Metal Chalcogenide–Hydroxide Hybrids as an Emerging Family of Two-Dimensional Heterolayered Materials: An Early Review
Abstract
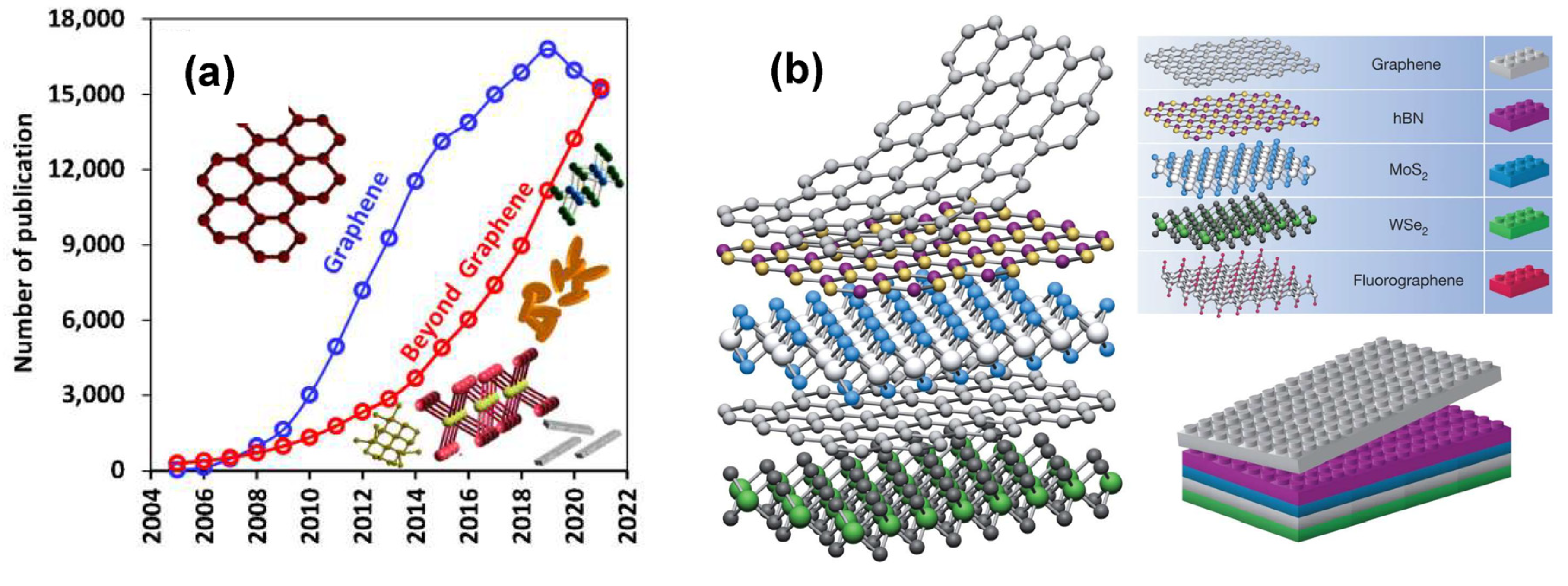
:1. Introduction
2. Natural Two-Dimensional Layered Minerals
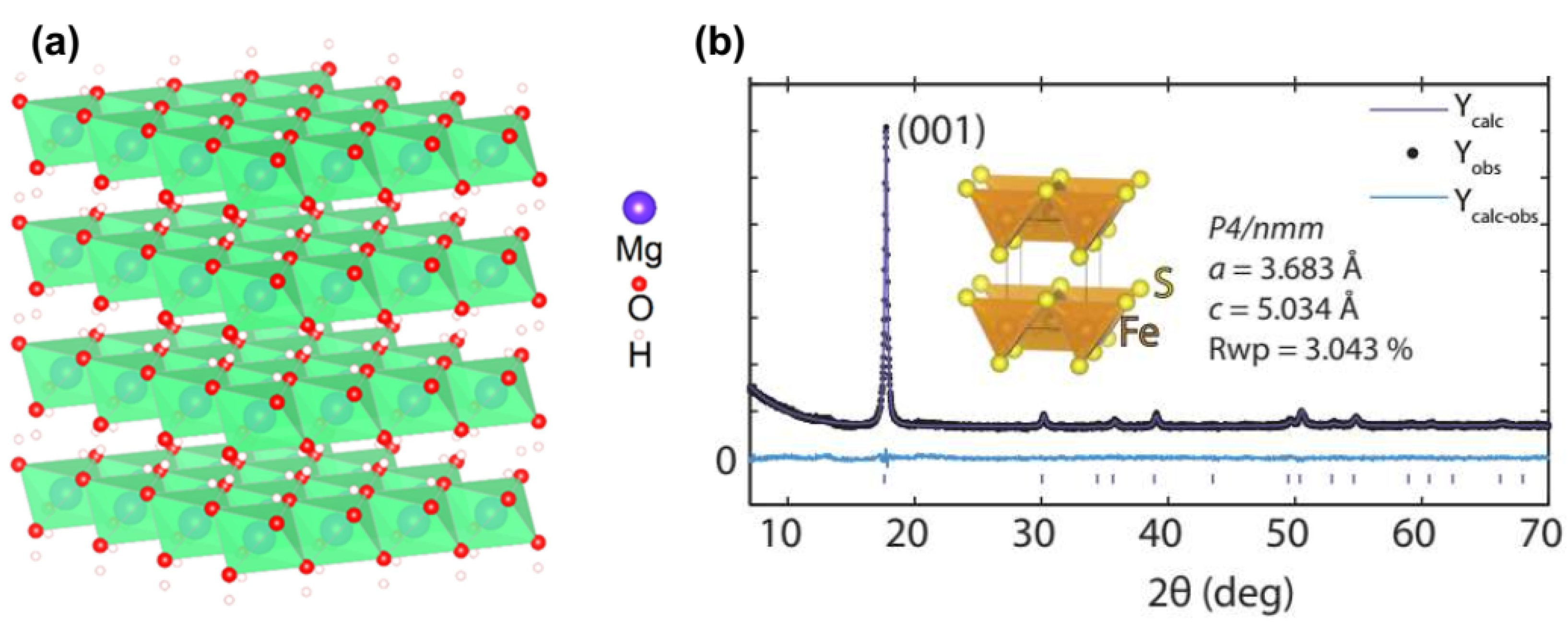
2.1. Brucite and Mackinawite
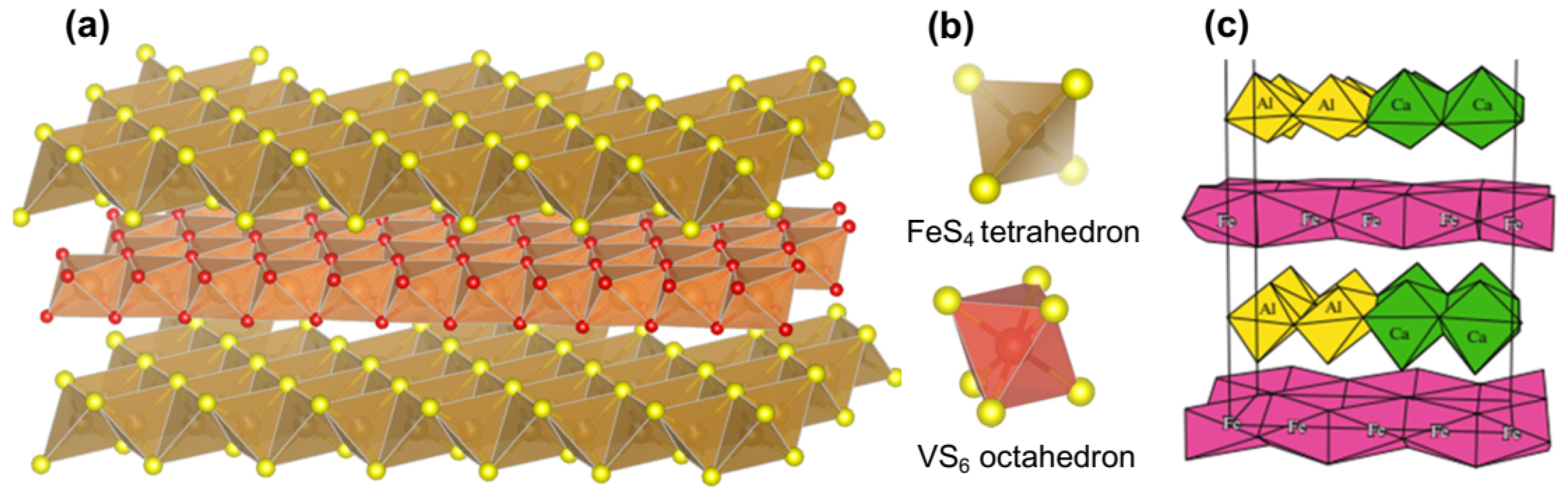
2.2. Valleriites
2.3. Tochilinite
2.4. Other Minerals of Valleriite Group
3. Synthesis of Heterolayered Materials
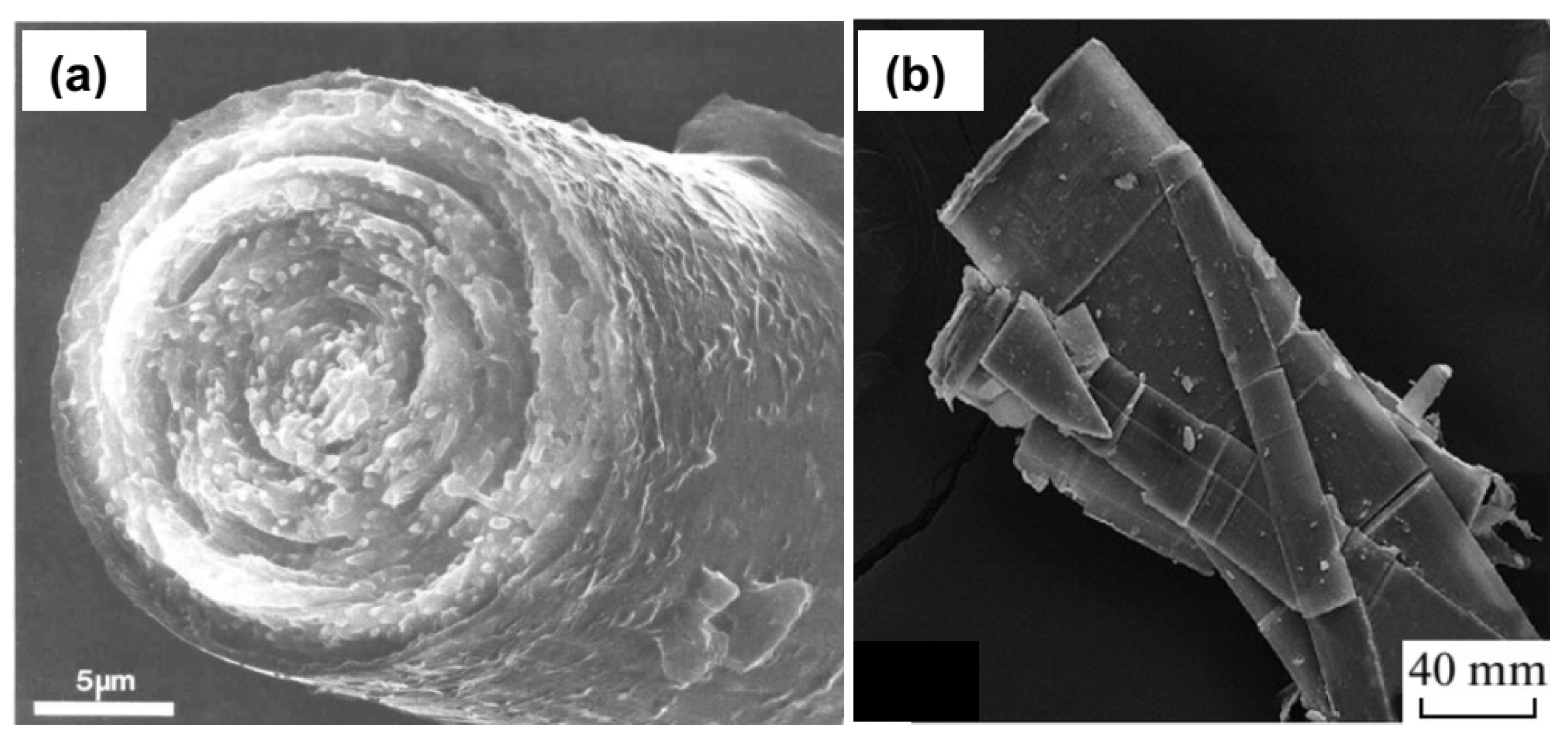
3.1. Syntheses of Valleriites
3.2. Tochilinite-Type Materials
3.3. Layered Superconductors of FeSe·(Li,Fe)(OH) Group
3.4. Summary on the Synthesis
4. Spectroscopic Characterization and Properties
4.1. Mössbauer, XPS, X-ray Absorption Spectra of Valleriite
4.2. Mössbauer and XPS Investigation of Tochilinite
4.3. Raman, IR Spectroscopy, UV-Vis-NIR Spectroscopy
4.4. Physical and Chemical Properties
5. New Characteristics and Potential Applications of Chalcogenide–Hydroxide 2D Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 67–69. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Urade, A.R.; Lahiri, I.; Suresh, K.S. Graphene Properties, Synthesis and Applications: A Review. JOM 2023, 75, 614–630. [Google Scholar] [CrossRef] [PubMed]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef]
- Xia, Y.; Gao, W.; Gao, C. A Review on Graphene-Based Electromagnetic Functional Materials: Electromagnetic Wave Shielding and Absorption. Adv. Funct. Mater. 2022, 32, 2204591. [Google Scholar] [CrossRef]
- Saeed, M.; Palacios, P.; Wei, M.D.; Baskent, E.; Fan, C.Y.; Uzlu, B.; Wang, K.T.; Hemmetter, A.; Wang, Z.; Neumaier, D.; et al. Graphene-Based Microwave Circuits: A Review. Adv. Mater. 2022, 34, 2108473. [Google Scholar] [CrossRef]
- Derakhshi, M.; Daemi, S.; Shahini, P.; Habibzadeh, A.; Mostafavi, E.; Ashkarran, A.A. Two-Dimensional Nanomaterials beyond Graphene for Biomedical Applications. J. Funct. Biomater. 2022, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Zhang, X.; Puthirath, A.B.; Meiyazhagan, A.; Bhattacharyya, S.; Rahman, M.M.; Babu, G.; Susarla, S.; Saju, S.K.; Tran, M.K.; et al. Structure, Properties and Applications of Two-Dimensional Hexagonal Boron Nitride. Adv. Mater. 2021, 33, 2101589. [Google Scholar] [CrossRef]
- Moon, S.; Kim, J.; Park, J.; Im, S.; Kim, J.; Hwang, I.; Kim, J.K. Hexagonal Boron Nitride for Next-Generation Photonics and Electronics. Adv. Mater. 2023, 35, 2204161. [Google Scholar] [CrossRef]
- Tedstone, A.A.; Lewis, D.J.; O’Brien, P. Synthesis, properties, and applications of transition metal-doped layered transition metal dichalcogenides. Chem. Mater. 2016, 28, 1965–1974. [Google Scholar] [CrossRef]
- He, Z.; Que, W. Molybdenum disulfide nanomaterials: Structures, properties, synthesis and recent progress on hydrogen evolution reaction. Appl. Mater. Today 2016, 3, 23–56. [Google Scholar] [CrossRef]
- Mak, K.F.; Shan, J. Photonics and optoelectronics of 2D semiconductor transition metal dichalcogenides. Nat. Photonics 2016, 10, 216–226. [Google Scholar] [CrossRef]
- Gao, T.; Zhang, Q.; Li, L.; Zhou, X.; Li, L.; Li, H.; Zhai, T. 2D ternary chalcogenides. Adv. Optical Mater. 2018, 6, 1800058. [Google Scholar] [CrossRef]
- Long, M.; Wang, P.; Fang, H.; Hu, W. Progress, Challenges, and Opportunities for 2D Material Based Photodetectors. Adv. Funct. Mater. 2019, 29, 1803807. [Google Scholar] [CrossRef]
- Verre, R.; Baranov, D.G.; Munkhbat, B.; Cuadra, J.; Käll, M.; Shegai, T. Transition metal dichalcogenide nanodisks as high-index dielectric Mie nanoresonators. Nat. Nanotechnol. 2019, 14, 679–683. [Google Scholar] [CrossRef]
- Monga, D.; Sharma, S.; Shetti, N.P.; Basu, S.; Reddy, K.R.; Aminabhavi, T.M. Advances in transition metal dichalcogenide-based two-dimensional nanomaterials. Mater. Today Chem. 2021, 19, 100399. [Google Scholar] [CrossRef]
- Yang, R.; Fan, Y.; Zhang, Y.; Mei, L.; Zhu, R.; Qin, J.; Hu, J.; Chen, Z.; Ng, Y.H.; Voiry, D.; et al. 2D Transition Metal Dichalcogenides for Photocatalysis. Ang. Chem.Int. Ed. 2023, 62, e202218016. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef]
- Verger, L.; Natu, V.; Carey, M.; Barsoum, M.W. MXenes: An Introduction of Their Synthesis, Select Properties, and Applications. Trends Chem. 2019, 1, 656–669. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Dai, J.H.; Zhang, Y.M.; Song, Y. Two-Dimensional, Ordered, Double Transition Metal Carbides (MXenes): A New Family of Promising Catalysts for the Hydrogen Evolution Reaction. J. Phys. Chem. C 2018, 122, 28113–28122. [Google Scholar] [CrossRef]
- Vahid Mohammadi, A.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, eabf1581. [Google Scholar] [CrossRef]
- Jiang, J.; Bai, S.; Zou, J.; Liu, S.; Hsu, J.P.; Li, N.; Zhu, G.; Zhuang, Z.; Kang, Q.; Zhang, Y. Improving stability of MXenes. Nano Res. 2022, 15, 6551–6567. [Google Scholar] [CrossRef]
- Kim, H.; Alshareef, H.N. MXetronics: MXene-Enabled Electronic and Photonic Devices. ACS Mater. Lett. 2020, 2, 55–70. [Google Scholar] [CrossRef]
- Kamysbayev, V.; Filatov, A.S.; Hu, H.; Rui, X.; Lagunas, F.; Wang, D.; Klie, R.F.; Talapin, D.V. Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes. Science 2020, 369, 979–983. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Hofmeister, W.; Von Platen, H. Crystal Chemistry and Atomic Order in Brucite-related Double-layer Structures. Crystallogr. Rev. 1992, 3, 3–26. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Hartman, H. Clays and the Origin of Life: The Experiments. Life 2022, 12, 259. [Google Scholar] [CrossRef] [PubMed]
- Tronto, J.; Bordonal, A.C.; Naal, Z.; Valim, J.B. Conducting polymers/layered double hydroxides intercalated nanocomposites. In Materials Science—Advanced Topics; Mastai, Y., Ed.; IntechOpen: London, UK, 2013; ISBN 978-953-51-6345-9. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Zhao, Y.; Bai, H.; Wen, T.; Kang, S.; Song, G.; Song, S.; Komarneni, S. Removal of heavy metals and dyes by clay-based adsorbents: From natural clays to 1D and 2D nanocomposites. Chem. Eng. J. 2020, 420, 127574. [Google Scholar] [CrossRef]
- Awad, A.M.; Shaikh, S.M.R.; Jalab, R.; Gulied, M.H.; Nasser, M.S.; Benamor, A.; Adham, S. Adsorption of Organic Pollutants by Natural and Modified Clays: A Comprehensive Review. Sep. Purif. Technol. 2019, 228, 115719. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J. Mater. Chem. A 2016, 4, 10744–10766. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Han, J.; Lu, L.; Qiu, G.; Wang, Z.L.; Sun, C. Fe2+-doped layered double (Ni, Fe) hydroxides as efficient electrocatalysts for water splitting and self-powered electrochemical systems. Small 2019, 15, 1902551. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Castro Neto, A.H. 2D materials and van der Waals heterostructures. Science 2016, 353, aac9439. [Google Scholar] [CrossRef]
- Li, M.-Y.; Chen, C.-H.; Shi, Y.; Li, L.-J. Heterostructures based on two-dimensional layered materials and their potential applications. Mater. Today 2016, 19, 322–335. [Google Scholar] [CrossRef]
- Jit, S.; Das, S. (Eds.) 2D Nanoscale Heterostructured Materials; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128176788. [Google Scholar]
- Pomerantseva, E.; Gogotsi, Y. Two-dimensional heterostructures for energy storage. Nat. Energy 2017, 2, 17089. [Google Scholar] [CrossRef]
- Wang, J.; Malgras, V.; Sugahara, Y.; Yamauchi, Y. Electrochemical energy storage performance of 2D nanoarchitectured hybrid materials. Nat. Commun. 2021, 12, 3563. [Google Scholar] [CrossRef]
- Blomstrand, C.W. On some new Swedish minerals and the composition of pyrrhotite. Öfversigt Af Kongl. Vetensk. Akad. Forh. 1870, 27, 19–27. [Google Scholar]
- Evans, H.T.; Allmann, R. The crystal structure and crystal chemistry of valleriite. Z. Flir Krist. 1968, 127, 73–93. [Google Scholar] [CrossRef]
- Harris, D.C.; Vaughan, D.J. Two fibrous iron sulfides and valleriite from Cupros, with new data on valleriite. Am. Mineral. 1972, 57, 1037–1053. [Google Scholar]
- Organova, N.I.; Drits, V.A.; Dmitrik, A.L. Structural study of tochilinite: Part I. The isometric variety. Sov. Phys. Crystallogr. 1973, 17, 667–671. [Google Scholar]
- Organova, N.I.; Drits, V.A.; Dmitrik, A.L. Structural study of tochilinite: Part II. Acicular variety: Unusual diffraction patterns. Sov. Phys. Crystallogr. 1974, 18, 606–609. [Google Scholar]
- Kato, Y.; Takahashi, R.; Sekiguchi, T.; Ryu, J. Study on medium-temperature chemical heat storage using mixed hydroxides. Int. J. Refrig. 2009, 32, 661–666. [Google Scholar] [CrossRef]
- Kurosawa, R.; Takeuchi, M.; Ryu, J. Comparison of the Effect of Coaddition of Li Compounds and Addition of a Single Li Compound on Reactivity and Structure of Magnesium Hydroxide. ACS Omega 2019, 4, 17752–17761. [Google Scholar] [CrossRef]
- Kondo, A.; Kurosawa, R.; Ryu, J.; Matsuoka, M.; Takeuchi, M. Investigation on the Mechanisms of Mg(OH)2 Dehydration and MgO Hydration by Near-Infrared Spectroscopy. J. Phys. Chem. C 2021, 125, 10937–10947. [Google Scholar] [CrossRef]
- Kosmulski, M. Isoelectric points and points of zero charge of metal (hydr)oxides: 50 years after Parks’ review. Adv. Colloid Interf. Sci. 2016, 238, 1–61. [Google Scholar] [CrossRef]
- Milton, C.; Milton, D.J. Nickel-gold ore of the Mackinaw Mine, Snohomish County, Washington. Econ. Geol. 1958, 53, 426–447. [Google Scholar] [CrossRef]
- Meyer, F.H.; Riggs, O.L.; McGlasson, R.L.; Sudbury, J.D. Corrosion products of mild steel in hydrogen sulfide environments. Corrosion 1958, 14, 69–75. [Google Scholar] [CrossRef]
- Kouvo, O.; Vuorelainen, Y.; Long, J.V.P. A tetragonal iron sulfide. Am. Mineral. 1963, 48, 511–524. [Google Scholar]
- Evans, H.T.; Milton, C.; Chao, E.C.T.; Adler, I.; Mead, C.; Ingram, B.; Berner, R.A. Valleriite and the new iron sulfide, mackinawite. US Geol. Surv. Prof. Pap. 1964, 475, 64–69. [Google Scholar]
- Borg, C.K.H.; Zhou, X.; Eckberg, C.; Campbell, D.J.; Saha, S.R.; Paglione, J.; Rodrigue, E.E. Strong anisotropy in nearly ideal tetrahedral superconducting FeS single crystals. Phys. Rev. B 2016, 93, 094522. [Google Scholar] [CrossRef]
- Bertaut, E.F.; Burlet, P.; Chappert, J. Sur l’absence d’ordre magnetique dans la forme quadratique de FeS. Solid State Commun. 1965, 3, 335–338. [Google Scholar] [CrossRef]
- Mücke, A. Review on Mackinawite and Valleriite: Formulae, Localities, Associations and Intergrowths of the Minerals, Mode of Formation and Optical Features in Reflected Light. J. Earth Sci. Clim. Change 2017, 8, 11. [Google Scholar] [CrossRef]
- Harmer, C.P.; Pak, C.H.; Greenfield, J.T.; Adeyemi, A.N.; Gamage, E.H.; Kovnir, K. Non-innocent intercalation of diamines into tetragonal FeS superconductor. ACS Appl. Energy Mater. 2021, 4, 42–46. [Google Scholar] [CrossRef]
- Boursiquot, S.; Mullet, M.; Abdelmoula, M.; Génin, J.-M.; Ehrhardt, J.-J. The dry oxidation of tetragonal FeS1-x mackinawite. Phys. Chem. Min. 2001, 28, 600–611. [Google Scholar] [CrossRef]
- Schröder, C.; Wan, M.; Butler, I.B.; Tait, A.; Peiffer, S.; McCammon, C.A. Identification of Mackinawite and Constraints on Its Electronic Configuration Using Mössbauer Spectroscopy. Minerals 2020, 10, 1090. [Google Scholar] [CrossRef]
- Hsu, F.C.; Luo, J.Y.; The, K.W.; Chen, T.K.; Huang, T.W.; Wu, P.M.; Lee, Y.C.; Huang, Y.L.; Chu, Y.Y.; Yan, D.C.; et al. Superconductivity in the PbO-type structure α-FeSe. Proc. Natl. Acad. Sci. USA 2008, 105, 14262–14264. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Takano, Y. Review of Fe chalcogenides as the simplest Fe-based superconductor. J. Phys. Soc. Jpn. 2010, 79, 102001. [Google Scholar] [CrossRef]
- Paglione, J.; Greene, R.L. High-temperature superconductivity in iron-based materials. Nat. Phys. 2010, 6, 645–658. [Google Scholar] [CrossRef]
- Pekov, I.V.; Sereda, E.V.; Yapaskurt, V.O.; Polekhovsky, Y.S.; Britvin, S.N.; Chukanov, N.V. Ferrovalleriite, 2(Fe,Cu)S·1.5Fe(OH)2: Validation as a mineral species and new data. Geol. Ore Depos. 2013, 55, 637–647. [Google Scholar] [CrossRef]
- Nickel, E.H.; Hudson, D.R. The replacement of chrome spinel by chromian valleriite in sulphide-bearing ultramafic rocks in Western Australia. Contrib. Mineral. Petrol. 1976, 55, 265–277. [Google Scholar] [CrossRef]
- Organova, N.I. Crystallochemistry of modulated and incommensurate structures in minerals. Int. Geol. Rev. 1986, 28, 802–814. [Google Scholar] [CrossRef]
- Organova, N.I.; Genkin, A.D.; Dmitrik, A.L.; Evstigneeva, T.L.; Laputina, I.P. On Structural Features and Isomorphism of Minerals of the Vallerite Group: Isomorphism in Minerals; Nauka: Moscow, Russia, 1975; pp. 150–162. (In Russian) [Google Scholar]
- Rice, C.M.; Atkin, D.; Bowles, J.F.; Criddle, A.J. Nukundamite, a new mineral, and idaite. Mineral. Mag. 1979, 43, 193–200. [Google Scholar] [CrossRef]
- Sugaki, A.; Shima, H.; Kitakaze, A.; Mizota, T. Hydrothermal synthesis of nukundamite and its crystal structure. Am. Mineral. 1981, 66, 398–402. [Google Scholar]
- Mikhlin, Y.L.; Likhatski, M.N.; Bayukov, O.A.; Knyazev, Y.V.; Velikanov, D.A.; Tomashevich, Y.V.; Romanchenko, A.S.; Vorobyev, S.A.; Volochaev, M.V.; Meira, D.M. Valleriite, a Natural Two-Dimensional Composite: X-ray Absorption, Photoelectron, and Mössbauer Spectroscopy, and Magnetic Characterization. ACS Omega 2021, 6, 7533–7543. [Google Scholar] [CrossRef]
- Genkin, A.D.; Distler, V.V.; Gladyshev, G.D. Sul’fidnye Medno-Nikelevye Rudy Noril’skikh Mestorozhdenii (Sulphidic Copper-Nickel Ores of Noril’sk Deposits); Nauka: Moscow, Russia, 1981. (In Russian) [Google Scholar]
- Dodin, D.A. Metallogeniya Taimyro-Noril’skogo Regiona (Metallogeny of Taimyr-Noril’sk Region); Nauka: St. Petersburg, Russia, 2002. (In Russian) [Google Scholar]
- Laptev, Y.V.; Shevchenko, V.S.; Urakaev, F.K. Sulphidation of Valleriite in SO2 Solutions. Hydrometallurgy 2009, 98, 201–205. [Google Scholar] [CrossRef]
- Chernyshov, N.M.; Molotkov, S.P. Some features of sulfide copper-nickel ore occurrences in the southeastern part of the Voronezh anteclise. In Proceedings of the Third Meeting on the Problems of Studying the Voronezh Anteclise, Voronezh, Russia, 7–11 April 1966. (In Russian). [Google Scholar]
- Jambor, J.L. Coalingite from the Muskox Intrusion, Northwest Territories. Am. Mineral. 1969, 54, 437–447. [Google Scholar]
- Clark, A.H. A probable second occurance of Jambors “fibrous iron sulfide”, Cornwall, England. Am. Mineral. 1970, 55, 283–286. [Google Scholar]
- Organova, N.I.; Genkin, A.D.; Drits, V.A.; Molotkov, S.P.; Kuz’mina, O.V.; Dmitrik, A.L. Tochilinite, a new iron and magnesium sulfide–hydroxide. Zap. Vsesoyuznogo Mineral. Obs. 1971, 100, 477–487. (In Russian) [Google Scholar]
- MacKinnon, I.D.R.; Zolensky, M.E. Proposed structures for poorly characterized phases in C2M carbonaceous chondrite matrix. Nature 1984, 309, 240–242. [Google Scholar] [CrossRef]
- Zolensky, M.E.; Mackinnon, J.D.R. Microstructures of cylindrical tochilinites. Am. Miner. 1986, 71, 1201–1209. [Google Scholar]
- Vacher, L.G.; Truche, L.; Faure, F.; Tissandier, L.; Mosser-Ruck, R.; Marrocchi, Y. Deciphering the conditions of tochilinite and cronstedtite formation in CM chondrites from low temperature hydrothermal experiments. Meteor. Planet. Sci. 2019, 54, 1870–1889. [Google Scholar] [CrossRef]
- Pekov, I.V.; Sereda, E.V.; Polekhovsky, Y.S.; Britvin, S.N.; Chukanov, N.V.; Yapaskurt, V.O.; Bryzgalov, I.A. Ferrotochilinite, 6FeS·5Fe(OH)2, a new mineral from the Oktyabr’sky deposit, Noril’sk district, Siberia, Russia. Geol. Ore Depos. 2013, 55, 567–574. [Google Scholar] [CrossRef]
- Muhma, M.; Vuorelainen, Y.; Häkli, T.A.; Papunen, H. Haapalaite, a new nickel-iron sulphide of the valleriite type from East Finland. Bull. Geol. Soc. Finl. 1973, 45, 103–106. [Google Scholar]
- Makeev, A.B.; Evstigneeva, T.L.; Troneva, N.V.; Vyalsov, L.N.; Gorshkov, A.I.; Trubkin, N.V. Yushkinite V1−xS·n[(Mg,Al)(OH)2]—A new mineral. Mineral. Zhurnal 1984, 6, 91–98. [Google Scholar]
- Soboleva, S.V.; Evstigneeva, T.E.; Boeva, N.M.; Bortnikov, N.S. Crystal Structure of Yushkinite [(Mg0.60Al0.30V0.10)Σ1(OH)2][V0.875S2]: An Example of a Commensurate Combination of Brucite and Sulfide Layers. Dokl. Earth Sci. 2020, 491, 210–213. [Google Scholar] [CrossRef]
- Evstigneeva, T.L.; Genkin, A.D.; Sandomirskaya, S.M.; Trubkin, N.V. Vyalsovite, a new sulfide-hydroxide of iron, calcium, and aluminum. Am. Mineral. 1992, 77, 201–206. [Google Scholar]
- Soboleva, S.V.; Boeva, N.M.; Evstigneeva, T.E.; Bortnikov, N.S. The Crystal Structure of Vyalsovite FeCaAlS(OH)5: First Example of the Commensurate Combination of Iron Sulfide and Hydroxide Layers. Dokl. Earth Sci. 2022, 503, 164–167. [Google Scholar] [CrossRef]
- Pekov, I.V.; Yapaskurt, V.O.; Polekhovsky, Y.S.; Vigasina, M.F.; Siidra, O.I. Ekplexite (Nb,Mo)S2∙(Mg1−xAlx)(OH)2+x, kaskasite (Mo,Nb)S2∙(Mg1−xAlx)(OH)2+x and manganokaskasite (Mo,Nb)S2∙(Mn1−xAlx)(OH)2+x, three new valleriite-group mineral species from the Khibiny alkaline complex, Kola peninsula, Russia. Mineral. Mag. 2014, 78, 663–679. [Google Scholar] [CrossRef]
- Iishi, K.; Kato, T.; Takeno, S. Syntheses of valleriite. Am. Mineral. 1970, 55, 2107–2110. [Google Scholar]
- Takeno, S.; Moh, G.H. Syntheses of selenian valleriite. Mineral. Petrol. 1994, 50, 209–218. [Google Scholar] [CrossRef]
- Hughes, A.E.; Kakos, G.A.; Turney, T.W.; Williams, T.B. Synthesis and structure of valleriite, a layered metal hydroxide/sulfide composite. J. Solid State Chem. 1993, 104, 422–436. [Google Scholar] [CrossRef]
- Chistyakova, N.I.; Gubaidulina, T.V.; Rusakov, V.S. Mössbauer investigations of natural and synthetic tochilinite and valleriite. Czech. J. Phys. 2006, 56, E123–E131. [Google Scholar] [CrossRef]
- Gubaidulina, T.V.; Chistyakova, N.I.; Rusakov, V.S. Mössbauer study of layered iron hydroxysulfides: Tochilinite and valleriite. Bull. Russ. Acad. Sci. Phys. 2007, 71, 1269–1272. [Google Scholar] [CrossRef]
- Chistyakova, N.I.; Rusakov, V.S.; Gubaidulina, T.V.; Gapochka, A.M.; Bychkov, A.Y. Mössbauer investigations of synthetic valleriit. Hyperfine Interact. 2012, 208, 99–104. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Borisov, R.V.; Vorobyev, S.A.; Tomashevich, Y.V.; Romanchenko, A.S.; Likhatski, M.N.; Karacharov, A.A.; Bayukov, O.A.; Knyazev, Y.V.; Velikanov, D.A.; et al. Synthesis and characterization of nanoscale composite particles formed by 2D layers of Cu–Fe sulfide and Mg-based hydroxide. J. Mater. Chem. A 2022, 10, 9621–9634. [Google Scholar] [CrossRef]
- Kakos, G.A.; Turney, T.W.; Williams, T.B. Synthesis and structure of tochilinite: A layered metal hydroxide/sulfide composite. J. Solid State Chem. 1994, 108, 102–111. [Google Scholar] [CrossRef]
- Kozerenko, S.V.; Organova, N.J.; Fadeev, V.V.; Magazina, L.O.; Kolpakova, N.N.; Kopneva, L.A. Tochilinite produced in laboratory. Lunar Planet. Sci. Conf. 1996, 27, 695–696. [Google Scholar]
- Moroz, L.V.; Kozerenko, S.V.; Fadeev, V.V. The reflectance spectrum of synthetic tochilinite. Lunar Planet. Sci. Conf. 1997, 28, 983. [Google Scholar]
- Kozerenko, S.V.; Fadeev, V.V.; Organova, N.I.; Rusakov, V.S.; Chistyakova, N.I.; Kolpakova, N.N.; Senin, V.G. Synthesis, formation conditions and crystallochemistry of tochilinites-iron, magnesium and sodium hydroxide-sulfides. Exp. Geosci. 2001, 10, 57–58. [Google Scholar]
- Peng, Y.; Xu, L.; Xi, G.; Zhong, C.; Lu, J.; Meng, Z.; Li, G.; Zhang, S.; Zhang, G.; Qian, Y. An experimental study on the hydrothermal preparation of tochilinite nanotubes and tochilinite-serpentine-intergrowth nanotubes from metal particles. Geochim. Cosmochim. 2007, 71, 2858–2875. [Google Scholar] [CrossRef]
- Peng, Y.; Xi, G.; Zhong, C.; Wang, L.; Lu, J.; Sun, X.; Zhu, L.; Han, Q.; Chen, L.; Shi, L.; et al. An experimental study on the preparation of tochilinite-originated intercalation compounds comprised of Fe1−xS host layers and various kinds of guest layers. Geochim. Cosmochim. Acta 2009, 73, 4862–4878. [Google Scholar] [CrossRef]
- Peng, Y.; Jing, Y. Hydrothermal preparation of analogous matrix minerals of CM carbonaceous chondrites from metal alloy particles. Earth Planet. Sci. Lett. 2014, 408, 252–262. [Google Scholar] [CrossRef]
- Bolney, R.; Grosch, M.; Winkler, M.; van Slageren, J.; Weigand, W.; Robl, C. Facile Synthesis and Characterization of Pure Tochilinite-like Materials from Nanoparticulate FeS. Z. Für Anorg. Und Allg. Chem. 2022, 648, e202200219. [Google Scholar] [CrossRef]
- Bolney, R.; Grosch, M.; Winkler, M.; van Slageren, J.; Weigand, W.; Robl, C. Mackinawite formation from elemental iron and sulfur. RSC Adv. 2021, 11, 32464–32475. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Borisov, R.V.; Likhatski, M.N.; Bajukov, O.A.; Knyazev, Y.V.; Zharkov, S.M.; Vorobyev, S.A.; Tomashevich, Y.V.; Ivaneeva, A.D.; Karacharov, A.A.; et al. Facile synthesis and selected characteristics of two-dimensional material composed of iron sulfide and magnesium-based hydroxide layers (tochilinite). New J. Chem. 2023, 47, 11869–11881. [Google Scholar] [CrossRef]
- Ge, J.-F.; Liu, Z.-L.; Liu, C.; Gao, C.-L.; Qian, D.; Xue, Q.-K.; Liu, Y.; Jia, J.-F. Superconductivity above 100 K in single-layer FeSe films on doped SrTiO3. Nat. Mater. 2015, 14, 285–289. [Google Scholar] [CrossRef]
- Krzton-Maziopa, A.; Svitlyk, V.; Pomjakushina, E.; Puzniak, R.; Conder, K. Superconductivity in alkali metal intercalated iron selenides. J. Phys. Cond. Matt. 2016, 28, 293002. [Google Scholar] [CrossRef]
- Krzton-Maziopa, A. Intercalated Iron Chalcogenides: Phase Separation Phenomena and Superconducting Properties. Front. Chem. 2021, 9, 640361. [Google Scholar] [CrossRef]
- Lu, X.F.; Wang, N.Z.; Wu, H.; Wu, Y.P.; Zhao, D.; Zeng, X.Z.; Luo, X.G.; Wu, T.; Bao, W.; Zhang, G.H.; et al. Coexistence of superconductivity and antiferromagnetism in (Li0.8Fe0.2)OHFeSe. Nat. Mater. 2015, 14, 325–329. [Google Scholar] [CrossRef]
- Lu, X.F.; Wang, N.Z.; Zhang, G.H.; Luo, X.G.; Ma, Z.M.; Lei, B.; Huang, F.Q.; Chen, X.H. Superconductivity in LiFeOFeSe with anti-PbO-type spacer layers. Phys. Rev. B 2014, 89, 020507. [Google Scholar] [CrossRef]
- Pachmayr, U.; Nitsche, F.; Luetkens, H.; Kamusella, S.; Brückner, F.; Sarkar, R.; Klauss, H.H.; Johrendt, D. Coexistence of 3d-Ferromagnetism and Superconductivity in [(Li1−xFex) OH](Fe1−yLiy)Se. Angew. Chem. Int Ed. 2015, 54, 293–297. [Google Scholar] [CrossRef]
- Pachmayr, U.; Johrendt, D. [(Li0.8Fe0.2) OH]FeS and the ferromagnetic superconductors [(Li0.8Fe0.2) OH]Fe(S1−xSex)(0 < x ≤ 1). Chem. Commun. 2015, 51, 4689–4692. [Google Scholar] [CrossRef]
- Zhang, X.; Lai, X.; Yi, N.; He, J.; Chen, H.; Zhang, H.; Lin, J.; Huang, F. Synthesis, crystal structure and physical properties of [Li0.85Fe0.15OH][FeS]. RSC Adv. 2015, 5, 38248–38253. [Google Scholar] [CrossRef]
- Chen, W.; Zeng, C.; Kaxiras, E.; Zhang, Z. Dual role of Fe dopants in enhancing stability and charge transfer in (Li0.8Fe0.2)OHFeSe superconductors. Phys. Rev. B 2016, 93, 064517. [Google Scholar] [CrossRef]
- Zhou, X.; Eckberg, C.; Wilfong, B.; Liou, S.C.; Vivanco, H.K.; Paglione, J.; Rodriguez, E.E. Superconductivity and magnetism in iron sulfides intercalated by metal hydroxides. Chem. Sci. 2017, 8, 3781–3788. [Google Scholar] [CrossRef]
- Du, Z.; Yang, X.; Altenfeld, D.; Gu, Q.; Yang, H.; Eremin, I.; Hirschfeld, P.J.; Mazin, I.I.; Lin, H.; Zhu, X.; et al. Sign reversal of the order parameter in (Li1−xFex) OHFe1−yZnySe. Nat. Phys. 2018, 14, 134–139. [Google Scholar] [CrossRef]
- Dong, X.; Jin, K.; Yuan, D.; Zhou, H.; Yuan, J.; Huang, Y.; Hua, W.; Sun, J.; Zheng, P.; Hu, W.; et al. (Li0.84Fe0.16) OHFe0.98Se superconductor: Ion-exchange synthesis of large single-crystal and highly two-dimensional electron properties. Phys. Rev. B 2015, 92, 064515. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, G.Y.; Ryu, G.H.; Lin, C.T. Structure and superconductivity of (Li1−xFex)OHFeSe single crystals grown using AxFe2−ySe2 (A = K, Rb, and Cs) as precursors. J. Phys. Condens. Matter 2016, 28, 015701. [Google Scholar] [CrossRef]
- Guo, M.; Lai, X.; Deng, J.; He, L.; Hao, J.; Tan, X.; Ren, Y.; Jian, J. NaOH-Intercalated Iron Chalcogenides (Na1−xOH)Fe1−yX (X = Se, S): Ion-Exchange Synthesis and Physical Properties. Inorg. Chem. 2021, 60, 8742–8753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Chou, M.M.C.; Lin, C.T. (Li1−xFex)OHFeSe superconductors: Crystal growth, structure, and electromagnetic properties. Crystals 2017, 7, 167. [Google Scholar] [CrossRef]
- Waanders, F.B.; Pollak, H. Mössbauer Spectroscopy to Characterize Iron Sulphides. S. Afr. J. Sci. 1999, 95, 387–390. [Google Scholar]
- Mikhlin, Y.L.; Romanchenko, A.S.; Tomashevich, E.V.; Volochaev, M.N.; Laptev, Y.V. XPS and XANES study of layered mineral valleriite. J. Struct. Chem. 2017, 58, 1137–1143. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Likhatski, M.N.; Romanchenko, A.S.; Vorobyev, S.A.; Tomashevich, Y.V.; Fetisova, O.Y. Valleriite-containing ore from Kingash deposit (Siberia, Russia): Mössbauer and X-ray photoelectron spectroscopy characterization, thermal and interfacial properties. J. Sib. Fed. Univ. Chem. 2022, 15, 303–317. [Google Scholar] [CrossRef]
- Burns, R.G.; Fisher, D.S. Nanophase mixed-valence iron minerals in meteorites identified by cryogenic Mössbauer spectroscopy. Hyperfine Interact. 1994, 91, 571–576. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, Q.-H.; Wu, J.-B.; Shi, W.; Tan, P.-H. Review on the Raman spectroscopy of different types of layered materials. Nanoscale 2016, 8, 6435–6450. [Google Scholar] [CrossRef]
- Available online: https://rruff.info/valleriite/names/asc/R080119 (accessed on 25 August 2023).
- Available online: https://rruff.info/chalcopyrite/names/asc/R050018 (accessed on 25 August 2023).
- Ohrendorf, F.W.; Haeuseler, H. Lattice dynamics of chalcopyrite type compounds. Part I. Vibrational frequencies. Cryst. Res. Technol. 1999, 34, 339–349. [Google Scholar] [CrossRef]
- Genchev, G.; Erbe, A. Raman Spectroscopy of Mackinawite FeS in Anodic Iron Sulfide Corrosion Products. J. Electrochem. Soc. 2016, 163, C333–C340. [Google Scholar] [CrossRef]
- Baum, A.; Milosavljević, A.; Lazarević, N.; Radonjić, M.M.; Nikolić, B.; Mitschek, M.; Inanloo Maranloo, Z.; Šćepanović, M.; Grujić-Brojčin, M.; Stojilović, N.; et al. Phonon anomalies in FeS. Phys. Rev. B 2018, 97, 054306. [Google Scholar] [CrossRef]
- Agrawal, A.; Cho, S.H.; Zandi, O.; Ghosh, S.; Johns, R.W.; Milliron, D.J. Localized surface plasmon resonance in semiconductor nanocrystals. Chem. Rev. 2018, 118, 3121–3207. [Google Scholar] [CrossRef]
- Gaspari, R.; Della Valle, G.; Ghosh, S.; Kriegel, I.; Scotognella, F.; Cavalli, A.; Manna, L. Quasi-static resonances in the visible spectrum from all-dielectric intermediate band semiconductor nanocrystals. Nano Lett. 2017, 17, 7691–7695. [Google Scholar] [CrossRef]
- Lee, S.; Ghosh, S.; Hoyer, C.E.; Liu, H.; Li, X.; Holmberg, V.C. Iron-content-dependent, quasi-static dielectric resonances and oxidative transitions in bornite and chalcopyrite copper iron sulfide nanocrystals. Chem. Mater. 2021, 33, 1821–1831. [Google Scholar] [CrossRef]
- Kuznetsov, A.I.; Miroshnichenko, A.E.; Brongersma, M.L.; Kivshar, Y.S.; Lukanchuk, B. Optically resonant dielectric nanostructures. Science 2016, 354, 6314. [Google Scholar] [CrossRef] [PubMed]
- Zywietz, U.; Evlyukhin, A.B.; Reinhardt, C.; Chichkov, B.N. Laser printing of silicon nanoparticles with resonant optical electric and magnetic responses. Nat. Commun. 2014, 5, 3402. [Google Scholar] [CrossRef]
- Valer, L.; Rossetto, D.; Scintilla, S.; Hu, Y.J.; Tomar, A.; Nader, S.; Betinol, I.O.; Mansy, S.S. Methods to identify and characterize iron–sulfur oligopeptides in water. Can. J. Chem. 2022, 100, 475–483. [Google Scholar] [CrossRef]
- Kubas, A. Characterization of charge transfer excited states in [2Fe–2S] iron–sulfur clusters using conventional configuration interaction techniques. Theor. Chem. Acc. 2020, 139, 120. [Google Scholar] [CrossRef]
- Likhatski, M.N.; Borisov, R.V.; Fetisova, O.Y.; Ivaneeva, A.D.; Karpov, D.V.; Tomashevich, Y.V.; Karacharov, A.A.; Vorobyev, S.A.; Mazurova, E.V.; Mikhlin, Y.L. Specificity of thermal stability and reactivity of two-dimensional layered Cu-Fe sulfide-Mg-based hydroxide compounds (valleriites). ACS Omega 2023. [Google Scholar] [CrossRef]
- Li, R.; Cui, L. Investigations on valleriite from Western China: Crystal chemistry and separation properties. Int. J. Miner. Process. 1994, 41, 271–283. [Google Scholar] [CrossRef]
- Romanchenko, A.; Likhatski, M.; Mikhlin, Y. X-ray Photoelectron Spectroscopy (XPS) Study of the Products Formed on Sulfide Minerals Upon the Interaction with Aqueous Platinum (IV) Chloride Complexes. Minerals 2018, 8, 578. [Google Scholar] [CrossRef]
- Karacharov, A.A.; Borisov, R.V.; Mikhlin, Y.L.; Likhatski, M.N.; Teremova, M.I.; Gurevich, Y.L. The Study of Bacterial Leaching of Synthetic Valleriite-Containing Materials. J. Sib. Fed. Univ. Chem. 2023, 16, 300–311. (In Russian) [Google Scholar]
- Allmann, R.; Lohse, H.N.; Hellner, E. Die kristallstruktur des koenenits, eine doppelschichtstruktur mit zwei inkommensurablen teilgittern. Z. Fur Krist. 1968, 126, 7–22. [Google Scholar] [CrossRef]



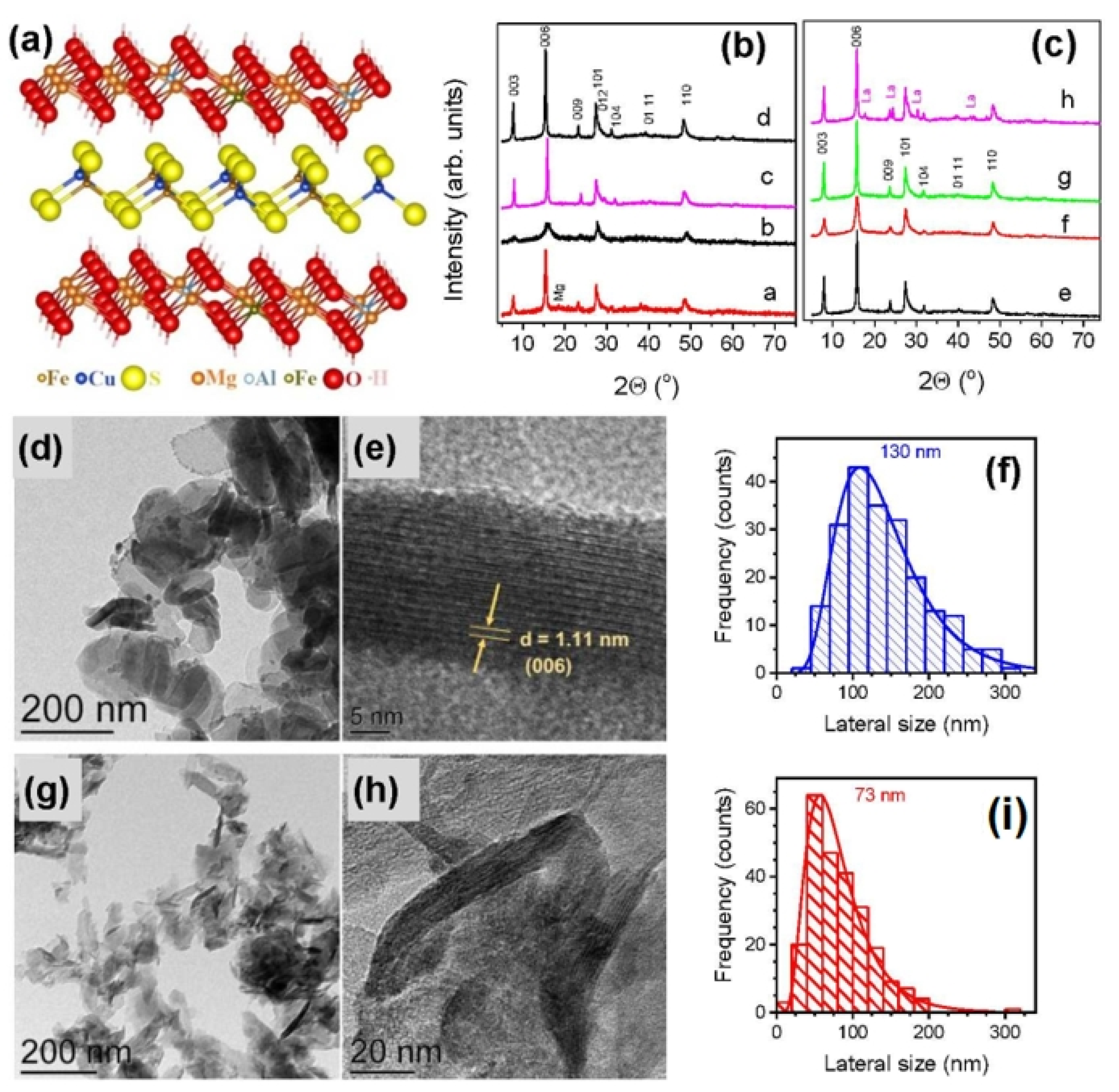

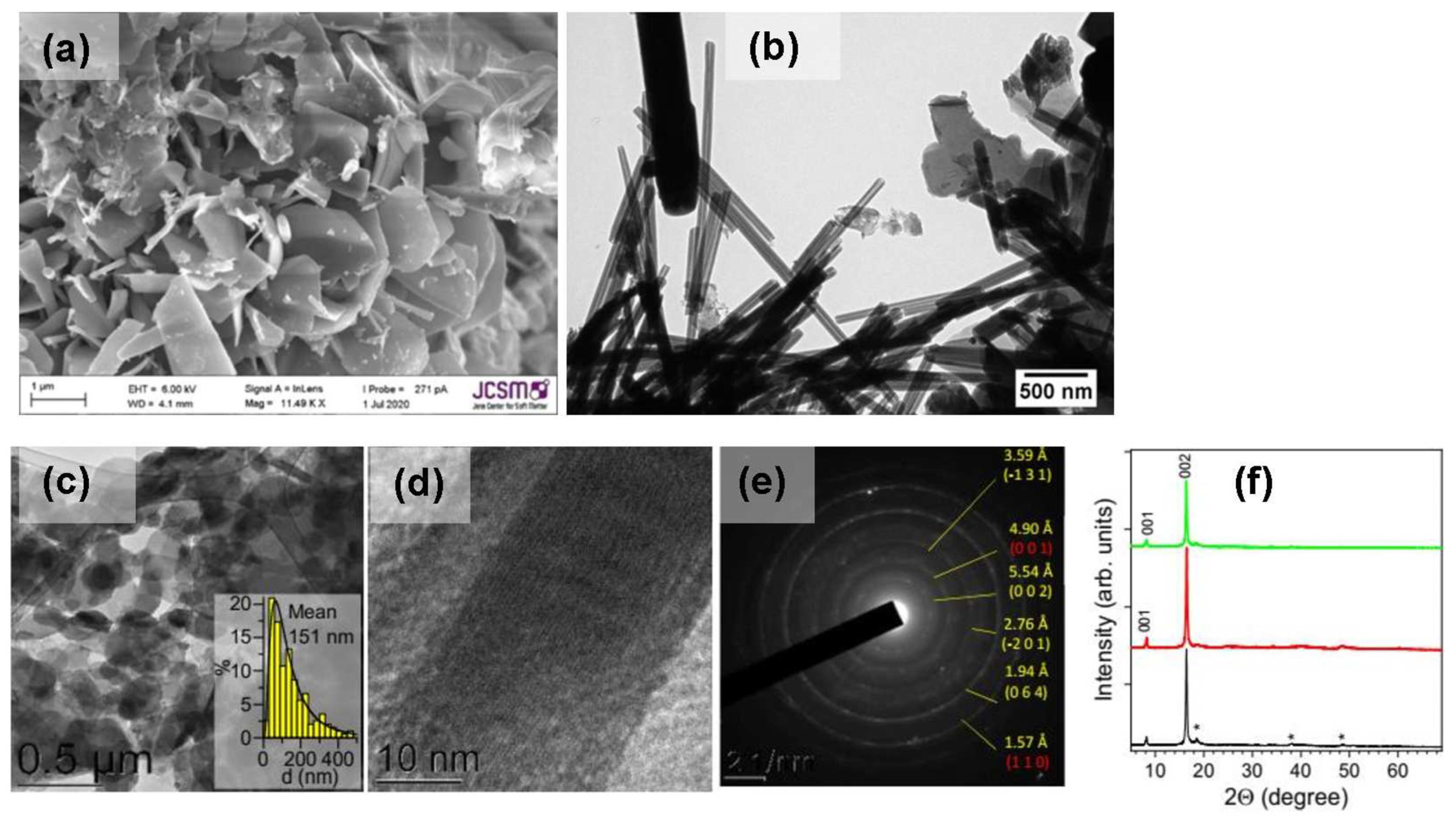
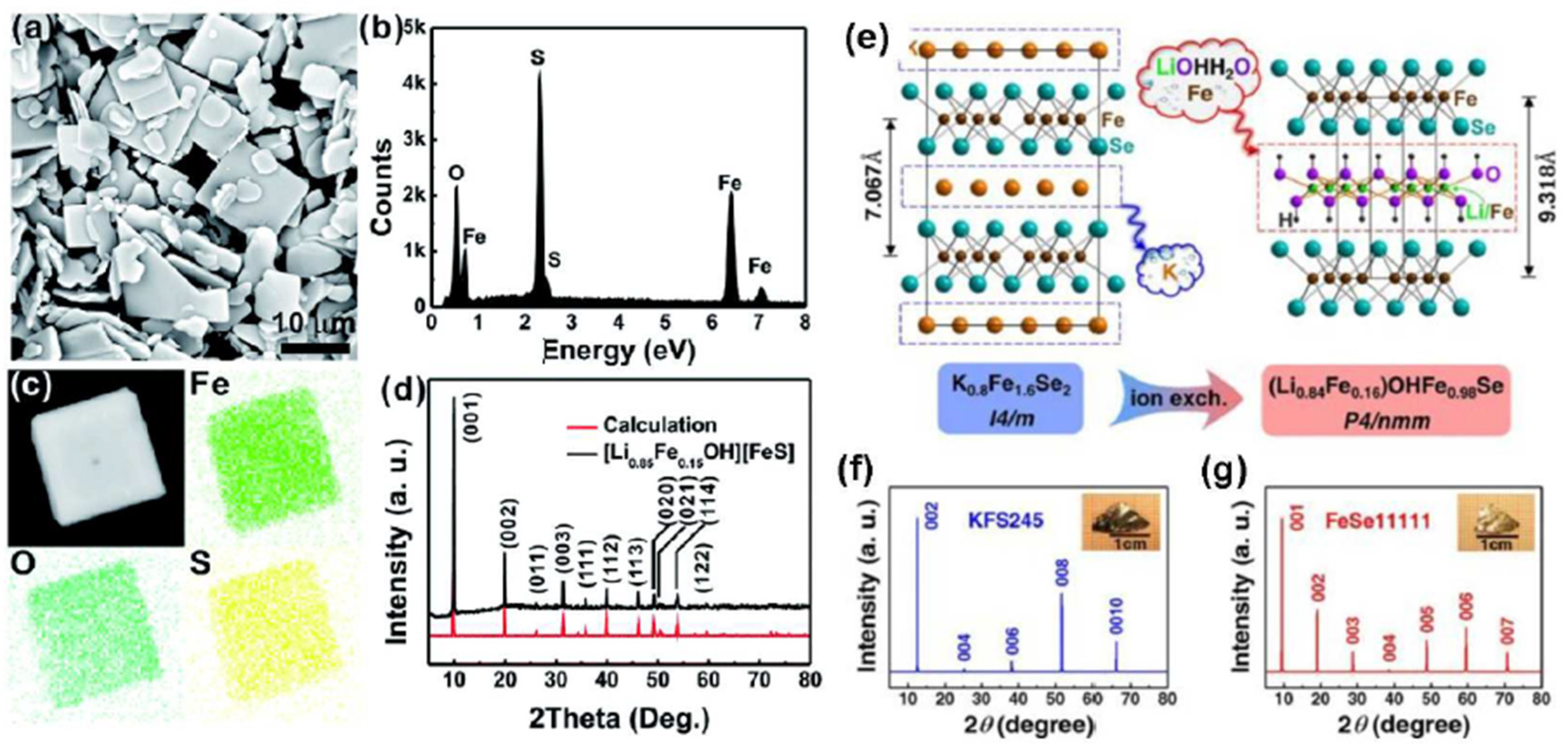
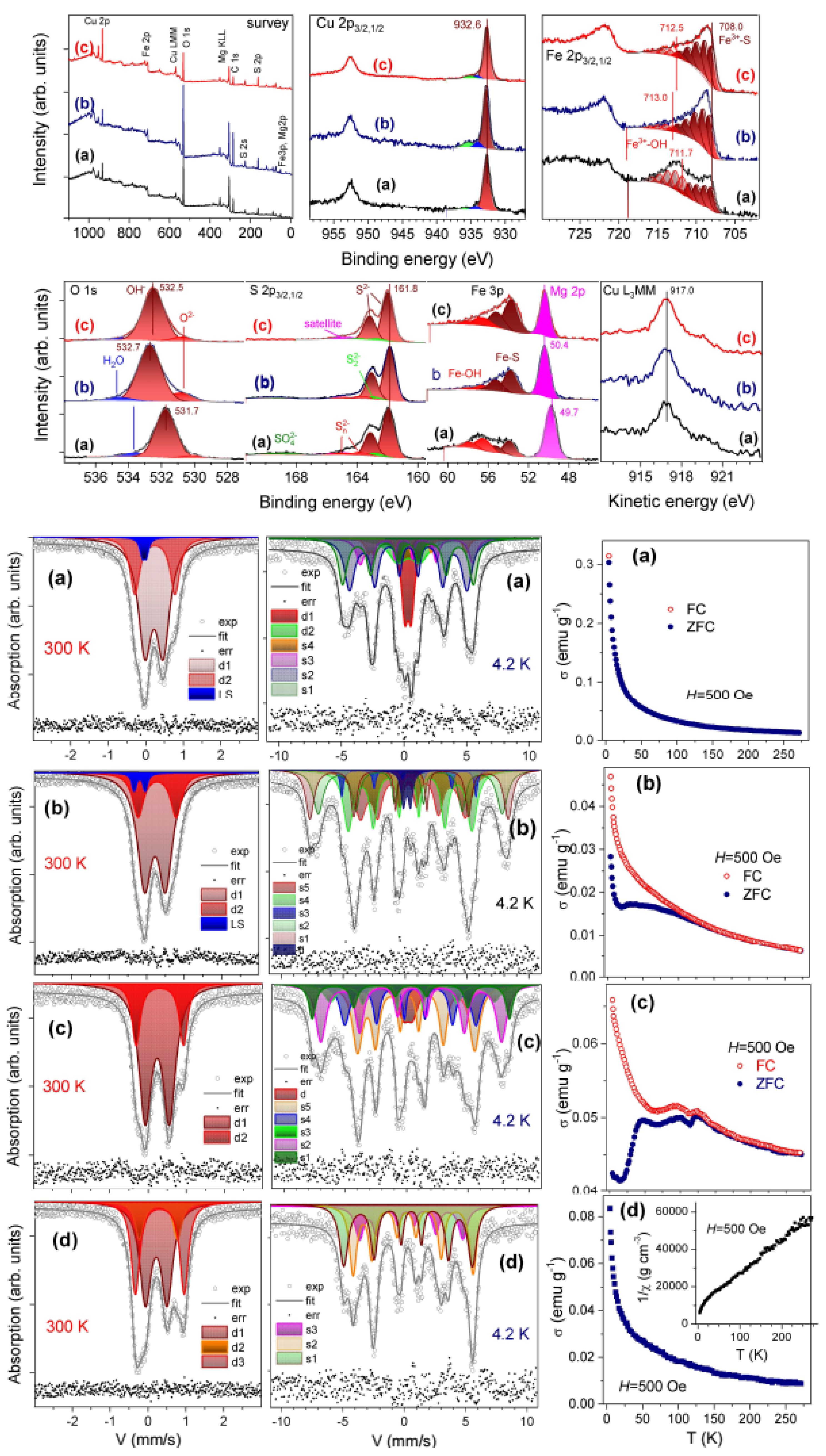



| Products of Synthesis | Starting Materials | Reaction Conditions | Reference(s) |
|---|---|---|---|
| Valleriite, chalcopyrite, brucite, pyrrhotite, covellite, boehmite | CuFeS2, MgO, γ-Al2O3 | 1000 bar, 5–20 days, 400–700 °C | K. Iiishi et al. [87] (1970) |
| Selenian valleriite, valleriite, metal sulfides (selenides), korshunskite | CuFeSe2, CuFe2S3, MgO and Al2O3 | 1000 bar, 10–19 days, 450 °C | S. Takeno and G. Moh [88] (1994) |
| Valleriite as thin platy crystals (100–200 nm) | Fe2+/Cu2+, excessive (NH4)2S Mg2+/Al3+ + 25% NH4OH | pH 8.5–9.5, H2 pressure of 10 Bar, 1–25 days, 110–300 °C | A. Hughes et al. [89] (1993) |
| Valleriite (<50%), chalcopyrite, Fe (hydr)oxides | FeSO4, CuSO4 and MgSO4 solutions, H2S (or Na2S), NaOH | Varying ratio Cu:Fe:Mg 30 days, 150 °C, 180 °C | N.Chistyakova et al. [90,91,92] (2006), (2006), (2012) |
| Valleriite, valleriite doped with Al, Li, Ni, Cr, Co, La, 100–200 nm flakes 10–20 nm thick | Sulfates of Fe, Cu and doping elements, excessive Na2S, sulfates of Mg, Al, NH4OH | 160 °C, 10–80 h, initial pH 9.5, final pH 12–12.5 | Y. Mikhlin et al. [93] (2022) |
| Tochilinite as plate- and tube-like sub-μm particles | Aqueous Fe2+, excess of (NH4)2S (Mg,Al)-hydroxide gel, NH4OH | pH 8.5–9.5, H2 pressure of 2 MBar 2 days, 200 °C | G. Kakos et al. [94] (1994) |
| Fe-tochilinite, magnetite, mackinawite, pyrrhotite | Aqueous Fe oxyhydroxide, H2S, NaOH | pH 7.8 and 11.5, 30–150 days 80 °C | S. Kozerenko et al. [95] (1996) |
| Tochilinite, Fe-tochilinite, magnetite, mackinawite | Aqueous Fe2+, metallic Mg, NaOH, H2S | pH > 12 10–45 days 120–140 °C | L. Moroz et al. [96] (1997), S. Kozerenko et al. [97] (2001) |
| Tochilinite (low yield), magnetite, troilite and pyrite | Fe(OH)2, H2S, Mg | Medium-alkaline no data of synthesis times 160–180 °C | N. Chistyakova, T. Gubaidulina et al. [90,91] (2006, 2007) |
| Tochilinite (<40%) in the mixtures characteristic of meteorites | FeMgAl alloy particles, aqueous solutions of Na2S or (NH4)2S, or S0, NaOH | N2 or Ar atmosphere, pH 13–14, 4–120 (typically 40–60) days, 105–160 °C | Y. Peng et al. [98,99,100] (2007, 2009, 2014) |
| Mg,Fe-Tochilinite, Al,Fe-tochilinite, Na-tochilinite, sub-μm platelets few nm thick, nanotubes | Elemental Fe and S (Fe/S ≥ 1), Al, Mg, Na hydroxides | 3–4 days, H2 130–160 °C | R. Bolney et al. [101] (2022) |
| Tochilinite, Al-, Li-doped, flakes ~150 nm and 10–20 nm thick | aqueous sulfates of Fe, Mg, Al, Na2S, NH4OH | pH after synthesis 12–12.5 40 h, 160 °C | Y. Mikhlin et al. [103] (2023) |
| FeSe·(Li,Fe)OH powder (μm crystals) | Fe metal powder, excess of selenourea, LiOH | 3–10 days 160 °C | X.F. Lu [107] (2015) |
| FeS·(Li,Fe)OH, powder of ~10–50 μm | Fe metal powder, thiourea, LiOH | 3 days, 200 °C | X Zhang et al. [111] (2015) |
| FeSe·(Li,Fe)OH, 1–2 cm single crystals | AxFe2−ySe2 (A = K, Rb, Cs) single crystal precursors, Fe metal powder, selenourea, LiOH | 2 days 120–200 °C | X. Dong et al. [115] (2015), G. Yu et al. [116] (2016) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhlin, Y.; Likhatski, M.; Borisov, R.; Karpov, D.; Vorobyev, S. Metal Chalcogenide–Hydroxide Hybrids as an Emerging Family of Two-Dimensional Heterolayered Materials: An Early Review. Materials 2023, 16, 6381. https://doi.org/10.3390/ma16196381
Mikhlin Y, Likhatski M, Borisov R, Karpov D, Vorobyev S. Metal Chalcogenide–Hydroxide Hybrids as an Emerging Family of Two-Dimensional Heterolayered Materials: An Early Review. Materials. 2023; 16(19):6381. https://doi.org/10.3390/ma16196381
Chicago/Turabian StyleMikhlin, Yuri, Maxim Likhatski, Roman Borisov, Denis Karpov, and Sergey Vorobyev. 2023. "Metal Chalcogenide–Hydroxide Hybrids as an Emerging Family of Two-Dimensional Heterolayered Materials: An Early Review" Materials 16, no. 19: 6381. https://doi.org/10.3390/ma16196381
APA StyleMikhlin, Y., Likhatski, M., Borisov, R., Karpov, D., & Vorobyev, S. (2023). Metal Chalcogenide–Hydroxide Hybrids as an Emerging Family of Two-Dimensional Heterolayered Materials: An Early Review. Materials, 16(19), 6381. https://doi.org/10.3390/ma16196381