The Corrosion Resistance of Reinforced Reactive Powder Concrete with Secondary Aluminum Ash Exposed to NaCl Action
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
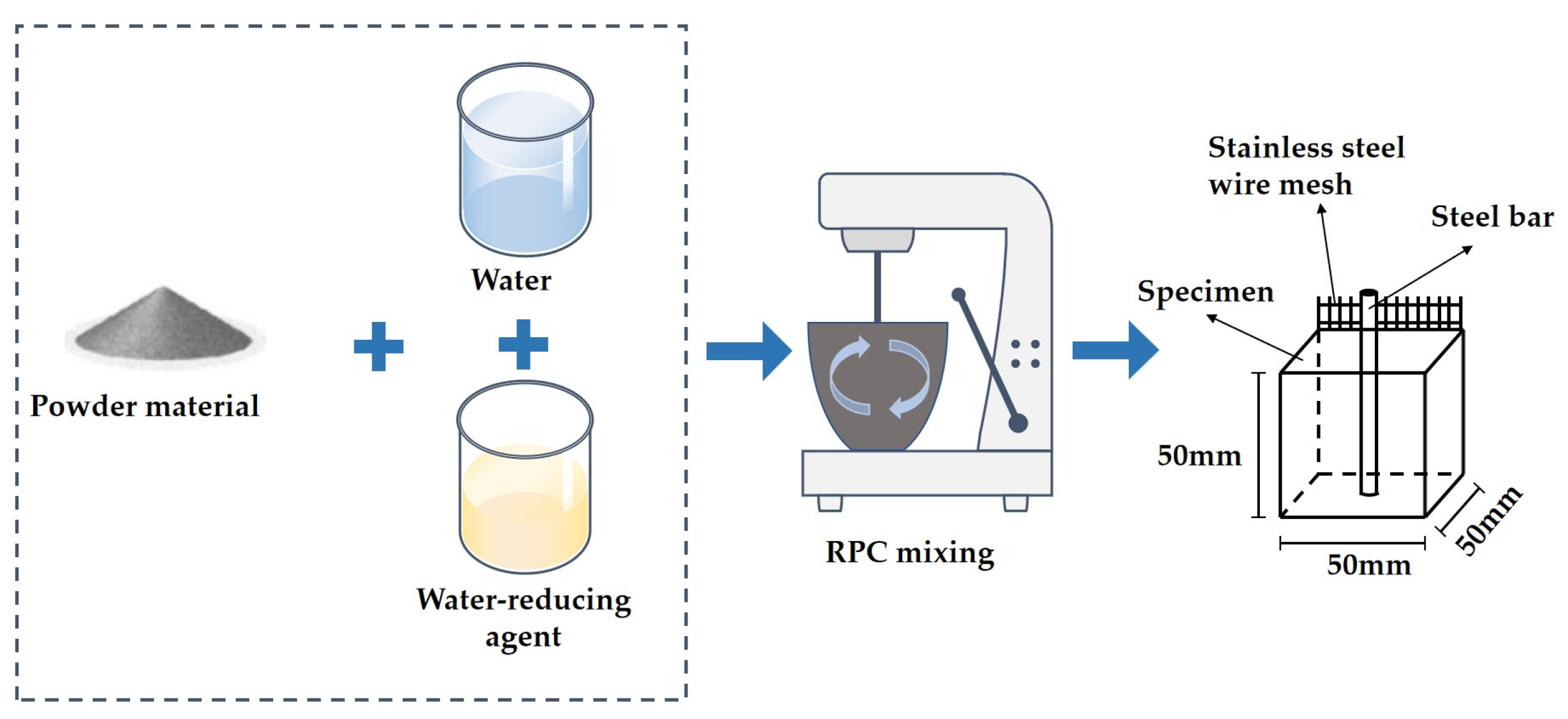
2.2. The Manufacturing Process of Specimens
2.3. NaCl Action on RPC
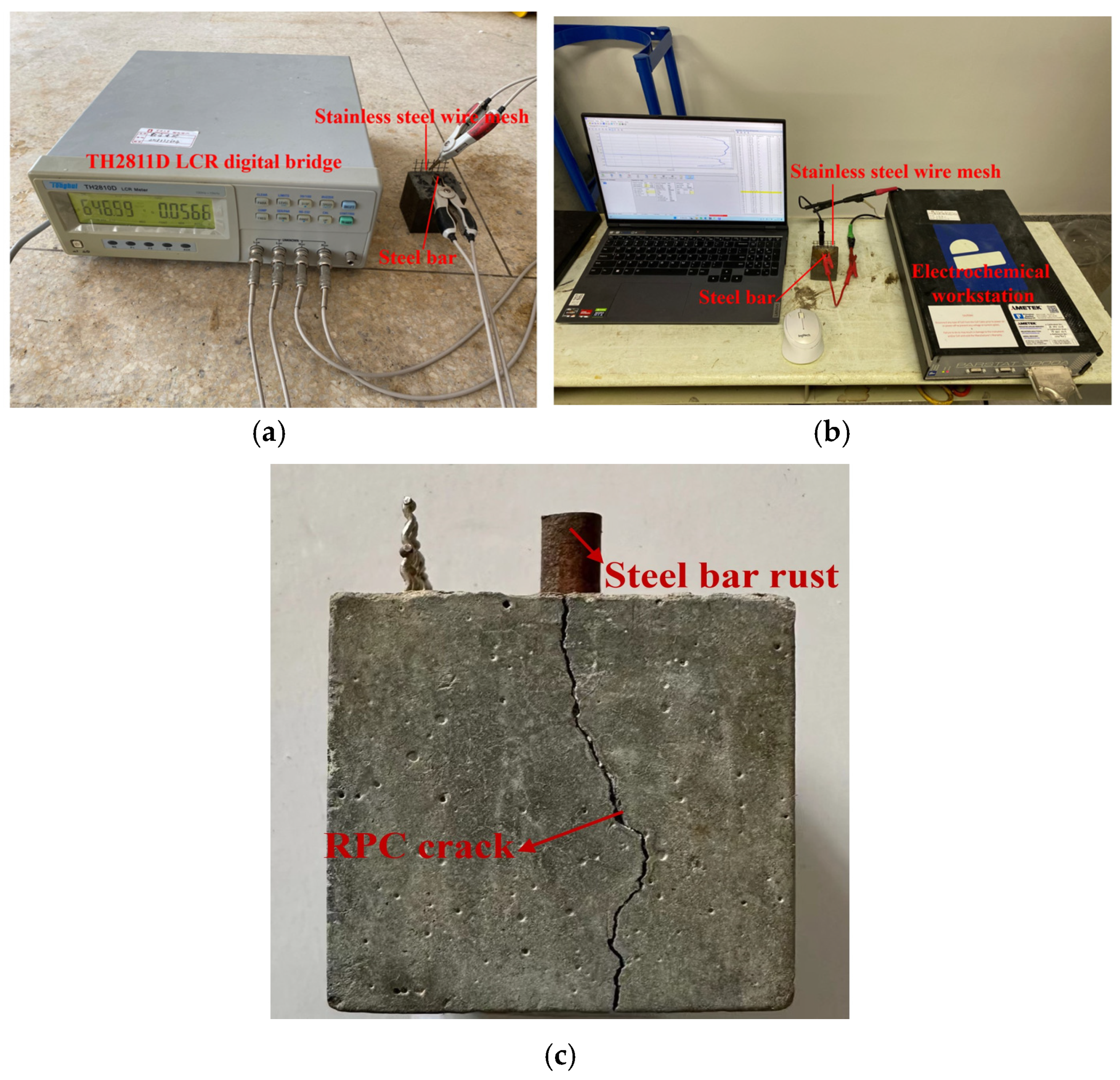
2.4. The Corrosion Resistance Parameters
3. Results and Discussion
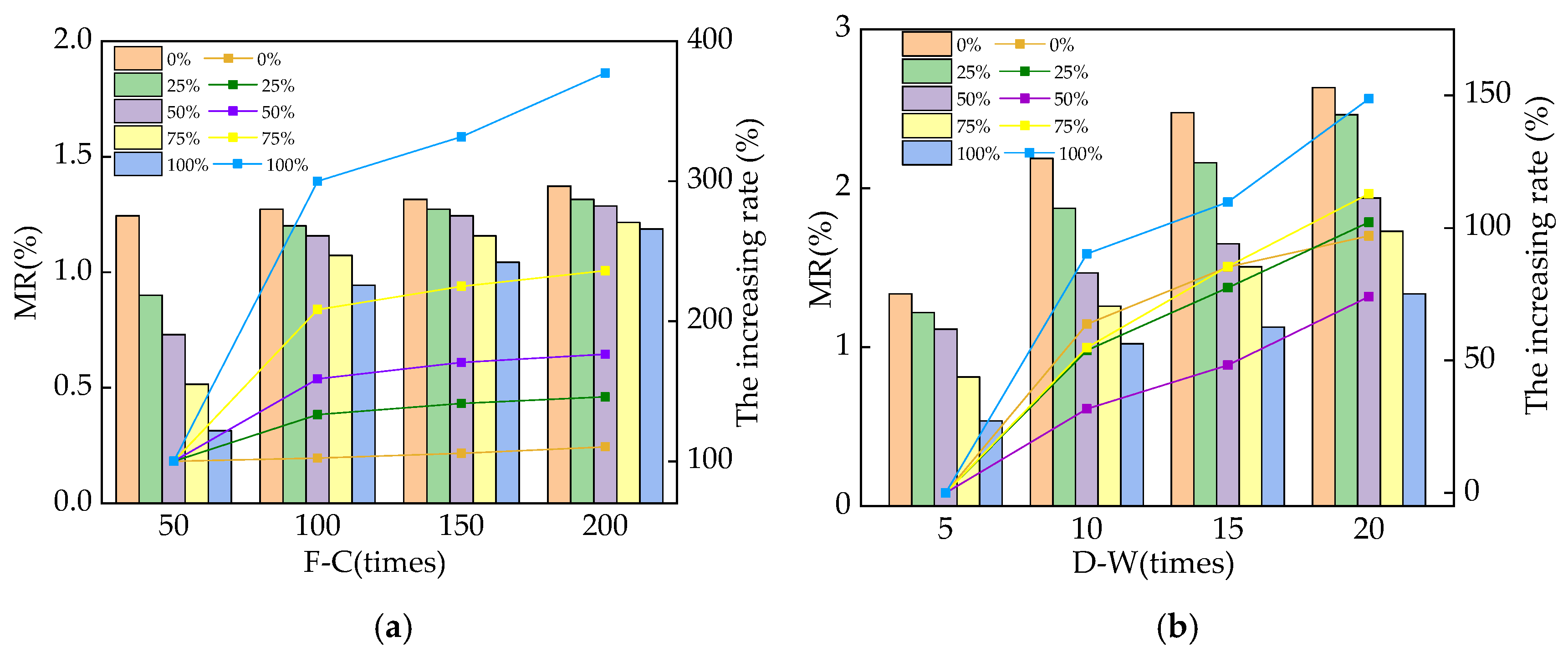
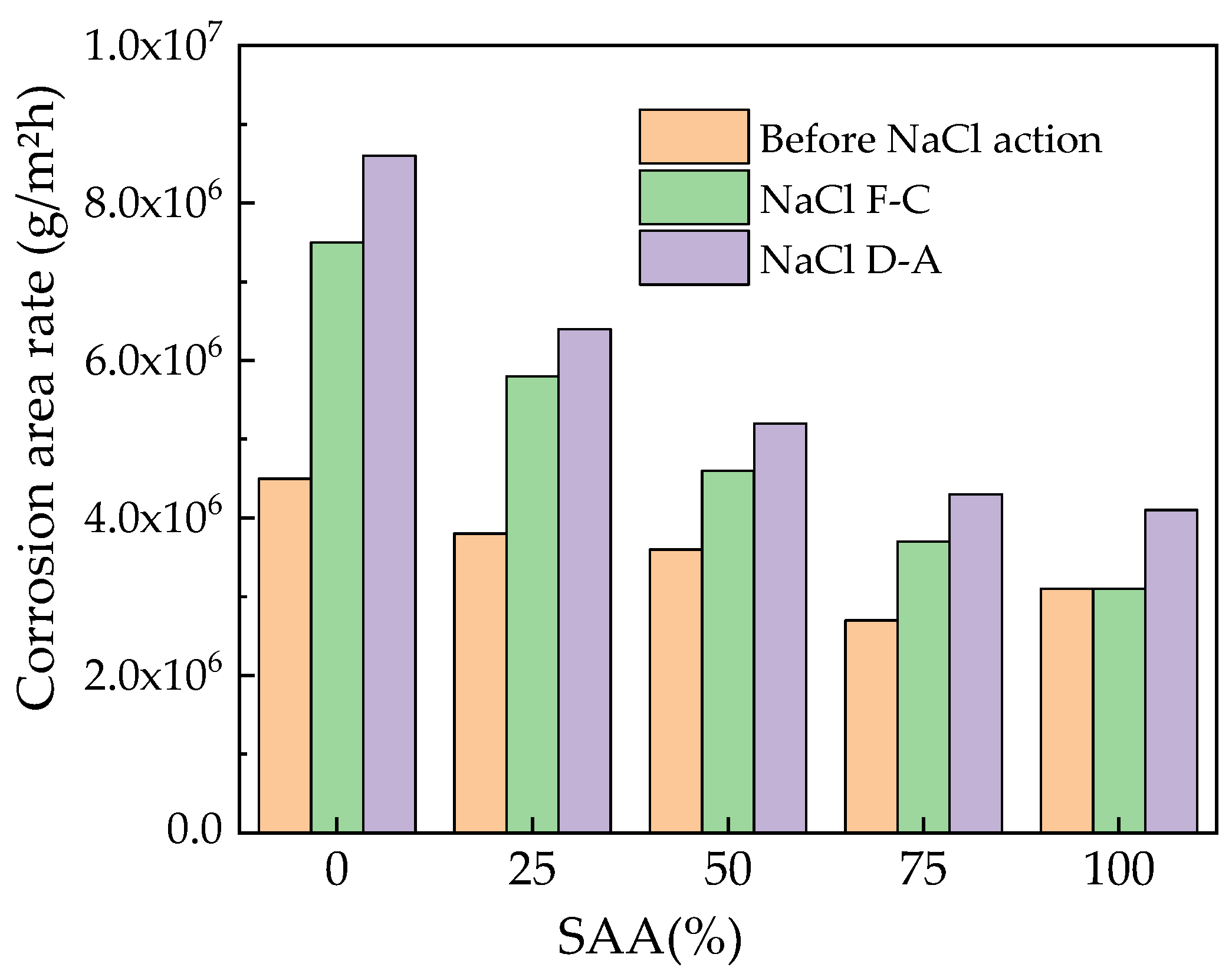
3.1. The Mass Loss Rate
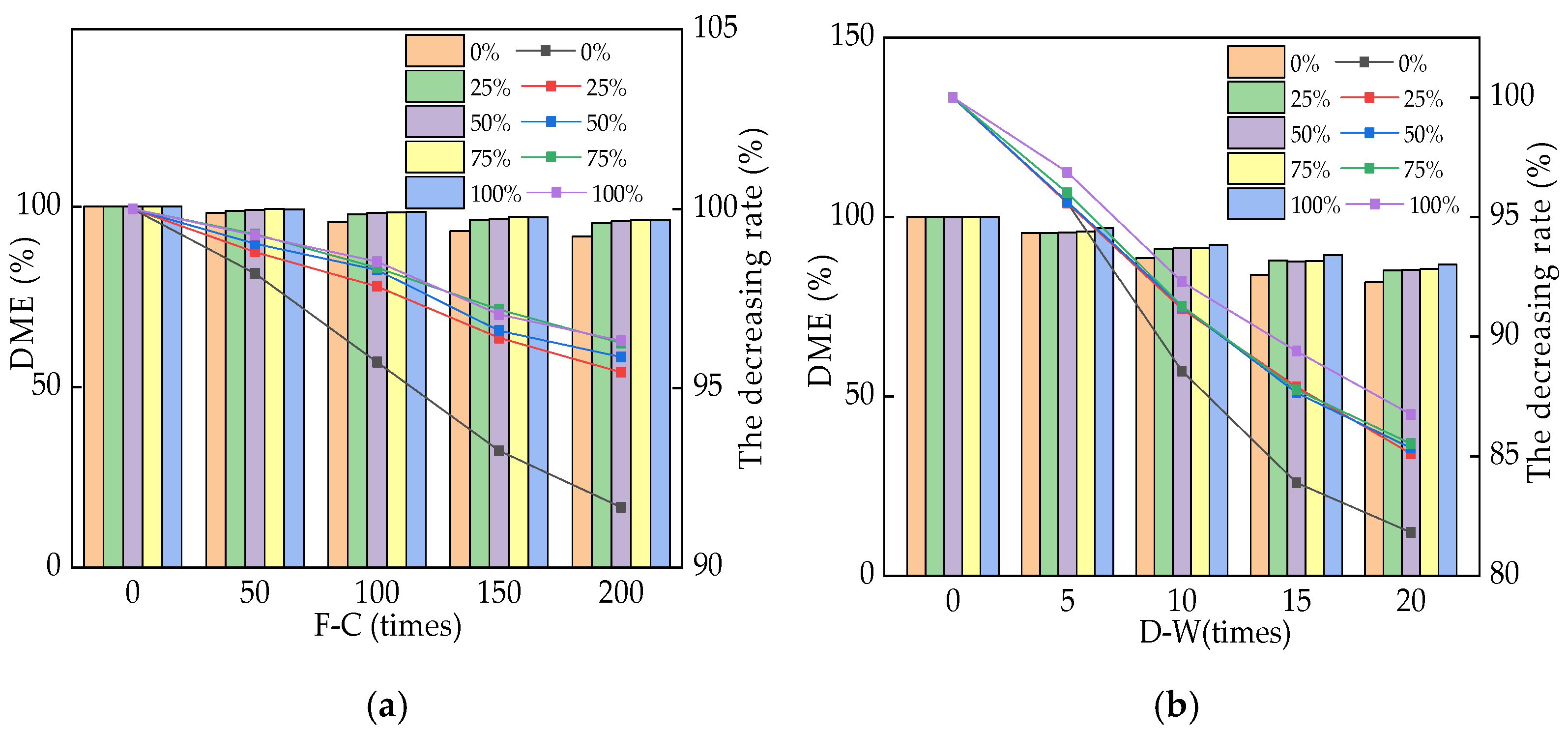
3.2. The Dynamic Modulus of Elasticity of Reinforced RPC
3.3. The Electrical Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OPC | Ordinary Portland cement |
| F-C | Freeze–thaw cycle |
| D-A | Dry–wet alternation |
| MR | Mass loss rate |
| EIS | Electrochemical impedance spectroscopy |
| DME | Dynamic modulus of elasticity |
| R | AC electrical resistance |
| SAA | Secondary aluminum ash |
| BFS | Blast furnace slag |
| FA | Fly ash |
References
- Palma-Rojas, S.; Caldeira-Pires, A.; Nogueira, J.M. Environmental and economic hybrid life cycle assessment of bagasse-derived ethanol produced in Brazil. Int. J. Life Cycle Assess 2015, 22, 317–327. [Google Scholar] [CrossRef]
- Russo, I.; Confente, I.; Scarpi, D.; Hazen, B.T. From trash to treasure: The impact of consumer perception of bio-waste products in closed-loop supply chains. J. Clean. Prod. 2019, 218, 966–974. [Google Scholar] [CrossRef]
- Padamata, S.K.; Andrey, Y. A Review of Secondary Aluminum Production and Its Byproducts. JOM 2021, 73, 2603–2614. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Ruiz-Molina, S.; Pérez-Villarejo, L.; Castro, E.; Sánchez-Soto, P.J. Dust filter of secondary aluminium industry as raw material of geopolymer foams. J. Build. Eng. 2020, 32, 101656. [Google Scholar] [CrossRef]
- Kumar, M.S.; Vasumathi, M.; Begum, S.R.; Luminita, S.M.; Vlase, S.; Pruncu, C.I. Influence of B4C and industrial waste fly ash reinforcement particles on the micro structural characteristics and mechanical behavior of aluminium (Al–Mg–Si-T6) hybrid metal matrix composite. J. Mater. Res. Technol. 2021, 15, 1201–1216. [Google Scholar] [CrossRef]
- Tenorio, J.A.S.; Espinosa, D.C.R. Effect of salt/oxide interaction on the process of aluminum recycling. J. Light Metals. 2002, 2, 89–93. [Google Scholar] [CrossRef]
- Ünlü, N.; Drouet, M.G. Comparison of salt-free aluminum dross treatment processes. Resour. Conserv. Recycl. 2002, 36, 61–72. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Allahverdi, A. A promising green process for synthesis of high purity activated-alumina nanopowder from secondary aluminum dross. J. Clean. Prod. 2018, 179, 93–102. [Google Scholar] [CrossRef]
- Shriraksha, J.; Chandrashekar, A.R.; Sujay, R.N. Eco-concrete for sustainability: Utilizing AD and iron slag as partial replacement materials. Clean Tech. Environ. Policy 2017, 19, 2291–2304. [Google Scholar]
- Shen, H.L.; Liu, B.; Ekberg, C. Harmless disposal and resource utilization for secondary aluminum dross: A review. Total Environ. 2021, 760, 14368. [Google Scholar] [CrossRef]
- Ozerkan, N.G.; Maki, O.L.; Anayeh, M.W.; Tangen, S. The effect of aluminium dross on mechanical and corrosion properties of concrete. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 9912–9922. [Google Scholar]
- Maria, G.; Nastasia, P.; Lidia, R.; Romanita, T.; Maria, R. Some contributions regarding technical and ecological compatibility of the secondary aluminium slags, with portland cement matrix. Rev. Rom. Mater. 2007, 37, 94–103. [Google Scholar]
- Arimanwa, J.I.; Onwuka, D.O.; Arimanwa, M.C.; Onwuka, U.S. Prediction of the compressive strength of aluminum waste–cement concrete using Scheffe’s theory. J. Mater. Civ. Eng. 2012, 24, 177–183. [Google Scholar] [CrossRef]
- Meshram, A.; Singh, K.K. Recovery of valuable products from hazardous aluminum dross: A review. Resour. Conserv. Recycl. 2018, 130, 95–108. [Google Scholar] [CrossRef]
- Sunwoo, P.; Lian, M.M.; Lee, D.Y. Chemical and physical characteristics of hybrid alkaline cement composite after laser interaction. J. Build. 2023, 68, 106131. [Google Scholar]
- Hang, T.; Luca, S.; David, B. Development of sustainable ultra-high performance concrete recycling aluminum production waste. Constr. Build. Mater. 2023, 371, 130212. [Google Scholar]
- Satish Reddy, M.; Neeraja, D. Mechanical and durability aspects of concrete incorporating secondary aluminium slag. Resour. Effic. Technol. 2016, 2, 225–232. [Google Scholar] [CrossRef]
- Ewais, E.M.M.; Khalil, N.M.; Amin, M.S. Utilization of aluminum sludge and aluminum slag (dross) for the manufacture of calcium aluminate cement. Ceram. Int. 2009, 35, 3381–3388. [Google Scholar] [CrossRef]
- Kočí, V.; Vejmelková, E.; Koňáková, D.; Pommer, V.; Grzeszczyk, S.; Matuszek-Chmurowska, A.; Mordak, A.; Černý, R. Basic physical, mechanical, thermal and hygric properties of reactive powder concrete with basalt and polypropylene fibers after high-temperature exposure. Constr. Build. Mater. 2023, 374, 130922. [Google Scholar] [CrossRef]
- Yang, H. Influence of Mineral Powder and Fiber on Work and Mechanical Properties of Reactive Powder Concrete. Multipurp. Util. Miner. Resour. 2023, 2, 197–204. [Google Scholar]
- Ting, M.; Wong, K.; Rahman, M.; Joo, M.S. Mechanical and durability performance of marine sand and seawater concrete incorporating silicomanganese slag as coarse aggregate. Constr. Build. Mater. 2020, 254, 119195. [Google Scholar] [CrossRef]
- Alderete, N.; Joseph, A.; Van den Heede, P.; Matthys, S.; De Belie, N. Effective and sustainable use of municipal solid waste incineration bottom ash in concrete regarding strength and durability. Resour. Conserv. Recycl. 2021, 167, 105356. [Google Scholar] [CrossRef]
- Zuo, Z.P.; Lv, H.; Li, R.B. A new approach to recover the valuable elements in black aluminum dross. Resour. Conserv. Recycl. 2021, 174, 105768. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, S.; Song, L.; Qu, Z.; Wang, H. Influence of Carbon Dioxide Curing on the Corrosion Resistance of Reinforced Cement Mortar under the External Erosion of NaCl Freeze–Thaw Cycle. Appl. Sci. 2022, 12, 5061. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Zanoletti, A.; Ponti, J.; Valsesia, A.; La Spina, R.; Zacco, A.; Bontempi, E. Zero-waste approach in municipal solid waste incineration: Reuse of bottom ash to stabilize fly ash. J. Clean. Prod. 2020, 245, 118779. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Tian, X. The Effect of Secondary Aluminum Ash on the Properties of Reactive Powder Concrete. Materials 2023, 16, 5265. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, M.; Chen, G.; Han, W. Experimental study of the effects of graphene nanoplatelets on microstructure and compressive properties of concrete under chloride ion corrosion. Constr. Build. Mater. 2022, 360, 129564. [Google Scholar] [CrossRef]
- GB/T50082-2009; Standard for Test Method of Long-Term Performance and Durability of Ordinary Concrete. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2009.
- Tan, Y.; Chen, H.; Wang, Z.; Xue, C.; He, R. Performances of Cement Mortar Incorporating Superabsorbent Polymer (SAP) Using Different Dosing Methods. Materials 2019, 12, 1619. [Google Scholar] [CrossRef]
- Ohtsu, M. Nondestructive evaluation of damaged concrete due to freezing and thawing by elastic-wave method. J. Adv. Concr. Technol. 2005, 3, 333–341. [Google Scholar] [CrossRef][Green Version]
- Cao, Z.; Wang, K.; Peng, X.; Wang, H.; Huang, R. Influence of NaCl Solution External Erosion on Corrosion Resistance of RPC Reinforced with Straw Fiber. Coatings 2023, 13, 1308. [Google Scholar] [CrossRef]
- Heidari, A.; Shourabi, F.N. Mechanical properties of ultra-high performance concrete based on reactive powder concrete: Effect of sand-to-cement ratio, adding glass fiber and calcium carbonate. Constr. Build. Mater. 2023, 368, 130108. [Google Scholar] [CrossRef]
- David, O.; Opeyemi, J.; Adekunle, M.; Babatunde, F. Influence of secondary aluminum dross (SAD) on compressive strength and water absorption capacity properties of sandcrete block. Cogent. Eng. 2019, 6, 1608687. [Google Scholar]
- Sreekumaran, S.; Mohan, K. Low-Velocity impact resistance of reactive powder concrete modified using ground granulated blast furnace slag and rice husk ash. Constr. Build. Mater. 2022, 341, 127891. [Google Scholar] [CrossRef]
- Abdulkareem, O.M.; Fraj, A.B.; Bouasker, M. Microstructural investigation of slag-blended UHPC: The effects of slag content and chemical/thermal activation. Constr. Build. Mater. 2021, 292, 123455. [Google Scholar] [CrossRef]
- Liu, J.; Zang, S.; Yang, F.; Zhang, M.; Li, A. Fracture Mechanical Properties of Steel Fiber Reinforced Self-Compacting Concrete under Dry–Wet Cycle Sulfate Attack. Buildings 2022, 12, 1623. [Google Scholar] [CrossRef]
- Zhang, J.; Shu, Y.; Fang, A.; Qin, R.; Wu, Y.; Zhang, J. Effect of dry-wet cycle on dynamic mechanical characteristics of EPS cement soil under different chloride salt environments. Case. Stud. Constr. Mat. 2023, 18, e02151. [Google Scholar] [CrossRef]
- Wang, L.; Yin, S.; Wang, F. Interfacial bonding performance of textile reinforced ECC and seawater sea-sand concrete under a dry-wet environment. Constr. Build. Mater. 2023, 368, 130384. [Google Scholar] [CrossRef]
- Zhang, Z.; Gong, R.; Zhang, H.; He, W. The Sustainability Performance of Reinforced Concrete Structures in Tunnel Lining Induced by Long-Term Coastal Environment. Sustainability 2020, 12, 3946. [Google Scholar] [CrossRef]
- Liang, J.; Zhu, H.; Chen, L.; Qinglin, X.; Ying, G. Rebar corrosion investigation in rubberaggregate concrete via the chloride electro-accelerated test. Materials 2019, 12, 862. [Google Scholar] [CrossRef]
- Mi, T.; Li, Y.; Liu, W.; Dong, Z. The effect of carbonation on chloride redistribution and corrosion of steel reinforcement. Constr. Build. Mater. 2023, 363, 129641. [Google Scholar] [CrossRef]
- Kępniak, M.; Woyciechowski, P.; Łukowski, P.; Kuziak, J.; Kobyłka, R. The Durability of Concrete Modified by Waste Limestone Powder in the Chemically Aggressive Environment. Materials 2019, 12, 1693. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Yin, S.; Lin, F.; Dong, P. Study on bond performance between seawater sea-sand concrete and BFRP bars under chloride corrosion. Constr. Build. Mater. 2023, 371, 130718. [Google Scholar]
- Shi, X.; Fay, L.; Peterson, M.; Yang, Z. Freeze-thaw damage and chemical change of a portland cement concrete in the presence of diluted deicers. Mater. Struct. 2010, 43, 933–946. [Google Scholar] [CrossRef]
- Zhang, J. Electrochemical Measurement Technology; Chemical Industry Press: Beijing, China, 2010. [Google Scholar]
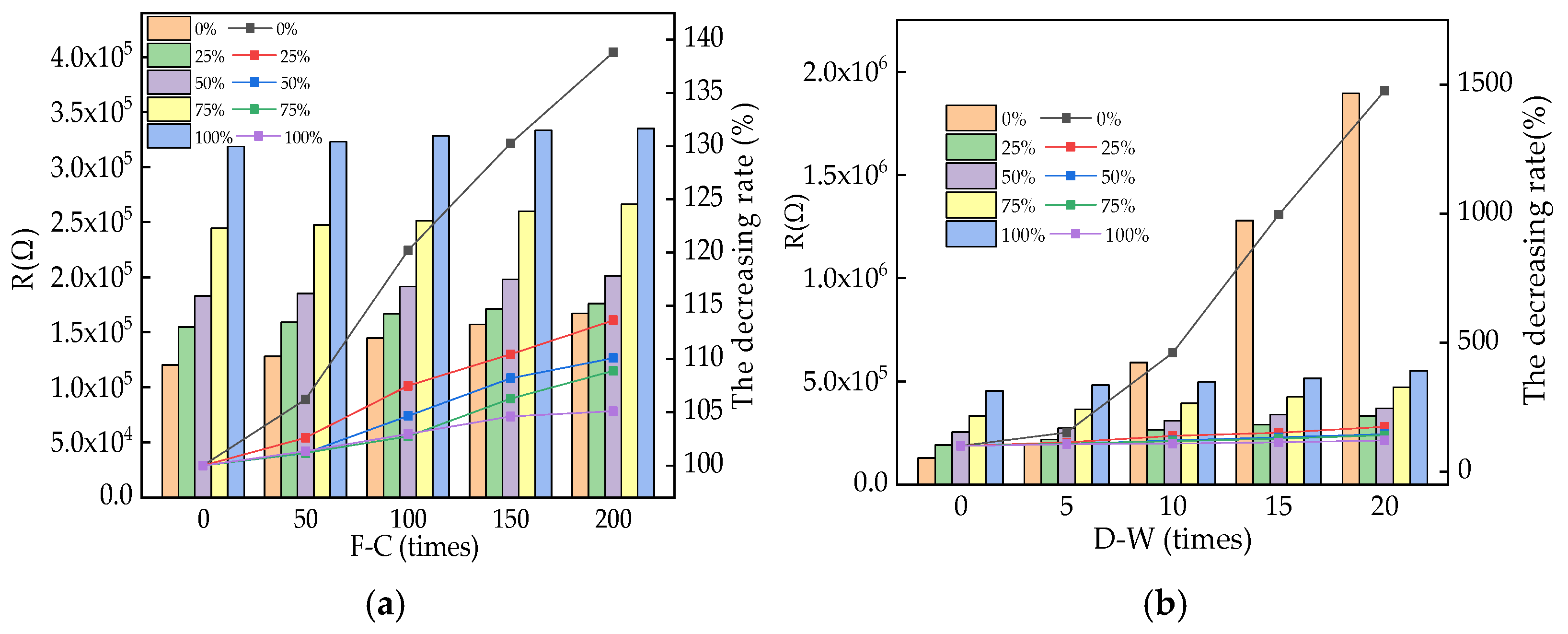
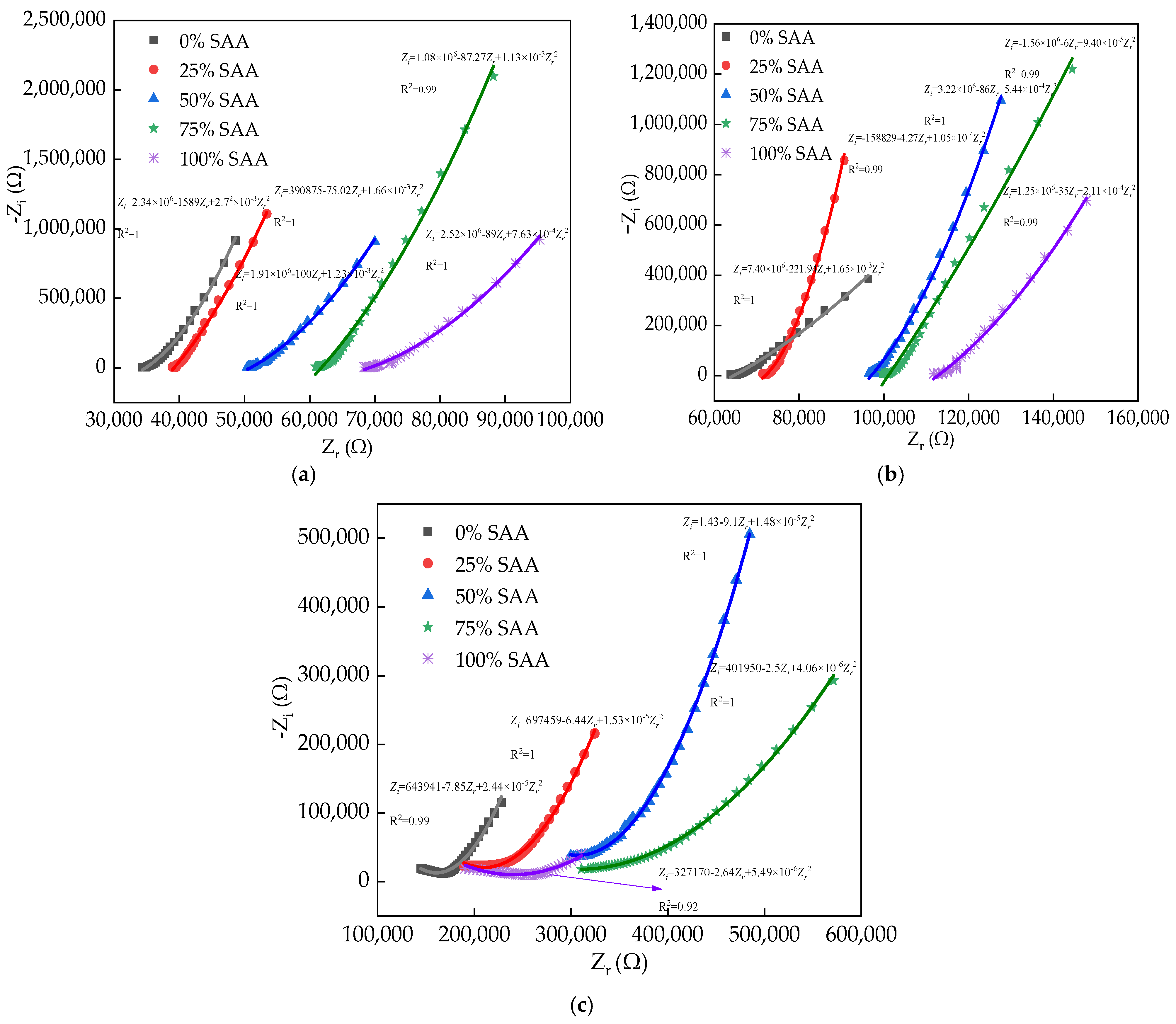

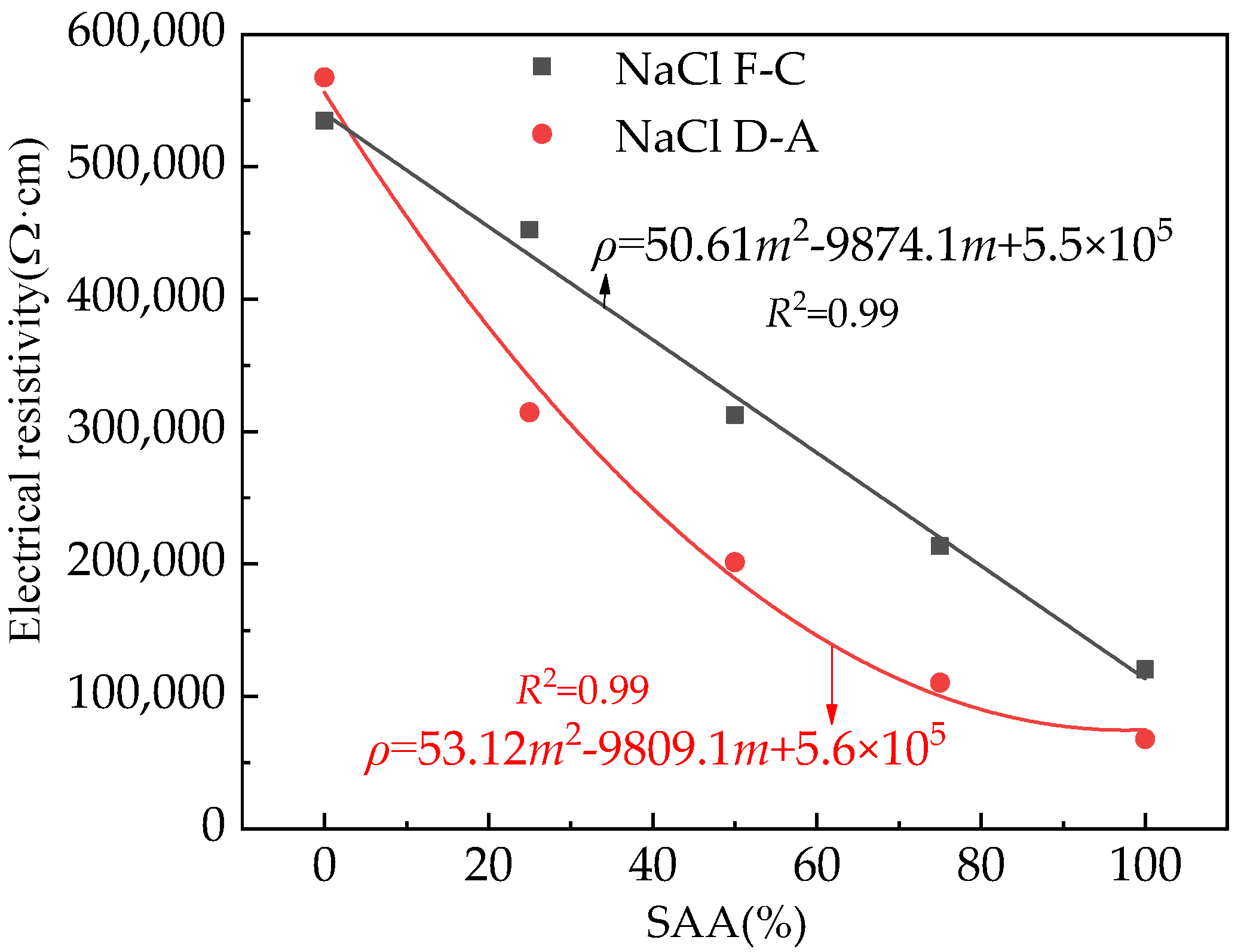
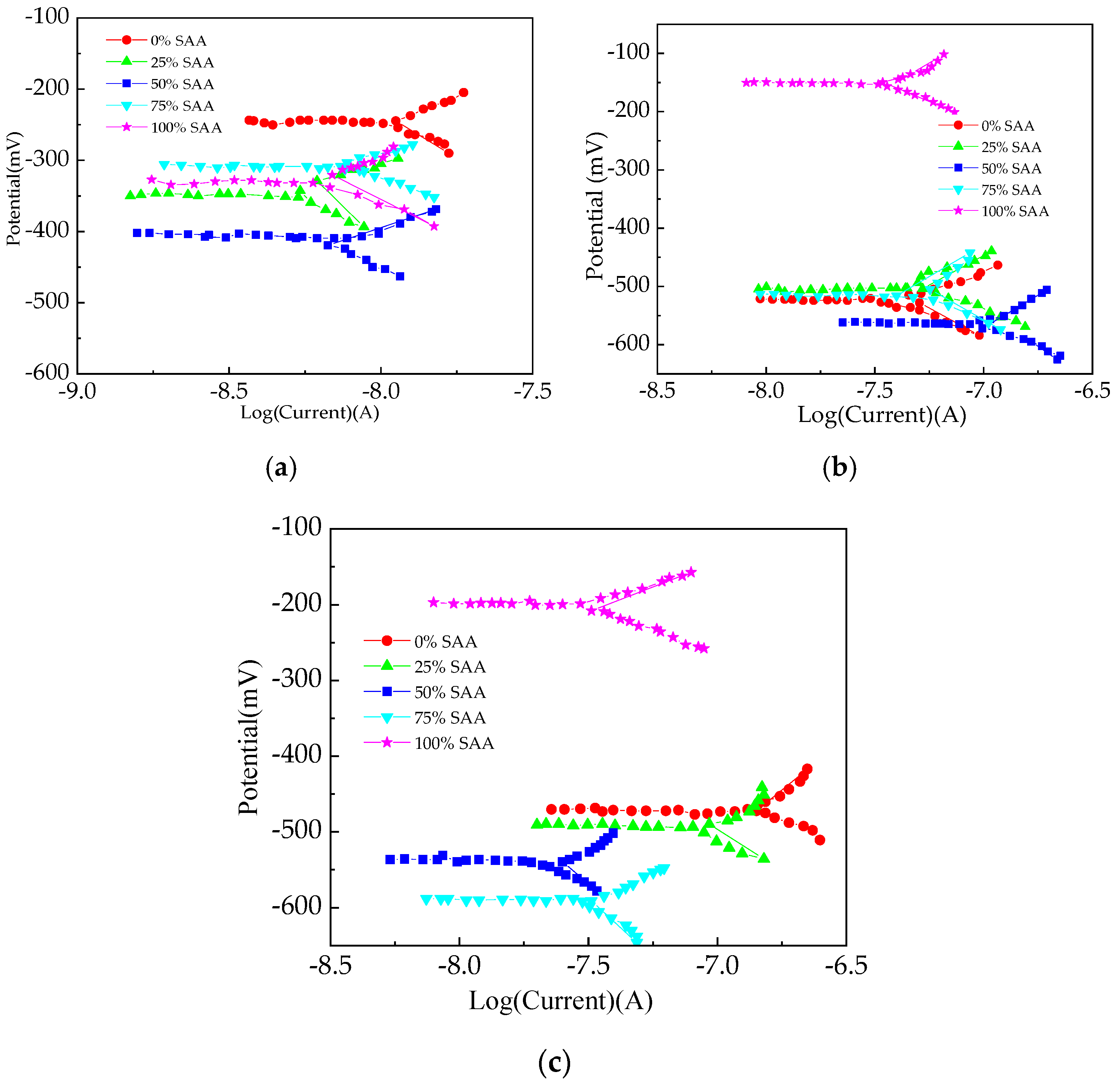
| Types | Particle Size/μm | ||||||
|---|---|---|---|---|---|---|---|
| 0.3 | 0.6 | 1 | 4 | 8 | 64 | 360 | |
| OPC | 0.13 | 0.33 | 2.36 | 15.22 | 28.25 | 93.11 | 100.00 |
| BFS | 0.04 | 0.12 | 3.25 | 19.31 | 35.08 | 97.86 | 100.00 |
| FA | 31.14 | 58.33 | 82.17 | 100.00 | 100.00 | 100.00 | 100.00 |
| Quartz sand | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 23.93 | 100.00 |
| SAA | 0.06 | 0.24 | 0.56 | 1.15 | 3.92 | 25.9 | 87.25 |
| Types | SiO2 | Al2O3 | FexOy | MgO | CaO | SO3 | K2O | Na2O | Ti2O | Loss on Ignition |
|---|---|---|---|---|---|---|---|---|---|---|
| OPC | 20.77 | 5.47 | 3.88 | 1.69 | 61.83 | 2.68 | - | - | - | 3.08 |
| BFS | 33.8 | 15.1 | 0.6 | 9.7 | 36.7 | 0.4 | 3.7 | - | - | - |
| FA | 90.60 | 0.23 | 0.61 | 0.25 | 0.43 | 0.20 | 7.50 | - | - | - |
| Quartz sand | 99.9 | - | 0.1 | - | - | - | - | - | - | - |
| SAA | 4.6 | 79.3 | 3.9 | 5.7 | 1.5 | - | - | 0.9 | - | - |
| Water | OPC | SAA | FA | BFS | Quartz Sand | Water Reducer |
|---|---|---|---|---|---|---|
| 241.96 | 733.29 | 0.00 | 366.60 | 109.99 | 968.12 | 16.14 |
| 241.96 | 733.29 | 91.67 | 274.92 | 109.99 | 968.12 | 16.14 |
| 241.96 | 733.29 | 183.35 | 183.35 | 109.99 | 968.12 | 16.14 |
| 241.96 | 733.29 | 274.92 | 91.67 | 109.99 | 968.12 | 16.14 |
| 241.96 | 733.29 | 366.60 | 0.00 | 109.99 | 968.12 | 16.14 |
| Measuring Parameters | Number of Specimens | Standard Deviation |
|---|---|---|
| MR | 6 | 0.091 |
| DME | 6 | 0.087 |
| R | 6 | 0.093 |
| EIS | 6 | 0.098 |
| Tafel experiment | 6 | 0.093 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Wang, K.; Wang, H. The Corrosion Resistance of Reinforced Reactive Powder Concrete with Secondary Aluminum Ash Exposed to NaCl Action. Materials 2023, 16, 5615. https://doi.org/10.3390/ma16165615
Jiang H, Wang K, Wang H. The Corrosion Resistance of Reinforced Reactive Powder Concrete with Secondary Aluminum Ash Exposed to NaCl Action. Materials. 2023; 16(16):5615. https://doi.org/10.3390/ma16165615
Chicago/Turabian StyleJiang, Hong, Kewei Wang, and Hui Wang. 2023. "The Corrosion Resistance of Reinforced Reactive Powder Concrete with Secondary Aluminum Ash Exposed to NaCl Action" Materials 16, no. 16: 5615. https://doi.org/10.3390/ma16165615
APA StyleJiang, H., Wang, K., & Wang, H. (2023). The Corrosion Resistance of Reinforced Reactive Powder Concrete with Secondary Aluminum Ash Exposed to NaCl Action. Materials, 16(16), 5615. https://doi.org/10.3390/ma16165615






