Solidification and Liquation Cracking in Welds of High Entropy CoCrFeNiCux Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
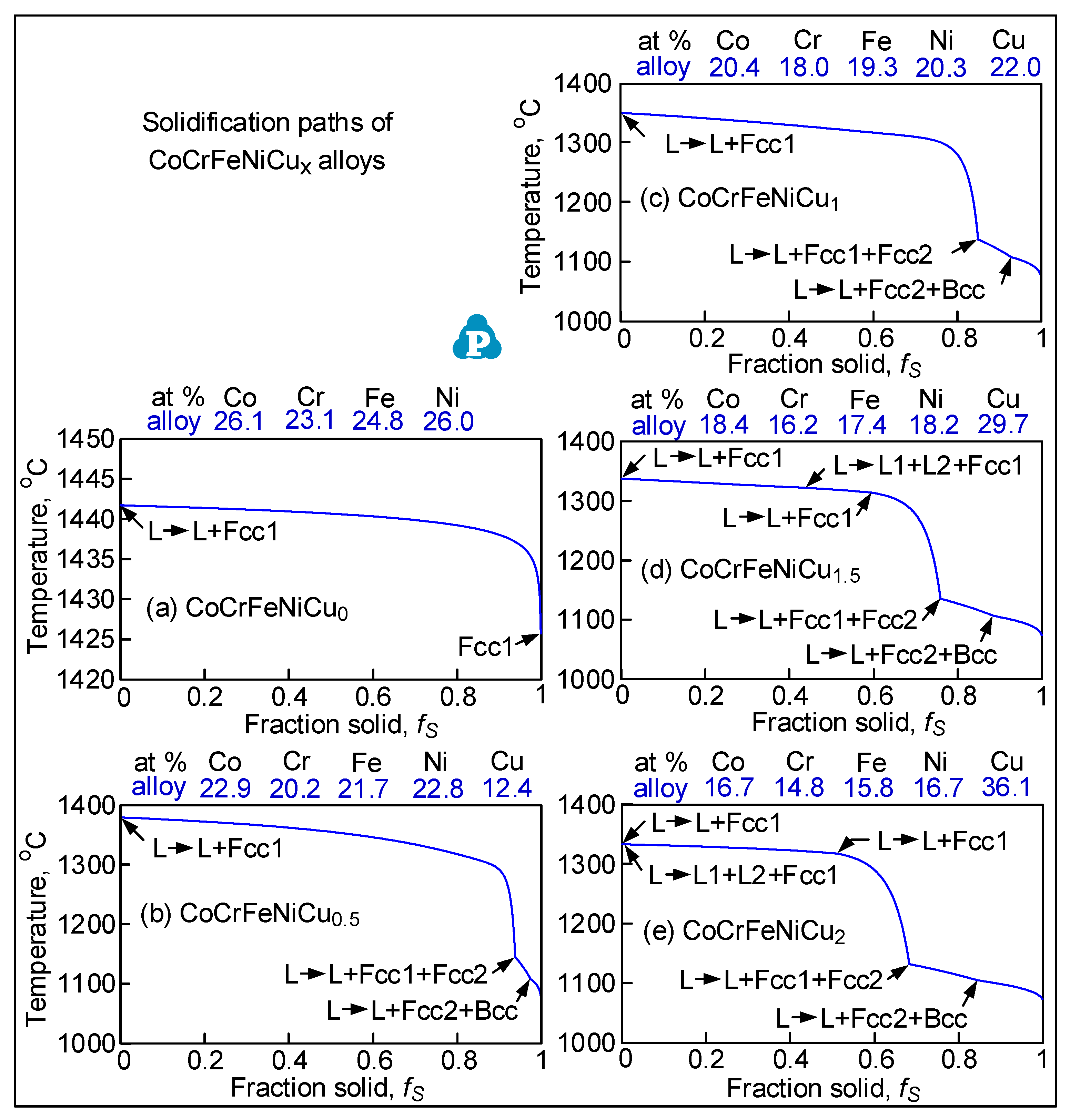
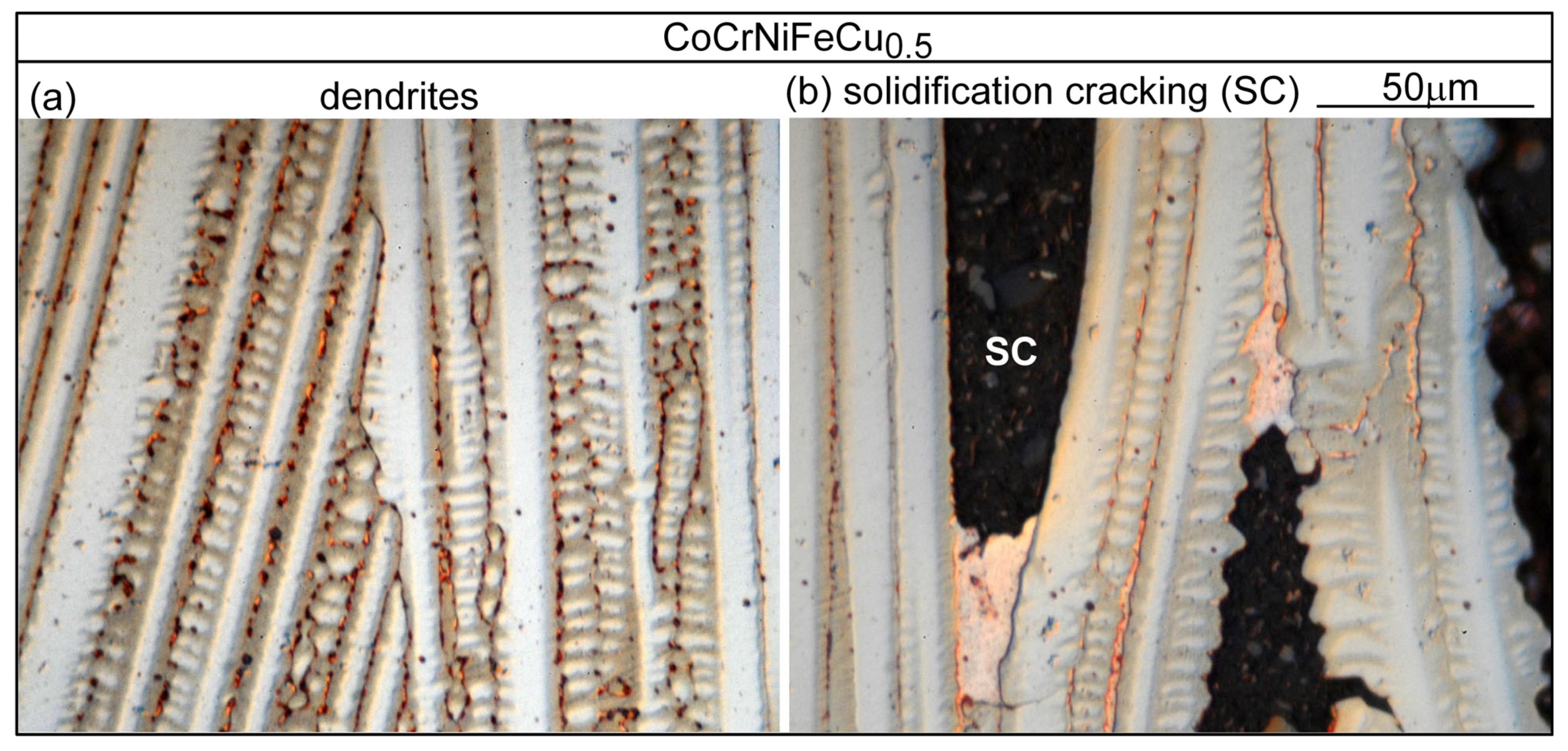
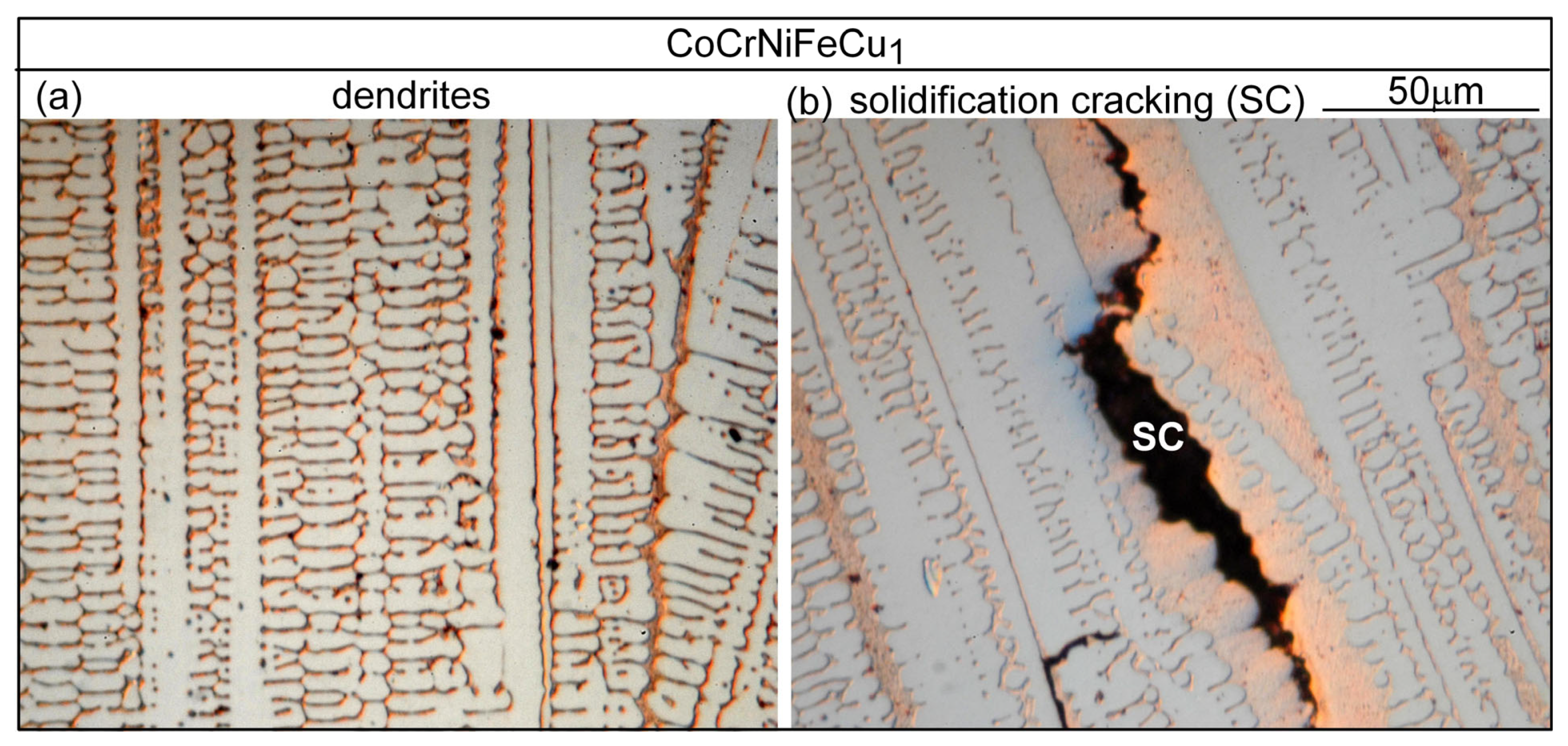
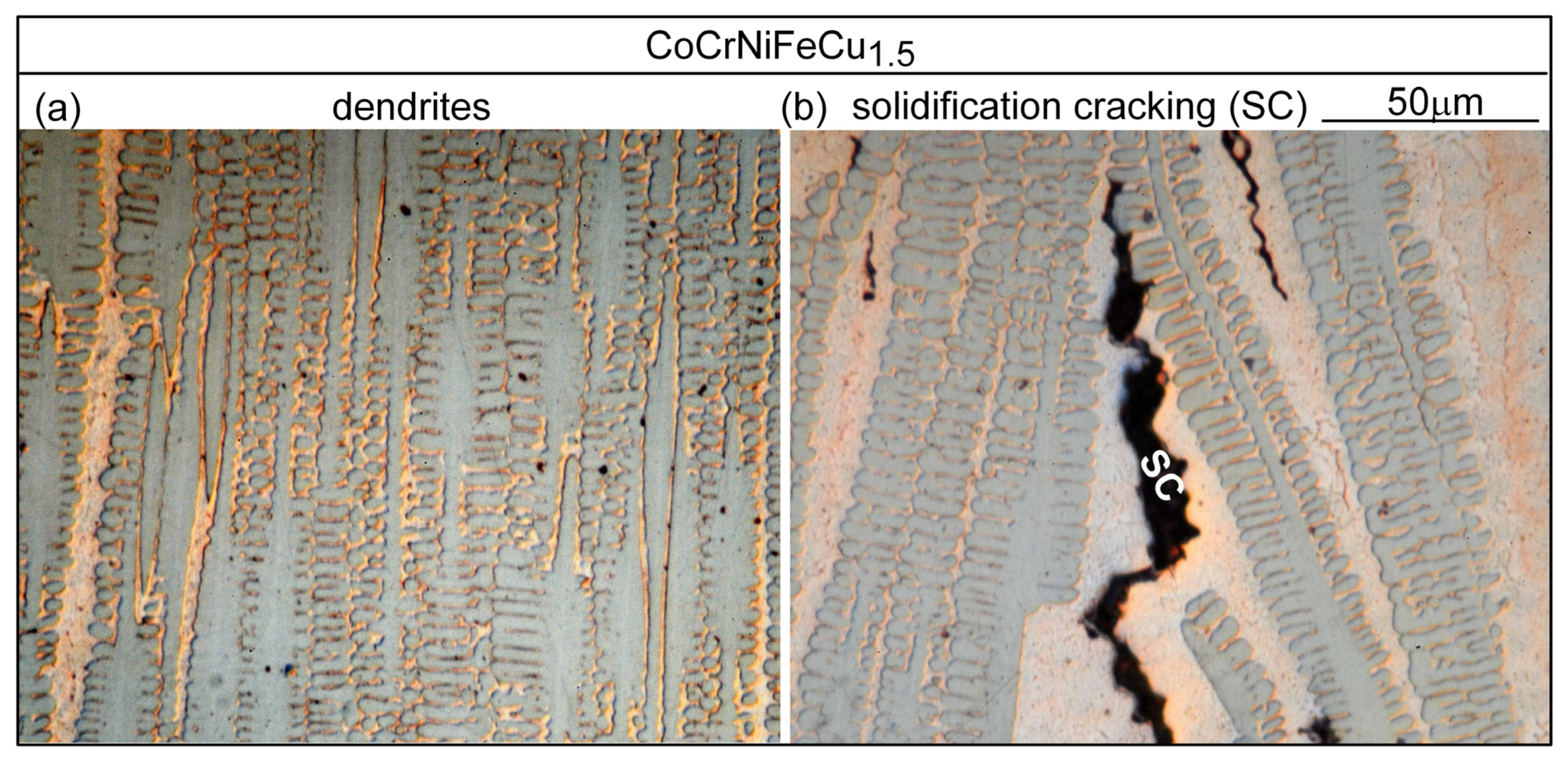
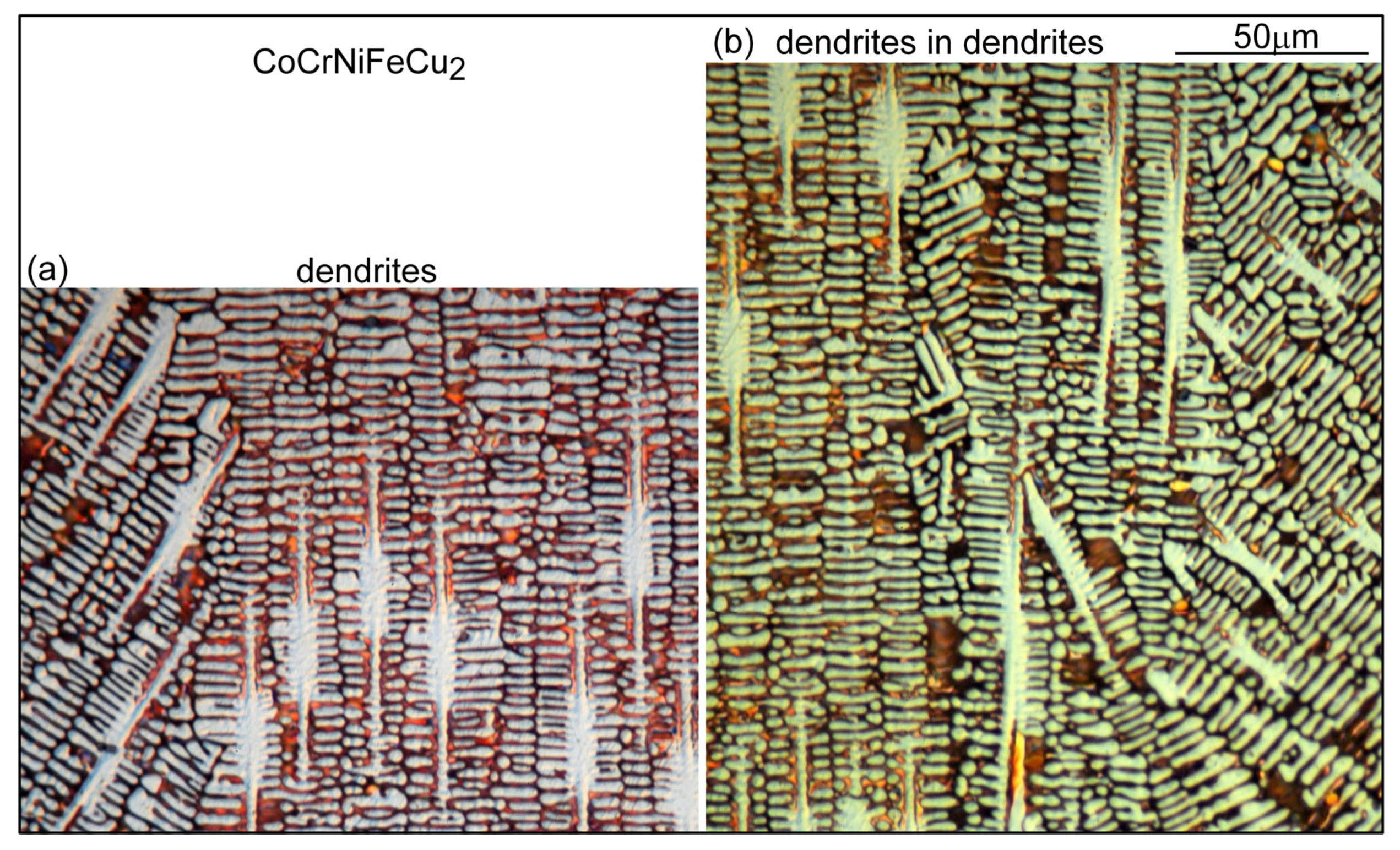
3.1. Solidification Cracking
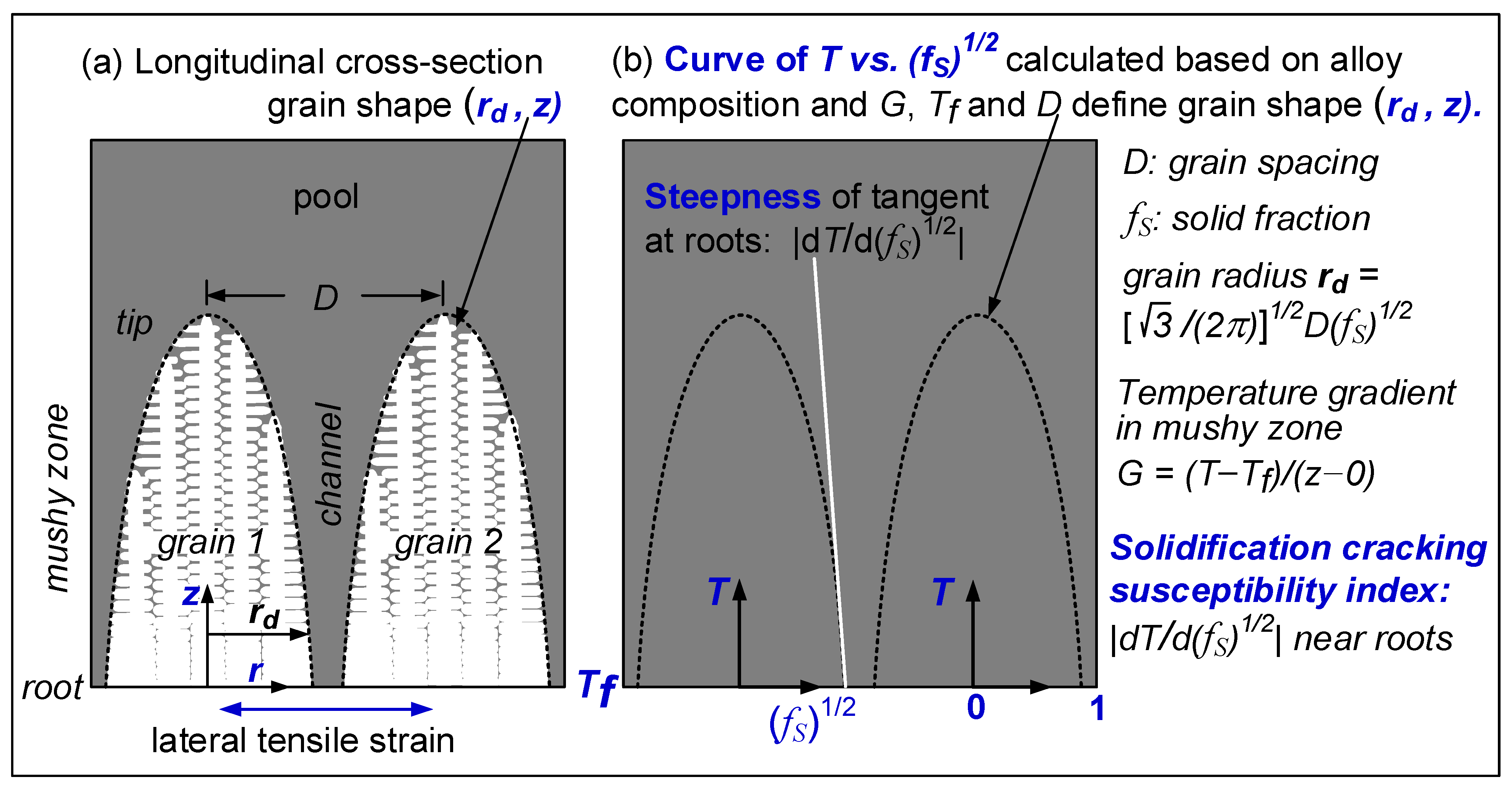
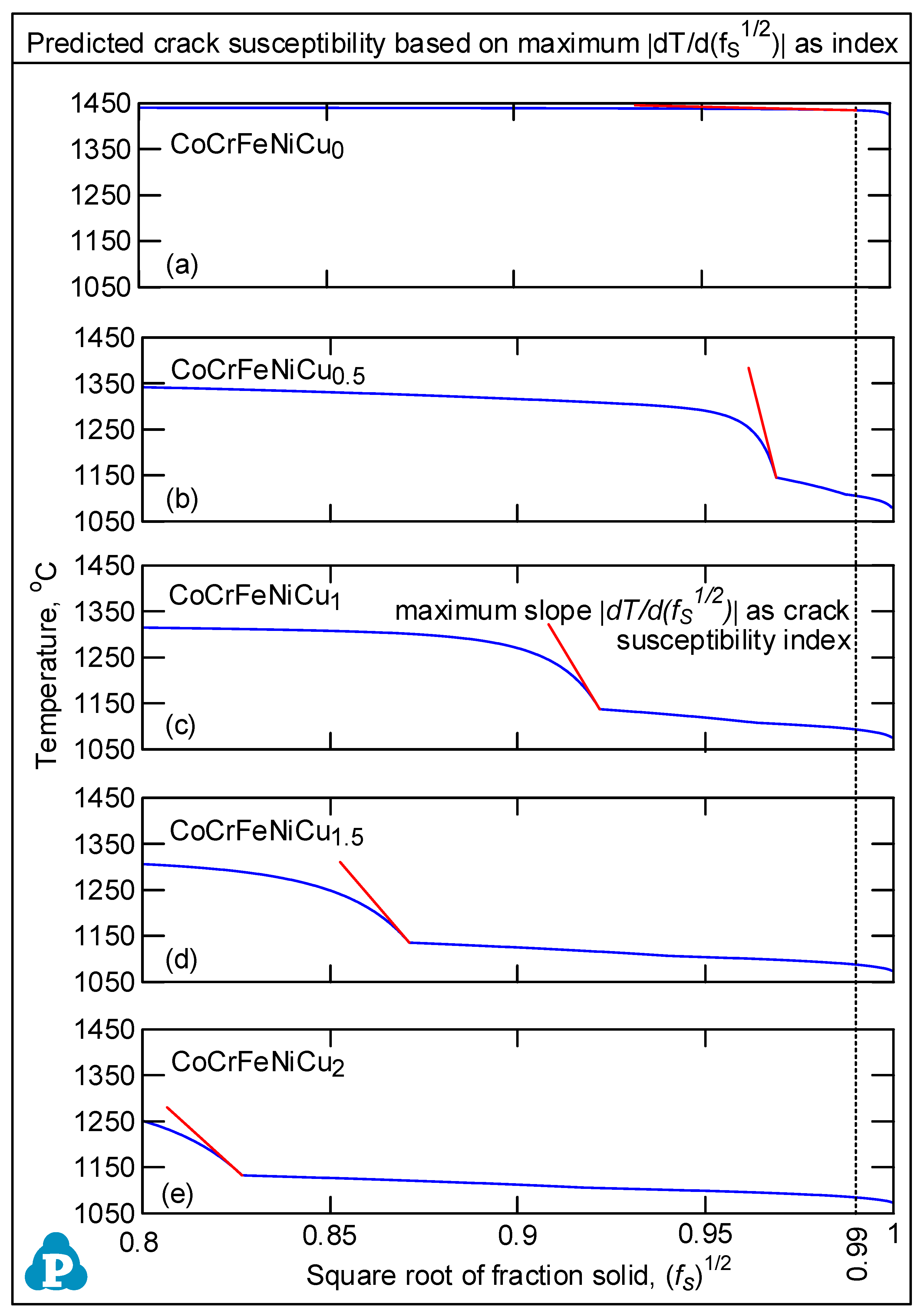
3.2. Solidification Cracking Susceptibility Index
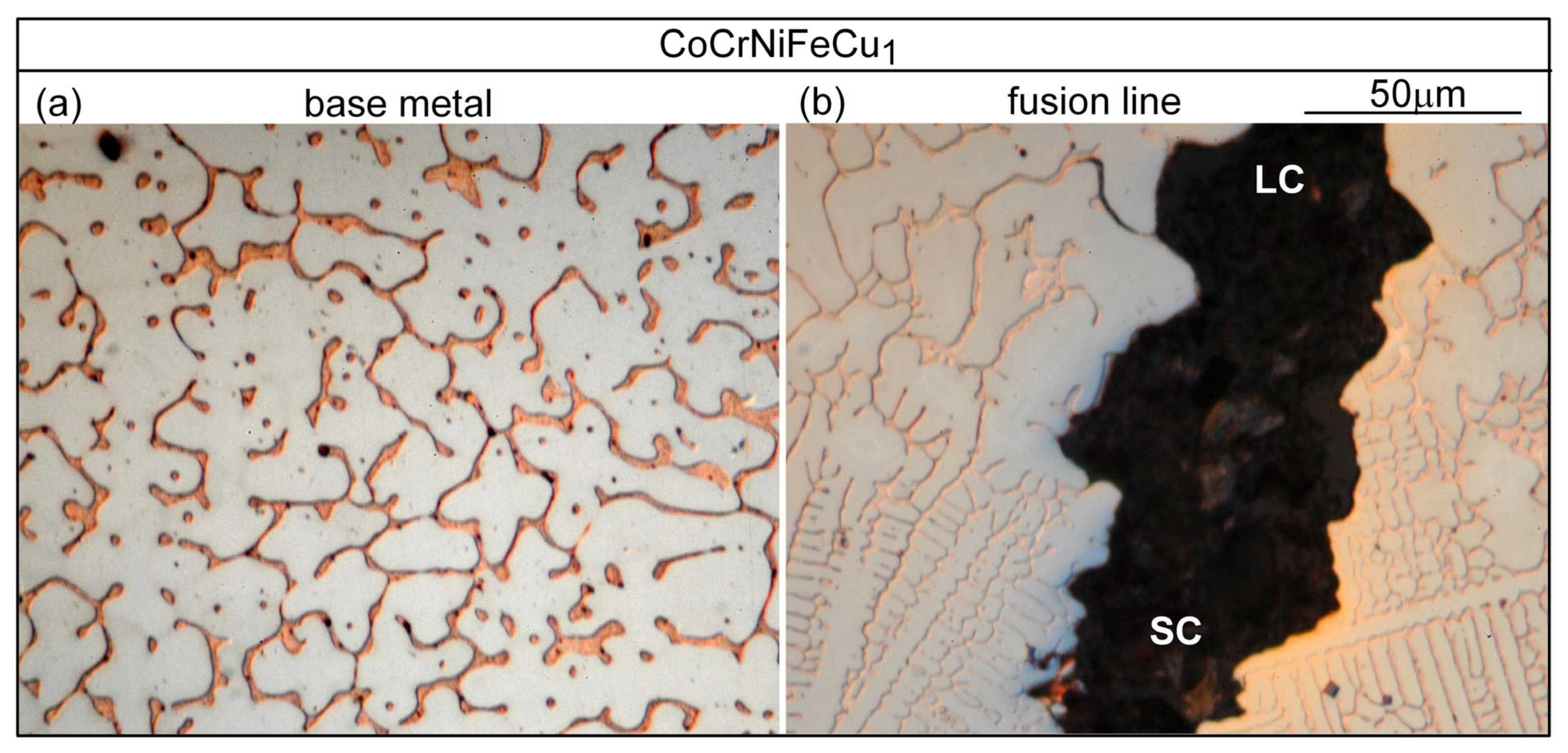
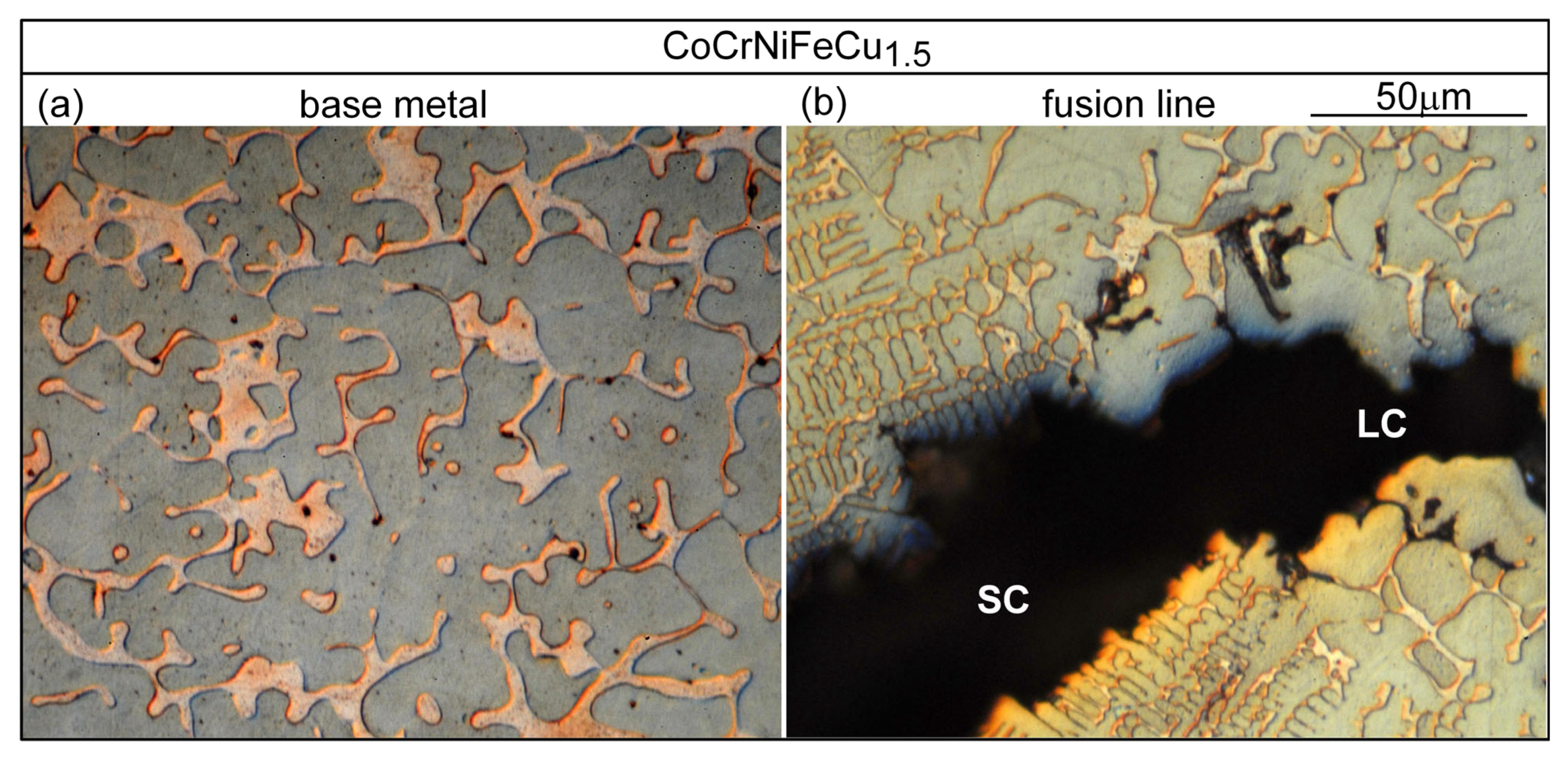
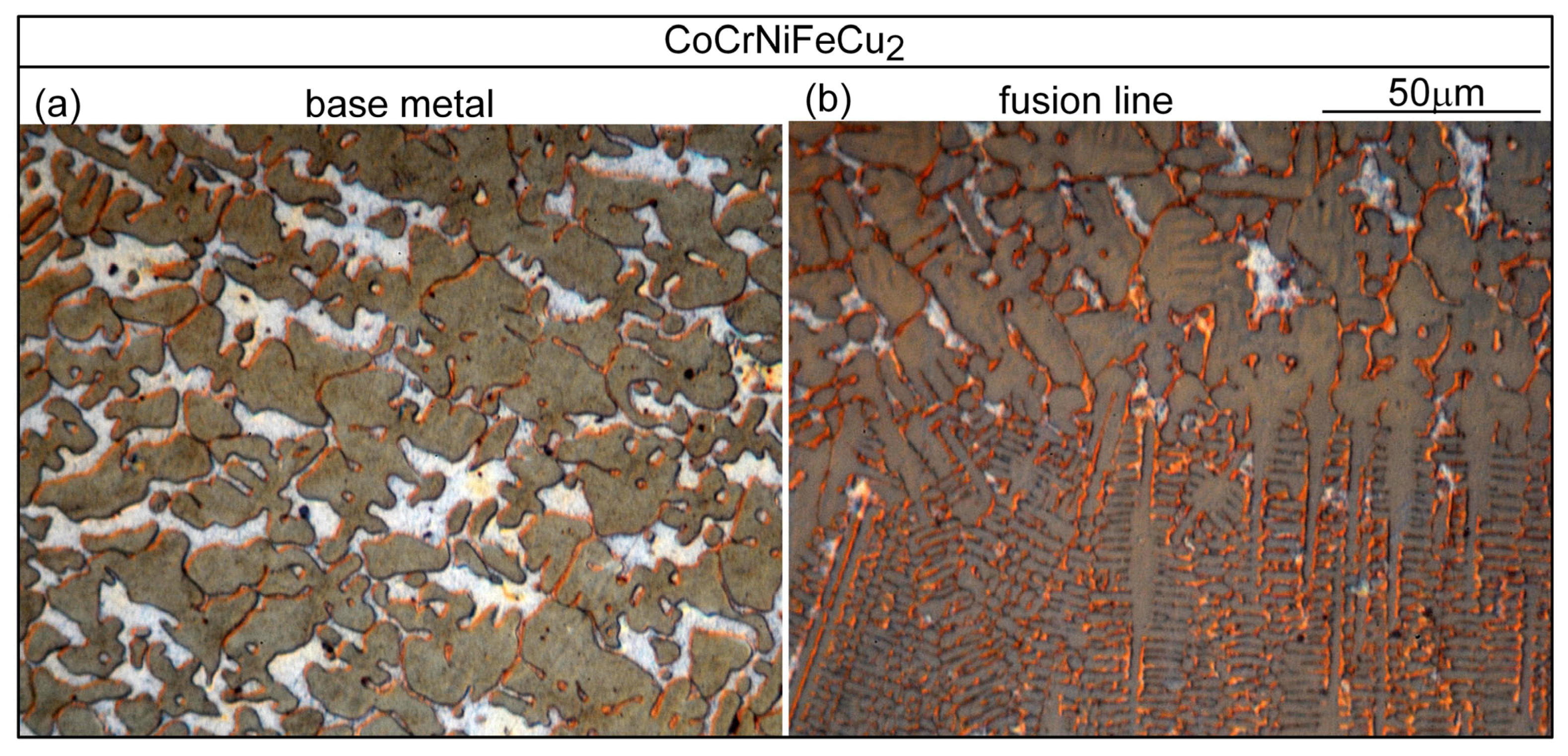
3.3. Liquation Cracking
4. Micrographs of Welds
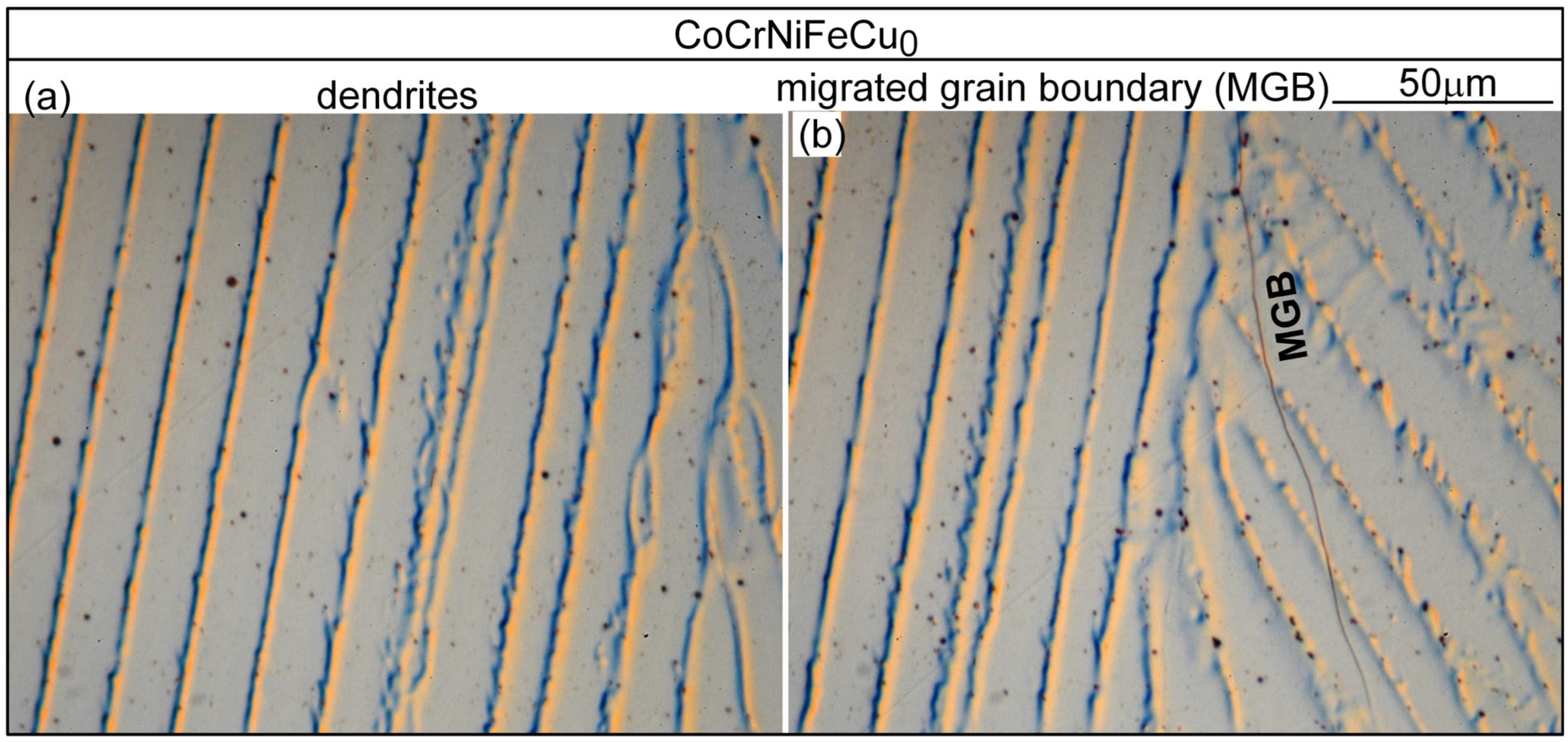
4.1. Solidification Cracking
4.2. Liquation Cracking
5. Conclusions
- (1)
- Based on cracks observed in both the fusion zone and the crater, the ranking of the susceptibility to solidification cracking in arc welding appears to be CoCrFeNiCu0.5 > CoCrFeNiCu1 > CoCrFeNiCu1.5 > CoCrFeNiCu2 > CoCrFeNiCu0. It can be said, at least, that alloys CoCrFeNiCu0.5, CoCrFeNiCu1 and CoCrFeNiCu1.5 are most susceptible to solidification cracking, followed by CoCrFeNiCu2, with CoCrFeNiCu0 being the least susceptible. The same ranking can be shown based on the maximum |dT/d(fS)1/2| up to (fS)1/2 = 0.99 as the index for the susceptibility to solidification cracking.
- (2)
- Solidification cracks in the fusion zone often show Cu-rich intergranular liquid near cracks; the higher the Cu content of the alloy, the greater the amount of the liquid.
- (3)
- Liquation cracking can occur in the PMZ near the fusion boundary and propagate into the fusion zone as solidification cracking. Similar to solidification cracking, the ranking of the susceptibility to liquation cracking appears to be CoCrFeNiCu0.5 > CoCrFeNiCu1 > CoCrFeNiCu1.5 > CoCrFeNiCu2 > CoCrFeNiCu0.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Guo, S.M. A thermodynamic study of corrosion behaviors for CoCrFeNi-based high-entropy alloys. J. Mater. Sci. 2018, 53, 14729–14738. [Google Scholar] [CrossRef]
- Park, N.; Watanabe, I.; Terada, D.; Yokoyama, Y.; Liaw, P.K.; Tsuji, N. Recrystallization behavior of CoCrCuFeNi high-entropy alloy. Metall. Mater. Trans. A 2015, 46, 1481–1487. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Xie, Z.; Huang, J.; Lin, J.; Yang, Y.; Jiang, L.; Huang, J.; Ye, G.; Zhao, C.; Yang, S.; et al. Microstructures and thermodynamic properties of high-entropy alloys CoCrCuFeNi. Intermetallics 2018, 93, 40–46. [Google Scholar] [CrossRef]
- Zhang, L.J.; Fan, J.T.; Liu, D.J.; Zhang, M.D.; Yu, P.F.; Jing, Q.; Ma, M.Z.; Liaw, P.K.; Li, G.; Liu, R.P. The microstructural evolution and hardness of the equiatomic CoCrCuFeNi high-entropy alloy in the semi-solid state. J. Alloys Compd. 2018, 745, 75–83. [Google Scholar] [CrossRef]
- Rogal, Ł. Semi-solid processing of the CoCrCuFeNi high entropy alloy. Mater. Des. 2017, 119, 406–416. [Google Scholar] [CrossRef]
- Cui, H.B.; Zheng, L.F.; Wang, J.Y. Microstructure evolution and corrosion behavior of directionally solidified FeCoNiCrCu high entropy alloy. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2011; Volume 66, pp. 146–149. [Google Scholar]
- Wang, G.; Yang, Y.; He, R.; Tan, C.; Huttula, M.; Cao, W. A novel high entropy CoFeCrNiCu alloy filler to braze SiC ceramics. J. Eur. Ceram. Soc. 2020, 40, 3391–3398. [Google Scholar] [CrossRef]
- Wu, Z.; David, S.A.; Leonard, D.N.; Feng, Z.; Bei, H. Microstructures and mechanical properties of a welded CoCrFeMnNi high-entropy alloy. Sci. Technol. Weld. Join. 2018, 23, 585–595. [Google Scholar] [CrossRef]
- Wang, W.L.; Hu, L.; Luo, S.B.; Meng, L.J.; Geng, D.L.; Wei, B. Liquid phase separation and rapid dendritic growth of high-entropy CoCrCuFeNi alloy. Intermetallics 2016, 77, 41–45. [Google Scholar] [CrossRef]
- Fan, Y.; Li, P.; Chen, K.; Fu, L.; Shan, A.; Chen, Z. Effect of fiber laser welding on solute segregation and proprieties of CoCrCuFeNi high entropy alloy. J. Laser Appl. 2020, 32, 022005. [Google Scholar] [CrossRef]
- Li, J.; Zhao, H.; Zhou, N.; Zhang, Y.; Qin, Q.; Wang, D.; Jiao, J.; Tang, G.; Li, Y. Study on Microstructure of Fiber Laser Welding of CoCrCuFeNi High Entropy Alloy. Materials 2022, 15, 8777. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Tsai, H.L. Equilibrium phase of high-entropy FeCoNiCrCu0.5 alloy at elevated temperature. J. Alloys Compd. 2010, 489, 30–35. [Google Scholar] [CrossRef]
- Kou, S. A criterion for cracking during solidification. Acta Mater. 2015, 88, 366–374. [Google Scholar] [CrossRef]
- Pandat2019, Phase Diagram Calculation Software Package for Multicomponent Systems; CompuTherm LLC: Madison, WI, USA, 2019.
- PanHEA2019, Thermodynamic Database for High Entropy Alloys; CompuTherm LLC: Madison, WI, USA, 2019.
- Dowd, J.D. Weld cracking of aluminum alloys. Weld. J. 1952, 31, 448s–456s. [Google Scholar]
- Dudas, J.H.; Collins, F.R. Preventing weld cracks in high-strength aluminum alloys. Weld. J. 1966, 45, 241s–249s. [Google Scholar]
- Kou, S. A Simple Index for Predicting the Susceptibility to Solidification Cracking. Weld. J. 2015, 94, 374s–388s. [Google Scholar]
- Shankar, V.; Devletian, J.H. Solidification cracking in low alloy steel welds. Sci. Technol. Weld. Join. 2005, 10, 236–243. [Google Scholar] [CrossRef]
- Fukuhisa, M.; Nakagawa, H.; Nakata, K.; Kohmoto, H.; Honda, Y. Quantitative Evaluation of Solidification Brittleness of Weld Metal During Solidification by Means of In Situ Observation and Measurement. Pt. 1. Development of the MISO Technique. Trans. JWRI 1983, 12, 65–72. [Google Scholar]
- Xia, C.; Kou, S. Calculating the susceptibility of carbon steels to solidification cracking during welding. Metall. Mater. Trans. B 2021, 52, 460–469. [Google Scholar] [CrossRef]
- Savage, W.F.; Lundin, C.D. The varestraint test. Weld. J. 1965, 44, 433s–442s. [Google Scholar]
- Soysal, T.; Kou, S. A simple test for assessing solidification cracking susceptibility and checking validity of susceptibility prediction. Acta Mater. 2018, 143, 181–197. [Google Scholar] [CrossRef]
- Kou, S. Welding Metallurgy, 3rd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Matsuda, F.; Nakata, K.; Arai, K.; Tsukamoto, K. Comparison of weld crack susceptibility of recent aluminum alloys. Trans. JWRI 1981, 10, 71–79. [Google Scholar]
- Matsuda, F.; Nakata, K.; Arai, K.; Tsukamoto, K. Assessment of solidification cracking test for aluminium alloy welds. Trans. JWRI 1982, 11, 67–77. [Google Scholar]


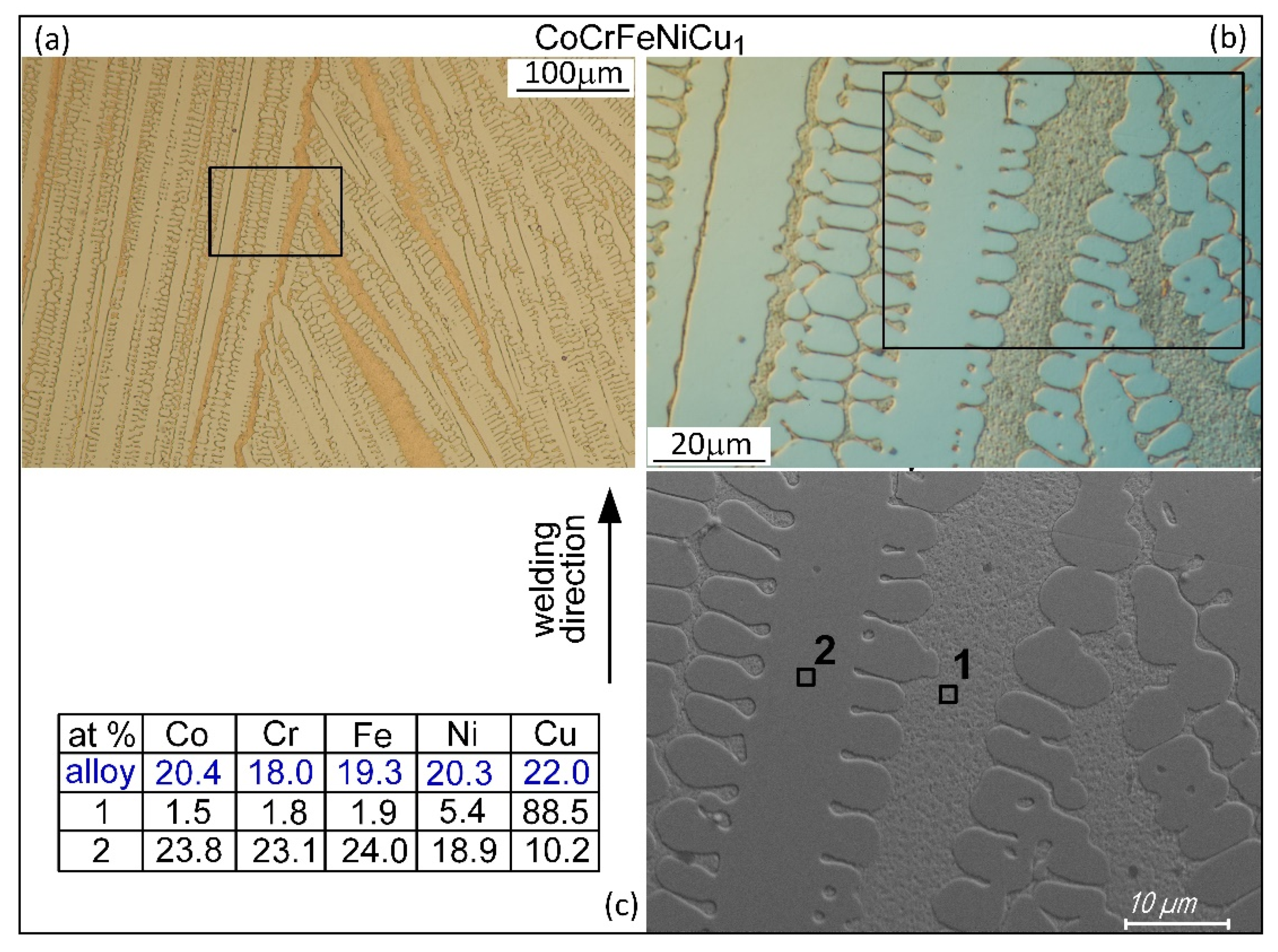
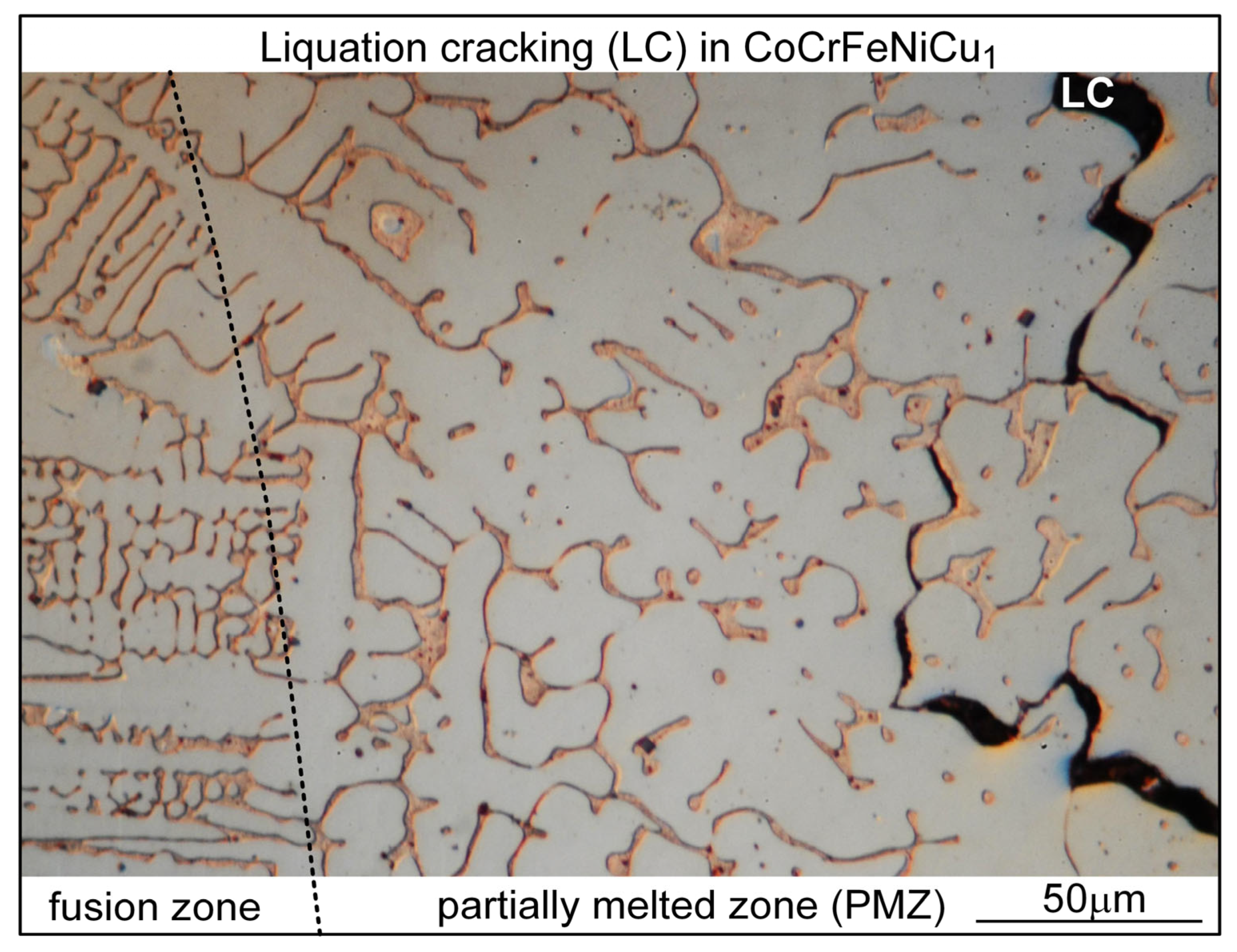
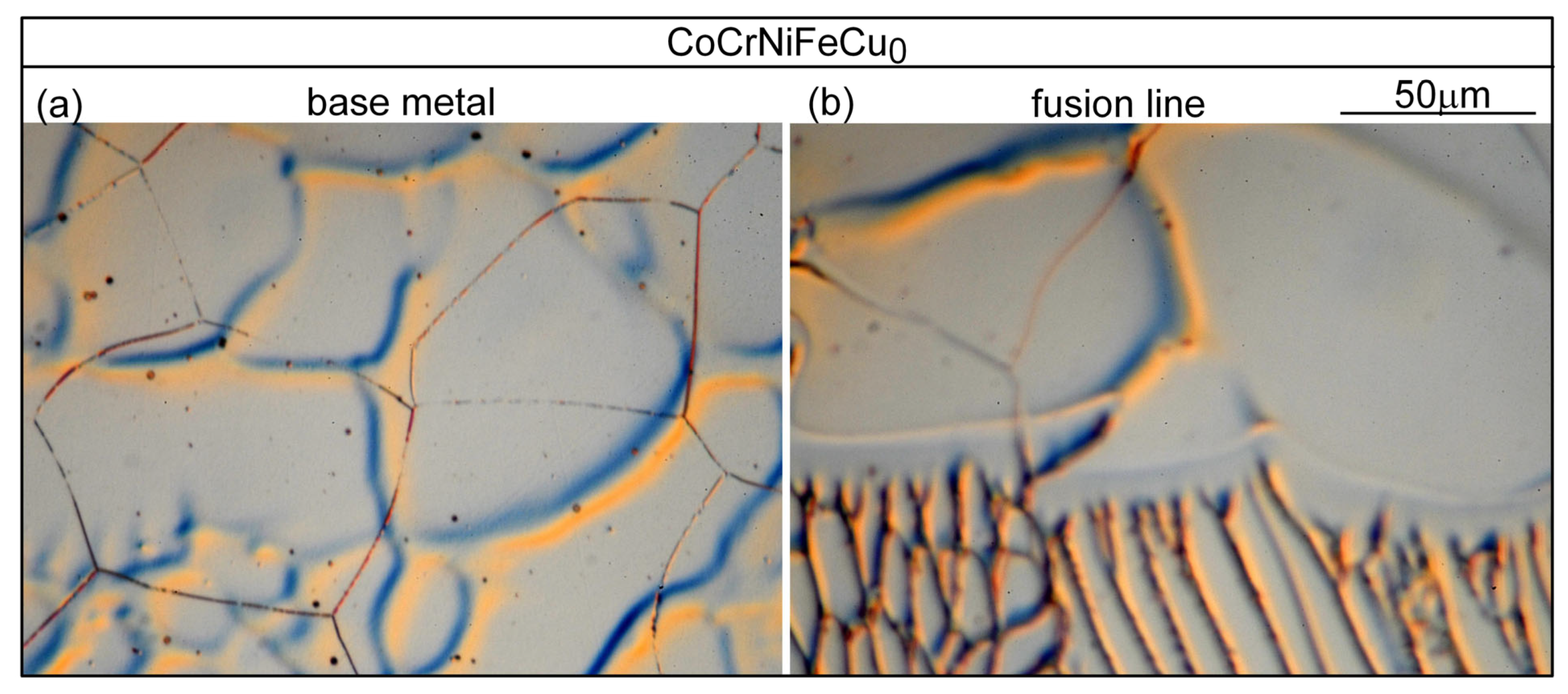
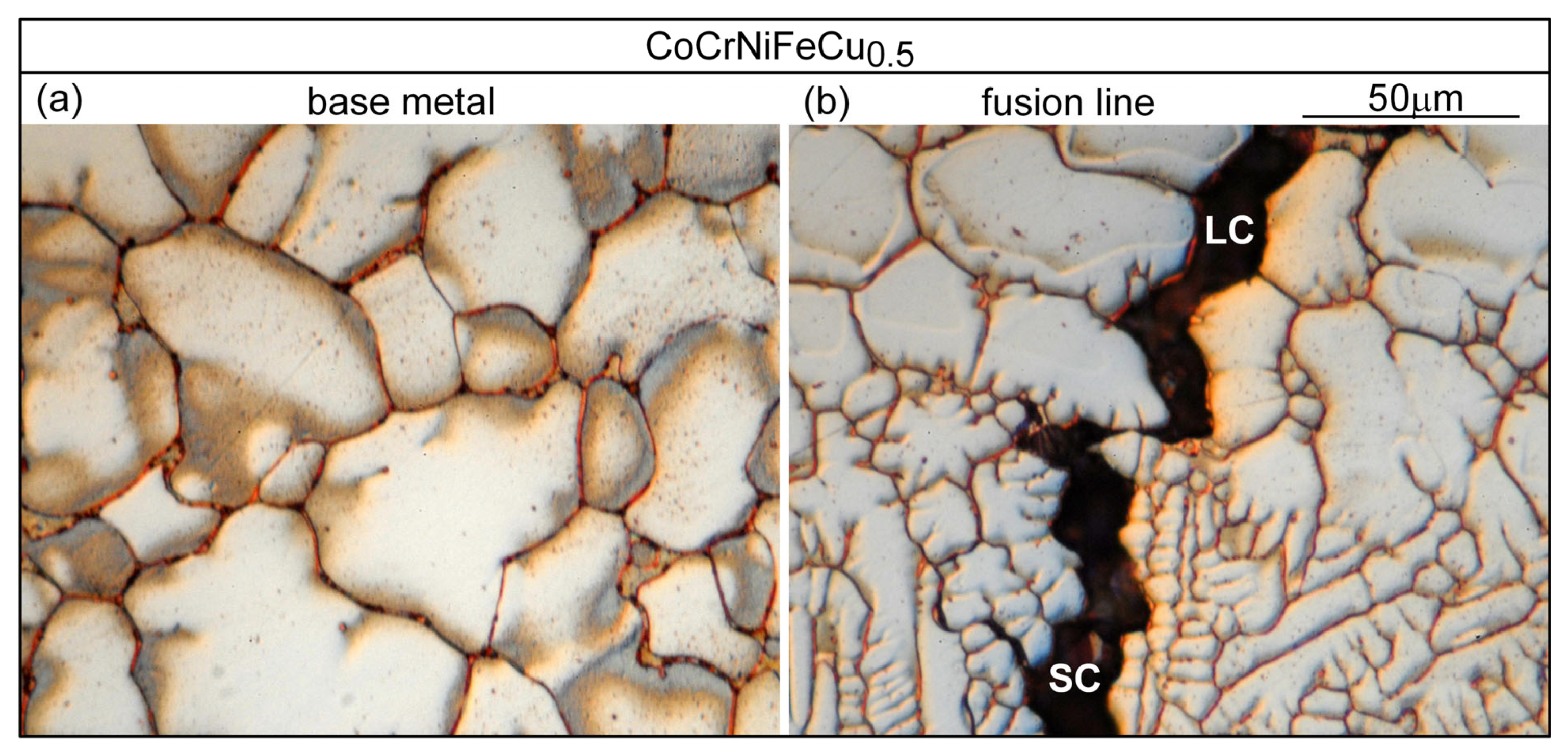
| Alloy | Co | Cr | Fe | Ni | Cu |
|---|---|---|---|---|---|
| CoCrFeNiCu0 | 26.14 | 23.06 | 24.77 | 26.03 | 0 |
| CoCrFeNiCu0.5 | 22.91 | 20.21 | 21.71 | 22.82 | 12.35 |
| CoCrFeNiCu1 | 20.39 | 17.99 | 19.32 | 20.31 | 21.99 |
| CoCrFeNiCu1.5 | 18.37 | 16.21 | 17.41 | 18.23 | 29.71 |
| CoCrFeNiCu2 | 16.72 | 14.75 | 15.84 | 16.65 | 36.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, P.; Kou, S.; Lin, C.-M. Solidification and Liquation Cracking in Welds of High Entropy CoCrFeNiCux Alloys. Materials 2023, 16, 5621. https://doi.org/10.3390/ma16165621
Yu P, Kou S, Lin C-M. Solidification and Liquation Cracking in Welds of High Entropy CoCrFeNiCux Alloys. Materials. 2023; 16(16):5621. https://doi.org/10.3390/ma16165621
Chicago/Turabian StyleYu, Ping, Sindo Kou, and Chun-Ming Lin. 2023. "Solidification and Liquation Cracking in Welds of High Entropy CoCrFeNiCux Alloys" Materials 16, no. 16: 5621. https://doi.org/10.3390/ma16165621
APA StyleYu, P., Kou, S., & Lin, C.-M. (2023). Solidification and Liquation Cracking in Welds of High Entropy CoCrFeNiCux Alloys. Materials, 16(16), 5621. https://doi.org/10.3390/ma16165621







