Controlled Delivery of 2-Mercapto 1-Methyl Imidazole by Metal–Organic Framework for Efficient Inhibition of Copper Corrosion in NaCl Solution
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Solutions
2.2. Preparation of AMOF and AMOF@MMI
2.3. Characterization of AMOF and AMOF@MMI
2.4. Electrochemical Measurements
2.5. Quantum Chemical Calculations
3. Results
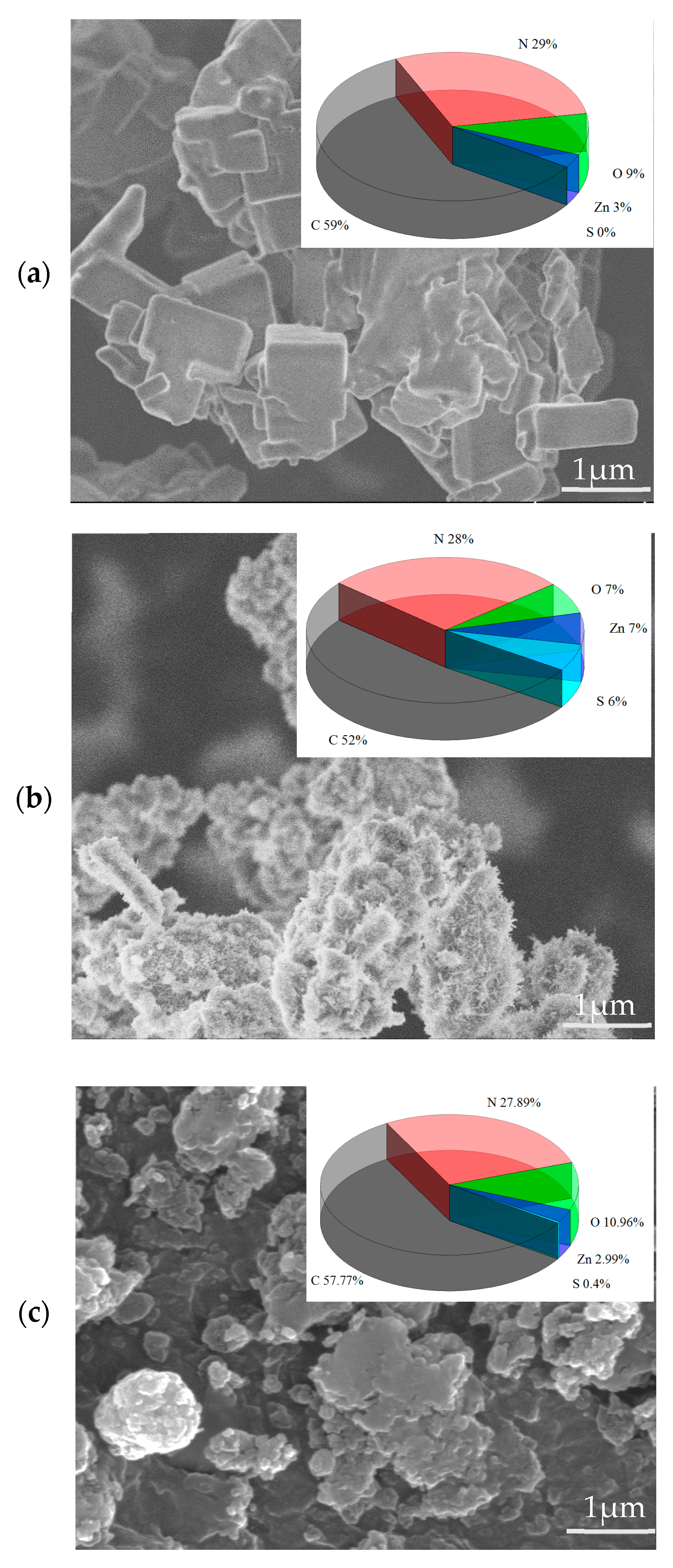
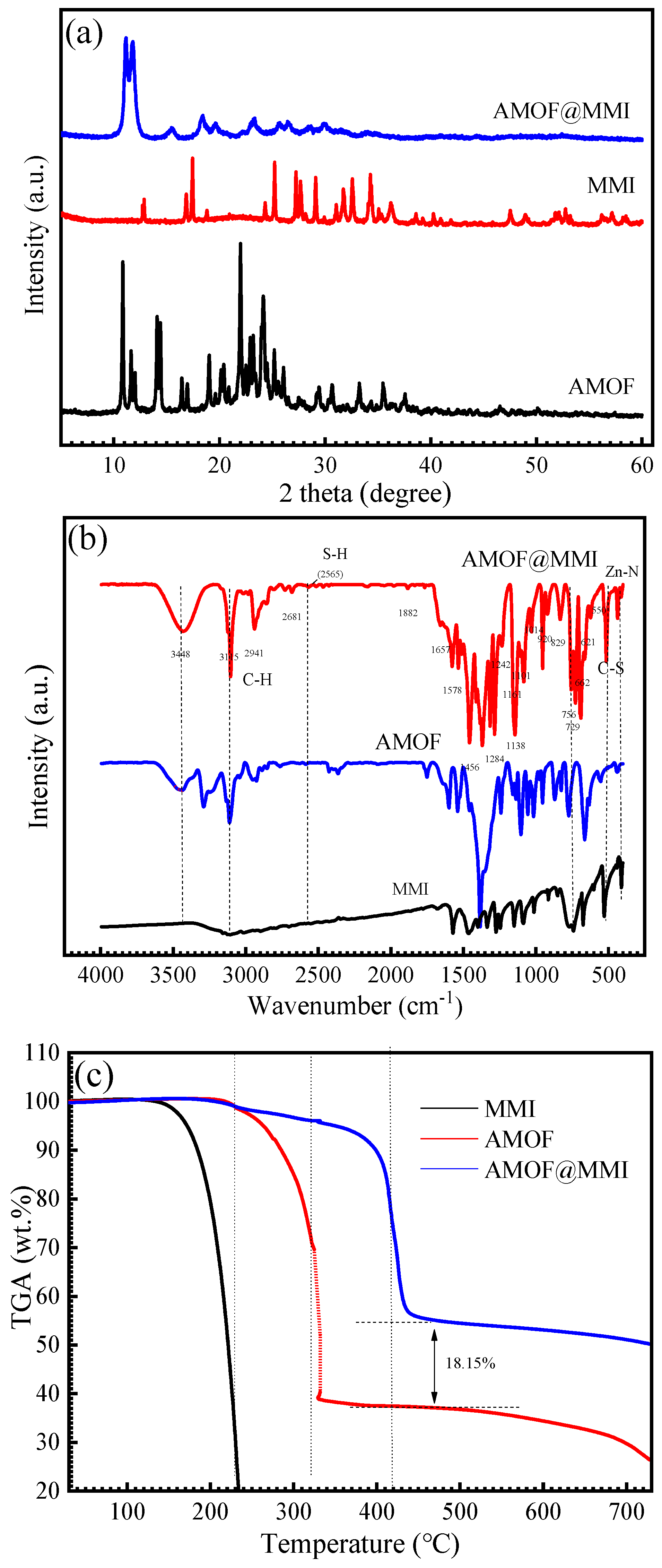
3.1. Characterization of AMOF and AMOF@MMI
3.2. Release of AMOF@MMI
3.3. Potentiodynamic Polarization
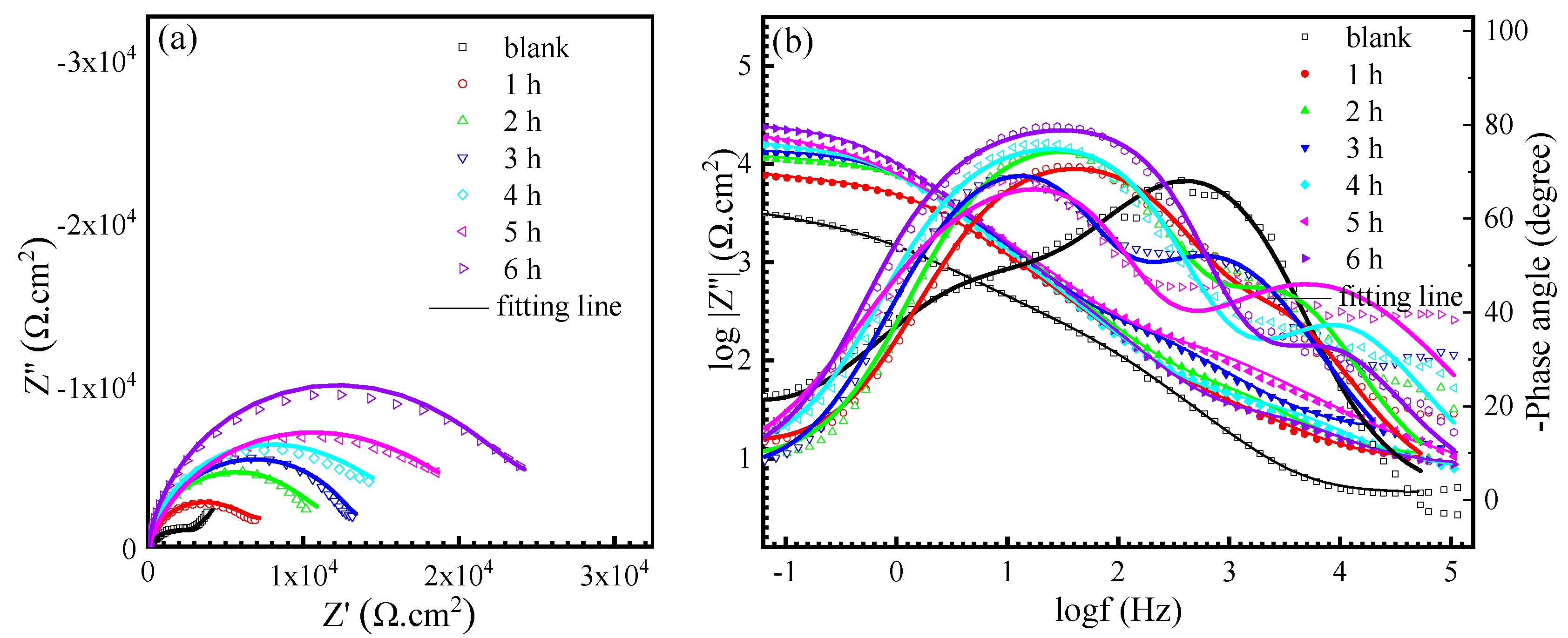
3.4. EIS Measurements
3.5. SEM and EDS Analyses
3.6. FTIR Analysis
3.7. XPS Analysis
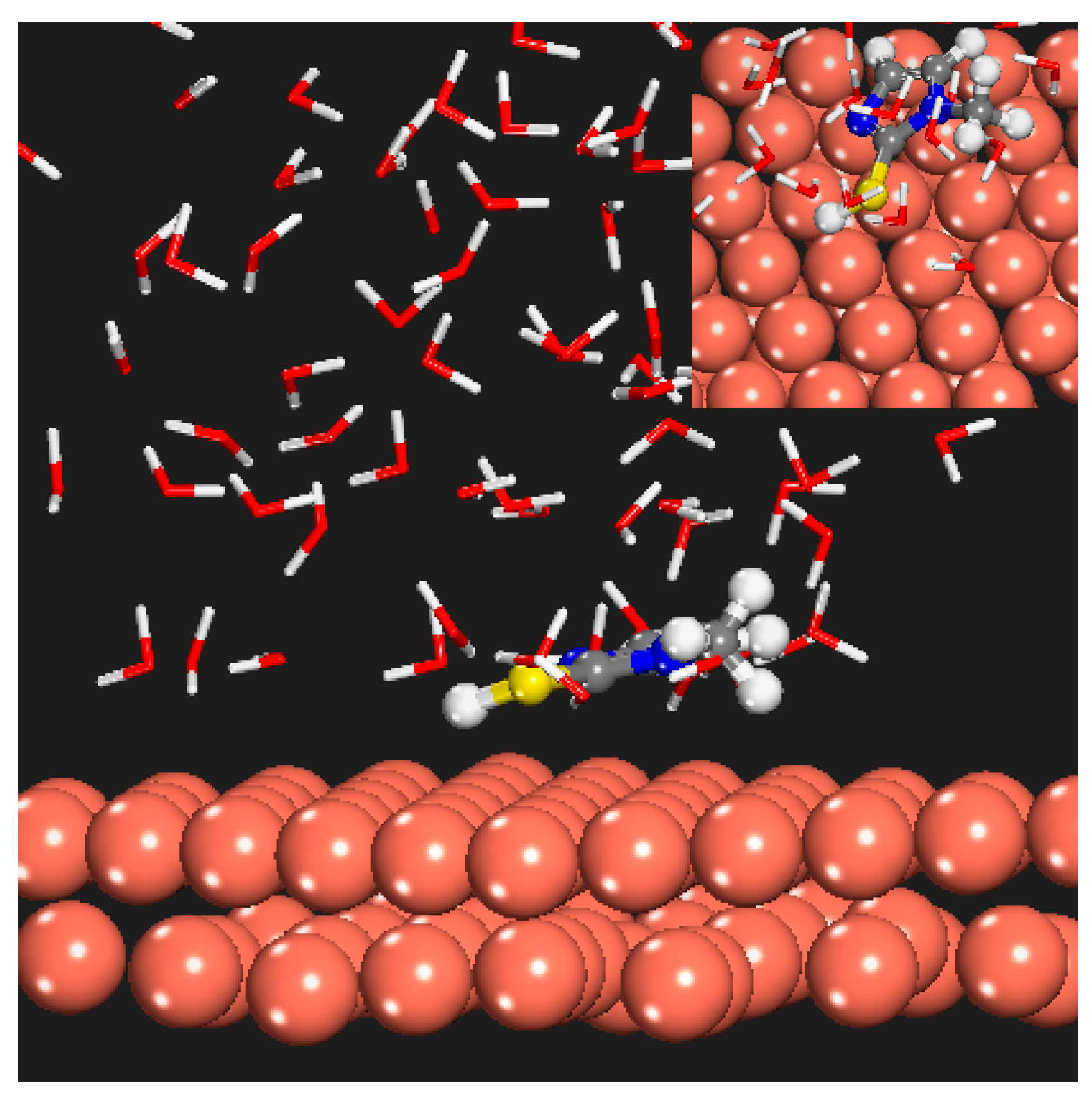
3.8. Theoretical Calculation Results
4. Discussions
4.1. Interaction Mechanism of MMI and AMOF
4.2. Corrosion Inhibition Mechanism of Released MMI
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chauhan, D.S.; Quraishi, M.; Qurashi, A. Recent trends in environmentally sustainable Sweet corrosion inhibitors. J. Mol. Liq. 2021, 326, 115117. [Google Scholar] [CrossRef]
- Haque, J.; Srivastava, V.; Quraishi, M.A.; Chauhan, D.S.; Lgaz, H.; Chung, I.-M. Polar group substituted imidazolium zwitterions as eco-friendly corrosion inhibitors for mild steel in acid solution. Corros. Sci. 2020, 172, 108665. [Google Scholar] [CrossRef]
- Lashgari, S.M.; Yari, H.; Mahdavian, M.; Ramezanzadeh, B.; Bahlakeh, G.; Ramezanzadeh, M. Unique 2-methylimidazole based Inorganic Building Brick nano-particles (NPs) functionalized with 3-aminopropyltriethoxysilane with excellent controlled corrosion inhibitors delivery performance; Experimental coupled with molecular/DFT-D simulations. J. Taiwan Inst. Chem. Eng. 2020, 117, 209–222. [Google Scholar] [CrossRef]
- Quraishi, M.; Chauhan, D.S.; Ansari, F.A. Development of environmentally benign corrosion inhibitors for organic acid environments for oil-gas industry. J. Mol. Liq. 2021, 329, 115514. [Google Scholar] [CrossRef]
- Yabuki, A.; Kanagaki, M.; Nishikawa, C.; Lee, J.H.; Fathona, I.W. Effective release of corrosion inhibitor by cellulose nanofibers and zeolite particles in self-healing coatings for corrosion protection. Prog. Org. Coatings 2021, 154, 106194. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Zhang, Y.; Tan, B.; Zheng, X.; Li, W. Combined electrochemical/surface and theoretical assessments of Rosa laevigata extract as an eco-friendly corrosion inhibitor for copper in acidic medium. J. Taiwan Inst. Chem. Eng. 2022, 136, 104408. [Google Scholar] [CrossRef]
- Kumar, D.; Jain, V.; Rai, B. Imidazole derivatives as corrosion inhibitors for copper: A DFT and reactive force field study. Corros. Sci. 2020, 171, 108724. [Google Scholar] [CrossRef]
- Fakhry, H.; El Faydy, M.; Benhiba, F.; Laabaissi, T.; Bouassiria, M.; Allali, M.; Lakhrissi, B.; Oudda, H.; Guenbour, A.; Warad, I.; et al. A newly synthesized quinoline derivative as corrosion inhibitor for mild steel in molar acid medium: Characterization (SEM/EDS), experimental and theoretical approach. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 610, 125746. [Google Scholar] [CrossRef]
- Berdimurodov, E.; Kholikov, A.; Akbarov, K.; Obot, I.; Guo, L. Thioglycoluril derivative as a new and effective corrosion inhibitor for low carbon steel in a 1 M HCl medium: Experimental and theoretical investigation. J. Mol. Struct. 2021, 1234, 130165. [Google Scholar] [CrossRef]
- Farahati, R.; Ghaffarinejad, A.; Mousavi-Khoshdel, S.M.; Rezania, J.; Behzadi, H.; Shockravi, A. Synthesis and potential applications of some thiazoles as corrosion inhibitor of copper in 1 M HCl: Experimental and theoretical studies. Prog. Org. Coatings 2019, 132, 417–428. [Google Scholar] [CrossRef]
- Kumar, D.; Jain, N.; Jain, V.; Rai, B. Amino acids as copper corrosion inhibitors: A density functional theory approach. Appl. Surf. Sci. 2020, 514, 145905. [Google Scholar] [CrossRef]
- Salmasifar, A.; Edraki, M.; Alibakhshi, E.; Ramezanzadeh, B.; Bahlakeh, G. Theoretical design coupled with experimental study of the effectiveness of the inhibitive molecules based on Cynara scolymus L extract toward chloride-induced corrosion of steel. J. Mol. Liq. 2021, 332, 115742. [Google Scholar] [CrossRef]
- Gao, L.; Peng, S.; Huang, X.; Gong, Z. A combined experimental and theoretical study of papain as a biological eco-friendly inhibitor for copper corrosion in H2SO4 medium. Appl. Surf. Sci. 2020, 511, 145446. [Google Scholar] [CrossRef]
- Kovačević, N.; Milošev, I.; Kokalj, A. The roles of mercapto, benzene, and methyl groups in the corrosion inhibition of imidazoles on copper: II. Inhibitor–copper bonding. Corros. Sci. 2015, 98, 457–470. [Google Scholar] [CrossRef]
- Abdullayev, E.; Price, R.; Shchukin, D.; Lvov, Y. Halloysite Tubes as Nanocontainers for Anticorrosion Coating with Benzotriazole. ACS Appl. Mater. Interfaces 2009, 1, 1437–1443. [Google Scholar] [CrossRef]
- Javidparvar, A.A.; Naderi, R.; Ramezanzadeh, B. Designing a potent anti-corrosion system based on graphene oxide nanosheets non-covalently modified with cerium/benzimidazole for selective delivery of corrosion inhibitors on steel in NaCl media. J. Mol. Liq. 2019, 284, 415–430. [Google Scholar] [CrossRef]
- Nikpour, B.; Ramezanzadeh, B.; Bahlakeh, G.; Mahdavian, M. Synthesis of graphene oxide nanosheets functionalized by green corrosion inhibitive compounds to fabricate a protective system. Corros. Sci. 2017, 127, 240–259. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Bahlakeh, G.; Ramezanzadeh, M. Polyaniline-cerium oxide (PAni-CeO2) coated graphene oxide for enhancement of epoxy coating corrosion protection performance on mild steel. Corros. Sci. 2018, 137, 111–126. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Hu, W. Facile synthesis of novel hierarchical core@shell structural magnetic nanovehicle Fe3O4@ZnAlCe-MoO4-LDHs for carbon steel protection. J. Magn. Magn. Mater. 2021, 523, 167576. [Google Scholar] [CrossRef]
- Zahidah, K.A.; Kakooei, S.; Ismail, M.C.; Raja, P.B. Halloysite nanotubes as nanocontainer for smart coating application: A review. Prog. Org. Coatings 2017, 111, 175–185. [Google Scholar] [CrossRef]
- Poznyak, S.; Tedim, J.; Rodrigues, L.M.; Salak, A.; Zheludkevich, M.; Dick, L.F.P.; Ferreira, M.G.S. Novel Inorganic Host Layered Double Hydroxides Intercalated with Guest Organic Inhibitors for Anticorrosion Applications. ACS Appl. Mater. Interfaces 2009, 1, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, A.; Kalagi, S.; Viana, E.L.; Martins, V.M.; Duarte, S.; Gregory, R.L.; Youngblood, J.P.; Platt, J.A.; Feitosa, S. Resin-based dental materials containing 3-aminopropyltriethoxysilane modified halloysite-clay nanotubes for extended drug delivery. Dent. Mater. 2021, 37, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Guo, T.; Wang, C.; Zhou, Y.; Xiong, T.; Wu, L.; Li, X.; Mittal, P.; Shi, S.; Gref, R.; et al. Glycoside scutellarin enhanced CD-MOF anchoring for laryngeal delivery. Acta Pharm. Sin. B 2020, 10, 1709–1718. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Wan, J.; Yu, C. MOF-on-MOF hybrids: Synthesis and applications. Coord. Chem. Rev. 2021, 432, 213743. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, F.; Chen, Y.-Q.; Hu, J.-M. Coatings embedded with GO/MOFs nanocontainers having both active and passive protecting properties. Corros. Sci. 2020, 168, 108563. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Y.; Zhang, H.; Chen, Z.; Ling, Y.; Yang, Y.; Liu, X.; Jia, Y.; Zhou, Y. TEA-assistant synthesis of MOF-74 nanorods for drug delivery and in-vitro magnetic resonance imaging. Microporous Mesoporous Mater. 2021, 315, 110900. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, W.; Ye, L.; Deng, Y.; Xie, Q.; Jiang, Y. Facile preparation of succinylated-zein-ZIF-8 hybrid for enhanced stability and pH-responsive drug delivery. Chem. Eng. Sci. 2020, 228, 115981. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, H.; Shao, G.; Yang, L.; Zhu, L.; Li, J.; Zhou, D.; Song, H.; Liu, H.; Zhu, D.; et al. Microstructural analysis of ZIF-8 particles using soy hull polysaccharide gel as a coating induced by different metal cations. Microporous Mesoporous Mater. 2020, 306, 110408. [Google Scholar] [CrossRef]
- Kaur, H.; Mohanta, G.C.; Gupta, V.; Kukkar, D.; Tyagi, S. Synthesis and characterization of ZIF-8 nanoparticles for controlled release of 6-mercaptopurine drug. J. Drug Deliv. Sci. Technol. 2017, 41, 106–112. [Google Scholar] [CrossRef]
- Yang, C.; Xu, W.; Meng, X.; Shi, X.; Shao, L.; Zeng, X.; Yang, Z.; Li, S.; Liu, Y.; Xia, X. A pH-responsive hydrophilic controlled release system based on ZIF-8 for self-healing anticorrosion application. Chem. Eng. J. 2021, 415, 128985. [Google Scholar] [CrossRef]
- Tian, H.; Li, W.; Liu, A.; Gao, X.; Han, P.; Ding, R.; Yang, C.; Wang, D. Controlled delivery of multi-substituted triazole by metal-organic framework for efficient inhibition of mild steel corrosion in neutral chloride solution. Corros. Sci. 2018, 131, 1–16. [Google Scholar] [CrossRef]
- Xiao, S.; Cao, X.; Dong, Z.; Ma, X.; Zhang, X.; Cai, G. A pH-responsive cerium-imidazole decorated ZIF-8 to achieve self-healing barrier property for epoxy coating on Al alloy by controlled release. Prog. Org. Coat. 2022, 163, 106640. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Asghari, M.; Ramezanzadeh, B.; Bahlakeh, G. Fabrication of an efficient system for Zn ions removal from industrial wastewater based on graphene oxide nanosheets decorated with highly crystalline polyaniline nanofibers (GO-PANI): Experimental and ab initio quantum mechanics approaches. Chem. Eng. J. 2018, 337, 385–397. [Google Scholar] [CrossRef]
- Javidparvar, A.A.; Naderi, R.; Ramezanzadeh, B.; Bahlakeh, G. Graphene oxide as a pH-sensitive carrier for targeted delivery of eco-friendly corrosion inhibitors in chloride solution: Experimental and theroretical investigations. J. Ind. Eng. Chem. 2019, 72, 196–213. [Google Scholar] [CrossRef]
- Sun, W.; Li, H.; Li, H.; Li, S.; Cao, X. Adsorption mechanisms of ibuprofen and naproxen to UiO-66 and UiO-66-NH2: Batch experiment and DFT calculation. Chem. Eng. J. 2019, 360, 645–653. [Google Scholar] [CrossRef]
- Lashgari, S.M.; Yari, H.; Mahdavian, M.; Ramezanzadeh, B.; Bahlakeh, G.; Ramezanzadeh, M. Synthesis of graphene oxide nanosheets decorated by nanoporous zeolite-imidazole (ZIF-67) based metal-organic framework with controlled-release corrosion inhibitor performance: Experimental and detailed DFT-D theoretical explorations. J. Hazard. Mater. 2021, 404, 124068. [Google Scholar] [CrossRef]
- Yang, C.; Yun, S.; Shi, J.; Sun, M.; Zafar, N.; Arshad, A.; Zhang, Y.; Zhang, L. Tailoring the supercapacitive behaviors of Co/Zn-ZIF derived nanoporous carbon via incorporating transition metal species: A hybrid experimental-computational exploration. Chem. Eng. J. 2021, 419, 129636. [Google Scholar] [CrossRef]
- Wu, L.; Xiao, J.; Wu, Y.; Xian, S.; Miao, G.; Wang, H.; Li, Z. A Combined Experimental/Computational Study on the Adsorption of Organosulfur Compounds over Metal–Organic Frameworks from Fuels. Langmuir 2014, 30, 1080–1088. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, L.-Y.; Guo, W.-L.; Luo, S.-Z.; Chai, Z.-F.; Shi, W.-Q. Extending the Use of Highly Porous and Functionalized MOFs to Th(IV) Capture. ACS Appl. Mater. Interfaces 2017, 9, 25216–25224. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.-Q.; Shi, N.; Wang, T.; Liu, J.; Pan, W.-P. Porous carbon with uniformly distributed cobalt nanoparticles derived from ZIF-67 for efficient removal of vapor elemental mercury: A combined experimental and DFT study. Chem. Eng. J. 2022, 428, 132095. [Google Scholar] [CrossRef]
- Sun, L.; Chen, D.; Wan, S.; Yu, Z. Performance, kinetics, and equilibrium of methylene blue adsorption on biochar derived from eucalyptus saw dust modified with citric, tartaric, and acetic acids. Bioresour. Technol. 2015, 198, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Bu, F. Correlations between the inhibition performances and the inhibitor structures of some azoles on the galvanic corrosion of copper coupled with silver in artificial seawater. Corros. Sci. 2020, 165, 108413. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, X.; Yan, X.; Kong, L.; Zhang, G.; Liu, H.; Qiu, J.; Yeung, K.L. Synthesis of Fe3O4@ZIF-8 magnetic core–shell microspheres and their potential application in a capillary microreactor. Chem. Eng. J. 2013, 228, 398–404. [Google Scholar] [CrossRef]
- Ren, B.; Chen, Y.; Li, Y.; Li, W.; Gao, S.; Li, H.; Cao, R. Rational design of metallic anti-corrosion coatings based on zinc gluconate@ZIF-8. Chem. Eng. J. 2020, 384, 123389. [Google Scholar] [CrossRef]
- Balachandran, V.; Lakshmi, A.; Janaki, A. Ab initio, DFT, HOMO–LUMO and Natural Bond Orbital analyses of the electronic structure of 2-mercapto-1-methylimidazole. J. Mol. Struct. 2011, 1006, 395–401. [Google Scholar] [CrossRef]
- Flakus, H.T.; Miros, A.; Jones, P.G. Polarization IR spectra of model crystals containing cyclic N H…S bonded dimers: 2-mercaptothiazoline and 2-mercapto-1-methylimidazole. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2002, 58, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, M.; Ramezanzadeh, M.; Ramezanzadeh, B.; Mahdavian, M. One-pot synthesis and construction of a high performance metal-organic structured nano pigment based on nanoceria decorated cerium (III)-imidazole network (NC/CIN) for effective epoxy composite coating anti-corrosion and thermo-mechanical properties improvement. Chem. Eng. J. 2020, 382, 122820. [Google Scholar]
- Arabkhani, P.; Javadian, H.; Asfaram, A.; Ateia, M. Decorating graphene oxide with zeolitic imidazolate framework (ZIF-8) and pseudo-boehmite offers ultra-high adsorption capacity of diclofenac in hospital effluents. Chemosphere 2021, 271, 129610. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Zhao, S.; Serdechnova, M.; Blawert, C.; Wang, H.; Zheludkevich, M.L.; Chen, F. Double-Ligand Strategy to Construct an Inhibitor-Loaded Zn-MOF and Its Corrosion Protection Ability for Aluminum Alloy 2A12. ACS Appl. Mater. Interfaces 2021, 13, 51685–51694. [Google Scholar] [CrossRef]
- Jing, C.; Wang, Z.; Gong, Y.; Huang, H.; Ma, Y.; Xie, H.; Li, H.; Zhang, S.; Gao, F. Photo and thermally stable branched corrosion inhibitors containing two benzotriazole groups for copper in 3.5 wt% sodium chloride solution. Corros. Sci. 2018, 138, 353–371. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, S.; Tan, B.; Feng, L.; Xiang, B.; Chen, F.; Li, W.; Xiong, B.; Song, T. Phenothiazine drugs as novel and eco-friendly corrosion inhibitors for copper in sulfuric acid solution. J. Taiwan Inst. Chem. Eng. 2020, 113, 253–263. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Erbe, A. The multiple roles of an organic corrosion inhibitor on copper investigated by a combination of electrochemistry-coupled optical in situ spectroscopies. Corros. Sci. 2018, 145, 232–238. [Google Scholar] [CrossRef]
- Finšgar, M.; Merl, D.K. An electrochemical, long-term immersion, and XPS study of 2-mercaptobenzothiazole as a copper corrosion inhibitor in chloride solution. Corros. Sci. 2014, 83, 164–175. [Google Scholar] [CrossRef]
- Finšgar, M. EQCM and XPS analysis of 1,2,4-triazole and 3-amino-1,2,4-triazole as copper corrosion inhibitors in chloride solution. Corros. Sci. 2013, 77, 350–359. [Google Scholar] [CrossRef]
- Finšgar, M. Surface analysis of 2-mercapto-1-methylimidazole adsorbed on copper by X-ray photoelectron spectroscopy. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2018, 190, 290–297. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Huang, Y.; Qi, Y. The polymeric nanofilm of triazinedithiolsilane fabricated by self-assembled technique on copper surface. Part 2: Characterization of composition and morphology. Appl. Surf. Sci. 2015, 356, 191–202. [Google Scholar] [CrossRef]
- Milošev, I.; Kovačević, N.; Kovač, J.; Kokalj, A. The roles of mercapto, benzene and methyl groups in the corrosion inhibition of imidazoles on copper: I. Experimental characterization. Corros. Sci. 2015, 98, 107–118. [Google Scholar] [CrossRef]
- Farhadian, A.; Rahimi, A.; Safaei, N.; Shaabani, A.; Abdouss, M.; Alavi, A. A theoretical and experimental study of castor oil-based inhibitor for corrosion inhibition of mild steel in acidic medium at elevated temperatures. Corros. Sci. 2020, 175, 108871. [Google Scholar] [CrossRef]
- Wang, D.; Xiang, B.; Liang, Y.; Song, S.; Liu, C. Corrosion control of copper in 3.5wt.% NaCl Solution by Domperidone: Experimental and Theoretical Study. Corros. Sci. 2014, 85, 77–86. [Google Scholar] [CrossRef]
- Kazansky, L.P.; Pronin, Y.E.; Arkhipushkin, I.A. XPS study of adsorption of 2-mercaptobenzothiazole on a brass surface. Corros. Sci. 2014, 89, 21–29. [Google Scholar] [CrossRef]
- Benali, O.; Larabi, L.; Harek, Y. Inhibiting effects of 2-mercapto-1-methylimidazole on copper corrosion in 0.5M sulfuric acid. J. Saudi Chem. Soc. 2010, 14, 231–235. [Google Scholar] [CrossRef]
- Dafali, A.; Hammouti, B.; Aouniti, A.; Mokhlisse, R.; Kertit, S.; Elkacemi, K. 2-Mercapto-1-methylimidazole as corrosion inhibitor of copper in aerated 3% NaCl solution. Ann. Chim. Sci. Matériaux 2000, 25, 437–446. [Google Scholar] [CrossRef]
- Larabi, L.; Benali, O.; Mekelleche, S.; Harek, Y. 2-Mercapto-1-methylimidazole as corrosion inhibitor for copper in hydrochloric acid. Appl. Surf. Sci. 2006, 253, 1371–1378. [Google Scholar] [CrossRef]
- Etaiw, S.E.-D.H.; Fouda, A.E.-A.S.; Abdou, S.N.; El-bendary, M.M. Structure, characterization and inhibition activity of new metal–organic framework. Corros. Sci. 2011, 53, 3657–3665. [Google Scholar] [CrossRef]
- Finšgar, M.; Milošev, I. Inhibition of copper corrosion by 1,2,3-benzotriazole: A review. Corros. Sci. 2010, 52, 2737–2749. [Google Scholar] [CrossRef]











| Time (h) | Ecorr (mV vs. SCE) | ba (mV/dec) | bc (mV/dec) | icorr (A/cm2) | η (%) |
|---|---|---|---|---|---|
| 0 | −218 ± 2 | 53.5 ± 1.4 | −112.0 ± 6.1 | (3.22 ± 0.14) × 10−5 | / |
| 1 | −209 ± 1 | 60.6 ± 3.3 | −119.2 ± 10.5 | (1.45 ± 0.26) × 10−5 | 55.2 ± 10.1 |
| 2 | −192 ± 3 | 55.0 ± 2.8 | −125.0 ± 29.0 | (7.32 ± 0.83) × 10−6 | 77.3 ± 2.6 |
| 3 | −181 ± 2 | 52.1 ± 3.6 | −127.8 ± 13.7 | (6.31 ± 0.74) × 10−6 | 80.4 ± 3.2 |
| 4 | −176 ± 4 | 53.0 ± 4.3 | −126.8 ± 19.0 | (4.62 ± 0.53) × 10−6 | 85.7 ± 2.2 |
| 5 | −163 ± 2 | 61.7 ± 7.3 | −129.9 ± 19.5 | (3.93 ± 0.81) × 10−6 | 87.8 ± 1.8 |
| 6 | −144 ± 3 | 55.2 ± 3.1 | −179.0 ± 45.7 | (3.80 ± 0.16) × 10−6 | 88.2 ± 1.4 |
| Time (h) | Rs (Ω·cm2) | CPEdl-T (Sn1·Ω−1·cm−2) | n1 | Rct (Ω·cm2) | CPEf-T (Sn2·Ω−1·cm−2) | n2 | Rf (Ω·cm2) |
|---|---|---|---|---|---|---|---|
| blank | 4.3 ± 0.4 | (2.26 ± 0.22) × 10−5 | 0.88 ± 0.03 | 1278.8 ± 16.1 | (1.42 ± 0.13) × 10−4 | 0.65 ± 0.07 | 2276 ± 48.7 |
| 1 | 9.4 ± 0.6 | (1.53 ± 0.79) × 10−5 | 0.82 ± 0.04 | 2612.1 ± 34.0 | (8.19 ± 0.04) × 10−6 | 0.90 ± 0.04 | 4206 ± 40.2 |
| 2 | 10.0 ± 1.2 | (1.45 ± 0.25) × 10−5 | 0.83 ± 0.06 | 4194.5 ± 37.1 | (5.55 ± 0.05) × 10−6 | 0.96 ± 0.01 | 8262 ± 89.0 |
| 3 | 17.6 ± 0.2 | (1.26 ± 0.43) × 10−5 | 0.83 ± 0.06 | 5474.7 ± 56.1 | (4.41 ± 0.03) × 10−6 | 0.99 ± 0.05 | 9384 ± 89.0 |
| 4 | 11.3 ± 1.5 | (1.10 ± 0.35) × 10−5 | 0.83 ± 0.06 | 7408.3 ± 72.1 | (2.82 ± 0.10) × 10−6 | 0.94 ± 0.04 | 11,308 ± 89.0 |
| 5 | 12.0 ± 1.0 | (1.14 ± 0.36) × 10−5 | 0.77 ± 0.06 | 9378.5 ± 191.8 | (2.83 ± 0.04) × 10−6 | 0.97 ± 0.08 | 12,014 ± 301.4 |
| 6 | 8.1 ± 1.5 | (1.09 ± 0.29) × 10−5 | 0.81 ± 0.08 | 11,330.8 ± 305.4 | (2.55 ± 0.06) × 10−6 | 0.99 ± 0.02 | 13,633 ± 246.8 |
| Sample | C 1s | O 1s | N 1s | S 2p | Cl 2p | Cu 2p | Zn 2p |
|---|---|---|---|---|---|---|---|
| blank | 37.59 | 40.72 | 1 | 0.32 | 4.36 | 15.36 | 0.65 |
| 3 h | 44.19 | 24.39 | 10.24 | 5.21 | 2 | 12.83 | 1.15 |
| 6 h | 44.74 | 12.85 | 16.77 | 8.07 | 2.54 | 11.27 | 3.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Zhang, X. Controlled Delivery of 2-Mercapto 1-Methyl Imidazole by Metal–Organic Framework for Efficient Inhibition of Copper Corrosion in NaCl Solution. Materials 2023, 16, 6712. https://doi.org/10.3390/ma16206712
Zhu Z, Zhang X. Controlled Delivery of 2-Mercapto 1-Methyl Imidazole by Metal–Organic Framework for Efficient Inhibition of Copper Corrosion in NaCl Solution. Materials. 2023; 16(20):6712. https://doi.org/10.3390/ma16206712
Chicago/Turabian StyleZhu, Zhishun, and Xiulan Zhang. 2023. "Controlled Delivery of 2-Mercapto 1-Methyl Imidazole by Metal–Organic Framework for Efficient Inhibition of Copper Corrosion in NaCl Solution" Materials 16, no. 20: 6712. https://doi.org/10.3390/ma16206712
APA StyleZhu, Z., & Zhang, X. (2023). Controlled Delivery of 2-Mercapto 1-Methyl Imidazole by Metal–Organic Framework for Efficient Inhibition of Copper Corrosion in NaCl Solution. Materials, 16(20), 6712. https://doi.org/10.3390/ma16206712





