FeIII Chelated with Humic Acid with Easy Synthesis Conditions and Good Performance as Anode Materials for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
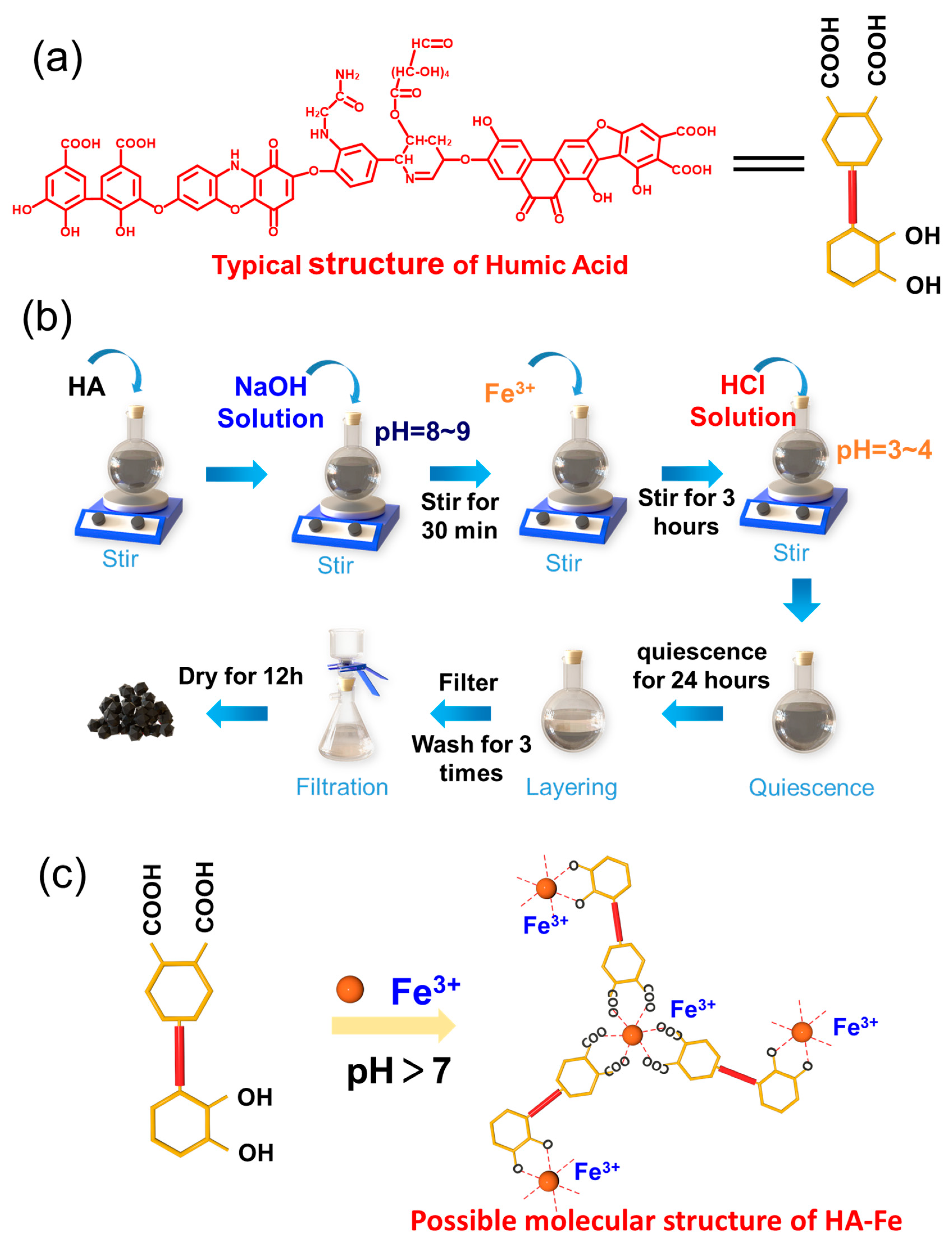
2.2. Preparation Procedure of HA-Fe Materials
2.3. Characterization and Electrochemical Properties of Materials
3. Results and Discussion
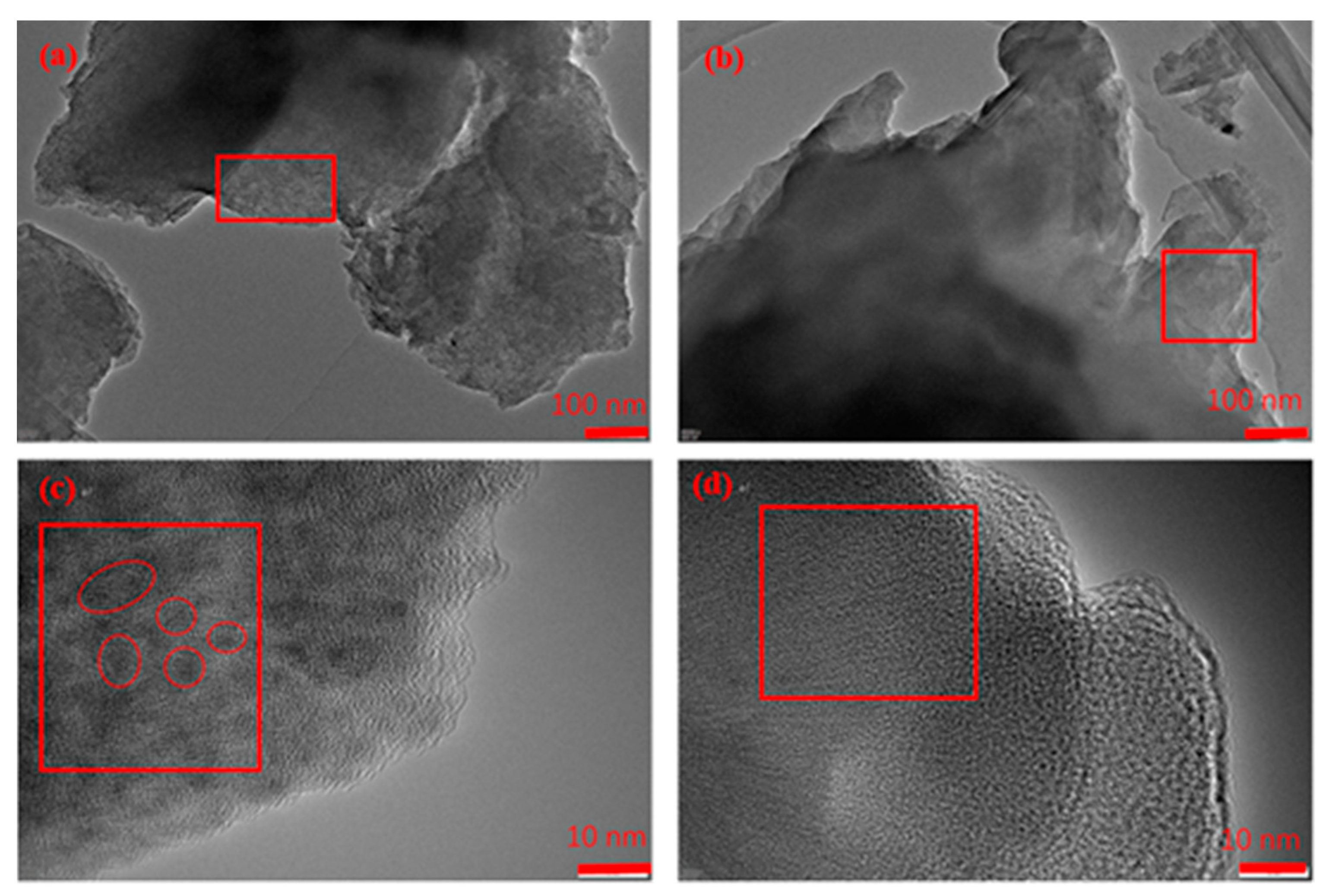
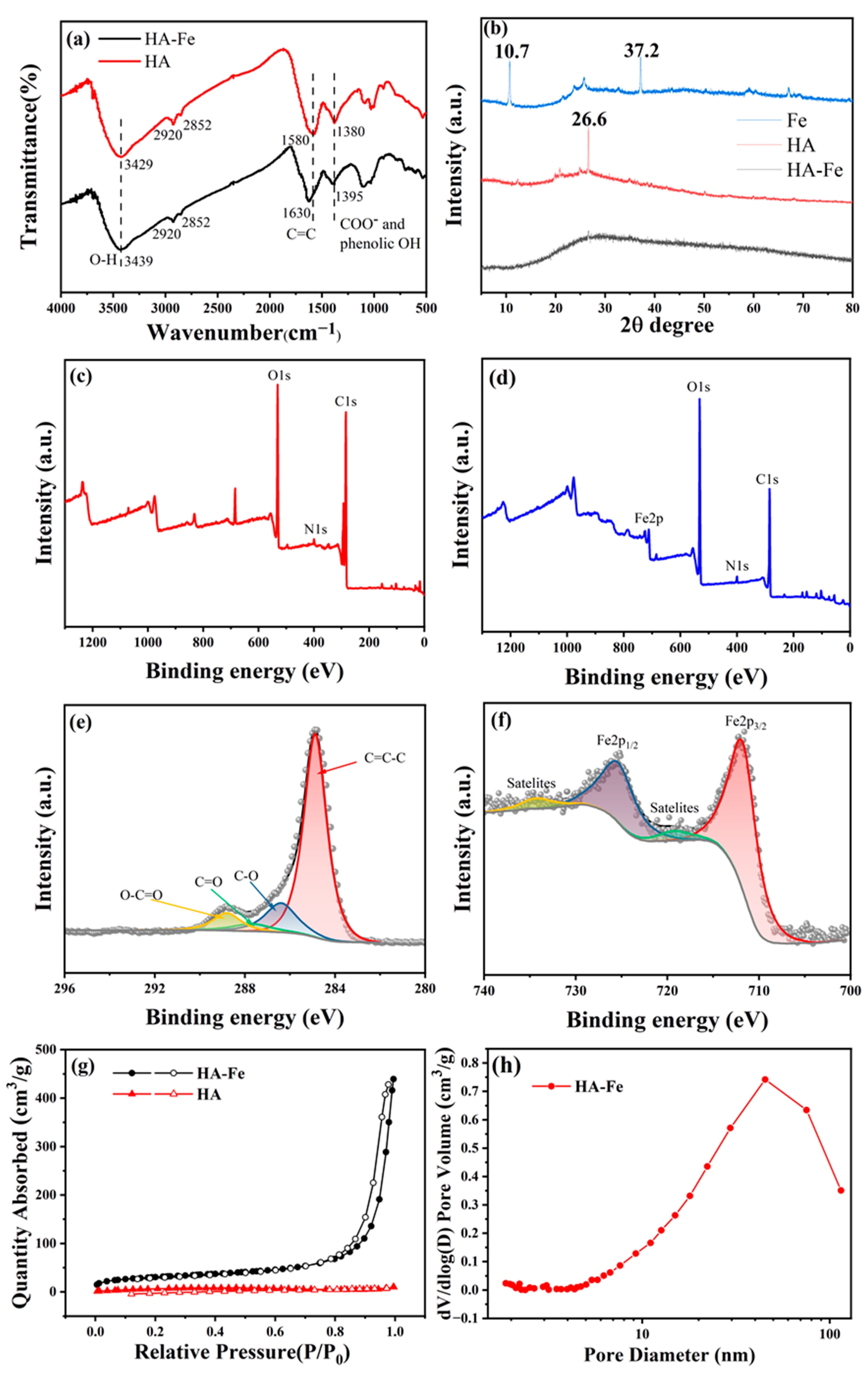
3.1. Characterization Results of HA and HA-Fe Materials
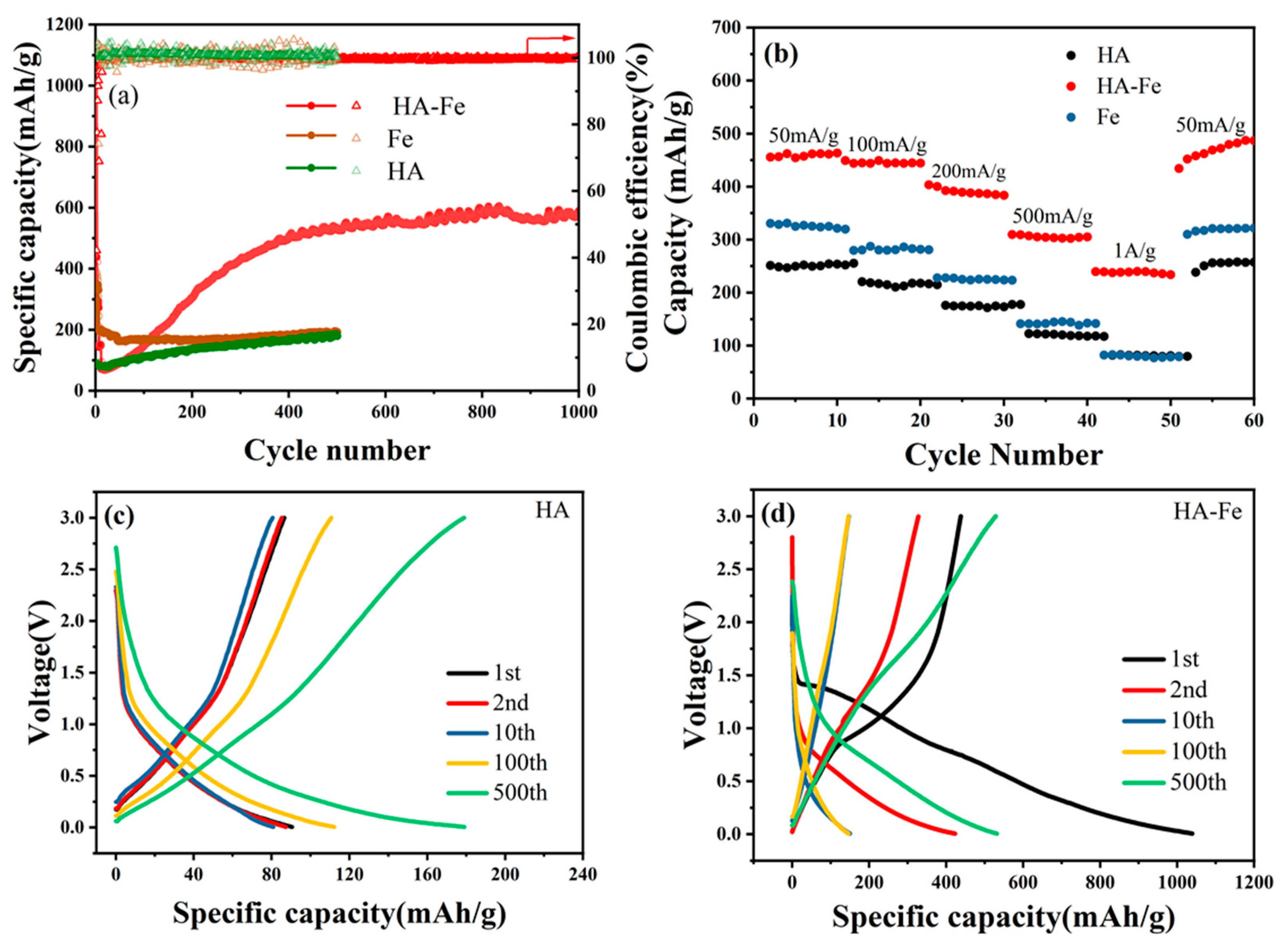
3.2. Electrochemical Properties Investigation of HA-Fe Material
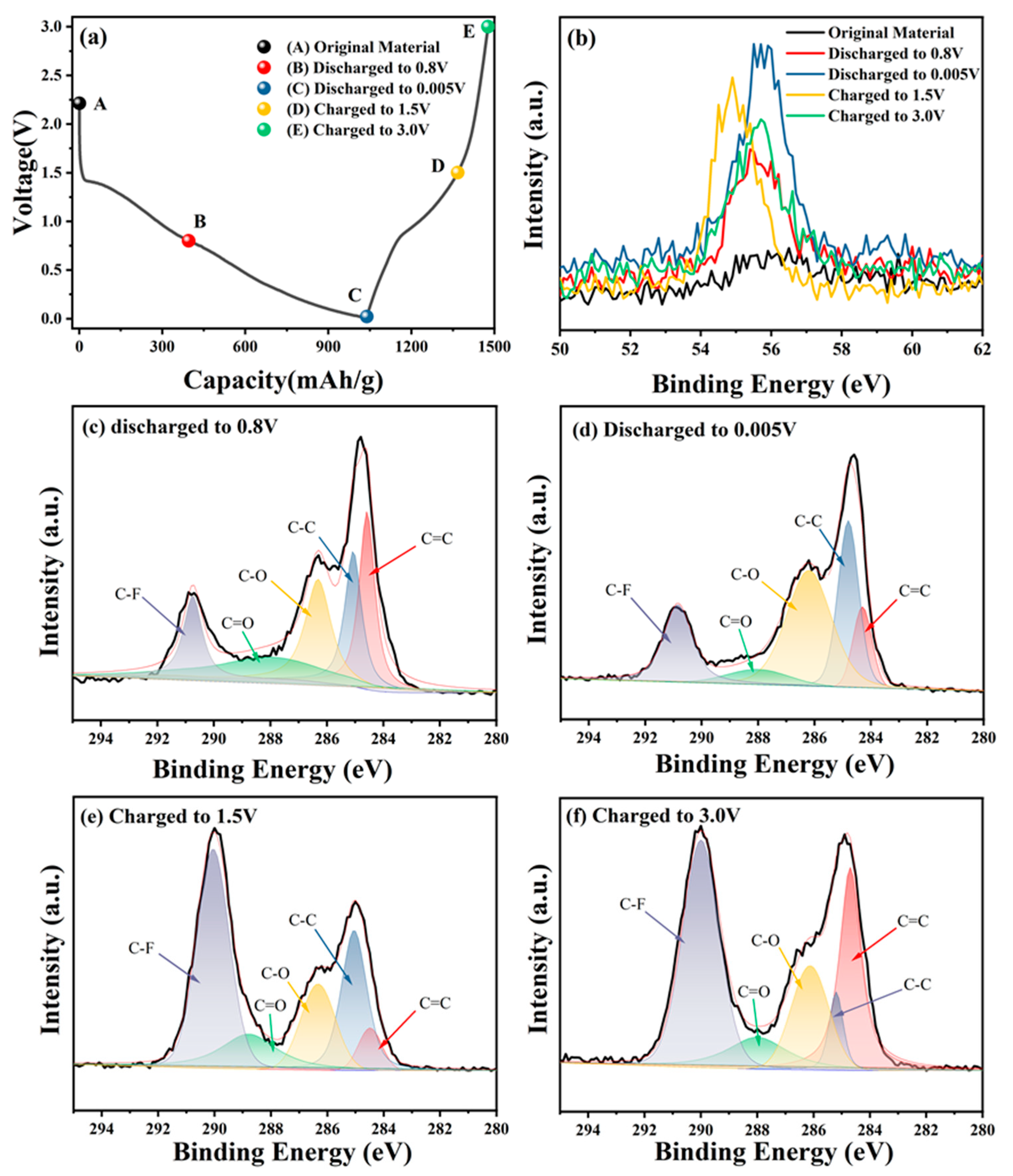
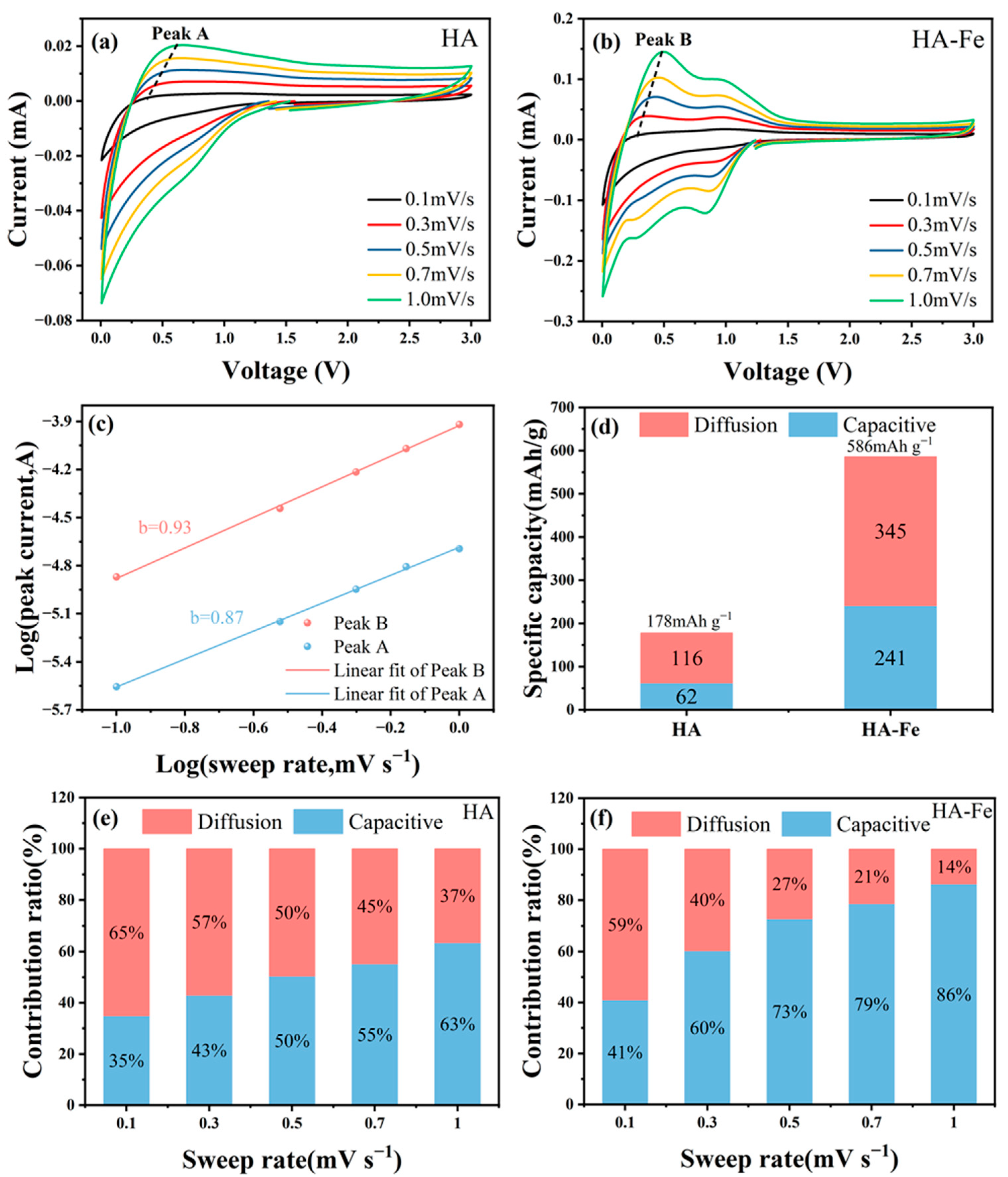
3.3. Lithium Storage Mechanism Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, L.; Fang, Z.; Zhu, Y.; Yan, C.; Yu, G. Holey 2D nanomaterials for electrochemical energy storage. Adv. Energy Mater. 2018, 8, 1702179. [Google Scholar] [CrossRef]
- Abdah, M.A.A.M.; Mokhtar, M.; Khoon, L.T.; Sopian, K.; Dzulkurnain, N.A.; Ahmad, A.; Sulaiman, Y.; Bella, F.; Su’ait, M.S. Synthesis and electrochemical characterizations of poly (3,4-ethylenedioxythiophene/manganese oxide coated on porous carbon nanofibers as a potential anode for lithium-ion batteries. Energy Rep. 2021, 7, 8677–8687. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Liang, F.; Wu, D.; Jiang, L.; Zhang, Z.; Zhang, W.; Rui, Y.; Tang, B.; Liu, F. Layered niobium oxide hydrate anode with excellent performance for lithium-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 51057–51065. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Liu, X.; Li, Z.; Feng, W. Synthesis of manganese coordination polymer microspheres for lithium-ion batteries with good cycling performance. Electrochim. Acta 2015, 174, 1088–1095. [Google Scholar] [CrossRef]
- Dai, Y.; Mo, D.-C.; Qu, Z.-T.; Wang, W.-K.; Lyu, S.-S. Organic—Inorganic Hybrid Interfaces Enable the Preparation of Nitrogen-Doped Hollow Carbon Nanospheres as High-Performance Anodes for Lithium and Potassium-Ion Batteries. Materials 2023, 16, 4936. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Lin, F.; Markus, I.M.; Nordlund, D.; Weng, T.-C.; Asta, M.D.; Xin, H.L.; Doeff, M.M. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 2014, 5, 3529. [Google Scholar] [CrossRef]
- Gauthier, M.; Carney, T.J.; Grimaud, A.; Giordano, L.; Pour, N.; Chang, H.-H.; Fenning, D.P.; Lux, S.F.; Paschos, O.; Bauer, C. Electrode-electrolyte interface in Li-ion batteries: Current understanding and new insights. J. Phys. Chem. Lett. 2015, 6, 4653–4672. [Google Scholar] [CrossRef]
- Liu, W.; Zong, K.; Li, Y.; Deng, Y.; Hussain, A.; Cai, X. Nano-Graphite Prepared by Rapid Pulverization as Anode for Lithium-Ion Batteries. Materials 2022, 15, 5148. [Google Scholar] [CrossRef]
- Lee, S.W.; Gallant, B.M.; Byon, H.R.; Hammond, P.T.; Shao-Horn, Y. Nanostructured carbon-based electrodes: Bridging the gap between thin-film lithium-ion batteries and electrochemical capacitors. Energy Environ. Sci. 2011, 4, 1972–1985. [Google Scholar] [CrossRef]
- Wang, D.; Yu, Y.; He, H.; Wang, J.; Zhou, W.; Abruna, H.D. Template-free synthesis of hollow-structured Co3O4 nanoparticles as high-performance anodes for lithium-ion batteries. ACS Nano 2015, 9, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, M.E.; Chae, S.; Kim, J.M.; Choi, J.-Y. Organic small molecules and polymers as an electrode material for rechargeable lithium ion batteries. J. Mater. Chem. A 2018, 6, 19885–19911. [Google Scholar] [CrossRef]
- Zhuang, J.; Xu, X.; Peleckis, G.; Hao, W.; Dou, S.X.; Du, Y. Silicene: A promising anode for lithium-ion batteries. Adv. Mater. 2017, 29, 1606716. [Google Scholar] [CrossRef]
- Chae, S.; Kim, N.; Ma, J.; Cho, J.; Ko, M. One-to-one comparison of graphite-blended negative electrodes using silicon nanolayer-embedded graphite versus commercial benchmarking materials for high-energy lithium-ion batteries. Adv. Energy Mater. 2017, 7, 1700071. [Google Scholar] [CrossRef]
- Min, X.; Xu, G.; Xie, B.; Guan, P.; Sun, M.; Cui, G. Challenges of prelithiation strategies for next generation high energy lithium-ion batteries. Energy Storage Mater. 2022, 47, 297–318. [Google Scholar] [CrossRef]
- Nzabahimana, J.; Chang, P.; Hu, X. Porous carbon-coated ball-milled silicon as high-performance anodes for lithium-ion batteries. J. Mater. Sci. 2019, 54, 4798–4810. [Google Scholar] [CrossRef]
- Croguennec, L.; Palacin, M.R. Recent achievements on inorganic electrode materials for lithium-ion batteries. J. Am. Chem. Soc. 2015, 137, 3140–3156. [Google Scholar] [CrossRef]
- Li, W.; Song, B.; Manthiram, A. High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 2017, 46, 3006–3059. [Google Scholar] [CrossRef]
- Lupi, C.; Pasquali, M.; Dell’Era, A. Nickel and cobalt recycling from lithium-ion batteries by electrochemical processes. Waste Manag. 2005, 25, 215–220. [Google Scholar] [CrossRef]
- Yu, Q.; Tang, W.; Hu, Y.; Gao, J.; Wang, M.; Liu, S.; Lai, H.; Xu, L.; Fan, C. Novel low-cost, high-energy-density (>700 Wh kg−1) Li-rich organic cathodes for Li-ion batteries. Chem. Eng. J. 2021, 415, 128509. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, J.; Huang, S.; Chen, Z.; Lu, C.; Yang, C.; Zhai, G.; Zhu, J.; Zhuang, X. Porphyrinic conjugated microporous polymer anode for Li-ion batteries. J. Power Sources 2022, 531, 231340. [Google Scholar] [CrossRef]
- Yu, J.; Li, N.; Wang, H.-G.; Gao, B.; Wang, B.; Li, Z. Unraveling the superior anodic lithium storage behavior in the redox-active porphyrinic triazine frameworks. Chem. Eng. J. 2023, 463, 142434. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, J.; Hou, S.; Lin, H.; Chen, S.; Wang, Q.; Wei, H.; Zhou, J.; Zhuo, S. Thiophene-diketopyrrolopyrrole-based polymer derivatives/reduced graphene oxide composite materials as organic anode materials for lithium-ion batteries. Chem. Eng. J. 2022, 438, 135540. [Google Scholar] [CrossRef]
- Araujo, R.B.; Banerjee, A.; Panigrahi, P.; Yang, L.; Strømme, M.; Sjödin, M.; Araujo, C.M.; Ahuja, R. Designing strategies to tune reduction potential of organic molecules for sustainable high capacity battery application. J. Mater. Chem. A 2017, 5, 4430–4454. [Google Scholar] [CrossRef]
- Yu, J.; Chen, X.; Wang, H.-G.; Gao, B.; Han, D.; Si, Z. Conjugated ladder-type polymers with multielectron reactions as high-capacity organic anode materials for lithium-ion batteries. Sci. China Mater. 2022, 65, 2354–2362. [Google Scholar] [CrossRef]
- Sang, P.; Chen, Q.; Wang, D.-Y.; Guo, W.; Fu, Y. Organosulfur materials for rechargeable batteries: Structure, mechanism, and application. Chem. Rev. 2023, 123, 1262–1326. [Google Scholar] [CrossRef]
- Chen, Q.; Li, L.; Wang, W.; Li, X.; Guo, W.; Fu, Y. Thiuram Monosulfide with Ultrahigh Redox Activity Triggered by Electrochemical Oxidation. J. Am. Chem. Soc. 2022, 144, 18918–18926. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Guo, W.; Fu, Y. Organosulfides: An emerging class of cathode materials for rechargeable lithium batteries. Acc. Chem. Res. 2019, 52, 2290–2300. [Google Scholar] [CrossRef]
- Wu, M.; Cui, Y.; Bhargav, A.; Losovyj, Y.; Siegel, A.; Agarwal, M.; Ma, Y.; Fu, Y. Organotrisulfide: A high capacity cathode material for rechargeable lithium batteries. Angew. Chem. 2016, 128, 10181–10185. [Google Scholar] [CrossRef]
- Du, W.; Du, X.; Ma, M.; Huang, S.; Sun, X.; Xiong, L. Polymer electrode materials for lithium-ion batteries. Adv. Funct. Mater. 2022, 32, 2110871. [Google Scholar] [CrossRef]
- Choi, W.; Ohtani, S.; Oyaizu, K.; Nishide, H.; Geckeler, K.E. Radical polymer-wrapped SWNTs at a molecular level: High-rate redox mediation through a percolation network for a transparent charge-storage material. Adv. Mater. 2011, 23, 4440–4443. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Yin, Y.-X.; Xin, S.; Guo, Y.-G.; Wan, L.-J. Superior radical polymer cathode material with a two-electron process redox reaction promoted by graphene. Energy Environ. Sci. 2012, 5, 5221–5225. [Google Scholar] [CrossRef]
- Ou, Y.; Zhang, Y.; Xiong, Y.; Hu, Z.; Dong, L. Three-dimensional porous radical polymer/reduced graphene oxide composite with two-electron redox reactions as high-performance cathode for lithium-ion batteries. Eur. Polym. J. 2021, 143, 110191. [Google Scholar] [CrossRef]
- Mao, P.; Fan, H.; Zhou, G.; Arandiyan, H.; Liu, C.; Lan, G.; Wang, Y.; Zheng, R.; Wang, Z.; Bhargava, S.K. Graphite-like structured conductive polymer anodes for high-capacity lithium storage with optimized voltage platform. J. Colloid Interface Sci. 2023, 634, 63–73. [Google Scholar] [CrossRef]
- Zhang, B.; Dong, Y.; Han, J.; Zhen, Y.; Hu, C.; Liu, D. Physicochemical Dual Crosslinking Conductive Polymeric Networks Combining High Strength and High Toughness Enable Stable Operation of Silicon Microparticles Anodes. Adv. Mater. 2023, 35, 2301320. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Dong, H.; Jiang, L.; Hu, W. Organic semiconductor crystals. Chem. Soc. Rev. 2018, 47, 422–500. [Google Scholar] [CrossRef]
- Acker, P.; Rzesny, L.; Marchiori, C.F.; Araujo, C.M.; Esser, B. π-conjugation enables ultra-high rate capabilities and cycling stabilities in phenothiazine copolymers as cathode-active battery materials. Adv. Funct. Mater. 2019, 29, 1906436. [Google Scholar] [CrossRef]
- Li, S.; Lin, J.; Zhang, Y.; Zhang, S.; Jiang, T.; Hu, Z.; Liu, J.; Wu, D.Y.; Zhang, L.; Tian, Z. Eight-electron redox cyclohexanehexone anode for high-rate high-capacity lithium storage. Adv. Energy Mater. 2022, 12, 2201347. [Google Scholar] [CrossRef]
- Ba, Z.; Wang, Z.; Zhou, Y.; Li, H.; Dong, J.; Zhang, Q.; Zhao, X. Electrochemical Properties of a Multicarbonyl Polyimide Superstructure as a Hierarchically Porous Organic Anode for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 13161–13171. [Google Scholar] [CrossRef]
- Wang, J.; Shen, Z.; Yi, M.; Zhang, X. Muconic acid as high-performance organic anode for lithium ion batteries. J. Alloys Compd. 2021, 865, 158573. [Google Scholar] [CrossRef]
- Li, K.; Yu, J.; Si, Z.; Gao, B.; Wang, H.-G.; Wang, Y. One-dimensional π-d conjugated coordination polymer with double redox-active centers for all-organic symmetric lithium-ion batteries. Chem. Eng. J. 2022, 450, 138052. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Chen, Y.; Tang, H.; Tang, M.; Xu, S.; Fan, K.; Zhang, C.; Ma, J.; Wang, C. Heterochelation boosts sodium storage in π-d conjugated coordination polymers. Energy Environ. Sci. 2021, 14, 6514–6525. [Google Scholar] [CrossRef]
- Yang, H.; Cui, W.; Han, Y.; Wang, B. Porous nanocomposite derived from Zn, Ni-bimetallic metal-organic framework as an anode material for lithium-ion batteries. Chin. Chem. Lett. 2018, 29, 842–844. [Google Scholar] [CrossRef]
- Zhu, H.; Yin, J.; Zhao, X.; Wang, C.; Yang, X. Humic acid as promising organic anodes for lithium/sodium ion batteries. Chem. Commun. 2015, 51, 14708–14711. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luo, M.; Ba, Z.; Wang, Z.; Chen, L.; Li, Y.; Li, M.; Li, H.-B.; Dong, J.; Zhao, X. Hierarchical multicarbonyl polyimide architectures as promising anode active materials for high-performance lithium/sodium ion batteries. J. Mater. Chem. A 2019, 7, 19112–19119. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Liu, H.; Liu, H.; Li, B.; Guan, S. A Novel High-Capacity Anode Material Derived from Aromatic Imides for Lithium-Ion Batteries. Small 2018, 14, 1704094. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Q.; Li, L.; Niu, Z.; Chen, J. Design strategies toward enhancing the performance of organic electrode materials in metal-ion batteries. Chem 2018, 4, 2786–2813. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Z.; Zhao, Y.; Li, Y.; Shi, J. A novel raspberry-like yolk-shell structured Si/C micro/nano-spheres as high-performance anode materials for lithium-ion batteries. J. Alloys Compd. 2020, 844, 156201. [Google Scholar] [CrossRef]
- Ravikumar, M.M.; Shetty, V.R.; Suresh, G.S. Synthesis and Applications of Aurin Tricarboxylic Acid-Copper Metal Organic Framework for Rechargeable Lithium-Ion Batteries. J. Electrochem. Soc. 2020, 167, 100533. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, H.; Deng, X.; Yang, Y.; Zhang, T.; Wang, J.; Zeng, H.; Wang, C.; Deng, Y. Metal chelation based supramolecular self-assembly enables a high-performance organic anode for lithium ion batteries. Chem. Eng. J. 2021, 413, 127525. [Google Scholar] [CrossRef]
- Wang, J.; Yao, H.; Du, C.; Guan, S. Polyimide schiff base as a high-performance anode material for lithium-ion batteries. J. Power Sources 2021, 482, 228931. [Google Scholar] [CrossRef]
- Xie, L.; Shang, C. Role of humic acid and quinone model compounds in bromate reduction by zerovalent iron. Environ. Sci. Technol. 2005, 39, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yang, Y.; Zhang, T.; Xu, D.; Lei, Z.; Wang, C.; Liu, G.; Deng, Y. FeIII chelated organic anode with ultrahigh rate performance and ultra-long cycling stability for lithium-ion batteries. Energy Storage Mater. 2020, 24, 432–438. [Google Scholar] [CrossRef]
- Wang, X.; Lv, L.; Cheng, Z.; Gao, J.; Dong, L.; Hu, C.; Qu, L. High-Density Monolith of N-Doped Holey Graphene for Ultrahigh Volumetric Capacity of Li-Ion Batteries. Adv. Energy Mater. 2016, 6, 1502100. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Z.; Wang, H.; Nie, Y.; Yan, J. Porous Fe2O3 nanoparticles as lithium-ion battery anode materials. ACS Appl. Nano Mater. 2021, 4, 8744–8752. [Google Scholar] [CrossRef]
- Yao, J.; Jin, T.; Li, Y.; Xiao, S.; Huang, B.; Jiang, J. Electrochemical performance of Fe2(SO4)3 as a novel anode material for lithium-ion batteries. J. Alloys Compd. 2021, 886, 161238. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic-inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Wu, S.; Lu, M.; Tian, X.; Jiang, C. A facile route to graphene-covered and carbon-encapsulated CoSO4 nanoparticles as anode materials for lithium-ion batteries. Chem. Eng. J. 2017, 313, 610–618. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, C.; Guo, X.; Zhao, J.; Zhang, Y.; Wang, B.; Dong, Y.; Liu, L. Electrochemical performance and storage mechanism study of conjugate donor-acceptor organic polymers as anode materials of lithium-ion battery. Energy Rep. 2020, 6, 2094–2105. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, W.; Yuan, Q.; Zhao, J.; Li, Y.; Xie, Y. The fabrication of hierarchical porous nano-SnO2@carbon@humic acid ternary composite for enhanced capacity and stability as anode material for lithium ion battery. Colloids Surf. A 2022, 650, 129560. [Google Scholar] [CrossRef]
- Zhao, P.-Y.; Yu, B.-J.; Sun, S.; Guo, Y.; Chang, Z.-Z.; Li, Q.; Wang, C.-Y. High-performance anode of sodium ion battery from polyacrylonitrile/humic acid composite electrospun carbon fibers. Electrochim. Acta 2017, 232, 348–356. [Google Scholar] [CrossRef]
- Huang, G.; Kong, Q.; Yao, W.; Wang, Q. Poly tannic acid carbon rods as anode materials for high performance lithium and sodium ion batteries. J. Colloid Interface Sci. 2023, 629, 832–845. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, Z.; Chou, S.; Liang, X. Novel Biomass-derived Hollow Carbons as Anode Materials for Lithium-ion Batteries. Chem. Res. Chin. Univ. 2023, 39, 283–289. [Google Scholar] [CrossRef]
- Yu, K.; Wang, J.; Wang, X.; Liang, J.; Liang, C. Sustainable application of biomass by-products: Corn straw-derived porous carbon nanospheres using as anode materials for lithium ion batteries. Mater. Chem. Phys. 2020, 243, 122644. [Google Scholar] [CrossRef]
- Zhang, C.; He, Y.; Mu, P.; Wang, X.; He, Q.; Chen, Y.; Zeng, J.; Wang, F.; Xu, Y.; Jiang, J.X. Toward high performance thiophene-containing conjugated microporous polymer anodes for lithium-ion batteries through structure design. Adv. Funct. Mater. 2018, 28, 1705432. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Sathiya, M.; Prakash, A.; Ramesha, K.; Tarascon, J.M.; Shukla, A.K. V2O5-anchored carbon nanotubes for enhanced electrochemical energy storage. J. Am. Chem. Soc. 2011, 133, 16291–16299. [Google Scholar] [CrossRef]
- Brezesinski, T.; Wang, J.; Tolbert, S.H.; Dunn, B. Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 2010, 9, 146–151. [Google Scholar] [CrossRef]
- Guo, X.; Yuan, Q.; Li, C.; Du, H.; Zhao, J.; Liu, L.; Li, Y.; Xie, Y.; Vaidya, V. The synthesis of alternating donor–acceptor polymers based on pyrene-4, 5, 9, 10-tetraone and thiophene derivatives, their composites with carbon, and their lithium storage performances as anode materials. RSC Adv. 2021, 11, 15044–15053. [Google Scholar] [CrossRef]
- Lian, L.; Li, K.; Ren, L.; Han, D.; Lv, X.; Wang, H.-G. Imine-linked triazine-based conjugated microporous polymers/carbon nanotube composites as organic anode materials for lithium-ion batteries. Colloids Surf. A 2023, 657, 130496. [Google Scholar] [CrossRef]
- Li, C.; Kong, L.; Zhao, J.; Liang, B. Preparation of DAD conjugated polymers based on [1,2,5] thiadiazolo[3,4-c]pyridine and thiophene derivatives and their electrochemical properties as anode materials for lithium-ion batteries. Colloids Surf. A 2022, 651, 129707. [Google Scholar] [CrossRef]
| Mass Fractions of Different Element on Surface Spectrum (wt%) | C | O | Fe |
|---|---|---|---|
| HA | 72.3 | 27.7 | <0.1 |
| HA-Fe | 44.0 | 26.8 | 29.2 |
| Sample | Rs (Ω) | Rct (Ω) | ||||
|---|---|---|---|---|---|---|
| Before Cycling | After 100 Cycles | After 400 Cycles | Before Cycling | After 100 Cycles | After 400 Cycles | |
| HA | 9.39 | 2.08 | 2.55 | 1403.6 | 240.7 | 162.0 |
| HA-Fe | 4.96 | 9.06 | 1.39 | 389.6 | 21.3 | 13.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Wang, Y.; Zhao, R.; Kou, M.; Guo, M.; Xu, K.; Tian, G.; Wei, X.; Jiang, S.; Yuan, Q.; et al. FeIII Chelated with Humic Acid with Easy Synthesis Conditions and Good Performance as Anode Materials for Lithium-Ion Batteries. Materials 2023, 16, 6477. https://doi.org/10.3390/ma16196477
Zhang H, Wang Y, Zhao R, Kou M, Guo M, Xu K, Tian G, Wei X, Jiang S, Yuan Q, et al. FeIII Chelated with Humic Acid with Easy Synthesis Conditions and Good Performance as Anode Materials for Lithium-Ion Batteries. Materials. 2023; 16(19):6477. https://doi.org/10.3390/ma16196477
Chicago/Turabian StyleZhang, Hao, Youkui Wang, Ruili Zhao, Meimei Kou, Mengyao Guo, Ke Xu, Gang Tian, Xinting Wei, Song Jiang, Qing Yuan, and et al. 2023. "FeIII Chelated with Humic Acid with Easy Synthesis Conditions and Good Performance as Anode Materials for Lithium-Ion Batteries" Materials 16, no. 19: 6477. https://doi.org/10.3390/ma16196477
APA StyleZhang, H., Wang, Y., Zhao, R., Kou, M., Guo, M., Xu, K., Tian, G., Wei, X., Jiang, S., Yuan, Q., & Zhao, J. (2023). FeIII Chelated with Humic Acid with Easy Synthesis Conditions and Good Performance as Anode Materials for Lithium-Ion Batteries. Materials, 16(19), 6477. https://doi.org/10.3390/ma16196477






