Picosecond Pulsed Laser Deposition of Metals and Metal Oxides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental
2.2. Theoretical
2.3. Sample Characterization
3. Results
3.1. Experimental Part
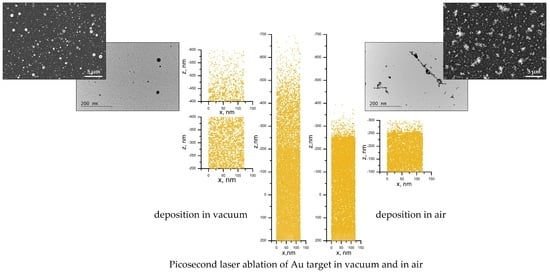
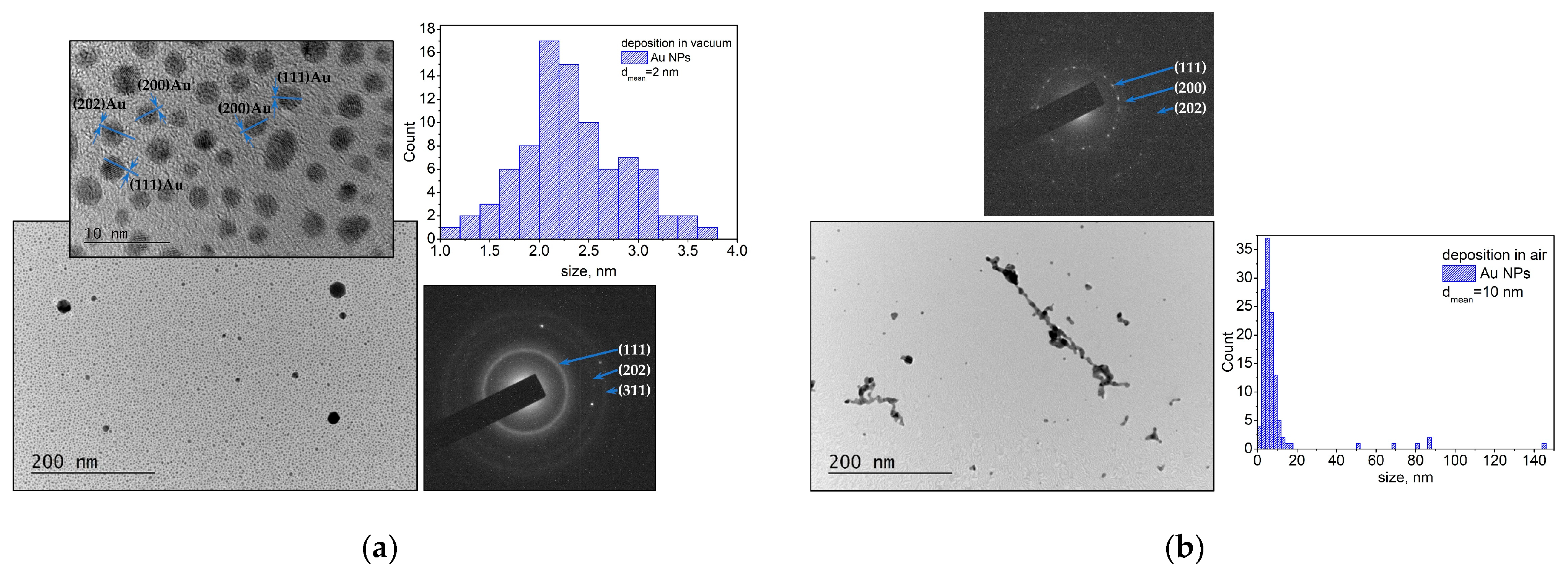
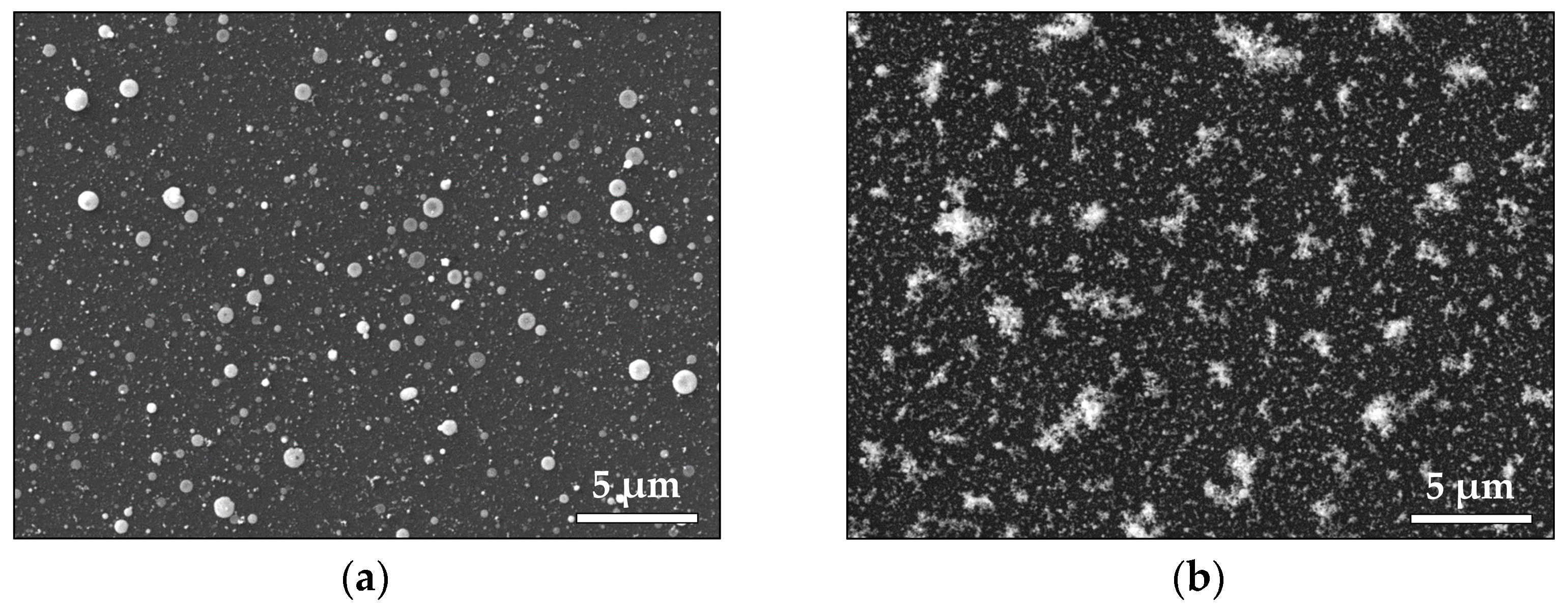
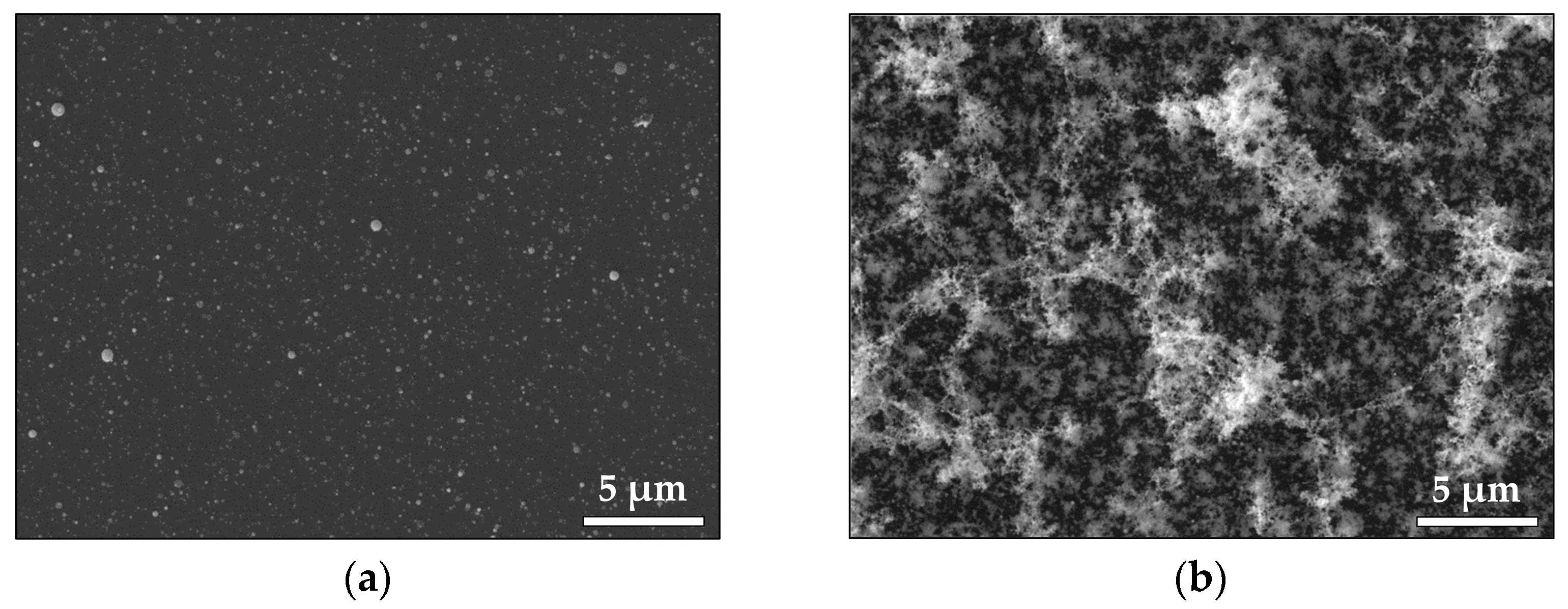
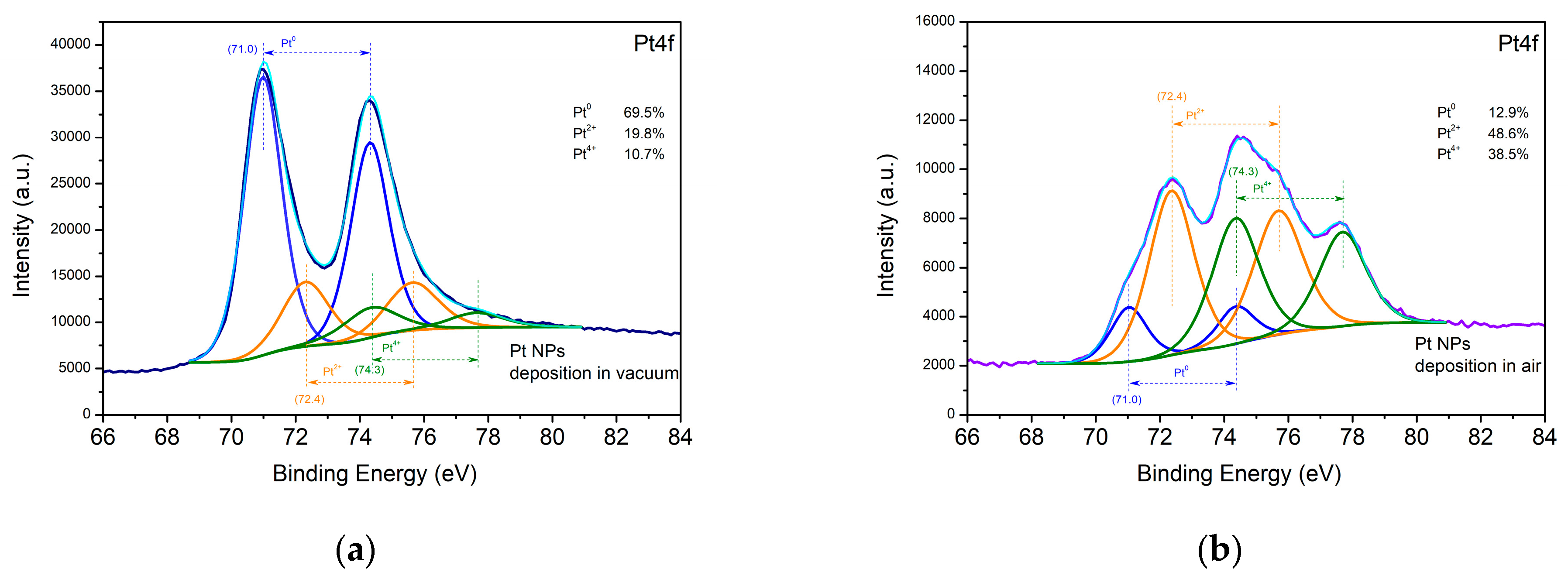
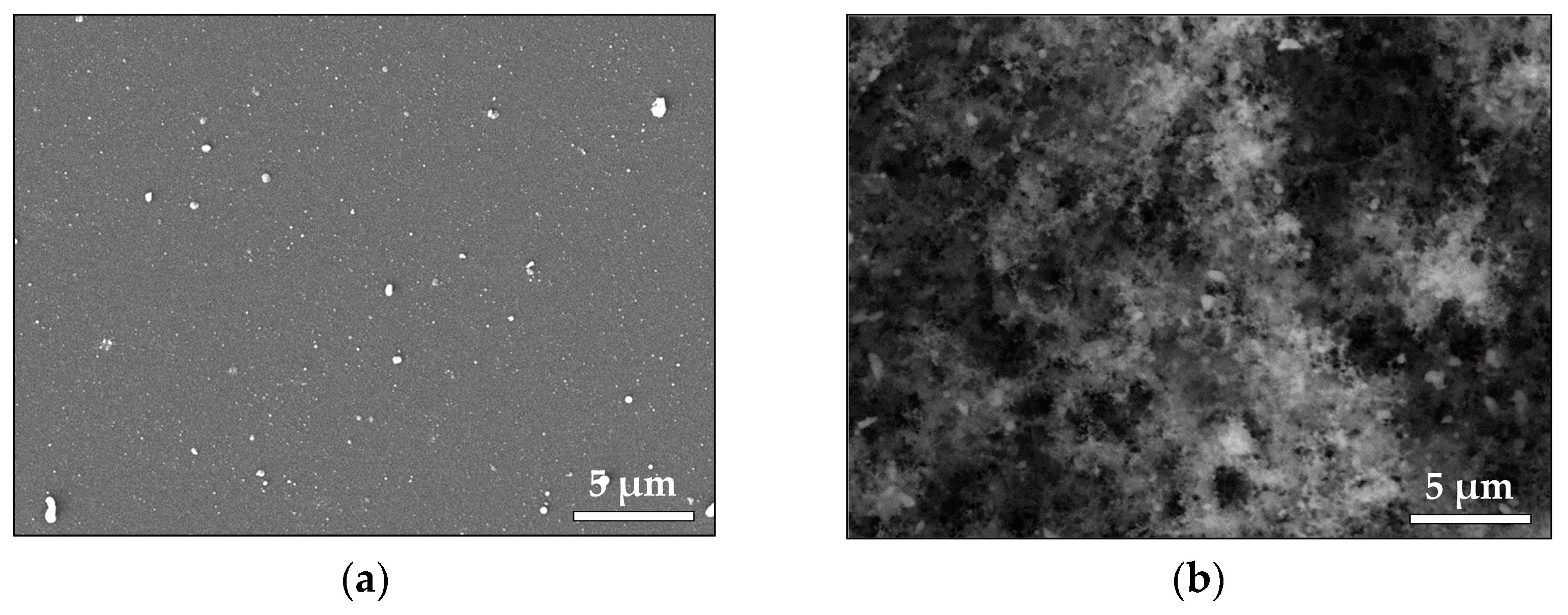
3.1.1. Noble Metals—Au and Pt
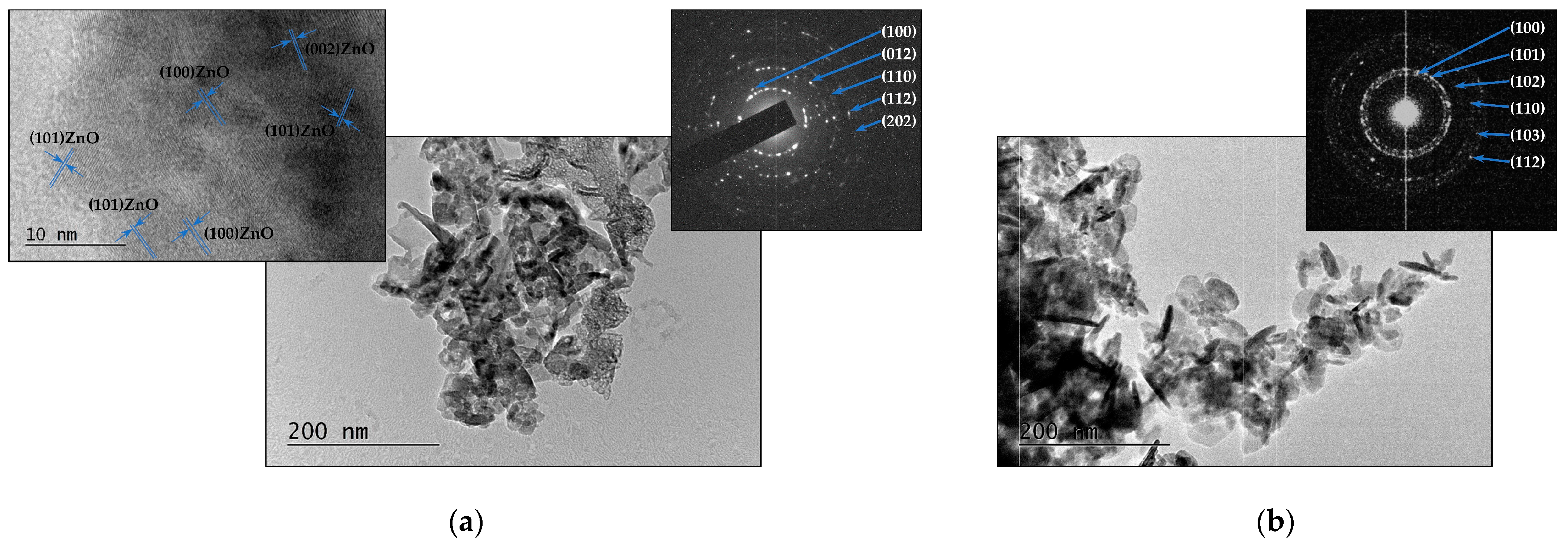
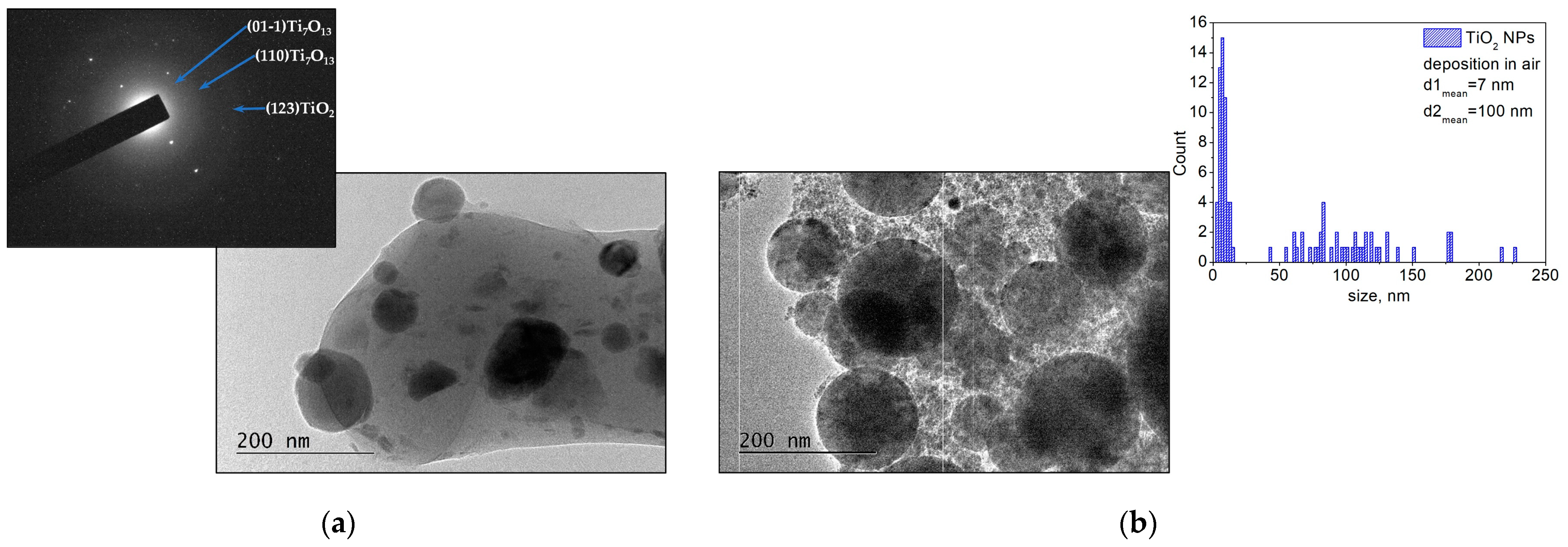
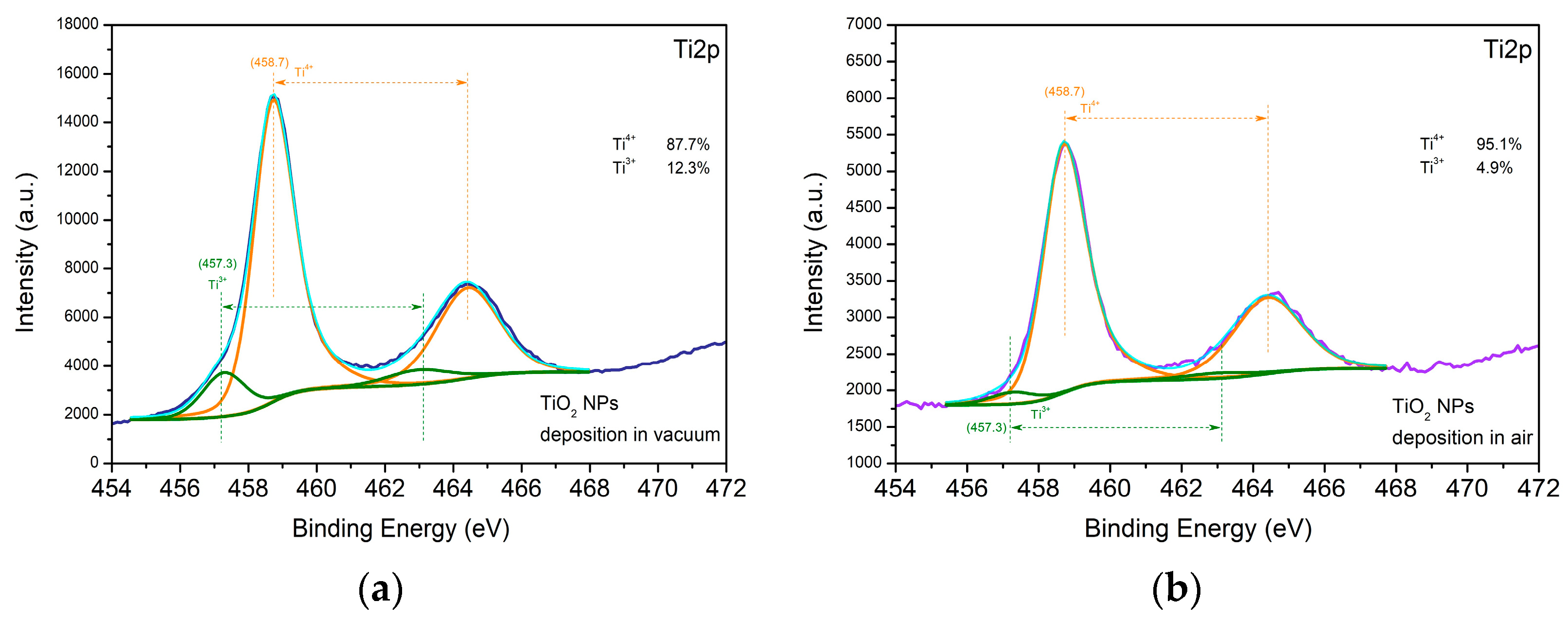
3.1.2. Metal Oxides—ZnO and TiO2
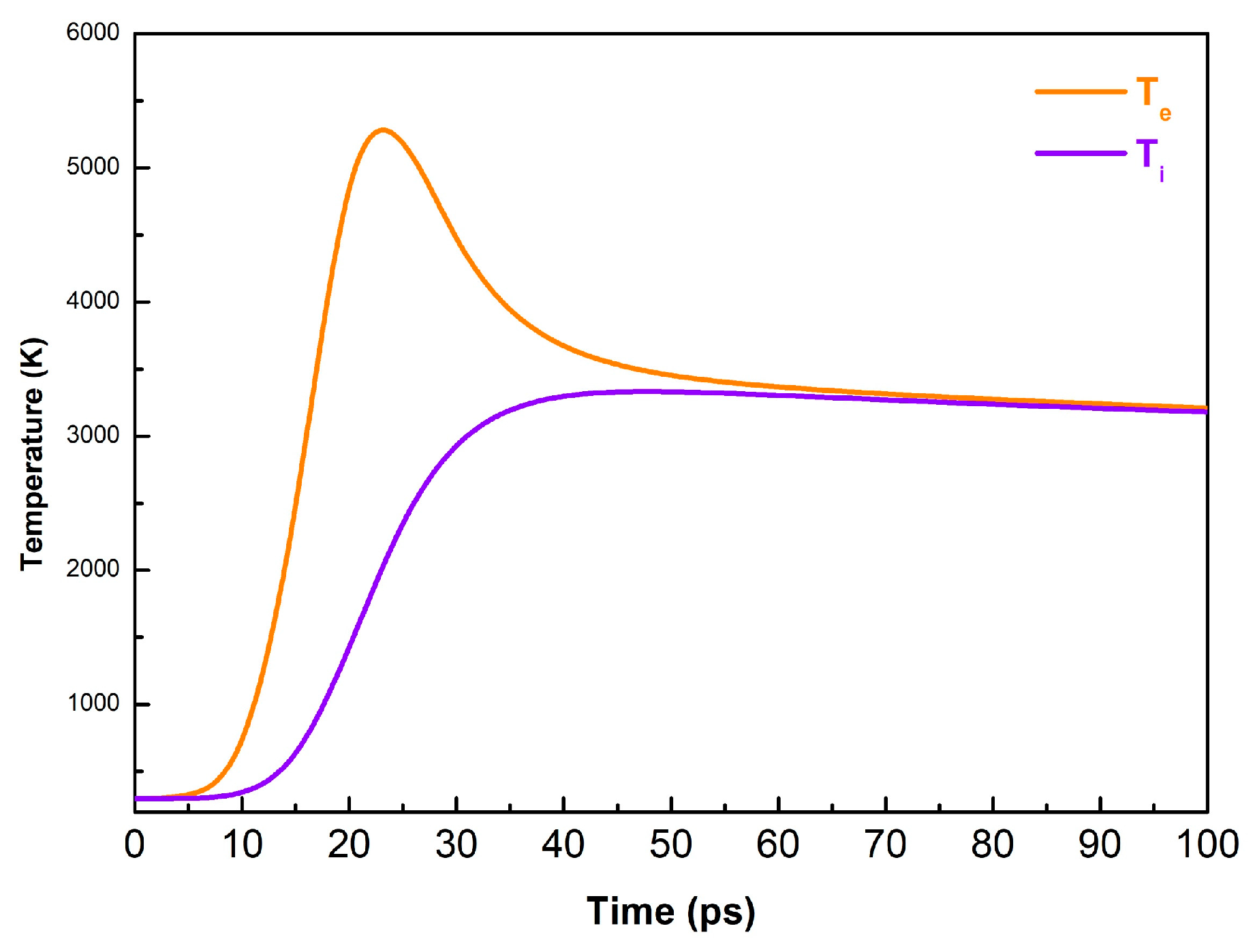
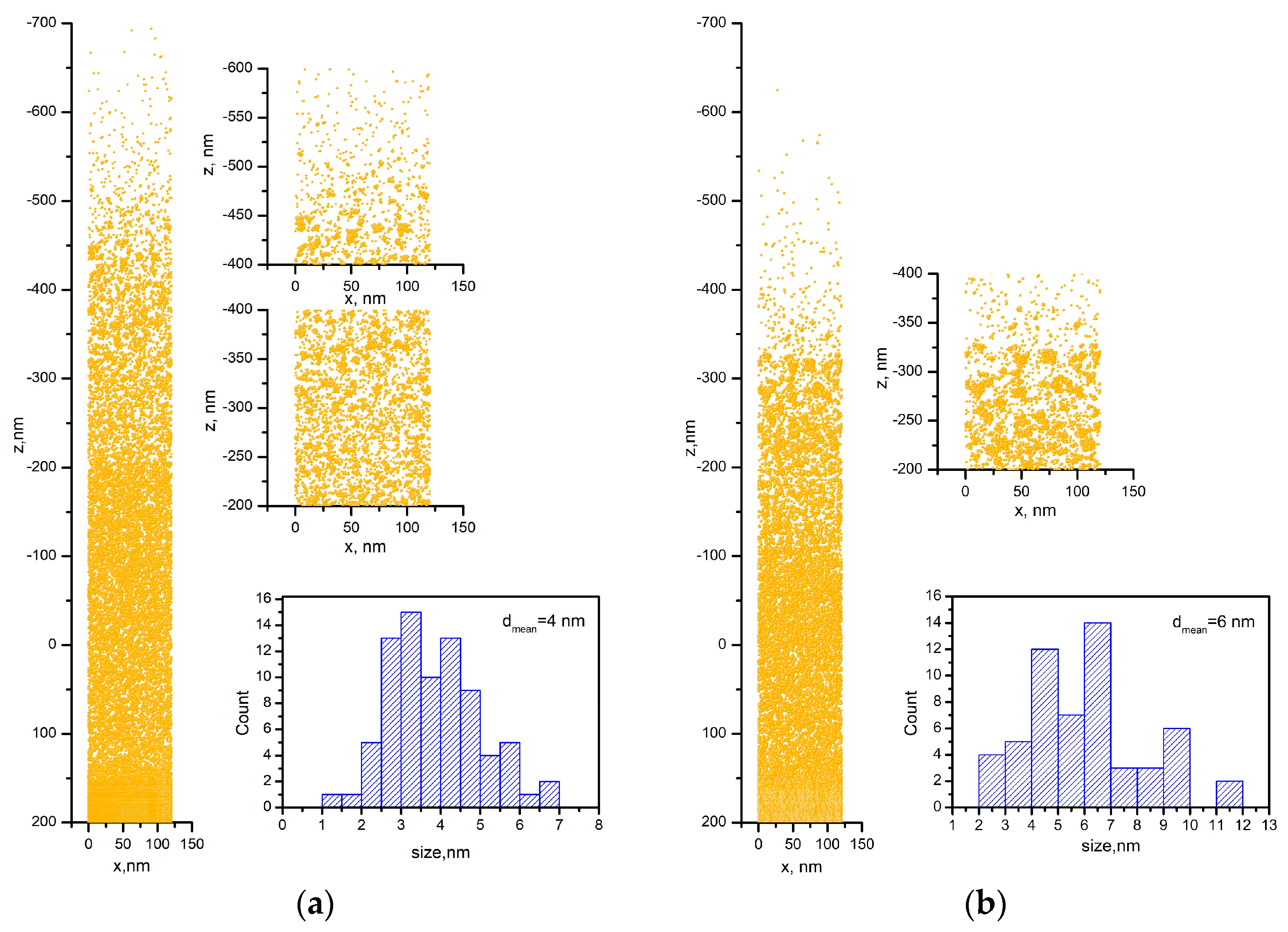
3.2. Theoretical Part
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sibbett, W.; Lagatsky, A.A.; Brown, C.T.A. The development and application of femtosecond laser systems. Opt. Express 2012, 20, 6989–7001. [Google Scholar] [CrossRef]
- Guo, B.; Sun, J.; Hua, Y.; Zhan, N.; Jia, J.; Chu, K. Femtosecond Laser Micro/Nano-manufacturing: Theories, Measurements, Methods, and Applications. Nanomanuf. Metrol. 2020, 3, 26–67. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Li, P.; Zhang, Y.; Wen, Y.; Qiu, Y.; Liu, Z.; Li, Y.P.; Liu, L. Femtosecond laser-based processing methods and their applications in optical device manufacturing: A review. Opt. Laser Technol. 2021, 135, 106687. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Direct femtosecond laser surface nano/microstructuring and its applications. Laser Photonics Rev. 2013, 7, 385–407. [Google Scholar] [CrossRef]
- Andreeva, Y.; Sharma, N.; Rudenko, A.; Mikhailova, J.; Sergeev, M.; Veiko, V.P.; Vocanson, F.; Lefkir, Y.; Destouches, N.; Itina, T.E. Insights into Ultrashort Laser-Driven Au:TiO2 Nanocomposite Formation. J. Phys. Chem. C 2020, 124, 10209–10219. [Google Scholar] [CrossRef]
- Cheng, J.; Perrie, W.; Sharp, M.; Edwardson, S.P.; Semaltianos, N.G.; Dearden, G.; Watkins, K.G. Single-pulse drilling study on Au, Al and Ti alloy by using a picosecond laser. Appl. Phys. A 2009, 95, 739–746. [Google Scholar] [CrossRef]
- Zhang, H.; Di, J.; Zhou, M.; Yan, Y.; Wang, R. An investigation on the hole quality during picosecond laser helical drilling of stainless steel 304. Appl. Phys. A 2015, 119, 745–752. [Google Scholar] [CrossRef]
- Shestakov, D.; Khairullina, E.; Shishov, A.; Khubezhov, S.; Makarov, S.; Tumkin, I.; Logunov, L. Picosecond laser writing of highly conductive copper micro-contacts from deep eutectic solvents. Opt. Laser Technol. 2023, 167, 109777. [Google Scholar] [CrossRef]
- Spiro, A.; Lowe, M.; Pasmanik, G. Drilling rate of five metals with picosecond laser pulses at 355, 532, and 1064 nm. Appl. Phys. A 2012, 107, 801–808. [Google Scholar] [CrossRef]
- Preißa, E.M.; Krauß, A.; Kekkonen, V.; Barsan, N.; Seidel, H. Characterization of WO3 thin films prepared by picosecond laser deposition for gas sensing. Sens. Act. B 2017, 248, 153–159. [Google Scholar] [CrossRef]
- Huotari, J.; Kekkonen, V.; Haapalainen, T.; Leidinger, M.; Sauerwald, T.; Puustinen, J.; Liimatainen, J.; Lappalainen, J. Pulsed laser deposition of metal oxide nanostructures for highly sensitive gas sensor applications. Sens. Act. B Chem. 2016, 236, 978–987. [Google Scholar] [CrossRef]
- Kekkonen, V.; Chaudhuri, S.; Clarke, F.; Kaisto, J.; Liimatainen, J.; Pandian, S.K.; Piirto, J.; Siltanen, M.; Zolotukhin, A. Picosecond pulsed laser deposition of metal-oxide sensing layers with controllable porosity for gas sensor applications. Appl. Phys. A 2016, 122, 233. [Google Scholar] [CrossRef]
- Pervolaraki, M.; Mihailescu, C.N.; Luculescu, C.R.; Ionescu, P.; Dracea, M.D.; Pantelica, D.; Giapintzakis, J. Picosecond ultrafast pulsed laser deposition of SrTiO3. Appl. Surf. Sci. 2015, 336, 278–282. [Google Scholar] [CrossRef]
- Salminen, T.; Hahtala, M.; Seppälä, I.; Niemi, T.; Pessa, M. Pulsed laser deposition of yttria-stabilized zirconium dioxide with a high repetition rate picosecond fiber laser. Appl. Phys. A 2010, 98, 487–490. [Google Scholar] [CrossRef]
- Mao, S.S.; Mao, X.; Greif, R.; Russo, R.E. Dynamics of an air breakdown plasma on a solid surface during picosecond laser ablation. Appl. Phys. Lett. 2000, 76, 31–33. [Google Scholar] [CrossRef]
- Mao, S.S.; Mao, X.; Greif, R.; Russo, R.E. Initiation of an early-stage plasma during picosecond laser ablation of solids. Appl. Phys. Lett. 2000, 77, 2464–2466. [Google Scholar] [CrossRef]
- Kravets, V.G.; Kabashin, A.V.; Barnes, W.L.; Grigorenko, A.N. Plasmonic Surface Lattice Resonances: A Review of Properties and Applications. Chem. Rev. 2018, 118, 5912–5951. [Google Scholar] [CrossRef]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A review on ZnO: Fundamental properties and applications. Mater. Today Proc. 2022, 49, 3028–3035. [Google Scholar] [CrossRef]
- Ahmed, R.M.; Hasan, I. A review on properties and applications of TiO2 and associated nanocomposite materials. Mater. Today Proc. 2023, 81, 1073–1078. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Clarendon: Oxford, UK, 1987. [Google Scholar] [CrossRef]
- Mohammed, K.; Shukla, M.M.; Milstein, F.; Merz, J.L. Lattice dynamics of face-centered-cubic metals using the ionic Morse potential immersed in the sea of free-electron gas. Phys. Rev. B 1984, 29, 3117. [Google Scholar] [CrossRef]
- Anisimov, S.I.; Kapelovich, B.L.; Perel’man, T.L. Electron emission from metal surfaces exposed to ultrashort laser pulses. Sov. Phys. JETP 1974, 39, 375–377. [Google Scholar]
- Amoruso, S.; Nedyalkov, N.N.; Wang, X.; Ausanio, G.; Bruzzese, R.; Atanasov, P.A. Ultrashort-pulse laser ablation of gold thin film targets: Theory and experiment. Thin Solid Films 2014, 550, 190–198. [Google Scholar] [CrossRef]
- Farid, N.; Harilal, S.S.; Ding, H.; Hassanein, A. Emission features and expansion dynamics of nanosecond laser ablation plumes at different ambient pressures. J. Appl. Phys. 2014, 115, 033107. [Google Scholar] [CrossRef]
- Bai, X.; Ma, Q.; Perrier, M.; Motto-Ros, V.; Sabourdy, D.; Nguyen, L.; Jalocha, A.; Yu, J. Experimental study of laser-induced plasma: Influence of laser fluence and pulse duration. Spectrochim. Acta Part B 2013, 87, 27–35. [Google Scholar] [CrossRef]
- Borchert, H.; Dar´ee, K.; Hugenschmidt, M. Plasma formation during the interaction of picosecond and nanosecond laser pulses with BK7 glass. J. Phys. D Appl. Phys. 2005, 38, 300–305. [Google Scholar] [CrossRef]
- Ihnatiuk, D.; Tossi, C.; Tittonen, I.; Linnik, O. Effect of Synthesis Conditions of Nitrogen and Platinum Co-Doped Titania Films on the Photocatalytic Performance under Simulated Solar Light. Catalysts 2020, 10, 1074. [Google Scholar] [CrossRef]
- Claros, M.; Setka, M.; Jimenez, Y.P.; Vallejos, S. AACVD Synthesis and Characterization of Iron and Copper Oxides Modified ZnO Structured Films. Nanomaterials 2020, 10, 471. [Google Scholar] [CrossRef]
- Tomić, M.; Claros, M.; Gràcia, I.; Figueras, E.; Cané, C.; Vallejos, S. ZnO Structures with Surface Nanoscale Interfaces Formed by Au, Fe2O3, or Cu2O Modifier Nanoparticles: Characterization and Gas Sensing Properties. Sensors 2021, 21, 4509. [Google Scholar] [CrossRef]
- Konatu, R.T.; Domingues, D.D.; França, R.; Alves, A.P.R. XPS Characterization of TiO2 Nanotubes Growth on the Surface of the Ti15Zr15Mo Alloy for Biomedical Applications. J. Funct. Biomater. 2023, 14, 353. [Google Scholar] [CrossRef]
- Pallotti, D.K.; Ni, X.; Fittipaldi, R.; Wang, X.; Lettieri, S.; Vecchione, A.; Amoruso, S. Laser ablation and deposition of titanium dioxide with ultrashort pulses at 527 nm. Appl. Phys. B 2015, 119, 445–452. [Google Scholar] [CrossRef]
- Sang, L.; Zhang, H.; Ni, X.; Anoop, K.K.; Fittipaldi, R.; Wang, X.; Amoruso, S. Hydrogen-evolving photoanode of TiO2 nanoparticles film deposited by a femtosecond laser. Int. J. Hydrogen Energy 2015, 40, 779–785. [Google Scholar] [CrossRef]
- Wu, C.; Zhigilei, L.V. Microscopic mechanisms of laser spallation and ablation of metal targets from large-scale molecular dynamics simulations. Appl. Phys. A 2014, 114, 11–32. [Google Scholar] [CrossRef]
- Lorazo, P.; Lewis, L.J.; Meunier, M. Short-Pulse Laser Ablation of Solids: From Phase Explosion to Fragmentation. Phys. Rev. Lett. 2003, 91, 225502. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, K.W.; Gupta, M.C.; Zhigilei, L.V. Plume and Nanoparticle Formation During Laser Ablation. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Wandelt, K., Ed.; Elsevier: Oxford, UK, 2018; Volume 2, pp. 594–603. [Google Scholar] [CrossRef]
- Nedyalkov, N.; Nikov, R.; Dikovska, A.; Atanasova, G.; Nakajima, Y.; Terakawa, M. Gold nanostructures deposition by laser ablation in air using nano- and femtosecond laser pulses. Appl. Phys. A 2017, 123, 306. [Google Scholar] [CrossRef]
- Nikov, R.G.; Dikovska, A.O.; Nedyalkov, N.N.; Atanasov, P.A. Fabrication of Au nanostructures by pulsed laser deposition in air. Proc. SPIE 2017, 10226, 119–125. [Google Scholar] [CrossRef]
- Nikov, R.G.; Dikovska, A.O.; Nedyalkov, N.N.; Avdeev, G.V.; Atanasov, P.A. Au nanostructure fabrication by pulsed laser deposition in open air: Influence of the deposition geometry. Beilstein J. Nanotechnol. 2017, 8, 2438–2445. [Google Scholar] [CrossRef]
- Nikov, R.G.; Dikovska, A.O.; Nedyalkov, N.N.; Atanasov, P.A.; Atanasova, G.; Hirsch, D.; Rauschenbach, B. ZnO nanostructures produced by pulsed laser deposition in open air. Appl. Phys. A 2017, 123, 657. [Google Scholar] [CrossRef]
- Bonis, A.D.; Teghil, R. Ultra-Short Pulsed Laser Deposition of Oxides, Borides and Carbides of Transition Elements. Coatings 2020, 10, 501. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dikovska, A.; Atanasova, G.; Dilova, T.; Baeva, A.; Avdeev, G.; Atanasov, P.; Nedyalkov, N. Picosecond Pulsed Laser Deposition of Metals and Metal Oxides. Materials 2023, 16, 6364. https://doi.org/10.3390/ma16196364
Dikovska A, Atanasova G, Dilova T, Baeva A, Avdeev G, Atanasov P, Nedyalkov N. Picosecond Pulsed Laser Deposition of Metals and Metal Oxides. Materials. 2023; 16(19):6364. https://doi.org/10.3390/ma16196364
Chicago/Turabian StyleDikovska, Anna, Genoveva Atanasova, Tina Dilova, Aleksandra Baeva, Georgi Avdeev, Petar Atanasov, and Nikolay Nedyalkov. 2023. "Picosecond Pulsed Laser Deposition of Metals and Metal Oxides" Materials 16, no. 19: 6364. https://doi.org/10.3390/ma16196364
APA StyleDikovska, A., Atanasova, G., Dilova, T., Baeva, A., Avdeev, G., Atanasov, P., & Nedyalkov, N. (2023). Picosecond Pulsed Laser Deposition of Metals and Metal Oxides. Materials, 16(19), 6364. https://doi.org/10.3390/ma16196364