Perspectives on Corrosion Inhibition Features of Novel Synthesized Gemini-Fluorinated Cationic Surfactants Bearing Varied Spacers for Acid Pickling of X60-Steel: Practical, and In Silico Calculations
Abstract
1. Introduction
2. Materials and Methods
2.1. Solutions and Materials
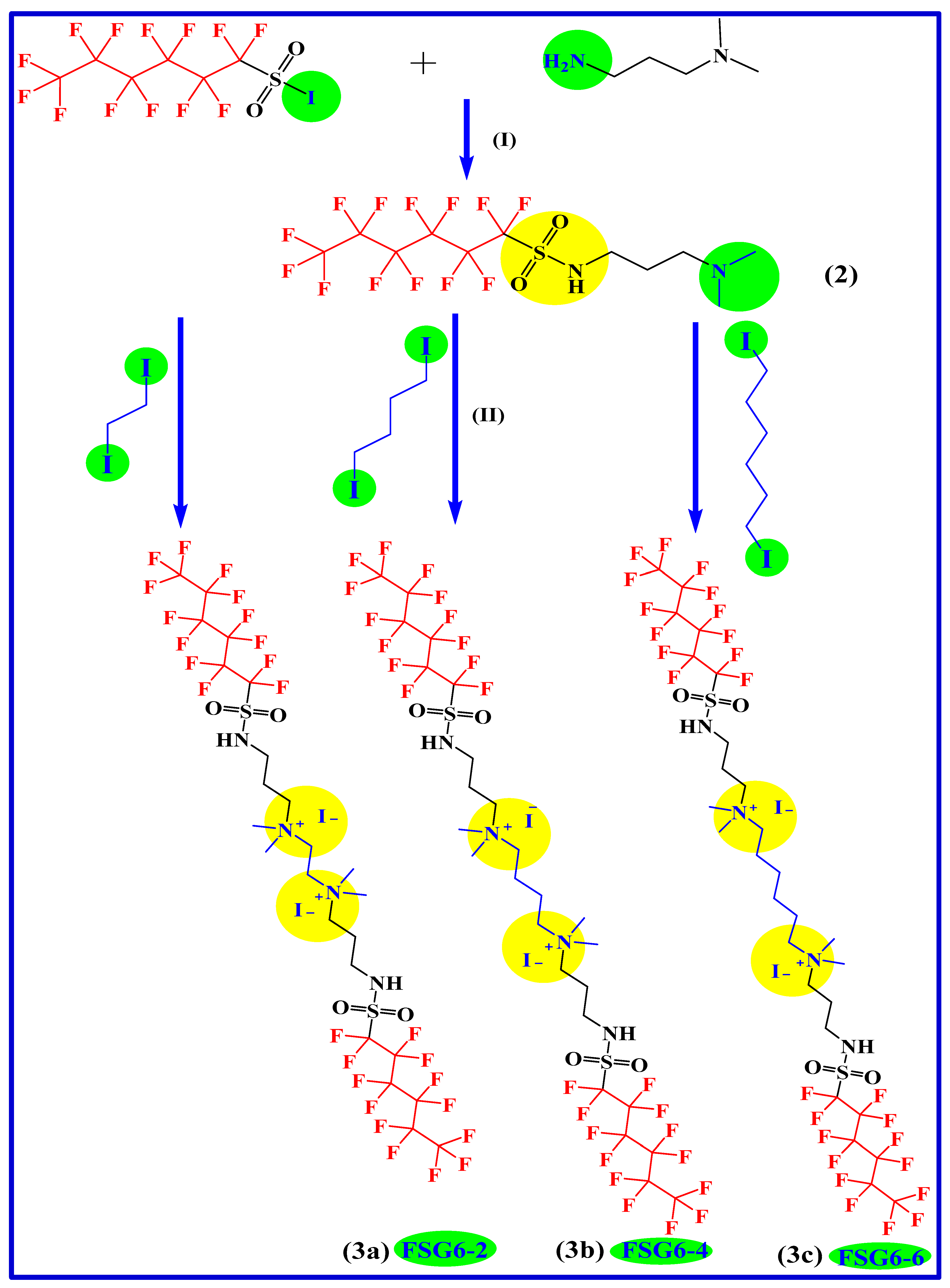
2.2. Synthetic Procedure and Characterizations
2.2.1. First Step: Preparation of N-(3-(dimethylamino)propyl)-1,1,2,2,3,3,4,4,5,5,6,6,6-tridecafluorohexane-1-sulfonamide
N-(3-(dimethylamino)propyl)-1,1,2,2,3,3,4,4,5,5,6,6,6-tridecafluorohexane-1-sulfonamide (Figure S1)
2.2.2. Second Step: Preparation of N1,N1,N2,N2-tetramethyl-N1,N2-bis(3-((perfluoro-alkyl)-sulfonamido)propyl)ethane-1,2-diaminium Iodide (3a–c)
N1,N1,N2,N2-tetramethyl-N1,N2-bis(3-((perfluorohexyl)sulfonamido)propyl)ethane-1,2-diaminium Iodide (3a, FSG6-2) (Figure S2)
N1,N1,N4,N4-tetramethyl-N1,N4-bis(3-((perfluorohexyl)sulfonamido)propyl)butane-1,4-diaminium Iodide (3b, FSG6-4) (Figure S3)
N1,N1,N6,N6-tetramethyl-N1,N6-bis(3-((perfluorohexyl)sulfonamido)propyl)hexane-1,6-diaminium Iodide (3c, FSG6-6) (Figure S4)
2.3. Measurements of Surface Tension
2.4. Electrochemical Examinations
2.5. Surface Characterization
2.6. Computational Details
3. Results and Discussion
3.1. Chemistry
3.2. Surface-Active Properties
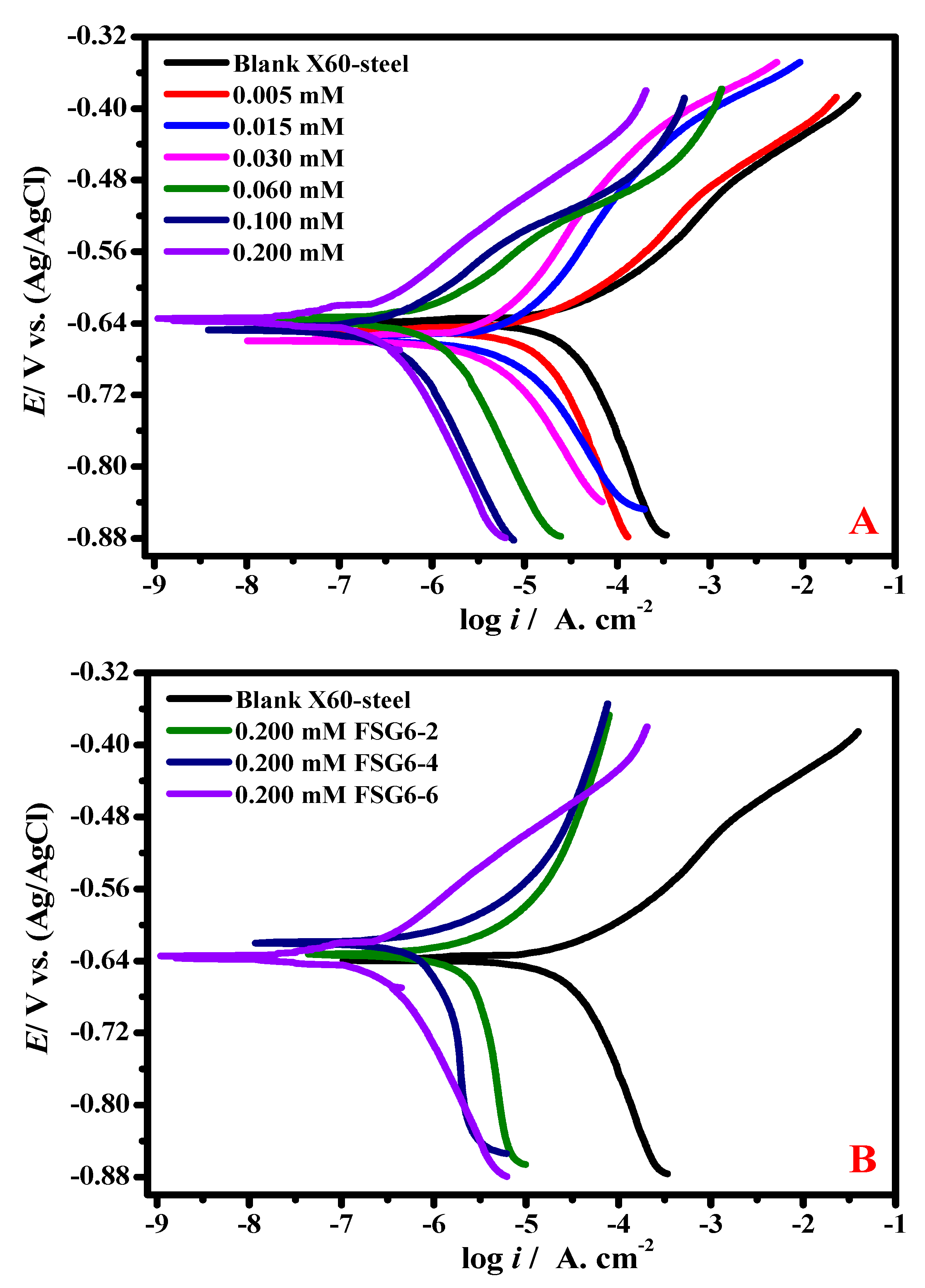
3.3. Polarization Measurements
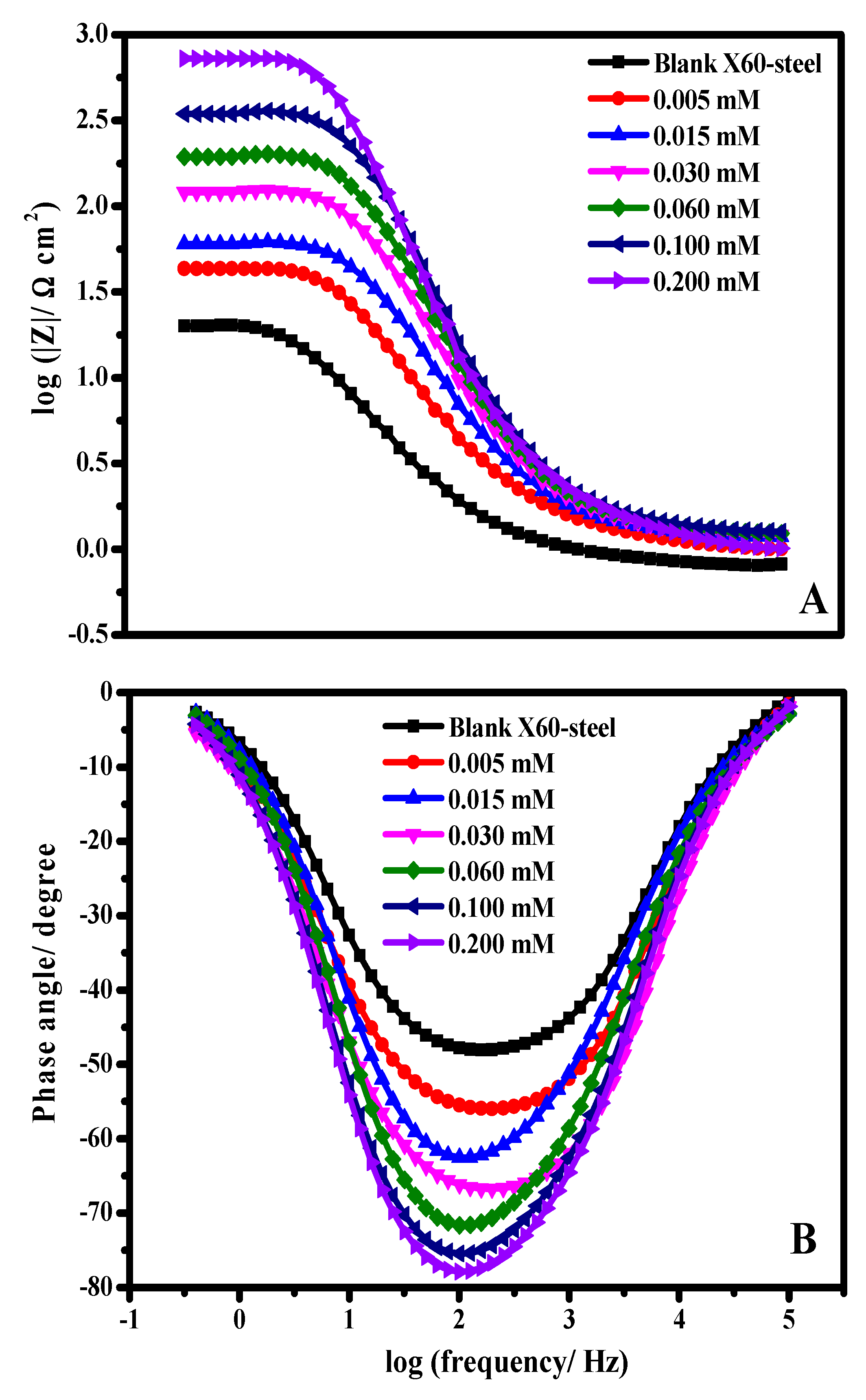
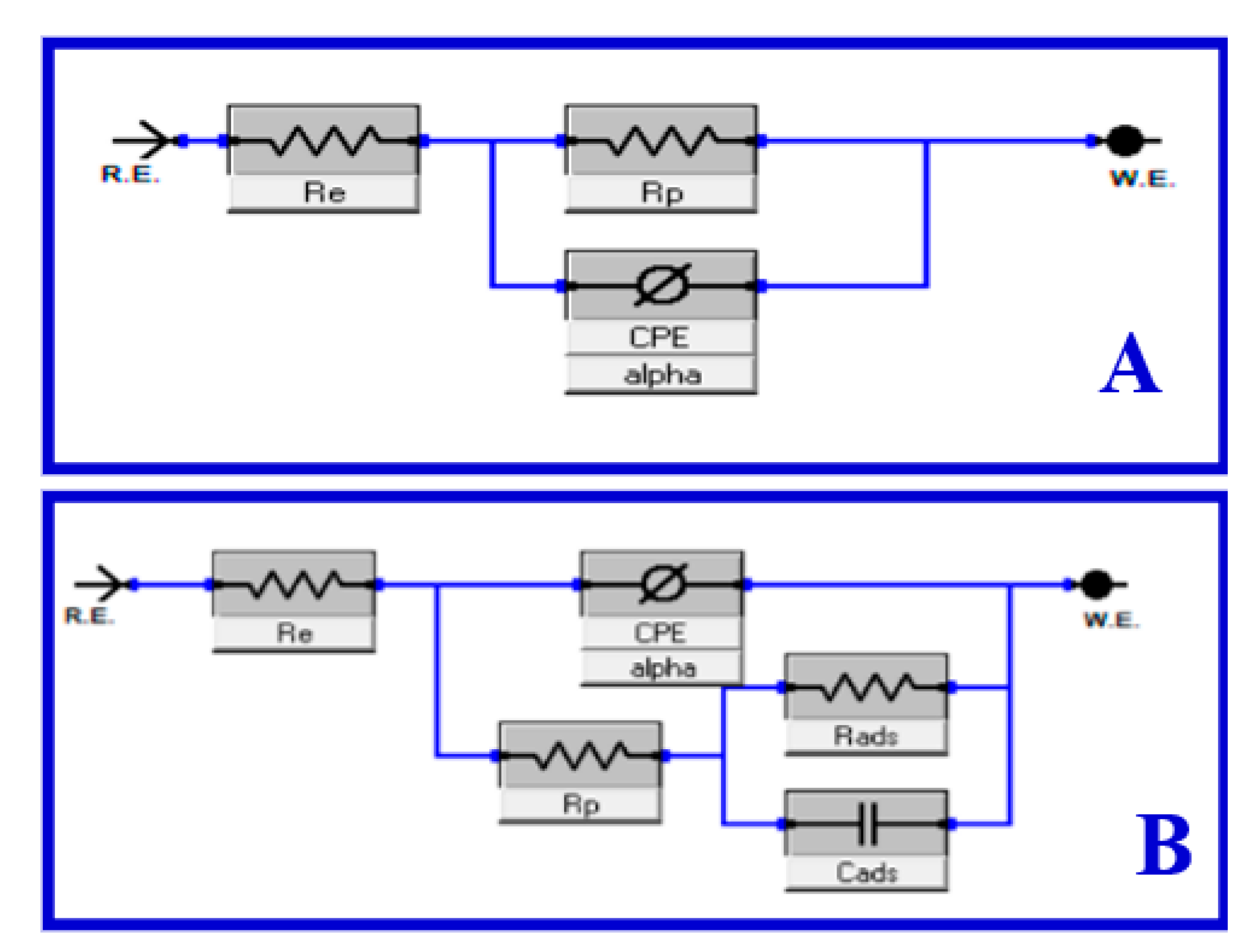
3.4. EIS Studies
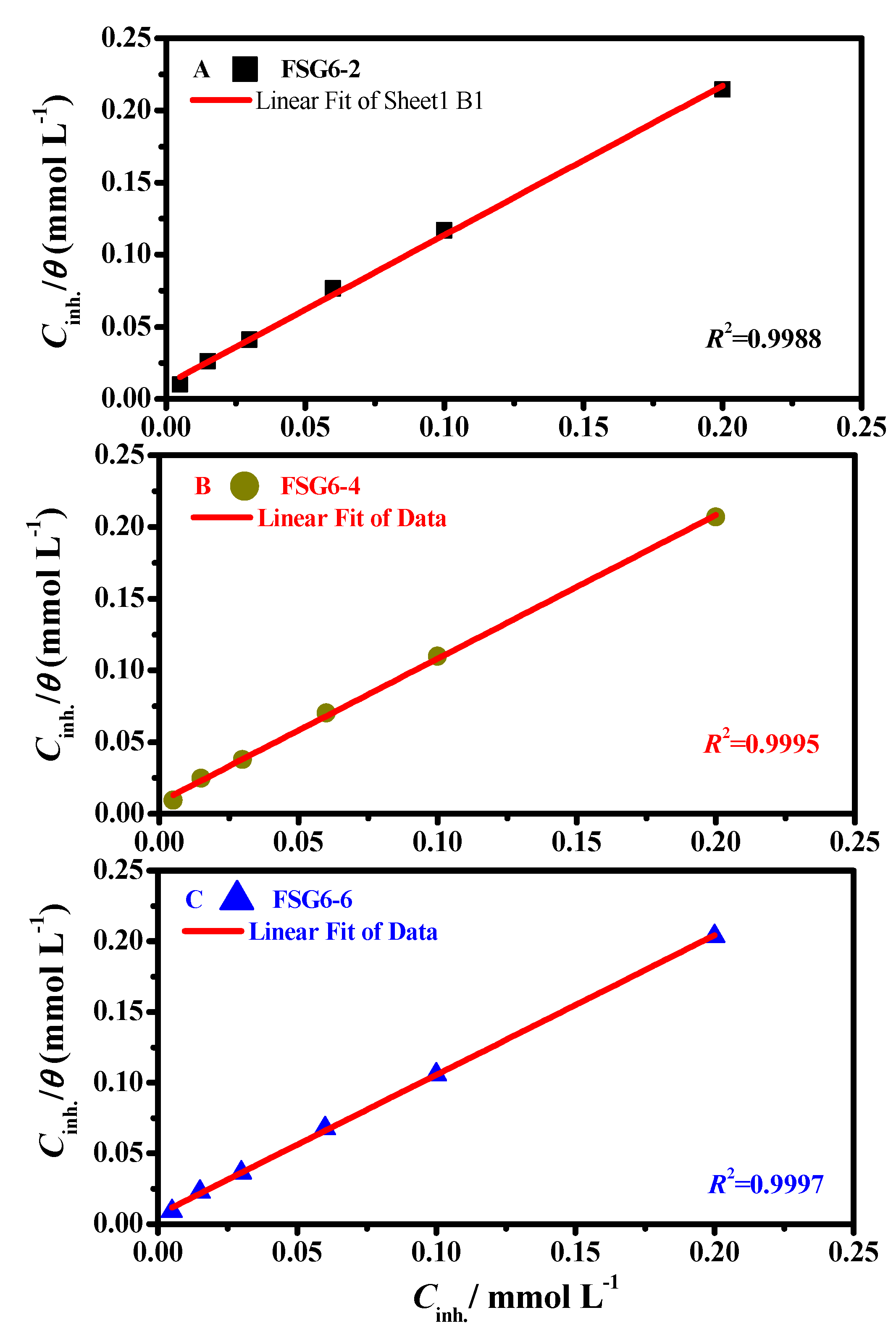
3.5. Thermodynamic Parameters and Adsorption Isotherms
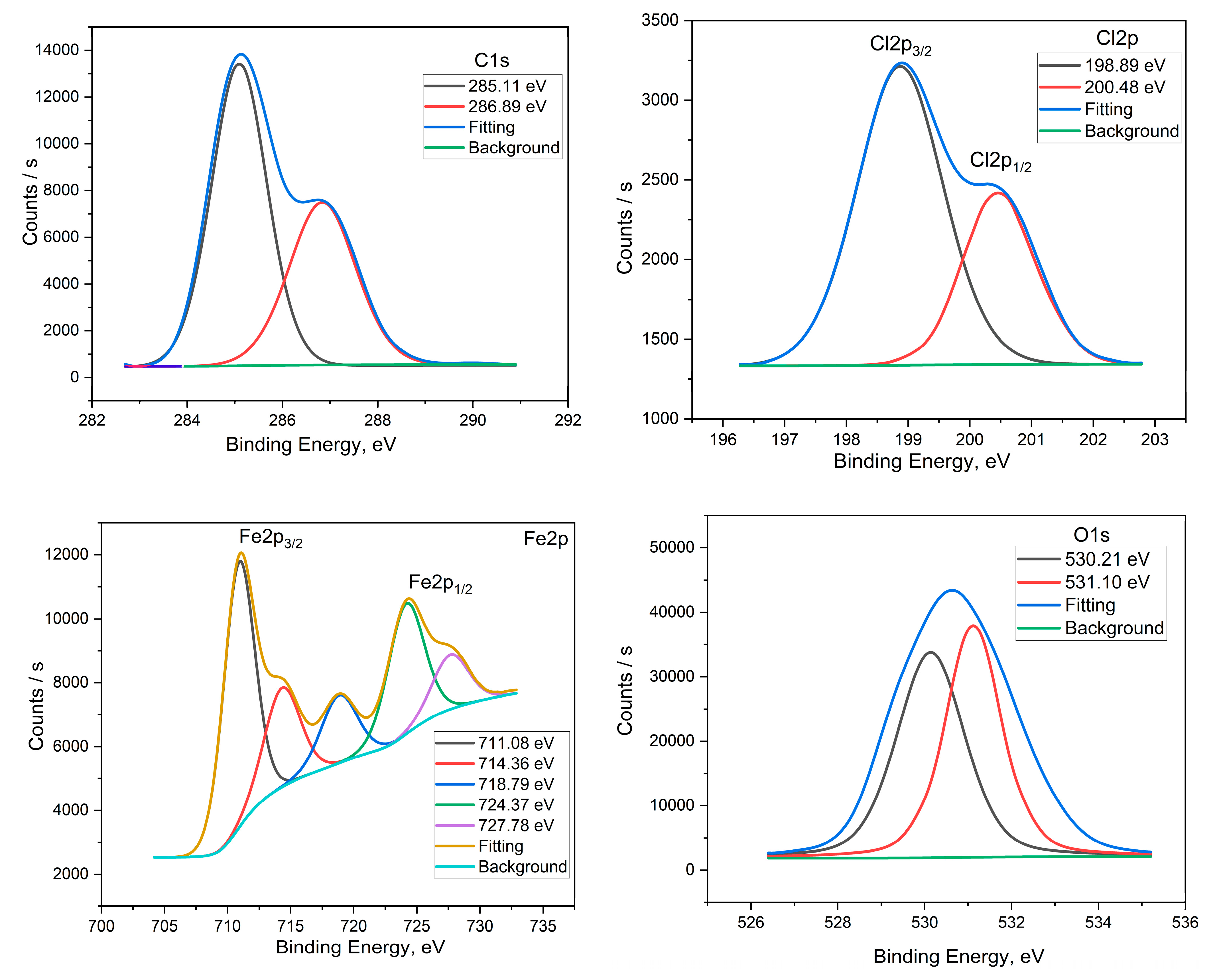
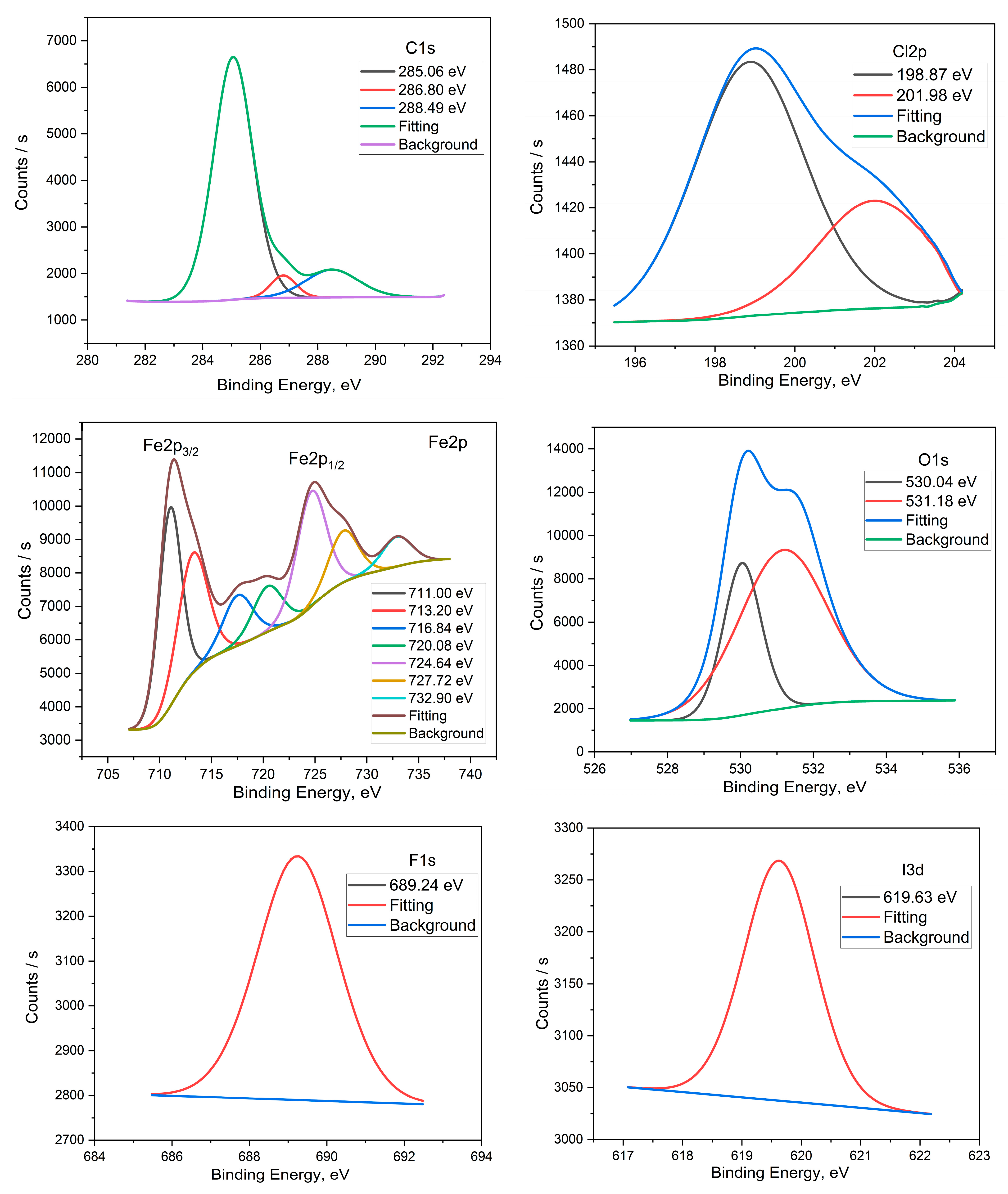
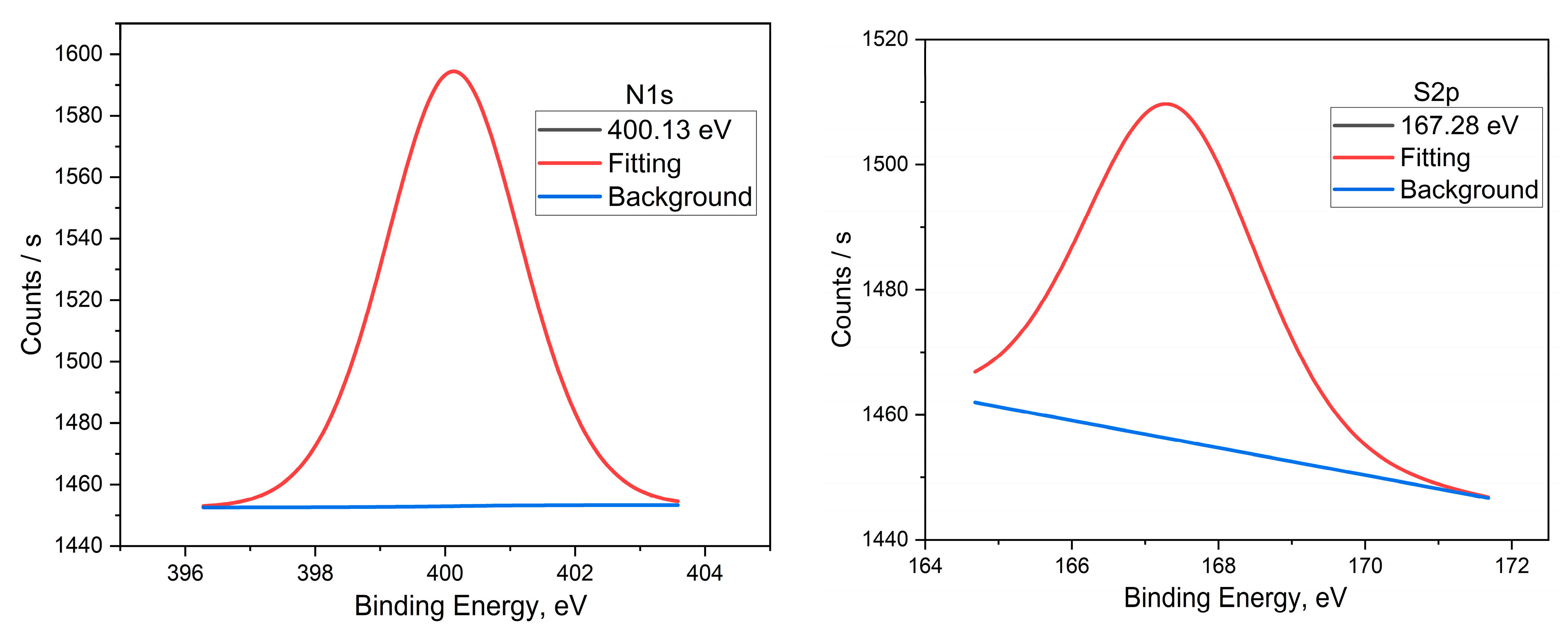
3.6. Surface Morphology by XPS Studies
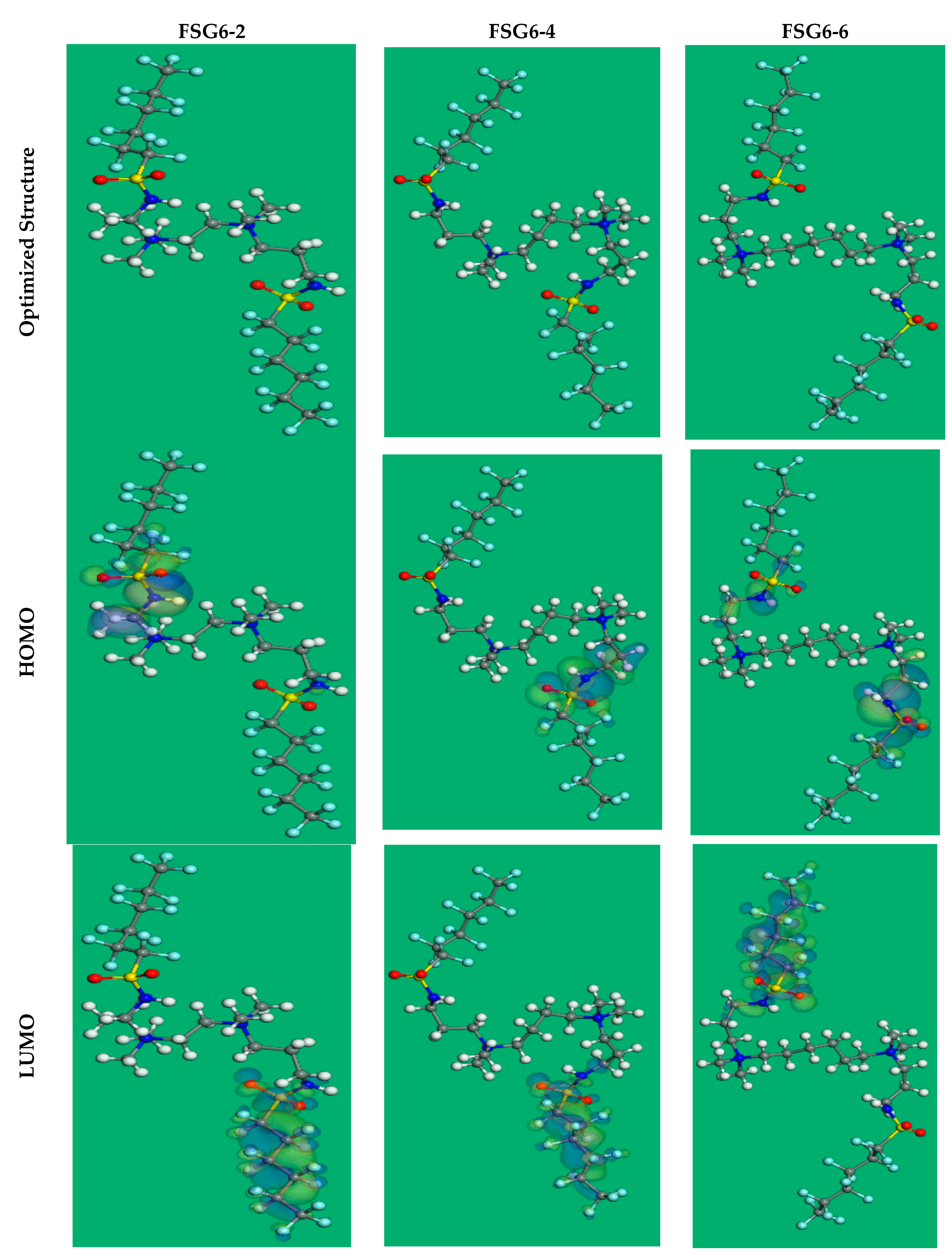
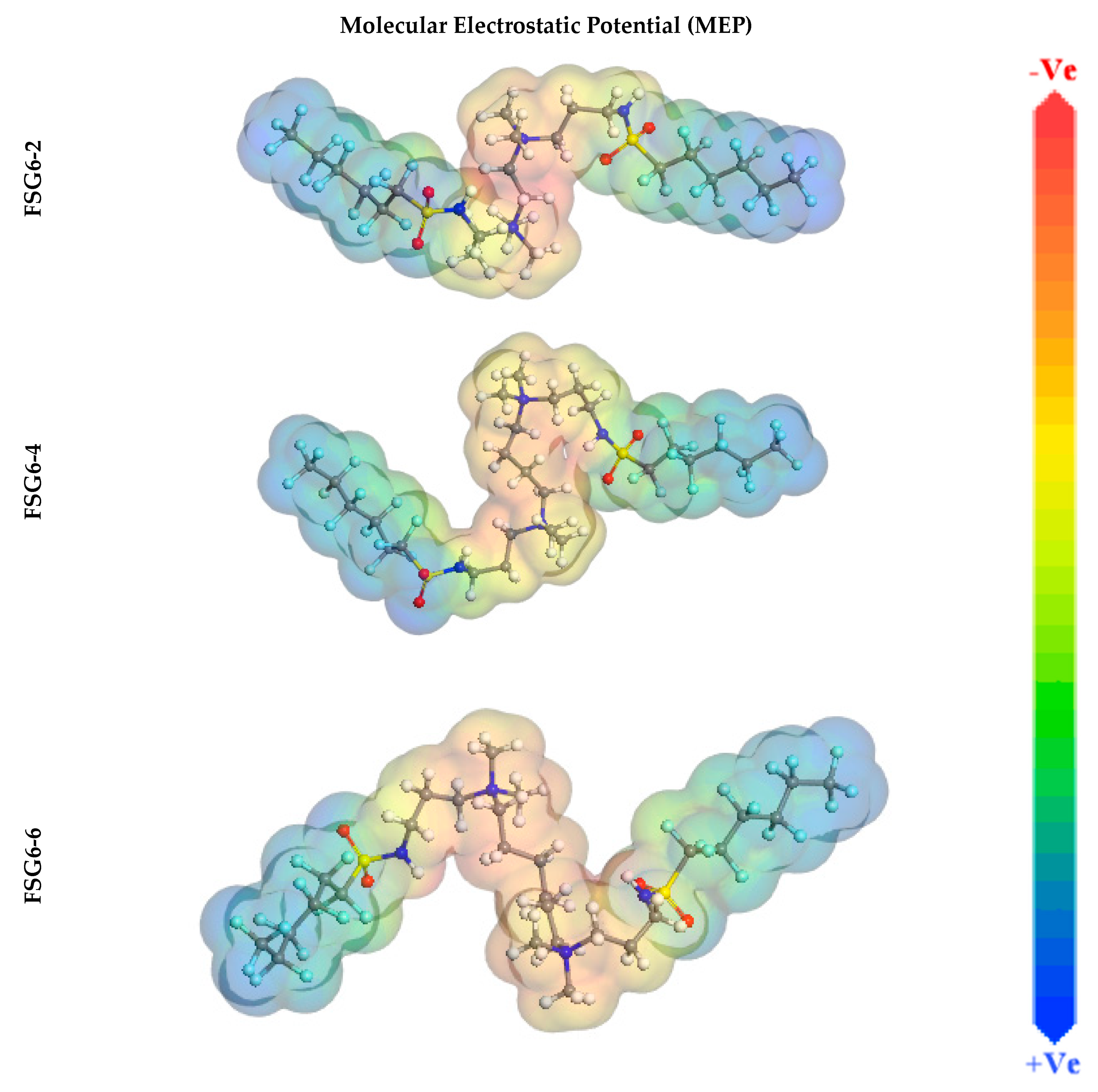
3.7. Computational Calculations (DFT)
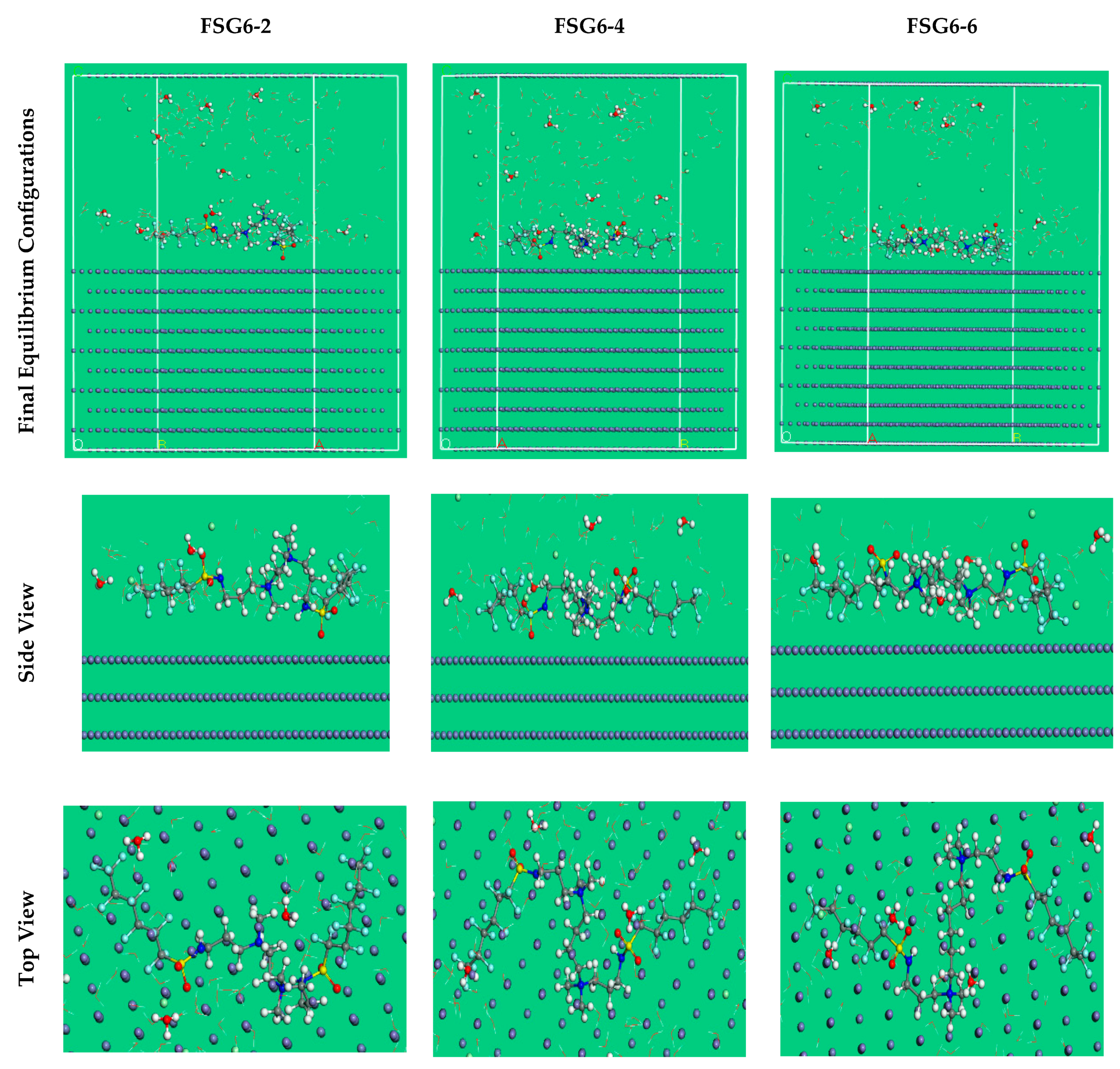
3.8. MC Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wazzan, N.; Obot, I.B.; Faidallah, H. Oxazolidine Derivatives as Corrosion Inhibitors for API X60 Steel in 1 M HCl Solution: Experimental and Theoretical Studies. Int. J. Electrochem. Sci. 2019, 14, 7450–7469. [Google Scholar] [CrossRef]
- Kanwal, M.; Khushnood, R.A.; Shahid, M.; Wattoo, A.G. An integrated and eco-friendly approach for corrosion inhibition and microstructural densification of reinforced concrete by immobilizing Bacillus subtilis in pyrolytic sugarcane-bagasse. J. Clean. Prod. 2022, 355, 131785. [Google Scholar] [CrossRef]
- Bolzoni, F.; Brenna, A.; Ormellese, M. Recent advances in the use of inhibitors to prevent chloride-induced corrosion in reinforced concrete. Cem. Concr. Res. 2022, 154, 106719. [Google Scholar] [CrossRef]
- Shehnazdeep; Pradhan, B. A study on effectiveness of inorganic and organic corrosion inhibitors on rebar corrosion in concrete: A review. Mater. Today Proc. 2022, 65, 1360–1366. [Google Scholar] [CrossRef]
- En-Nylly, M.; Skal, S.; El Aoufir, Y.; Lgaz, H.; Adnin, R.J.; Alrashdi, A.A.; Bellaouchou, A.; Al-Hadeethi, M.R.; Benali, O.; Guedira, T.; et al. Performance evaluation and assessment of the corrosion inhibition mechanism of carbon steel in HCl medium by a new hydrazone compound: Insights from experimental, DFT and first-principles DFT simulations. Arab. J. Chem. 2023, 16, 104711. [Google Scholar] [CrossRef]
- Eid, A.M.; Shaaban, S.; Shalabi, K. Tetrazole-based organoselenium bi-functionalized corrosion inhibitors during oil well acidizing: Experimental, computational studies, and SRB bioassay. J. Mol. Liq. 2020, 298, 111980. [Google Scholar] [CrossRef]
- Yang, X.; He, G.; Dong, W.; Yu, L.; Li, X. Synthesis, electrochemistry, DFT studies and MD simulations of novel dopamine derivatives for corrosion inhibition of Q235 steel in 1 M HCl solution. J. Environ. Chem. Eng. 2023, 11, 109846. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Sayed, A.R.; Shalabi, K. Synthesis and theoretical studies of novel conjugated polyazomethines and their application as efficient inhibitors for C1018 steel pickling corrosion behavior. Surf. Interfaces 2021, 23, 101037. [Google Scholar] [CrossRef]
- Gadow, H.S.; Fawzy, A.; Khairy, M.; Sanad, M.M.S.; Toghan, A. Experimental and theoretical approaches to the inhibition of carbon steel corrosion by thiophene derivative in 1 M HCl. Int. J. Electrochem. Sci. 2023, 18, 100174. [Google Scholar] [CrossRef]
- Solmaz, R.; Salcı, A.; Dursun, Y.A.; Kardaş, G. A comprehensive study on the adsorption, corrosion inhibition efficiency and stability of acriflavine on mild steel in 1 M HCl solution. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131908. [Google Scholar] [CrossRef]
- Yoshimura, T.; Bong, M.; Matsuoka, K.; Honda, C.; Endo, K. Surface properties and aggregate morphology of partially fluorinated carboxylate-type anionic gemini surfactants. J. Colloid Interface Sci. 2009, 339, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Blankschtein, D. Effect of Counterion Binding on Micellar Solution Behavior: 1. Molecular−Thermodynamic Theory of Micellization of Ionic Surfactants. Langmuir 2003, 19, 9932–9945. [Google Scholar] [CrossRef]
- Downes, N.; Ottewill, G.A.; Ottewill, R.H. An investigation of the behaviour of ammonium perfluoro-octanoate at the air/water interface in the absence and presence of salts. Colloids Surf. A Physicochem. Eng. Asp. 1995, 102, 203–211. [Google Scholar] [CrossRef]
- Schneider, J.; Erdelen, C.; Ringsdorf, H.; Rabolt, J.F. Structural studies of polymers with hydrophilic spacer groups. 2. Infrared spectroscopy of Langmuir-Blodgett multilayers of polymers with fluorocarbon side chains at ambient and elevated temperatures. Macromolecules 2002, 22, 3475–3480. [Google Scholar] [CrossRef]
- Krafft, M.P.; Riess, J.G. Selected physicochemical aspects of poly- and perfluoroalkylated substances relevant to performance, environment and sustainability-part one. Chemosphere 2015, 129, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Al Sabagh, A.M.; Kandil, N.G.; Badawi, A.M.; El-Sharkawy, H. Surface activity and thermodynamic of micellization and adsorption for isooctylphenol ethoxylates, phosphate esters and their mixtures with N-diethoxylated perfluorooctanamide. Colloids Surf. A Physicochem. Eng. Asp. 2000, 170, 127–136. [Google Scholar] [CrossRef]
- Yoshimura, T.; Ohno, A.; Esumi, K. Equilibrium and dynamic surface tension properties of partially fluorinated quaternary ammonium salt gemini surfactants. Langmuir 2006, 22, 4643–4648. [Google Scholar] [CrossRef]
- Szymczyk, K.; Zdziennicka, A.; Janczuk, B. Properties of some nonionic fluorocarbon surfactants and their mixtures with hydrocarbon ones. Adv. Colloid Interface Sci. 2021, 292, 102421. [Google Scholar] [CrossRef]
- Lin, I.J. Hydrophile-lipophile balance (hlb) of fluorocarbon surfactants and its relation to the critical micelle concentration (cmc). J. Phys. Chem. 2002, 76, 2019–2023. [Google Scholar] [CrossRef]
- Kunieda, H.; Shinoda, K. Krafft points, critical micelle concentrations, surface tension, and solubilizing power of aqueous solutions of fluorinated surfactants. J. Phys. Chem. 2002, 80, 2468–2470. [Google Scholar] [CrossRef]
- Peng, Y.-Y.; Lu, F.; Tong, Q.-X. One-step synthesis, wettability and foaming properties of high-performance non-ionic hydro-fluorocarbon hybrid surfactants. Appl. Surf. Sci. 2018, 433, 264–270. [Google Scholar] [CrossRef]
- Zhang, D.; Sha, M.; Pan, R.; Lin, X.; Xing, P.; Jiang, B. CF3CF2CF2C(CF3)2-based fluorinated surfactants with high surface activity. Chem. Pap. 2019, 73, 1499–1508. [Google Scholar] [CrossRef]
- Munoz, G.; Desrosiers, M.; Duy, S.V.; Labadie, P.; Budzinski, H.; Liu, J.; Sauve, S. Environmental Occurrence of Perfluoroalkyl Acids and Novel Fluorotelomer Surfactants in the Freshwater Fish Catostomus commersonii and Sediments following Firefighting Foam Deployment at the Lac-Megantic Railway Accident. Environ. Sci. Technol. 2017, 51, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Fanga, C.; Megharaj, M.; Naidu, R. Electrochemical Detection of Thioether-Based Fluorosurfactants in Aqueous Film-Forming Foam (AFFF). Electroanalysis 2017, 29, 1095–1102. [Google Scholar] [CrossRef]
- Dichiarante, V.; Milani, R.; Metrangolo, P. Natural surfactants towards a more sustainable fluorine chemistry. Green Chem. 2018, 20, 13–27. [Google Scholar] [CrossRef]
- Shen, Y.; Jin, Y.; Lai, S.; Shi, L.; Du, W.; Zhou, R. Synthesis, surface properties and cytotoxicity evaluation of nonionic urethane fluorinated surfactants with double short fluoroalkyl chains. J. Mol. Liq. 2019, 296, 111851. [Google Scholar] [CrossRef]
- Vieira, N.S.M.; Bastos, J.C.; Hermida-Merino, C.; Pastoriza-Gallego, M.J.; Rebelo, L.P.N.; Piñeiro, M.M.; Araújo, J.M.M.; Pereiro, A.B. Aggregation and phase equilibria of fluorinated ionic liquids. J. Mol. Liq. 2019, 285, 386–396. [Google Scholar] [CrossRef]
- Smithwick, M.; Mabury, S.A.; Solomon, K.R.; Sonne, C.; Martin, J.W.; Born, E.W.; Dietz, R.; Derocher, A.E.; Letcher, R.J.; Evans, T.J.; et al. Circumpolar study of perfluoroalkyl contaminants in polar bears (Ursus maritimus). Environ. Sci. Technol. 2005, 39, 5517–5523. [Google Scholar] [CrossRef]
- Nuer, M.; Duan, J.; Wei, Z.; Wu, W.; Ma, J.; Zhang, A. Fluorocarbon-hydrocarbon hybrid cationic surfactants: Synthesis, surface-activity properties and anti-corrosion performance. J. Mol. Liq. 2020, 306, 112897. [Google Scholar] [CrossRef]
- Krafft, M.P.; Riess, J.G. Per- and polyfluorinated substances (PFASs): Environmental challenges. Curr. Opin. Colloid Interface Sci. 2015, 20, 192–212. [Google Scholar] [CrossRef]
- Becker, A.M.; Gerstmann, S.; Frank, H. Perfluorooctanoic acid and perfluorooctane sulfonate in the sediment of the Roter Main river, Bayreuth, Germany. Environ. Pollut. 2008, 156, 818–820. [Google Scholar] [CrossRef] [PubMed]
- Sha, M.; Xing, P.; Jiang, B. Strategies for synthesizing non-bioaccumulable alternatives to PFOA and PFOS. Chin. Chem. Lett. 2015, 26, 491–498. [Google Scholar] [CrossRef]
- Schuster, T.; Krumpfer, J.W.; Schellenberger, S.; Friedrich, R.; Klapper, M.; Mullen, K. Effects of chemical structure on the dynamic and static surface tensions of short-chain, multi-arm nonionic fluorosurfactants. J. Colloid Interface Sci. 2014, 428, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Lehmler, H.J. Synthesis of environmentally relevant fluorinated surfactants—A review. Chemosphere 2005, 58, 1471–1496. [Google Scholar] [CrossRef]
- Renner, R. The long and the short of perfluorinated replacements. Environ. Sci. Technol. 2006, 40, 12–13. [Google Scholar] [CrossRef]
- Bodduri, V.D.V.; Chirumarry, S.; Lim, J.-M.; Lee, Y.-I.; Jang, K.; Choi, B.-I.; Chung, S.-Y.; Shin, D.-S. Synthesis and properties of hemifluorinated disodium alkanesulfonates. J. Fluor. Chem. 2014, 163, 42–45. [Google Scholar] [CrossRef]
- Schuster, T.; Schellenberger, S.; Friedrich, R.; Klapper, M.; Müllen, K. Branched fluorinated amphiphiles based on carbohydrates. J. Fluor. Chem. 2013, 154, 30–36. [Google Scholar] [CrossRef]
- Zhang, D.; Xing, P.; Pan, R.; Lin, X.; Sha, M.; Jiang, B. Preparation and Surface Properties Study of Novel Fluorine-Containing Methacrylate Polymers for Coating. Materials 2018, 11, 2258. [Google Scholar] [CrossRef]
- Zana, R. Dimeric (gemini) surfactants: Effect of the spacer group on the association behavior in aqueous solution. J. Colloid Interface Sci. 2002, 248, 203–220. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Abo-Riya, M.A.; Tantawy, A.H. Empirical and quantum chemical studies on the corrosion inhibition performance of some novel synthesized cationic gemini surfactants on carbon steel pipelines in acid pickling processes. Corros. Sci. 2016, 108, 94–110. [Google Scholar] [CrossRef]
- Abo-Riya, M.; Tantawy, A.H.; El-Dougdoug, W. Synthesis and evaluation of novel cationic gemini surfactants based on Guava crude fat as petroleum-collecting and dispersing agents. J. Mol. Liq. 2016, 221, 642–650. [Google Scholar] [CrossRef]
- Jain, T.; Tehrani-Bagha, A.R.; Shekhar, H.; Crawford, R.; Johnson, E.; Nørgaard, K.; Holmberg, K.; Erhart, P.; Moth-Poulsen, K. Anisotropic growth of gold nanoparticles using cationic gemini surfactants: Effects of structure variations in head and tail groups. J. Mater. Chem. C 2014, 2, 994–1003. [Google Scholar] [CrossRef]
- Peng, F.; Chen, Y.; Liu, J.; Xing, Z.; Fan, J.; Zhang, W.; Qiu, F. Facile design of gemini surfactant-like peptide for hydrophobic drug delivery and antimicrobial activity. J. Colloid Interface Sci. 2021, 591, 314–325. [Google Scholar] [CrossRef]
- Kumar, A.; Mandal, A. Synthesis and physiochemical characterization of zwitterionic surfactant for application in enhanced oil recovery. J. Mol. Liq. 2017, 243, 61–71. [Google Scholar] [CrossRef]
- Massi, L.; Guittard, F.; Levy, R.; Duccini, Y.; Geribaldi, S. Preparation and antimicrobial behaviour of gemini fluorosurfactants. Eur. J. Med. Chem. 2003, 38, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Siddiq, A.M.; Natarajan, D.; Kiran, M.S.; Baskar, G. Physicochemical properties and bioactivity studies of synthesized counterion coupled (COCO) gemini surfactant, 1,6-bis(N,N-hexadecyldimethylammonium) adipate. J. Mol. Liq. 2019, 273, 16–26. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Tantawy, A.H. Synthesis and evaluation of novel series of Schiff base cationic surfactants as corrosion inhibitors for carbon steel in acidic/chloride media: Experimental and theoretical investigations. RSC Adv. 2016, 6, 8681–8700. [Google Scholar] [CrossRef]
- Bousba, S.; Allal, H.; Damous, M.; Maza, S. Computational DFT analysis and molecular modeling on imidazole derivatives used as corrosion inhibitors for aluminum in acidic media. Comput. Theor. Chem. 2023, 1225, 114168. [Google Scholar] [CrossRef]
- Dehghani, A.; Mostafatabar, A.H.; Bahlakeh, G.; Ramezanzadeh, B. A detailed study on the synergistic corrosion inhibition impact of the Quercetin molecules and trivalent europium salt on mild steel; electrochemical/surface studies, DFT modeling, and MC/MD computer simulation. J. Mol. Liq. 2020, 316, 113914. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, S.; Guo, W.; Zhang, Y.; Liu, G. Inhibition of iron corrosion by 5,10,15,20-tetraphenylporphyrin and 5,10,15,20-tetra-(4-chlorophenyl)porphyrin adlayers in 0.5M H2SO4 solutions. J. Electroanal. Chem. 2007, 602, 115–122. [Google Scholar] [CrossRef]
- Abd-Elaal, A.A.; Elbasiony, N.M.; Shaban, S.M.; Zaki, E.G. Studying the corrosion inhibition of some prepared nonionic surfactants based on 3-(4-hydroxyphenyl) propanoic acid and estimating the influence of silver nanoparticles on the surface parameters. J. Mol. Liq. 2018, 249, 304–317. [Google Scholar] [CrossRef]
- Kamboj, R.; Singh, S.; Bhadani, A.; Kataria, H.; Kaur, G. Gemini imidazolium surfactants: Synthesis and their biophysiochemical study. Langmuir 2012, 28, 11969–11978. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Fang, S.; Hu, M.; He, X.; Zhao, M.; Wu, X.; Yang, S.; Wu, Y. Synthesis, surface adsorption and micelle formation of a class of morpholinium gemini surfactants. J. Ind. Eng. Chem. 2017, 54, 226–233. [Google Scholar] [CrossRef]
- Tawfik, S.M.; Abd-Elaal, A.A.; Shaban, S.M.; Roshdy, A.A. Surface, thermodynamic and biological activities of some synthesized Gemini quaternary ammonium salts based on polyethylene glycol. J. Ind. Eng. Chem. 2015, 30, 112–119. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Soliman, K.A.; Tantawy, A.H. Novel synthesized Schiff Base-based cationic gemini surfactants: Electrochemical investigation, theoretical modeling and applicability as biodegradable inhibitors for mild steel against acidic corrosion. J. Mol. Liq. 2017, 232, 478–498. [Google Scholar] [CrossRef]
- Rub, M.A.; Khan, F.; Sheikh, M.S.; Azum, N.; Asiri, A.M. Tensiometric, fluorescence and 1 H NMR study of mixed micellization of non-steroidal anti-inflammatory drug sodium salt of ibuprofen in the presence of non-ionic surfactant in aqueous/urea solutions. J. Chem. Thermodyn. 2016, 96, 196–207. [Google Scholar] [CrossRef]
- Carla de Santana de Lima, K.; Magno Paiva, V.; Gustavo Araujo Schwarz, M.; Martins Bomfim Barreto, N.; Perrone, D.; D’Elia, E. The role of soybean meal extracts proteins in the inhibition of mild steel acid corrosion. Sustain. Chem. Pharm. 2023, 35, 101174. [Google Scholar] [CrossRef]
- Kaya, F.; Solmaz, R.; Geçibesler, İ.H. Investigation of adsorption, corrosion inhibition, synergistic inhibition effect and stability studies of Rheum ribes leaf extract on mild steel in 1 M HCl solution. J. Taiwan Inst. Chem. Eng. 2023, 143, 104712. [Google Scholar] [CrossRef]
- Ran, B.; Wei, Z.; Yu, S.; Zhi, H.; Yan, S.; Cai, S.; Wen, L.; Fan, B.; Wang, J.; Wang, K.; et al. The study on corrosion inhibition effect of 2-Phenylbenzimidazole for X70 steel in HCl solution at 308 K. Int. J. Electrochem. Sci. 2023, 18, 100032. [Google Scholar] [CrossRef]
- Cao, S.; Liu, D.; Ding, H.; Wang, J.; Lu, H.; Gui, J. Corrosion inhibition effects of a novel ionic liquid with and without potassium iodide for carbon steel in 0.5 M HCl solution: An experimental study and theoretical calculation. J. Mol. Liq. 2019, 275, 729–740. [Google Scholar] [CrossRef]
- Alhaffar, M.T.; Umoren, S.A.; Obot, I.B.; Ali, S.A.; Solomon, M.M. Studies of the anticorrosion property of a newly synthesized Green isoxazolidine for API 5L X60 steel in acid environment. J. Mater. Res. Technol. 2019, 8, 4399–4416. [Google Scholar] [CrossRef]
- Roque, J.M.; Pandiyan, T.; Cruz, J.; García-Ochoa, E. DFT and electrochemical studies of tris(benzimidazole-2-ylmethyl)amine as an efficient corrosion inhibitor for carbon steel surface. Corros. Sci. 2008, 50, 614–624. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Njoku, V.O.; Enenebeaku, C.K.; Akalezi, C.O.; Obi, C. Effect of hexamethylpararosaniline chloride (crystal violet) on mild steel corrosion in acidic media. Corros. Sci. 2008, 50, 3480–3486. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Shaaban, S.; Khalaf, M.M.; Toghan, A.; Shalabi, K. Synthesis, experimental, and computational studies of water soluble anthranilic organoselenium compounds as safe corrosion inhibitors for J55 pipeline steel in acidic oilfield formation water. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126894. [Google Scholar] [CrossRef]
- Ouici, H.; Tourabi, M.; Benali, O.; Selles, C.; Jama, C.; Zarrouk, A.; Bentiss, F. Adsorption and corrosion inhibition properties of 5-amino 1,3,4-thiadiazole-2-thiol on the mild steel in hydrochloric acid medium: Thermodynamic, surface and electrochemical studies. J. Electroanal. Chem. 2017, 803, 125–134. [Google Scholar] [CrossRef]
- Fan, G.; Liu, H.; Fan, B.; Ma, Y.; Hao, H.; Yang, B. Trazodone as an efficient corrosion inhibitor for carbon steel in acidic and neutral chloride-containing media: Facile synthesis, experimental and theoretical evaluations. J. Mol. Liq. 2020, 311, 113302. [Google Scholar] [CrossRef]
- Bouanis, M.; Tourabi, M.; Nyassi, A.; Zarrouk, A.; Jama, C.; Bentiss, F. Corrosion inhibition performance of 2,5-bis(4-dimethylaminophenyl)-1,3,4-oxadiazole for carbon steel in HCl solution: Gravimetric, electrochemical and XPS studies. Appl. Surf. Sci. 2016, 389, 952–966. [Google Scholar] [CrossRef]
- Solomon, M.M.; Umoren, S.A.; Quraishi, M.A.; Salman, M. Myristic acid based imidazoline derivative as effective corrosion inhibitor for steel in 15% HCl medium. J. Colloid Interface Sci. 2019, 551, 47–60. [Google Scholar] [CrossRef]
- Hashim, N.Z.N.; Anouar, E.H.; Kassim, K.; Zaki, H.M.; Alharthi, A.I.; Embong, Z. XPS and DFT investigations of corrosion inhibition of substituted benzylidene Schiff bases on mild steel in hydrochloric acid. Appl. Surf. Sci. 2019, 476, 861–877. [Google Scholar] [CrossRef]
- Bommersbach, P.; Alemany-Dumont, C.; Millet, J.P.; Normand, B. Formation and behaviour study of an environment-friendly corrosion inhibitor by electrochemical methods. Electrochim. Acta 2005, 51, 1076–1084. [Google Scholar] [CrossRef]
- Temesghen, W.; Sherwood, P.M. Analytical utility of valence band X-ray photoelectron spectroscopy of iron and its oxides, with spectral interpretation by cluster and band structure calculations. Anal. Bioanal. Chem. 2002, 373, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, A.; Hammouti, B.; Lakhlifi, T.; Traisnel, M.; Vezin, H.; Bentiss, F. New 1 H-pyrrole-2,5-dione derivatives as efficient organic inhibitors of carbon steel corrosion in hydrochloric acid medium: Electrochemical, XPS and DFT studies. Corros. Sci. 2015, 90, 572–584. [Google Scholar] [CrossRef]
- Mourya, P.; Singh, P.; Rastogi, R.B.; Singh, M.M. Inhibition of mild steel corrosion by 1,4,6-trimethyl-2-oxo-1,2-dihydropyridine-3-carbonitrile and synergistic effect of halide ion in 0.5 M H2SO4. Appl. Surf. Sci. 2016, 380, 141–150. [Google Scholar] [CrossRef]
- Kharbach, Y.; Qachchachi, F.Z.; Haoudi, A.; Tourabi, M.; Zarrouk, A.; Jama, C.; Olasunkanmi, L.O.; Ebenso, E.E.; Bentiss, F. Anticorrosion performance of three newly synthesized isatin derivatives on carbon steel in hydrochloric acid pickling environment: Electrochemical, surface and theoretical studies. J. Mol. Liq. 2017, 246, 302–316. [Google Scholar] [CrossRef]
- Li, Y.; Bettge, M.; Bareño, J.; Trask, S.E.; Abraham, D.P. Exploring Electrochemistry and Interface Characteristics of Lithium-Ion Cells with Li1.2Ni0.15Mn0.55Co0.1O2 Positive and Li4Ti5O12 Negative Electrodes. J. Electrochem. Soc. 2015, 162, A7049. [Google Scholar] [CrossRef]
- Usman, B.J.; Umoren, S.A.; Gasem, Z.M. Inhibition of API 5L X60 steel corrosion in CO2-saturated 3.5% NaCl solution by tannic acid and synergistic effect of KI additive. J. Mol. Liq. 2017, 237, 146–156. [Google Scholar] [CrossRef]
- Feng, J.; Wen, G.; Huang, W.; Kang, E.-T.; Neoh, K.G. Influence of oxygen plasma treatment on poly(ether sulphone) films. Polym. Degrad. Stab. 2006, 91, 12–20. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Shalabi, K.; Tantawy, A.H. Corrosion inhibition and adsorption features of novel bioactive cationic surfactants bearing benzenesulphonamide on C1018-steel under sweet conditions: Combined modeling and experimental approaches. J. Mol. Liq. 2020, 320, 114564. [Google Scholar] [CrossRef]
- Palaniappan, N.; Cole, I.S.; Kuznetsov, A.E. Experimental and computational studies of graphene oxide covalently functionalized by octylamine: Electrochemical stability, hydrogen evolution, and corrosion inhibition of the AZ13 Mg alloy in 3.5% NaCl. RSC Adv. 2020, 10, 11426–11434. [Google Scholar] [CrossRef]
- Lukovits, I.; Kálmán, E.; Zucchi, F. Corrosion Inhibitors—Correlation between Electronic Structure and Efficiency. Corrosion 2001, 57, 3–8. [Google Scholar] [CrossRef]
- Yesudass, S.; Olasunkanmi, L.O.; Bahadur, I.; Kabanda, M.M.; Obot, I.B.; Ebenso, E.E. Experimental and theoretical studies on some selected ionic liquids with different cations/anions as corrosion inhibitors for mild steel in acidic medium. J. Taiwan Inst. Chem. Eng. 2016, 64, 252–268. [Google Scholar] [CrossRef]
- Debab, H. Electrochemical and Quantum Chemical Studies of Adsorption and Corrosion Inhibition of Two New Schiff Bases on Carbon Steel in Hydrochloric Acid Media. Int. J. Electrochem. Sci. 2018, 13, 6958–6977. [Google Scholar] [CrossRef]
- Gao, G.; Liang, C. Electrochemical and DFT studies of β-amino-alcohols as corrosion inhibitors for brass. Electrochim. Acta 2007, 52, 4554–4559. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Shalabi, K.; Tantawy, A.H. Corrosion inhibition of carbon steel in hydrochloric acid solution using newly synthesized urea-based cationic fluorosurfactants: Experimental and computational investigations. New J. Chem. 2020, 44, 17791–17814. [Google Scholar] [CrossRef]
- Goyal, M.; Vashist, H.; Kumar, S.; Bahadur, I.; Benhiba, F.; Zarrouk, A. Acid corrosion inhibition of ferrous and non-ferrous metal by nature friendly Ethoxycarbonylmethyltriphenylphosphonium Bromide (ECMTPB): Experimental and MD simulation evaluation. J. Mol. Liq. 2020, 315, 113705. [Google Scholar] [CrossRef]
- Abdallah, Y.M.; El-Gammal, O.A.; Abd El-Lateef, H.M.; Shalabi, K. Synthesis and characterization of novel dicarbohydrazide derivatives with electrochemical and theoretical approaches as potential corrosion inhibitors for N80 steel in a 3.5% NaCl solution. RSC Adv. 2022, 12, 14665–14685. [Google Scholar] [CrossRef]
- Oyebamiji, A.K.; Adeleke, B.B. Quantum chemical studies on inhibition activities of 2,3-dihydroxypropyl-sulfanyl derivative on carbon steel in acidic media. Int. J. Corros. Scale Inhib. 2018, 7, 498–508. [Google Scholar] [CrossRef]
- Gece, G.; Bilgiç, S. Quantum chemical study of some cyclic nitrogen compounds as corrosion inhibitors of steel in NaCl media. Corros. Sci. 2009, 51, 1876–1878. [Google Scholar] [CrossRef]
- Madkour, L.H.; Kaya, S.; Obot, I.B. Computational, Monte Carlo simulation and experimental studies of some arylazotriazoles (AATR) and their copper complexes in corrosion inhibition process. J. Mol. Liq. 2018, 260, 351–374. [Google Scholar] [CrossRef]
- Shalabi, K.; Helmy, A.M.; El-Askalany, A.H.; Shahba, M.M. New pyridinium bromide mono-cationic surfactant as corrosion inhibitor for carbon steel during chemical cleaning: Experimental and theoretical studies. J. Mol. Liq. 2019, 293, 111480. [Google Scholar] [CrossRef]
- Özcan, M.; Dehri, İ.; Erbil, M. Organic sulphur-containing compounds as corrosion inhibitors for mild steel in acidic media: Correlation between inhibition efficiency and chemical structure. Appl. Surf. Sci. 2004, 236, 155–164. [Google Scholar] [CrossRef]
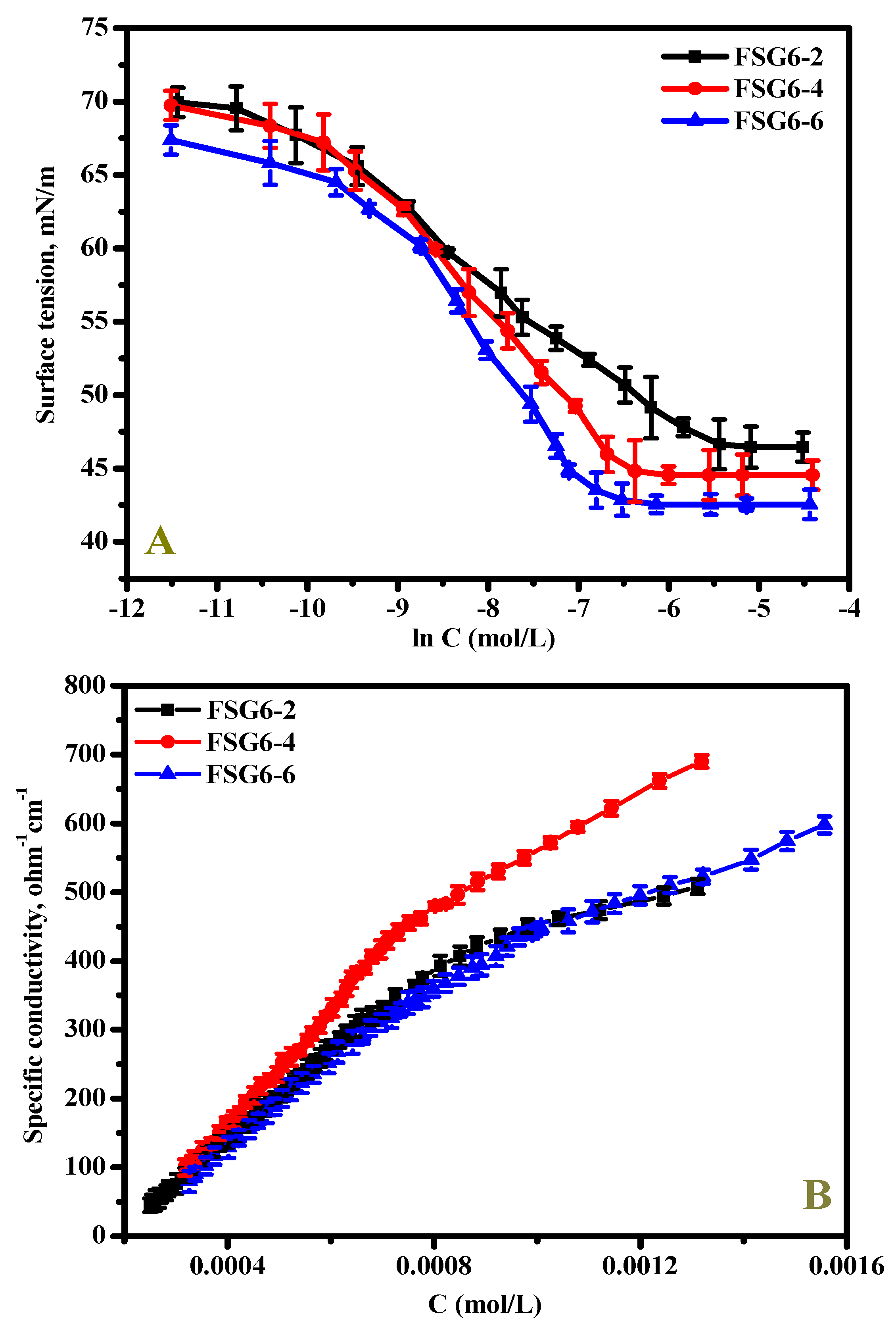

| Compounds | CMC a/ mM | CMC b/ mM | γCMC/ mN m−1 | ΠCMC/ mN m−1 | β | α | Γmax × 1011/ mol/cm−2 | Amin/ nm2 | KJ mol−1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| FSG6-2 | 1.11 | 1.05 | 46.63 | 25.4 | 0.51 | 0.49 | 5.40 | 3.07 | −25.48 | −30.15 |
| FSG6-4 | 0.83 | 0.89 | 44.54 | 27.46 | 0.58 | 0.42 | 5.02 | 3.30 | −27.54 | −32.97 |
| FSG6-6 | 0.72 | 0.76 | 42.55 | 29.45 | 0.63 | 0.37 | 3.78 | 4.39 | −29.22 | −36.97 |
| Inhibitors Code | Cinh./ mol L−1 | icor/ µAcm−2 ±SD | Ecor/ mV (Ag/AgCl) ±SD | βa/ mV dec −1 | −βc/ mV dec −1 | θ | ZPDP/% |
|---|---|---|---|---|---|---|---|
| Blank | 0.0 | 325.7 ± 24.2 | −640 | 95.2 | 170.2 | - | - |
| FSG6-2 | 5 × 10−6 | 164.31 ± 14.5 | −651 | 100.8 | 166.8 | 0.495 | 49.55 |
| 1.5 × 10−5 | 138.71 ± 10.1 | −660 | 97.4 | 178.8 | 0.574 | 57.41 | |
| 3.0 × 10−5 | 87.09 ± 7.6 | −658 | 98.5 | 181.4 | 0.732 | 73.26 | |
| 6.0 × 10−5 | 70.51 ± 5.9 | −635 | 99.6 | 160.1 | 0.783 | 78.35 | |
| 1.0 × 10−4 | 46.60 ± 4.2 | −648 | 106.4 | 152.3 | 0.856 | 85.69 | |
| 2.0 × 10−4 | 21.59 ± 2.3 | −636 | 103.1 | 174.7 | 0.933 | 93.37 | |
| FSG6-4 | 5 × 10−6 | 153.86 ± 13.7 | −645 | 99.7 | 180.3 | 0.527 | 52.76 |
| 1.5 × 10−5 | 127.38 ± 11.3 | −652 | 96.3 | 174.7 | 0.608 | 60.89 | |
| 3.0 × 10−5 | 67.77 ± 5.8 | −638 | 90.7 | 169.1 | 0.791 | 79.19 | |
| 6.0 × 10−5 | 47.19 ± 4.9 | −657 | 92.9 | 170.2 | 0.855 | 85.51 | |
| 1.0 × 10−4 | 28.75 ± 2.4 | −639 | 98.8 | 180.3 | 0.911 | 91.17 | |
| 2.0 × 10−4 | 10.61 ± 1.5 | −633 | 97.4 | 174.7 | 0.967 | 96.74 | |
| FSG6-6 | 5 × 10−6 | 141.94 ± 13.6 | −642 | 98.5 | 162.4 | 0.564 | 56.42 |
| 1.5 × 10−5 | 110.86 ± 9.8 | −652 | 95.2 | 165.7 | 0.659 | 65.96 | |
| 3.0 × 10−5 | 54.97 ± 4.7 | −647 | 96.3 | 170.2 | 0.831 | 83.12 | |
| 6.0 × 10−5 | 36.93 ± 3.1 | −627 | 92.9 | 182.5 | 0.886 | 88.66 | |
| 1.0 × 10−4 | 17.81 ± 1.2 | −630 | 100.8 | 175.8 | 0.945 | 94.53 | |
| 2.0 × 10−4 | 5.31 ± 1.0 | −618 | 97.6 | 172.4 | 0.983 | 98.37 |
| Inhibitor Code | Cinh/ mol L−1 | Rs/ Ω cm2 | RP/ Ω cm2 ±SD Rp = Rd +Rf + Ra + Rct | Cdl/ µF cm−2 | QCPE | θ | ηi/% | |
|---|---|---|---|---|---|---|---|---|
| Y0/ μΩ−1 sn cm−2 | n | |||||||
| Blank | 0.0 | 0.22 | 22.1 ± 2.1 | 232.97 | 3.802 | 0.793 | -- | -- |
| FSG6-2 | 5 × 10−6 | 0.51 | 31.3 ± 3.1 | 143.42 | 1.570 | 0.870 | 0.293 | 29.39 |
| 1.5 × 10−5 | 1.15 | 53.2 ± 3.9 | 103.15 | 0.956 | 0.880 | 0.584 | 58.45 | |
| 3.0 × 10−5 | 1.21 | 87.9 ± 7.8 | 78.55 | 0.658 | 0.870 | 0.748 | 74.85 | |
| 6.0 × 10−5 | 2.49 | 134.2 ± 11.8 | 67.29 | 0.309 | 0.889 | 0.835 | 83.53 | |
| 1.0 × 10−4 | 2.64 | 257.7 ± 21.3 | 37.48 | 0.189 | 0.890 | 0.914 | 91.42 | |
| 2.0 × 10−4 | 2.84 | 487.4 ± 41.2 | 28.38 | 0.118 | 0.883 | 0.954 | 95.46 | |
| FSG6-4 | 5 × 10−6 | 0.42 | 39.2 ± 2.4 | 122.88 | 1.206 | 0.851 | 0.436 | 43.62 |
| 1.5 × 10−5 | 0.47 | 62.9 ± 4.5 | 98.64 | 0.728 | 0.841 | 0.648 | 64.86 | |
| 3.0 × 10−5 | 0.53 | 103.4 ± 8.8 | 75.94 | 0.502 | 0.869 | 0.786 | 78.62 | |
| 6.0 × 10−5 | 1.11 | 157.4 ± 12.5 | 66.21 | 0.248 | 0.879 | 0.859 | 85.95 | |
| 1.0 × 10−4 | 1.83 | 289.4 ± 18.9 | 32.16 | 0.134 | 0.838 | 0.923 | 92.36 | |
| 2.0 × 10−4 | 2.11 | 547.3 ± 43.2 | 24.05 | 0.897 | 0.852 | 0.959 | 95.96 | |
| FSG6-6 | 5 × 10−6 | 0.75 | 43.6 ± 3.6 | 113.15 | 0.931 | 0.842 | 0.493 | 49.31 |
| 1.5 × 10−5 | 0.64 | 71.5 ± 7.7 | 93.42 | 0.568 | 0.851 | 0.690 | 69.09 | |
| 3.0 × 10−5 | 0.94 | 124.9 ± 11.4 | 72.16 | 0.385 | 0.861 | 0.823 | 82.30 | |
| 6.0 × 10−5 | 1.89 | 199.2 ± 15.8 | 59.36 | 0.202 | 0.874 | 0.889 | 88.90 | |
| 1.0 × 10−4 | 2.91 | 351.4 ± 21.2 | 27.47 | 0.111 | 0.880 | 0.937 | 93.71 | |
| 2.0 × 10−4 | 3.11 | 725.1 ± 56.1 | 17.02 | 0.088 | 0.868 | 0.969 | 96.95 | |
| X60-Steel in the Pickling Solution | X60-Steel in the Pickling Solution with 2.0 × 10−4 M FSG6-6 | ||||
|---|---|---|---|---|---|
| Core Element | BE, eV | Assignments | Core Element | BE/eV | Assignments |
| C 1s | 285.11 | −C−C− | C 1s | 285.06 | −C−H, −C−C− |
| 286.86 | −C−Cl | 286.80 | −C−N, −C−Cl | ||
| 288.55 | −C−N+ | ||||
| Cl 2p | 198.89 | Cl 2p3/2 | Cl 2p | 198.87 | Cl 2p3/2 |
| 200.48 | Cl 2p1/2 | 201.98 | Cl 2p1/2 | ||
| Fe 2p | 711.08 | Fe 2p3/2 of Fe2+ | Fe 2p | 711.00 | Fe 2p3/2 of Fe2+ |
| 714.36 | Fe 2p3/2 of Fe3+ | 713.20 | Fe 2p3/2 of Fe3+ | ||
| 718.79 | Satellite Fe 2p3/2 of Fe2+ | 716.84 | Satellite Fe 2p3/2 of Fe2+ | ||
| 724.37 | Fe 2p1/2 of Fe2+ | 720.00 | Satellite Fe 2p3/2 of Fe3+ | ||
| 727.78 | Fe 2p1/2 of Fe3+ | 724.64 | Fe 2p1/2 of Fe2+ | ||
| 727.72 | Fe 2p1/2 of Fe3+ | ||||
| 732.90 | Satellite Fe 2p1/2 of Fe3+ | ||||
| O 1s | 530.21 | FeO, Fe2O3 | O 1s | 530.04 | FeO, Fe2O3 |
| 531.10 | FeOOH | 531.18 | FeOOH | ||
| N 1s | 400.13 | -NR2 | |||
| F 1s | 689.24 | −C−F | |||
| I 3d | 619.63 | I3− | |||
| S 2p | 167.28 | −SO2− | |||
| Parameters | FSG6-2 | FSG6-4 | FSG6-6 |
|---|---|---|---|
| EHOMO (eV) | −7.29 | −7.14 | −7.09 |
| ELUMO (eV) | −1.81 | −1.92 | −2.05 |
| ΔE = ELUMO − EHOMO (eV) | 5.48 | 5.22 | 5.04 |
| Electronegativity (χ) | 4.55 | 4.53 | 4.57 |
| Global hardness (η) | 2.74 | 2.61 | 2.52 |
| Global softness (σ) | 0.36 | 0.38 | 0.40 |
| The number of electrons transferred (ΔN) | 0.45 | 0.47 | 0.48 |
| ∆Eback-donation | −0.69 | −0.65 | −0.63 |
| Dipole moments (µ) Debye | 8.31 | 12.49 | 15.48 |
| Molecular surface area, Å2 | 677.41 | 709.96 | 757.18 |
| Corrosion Systems | Adsorption Energy/ kcal mol−1 | Rigid Adsorption Energy/ kcal mol−1 | Deformation Energy/ kcal mol−1 | dEads/dNi: Inhibitor kcal mol−1 | dEads/dNi: Cl− Ions kcal mol−1 | dEads/dNi: Hydronium kcal mol−1 | dEads/dNi: Water kcal mol−1 |
|---|---|---|---|---|---|---|---|
| Fe (110) | −1848.35 | −1630.31 | −218.04 | −206.14 | −105.65 | −52.17 | −15.05 |
| FSG6-2 | |||||||
| Water | |||||||
| Hydronium | |||||||
| Cl− ions | |||||||
| Fe (110) | −1900.63 | −1645.24 | −255.39 | −252.11 | −105.60 | −52.41 | −15.80 |
| FSG6-4 | |||||||
| Water | |||||||
| Hydronium | |||||||
| Cl− ions | |||||||
| Fe (110) | −1971.88 | −1662.44 | −309.43 | −317.05 | −106.35 | −51.73 | −15.27 |
| FSG6-6 | |||||||
| Water | |||||||
| Hydronium | |||||||
| Cl− ions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalabi, K.; Abd El-Lateef, H.M.; Hammouda, M.M.; Osman, A.M.A.; Tantawy, A.H.; Abo-Riya, M.A. Perspectives on Corrosion Inhibition Features of Novel Synthesized Gemini-Fluorinated Cationic Surfactants Bearing Varied Spacers for Acid Pickling of X60-Steel: Practical, and In Silico Calculations. Materials 2023, 16, 5192. https://doi.org/10.3390/ma16145192
Shalabi K, Abd El-Lateef HM, Hammouda MM, Osman AMA, Tantawy AH, Abo-Riya MA. Perspectives on Corrosion Inhibition Features of Novel Synthesized Gemini-Fluorinated Cationic Surfactants Bearing Varied Spacers for Acid Pickling of X60-Steel: Practical, and In Silico Calculations. Materials. 2023; 16(14):5192. https://doi.org/10.3390/ma16145192
Chicago/Turabian StyleShalabi, Kamal, Hany M. Abd El-Lateef, Mohamed M. Hammouda, Amany M. A. Osman, Ahmed H. Tantawy, and Mohamed A. Abo-Riya. 2023. "Perspectives on Corrosion Inhibition Features of Novel Synthesized Gemini-Fluorinated Cationic Surfactants Bearing Varied Spacers for Acid Pickling of X60-Steel: Practical, and In Silico Calculations" Materials 16, no. 14: 5192. https://doi.org/10.3390/ma16145192
APA StyleShalabi, K., Abd El-Lateef, H. M., Hammouda, M. M., Osman, A. M. A., Tantawy, A. H., & Abo-Riya, M. A. (2023). Perspectives on Corrosion Inhibition Features of Novel Synthesized Gemini-Fluorinated Cationic Surfactants Bearing Varied Spacers for Acid Pickling of X60-Steel: Practical, and In Silico Calculations. Materials, 16(14), 5192. https://doi.org/10.3390/ma16145192









