Investigation of Vegetable Oils and Their Derivatives for the Synthesis of Extreme Pressure Additives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Methods
- Appearance [Visual].
- Density at 20 °C, [ISO 12185:1998], g/cm3.
- Kinematic viscosity at 40 °C [ISO 3104:1996], mm2/s.
- Kinematic viscosity at 100 °C-on [ISO 3104:1996], mm2/s.
- Dynamic viscosity at 40 °C-on [calculated] [ISO 3104:1996], mPa·s.
- Acid value [ISO 660:2000], mg KOH/g.
- Saponification number [ISO 6293:1994], mg KOH/g.
- Iodine value [ISO 3961:2000], g I2/100g.
- Water content [ISO 12937:2001], wt%.
- Sediment content [IEC 60422], wt%.
- Four-ball test – weld load [DIN 51350-4:2015], N.
- Four-ball test – wear scar diameter [DIN 51350-5:2015], mm.
3. Results
4. Conclusions
- All basic physicochemical characteristics of the examined materials evaluated in this work present some relation with the properties of the tested vegetable oils and their derivates and olefins (where there is a relation between the viscosity and iodine value of materials).
- It was found that vegetable oil with a density above 0.913 g/cm3 is preferable for the synthesis of EP additives.
- The application of the upper phase of the used cooking oil is beneficial after sedimentation of its higher-density fraction.
- Although it is not possible to select vegetable oil and its derivatives based on their chemical composition only, it is preferable to use raw materials with a high iodine value, which is beneficial from the perspective of synthesis.
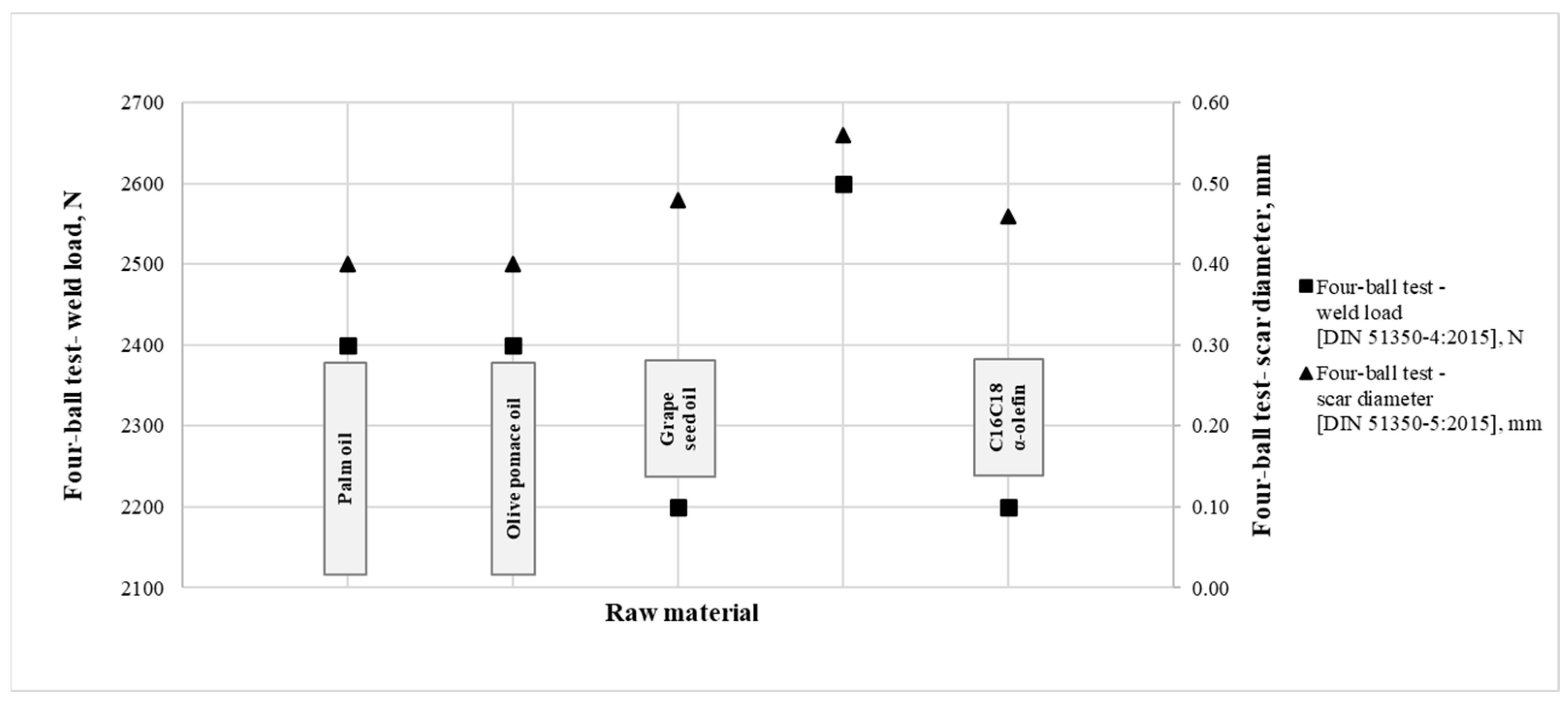
- It was found that additives made from palm oil, olive pomace oil, grapeseed oil and C16C18 α-olefin have the best wear scar-inhibiting effect. Therefore, the sulfurized derivatives of the investigated raw materials produced by the same technology resulted in effective EP additives (2100–2600 N).
- Based on the experimental data and the stated criteria, the examined raw materials were found to be suitable for the synthesis of EP additives. The most favourable outcomes in terms of the EP effect were achieved when utilizing palm oil, olive pomace oil, and C12C14 α-olefin. However, in the case of use, if a low wear scar diameter value is an important requirement for the additive, it is recommended to use the raw materials listed in Conclusion point 5.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Theo, M.; Wilfried, D. Lubricants and Lubrication, 2nd ed.; Wiley: Weinheim, Germany, 2007; pp. 88–119. [Google Scholar]
- Syed, Q.A.R. A Comprehensive Review of Lubricant Chemistry, Technology, Selection and Design; ASTM International: West Conshohocken, PH, USA, 2009; pp. 149–181. [Google Scholar]
- Leslie, R.R. Lubricant Additives. In Chemistry and Applications, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 211–278. [Google Scholar]
- Pradeep, L.M.; Sudeep, P.I.; Michael, N.; Satish, V.K.; Michael, R.L. Tribology for Scientists and Engineers; Springer: New York, NY, USA, 2013; pp. 317–318. [Google Scholar]
- Vámos, E. Friction, wear and lubrication of machines and machine parts. In Tribological Handbook; Technical Publishing House: Budapest, Hungary, 1983; pp. 36–116. [Google Scholar]
- Theo, M.; Kirsten, B.; Thorsten, B. Tribosystems, Friction, Wear and Surface Engineering, Lubrication. In Industrial Tribology; Wiley: Weinheim, Germany, 2011; pp. 320–335. [Google Scholar]
- Don, M.P.; Martin, W.; Ekkehard, D. Lubrication Fundamentals, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 35–60. [Google Scholar]
- Theo, M. Encyclopedia of Lubricants and Lubrication; Springer: Berlin/Heidelberg, Germany, 2014; pp. 131–146. [Google Scholar]
- Frank, L.E.; Phillip, S.L.; Robert, E.A. Vegetable Oil Derivatives as Lubricant Additives. U.S. Patent 4,970,010, 1990. [Google Scholar]
- Edward, W.B. Wax Esters of Vegetable Oil Fatty Acids Are Useful as Lubricants. U.S. Patent 4,152,278, 1979. [Google Scholar]
- Chiu, L.O.; Xuefeng, J.; Joon, C.J.; Nader, G.K.; Thorsten, H. Ashless and non-corrosive disulfide compounds as excellent extreme pressure additives in naphthenic oil. J. Mol. Liq. 2022, 351, 118553. [Google Scholar]
- Nor, A.M.; Mohamad, A.S.; Ghazali, O.; Muhammed, R.M.; Adzni, M.S.; Nurfaizey, A.H.; Mohamad, I.S.; Feng, D. Vegetable Oil as Bio-Lubricant and Natural Additive in Lubrication: A Review. Int. J. Nanoelectron. Mater. 2020, 13, 161–176. [Google Scholar]
- Abdel Hameed, R.S. Recycling of the Waste Cooking Oils as Non-ionic Surfactants. Mater. Sci. Indian J. 2017, 15, 104. [Google Scholar]
- Arjun, B.C.; KChris, W.; MRafiqul, I. Waste Cooking Oil as an Alternate Feedstock for Biodiesel Production. Energies 2008, 1, 3–18. [Google Scholar]
- Juliano, G.; Melissa, M.M.; Aline, O.; Aline, M. Grape Seed Oil Compounds: Biological and Chemical Actions for Health. Nutr. Metab. Insights 2016, 9, S32910. [Google Scholar]
- Hammond, E.W. Vegetable Oils, Types and Properties; Elsevier: Amsterdam, The Netherlands, 2003; pp. 5899–5904. [Google Scholar]
- Dimitrios, B.; Georgios, B.; Maria, T. Olive Oil Composition. In Olive Oil; AOCS Press: Urbana, IL, USA, 2006; pp. 41–72. [Google Scholar]
- Karak, N. Vegetable Oils and Their Derivatives; Woodhead Publishing Limited: Cambridge, UK, 2012; pp. 54–94. [Google Scholar]
- DIN 51350-4; Testing of Lubricants—Testing in the 4-Ball Tester—Part 4: Determination of the Welding Load of Consistent Lubricants. Deutsches Institut für Normung e. V., Beuth Verlag GmbH: Berlin, Germany, 2015.
- DIN 51350-5; Testing of Lubricants–Testing in The 4-Ball Tester–Part 5: Determination of the Wearing Characteristics of Consistent Lubricants. Deutsches Institut für Normung e. V., Beuth Verlag GmbH: Berlin, Germany, 2015.
- Emile, N.H. Novel Sulfur-Containing Compositions. U.S. Patent 3,926,822, 1974. [Google Scholar]
- Donald, A.L.; John, A.B. Extreme Pressure Additives for Lubricants. U.S. Patent 4,149,982, 1979. [Google Scholar]
- Shubhamita, B.; James, N.V.; Michael, S.W. Industrial gear lubricant additive package with biodegradable sulfur component. U.S. Patent 10,208,267, 2019. [Google Scholar]

| Properties | Rapeseed Oil (Food) 6404/2110 | Sunflower Oil (Industrial) 6405/2110 | Sunflower Oil (Food) 6331/2206 | UCO 1 6371/2109 | UCO (Upper Phase) 2 6194/2203 | Palm Oil 6202/2203 | Olive Pomace Oil 6133/2202 | Extra Virgin Olive Oil 6538/2209 | Grape Seed Oil 6539/2209 | Castor Oil 6552/2209 | Oleic Acid 210625/033 | Stearic Acid 18:65 6197/2203 | Fatty Acid Methyl Ester 6406/2110 | C12C14 α-Olefin 6326/2205 | C14C16 α-Olefin 6325/2205 | C16C18 α-Olefin 6324/2206 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trade name | Refined rapeseed oil | Raw sunflower oil | Refined sunflower oil | UCO | UCO | Palm frying oil | Olive pomace oil | Bio Organic extra virgin olive oil | Refined grapeseed oil | Castor oil | Palmera A1818 | Cremer AC Stearic acid 18:65 | FAME B-100 | Alpha Olefin C1214 | Alpha Olefin C1416 | Alpha Olefin C1618 |
| Producer or distributor | Bunge Ltd., Budapest, Hungary | Bunge Ltd., Budapest, Hungary | Bunge Ltd., Budapest, Hungary | Biofilter Ltd., Törökbálint, Hungary | Biofilter Ltd., Törökbálint, Hungary | Vog Ltd., Bük, Hungary | Fortis Holding Ltd., Budapest, Hungary | Borges S.A.U., Tàrrega, Spain | Virgin Oil Press Ltd., Budapest, Hungary | Thermo Fisher Scientific, Waltham, USA | Palm-Oleo Sdn. Bhd., Petaling Jaya, Malaysia | CREMER OLEO GmbH, Hamburg, Germany | Rossi Biofuel Ltd., Komárom, Hungary | INEOS Oligomers, Alvin, TX, USA | INEOS Oligomers, Alvin, TX, USA | INEOS Oligomers, Alvin, TX, USA |
| Area of application | food industry, fuels | lubricants, lubricant additives, fuels | food industry, fuels | biodiesel production | - | food industry, chemical synthesis | food industry | food industry, cosmetics | food industry, cosmetics | medications, cosmetics, lubricants, chemical synthesis | lubricants, cosmetics, plastics | detergents, cosmetics, lubricants, coating | mainly biodiesel | detergent intermediates | oilfield and paper chemicals | oilfield and paper chemicals |
| Purity, % | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | min. 70 | min. 65 | min. 99.7 | min. 99.7 | min. 99.5 | min. 99.2 |
| Raw Material | Carbon Atom Numbers and Double Bonds of Fatty Acids | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | C20:1 | Others 3 | |
| Rapeseed oil | 4–5 | 0.3 | 1–2 | 56–64 | 20–26 | 8–10 | 0.6 | 1.4 | 0–1 |
| Sunflower oil | 4.2–6.8 | 0.3 | 2.1–5 | 20–25 | 63–68 | 0.2 | 0.4–1 | - | 0.3 |
| Used cooking oil | 19–21 | 4–5 | 4–6 | 50–55 | 0–1.5 | 0–1 | 0–1 | 0–1.5 | 0–1.5 |
| Palm oil | 37–44 | 0.4 | 3–6 | 39–44 | 8–10 | 0.3 | 0.4 | - | 1 |
| Olive oil | 7.5–20 | 0.3–3.5 | 0.5–5 | 55–83 | 3.5–21 | 0.9 | 0.4 | 0.4 | 0–1 |
| Grape seed oil | 5–7 | 0–1 | 3–5 | 13–15 | 73–77 | 0–1 | 0–0.5 | - | 2–4 |
| Castor oil | 0.5–1 | - | 0.5–1 | 4–5 | 2–4 | 0.5–1 | - | - | 83–85 |
| Oleic acid | 5–6 | 1.2–1.8 | 78–82 | 12–13 | 0.1–0.3 | - | 0–1 | ||
| Stearic acid | 31–34 | - | 62–68 | 0–0.5 | - | - | 0–0.5 | - | 0–2 |
| Fatty acid methyl ester | 8–9 | 0.5 | 2–4 | 51–60 | 21–24 | 6–8 | 0.5 | 1.2 | 1–2 |
| Measurement | Four-Ball Weld Load | Four-Ball Wear Scar Diameter |
|---|---|---|
| Equipment | Four-ball tester | Four-ball tester |
| Method | DIN 51350-4 | DIN 51350-5 |
| Size, shape (static) | d = 12.7 mm, ball | d = 12.7 mm, ball |
| Rotational frequency, shape (dynamic) | 1500 rpm, ball | 1500 rpm, ball |
| Load | max. 12,000 N | 400 N |
| Measurement length | 60 s | 1 h |
| Measured property | weld load, N | wear scar diameter, mm |
| Contact type of surfaces | ball-ball | ball-ball |
| Method | Rapeseed Oil (Food) 6404/2110 | Sunflower Oil (Industrial) 6405/2110 | Sunflower Oil (Food) 6331/2206 | UCO 4 6371/2109 | UCO (Upper Phase) 5 6194/2203 | Palm Oil 6202/2203 | Olive Pomace Oil 6133/2202 | Extra Virgin Olive Oil 6538/2209 | Grape Seed Oil 6539/2209 | Castor Oil 6552/2209 | Oleic Acid 210625/033 | Stearic Acid 18:65 6197/2203 | Fatty Acid Methyl Ester 6406/2110 | C12C14 α-Olefin 6326/2205 | C14C16 α-Olefin 6325/2205 | C16C18 α-Olefin 6324/2206 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance [Visual] | yellow, clear liquid | yellow, slightly opalescent liquid | yellow, clear liquid | brown, opalescent liquid | brown, clear liquid | light yellow, solid | yellow, clear liquid | yellow, clear liquid | light yellow, clear liquid | light yellow, clear liquid | dark yellow, clear liquid | white, solid | brownish yellow, clear liquid | colourless, clear liquid | colourless, clear liquid | colourless, clear liquid |
| Density at 20 °C [ISO 12185:1998], g/cm3 | 0.9166 | 0.9195 | 0.9187 | 0.9198 | 0.9202 | 0.9185 | 0.9141 | 0.9163 | 0.9183 | 0.9578 | 0.8955 | not measurable | 0.8797 | 0.7634 | 0.7789 | 0.7862 |
| Kinematic viscosity at 40 °C [ISO 3104:1996], mm2/s | 35.44 | 33.21 | 32.28 | 73.96 | 41.40 | 72.01 | 40.42 | 38.28 | 31.01 | 250.30 | 19.77 | not measurable | 4.36 | 1.44 | 2.28 | 2.95 |
| Kinematic viscosity at 100 °C [ISO 3104:1996], mm2/s | 8.11 | 7.81 | 7.74 | 13.94 | 8.84 | 8.76 | 8.45 | 8.39 | 7.60 | 19.19 | 4.85 | not measurable | 1.98 | 0.74 | 1.04 | 1.26 |
| Dynamic viscosity at 40 °C [calculated] [ISO 3104:1996], mPa·s | 32.04 | 30.13 | 29.26 | 67.13 | 37.59 | 65.27 | 36.45 | 35.08 | 28.48 | 239.74 | 17.45 | not measurable | 3.78 | 1.08 | 1.74 | 2.27 |
| Acid value [ISO 660:2000], mg KOH/g | 0.12 | 1.58 | 0.07 | 2.92 | 3.51 | 0.19 | 0.19 | 0.21 | 0.28 | 0.54 | 200 | 208.23 | 0.44 | <0.01 | <0.01 | <0.01 |
| Saponification number [ISO 6293:1994], mg KOH/g | 191.9 | 191.1 | 191.2 | 194.0 | 194.2 | 198.8 | 189.1 | 198.4 | 204.1 | 178.2 | 199.1 | 209.68 | 193.8 | 0.0 | 0.0 | 0.0 |
| Iodine value [ISO 3961:2000], gI2/100g | 108.0 | 122.0 | 125.5 | 97.2 | 85.4 | 52.2 | 72.0 | 78.0 | 134.7 | 81.1 | 100.6 | 0.16 | 95.9 | 145.7 | 122.6 | 110.8 |
| Water content [KF-Coulometry] [ISO 12937:2001], wt% | 0.044 | 0.057 | 0.029 | 0.400 | 0.130 | <0.01 | 0.050 | 0.070 | 0.010 | 0.190 | 0.08 | not measurable | 0.064 | 0.010 | 0.010 | 0.010 |
| Sediment content [IEC 60422], wt% | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01- |
| Raw Material | Active Sulfur Content [ASTM D 1662], wt% | Four-Ball Test- Weld Load [DIN 51350-4], N | Four-Ball Test- Scar Diameter [DIN 51350], mm |
|---|---|---|---|
| Rapeseed oil, food grade | 1.37 | 2300 | 0.50 |
| Sunflower oil, industrial grade | 1.18 | 2300 | 0.51 |
| Sunflower oil, food grade | 1.23 | 2300 | 0.51 |
| Used cooking oil | 1.94 | 2100 | 0.52 |
| Used cooking oil (upper phase) | 2.61 | 2300 | 0.50 |
| Palm oil | 4.70 | 2400 | 0.40 |
| Olive pomace oil | 3.78 | 2400 | 0.40 |
| Extra-virgin olive oil | 4.79 | 2300 | 0.50 |
| Grape seed oil | 1.62 | 2200 | 0.48 |
| Castor oil | inhomogeneous solution | ||
| Oleic acid | 6.96 | 2300 | 0.58 |
| Vegetable stearic acid 18:65 | inhomogeneous solution | ||
| Fatty acid methyl ester | 4.72 | 2100 | 0.56 |
| C12C14 α-olefin | 9.37 | 2600 | 0.56 |
| C14C16 α-olefin | 7.89 | 2300 | 0.52 |
| C16C18 α-olefin | 6.45 | 2200 | 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, G.Z.; Nagy, R. Investigation of Vegetable Oils and Their Derivatives for the Synthesis of Extreme Pressure Additives. Materials 2023, 16, 6570. https://doi.org/10.3390/ma16196570
Nagy GZ, Nagy R. Investigation of Vegetable Oils and Their Derivatives for the Synthesis of Extreme Pressure Additives. Materials. 2023; 16(19):6570. https://doi.org/10.3390/ma16196570
Chicago/Turabian StyleNagy, Gábor Zoltán, and Roland Nagy. 2023. "Investigation of Vegetable Oils and Their Derivatives for the Synthesis of Extreme Pressure Additives" Materials 16, no. 19: 6570. https://doi.org/10.3390/ma16196570
APA StyleNagy, G. Z., & Nagy, R. (2023). Investigation of Vegetable Oils and Their Derivatives for the Synthesis of Extreme Pressure Additives. Materials, 16(19), 6570. https://doi.org/10.3390/ma16196570







