Palladium–Copper Bimetallic Aerogel as New Modifier for Highly Sensitive Determination of Bisphenol A in Real Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Solutions
2.2. Synthesis of Pd-Cu Aerogel
2.3. Preparation of Modified Electrode
2.4. Instrumentation
3. Results and Discussion
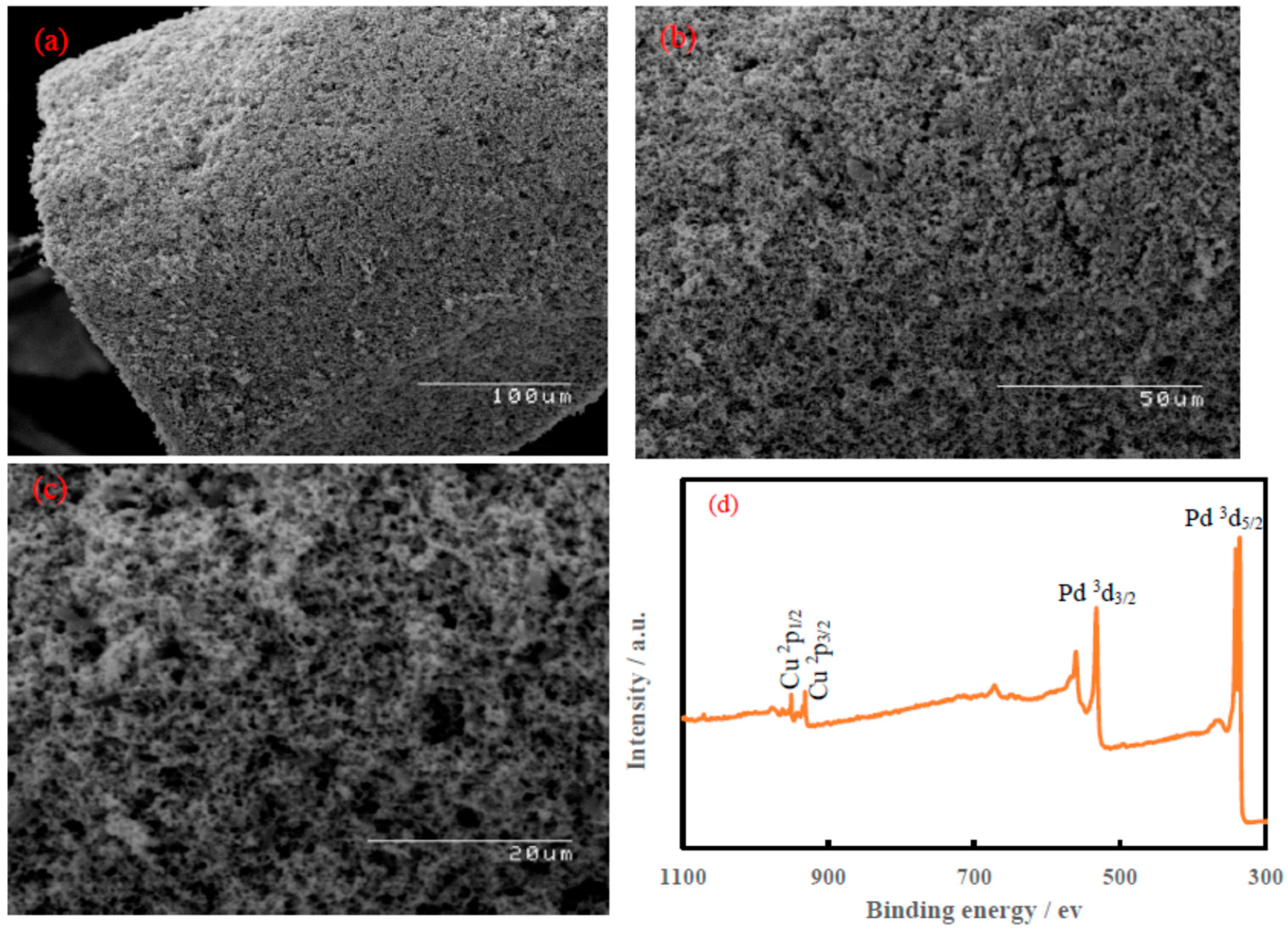
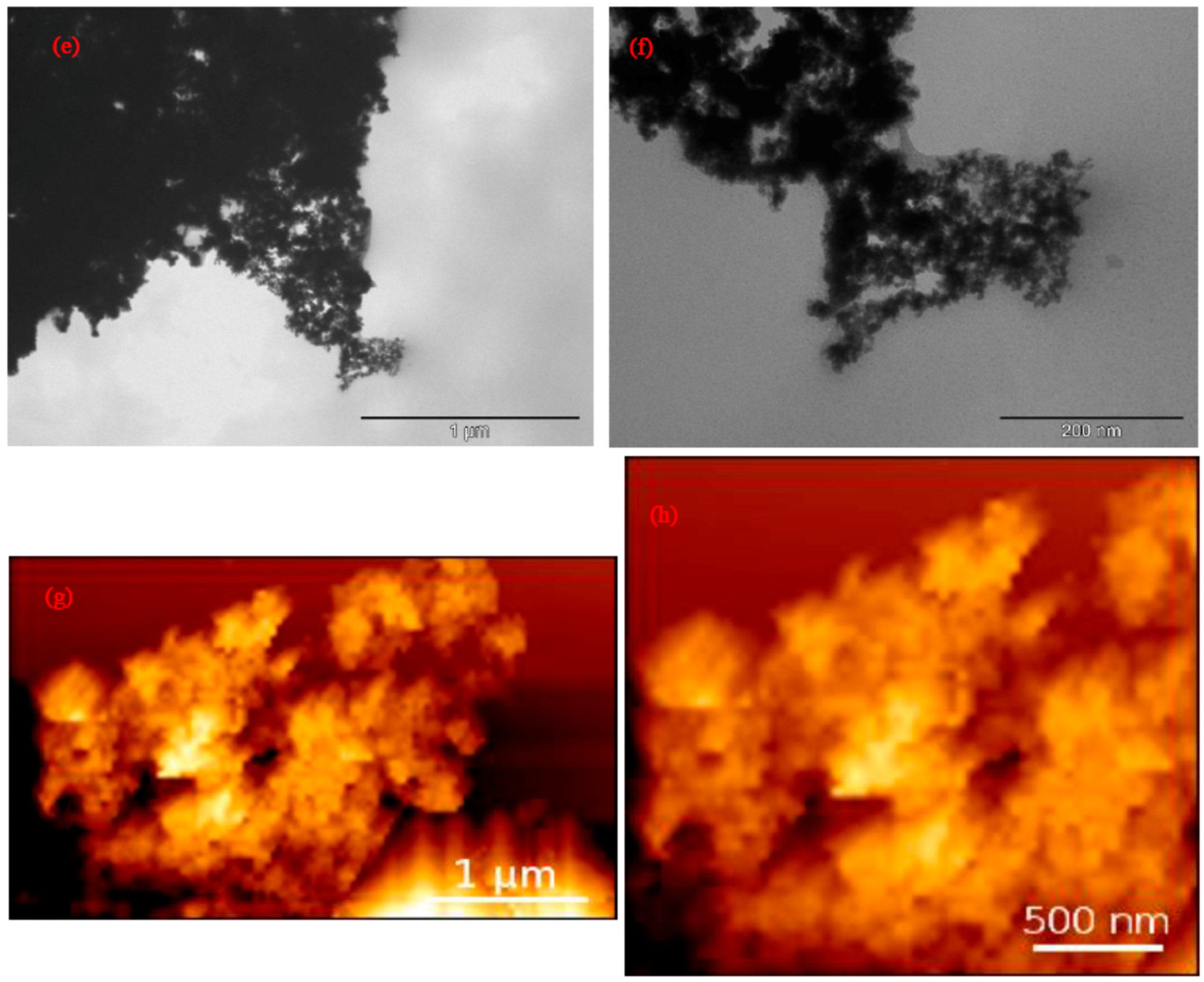
3.1. Characterization
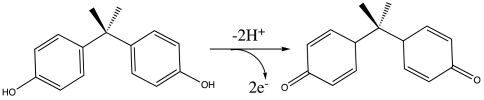
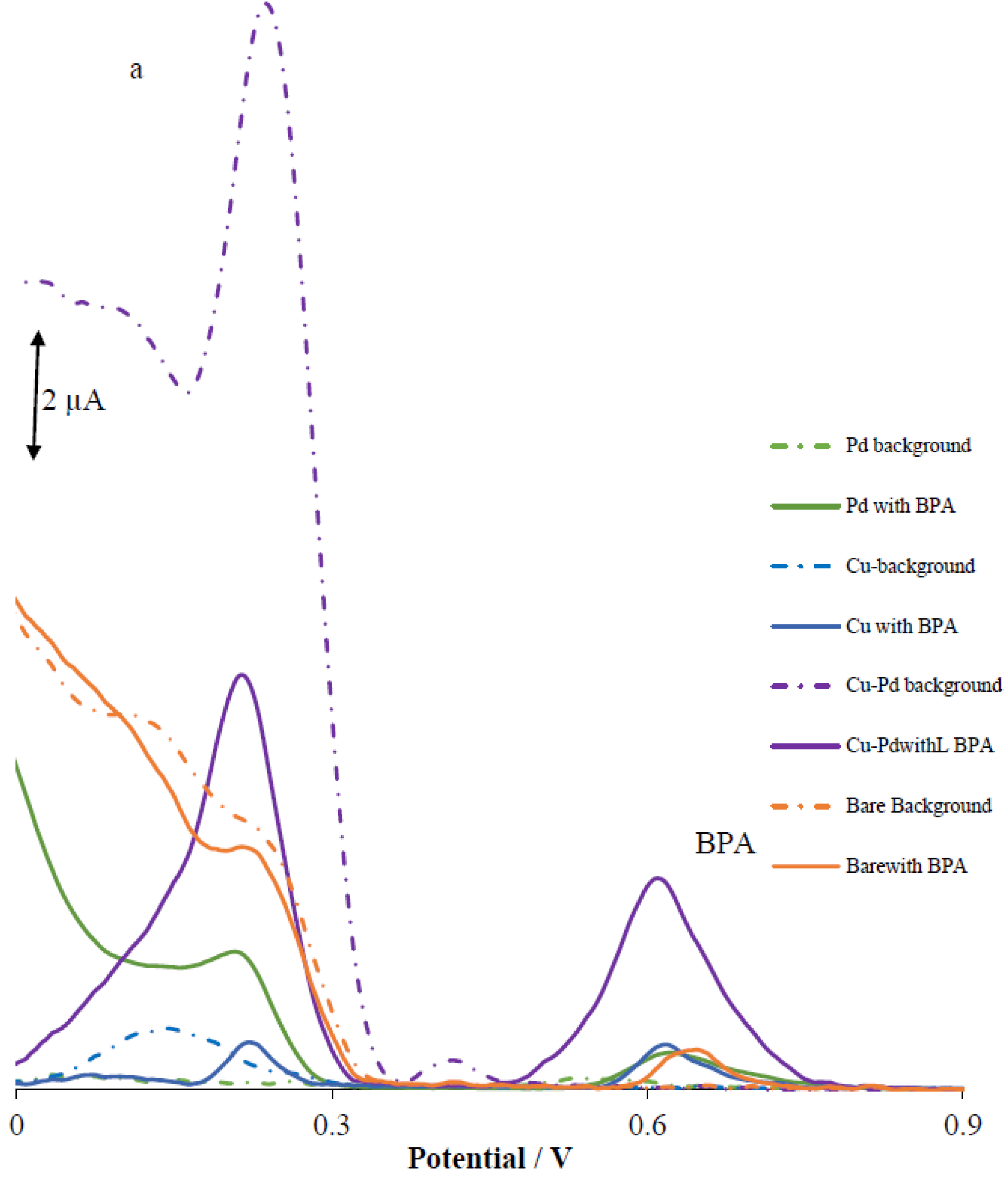
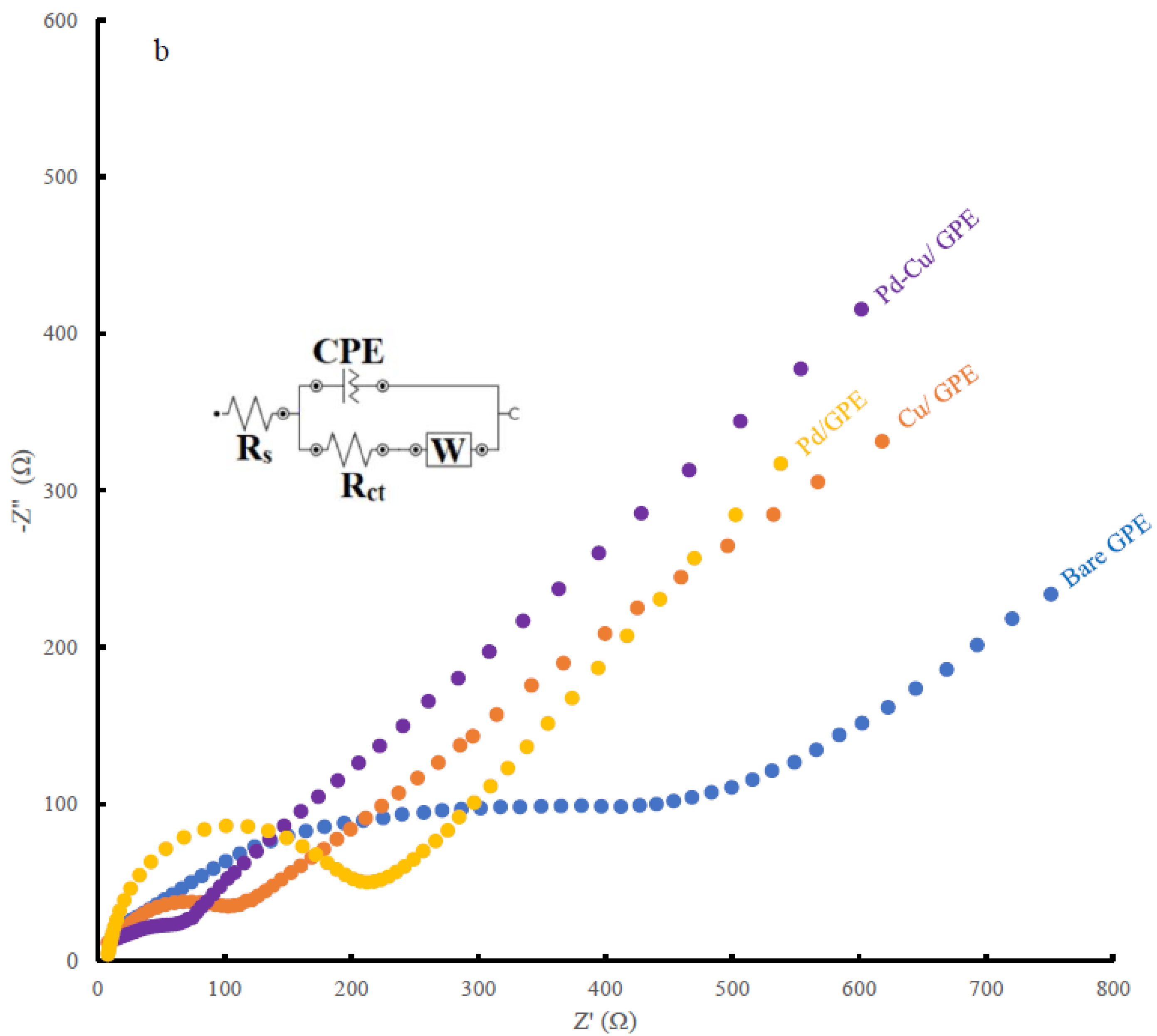
3.2. Analytical Performance of the Modified Electrodes
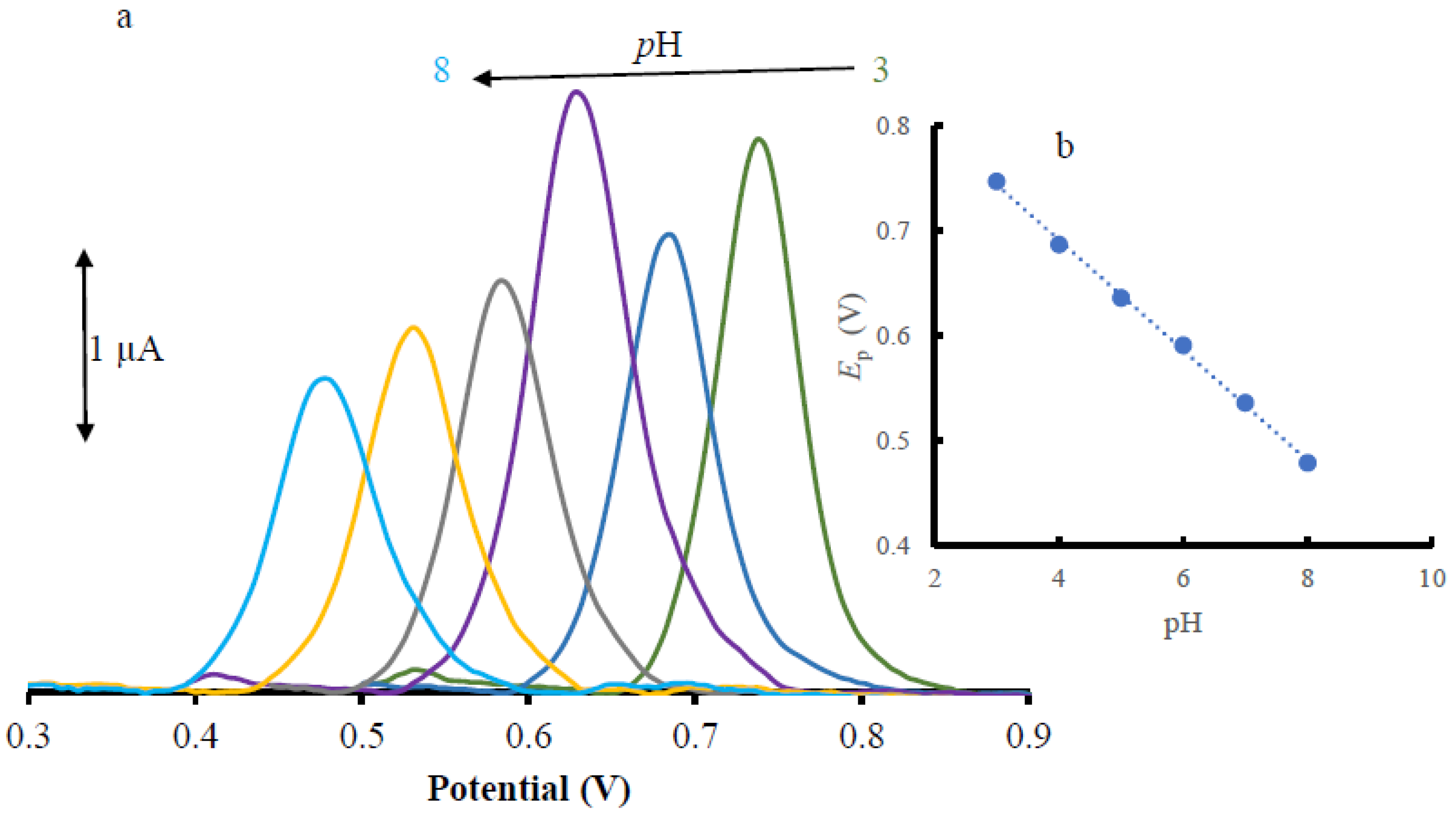
3.3. pH Study
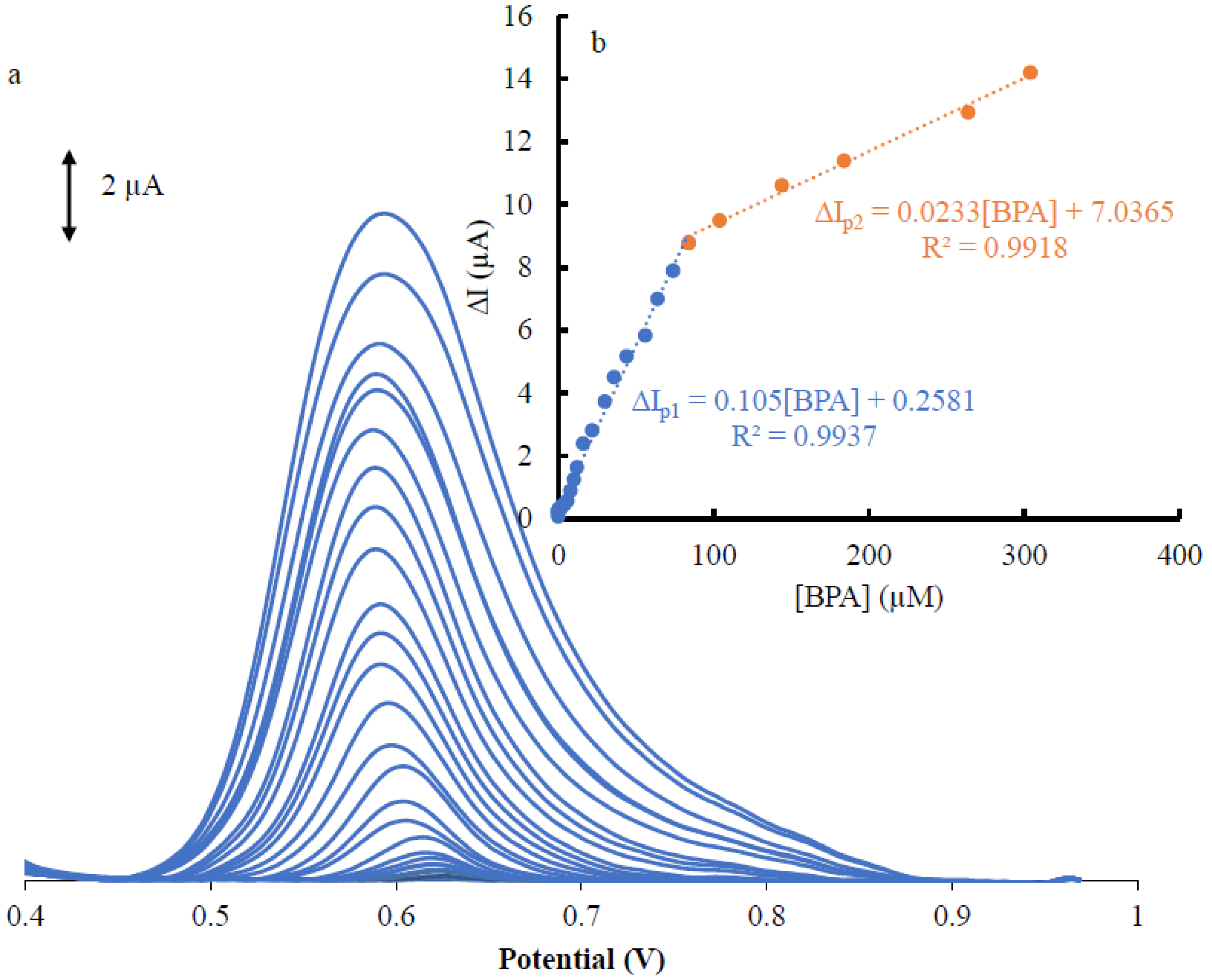
3.4. Calibration Curve
3.5. Real Sample
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almeida, S.; Raposo, A.; Almeida-González, M.; Carrascosa, C. Bisphenol A: Food Exposure and Impact on Human Health. Comp. Food Sci. Food Saf. 2018, 17, 1503–1517. [Google Scholar] [CrossRef] [PubMed]
- Gewurtz, S.B.; Tardif, G.; Power, M.; Backus, S.M.; Dove, A.; Dubé-Roberge, K.; Garron, C.; King, M.; Lalonde, B.; Letcher, R.J.; et al. Bisphenol A in the Canadian environment: A multimedia analysis. Sci. Total Environ. 2021, 755, 142472. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Neogi, S.; De, S. Mechanistic investigation of photocatalytic degradation of Bisphenol-A using MIL-88A(Fe)/MoS2 Z-scheme heterojunction composite assisted peroxymonosulfate activation. Chem. Eng. J. 2022, 428, 131028. [Google Scholar] [CrossRef]
- Abraham, A.; Chakraborty, P. A review on sources and health impacts of bisphenol A. Environ. Health 2020, 35, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Wang, L.; Zhou, Q.; Huang, X. Hazards of bisphenol A (BPA) exposure: A systematic review of plant toxicology studies. J. Hazard. Mater. 2020, 384, 121488. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.; et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef] [PubMed]
- Czarny, K.; Krawczyk, B.; Szczukocki, D. Toxic effects of bisphenol A and its analogues on cyanobacteria Anabaena variabilis and Microcystis aeruginosa. Chemosphere 2021, 263, 128299. [Google Scholar] [CrossRef] [PubMed]
- Tarafdar, A.; Sirohi, R.; Balakumaran, P.A.; Reshmy, R.; Madhavan, A.; Sindhu, R.; Binod, P.; Kumar, Y.; Kumar, D.; Sim, S.J. The hazardous threat of Bisphenol A: Toxicity, detection and remediation. J. Hazard. Mater. 2022, 423, 127097. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Zhang, K.; Le, Q.V.; Jang, H.W.; Kim, S.Y.; Shokouhimehr, M. Recent Advances in Electrochemical Sensors and Biosensors for Detecting Bisphenol A. Sensors 2020, 20, 3364. [Google Scholar] [CrossRef]
- Vilarinho, F.; Sendón, R.; van der Kellen, A.; Vaz, M.; Silva, A.S. Bisphenol A in food as a result of its migration from food packaging. Trends Food Sci. Technol. 2019, 91, 33–65. [Google Scholar] [CrossRef]
- Cimmino, I.; Fiory, F.; Perruolo, G.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. Potential Mechanisms of Bisphenol A (BPA) Contributing to Human Disease. Int. J. Mol. Sci. 2020, 21, 5761. [Google Scholar] [CrossRef] [PubMed]
- Geens, T.; Aerts, D.; Berthot, C.; Bourguignon, J.P.; Goeyens, L.; Lecomte, P.; Maghuin-Rogister, G.; Pironnet, A.; Pussemier, L.; Scippo, M.; et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.R.; Noroozifar, M.; Kerman, K. Review—Nanocomposite-Based Sensors for Voltammetric Detection of Hazardous Phenolic Pollutants in Water. J. Electrochem. Soc. 2020, 167, 037568. [Google Scholar] [CrossRef]
- Duenas-Moreno, J.; Mora, A.; Cervantes-Aviles, P.; Mahlknecht, J. Groundwater contamination pathways of phthalates and bisphenol A: Origin, characteristics, transport, and fate—A review. Environ. Int. 2022, 170, 107550. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Kang, L.; Xiang, X.; Li, H.; Luo, X.; Luo, R.; Lu, C.; Peng, X. Recent advances and progress in the detection of bisphenol A. Anal. Bioanal. Chem. 2016, 408, 6913–6927. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.H.; Huang, Y.Y.; Wang, L.; Shen, X.F.; Wang, Y.Y. Determination of bisphenol A and bisphenol S by a covalent organic framework electrochemical sensor. Environ. Pollut. 2020, 263, 114616. [Google Scholar]
- Chiriac, F.L.; Paun, I.; Pirvu, F.; Iancu, V.; Galaon, T. Fast and Sensitive LC-MS Detection of Bisphenol A and Butylhydroxyanisole in WWTP Sewage Sludge. Rev. Chim. 2019, 70, 2123–2127. [Google Scholar] [CrossRef]
- Yazdinezhad, S.R.; Ballesteros-Gómez, A.; Lunar, L.; Rubio, S. Single-step extraction and cleanup of bisphenol A in soft drinks by hemimicellar magnetic solid phase extraction prior to liquid chromatography/tandem mass spectrometry. Anal. Chim. Acta 2013, 778, 31–37. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Q.; Yan, X.; Liao, C.; Jiang, G. Occurrence, fate and risk assessment of BPA and its substituents in wastewater treatment plant: A review. Environ. Res. 2019, 178, 108732. [Google Scholar] [CrossRef]
- Alizadeh, T.; Hamidi, N.; Ganjali, M.R.; Rafiei, F. Determination of subnanomolar levels of mercury (II) by using a graphite paste electrode modified with MWCNTs and Hg(II)-imprinted polymer nanoparticles. Microchim. Acta 2018, 185, 16. [Google Scholar] [CrossRef]
- Charithra, M.M.; Manjunatha, J.G.G.; Raril, C. Surfactant Modified Graphite Paste Electrode as an Electrochemical Sensor for the Enhanced Voltammetric Detection of Estriol with Dopamine and Uric acid. Adv. Pharm. Bull. 2020, 10, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Douk, A.S.; Saravani, H.; Noroozifar, M.; Kim, K.H. Tuning the morphology of Pd aerogels for advanced electrocatalysis of formic acid. Microporous Mesoporous Mater. 2022, 344, 112206. [Google Scholar] [CrossRef]
- Yazdan-Abad, M.Z.; Noroozifar, M.; Douk, A.S.; Modarresi-Alam, A.R.; Saravani, H. Shape engineering of palladium aerogels assembled by nanosheets to achieve a high performance electrocatalyst. Appl. Catal. B Environ. 2019, 250, 242–249. [Google Scholar] [CrossRef]
- Lin, F.; Jiang, X.; Boreriboon, N.; Wang, Z.; Song, C.; Cen, K. Effects of supports on bimetallic Pd-Cu catalysts for CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2019, 585, 117210. [Google Scholar] [CrossRef]
- Ghouri, Z.K.; Barakat, N.A.M.; Kim, H.Y. Influence of copper content on the electrocatalytic activity toward methanol oxidation of CoχCuy alloy nanoparticles-decorated CNFs. Sci. Rep. 2015, 5, 16695. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Jeon, J.; Toyao, T.; Shimizu, K.I.; Furukawa, S. A Cu–Pd single-atom alloy catalyst for highly efficient NO reduction. Chem. Sci. 2019, 10, 8292–8298. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, G.; Asif, M.; Aziz, A.; Wang, Z.; Qiu, X.; Huang, Q.; Xiao, F.; Liu, H. Nanocomposites consisting of copper and copper oxide incorporated into MoS4 nanostructures for sensitive voltammetric determination of bisphenol A. Microchim. Acta 2019, 186, 337. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; ALOthman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud. Univ. Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Mo, F.; Xie, J.; Wu, T.; Liu, M.; Zhang, Y.; Yao, S. A sensitive electrochemical sensor for bisphenol A on the basis of the AuPd incorporated carboxylic multi-walled carbon nanotubes. Food Chem. 2019, 292, 253–259. [Google Scholar] [CrossRef]
- Douk, A.S.; Saravani, H.; Yazdan Abad, M.Z.; Noroozifar, M. Three-Dimensional Engineering of Nanoparticles To Fabricate a Pd-Au Aerogel as an Advanced Supportless Electrocatalyst for Low-Temperature Direct Ethanol Fuel Cells. ACS Appl. Energy Mater. 2020, 3, 7527–7534. [Google Scholar] [CrossRef]
- Douk, A.S.; Saravani, H.; Yazdan Abad, M.Z.; Noroozifar, M. Controlled organization of building blocks to prepare three-dimensional architecture of Pd–Ag aerogel as a high active electrocatalyst toward formic acid oxidation. Compos. Part B Eng. 2019, 172, 309–315. [Google Scholar] [CrossRef]
- UNE-EN 13130-13; Materials and Articles in Contact with Foodstuffs—Plastics Substances Subject to Limitation. Part 13: Determination of 2,2-Bis(4-Hydroxyphenyl)propane (Bisphenol A) in Food Simulants. European Standards: Brussels, Belgium, 2005.
- Venezia, A.M.; Rossi, A.; Duca, D.; Martorana, A.; Deganello, G. Particle size and metal-support interaction effects in pumice supported palladium catalysts. Appl. Catal. A Gen. 1995, 125, 113–128. [Google Scholar] [CrossRef]
- Swadzba-Kwasny, M.; Chancelier, L.; Ng, S.; Manyar, H.G.; Hardacre, C.; Nockemann, P. Facile in situ synthesis of nanofluids based on ionic liquids and copper oxide clusters and nanoparticles. Dalton Trans. 2012, 41, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.S.; Sun, C.; Zhou, Y.B.; Yang, X.D.; Yang, W.B. A facial electrochemical approach to determinate bisphenol A based on graphene-hypercrosslinked resin MN202 composite. Food Chem. 2014, 158, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, X.; Chen, L. An ultrasensitive electrochemical bisphenol A sensor based on hierarchical Ce-metal-organic framework modified with cetyltrimethylammonium bromide. Sens. Actuators B Chem. 2018, 261, 425–433. [Google Scholar] [CrossRef]
- Patel, B.R.; Hassan, Q.; Noroozifar, M.; Kerman, K. Simultaneous Square Wave Voltammetric Detection of Endocrine-Disrupting Agents Using a Nanocomposite of Magnetic Fe3O4 Nanorods and Poly(3,4-Methylenedioxy)aniline. ACS Sustain. Chem. Eng. 2020, 8, 15108–15119. [Google Scholar] [CrossRef]
- Noroozifar, M.; Khorasani-Motlagh, M.; Jahromi, F.Z.; Rostami, S. Sensitive and selective determination of uric acid in real samples by modified glassy carbon electrode with holmium fluoride nanoparticles/multi-walled carbon nanotube as a new biosensor. Sens. Actuators B Chem. 2013, 188, 65–72. [Google Scholar] [CrossRef]
- Santana, E.R.; Lima, C.A.D.; Piovesan, J.V.; Spinelli, A. An original ferro ferric oxide and gold nanoparticles-modified glassy carbon electrode for the determination of bisphenol A. Sens. Actuators B Chem. 2017, 240, 487–496. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Huang, C.; Jia, N. MWCNTs-PEI composites-based electrochemical sensor for sensitive detection of bisphenol A. Sens. Actuators B Chem. 2016, 235, 408–413. [Google Scholar] [CrossRef]
- Zhan, T.R.; Song, Y.; Tan, Z.W.; Hou, W.G. Electrochemical bisphenol A sensor based on exfoliated Ni2Al-layered double hydroxide nanosheets modified electrode. Sens. Actuators B Chem. 2017, 238, 962–971. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Wang, W.C.; Chen, Z.D. Electrochemical sensing of bisphenol A by a didodecyldimethylammonium bromide-modified expanded graphite paste electrode. J. AOAC Int. 2016, 99, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Z.; Yang, L.H.; Li, S.H.; Dang, Y. A novel electrochemical sensor for highly sensitive detection of bisphenol A based on the hydrothermal synthesized Na-doped WO3 nanorods. Sens. Actuators B 2017, 245, 238–246. [Google Scholar] [CrossRef]
- Allen, J.; Bard, L.R.F. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons Inc.: New York, NY, USA; Chichester, UK; Weinheim, Germany; Brisbane, Australia; Singapore; Toronto, ON, Canada, 2020. [Google Scholar]
- Zare, H.R.; Ghanbari, Z.; Nasirizadeh, N.; Benvidi, A. Simultaneous determination of adrenaline, uric acid, and cysteine using bifunctional electrocatalyst of ruthenium oxide nanoparticles. Comptes Rendus Chim. 2013, 16, 287–295. [Google Scholar] [CrossRef]
- Koyun, O.; Gorduk, S.; Gencten, M.; Sahin, Y. A novel copper(ıı) phthalocyanine-modified multiwalled carbon nanotube-based electrode for sensitive electrochemical detection of bisphenol A. New J. Chem. 2019, 43, 85–92. [Google Scholar] [CrossRef]
- Kim, M.; Song, Y.E.; Xiong, J.Q.; Kim, K.Y.; Jang, M.; Jeon, B.H.; Kim, J.R. Electrochemical detection and simultaneous removal of endocrine disruptor, bisphenol A using a carbon felt electrode. J. Electroanal. Chem. 2021, 880, 114907. [Google Scholar] [CrossRef]
- Baghayeri, M.; Amiri, A.; Fayazi, M.; Nodehi, M.; Esmaeelnia, A. Electrochemical detection of bisphenol a on a MWCNTs/CuFe2O4 nanocomposite modified glassy carbon electrode. Mater. Chem. Phys. 2021, 261, 124247. [Google Scholar] [CrossRef]
- Sanko, V.; Şenocak, A.; Tümay, S.O.; Orooji, Y.; Demirbas, E.; Khataee, A. An electrochemical sensor for detection of trace-level endocrine disruptor bisphenol A using Mo2Ti2AlC3 MAX phase/MWCNT composite modified electrode. Environ. Res. 2022, 212, 113071. [Google Scholar] [CrossRef]
- Jodar, L.V.; Orzari, L.O.; Ortolani, T.S.; Assumpção, M.H.M.T.; Vicentini, F.C.; Janegitz, B.C. Electrochemical Sensor Based on Casein and Carbon Black for Bisphenol A Detection. Electroanalysis 2019, 31, 2162–2170. [Google Scholar] [CrossRef]
- Baccarin, M.; Ciciliati, M.A.; Oliveira, O.N.; Cavalheiro, E.T.; Raymundo-Pereira, P.A. Pen sensor made with silver nanoparticles decorating graphite-polyurethane electrodes to detect bisphenol-A in tap and river water samples. Mater. Sci. Eng. C 2020, 114, 110989. [Google Scholar] [CrossRef]
- Jebril, S.; Cubillana-Aguilera, L.; Palacios-Santander, J.M.; Dridi, C. A novel electrochemical sensor modified with green gold sononanoparticles and carbon black nanocomposite for bisphenol A detection. Mater. Sci. Eng. B 2021, 264, 114951. [Google Scholar] [CrossRef]
- Zhou, Y.; Hao, C.; Dang, Y.; Chen, S. Study on Electrocatalysis of Environmental Hormone BPA Based on Fluent-Electron-Transfer Ce-Doped ZnO Nanorods. J. Electrochem. Soc. 2018, 165, H962–H968. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, C.; Zhuang, Q. An electrochemical sensor modified with nickel nanoparticle/nitrogen-doped carbon nanosheet nanocomposite for bisphenol A detection. J. Alloys Compd. 2020, 827, 154335. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Y.; Zhang, S.; Wang, T.; Zhuang, X.; Tian, C.; Luan, F.; Ni, S.Q.; Fu, X. Enhanced electrochemical sensor based on gold nanoparticles and MoS2 nanoflowers decorated ionic liquid-functionalized graphene for sensitive detection of bisphenol A in environmental water. Microchem. J. 2021, 161, 105769. [Google Scholar] [CrossRef]
- Furquim, F.; Santos, E.; Mercante, L.; Amaral, M.; Pavinatto, A.; Rodrigues, B. Green and low-cost electrospun membranes from polycaprolactone/graphene oxide for Bisphenol A sensing. Mater. Lett. 2020, 274, 128014. [Google Scholar] [CrossRef]
- Miralles, P.; Chisvert, A.; Salvador, A. Determination of Phenolic Endocrine Disruptors in Cosmetics by High-Performance Liquid Chromatography Mass Spectrometry. Anal. Lett. 2018, 51, 717–727. [Google Scholar] [CrossRef]
- Kempińska, D.; Kot-Wasik, A. The Use of RP-HPLC-QTOF-MS as a Powerful Tool for Wastewater Composition Profiling and Selection of Water Pollution Marker Specific to Wastewater Contamination. Monatshefte Chem. 2018, 149, 1595–1604. [Google Scholar] [CrossRef]
- Andrianou, X.D.; Gangler, S.; Piciu, A.; Charisiadis, P.; Zira, C.; Aristidou, K.; Piciu, D.; Hauser, R.; Makris, K.C. Human Exposures to Bisphenol A, Bisphenol F and Chlorinated Bisphenol a Derivatives and Thyroid Function. PLoS ONE 2016, 11, e0155237. [Google Scholar] [CrossRef]
| Electrode | Method | pH | Linear Range (µM) | LOD (nM) | Ref. |
|---|---|---|---|---|---|
| CuPc/MWCNT-COOH/PGE a | DPV | 2 | 0.1–27.5 | 18.9 | [46] |
| MWCNTs/CuFe2O4/GCE b | DPV | 7 | 0.01–120 | 3.2 | [47] |
| Carbon felt c | CV | 7 | 0.01–150 | 480 | [48] |
| Mo2Ti2AlC3MAX phase/MWCNT/GCE d | DPV | 7 | 0.01–8.5 | 2.7 | [49] |
| CAS-CB/GCE e | LSV | 5 | 0.49–24 | 250 | [50] |
| AgNPs/GPUE f | DPV | 7.4 | 2.5–15 | 240 | [51] |
| CB/AuSNPs/SNGCE g | DPV | 7 | 0.5–15 | 60 | [52] |
| Ce-doped ZnO/CPE h | DPV | 7 | 0.05–3.2, 3.2–62.0 | 18 | [53] |
| MNR-PMDAN/GPE i | SWV&DPV | 2 | 0.01–5.0, 5.0–40.0 | 4.3 | [37] |
| NiNPs/NCN/CS/GCE j | DPV | 5 | 0.1–2.5, 2.5–15 | 45 | [54] |
| AuNPs/MoS2-NFs/IL-graphene/GCE k | DPV | 6.5 | 0.05–0.8, 0.8–4 | 28 | [55] |
| PCL/GO membranes l | DPV | 7.4 | 0.025–1, 1–20 | 23 | [56] |
| Cu-Pd/GPE | DPV | 5 | 0.04–85, 85–305 | 20 | This Work |
| Sample | Pd-Cu/GPE | HPLC Method | |||||
|---|---|---|---|---|---|---|---|
| Detected (µM) | Spike (µM) | Found ± SD a (µM) | R b (%) | Detected (µM) | Found ± SD a (µM) | R b (%) | |
| Domestic wastewater | - | 10.0 | 10.2 ± 0.11 | 102.0 | - | 9.85 ± 0.08 | 98.5 |
| Tap water | - | 10.0 | 9.87 ± 0.07 | 98.7 | - | 10.4 ± 0.05 | 104.0 |
| Bottled water | - | 10.0 | 9.92 ± 0.05 | 99.2 | - | 10.3 ± 0.10 | 103.0 |
| 1% Commercial hair dye | 18.5 | 5.0 | 4.89 ± 0.11 | 97.8 | 18.9 | 4.85 ± 0.09 | 97.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Z.; Wang, J.; Xue, Y.; Khorasani Motlagh, M.; Noroozifar, M.; Kraatz, H.-B. Palladium–Copper Bimetallic Aerogel as New Modifier for Highly Sensitive Determination of Bisphenol A in Real Samples. Materials 2023, 16, 6081. https://doi.org/10.3390/ma16186081
Fang Z, Wang J, Xue Y, Khorasani Motlagh M, Noroozifar M, Kraatz H-B. Palladium–Copper Bimetallic Aerogel as New Modifier for Highly Sensitive Determination of Bisphenol A in Real Samples. Materials. 2023; 16(18):6081. https://doi.org/10.3390/ma16186081
Chicago/Turabian StyleFang, Zehao, Junyan Wang, Yilei Xue, Mozhgan Khorasani Motlagh, Meissam Noroozifar, and Heinz-Bernhard Kraatz. 2023. "Palladium–Copper Bimetallic Aerogel as New Modifier for Highly Sensitive Determination of Bisphenol A in Real Samples" Materials 16, no. 18: 6081. https://doi.org/10.3390/ma16186081
APA StyleFang, Z., Wang, J., Xue, Y., Khorasani Motlagh, M., Noroozifar, M., & Kraatz, H.-B. (2023). Palladium–Copper Bimetallic Aerogel as New Modifier for Highly Sensitive Determination of Bisphenol A in Real Samples. Materials, 16(18), 6081. https://doi.org/10.3390/ma16186081






