Mg-Doped PLA Composite as a Potential Material for Tissue Engineering—Synthesis, Characterization, and Additive Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solution Preparation and Thin Film Formation
2.3. Characterization
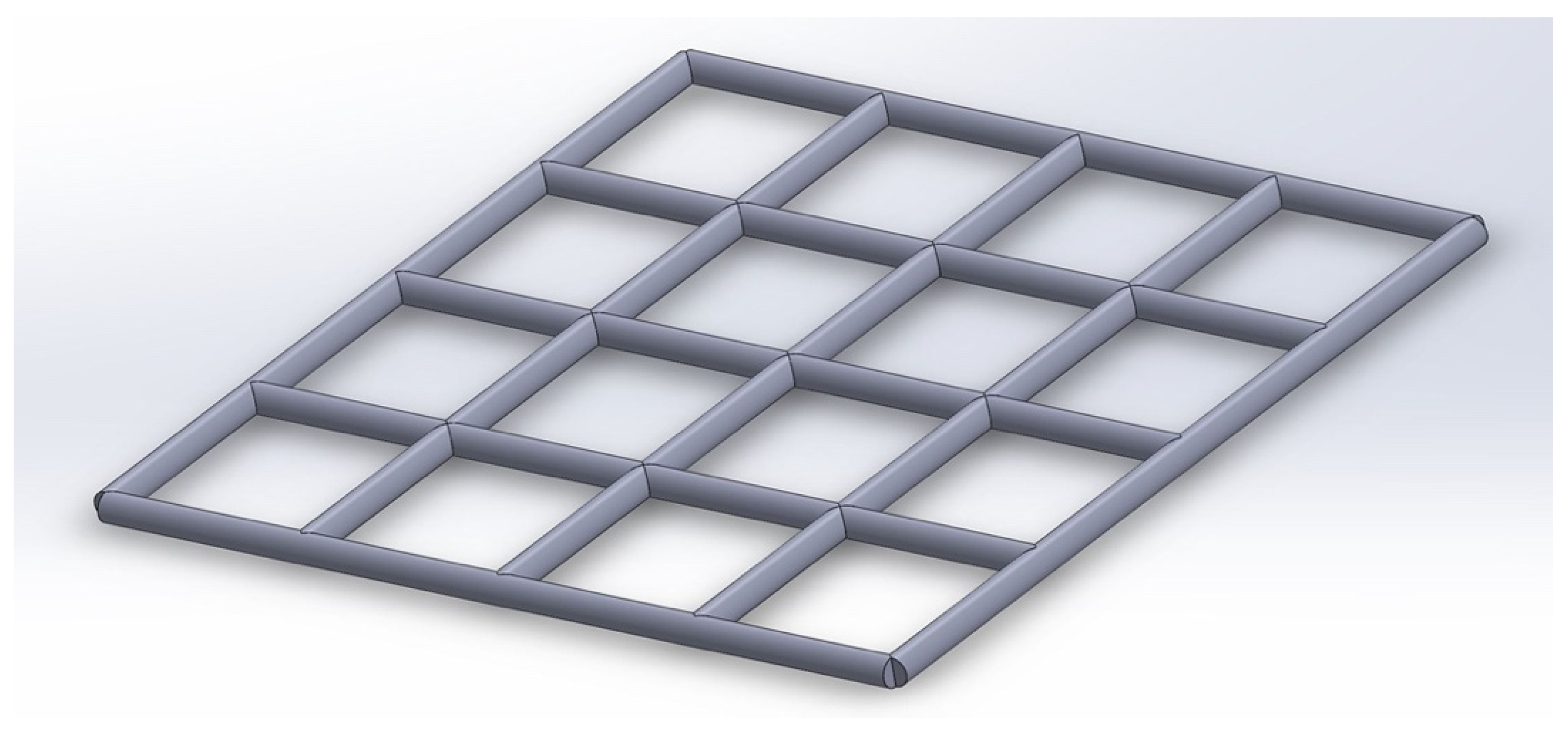
2.4. Direct Ink Writing (DIW) 3D Printing of PLA/Mg Ink
3. Results and Discussion
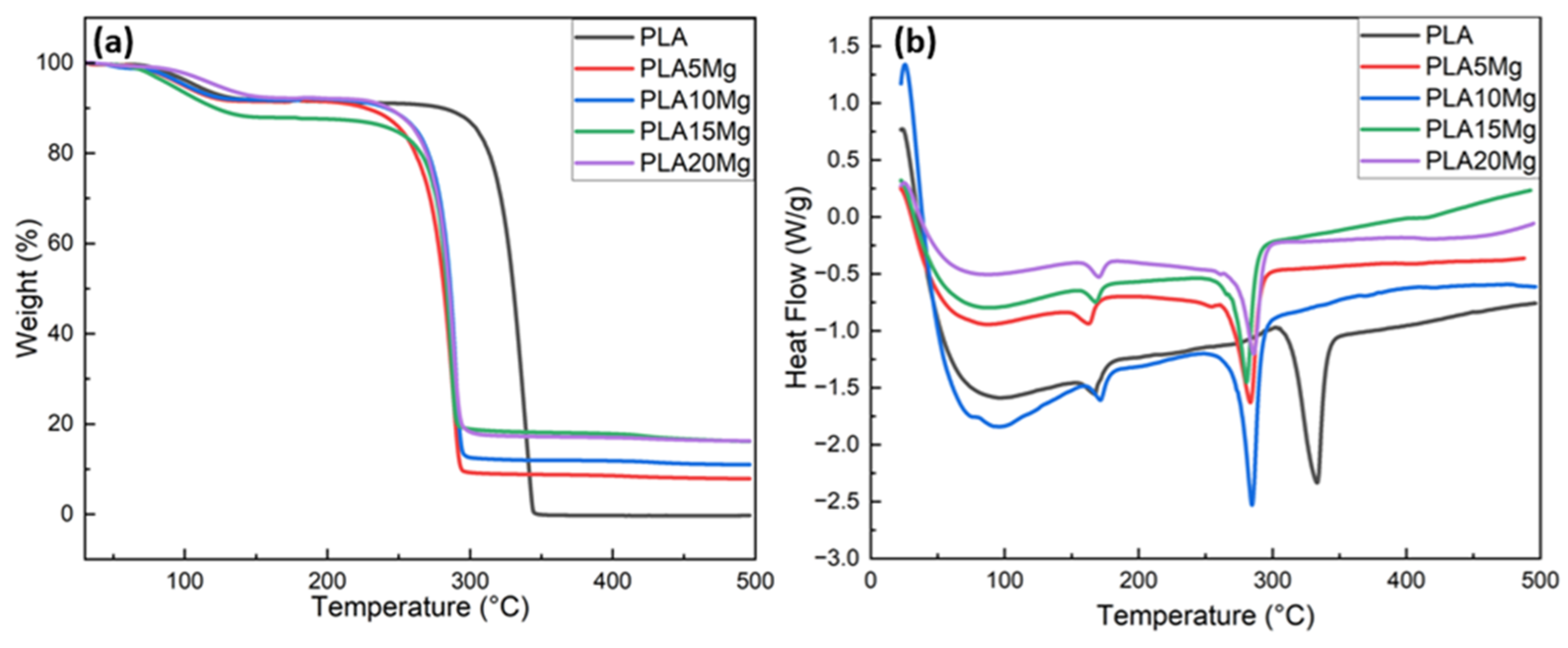
3.1. Thermogravimetric Analysis (TGA)
3.2. Differential Scanning Calorimetry (DSC)
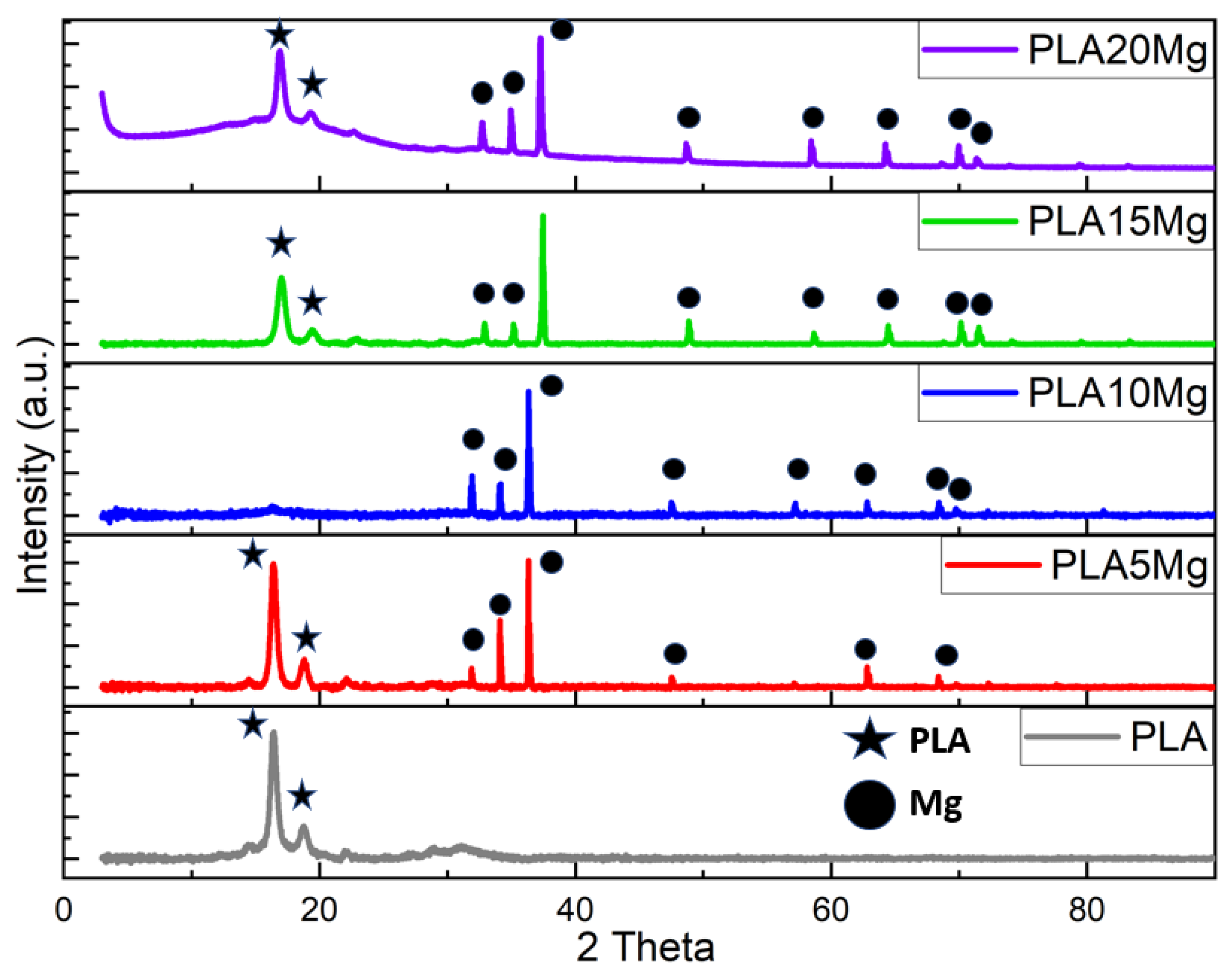
3.3. X-ray Diffraction (XRD)
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
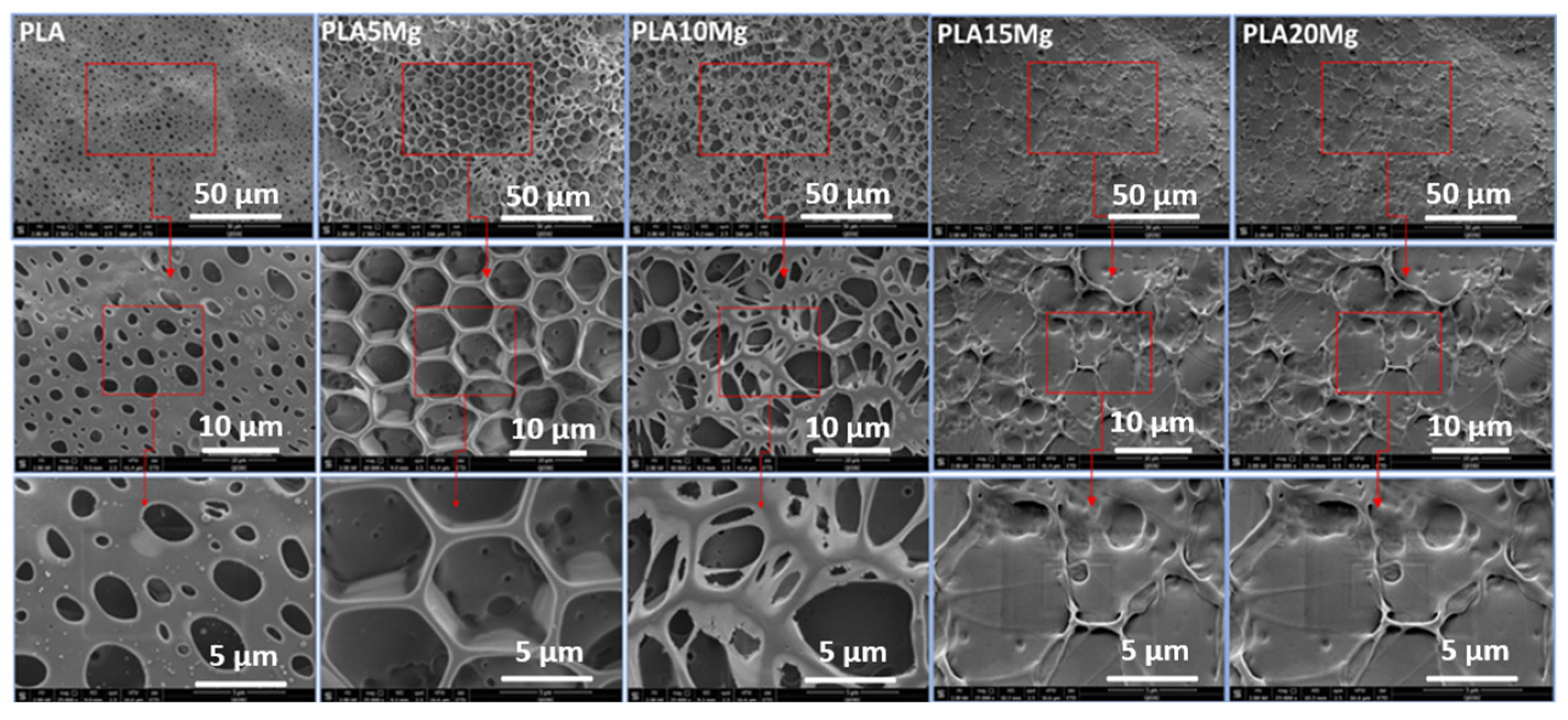
3.5. Scanning Electron Microscope (SEM)
3.6. Degradation of PLA/Mg Composites
3.7. PLA/Mg Mass Variation (Weight Gain/Loss)
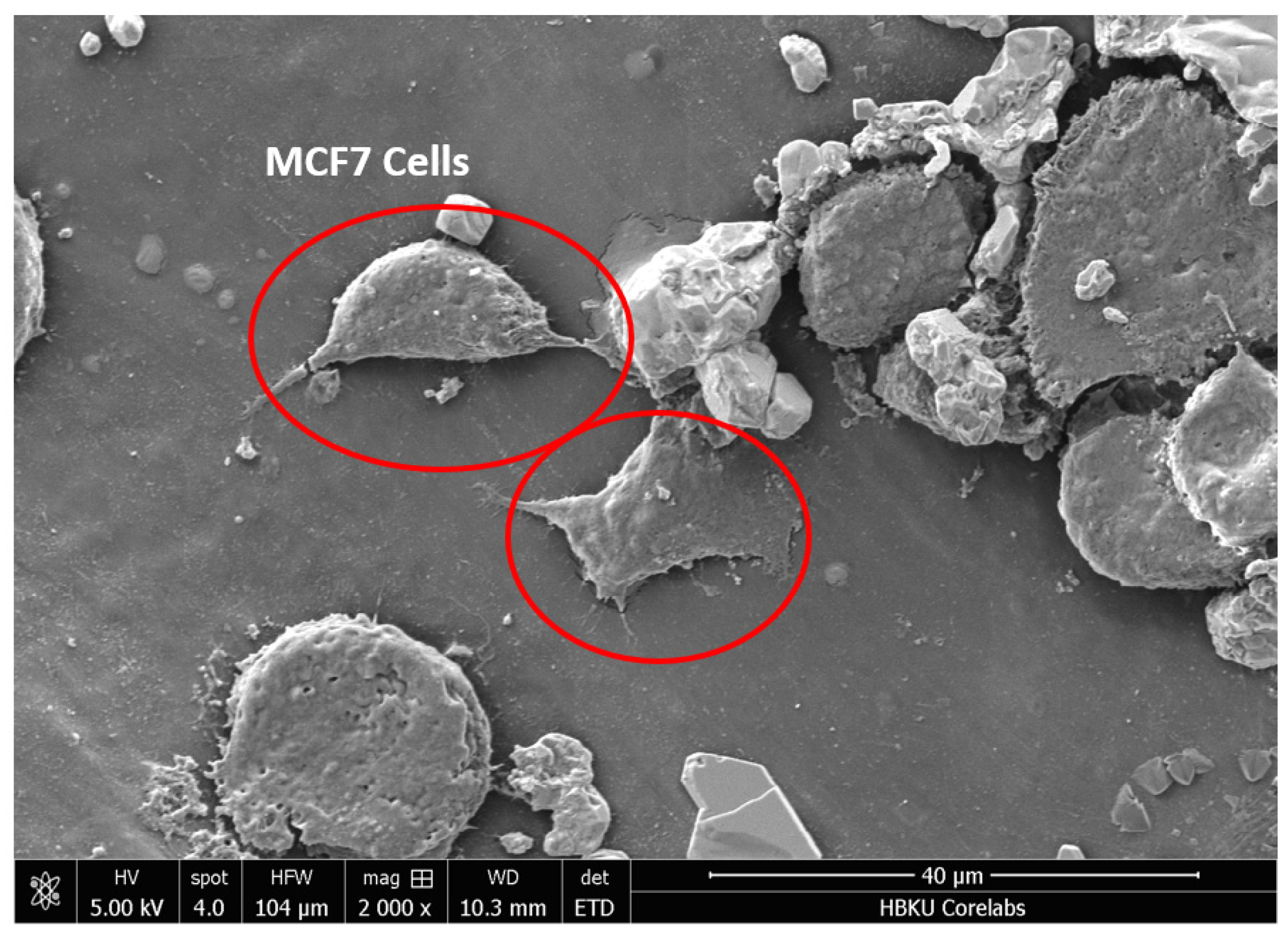
3.8. Cell Adhesion
3.9. Direct Ink Writing (3D Printing) of PLA/Mg Ink
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, F.; Kalva, S.N.; Koç, M. Additive Manufacturing of Polymer/Mg-Based Composites for Porous Tissue Scaffolds. Polymers 2022, 14, 5460. [Google Scholar] [CrossRef]
- Penumakala, P.K.; Santo, J.; Thomas, A. A critical review on the fused deposition modeling of thermoplastic polymer composites. Compos. Part B Eng. 2020, 201, 108336. [Google Scholar] [CrossRef]
- Rahmatabadi, D.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Baghani, M. 3D printing of PLA-TPU with different component ratios: Fracture toughness, mechanical properties, and morphology. J. Mater. Res. Technol. 2022, 21, 3970–3981. [Google Scholar] [CrossRef]
- Chaunier, L.; Guessasma, S.; Belhabib, S.; Della Valle, G.; Lourdin, D.; Leroy, E. Material extrusion of plant biopolymers: Opportunities & challenges for 3D printing. Addit. Manuf. 2018, 21, 220–233. [Google Scholar] [CrossRef]
- Freed, L.E.; Vunjak-Novakovic, G.; Biron, R.J.; Eagles, D.B.; Lesnoy, D.C.; Barlow, S.K.; Langer, R. Biodegradable Polymer Scaffolds for Tissue Engineering. Nat. Biotechnol. 1994, 12, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Sell, S.; Barnes, C.; Smith, M.; McClure, M.; Madurantakam, P.; Grant, J.; McManus, M.; Bowlin, G. Extracellular matrix regenerated: Tissue engineering via electrospun biomimetic nanofibers. Polym. Int. 2007, 56, 1349–1360. [Google Scholar] [CrossRef]
- Gregor, A.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A.; et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 1–21. [Google Scholar] [CrossRef]
- Athanasiou, K.; Agrawal, C.; Barber, F.; Burkhart, S. Orthopaedic applications for PLA-PGA biodegradable polymers. Arthrosc. J. Arthrosc. Relat. Surg. 1998, 14, 726–737. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 1997, 28, 5–24. [Google Scholar] [CrossRef]
- Chen, K.-J.; Hung, F.-Y.; Wang, Y.-T.; Yen, C.-W. Mechanical properties and biomedical application characteristics of degradable polylactic acid–Mg–Ca3(PO4)2 three-phase composite. J. Mech. Behav. Biomed. Mater. 2022, 125, 104949. [Google Scholar] [CrossRef] [PubMed]
- Akindoyo, J.O.; Beg, M.D.; Ghazali, S.; Heim, H.P.; Feldmann, M. Effects of surface modification on dispersion, mechanical, thermal and dynamic mechanical properties of injection molded PLA-hydroxyapatite composites. Compos. Part A Appl. Sci. Manuf. 2017, 103, 96–105. [Google Scholar] [CrossRef]
- Batakliev, T.; Petrova-Doycheva, I.; Angelov, V.; Georgiev, V.; Ivanov, E.; Kotsilkova, R.; Casa, M.; Cirillo, C.; Adami, R.; Sarno, M.; et al. Effects of Graphene Nanoplatelets and Multiwall Carbon Nanotubes on the Structure and Mechanical Properties of Poly(lactic acid) Composites: A Comparative Study. Appl. Sci. 2019, 9, 469. [Google Scholar] [CrossRef]
- Anugwom, I.; Lahtela, V.; Kallioinen, M.; Kärki, T. Lignin as a functional additive in a biocomposite: Influence on mechanical properties of polylactic acid composites. Ind. Crop. Prod. 2019, 140, 111704. [Google Scholar] [CrossRef]
- Cifuentes, S.; Gavilán, R.; Lieblich, M.; Benavente, R.; González-Carrasco, J. In vitro degradation of biodegradable polylactic acid/magnesium composites: Relevance of Mg particle shape. Acta Biomater. 2016, 32, 348–357. [Google Scholar] [CrossRef]
- Leonés, A.; Salaris, V.; Aranda, I.R.; Lieblich, M.; López, D.; Peponi, L. Thermal Properties and In Vitro Biodegradation of PLA-Mg Filaments for Fused Deposition Modeling. Polymers 2023, 15, 1907. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Wu, S.; Yeung, K.; Zheng, Y.; Chu, P.K. Design of magnesium alloys with controllable degradation for biomedical implants: From bulk to surface. Acta Biomater. 2016, 45, 2–30. [Google Scholar] [CrossRef]
- Tajvar, S.; Hadjizadeh, A.; Samandari, S.S. Scaffold degradation in bone tissue engineering: An overview. Int. Biodeterior. Biodegrad. 2023, 180, 105599. [Google Scholar] [CrossRef]
- Namdar, A.; Salahinejad, E. Advances in ion-doping of Ca-Mg silicate bioceramics for bone tissue engineering. Co-ord. Chem. Rev. 2023, 478, 215001. [Google Scholar] [CrossRef]
- Hu, J.; Shao, J.; Huang, G.; Zhang, J.; Pan, S. In Vitro and In Vivo Applications of Magnesium-Enriched Biomaterials for Vascularized Osteogenesis in Bone Tissue Engineering: A Review of Literature. J. Funct. Biomater. 2023, 14, 326. [Google Scholar] [CrossRef]
- Bairagi, D.; Mandal, S. A comprehensive review on biocompatible Mg-based alloys as temporary orthopaedic implants: Current status, challenges, and future prospects. J. Magnes. Alloy. 2021, 10, 627–669. [Google Scholar] [CrossRef]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg alloys for orthopedic implants—A review. J. Magnes. Alloy. 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Gollwitzer, H.; Thomas, P.; Diehl, P.; Steinhauser, E.; Summer, B.; Barnstorf, S.; Gerdesmeyer, L.; Mittelmeier, W.; Stemberger, A. Biomechanical and allergological characteristics of a biodegradable poly(D,L-lactic acid) coating for orthopaedic implants. J. Orthop. Res. 2005, 23, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, T.; Tsukegi, T.; Shirai, Y.; Nishida, H.; Endo, T. Effects of MgO catalyst on depolymerization of poly-l-lactic acid to l,l-lactide. Polym. Degrad. Stab. 2007, 92, 1350–1358. [Google Scholar] [CrossRef]
- Ferrández-Montero, A.; Lieblich, M.; González-Carrasco, J.; Benavente, R.; Lorenzo, V.; Detsch, R.; Boccaccini, A.; Ferrari, B. Development of biocompatible and fully bioabsorbable PLA/Mg films for tissue regeneration applications. Acta Biomater. 2019, 98, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Zerankeshi, M.M.; Sayedain, S.S.; Tavangarifard, M.; Alizadeh, R. Developing a novel technique for the fabrication of PLA-graphite composite filaments using FDM 3D printing process. Ceram. Int. 2022, 48, 31850–31858. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.; Cheon, K.-H.; Park, C.; Jang, T.-S.; Kim, H.-E.; Jung, H.-D. Fabrication of poly(lactic acid)/Ti composite scaffolds with enhanced mechanical properties and biocompatibility via fused filament fabrication (FFF)–based 3D printing. Addit. Manuf. 2019, 30, 100883. [Google Scholar] [CrossRef]
- Hasanpur, E.; Ghazavizadeh, A.; Sadeghi, A.; Haboussi, M. In vitro corrosion study of PLA/Mg composites for cardiovascular stent applications. J. Mech. Behav. Biomed. Mater. 2021, 124, 104768. [Google Scholar] [CrossRef]
- Antoniac, I.; Popescu, D.; Zapciu, A.; Antoniac, A.; Miculescu, F.; Moldovan, H. Magnesium Filled Polylactic Acid (PLA) Material for Filament Based 3D Printing. Materials 2019, 12, 719. [Google Scholar] [CrossRef]
- Zhu, Y.; Sheng, R.; Luo, T.; Li, H.; Sun, J.; Chen, S.; Sun, W.; Cao, A. Honeycomb-Structured Films by Multifunctional Amphiphilic Biodegradable Copolymers: Surface Morphology Control and Biomedical Application as Scaffolds for Cell Growth. ACS Appl. Mater. Interfaces 2011, 3, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ding, H.; Jiao, Q.; Shi, Y. Influence of Solvents on the Formation of Honeycomb Films by Water Droplets Templating. Macromol. Chem. Phys. 2006, 207, 545–553. [Google Scholar] [CrossRef]
- Zhao, X.; Cai, Q.; Shi, G.; Shi, Y.; Chen, G. Formation of ordered microporous films with water as templates from poly(D,L-lactic-co-glycolic acid) solution. J. Appl. Polym. Sci. 2003, 90, 1846–1850. [Google Scholar] [CrossRef]
- Tian, Y.; Ding, H.; Shi, Y.; Jiao, Q.; Wang, X. Water-assisted formation of honeycomb films of poly(L-lactic-co-glycolic acid). J. Appl. Polym. Sci. 2006, 100, 1013–1018. [Google Scholar] [CrossRef]
- Jana, A.; Das, M.; Balla, V.K. In vitro and in vivo degradation assessment and preventive measures of biodegradable Mg alloys for biomedical applications. J. Biomed. Mater. Res. Part A 2021, 110, 462–487. [Google Scholar] [CrossRef]
- Yabu, H. Fabrication of honeycomb films by the breath figure technique and their applications. Sci. Technol. Adv. Mater. 2018, 19, 802–822. [Google Scholar] [CrossRef]
- Shebi, A.; Lisa, S. Evaluation of biocompatibility and bactericidal activity of hierarchically porous PLA-TiO2 nanocomposite films fabricated by breath-figure method. Mater. Chem. Phys. 2019, 230, 308–318. [Google Scholar] [CrossRef]
- Kalva, S.N.; Ali, F.; Velasquez, C.A.; Koç, M. 3D-Printable PLA/Mg Composite Filaments for Potential Bone Tissue Engineering Applications. Polymers 2023, 15, 2572. [Google Scholar] [CrossRef]
- Ali, F.; Kalva, S.N.; Mroue, K.H.; Keyan, K.S.; Tong, Y.; Khan, O.M.; Koç, M. Degradation assessment of Mg-Incorporated 3D printed PLA scaffolds for biomedical applications. Bioprinting 2023, 35, e00302. [Google Scholar] [CrossRef]
- Asadollahi, M.; Gerashi, E.; Zohrevand, M.; Zarei, M.; Sayedain, S.S.; Alizadeh, R.; Labbaf, S.; Atari, M. Improving mechanical properties and biocompatibility of 3D printed PLA by the addition of PEG and titanium particles, using a novel incorporation method. Bioprinting 2022, 27, e00228. [Google Scholar] [CrossRef]
- Shuai, C.; Li, Y.; Feng, P.; Guo, W.; Yang, W.; Peng, S. Positive feedback effects of Mg on the hydrolysis of poly-l-lactic acid (PLLA): Promoted degradation of PLLA scaffolds. Polym. Test. 2018, 68, 27–33. [Google Scholar] [CrossRef]



| Material | Polymer Matrix (w%) | |
|---|---|---|
| PLA | Mg | |
| PLA | 100 | 0 |
| PLA/5Mg | 95 | 5 |
| PLA/10Mg | 90 | 10 |
| PLA/15Mg | 85 | 15 |
| PLA/20Mg | 80 | 20 |
| Mesh Design Specifications | |
|---|---|
| Overall Dimensions | 12.4 mm × 12.4 mm × 0.4 mm |
| Strut Thickness | 0.4 mm |
| Strut Spacing | 3 mm |
| 3DP Process Parameters | |
| Printing Speed | 2 mm/s |
| Layer Height | 0.1 mm |
| Infill Density | 100% |
| Materials | Tg | Tm | Td |
|---|---|---|---|
| PLA | 41 | 167 | 340 |
| PLA/5Mg | 48 | 172 | 295 |
| PLA/10Mg | 50 | 173 | 295 |
| PLA/15Mg | 47 | 170 | 290 |
| PLA/20Mg | 49 | 172 | 282 |
| Materials | pH Values | Weight Gain/Loss | |
|---|---|---|---|
| Weight Gain (%) | Weight Loss (%) | ||
| PLA | 7.9 | 0.9 | 0.1 |
| 5%Mg | 8.6 | 1.0 | 1.5 |
| 10%Mg | 8.8 | 3.0 | 5.0 |
| 15%Mg | 8.85 | 5.0 | 7.0 |
| 20%Mg | 8.89 | 5.2 | 7.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, F.; Al Rashid, A.; Kalva, S.N.; Koç, M. Mg-Doped PLA Composite as a Potential Material for Tissue Engineering—Synthesis, Characterization, and Additive Manufacturing. Materials 2023, 16, 6506. https://doi.org/10.3390/ma16196506
Ali F, Al Rashid A, Kalva SN, Koç M. Mg-Doped PLA Composite as a Potential Material for Tissue Engineering—Synthesis, Characterization, and Additive Manufacturing. Materials. 2023; 16(19):6506. https://doi.org/10.3390/ma16196506
Chicago/Turabian StyleAli, Fawad, Ans Al Rashid, Sumama Nuthana Kalva, and Muammer Koç. 2023. "Mg-Doped PLA Composite as a Potential Material for Tissue Engineering—Synthesis, Characterization, and Additive Manufacturing" Materials 16, no. 19: 6506. https://doi.org/10.3390/ma16196506
APA StyleAli, F., Al Rashid, A., Kalva, S. N., & Koç, M. (2023). Mg-Doped PLA Composite as a Potential Material for Tissue Engineering—Synthesis, Characterization, and Additive Manufacturing. Materials, 16(19), 6506. https://doi.org/10.3390/ma16196506








