Synthesis and Characterization of Doped Magnesium Hydroxide for Medium Heat Storage Application
Abstract
1. Introduction
2. Materials and Methods
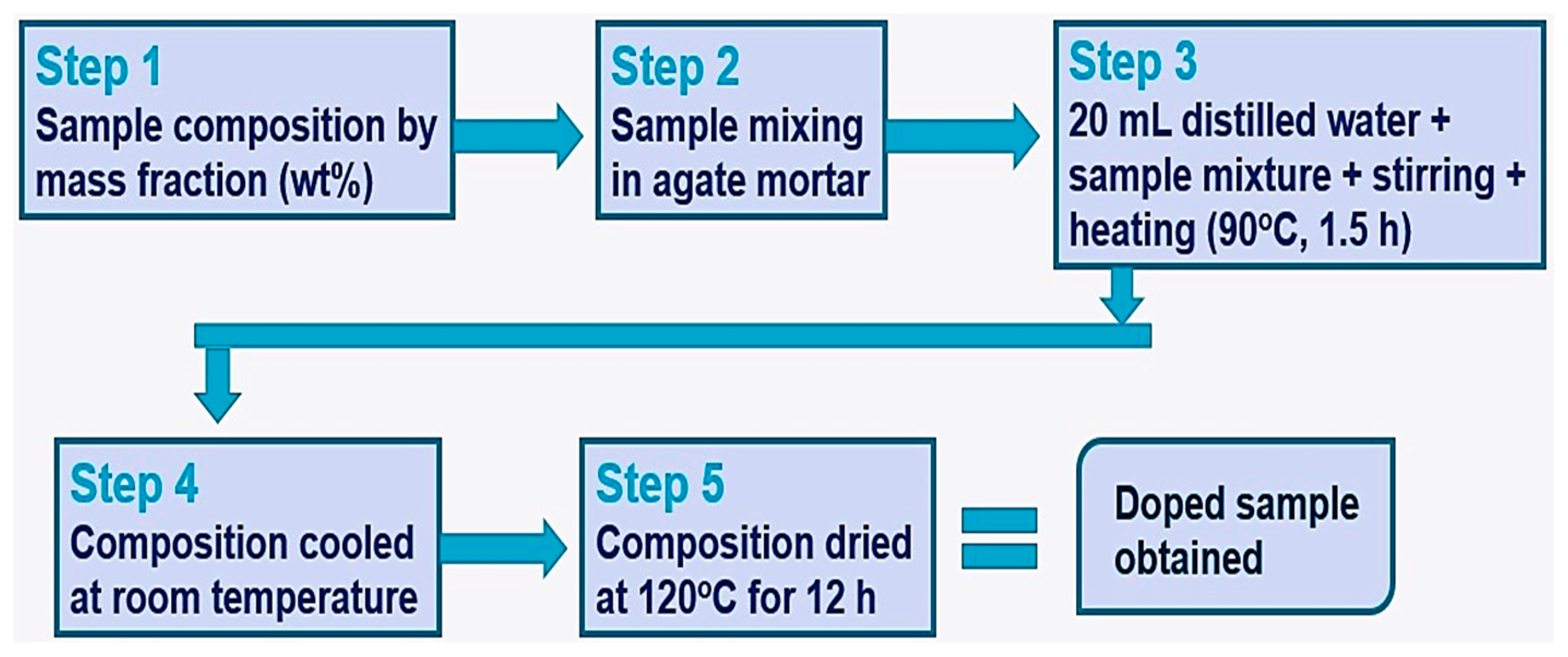
2.1. Material Development
2.2. Material Characterization
2.2.1. Powder X-ray Diffraction
2.2.2. Differential Scanning Calorimetry
2.2.3. Thermogravimetry Analysis
2.2.4. Brunauer–Emmett–Teller Analysis
2.2.5. Scanning Electron Microscopy
2.2.6. Energy-Dispersive X-ray Spectroscopy
3. Results and Discussion
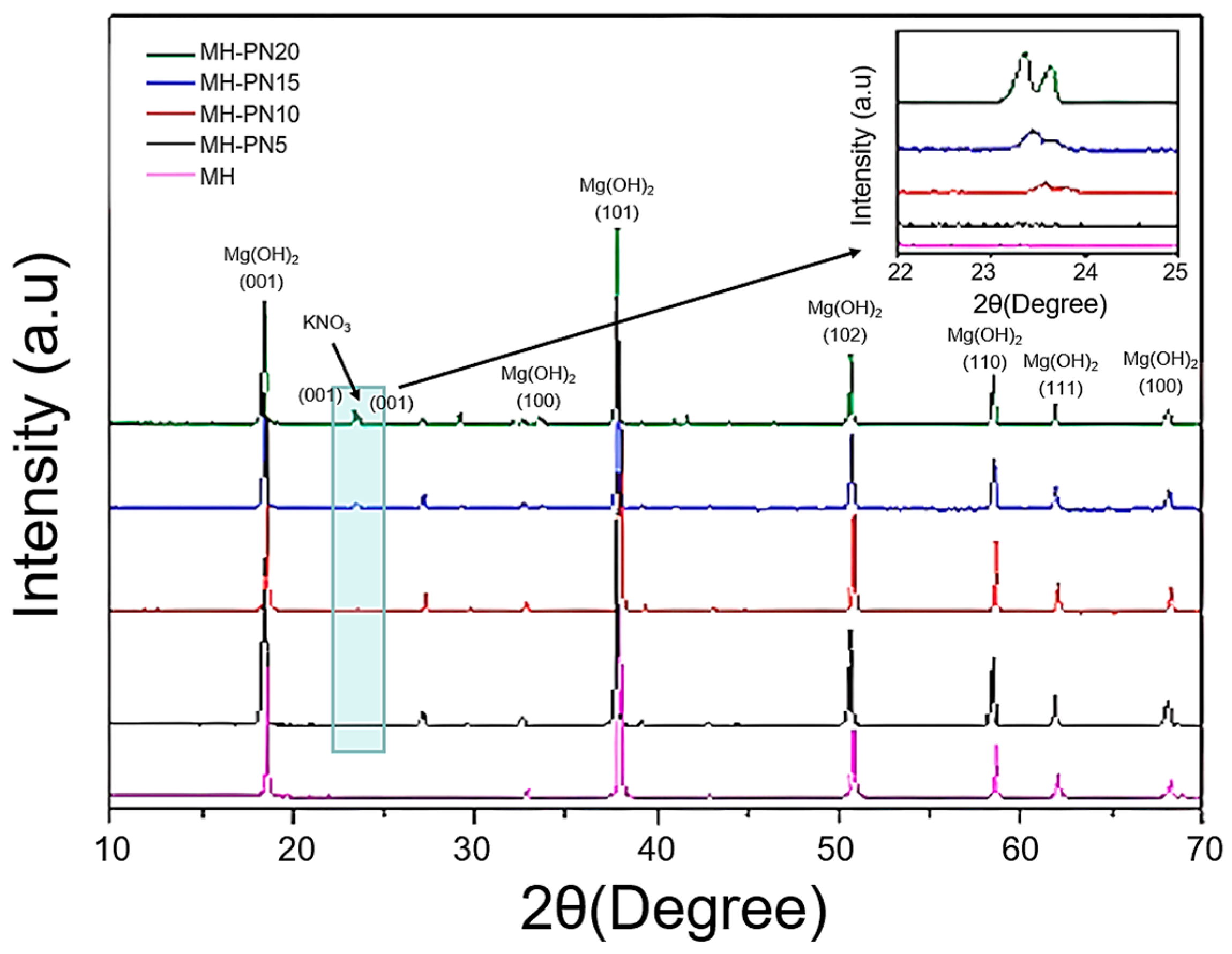
3.1. Powder XRD
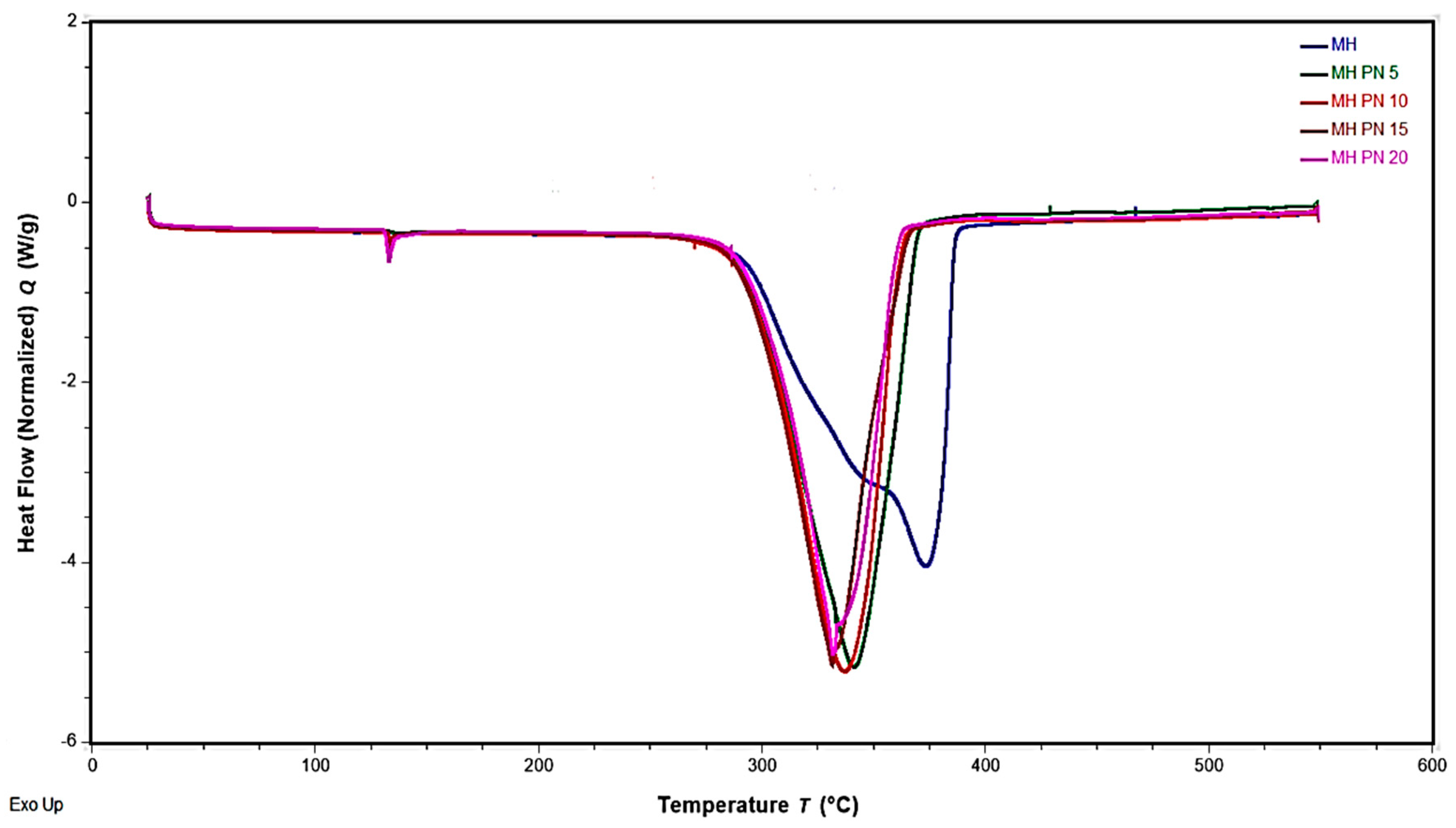
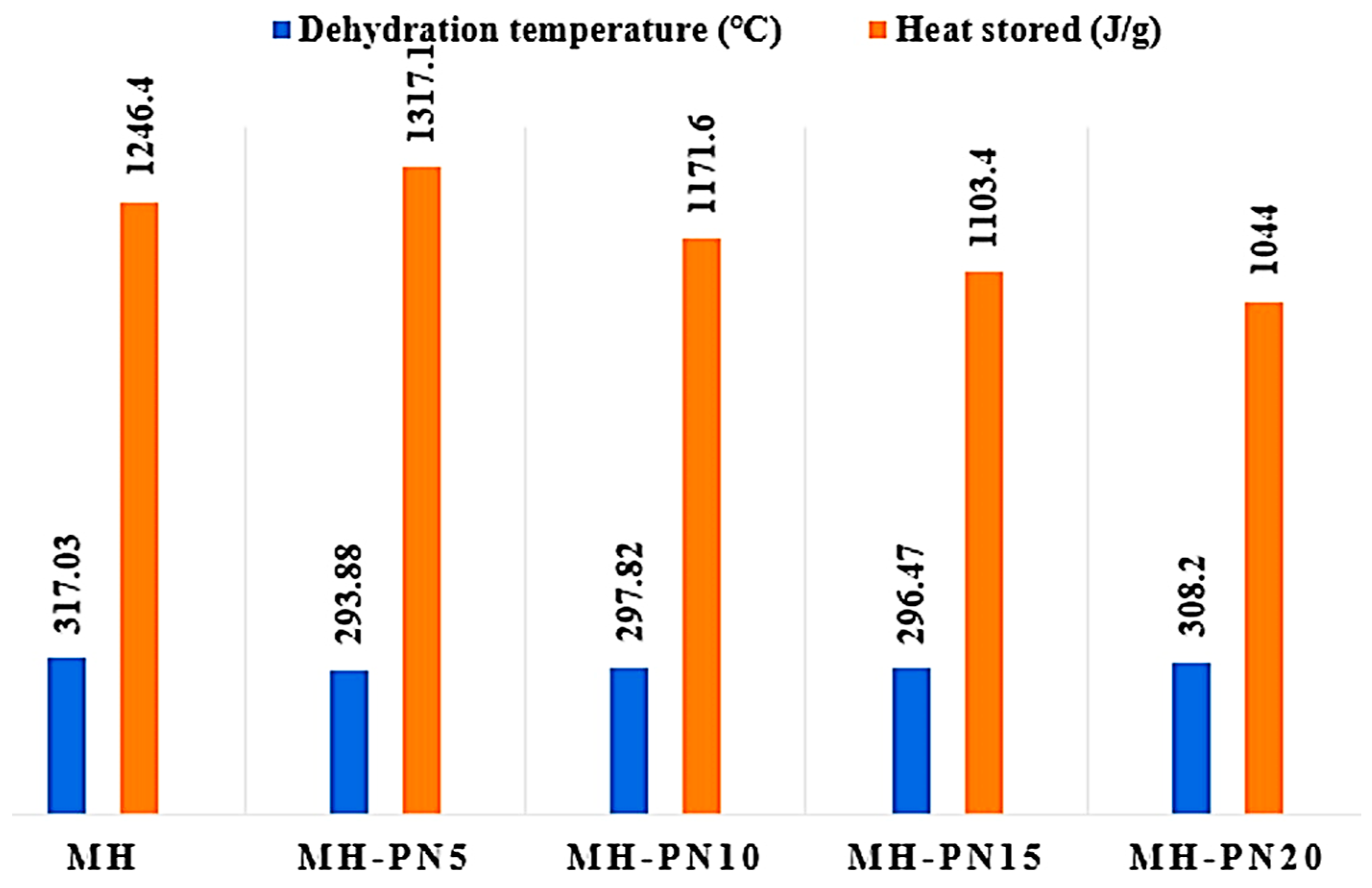
3.2. DSC
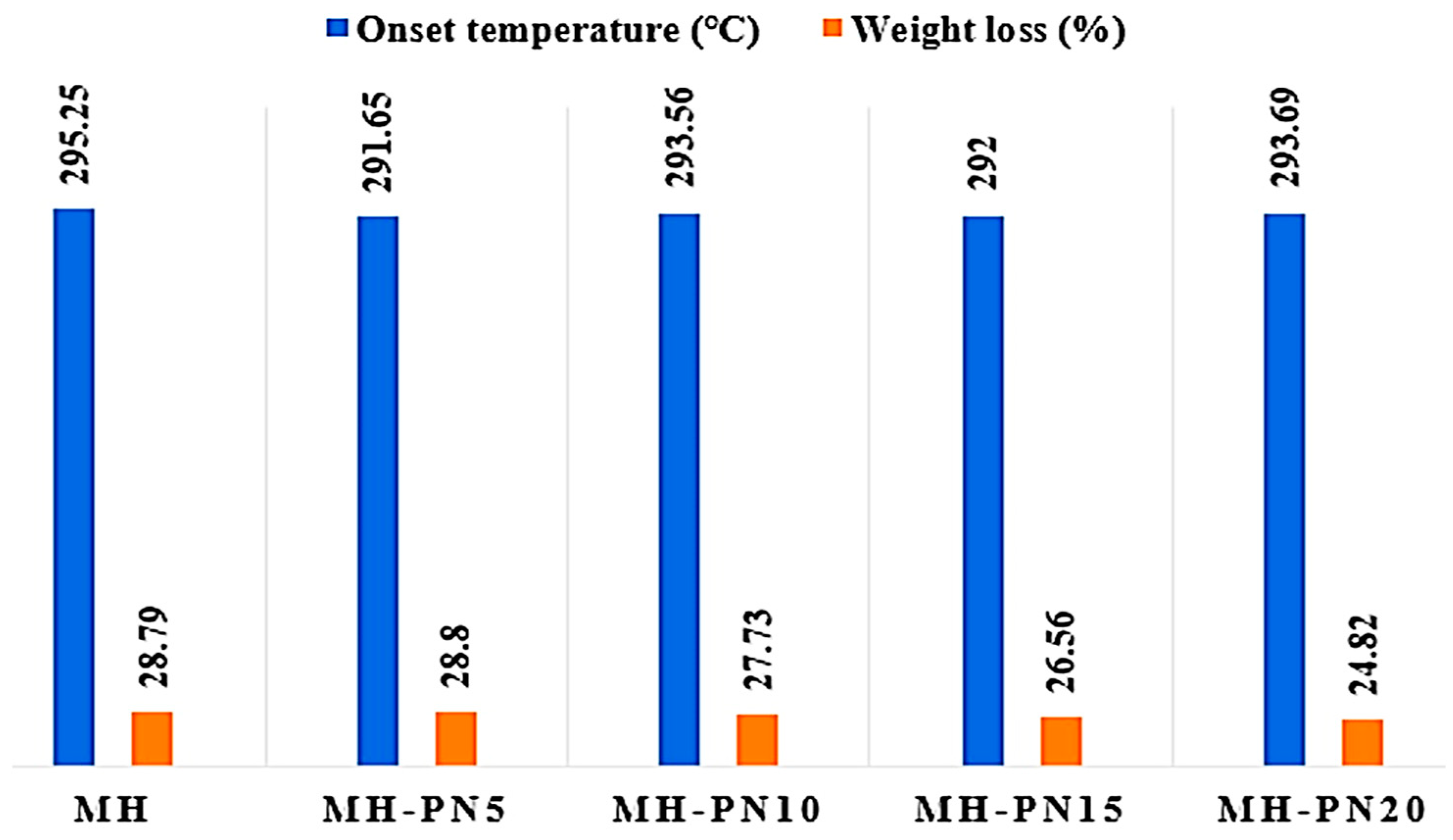
3.3. TGA
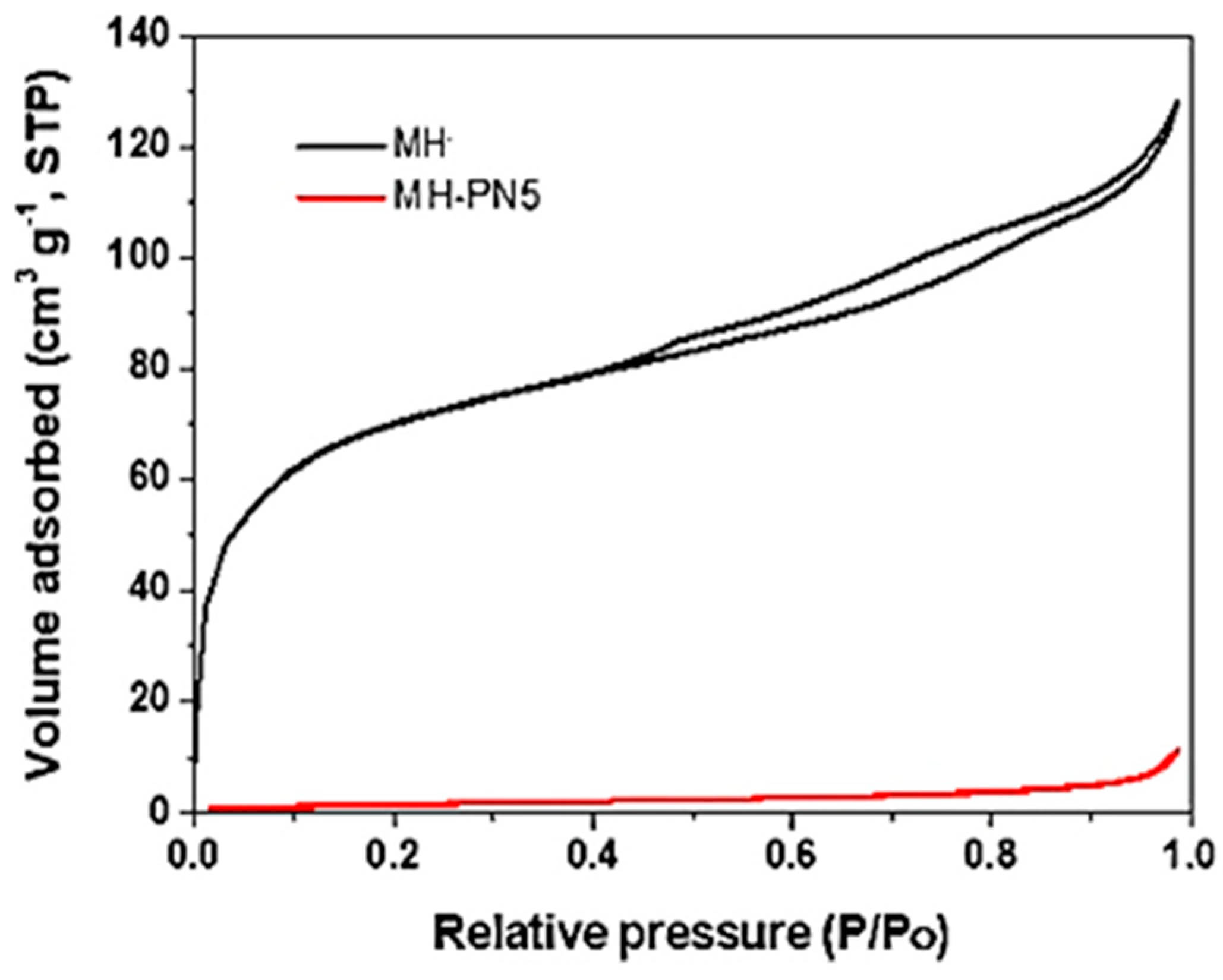
3.4. BET Analysis of MH-PN5
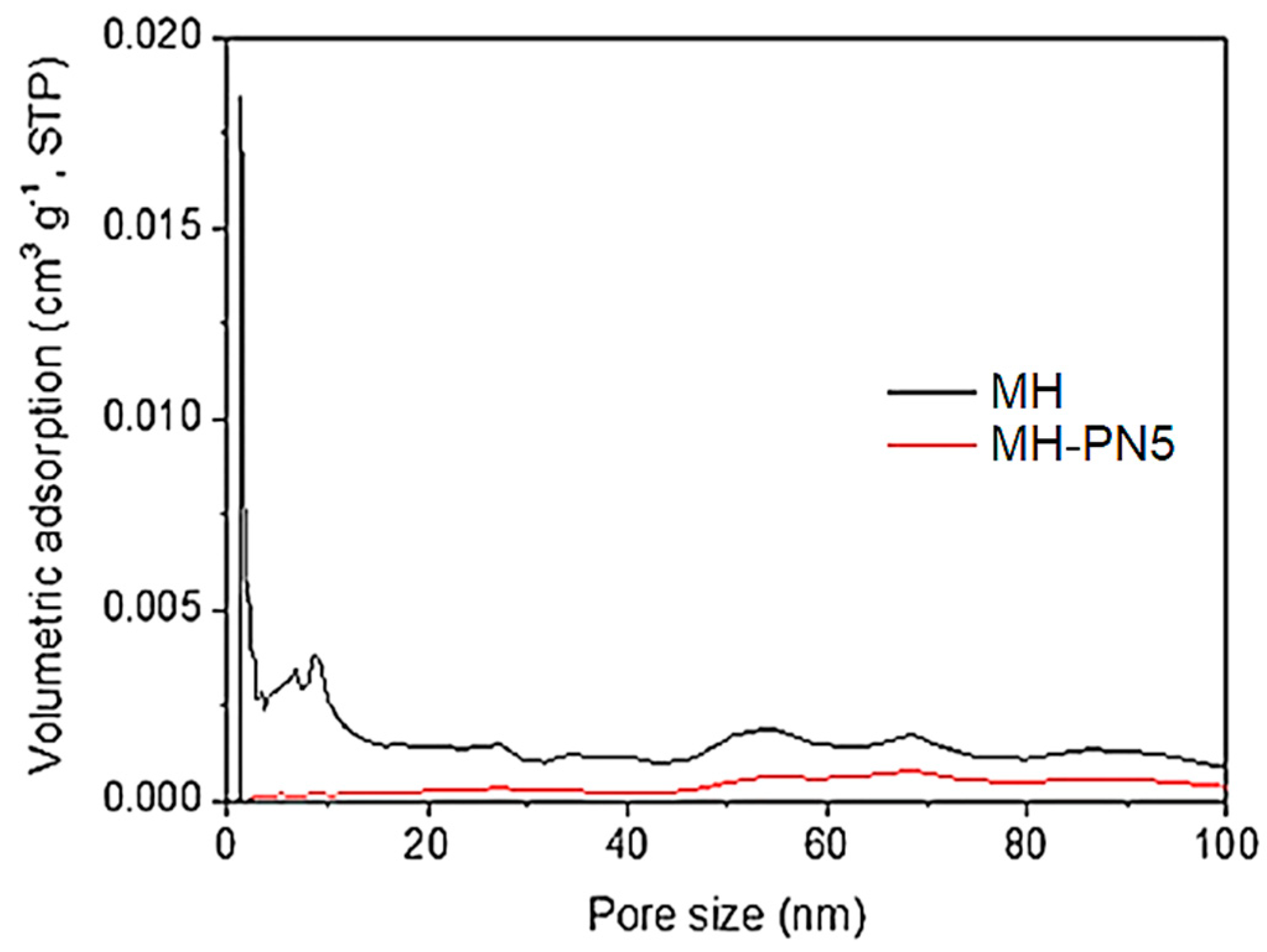
3.5. PSD Analysis of MH-PN5
3.6. SEM Analysis of MH-PN5
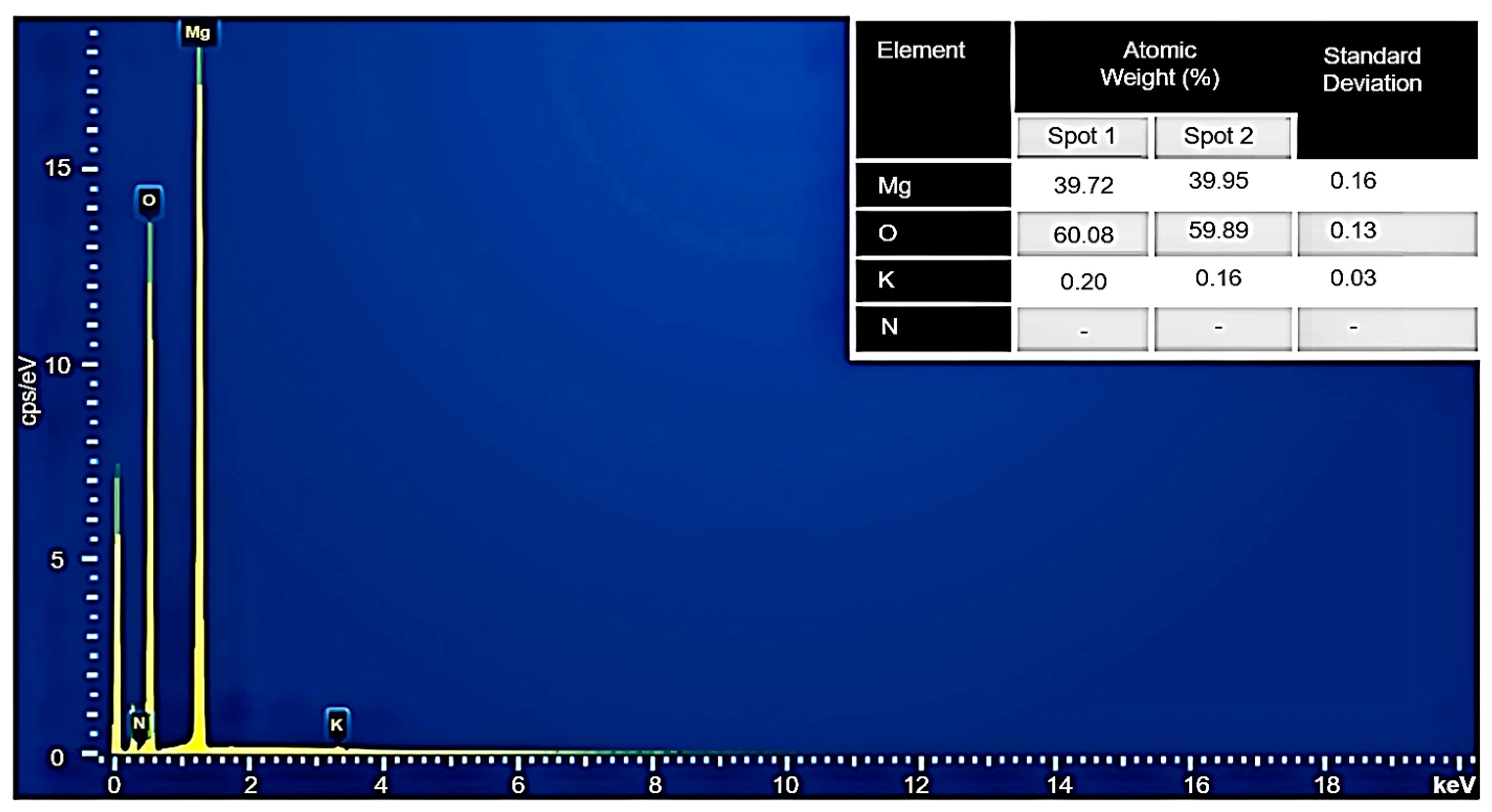
3.7. EDX Analysis of MH-PN5
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ritchie, H.; Roser, M.; Rosado, P. CO2 and Greenhouse Gas Emissions. Available online: https://ourworldindata.org/co2-and-greenhouse-gas-emissions (accessed on 20 February 2023).
- Adams, M.; Burrows, V.; Richardson, S. Bringing Embodied Carbon Upfront. Available online: https://worldgbc.s3.eu-west-2.amazonaws.com/wp-content/uploads/2022/09/22123951/WorldGBC_Bringing_Embodied_Carbon_Upfront.pdf (accessed on 20 February 2023).
- Watson, S.D.; Lomas, K.J.; Buswell, R.A. Decarbonising Domestic Heating: What Is the Peak GB Demand? Energy Policy 2019, 126, 533–544. [Google Scholar] [CrossRef]
- Stevenson, P.; Hyde, R. The Potential for Recovering and Using Surplus Heat from Industry. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/294900/element_energy_et_al_potential_for_recovering_and_using_surplus_heat_from_industry.pdf (accessed on 20 February 2023).
- Sadeghi, G. Energy Storage on Demand: Thermal Energy Storage Development, Materials, Design, and Integration Challenges. Energy Storage Mater. 2022, 46, 192–222. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Qi, C.; Ling, X.; Peng, H. State of the Art on the High-Temperature Thermochemical Energy Storage Systems. Energy Convers. Manag. 2018, 177, 792–815. [Google Scholar] [CrossRef]
- Srivastava, N.C.; Eames, I.W. A Review of Adsorbents and Adsorbates in Solid-Vapour Adsorption Heat Pump Systems. Appl. Therm. Eng. 1998, 18, 707–714. [Google Scholar] [CrossRef]
- Hongois, S.; Kuznik, F.; Stevens, P.; Roux, J.J. Development and Characterisation of a New MgSO4-Zeolite Composite for Long-Term Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2011, 95, 1831–1837. [Google Scholar] [CrossRef]
- Felderhoff, M.; Bogdanović, B. High Temperature Metal Hydrides as Heat Storage Materials for Solar and Related Applications. Int. J. Mol. Sci. 2009, 10, 325–344. [Google Scholar] [CrossRef] [PubMed]
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A Review on High Temperature Thermochemical Heat Energy Storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef]
- Papapetrou, M.; Kosmadakis, G.; Cipollina, A.; La Commare, U.; Micale, G. Industrial Waste Heat: Estimation of the Technically Available Resource in the EU per Industrial Sector, Temperature Level and Country. Appl. Therm. Eng. 2018, 138, 207–216. [Google Scholar] [CrossRef]
- Kato, Y.; Yamashita, N.; Yoshizawa, Y. Study of Chemical Heat Pump with Reaction System of Magnesium Oxide/Water. Jpn. Sci. Technol. Inf. Aggregator Electron. 1993, 19, 1213–1216. [Google Scholar] [CrossRef][Green Version]
- Kato, Y.; Takahashi, F.; Watanabe, A.; Yoshizawa, Y. Thermal Analysis of a Magnesium Oxide/Water Chemical Heat Pump for Cogeneration. Appl. Therm. Eng. 2001, 21, 1067–1081. [Google Scholar] [CrossRef]
- Ryu, J.; Hirao, N.; Takahashi, R.; Kato, Y. Dehydration Behavior of Metal-Salt-Added Magnesium Hydroxide as Chemical Heat Storage Media. Chem. Lett. 2008, 37, 1140–1141. [Google Scholar] [CrossRef]
- Shkatulov, A.; Aristov, Y. Modification of Magnesium and Calcium Hydroxides with Salts: An Efficient Way to Advanced Materials for Storage of Middle-Temperature Heat. Energy 2015, 85, 667–676. [Google Scholar] [CrossRef]
- Shkatulov, A.; Krieger, T.; Zaikovskii, V.; Chesalov, Y.; Aristov, Y. Doping Magnesium Hydroxide with Sodium Nitrate: A New Approach to Tune the Dehydration Reactivity of Heat-Storage Materials. ACS Appl. Mater. Interfaces 2014, 6, 19966–19977. [Google Scholar] [CrossRef] [PubMed]
- Shkatulov, A.I.; Aristov, Y. Thermochemical Energy Storage Using LiNO3-Doped Mg(OH)2: A Dehydration Study. Energy Technol. 2018, 6, 1844–1851. [Google Scholar] [CrossRef]
- Sun, L.; Wu, Q.; Zhang, L.; Li, Y.; Li, M.; Gao, T.; Guo, S.; Wang, D. Doping Magnesium Hydroxide with Ce(NO3)3: A Promising Candidate Thermochemical Energy Storage Materials. IOP Conf. Ser. Earth Environ. Sci. 2019, 295, 032068. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Tan, T.; Nie, J.; Zhang, H. Optimization of LiNO3–Mg(OH)2 Composites as Thermo-Chemical Energy Storage Materials. J. Environ. Manag. 2020, 262, 110258. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, N.; Yamashita, S.; Fukuda, Y.; Nakazawa, Y.; Konno, T. Mobility of Hydrated Alkali Metal Ions in Metallosupramolecular Ionic Crystals. Chem. Sci. 2019, 10, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Shkatulov, A.; Aristov, Y. Calcium Hydroxide Doped by KNO3 as a Promising Candidate for Thermochemical Storage of Solar Heat. RSC Adv. 2017, 7, 42929–42939. [Google Scholar] [CrossRef]
- Daud, F.D.M.; Vignesh, K.; Sreekantan, S.; Mohamed, A.R. Improved CO2 Adsorption Capacity and Cyclic Stability of CaO Sorbents Incorporated with MgO. New J. Chem. 2016, 40, 231–237. [Google Scholar] [CrossRef]


| Sample | SBET (m2/g) |
|---|---|
| MH | 250 |
| MH-PN5 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albeladi, N.; Kur, A.; Mokaya, R.; Darkwa, J.; Roger-Lund, S.; Worall, M.; Calautit, J.; Boukhanouf, R. Synthesis and Characterization of Doped Magnesium Hydroxide for Medium Heat Storage Application. Materials 2023, 16, 6296. https://doi.org/10.3390/ma16186296
Albeladi N, Kur A, Mokaya R, Darkwa J, Roger-Lund S, Worall M, Calautit J, Boukhanouf R. Synthesis and Characterization of Doped Magnesium Hydroxide for Medium Heat Storage Application. Materials. 2023; 16(18):6296. https://doi.org/10.3390/ma16186296
Chicago/Turabian StyleAlbeladi, Nawaf, Anti Kur, Robert Mokaya, Jo Darkwa, Sarah Roger-Lund, Mark Worall, John Calautit, and Rabah Boukhanouf. 2023. "Synthesis and Characterization of Doped Magnesium Hydroxide for Medium Heat Storage Application" Materials 16, no. 18: 6296. https://doi.org/10.3390/ma16186296
APA StyleAlbeladi, N., Kur, A., Mokaya, R., Darkwa, J., Roger-Lund, S., Worall, M., Calautit, J., & Boukhanouf, R. (2023). Synthesis and Characterization of Doped Magnesium Hydroxide for Medium Heat Storage Application. Materials, 16(18), 6296. https://doi.org/10.3390/ma16186296








