrochem. Soc. 2015, 162, F783–F788. [Google Scholar] [CrossRef]
- Prabhakaran, V.; Wang, G.; Parrondo, J.; Ramani, V. Contribution of electrocatalyst support to pem oxidative degradation in an operating PEFC. J. Electrochem. Soc. 2016, 163, F1611–F1617. [Google Scholar] [CrossRef]
- Cherevko, S.; Kulyk, N.; Mayrhofer, K. Durability of platinum-based fuel cell electrocatalysts: Dissolution of bulk and nanoscale platinum. Nano Energy 2016, 29, 275–298. [Google Scholar] [CrossRef]
- Chisaka, M.; Nagano, W.; Delgertsetseg, B.; Takeguchi, D. Inexpensive gram scale synthesis of porous Ti4O7 for high performance polymer electrolyte fuel cell electrodes. Chem. Commun. 2021, 57, 12772–12775. [Google Scholar] [CrossRef]
- Esfahani, R.; Ebralidze, I.; Specchia, S.; Easton, E. A fuel cell catalyst support based on doped titanium suboxides with enhanced conductivity, durability and fuel cell performance. J. Mater. Chem. A 2018, 6, 14805–14815. [Google Scholar] [CrossRef]
- Nguyen, S.; Lee, J.; Yang, Y.; Wang, X. Excellent durability of substoichiometric titanium oxide as a catalyst support for Pd in alkaline direct ethanol fuel cells. Ind. Eng. Chem. Res. 2012, 51, 9966–9972. [Google Scholar] [CrossRef]
- Won, J.; Kwak, D.; Han, S.; Park, H.; Park, J.; Ma, K.; Kim, D.; Park, K. PtIr/Ti4O7 as a bifunctional electrocatalyst for improved oxygen reduction and oxygen evolution reactions. J. Catal. 2018, 358, 287–294. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Y.; Huang, H.; Zhang, H.; Wang, Y.; Wang, Y. Ordered Ag@Pd alloy supported on Ti4O7 by ascorbic acid-assisted galvanic replacement for efficient oxygen reduction. J. Alloy. Compd. 2022, 929, 167251. [Google Scholar] [CrossRef]
- Chen, G.; Betterton, E.A.; Arnold, R.G.; Ela, W.P. Electrolytic reduction of trichloroethylene and chloroform at a Pt- or Pd-coated ceramic cathode. J. Appl. Electrochem. 2003, 33, 161–169. [Google Scholar] [CrossRef]
- Kearney, D.; Bejan, D.; Bunce, N. The use of Ebonex electrodes for the electrochemical removal of nitrate ion from water. Can. J. Chem. 2012, 90, 666–674. [Google Scholar] [CrossRef]
- Yang, P.; Wang, Y.; Lu, J.; Tishchenko, V.; Huang, Q. Electrochemical oxidation of perfluorooctanesulfonate by Magnéli phase Ti4O7 electrode in the presence of trichloroethylene. Adv. Environ. Eng. Res. 2020, 1, 6. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Oturan, N.; Raffy, S.; Cretin, M.; Esmilaire, R.; van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Sub-stoichiometric titanium oxide (Ti4O7) as a suitable ceramic anode for electro oxidation of organic pollutants: A case study of kinetics, mineralization and toxicity assessment of amoxicillin. Water Res. 2016, 106, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Liu, G.; Liang, J.; You, S. Electrochemical oxidation of sulfadiazine with titanium suboxide mesh anode. Electrochim. Acta 2019, 331, 135441. [Google Scholar] [CrossRef]
- Santos, M.C.; Elabd, Y.A.; Jing, Y.; Chaplin, B.P.; Fang, L. Highly porous Ti4O7 reactive electrochemical water filtration membranes fabricated via electrospinning/electrospraying. AIChE J. 2016, 62, 508–524. [Google Scholar] [CrossRef]
- Zaky, A.M.; Chaplin, B.P. Porous substoichiometric TiO2 anodes as reactive electrochemical membranes for water treatment. Environ. Sci. Technol. 2013, 47, 6554–6563. [Google Scholar] [CrossRef]
- Guo, L.; Jing, Y.; Chaplin, B.P. Development and characterization of ultrafiltration TiO2 Magnéli phase reactive electrochemical membranes. Environ. Sci. Technol. 2016, 50, 1428–1436. [Google Scholar] [CrossRef]
- Qi, G.; Wang, X.; Zhao, J.; Song, C.; Zhang, Y.; Ren, F.; Zhang, N. Fabrication and characterization of the porous Ti4O7 reactive electrochemical membrane. Front. Chem. 2022, 9, 833024. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Liu, B.; Gao, Y.; Wang, Y.; Tang, C.; Huang, Y. Monolithic porous Magnéli-phase Ti4O7 for electro-oxidation treatment of industrial wastewater. Electrochim. Acta 2016, 214, 326–335. [Google Scholar] [CrossRef]
- Geng, P.; Chen, G. Magnéli Ti4O7 modified ceramic membrane for electrically-assisted filtration with antifouling property. J. Membr. Sci. 2016, 498, 302–314. [Google Scholar] [CrossRef]
- Liang, S.; Lin, H.; Habteselassie, M.; Huang, Q. Electrochemical inactivation of bacteria with a titanium sub-oxide reactive membrane. Water Res. 2018, 145, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Tao, X.; Wang, J.; Ying, Z.; Cai, Q.; Zheng, G.; Gan, Y.; Huang, H.; Xia, Y.; Liang, C.; Zhang, W.; et al. Strong sulfur binding with conducting Magnéli-phase TinO2n−1 nanomaterials for improving lithium-sulfur batteries. Nano Lett. 2014, 14, 5288–5294. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, S.; Zhuang, R.; Luan, K.; Qian, X.; Xiang, J.; Shen, X.; Li, T.; Xiao, K.; Qin, S. Shape-controlled synthesis of Ti4O7 nanostructures under solvothermal-assisted heat treatment and its application in lithium-sulfur batteries. J. Alloys Compd. 2017, 729, 1136–1144. [Google Scholar] [CrossRef]
- Wu, X.; Li, S.; Yao, S.; Liu, M.; Pang, S.; Shen, X.; Li, T.; Qin, S. Nanosized Ti4O7 supported on carbon nanotubes composite modified separator for enhanced electrochemical properties of lithium sulfur battery. Int. J. Energy Res. 2021, 45, 4331–4344. [Google Scholar] [CrossRef]
- Yu, C.; Tsai, C. Ti4O7 as conductive additive in sulfur and graphene-sulfur cathodes for high-performance lithium-sulfur batteries with a facile preparation method. MRS Energy Sustain. 2022, 9, 369–377. [Google Scholar] [CrossRef]
- Ellis, K.; Hill, A.; Hill, J.; Loyns, A.; Partington, T. The performance of Ebonex® electrodes in bipolar lead-acid batteries. J. Power Sources 2004, 136, 366–371. [Google Scholar] [CrossRef]
- Huang, H.; Luo, Y.; Zhang, L.; Zhang, H.; Wang, Y. Cobalt-nickel alloys supported on Ti4O7 and embedded in N, S doped carbon nanofibers as an efficient and stable bifunctional catalyst for Zn-air batteries. J. Colloid. Interface Sci. 2023, 630, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Park, S.; Cowan, S.; Tong, M.; Cho, S.; Lee, K.; Heeger, A. Titanium suboxide as an optical spacer in polymer solar cells. Appl. Phys. Lett. 2009, 95, 013302. [Google Scholar] [CrossRef]
- Lee, B.; Coughlin, J.; Kim, G.; Bazan, G.; Lee, K. Efficient solution-processed small-molecule solar cells with titanium suboxide as an electric adhesive layer. Appl. Phys. 2014, 104, 213305. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.; Lee, K. Efficiency enhancement in polymer optoelectronic devices by introducing titanium sub-oxide layer. Curr. Appl. Phys. 2010, 10, S528–S531. [Google Scholar] [CrossRef]
- Arakawa, H.; Sayama, K.; Hara, K.; Sugihara, H.; Yamaguchi, T.; Yanagida, M.; Kawauchi, H.; Kashima, T.; Fujihashi, G.; Takano, S. Improvement of efficiency of dye-sensitized solar cell—Optimization of titanium oxide photoelectrode. In Proceedings of the 3rd World Conference on Photovoltaic Energy Conversion 2003, Osaka, Japan, 11–18 May 2003; Volume 1, pp. 19–22. [Google Scholar]
- Kang, D.; Ko, J.; Lee, C.; Kim, C.; Lee, D.; Kang, H.; Lee, Y.; Lee, H. Titanium oxide nanomaterials as an electron-selective contact in silicon solar cells for photovoltaic devices. Discov. Nano 2023, 18, 39. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Insights in the application of stoichiometric and non-stoichiometric titanium oxides for the design of sensors for the determination of gases and VOCs (TiO2−x and TinO2n−1 vs. TiO2). Sensors 2020, 20, 6833. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Tereshchenko, A.; Karpicz, R.; Ratautaite, V.; Bubniene, U.; Maneikis, A.; Jagminas, A.; Ramanavicius, A. TiO2−x/TiO2-structure based ‘Self-Heated’ sensor for the determination of some reducing gases. Sensors 2019, 20, 74. [Google Scholar] [CrossRef]
- Gardon, M.; Monereo, O.; Dosta, S.; Vescio, G.; Cirera, A.; Guilemany, J.M. New procedures for building-up the active layer of gas sensors on flexible polymers. Surf. Coat. Technol. 2013, 235, 848–852. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Ma, S.; Ye, J.; Zhang, X.; Wang, G.; Qiu, Y. The synthesis and gas sensitivity of the β-Ti3O5 powder: Experimental and DFT study. J. Alloy. Compd. 2015, 649, 939–948. [Google Scholar] [CrossRef]
- Lee, C.; Koo, S.; Oh, J.; Moon, K.; Lee, D. Selectable titanium-oxide-based critical and differential temperature sensor in a single devices. IEEE Electron. Device Lett. 2018, 39, 1058–1060. [Google Scholar] [CrossRef]
- Ohkoshi, S.I.; Tsunobuchi, Y.; Matsuda, T.; Hashimoto, K.; Namai, A.; Hakoe, F.; Tokoro, H. Synthesis of a metal oxide with a room-temperature photoreversible phase transition. Nat. Chem. 2010, 2, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Bletsa, E.; Merkl, P.; Thersleff, T.; Normark, S.; Henriques-Normark, B.; Sotiriou, G. Highly durable photocatalytic titanium suboxide–polymer nanocomposite films with visible light-triggered antibiofilm activity. Chem. Eng. J. 2023, 454, 139971. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Y.; Wang, Y.; Huang, H.; Zhang, L.; Zhang, H.; Wang, Y. Illumination enabling monoatomic Fe and Pt-based catalysts on NC/TiOx for efficient and stable oxygen reduction. Appl. Catal. B. Environ. 2022, 317, 121797. [Google Scholar] [CrossRef]
- Xiong, G.; Wang, Y.; Xu, F.; Tang, G.; Zhang, H.; Wang, F.; Wang, Y. Au(111)@Ti6O11 heterostructure composites with enhanced synergistic effects as efficient electrocatalysts for the hydrogen evolution reaction. Nanoscale 2022, 14, 3878–3887. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Zhang, K.; Li, Y.; Xu, B.; Liang, F.; Dai, Y.; Yao, Y. Enhancing the rate performance of high-capacity LiNi0.8Co0.15Al0.05O2 cathode materials by using Ti4O7 as a conductive additive. J. Energy Storage 2020, 28, 101182–101189. [Google Scholar] [CrossRef]
- Liu, R.; Yang, L.; Wang, Y.; Liu, H.; Zhang, X.; Zeng, C. Effects of carbon nanotubes, graphene and titanium suboxides on electrochemical properties of V2Al1−xCTz ceramic as an anode for lithium-ion batteries. Ceram. Int. 2021, 47, 35081–35088. [Google Scholar] [CrossRef]
- Liu, H.; Xiao, H.; Qiao, Y.; Luo, M.; Wang, C.; Yang, L.; Zeng, C.; Fu, C. Preparation, characterization, and electrochemical behavior of a novel porous Magneli phase Ti4O7-coated Ti electrode. Ceram. Int. 2023, 49, 20564–20575. [Google Scholar] [CrossRef]
| Synthesis Method | Process Conditions | Characterization | Ref. |
|---|---|---|---|
| H2 reduction | Pigmentary TiO2 reacts with H2 at 1050 °C | Monophasic Ti4O7 | [4] |
| H2 reduction | Anatase TiO2 reacts with H2 (99.99%) at 950 °C | Pure triclinic phase of Ti4O7, 0.5–1 µm | [2] |
| H2 reduction | TiO2 nanotube arrays react with H2 at 850 °C for two hours | Ti4O7 nanotube arrays | [19] |
| H2 reduction | TiO2 reacts with H2 | 70% Ti4O7, 30% Ti5O9 | [20] |
| H2 reduction | Rutile TiO2 was reduced in a mixture of N2 and H2 gases | Ti4O7, Ti5O9 and Ti6O11 | [21] |
| H2 reduction | TiO(NO3)2 reacts with H2 at 1000 °C for 6 h | Ti4O7, 250 nm | [22] |
| H2 reduction | H2TiO3 reacts with H2/Ar in a thermal plasma reactor | TinO2n−1 nanoparticles, 20–100 nm | [23] |
| H2 reduction | TiO2 + H2 in a combined catalytic and thermal reduction reaction | Ti8O15, Ti4O7, Ti3O5 | [24] |
| C reduction | The reduction of TiO2 by graphite or metallic titanium | Various phases | [25] |
| C reduction | TiO2 anatase (100 nm) reacts with carbon black at 1020 °C for 0.5–2 h | Ti4O7, 98.5% | [26] |
| C reduction | TiO2 reacts with poly(vinyl alcohol) at 1100 °C | Ti4O7, a few hundreds of nm in size | [27] |
| C reduction | TiO2 reacts with polymer PVP at 925 °C in a microwave furnace | Ti4O7 nanoparticles (25, 60, and 125 nm) | [28] |
| C reduction | Reduction of rutile TiO2 in a carbon black micro-environment | Titanium sub-oxide fibers | [29] |
| Metal reduction | Heating Ti and TiO2 in an electric arc furnace | Titanium sub-oxides | [12] |
| Metal reduction | Heating TiO2 and Ti metal in an evacuated silica tube at 1150 °C | Ti4O7 crystals | [30] |
| Metal reduction | Heating Ti and TiO2 in H2 | Ti4O7 | [31] |
| Metal reduction | Heating TiO2 and silicon powder or silicon/CaCl2 powder | Various titanium sub-oxide powders | [32] |
| Metal reduction | Reducing macroporous anatase TiO2 using a zirconium getter | TinO2n−1 (n = 2, 3, 4, 6) | [33] |
| Hydride reduction | Solid-phase reaction of TiO2 with TiH2 at relatively low temperature | Titanium sub-oxide nanoparticles | [34] |
| Hydride reduction | Heating TiO2 nanoparticles and CaH2 powder at 350 °C | Ti2O3 nanoparticles | [35] |
| Hydride reduction | TiO2 was embedded with CaH2 and heated at 360 to 500 °C. | TiOx thin films | [36] |
| Materials | Method | Ref. |
|---|---|---|
| Ti8O15 nanowires | Heating H2Ti3O7 nanowires in hydrogen at 850 °C | [53] |
| Ti8O15 nanowires | An evaporation–deposition synthesis method | [15] |
| Ti4O7 particles with diameters of 200–500 nm | Reduction of H4TiO5 with hydrogen at 850 °C | [57] |
| Ti4O7 crystals (8–20 nm) | Carbothermal reduction of cross-linked titanium ethoxide with polyethylene glycol at ~950 °C in Ar stream | [58] |
| Magnéli phases with specific surface areas from 55 to 300 m2 g−1 | The gels made from titanium (IV) ethoxide and amino- or ethoxy-containing oligomers or polymers were heated at different temperatures under N2 or Ar | [41] |
| Nanocrystalline Ti2O3, Ti3O5 and Ti4O7 | A combined sol-gel and vacuum-carbothermic processes | [44] |
| Ti4O7 particles (around 250 nm) | Reduction of TiO(NO3)2 in hydrogen at 1000 °C for 6 h | [22] |
| Ti4O7 nanopowder (115 ± 30 nm) | Reduction of titanium (III) oxalate particles in hydrogen | [62] |
| Ti2O3 nanoparticles | Heating TiO2 nanoparticles (10–30 nm) and CaH2 powder at 350 °C | [35] |
| Titanium sub-oxide nanoparticles | Irradiation of TiO2 particles dispersed in liquid with a pulsed UV laser | [64] |
| Titanium sub-oxide nanoparticles (20–100 nm) | By a thermal plasma method, using metatitanic acid (H2TiO3) as a starting material | [23] |
| Ti4O7 nanoparticles | Carbothermal reduction using a multimode microwave apparatus | [28] |
| Ti4O7 nanoparticles (60 nm) | Carbothermal reduction of TiO2 nanoparticles using microwave irradiation at 950 °C for 30 min | [65] |
| Titanium sub-oxides (30 nm) | A thermal-induced plasma process | [66] |
| Synthesis Methods | Advantages/Limitations | Characteristics |
|---|---|---|
| Hydrogen reduction | A simple, well established/handling reactive gas | For synthesis of multi-dimensional pure titanium sub-oxides |
| Carbon reduction | Use of various of carbon sources/uniform mixing of the reactants | For synthesis of various titanium sub-oxides by controlling mole ratio of carbon and TiO2 |
| Metal reduction | Without handling reactive gas/controlling reaction process | Usually a mixture of different titanium sub-oxides |
| Hydride reduction | Reaction at relatively low temperature/handling reactive starting reactant | For synthesis of titanium sub-oxides with smaller particle sizes |
| TinO2n−1 Phase | Electrical Conductivity (σ/S cm−1) | Log10 (σ/S cm−1) |
|---|---|---|
| Ti4O7 | 1995 | 3.3 |
| Ti5O9 | 631 | 2.8 |
| Ti6O11 | 63 | 1.8 |
| Ti8O15 | 25 | 1.4 |
| Area | Examples | Ref. |
|---|---|---|
| Electrodes | Electrodes for lead–acid batteries | [67] |
| Fuel cells | Conductive titanium sub-oxide support materials in fuel cells | [73,74,75,76,77,78,79,80,81,82,83,84,85,86] |
| Remediation of aqueous waste and contaminated water | Electrocatalytic degradation for wastewater treatment | [87,88,89,90,91] |
| Ti4O7 reactive membranes | Membranes for advanced electrochemical oxidation processes | [92,93,94,95,96,97,98] |
| Batteries | As a sulfur host in Li2S battery, and conductive additive for improving performance of Li2S battery | [43,60,99,100,101,102,103,104,105] |
| Solar cells | The TiO/TiOx layer can enhance the absorption of sunlight, thus increasing solar conversion efficiency | [106,107,108,109,110] |
| Sensors | Investigation of using nanostructured titanium sub-oxides as sensor materials for the determination of gaseous materials | [111,112,113,114,115] |
| Electronic and photonic materials | Nanostructured Ti4O7 in TiO2 resistive switching memory. Ti3O5 for light-triggered metal semiconductor transition. Titanium sub-oxides as thermoelectric materials | [3,7,16,116] |
| Biological applications | Coating material for medical devices | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Wang, H.; Wang, Y. A Review: Synthesis and Applications of Titanium Sub-Oxides. Materials 2023, 16, 6874. https://doi.org/10.3390/ma16216874
Wu X, Wang H, Wang Y. A Review: Synthesis and Applications of Titanium Sub-Oxides. Materials. 2023; 16(21):6874. https://doi.org/10.3390/ma16216874
Chicago/Turabian StyleWu, Xiaoping, Haibo Wang, and Yu Wang. 2023. "A Review: Synthesis and Applications of Titanium Sub-Oxides" Materials 16, no. 21: 6874. https://doi.org/10.3390/ma16216874
APA StyleWu, X., Wang, H., & Wang, Y. (2023). A Review: Synthesis and Applications of Titanium Sub-Oxides. Materials, 16(21), 6874. https://doi.org/10.3390/ma16216874






