Evaluation of Nitrogen Oxide Reduction Performance in Permeable Concrete Surfaces Treated with a TiO2 Photocatalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Permeable Concrete
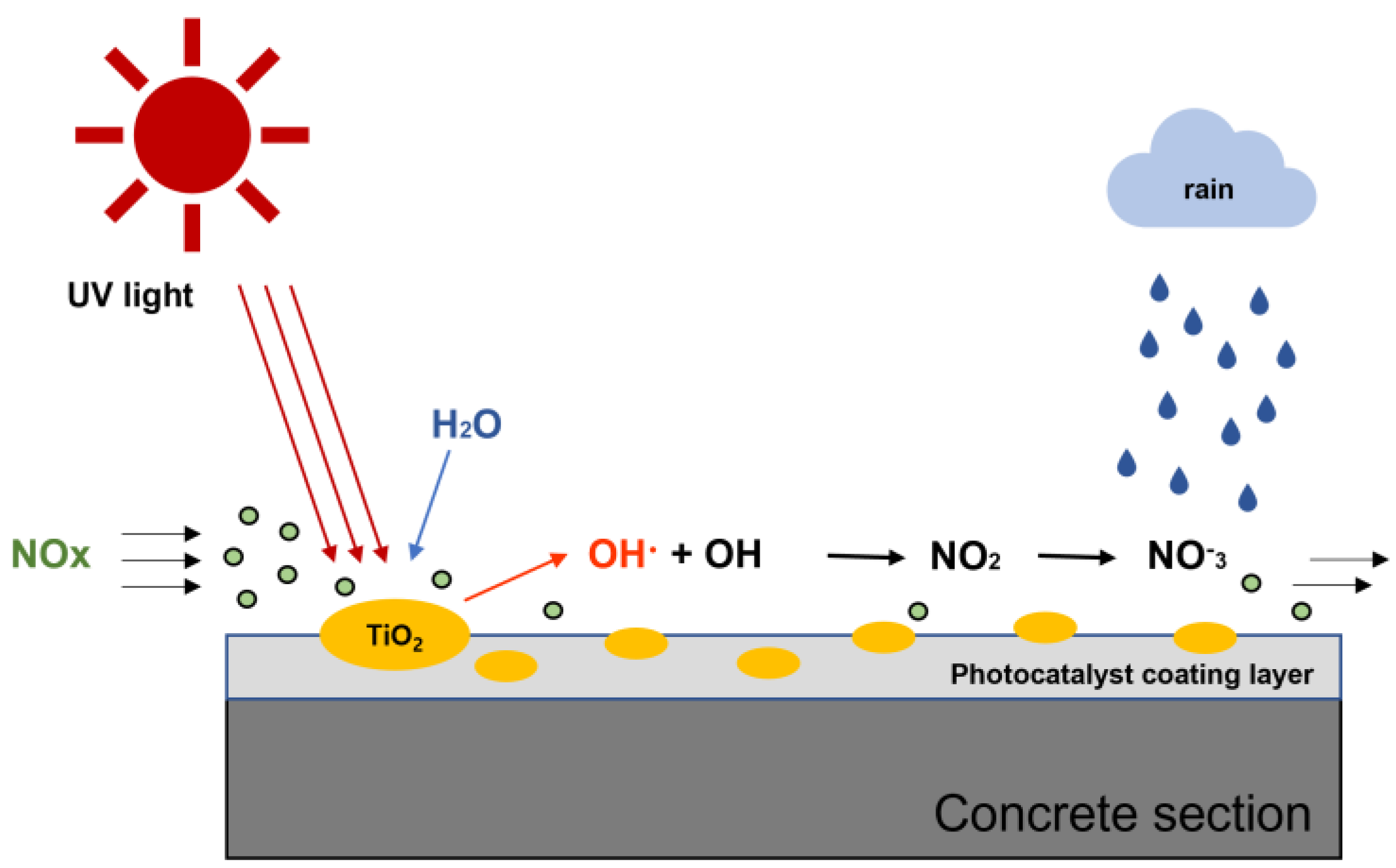
2.1.2. Photocatalyst
2.2. Experimental Variable
2.3. Characterization
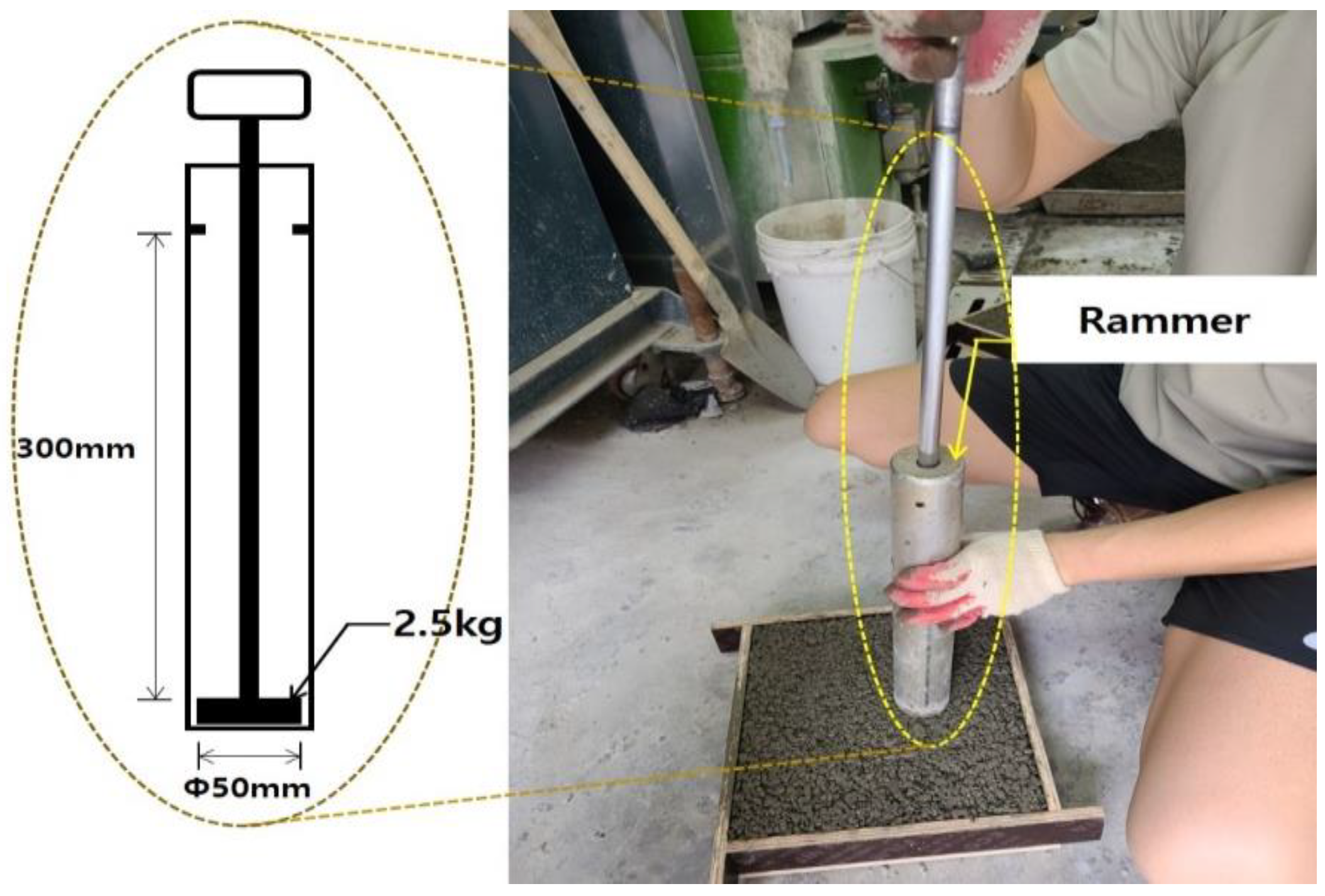
2.3.1. Mechanical and Durability Tests
2.3.2. NOx Reduction Performance Evaluation Test
2.3.3. Nitrate Assessment
3. Results and Discussion
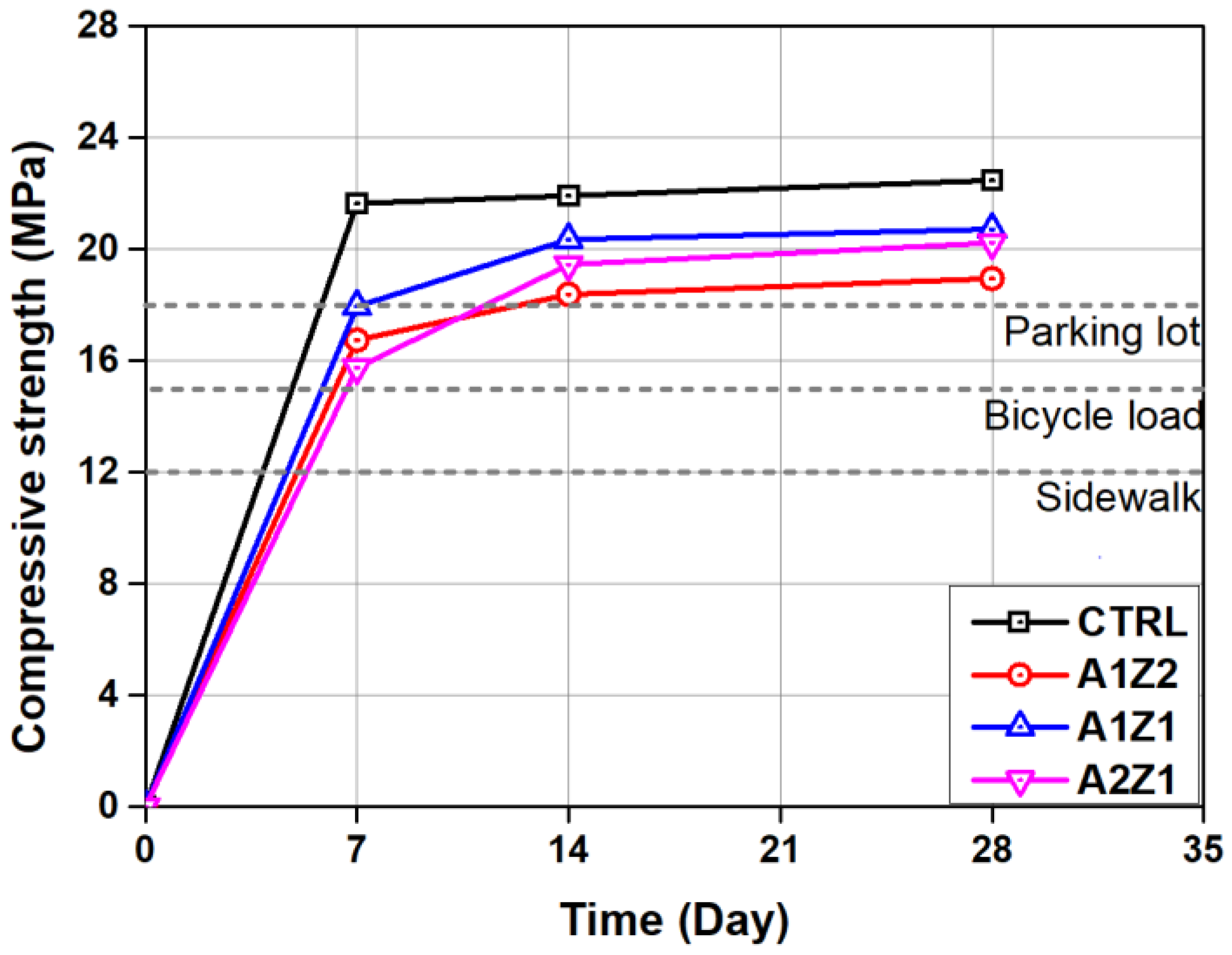
3.1. Mechanical Properties of Permeable Concretes
3.2. Durability of Permeable Concretes
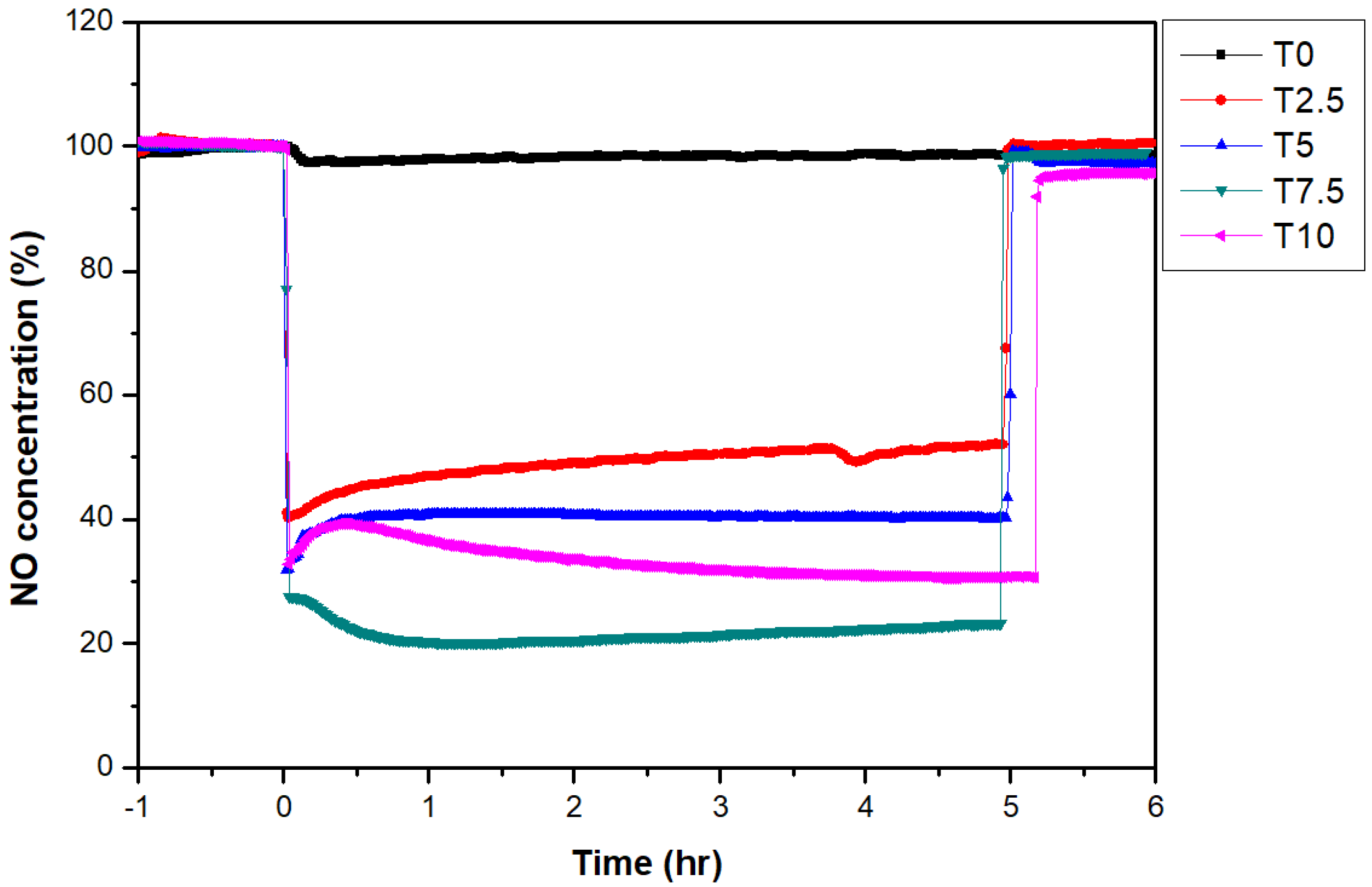
3.3. NOx Reduction Performance Evaluation According to TiO2 Content
3.4. Nitrate Analysis after Photolysis of Nitrogen Oxides
4. Conclusions
- 1.
- A decrease in the physical performance of permeable concrete that includes active loess and zeolite was observed in comparison to the CTRL, but they all satisfied the quality standard of permeable concrete for sidewalk and road pavement.
- 2.
- The skid resistance and permeability coefficient showed results that satisfy standards for sidewalk concrete in all permeable concrete specimens, but the quality standard was satisfied at 80% or above of residual compressive strength in only CTRL and A1Z1 specimens in the freezing and thawing experiment.
- 3.
- Considering the physical and durability performances of this study, A1Z1 mixture was determined as the optimal mixing ratio for permeable concrete for applying TiO2 photocatalyst.
- 4.
- The NOx reduction efficiency tended to increase according to the increase in TiO2 content. With 7.5% TiO2 content, a maximum NOx reduction efficiency of 77.5% was observed.
- 5.
- The tank photoreactor of the specimen using the compressive strength test piece also showed similar results to the NOx reduction tendency in the ISO standard photoreactor.
- 6.
- By assessing the nitrate concentration generated after NOx reduction assessment, it was found that a photocatalyst reaction occurred during UV irradiation on the surface of the photocatalyst-coated permeable concrete. Additionally, higher nitrate concentration and higher NOx reduction efficiency were observed with 7.5% TiO2 content than with 10% TiO2 content.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jang, A.-S. Impact of Particulate Matter on Health. J. Korean Med. Assoc. 2014, 57, 763–768. [Google Scholar] [CrossRef] [Green Version]
- Samet, J.M.; Dominici, F.; Curriero, F.C.; Coursac, I.; Zeger, S.L. Fine Particulate Air Pollution and Mortality in 20 U.S. Cities, 1987–1994. N. Engl. J. Med. 2000, 343, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Laden, F.; Schwartz, J.; Speizer, F.E.; Dockery, D.W. Reduction in Fine Particulate Air Pollution and Mortality: Extended Follow-up of the Harvard Six Cities Study. Am. J. Respir. Crit. Care Med. 2006, 173, 667–672. [Google Scholar] [CrossRef] [Green Version]
- Pope, C.A.; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung Cancer, Cardiopulmonary Mortality, and Long-Term Exposure to Fine Particulate Air Pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A. Outdoor Air Pollution a Leading Environmental Cause of Cancer Deaths; International Institution for Research on Cancer (IARC): Lyon, France, 2013; Volume 161, ISBN 978-92-832-2166-1. [Google Scholar]
- Kim, J.S.; Choi, Y.J.; Lee, K.B.; Kim, S. Do Relation with Activity of Road Mobile Source and Roadside Nitrogen Oxide Concentration. J. Korean Soc. Atmos. Environ. 2016, 32, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Nicolás, M.; Navarro-Blasco, I.; Fernández, J.M.; Alvarez, J.I. Atmospheric NOx Removal: Study of Cement Mortars with Iron- and Vanadium-Doped TiO2 as Visible Light–Sensitive Photocatalysts. Constr. Build. Mater. 2017, 149, 257–271. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.; Liu, H.; Li, W.; Lyu, X. Automobile Exhaust Removal Performance of Pervious Concrete with Nano TiO2 under Photocatalysis. Nanomaterials 2020, 10, 2088. [Google Scholar] [CrossRef]
- Park, T.S.; Cho, D.M.; Kim, D.C. Photocatalyst of Asphalt Mixture for Reducing Nitrogen Oxide (NOx) Application Case Literature Survey Study. J. Korean Asph. Inst. 2021, 11, 185–192. [Google Scholar]
- Kim, H.-J.; Park, J.-H.; Yoon, Y.-S.; Kwon, S.-J. Durability and Mechanical Performance in Activated Hwangtoh -Based Composite for NOx Reduction. Adv. Concr. Constr. 2021, 11, 307–314. [Google Scholar]
- Park, J.-H.; Kim, H.-J. Evaluation of NOx Reduction Performance by Photocatalytic (TiO2) Coating of Cement Mortar Mixed with Zeolite and Activate Hwangtoh. J. Korean Recycl. Constr. Resour. Inst. 2020, 8, 483–489. [Google Scholar]
- Dylla, H.; Hassan, M.M.; Schmitt, M.; Rupnow, T.; Mohammad, L.N. Laboratory Investigation of the Effect of Mixed Nitrogen Dioxide and Nitrogen Oxide Gases on Titanium Dioxide Photocatalytic Efficiency in Concrete Pavements. J. Mater. Civ. Eng. 2011, 23, 1087–1093. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, C.; Chen, Y. Experimental Study on Photocatalytic Degradation Efficiency of Mixed Crystal Nano-TiO2 Concrete. Nanotechnol. Rev. 2020, 9, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Gopala Krishna Sastry, K.V.S.; Sahitya, P.; Ravitheja, A. Influence of Nano TiO2 on Strength and Durability Properties of Geopolymer Concrete. Mater. Today Proc. 2021, 45, 1017–1025. [Google Scholar] [CrossRef]
- Kim, Y.K.; Hong, S.J.; Kim, H.B.; Lee, S.W. Evaluation of In-Situ NOx Removal Efficiency of Photocatalytic Concrete in Expressways. KSCE J. Civ. Eng. 2018, 22, 2274–2280. [Google Scholar] [CrossRef]
- Boonen, E.; Beeldens, A. Recent Photocatalytic Applications for Air Purification in Belgium. Coatings 2014, 4, 553–573. [Google Scholar] [CrossRef] [Green Version]
- Maggos, T.; Bartzis, J.G.; Liakou, M.; Gobin, C. Photocatalytic Degradation of NOx Gases Using TiO2-Containing Paint: A Real Scale Study. J. Hazard. Mater. 2007, 146, 668–673. [Google Scholar] [CrossRef]
- Guerrini, G.L. Photocatalytic Performances in a City Tunnel in Rome: NOx Monitoring Results. Constr. Build. Mater. 2012, 27, 165–175. [Google Scholar] [CrossRef]
- Ibrahim, I.; Kaltzoglou, A.; Athanasekou, C.; Katsaros, F.; Devlin, E.; Kontos, A.G.; Ioannidis, N.; Perraki, M.; Tsakiridis, P.; Sygellou, L.; et al. Magnetically Separable TiO2/CoFe2O4/Ag Nanocomposites for the Photocatalytic Reduction of Hexavalent Chromium Pollutant under UV and Artificial Solar Light. Chem. Eng. J. 2020, 381, 122730. [Google Scholar] [CrossRef]
- Sakkas, V.A.; Arabatzis, I.M.; Konstantinou, I.K.; Dimou, A.D.; Albanis, T.A.; Falaras, P. Metolachlor Photocatalytic Degradation Using TiO2 Photocatalysts. Appl. Catal. B 2004, 49, 195–205. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Cheng, B.; Yu, J.; Fan, J. Sulfur-Doped g-C3N4/TiO2 S-Scheme Heterojunction Photocatalyst for Congo Red Photodegradation. Chin. J. Catal. 2021, 42, 56–68. [Google Scholar] [CrossRef]
- Park, J.-Y.; Choi, K.-I.; Lee, J.-H.; Hwang, C.-H.; Choi, D.-Y.; Lee, J.-W. Fabrication and Characterization of Metal-Doped TiO2 Nanofibers for Photocatalytic Reactions. Mater. Lett. 2013, 97, 64–66. [Google Scholar] [CrossRef]
- Han, C.; Pelaez, M.; Likodimos, V.; Kontos, A.G.; Falaras, P.; O’Shea, K.; Dionysiou, D.D. Innovative Visible Light-Activated Sulfur Doped TiO2 Films for Water Treatment. Appl. Catal. B 2011, 107, 77–87. [Google Scholar] [CrossRef]
- Ibrahim, I.; Belessiotis, G.V.; Antoniadou, M.; Kaltzoglou, A.; Sakellis, E.; Katsaros, F.; Sygellou, L.; Arfanis, M.K.; Salama, T.M.; Falaras, P. Silver Decorated TiO2/g-C3N4 Bifunctional Nanocomposites for Photocatalytic Elimination of Water Pollutants under UV and Artificial Solar Light. Results Eng. 2022, 14, 100470. [Google Scholar] [CrossRef]
- Choi, H.-Y.; Kim, M.-H.; Kim, M.-H.; Hwang, H.-Z.; Choi, S.-W. Experimental Study on the Properties of Concrete by the Kinds of Admixture and the Replacement Ratios of Activated Hwangtoh. J. Korea Concr. Inst. 2001, 13, 123–129. [Google Scholar]
- Kwon, S.J.; Lim, H.S.; Kim, H.J.; Hyun, J.H. Evaluation of Mechanical Properties of Mortar Mixed with Zeolites and Active Hwangtoh. J. Korean Recycl. Constr. Resour. Inst. 2019, 7, 405–412. [Google Scholar]
- SPS-F-KSPIC-001-2006:2018; Soil Concrete. Korea Scenery Pavement Industry Cooperative: Daejeon, Republic of Korea, 2018. Available online: https://www.standard.go.kr/KSCI/ct/ptl/std/curstat/detail.do (accessed on 10 July 2023).
- ISO 22197-1:2016; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Air-Purification Performance of Semiconducting Photocatalytic Materials—Part 1: Removal of Nitric Oxide. International Organization for Standardization: Geneve, Switzerland, 2016. Available online: https://www.iso.org/standard/65416.html (accessed on 10 July 2023).
- Hassan, M.; Mohammad, L.N.; Asadi, S.; Dylla, H.; Cooper, S. Sustainable Photocatalytic Asphalt Pavements for Mitigation of Nitrogen Oxide and Sulfur Dioxide Vehicle Emissions. J. Mater. Civil. Eng. 2013, 25, 365–371. [Google Scholar] [CrossRef]
- Park, J.; Park, J.; Jung, M. Variation in Service Life on RC Structure According to Concrete Binder Type. Materials 2020, 13, 5430. [Google Scholar] [CrossRef]
- Lee, H.S.; Ismail, M.; Hussin, M.W. Durability Probabilistic Evaluation of RC Structures Subjected to Chloride Ion. Renew. Energy Sustain. Dev. 2015, 1, 160. [Google Scholar] [CrossRef]
- Mehta, P.K. Blended Cements in Construction. Cem. Concr. Compos. 1992, 14, 223–224. [Google Scholar] [CrossRef]
- Halstead, W.J.; Ozyildirim, C. Improved Concrete Quality with Combinations of Fly Ash and Silica Fume. ACI Mater. J. 1995, 91, 587–594. [Google Scholar] [CrossRef]








| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | Etc. |
|---|---|---|---|---|---|---|
| 62.79% | 21.74% | 5.00% | 3.17% | 2.97% | 1.67% | 1.37% |
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Etc. | |
|---|---|---|---|---|---|---|---|
| Active loess | 43.0% | 35.9% | 10.8% | 7.2% | 1.6% | 0.8% | 1.7% |
| Zeolite | 68.9% | 16.4% | 5.3% | 2.6% | 1.0% | 3.7% | 2.1% |
| Properties | Unit | Value |
|---|---|---|
| TiO2 type | - | Anatase |
| TiO2 content | % | 39–41 |
| Viscosity | MPa·s | ≤30 |
| pH value | - | 5.0–7.0 |
| Density at 20 °C | g/m3 | 1.41 |
| Particle size | nm | 70 |
| Specimen | W/B (%) | Water (kg) | Binder (kg) | Gravel (kg) | ||
|---|---|---|---|---|---|---|
| Cement | Active Loess | Zeolite | ||||
| CTRL | 36.4 | 120 | 330 | - | - | 1600 |
| A1Z2 | 280 | 16.5 | 33 | |||
| A1Z1 | 24.75 | 24.75 | ||||
| A2Z1 | 33 | 16.5 | ||||
| Photocatalyst Code | T0 | T2.5 | T5 | T7.5 | T10 |
|---|---|---|---|---|---|
| AERODISP®W740X (g) | - | 6.2 | 12.5 | 18.8 | 25.0 |
| DI water (g) | - | 93.8 | 87.3 | 81.2 | 75.0 |
| TiO2 content (%) | 0 | 2.5 | 5 | 7.5 | 10 |
| Sidewalk | Bicycle Load | Parking Lot | Standard | |
|---|---|---|---|---|
| Compressive strength (MPa) | 12 or more | 15 or more | 18 or more | KS F 2405 |
| Flexural strength (MPa) | 1.2 or more | 1.5 or more | 1.8 or more | KS F 2408 |
| Compressive strength after 100 cycles of freezing and thawing (%) | At least 80% of strength at 28 days | KS F 2456 KS F 2405 | ||
| Skid resistance (BPN) | 30 or more | 40 or more | 40 or more | KS F 2375 |
| Permeability coefficient (cm/s) | 1.0 × 10−3 | KS F 4001 | ||
| Specimen Size (mm) | Compaction Layer | Compaction Frequency per Layer | |
|---|---|---|---|
| Compressive strength | ϕ100 × 200 | 3 | 25 |
| Flexural strength | 150 × 150 × 530 | 2 | 80 |
| Compressive strength after 100 freeze–thaw cycles | ϕ100 × 200 | 3 | 25 |
| Skid resistance | 150 × 90 × 50 | 1 | 14 |
| Permeability coefficient | 300 × 300 × 60 | 1 | 90 |
| Sample | Compressive Strength (MPa) | Flexural Strength (MPa) | ||
|---|---|---|---|---|
| 7 Days | 14 Days | 28 Days | 28 Days | |
| CTRL | 21.64 | 22.04 | 33.47 | 3.88 |
| A1Z2 | 16.74 | 18.12 | 28.95 | 3.01 |
| A1Z1 | 17.95 | 20.35 | 20.72 | 3.63 |
| A2Z1 | 15.75 | 19.56 | 20.23 | 3.02 |
| Item | NO. | CTRL | A1Z2 | A1Z1 | A2Z1 |
|---|---|---|---|---|---|
| Skid resistance (BPN) | T1 | 46 | 51 | 46 | 51 |
| T2 | 50 | 52 | 48 | 48 | |
| T3 | 49 | 48 | 50 | 50 | |
| Average | 48.3 | 50.3 | 48.0 | 49.7 | |
| Permeability coefficient (×10−3 cm/s) | T1 | 7.0 | 7 | 6.1 | 6.3 |
| T2 | 6.9 | 7.3 | 6.3 | 6.2 | |
| T3 | 6.4 | 7.5 | 5.9 | 6.6 | |
| Average | 6.77 | 6.93 | 6.1 | 6.37 |
| Sample Code | Compressive Strength (MPa) | Freezing and Thawing Resistance (%) | |
|---|---|---|---|
| Before | After | ||
| CTRL | 22.47 | 19.6 | 87.1 |
| A1Z2 | 18.95 | - | - |
| A1Z1 | 20.72 | 16.8 | 82.4 |
| A2Z1 | 20.23 | - | - |
| Sample Code | Efficiency of NOx Reduction (%) |
|---|---|
| T0 | 1.6 |
| T2.5 | 49.8 |
| T5 | 59.6 |
| T7.5 | 77.5 |
| T10 | 67.7 |
| Sample Code | Efficiency of NOx Reduction (%) |
|---|---|
| T0 | 1.4 |
| T7.5 | 51.2 |
| T10 | 46.9 |
| Sample Code | Nitrate Concentration (mg/L) | |||
|---|---|---|---|---|
| ISO Standard Photoreactor | Tank Photoreactor | |||
| 1st | 2nd | 1st | 2nd | |
| T0 | 0.01 | 0.01 | 0 | 0.1 |
| T7.5 | 2.36 | 2.28 | 2.16 | 2.53 |
| T10 | 1.72 | 1.69 | 1.89 | 2.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-j.; Hong, K. Evaluation of Nitrogen Oxide Reduction Performance in Permeable Concrete Surfaces Treated with a TiO2 Photocatalyst. Materials 2023, 16, 5512. https://doi.org/10.3390/ma16165512
Kim H-j, Hong K. Evaluation of Nitrogen Oxide Reduction Performance in Permeable Concrete Surfaces Treated with a TiO2 Photocatalyst. Materials. 2023; 16(16):5512. https://doi.org/10.3390/ma16165512
Chicago/Turabian StyleKim, Hyeok-jung, and Kinam Hong. 2023. "Evaluation of Nitrogen Oxide Reduction Performance in Permeable Concrete Surfaces Treated with a TiO2 Photocatalyst" Materials 16, no. 16: 5512. https://doi.org/10.3390/ma16165512
APA StyleKim, H.-j., & Hong, K. (2023). Evaluation of Nitrogen Oxide Reduction Performance in Permeable Concrete Surfaces Treated with a TiO2 Photocatalyst. Materials, 16(16), 5512. https://doi.org/10.3390/ma16165512





