W-doped Lanthanum Molybdenum Oxide/Lithium-Sodium-Potassium Carbonate Composite Membranes for Carbon Dioxide Permeation
Abstract
1. Introduction
2. Materials and Methods
2.1. W-LAMOX Synthesis; Membrane Preparation
2.2. Thermal, Structural, and Electrical Analyses
3. Results and Discussion
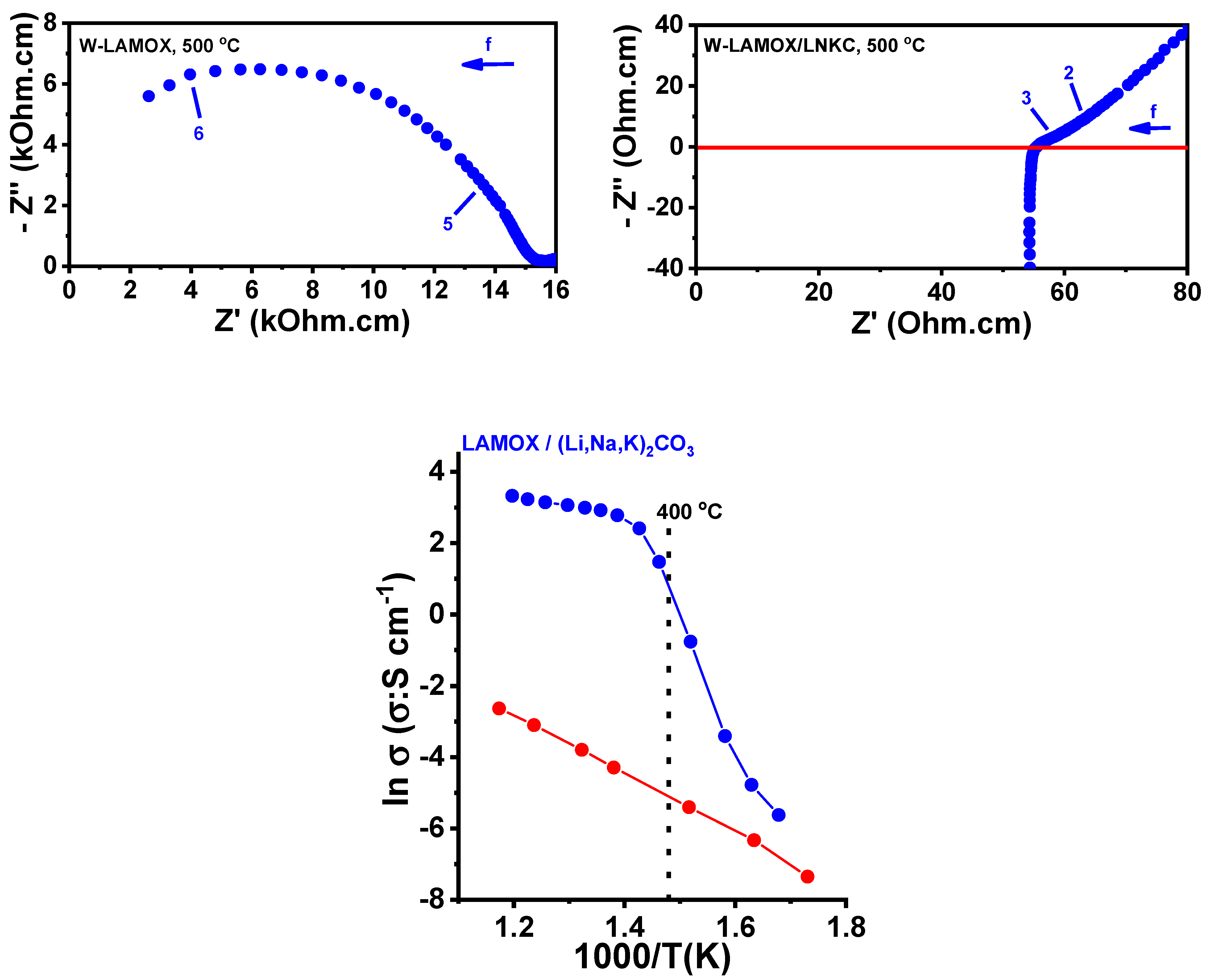
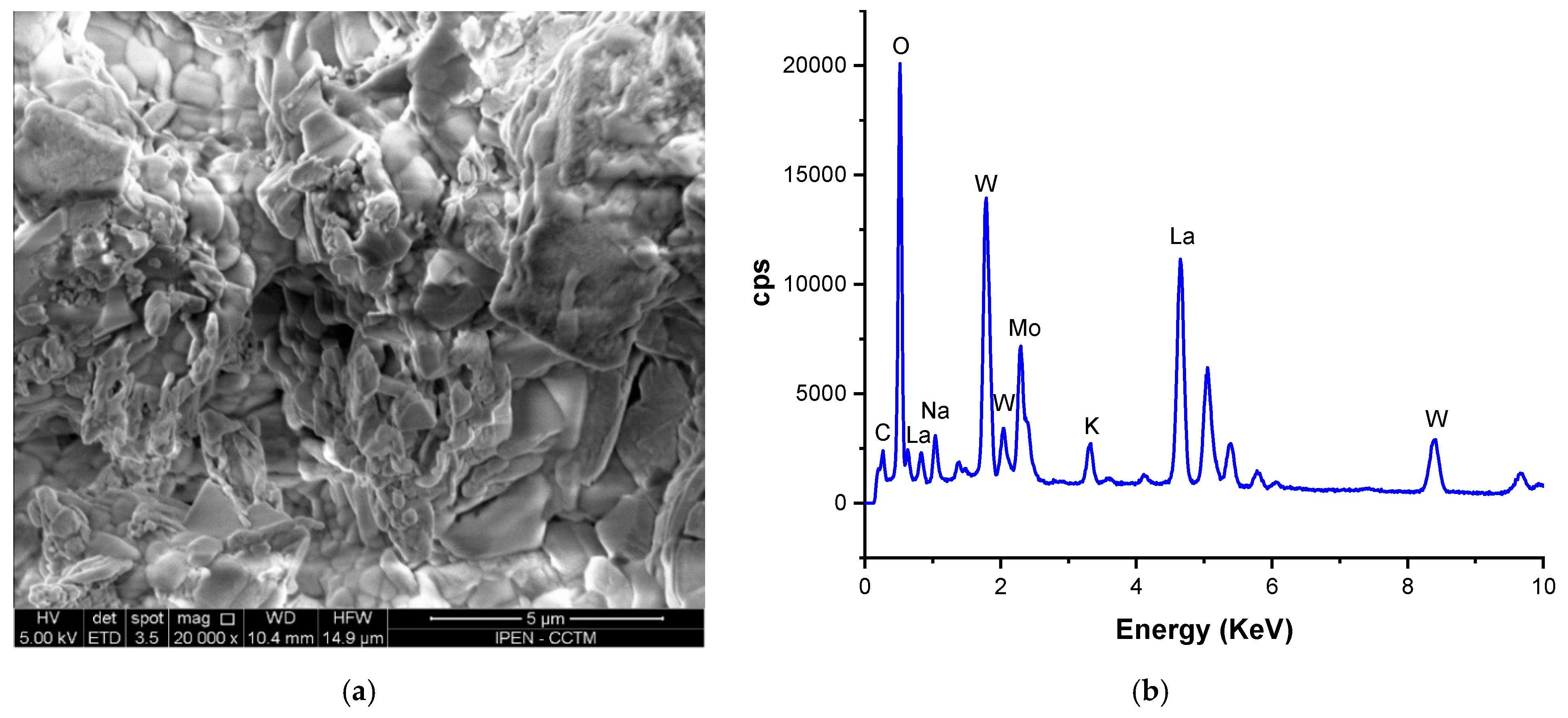
3.1. Dense W-LAMOX
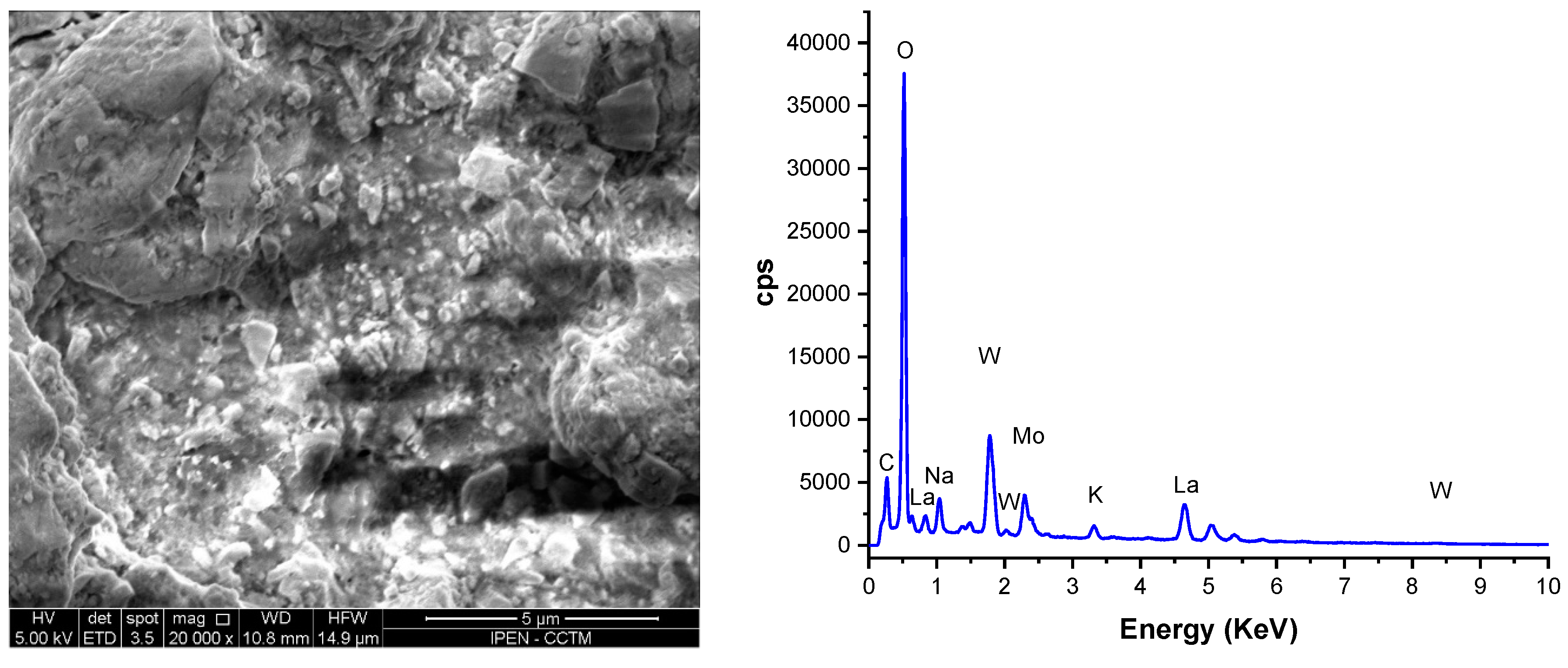
3.2. Porous W-LAMOX
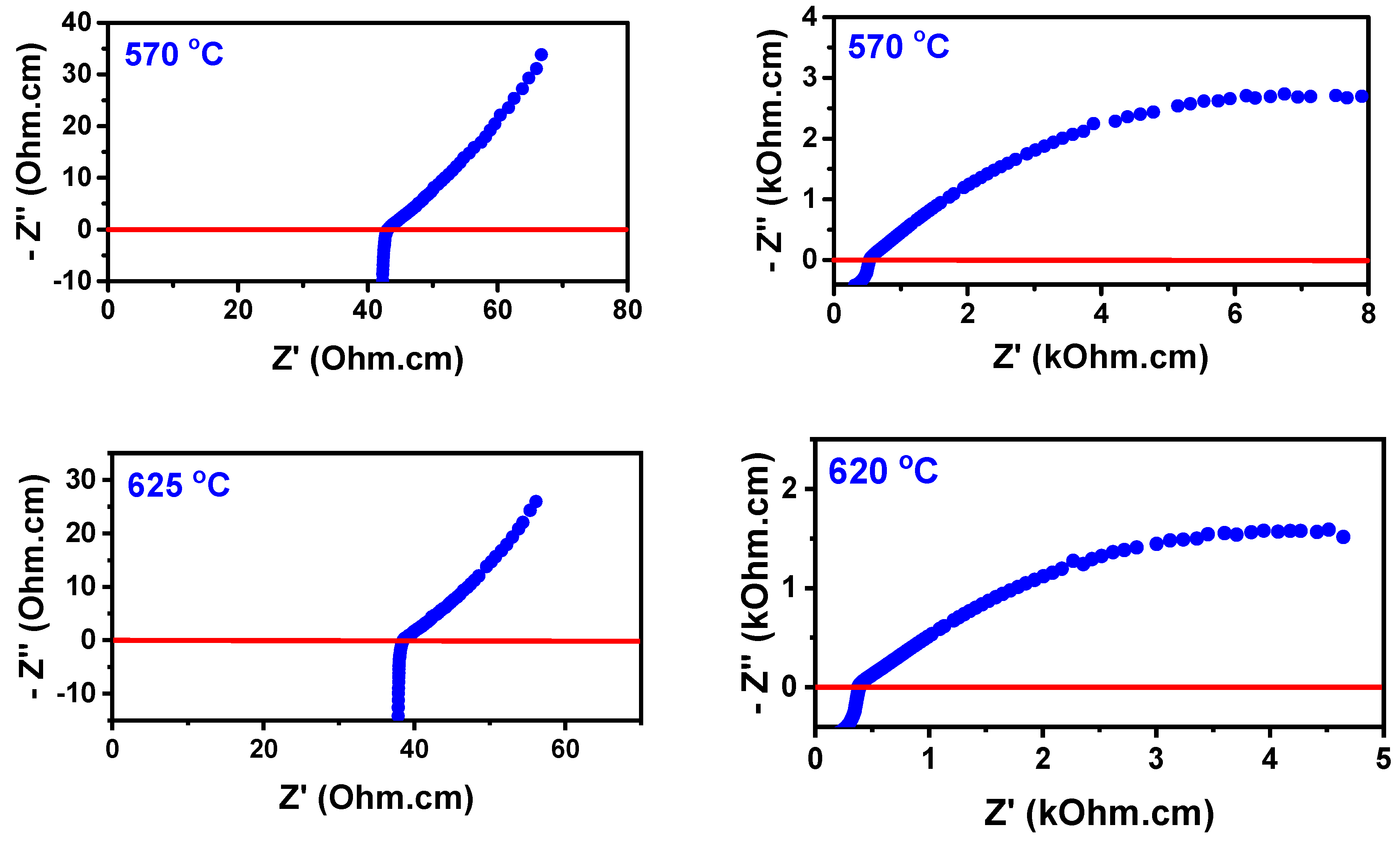
3.3. W-LAMOX/(Li,Na,K)2CO3 Composite Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goutenoire, F.; Isnard, O.; Retoux, R.; Lacorre, P. Crystal structure of La2Mo2O9, a new fast oxide-ion conductor. Chem. Mater. 2000, 12, 2575–2580. [Google Scholar] [CrossRef]
- Lacorre, P.; Goutenoire, F.; Bohnke, O.; Retoux, R.; Laligant, Y. Designing fast oxide-ion conductors based on La2Mo2O9. Nature 2000, 404, 856–858. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.A.; Muccillo, E.N.S. Influence of sintering on electrical properties and phase transition of La2Mo2O9. Mater. Sci. Forum 2006, 530–531, 520–525. [Google Scholar] [CrossRef]
- Marrero-López, D.; Ruiz-Morales, J.C.; Perez-Coll, D.; Núñez, P.; Abrantes, J.C.C.; Frade, J.R. Stability and transport properties of La2Mo2O9. J. Solid State Electrochem. 2004, 8, 638–643. [Google Scholar] [CrossRef]
- Marozau, I.P.; Marrero-Lopez, D.; Shaula, A.L.; Kharton, V.V.; Tsipis, E.V.; Nunez, P.; Frade, J.R. Ionic and electronic transport in stabilized β-La2Mo2O9 electrolytes. Electrochim. Acta 2004, 49, 3517–3524. [Google Scholar] [CrossRef]
- Marrero-López, D.; Canales-Vázquez, J.; Zhou, W.; Irvine, J.T.; Núñez, P. Structural studies on W6+ and Nd3+ substituted La2Mo2O9 materials. J. Solid State Chem. 2006, 179, 278–288. [Google Scholar] [CrossRef]
- Corbel, G.; Laligant, Y.; Goutenoire, F.; Suard, E.; Lacorre, P. Effects of partial substitution of Mo6+ by Cr6+ and W6+ on the crystal structure of the fast oxide-ion conductor structural effects of W6+. Chem. Mater. 2005, 17, 4678–4684. [Google Scholar] [CrossRef]
- Paul, T.; Tsur, Y. Influence of isovalent ‘W’ substitutions on the structure and electrical properties of La2Mo2O9 electrolyte for intermediate-temperature solid oxide fuel cells. Ceramics 2021, 4, 502–515. [Google Scholar] [CrossRef]
- Marrero-Lopez, D.; Canales-Vazquez, J.; Ruiz-Morales, J.C.; Irvine, J.T.; Nunez, P. Electrical conductivity and redox stability of La2Mo2−xWxO9 materials. Electrochim. Acta 2005, 50, 4385–4395. [Google Scholar] [CrossRef]
- Georges, S.; Bohnké, O.; Goutenoire, F.; Laligant, Y.; Fouletier, J.; Lacorre, P. Effects of tungsten substitution on the transport properties and mechanism of fast oxide-ion conduction in La2Mo2O9. Solid State Ion. 2006, 177, 1715–1720. [Google Scholar] [CrossRef]
- Pinet, P.; Fouletier, J.; Georges, S. Conductivity of reduced La2Mo2O9 based oxides: The effect of tungsten substitution. Mater. Res. Bull. 2007, 42, 935–942. [Google Scholar] [CrossRef]
- Subasri, R.; Matusch, D.; Näfe, H.; Aldinger, F. Synthesis and characterization of (La1−xMx)2Mo2O9−δ; M = Ca2+, Sr2+ or Ba2+. J. Eur. Ceram. Soc. 2004, 24, 129–137. [Google Scholar] [CrossRef]
- He, T.; Huang, Y.; He, Q.; Ji, Y.; Pei, L.; Liu, J.; Lu, Z. The effects on the structures and properties in the oxide-ion conductor La2Mo2O9 by partial substituting Ba for La. J. Alloys Compnd. 2005, 388, 145–152. [Google Scholar] [CrossRef]
- Yoo, K.S.; Jacobson, A.J. Effects of Sr and Cr doping on properties of La2Mo2O9 electrolytes. J. Mater Sci. 2005, 40, 4431–4434. [Google Scholar] [CrossRef]
- Paul, T.; Ghosh, A. Correlation between structure and oxygen ion dynamics in Y substituted La2Mo2O9 ionic conductors. AIP Adv. 2016, 6, 095015. [Google Scholar] [CrossRef]
- Corbel, G.; Suard, E.; Lacorre, P. Structural key of the thermal expansion and the oxide ionic conduction in derivatives of La2Mo2O9: A temperature-controlled neutron diffraction study of β-La1.7Bi0.3Mo2O9. Chem. Mater. 2011, 23, 1288–1298. [Google Scholar] [CrossRef]
- Du, N.; Park, H.B.; Robertson, G.P.; Dal-Cin, M.M.; Visser, T.; Scoles, L.; Guiver, M.D. Polymer nanosieve membranes for CO2-capture applications. Nat. Mater. 2011, 10, 372–375. [Google Scholar] [PubMed]
- Asaeda, M.; Yamasaki, S. Separation of inorganic/organic gas mixtures by porous silica membranes. Sep. Purif. Technol. 2001, 25, 151–159. [Google Scholar] [CrossRef]
- Kusakabe, K.; Kuroda, T.; Morooka, S. Separation of carbon dioxide from nitrogen using ion-exchanged faujasite-type zeolite membranes formed on porous support tubes. J. Membr. Sci. 1998, 148, 13–23. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, A.E.; Wright, I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef]
- Chung, S.J.; Park, J.H.; Li, D.; Ida, J.I.; Kumakiri, I.; Lin, J.Y. Dual-phase metal-carbonate membrane for high-temperature carbon dioxide separation. Ind. Eng. Chem. Res. 2005, 44, 7999–8006. [Google Scholar] [CrossRef]
- Anderson, M.; Lin, Y. Carbonate-ceramic dual-phase membrane for carbon dioxide separation. J. Membr. Sci. 2010, 357, 122–129. [Google Scholar] [CrossRef]
- Lu, B.; Lin, Y.S. Synthesis and characterization of thin ceramic-carbonate dual-phase membranes for carbon dioxide separation. J. Membr. Sci. 2013, 444, 402–411. [Google Scholar] [CrossRef]
- Norton, T.T.; Ortiz-Landeros, J.; Lin, Y.S. Stability of La–Sr–Co–Fe oxide–carbonate dual-phase membranes for carbon dioxide separation at high temperatures. Ind. Eng. Chem. Res. 2014, 53, 2432–2440. [Google Scholar] [CrossRef]
- Li, Y.; Rui, Z.; Xia, C.; Anderson, M.; Lin, Y.S. Performance of ionic-conducting ceramic/carbonate composite material as solid oxide fuel cell electrolyte and CO2 permeation membrane. Catal. Today 2009, 148, 303–309. [Google Scholar] [CrossRef]
- Carvalho, S.G.M.; Muccillo, E.N.S.; Fonseca, F.C.; Müller, M.; Schulze-Küppers, F.; Baumann, S.; Meulenberg, W.A.; Guillon, O.; Muccillo, R. Tape-casting and freeze-drying gadolinia-doped ceria composite membranes for carbon dioxide permeation. J. Membr. Sci. 2022, 648, 120355. [Google Scholar] [CrossRef]
- Kleitz, M.; Kennedy, J.H. Resolution of multicomponent impedance diagrams. In Fast Ion Transport in Solids; Mundy, J.N., Shenoy, G.K., Vashishta, P., Eds.; Elsevier: North Holland, The Netherlands, 1979; pp. 185–188. [Google Scholar]
- Corbel, G.; Durand, P.; Lacorre, P. Comprehensive survey of Nd3+ substitution In La2Mo2O9 oxide-ion conductor. J. Solid State Chem. 2009, 182, 1009–1016. [Google Scholar] [CrossRef]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Macdonald, J.R.; Johnson, W.B. Fundamentals of Impedance Spectroscopy. In Impedance Spectroscopy—Theory, Experimental and Applications; Barsoukov, E., Macdonald, J.B., Eds.; Wiley: Hoboken, NJ, USA, 2005; pp. 1–26. [Google Scholar]
- ImageJ Version 1.34N, Wayne Rasband, National Institute of Health, USA. Available online: https://imagej.nih.gov/ij/ (accessed on 1 January 2023).
- Cruz-Manzo, S.; Chen, R.; Rama, P. Inductive Effect on the Fuel Cell Cathode Impedance Spectrum at High Frequencies. J. Fuel Cell Sci. Technol. 2012, 9, 051002. [Google Scholar] [CrossRef]
- Frangini, S.; Masi, A. Molten carbonates for advanced and sustainable energy applications: Part I. Revisiting molten carbonate properties from a sustainable viewpoint. Int. J. Hydrog. Energy 2016, 41, 18739–18746. [Google Scholar] [CrossRef]
- Grilo, J.P.; Macedo, D.A.; Nascimento, R.M.; Marques, F.M. Electronic conductivity in Gd-doped ceria with salt additions. Electrochim. Acta 2019, 318, 977–988. [Google Scholar] [CrossRef]
- Nikolaeva, E.V.; Zakir’yanova, I.D.; Bove, A.L. Electric Conductivity of α-Al2O3 Suspensions in Carbonate and Carbonate-Chloride Melts. Russ. J. Electrochem. 2018, 54, 690–696. [Google Scholar] [CrossRef]
- Carvalho, S.G.M.; Muccillo, E.N.S.; Marques, F.M.B.; Muccillo, R. Electric field-assisted sintering (gadolinia-doped ceria/alkali salts) composite membranes. Materialia 2020, 11, 100679. [Google Scholar] [CrossRef]
- Zhadan, A.; Sarou-Kanian, V.; Del Campo, L.; Cosson, L.; Malki, M.; Bessada, C. Transport properties in molten carbonates: Self-diffusion and conductivity measurements at high temperature. Int. J. Hydrog. Energy 2021, 46, 15059–15065. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina, M.S.; Carvalho, S.G.M.; Tabuti, F.N.; Muccillo, E.N.S.; Fonseca, F.C.; Muccillo, R. W-doped Lanthanum Molybdenum Oxide/Lithium-Sodium-Potassium Carbonate Composite Membranes for Carbon Dioxide Permeation. Materials 2023, 16, 5128. https://doi.org/10.3390/ma16145128
Medina MS, Carvalho SGM, Tabuti FN, Muccillo ENS, Fonseca FC, Muccillo R. W-doped Lanthanum Molybdenum Oxide/Lithium-Sodium-Potassium Carbonate Composite Membranes for Carbon Dioxide Permeation. Materials. 2023; 16(14):5128. https://doi.org/10.3390/ma16145128
Chicago/Turabian StyleMedina, Midilane S., Sabrina G. M. Carvalho, Francisco N. Tabuti, Eliana N. S. Muccillo, Fábio C. Fonseca, and Reginaldo Muccillo. 2023. "W-doped Lanthanum Molybdenum Oxide/Lithium-Sodium-Potassium Carbonate Composite Membranes for Carbon Dioxide Permeation" Materials 16, no. 14: 5128. https://doi.org/10.3390/ma16145128
APA StyleMedina, M. S., Carvalho, S. G. M., Tabuti, F. N., Muccillo, E. N. S., Fonseca, F. C., & Muccillo, R. (2023). W-doped Lanthanum Molybdenum Oxide/Lithium-Sodium-Potassium Carbonate Composite Membranes for Carbon Dioxide Permeation. Materials, 16(14), 5128. https://doi.org/10.3390/ma16145128






