Electrochemical Performance of Corn Waste Derived Carbon Electrodes Based on the Intrinsic Biomass Properties
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
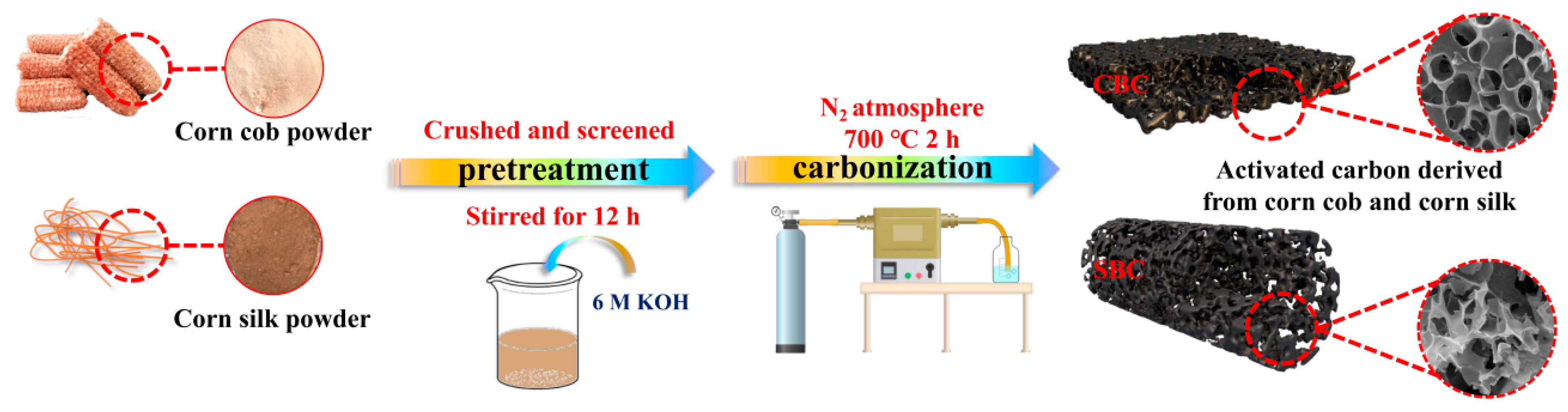
2.2. Synthesis of the CBC and SBC Materials
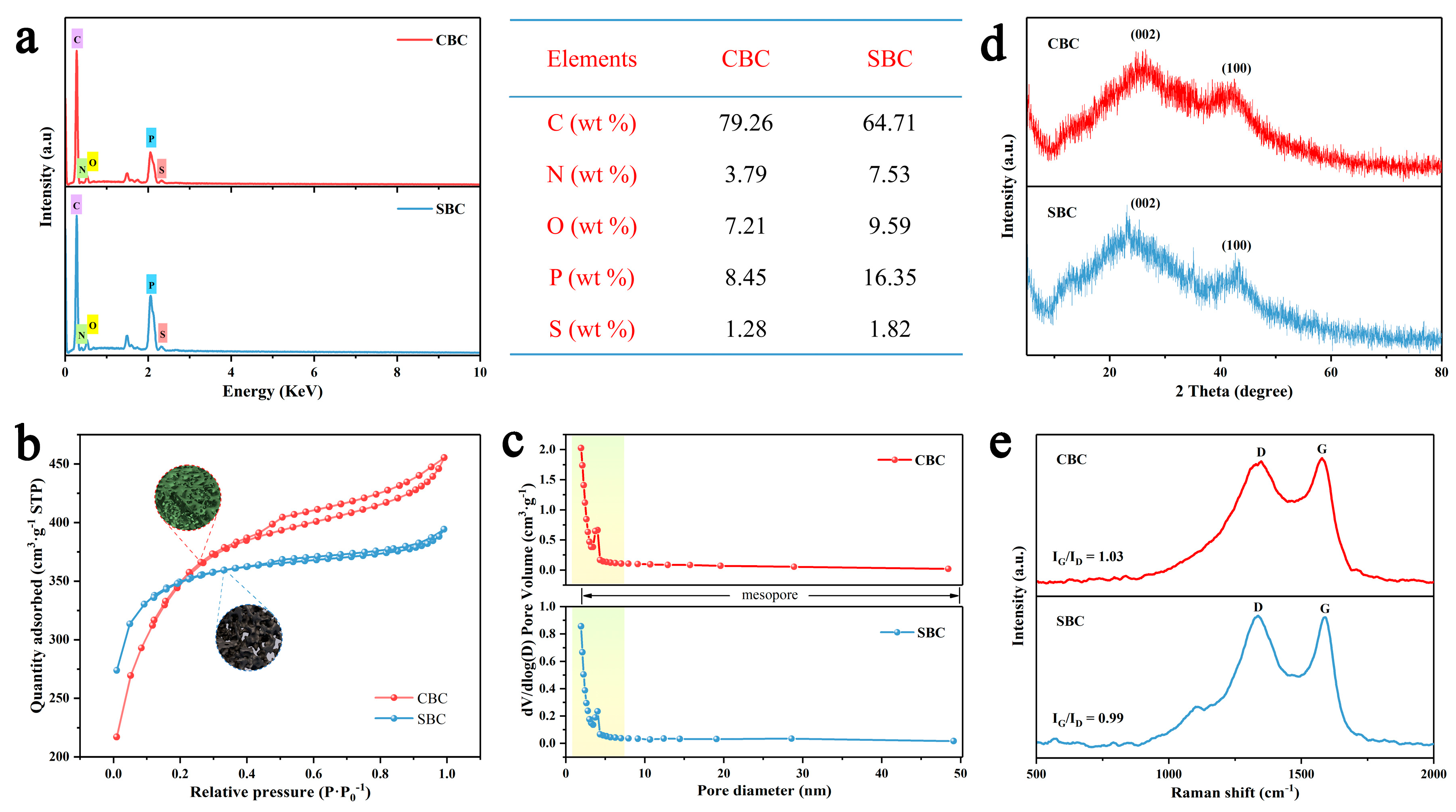
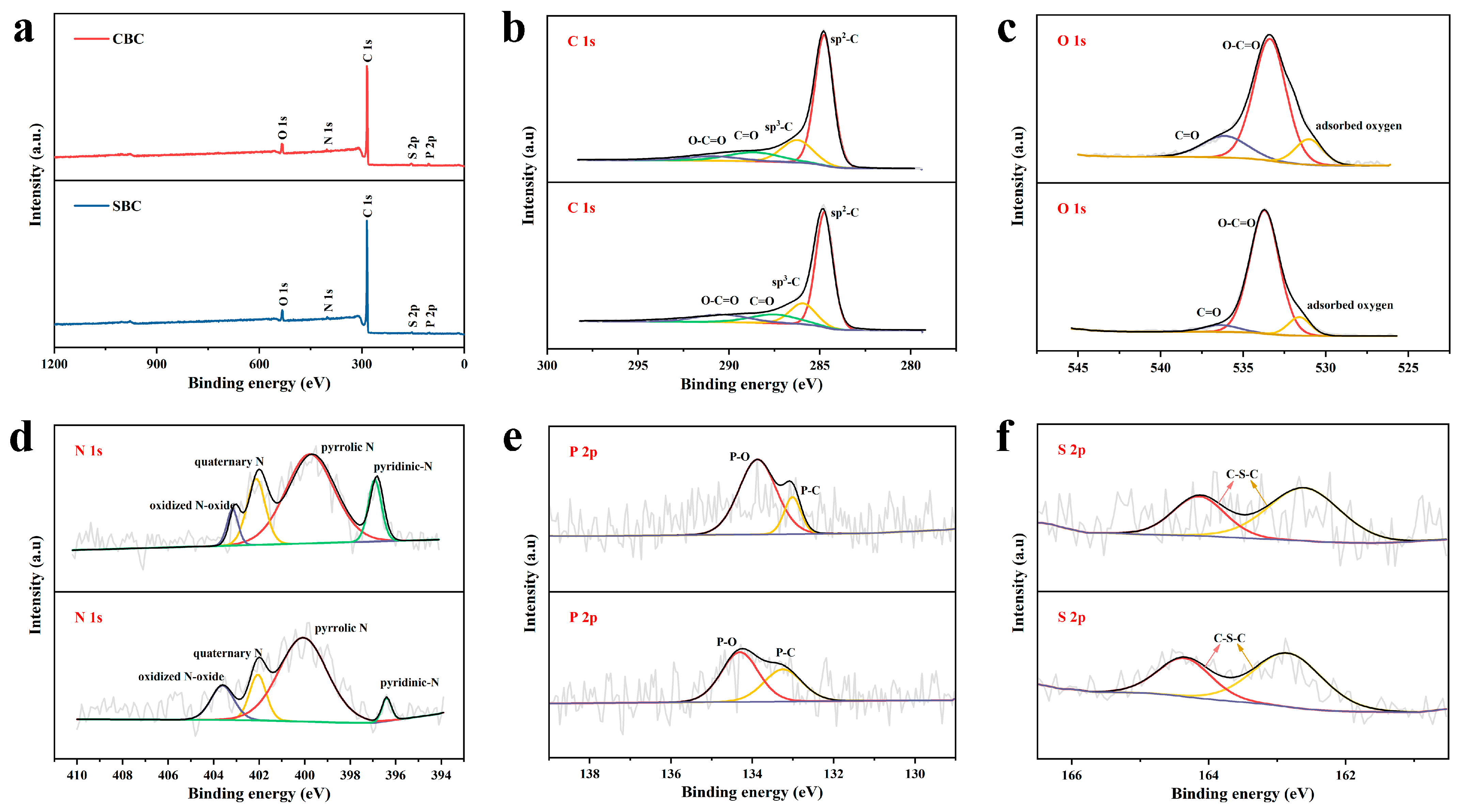
2.3. Characterization
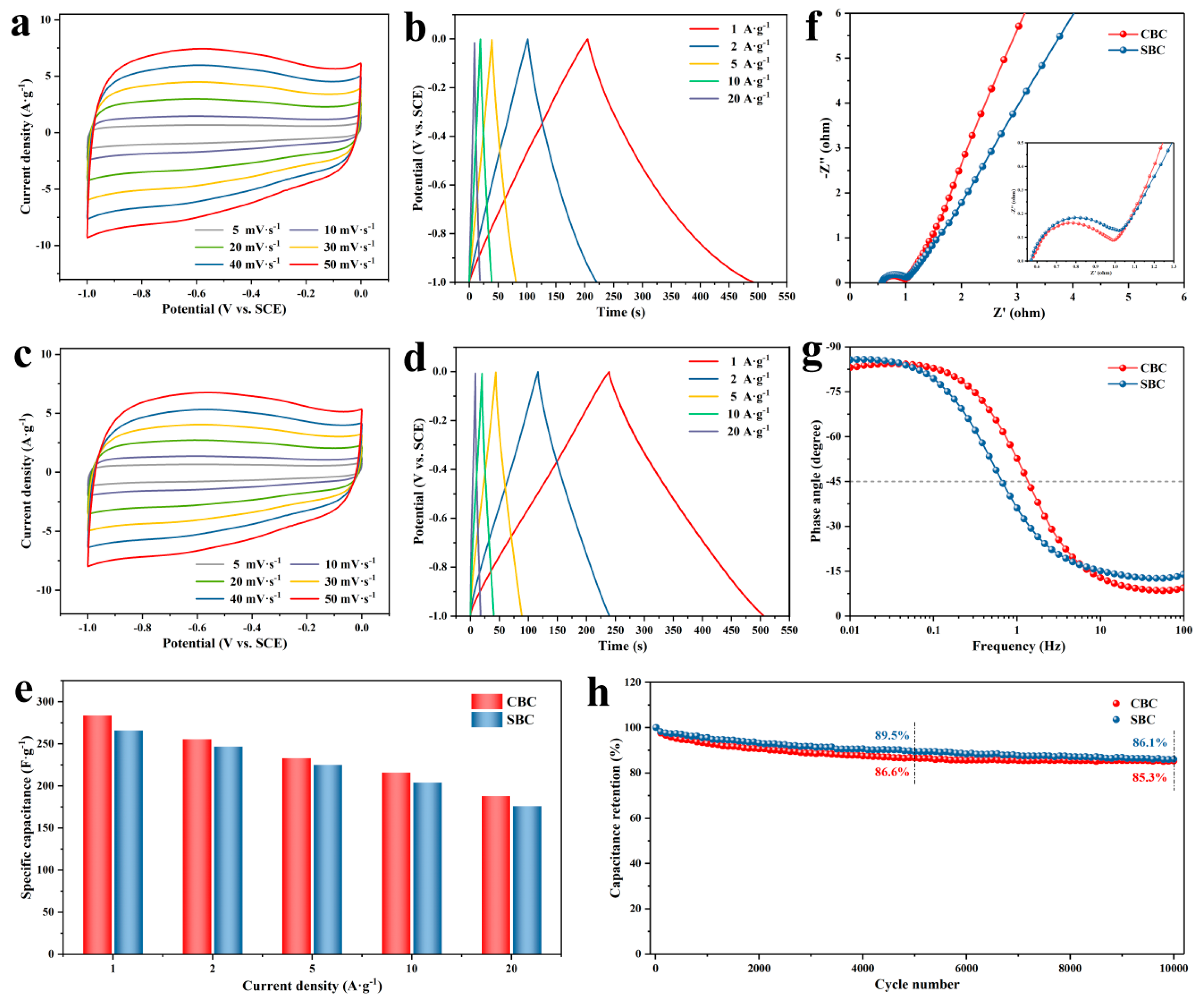
2.4. Electrochemical Characterization
2.5. Computation Methods
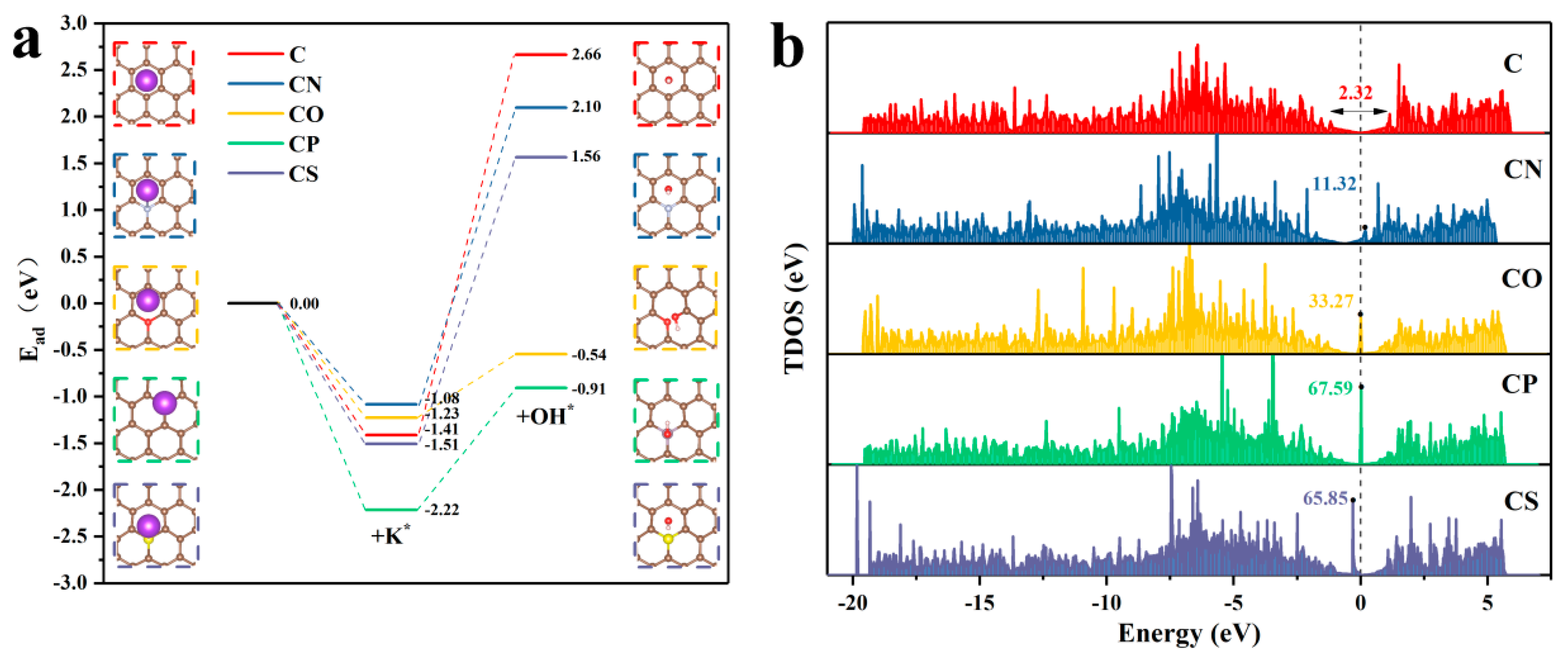
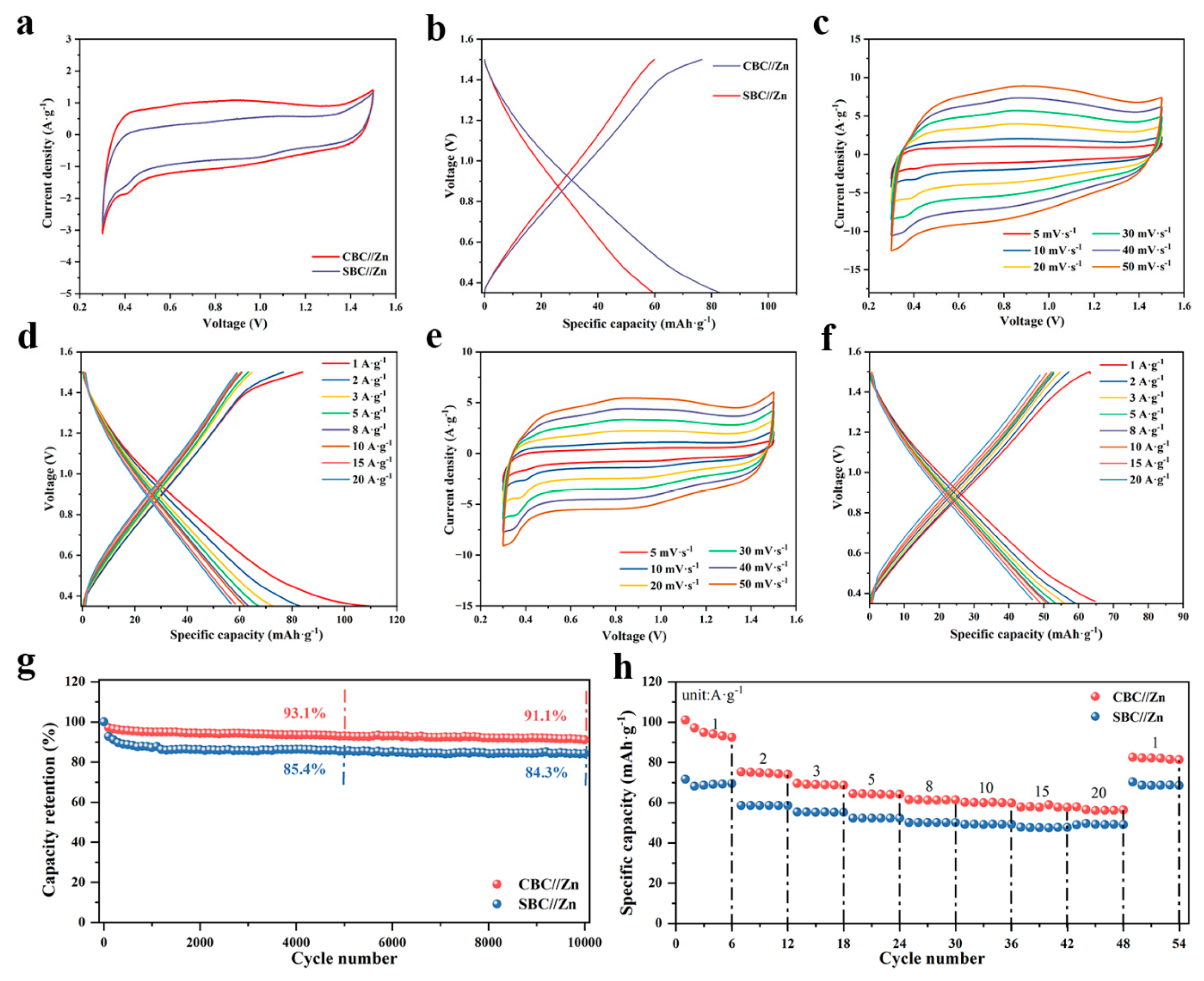
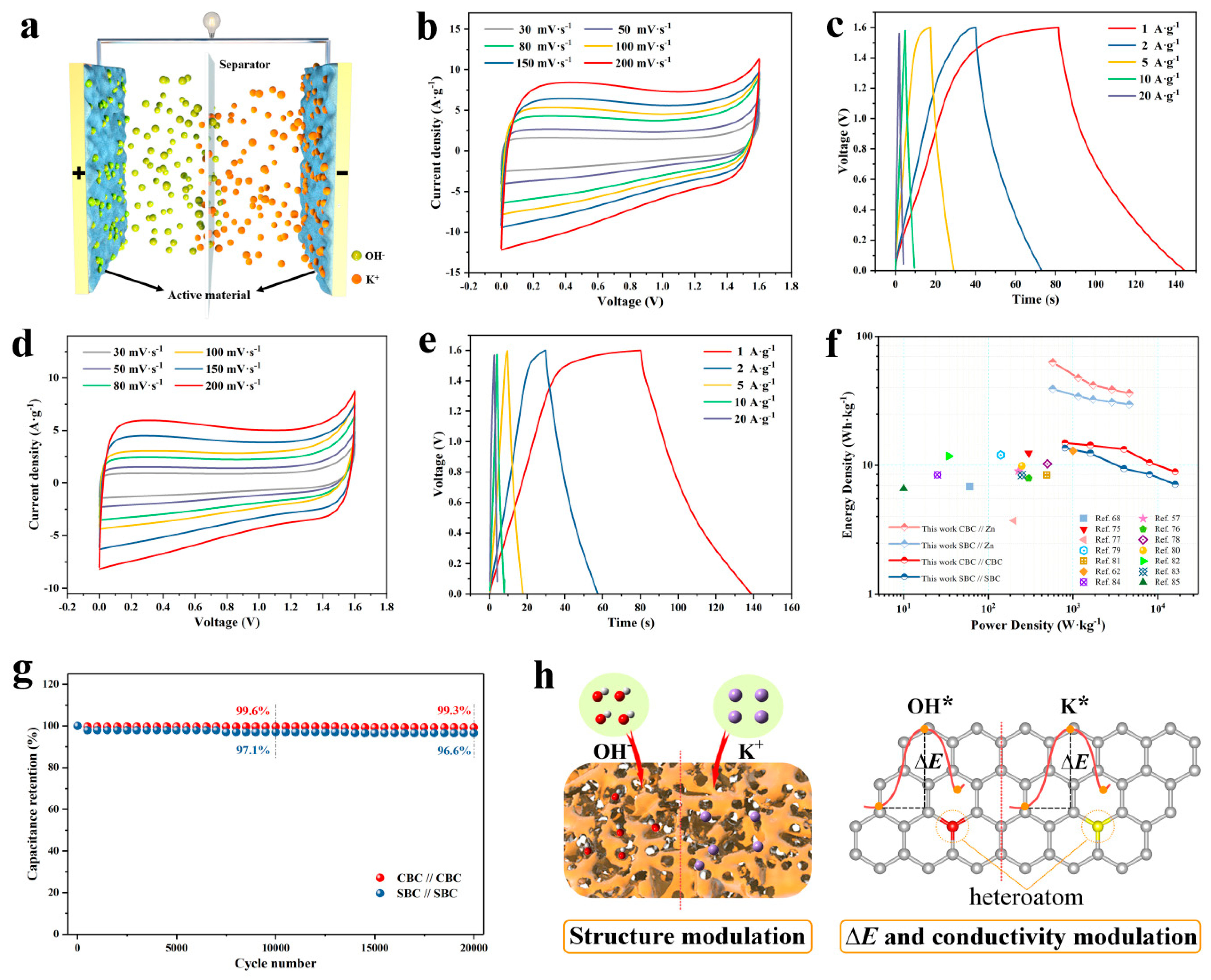
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, 6468. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, S.; Song, X.; Wang, N.; Peng, H.; Su, J.; Zeng, S.; Xu, X.; Yang, J. Suppressed dissolution and enhanced desolvation in core-shell MoO3@TiO2 nanorods as a high-rate and long-life anode material for proton batteries. Adv. Energy Mater. 2022, 12, 2200157. [Google Scholar] [CrossRef]
- Wang, J.; Yang, L.; Fu, Y.; Yin, P.; Guan, X.; Wang, G. Delicate control of crystallographic Cu2O derived Ni-Co amorphous double hydroxide nanocages for high-performance hybrid supercapacitors: An experimental and computational investigation. Nanoscale 2021, 13, 8562–8574. [Google Scholar] [CrossRef]
- Zhang, S.; Hao, J.; Luo, D.; Zhang, P.; Zhang, B.; Davey, K.; Lin, Z.; Qin, S. Dual-function electrolyte additive for highly reversible Zn anode. Adv. Energy Mater. 2021, 11, 2102010. [Google Scholar] [CrossRef]
- Yang, L.; Lu, X.; Wang, S.; Wang, J.; Guan, X.; Guan, X.; Wang, G. Designed synthesis of nickel-cobalt-based electrode materials for high-performance solid-state hybrid supercapacitors. Nanoscale 2020, 12, 1921–1938. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Yao, W.; Yao, R.; Sui, Y.; Zhu, H.; Wang, F.; Zhao, J.; Zhi, C.; Yang, C. Cations coordination-regulated reversibility enhancement for aqueous Zn-ion battery. Adv. Funct. Mater. 2021, 31, 2105736. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and mechanisms of asymmetric supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Tan, H.; Chao, D.; Fan, H. Recent advances in Zn-ion batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Wei, T.; Wei, X.; Gao, Y.; Li, H. Large scale production of biomass-derived nitrogen-doped porous carbon materials for supercapacitors. Electrochim. Acta 2015, 169, 186–194. [Google Scholar] [CrossRef]
- Kang, D.; Liu, Q.; Gu, J.; Su, Y.; Zhang, W.; Zhang, D. “Egg-box”-assisted fabrication of porous carbon with small mesopores for high-rate electric double layer capacitors. ACS Nano 2015, 9, 11225–11233. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, W.; Zhou, H.; Zhang, Z.; Zhao, M.; Liu, Q.; Yang, J.; Lu, X. Self-discharge of supercapacitors based on carbon nanotubes with different diameters. Electrochim. Acta 2020, 357, 136855. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, C.; Dai, Y.; Zhao, S.; Hu, X.; Zhao, F.; Zhang, W.; Chen, R.; Zong, W.; Du, Z.; et al. Three-dimensional manganese oxide@carbon networks as free-standing, high-loading cathodes for high-performance zinc-ion batteries. Small Struct. 2022, 4, 2200316. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, D.; Lau, A.; Ma, T.; Lin, H.; Jia, B. Hybridized graphene for supercapacitors: Beyond the limitation of pure graphene. Small 2021, 17, 2007311. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Y.; Zhao, X.; Shen, Q.; Zhao, W.; Tan, Q.; Zhang, N.; Li, P.; Jiao, L.; Qu, X. Sandwich-like heterostructures of MoS2/graphene with enlarged interlayer spacing and enhanced hydrophilicity as high-performance cathodes for aqueous zinc-ion batteries. Adv. Mater. 2021, 33, 2007480. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yin, C.; Zhang, M.; Xie, Y.; Hu, J.; Long, R.; Wu, X.; Wu, X. The intercalation cathode of MOFs-driven vanadium-based composite embedded in N-doped carbon for aqueous zinc ion batteries. Chem. Eng. J. 2023, 452, 139573. [Google Scholar] [CrossRef]
- Yu, L.; Hu, L.; Anasori, B.; Liu, Y.-T.; Zhu, Q.; Zhang, P.; Gogotsi, Y.; Xu, B. MXene-bonded activated carbon as a flexible electrode for high-performance supercapacitors. ACS Energy Lett. 2018, 3, 1597–1603. [Google Scholar] [CrossRef]
- Huo, Y.; Teng, Y.; Cai, K.; Chen, H. Honeycomb ZnO/N/C obtained from cornsilk and ZIF-8 dual induced method for long life aqueous zinc-ion batteries. J. Alloys Compd. 2021, 855, 157398. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Jia, M.; Cui, L.; Gao, J.; Jin, X.-J. Hydrothermal synthesis and characterization of litchi-like NiCo2Se4@carbon microspheres for asymmetric supercapacitors with high energy density. J. Electrochem. Soc. 2018, 165, E303. [Google Scholar] [CrossRef]
- Xie, L.; Su, F.; Xie, L.; Guo, X.; Wang, Z.; Kong, Q.; Sun, G.; Ahmad, A.; Li, X.; Yi, Z.; et al. Effect of pore structure and doping species on charge storage mechanisms in porous carbon-based supercapacitors. Mater. Chem. Front. 2020, 4, 2610–2634. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, H.; Li, Y.; Qi, D.; Wang, S.; Shen, K. Hierarchical porous carbon derived from carboxylated coal-tar pitch for electrical double-layer capacitors. RSC Adv. 2019, 9, 29131–29140. [Google Scholar] [CrossRef]
- Hou, J.; Cao, C.; Idrees, F.; Ma, X. Hierarchical porous nitrogen-doped carbon nanosheets derived from silk for ultrahigh-capacity battery anodes and supercapacitors. ACS Nano 2015, 9, 2556–2564. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Ji, Y.; Wu, H.; Chen, F. Enhanced electrochemical performance and high voltage window for supercapacitor based on multi-heteroatom modified porous carbon materials. Chem. Commun. 2019, 55, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, J.; Wang, S.; Guan, X.; Guan, X.; Wang, G. Biomass-derived multi-heteroatom-doped carbon materials for high-performance solid-state symmetric supercapacitors with superior long-term cycling stability. Ionics 2020, 26, 4141–4151. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Emerging applications of biochar-based materials for energy storage and conversion. Energy Environ. Sci. 2019, 12, 1751–1779. [Google Scholar] [CrossRef]
- Gao, M.; Wang, W.-K.; Zheng, Y.-M.; Zhao, Q.-B.; Yu, H.-Q. Hierarchically porous biochar for supercapacitor and electrochemical H2O2 production. Chem. Eng. J. 2020, 402, 126171. [Google Scholar] [CrossRef]
- Liu, X.; Shen, X.; Chen, T.; Xu, Q. The spinel MnFe2O4 grown in biomass-derived porous carbons materials for high-performance cathode materials of aqueous zinc-ion batteries. J. Alloys Compd. 2022, 904, 164002. [Google Scholar] [CrossRef]
- Ma, Z.-W.; Liu, H.-Q.; Lü, Q.-F. Porous biochar derived from tea saponin for supercapacitor electrode: Effect of preparation technique. J. Energy Storage 2021, 40, 102773. [Google Scholar] [CrossRef]
- Fu, M.; Chen, W.; Ding, J.; Zhu, X.; Liu, Q. Biomass waste derived multi-hierarchical porous carbon combined with CoFe2O4 as advanced electrode materials for supercapacitors. J. Alloys Compd. 2019, 782, 952–960. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; Gyenge, E.; Ellis, N. A novel method to tailor the porous structure of KOH-activated biochar and its application in capacitive deionization and energy storage. Biomass Bioenergy 2016, 87, 107–121. [Google Scholar] [CrossRef]
- Cuong, D.V.; Matsagar, B.M.; Lee, M.; Hossain, M.S.A.; Yamauchi, Y.; Vithanage, M.; Sarkar, B.; Ok, Y.S.; Wu, K.; Hou, C.-H. A critical review on biochar-based engineered hierarchical porous carbon for capacitive charge storage. Renew. Sustain. Energy Rev. 2021, 145, 111029. [Google Scholar] [CrossRef]
- Zou, K.; Guan, Z.; Deng, Y.; Chen, G. Nitrogen-rich porous carbon in ultra-high yield derived from activation of biomass waste by a novel eutectic salt for high performance Li-ion capacitors. Carbon 2020, 161, 25–35. [Google Scholar] [CrossRef]
- Ma, G.; Yang, Q.; Sun, K.; Peng, H.; Ran, F.; Zhao, X.; Lei, Z. Nitrogen-doped porous carbon derived from biomass waste for high-performance supercapacitor. Bioresour. Technol. 2015, 197, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, H.; Dang, B.; Wang, Z.; Shen, X.; Li, C.; Sun, Q. Ultrahigh yield of nitrogen doped porous carbon from biomass waste for supercapacitor. Renew. Energy 2020, 156, 370–376. [Google Scholar] [CrossRef]
- Purkait, T.; Singh, G.; Singh, M.; Kumar, D.; Dey, R.S. Large area few-layer graphene with scalable preparation from waste biomass for high-performance supercapacitor. Sci. Rep. 2017, 7, 15239. [Google Scholar] [CrossRef]
- Ma, G.; Hua, F.; Sun, K.; Zhang, Z.; Feng, E.; Peng, H.; Lei, Z. Porous carbon derived from sorghum stalk for symmetric supercapacitors. RSC Adv. 2016, 6, 103508–103516. [Google Scholar] [CrossRef]
- Han, J.; Cao, R.; Zhou, X.; Xu, Y. An integrated biorefinery process for adding values to corncob in co-production of xylooligosaccharides and glucose starting from pretreatment with gluconic acid. Bioresour. Technol. 2020, 307, 123200. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Pandey, N.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Pyrolysis of agricultural biomass residues: Comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour. Technol. 2017, 237, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Chen, X.; Zhou, D.; Liu, C. Biomass-derived hierarchical porous carbon/silicon carbide composite for electrochemical supercapacitor. Colloids Surf. A 2021, 620, 126567. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption isotherms, kinetics, thermodynamics and desorption studies of 2,4,6-trichlorophenol on oil palm empty fruit bunch-based activated carbon. J. Hazard. Mater. 2009, 164, 473–482. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Wang, G.-S.; Zhang, X.-J.; Gao, C. Porous carbon polyhedrons coupled with bimetallic CoNi alloys for frequency selective wave absorption at ultralow filler loading. J. Mater. Sci. Technol. 2022, 103, 34–41. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, K.; Li, S.; Li, M.; Li, J.; Ren, K. Synthesis of garlic skin-derived 3D hierarchical porous carbon for high-performance supercapacitors. Nanoscale 2018, 10, 2427–2437. [Google Scholar] [CrossRef]
- Tian, W.; Gao, Q.; Tan, Y.; Li, Z. Unusual interconnected graphitized carbon nanosheets as the electrode of high-rate ionic liquid-based supercapacitor. Carbon 2017, 119, 287–295. [Google Scholar] [CrossRef]
- Tian, W.; Gao, Q.; Tan, Y.; Yang, K.; Zhu, L.; Yang, C.; Zhang, H. Bio-inspired beehive-like hierarchical nanoporous carbon derived from bamboo-based industrial by-product as a high performance supercapacitor electrode material. J. Mater. Chem. A 2015, 3, 5656–5664. [Google Scholar] [CrossRef]
- Chin, S.F.; Pang, S.C.; Anderson, M.A. Self-assembled manganese dioxide nanowires as electrode materials for electrochemical capacitors. Mater. Lett. 2010, 64, 2670–2672. [Google Scholar] [CrossRef]
- Wu, F.; Gao, J.; Zhai, X.; Xie, M.; Sun, Y.; Kang, H.; Tian, Q.; Qiu, H. Hierarchical porous carbon microrods derived from albizia flowers for high performance supercapacitors. Carbon 2019, 147, 242–251. [Google Scholar] [CrossRef]
- Sankar, S.; Ahmed, A.T.A.; Inamdar, A.I.; Im, K.; Im, Y.B.; Lee, Y.; Kim, D.Y.; Lee, S. Biomass-derived ultrathin mesoporous graphitic carbon nanoflakes as stable electrode material for high-performance supercapacitors. Mater. Des. 2019, 169, 107688. [Google Scholar] [CrossRef]
- Li, J.; Gao, Y.; Han, K.; Qi, J.; Li, M.; Teng, Z. High performance hierarchical porous carbon derived from distinctive plant tissue for supercapacitor. Sci. Rep. 2019, 9, 17270. [Google Scholar] [CrossRef]
- Wang, D.-W.; Li, F.; Liu, M.; Lu, G.Q.; Cheng, H.-M. 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage. Angew. Chem. Int. Ed. 2010, 47, 1525. [Google Scholar]
- Zhang, J.; Li, Y.; Liang, X.; Liu, Q.; Chen, Q.; Chen, M. Sulfur vacancies-engineered Ni3S4−x hollow microspheres with optimized anionic adsorption energy for high-performance supercapacitor. Small 2022, 18, 2106074. [Google Scholar] [CrossRef]
- Jia, H.; Sun, J.; Xie, X.; Yin, K.; Sun, L. Cicada slough-derived heteroatom incorporated porous carbon for supercapacitor: Ultra-high gravimetric capacitance. Carbon 2019, 143, 309–317. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, L.; Sheng, L.; Zhou, Q.; Wei, T.; Zhang, B.; Fan, Z. Oxygen clusters distributed in graphene with “paddy land” structure: Ultrahigh capacitance and rate performance for supercapacitors. Adv. Funct. Mater. 2017, 28, 1705258. [Google Scholar] [CrossRef]
- Komolov, A.S.; Zhukov, Y.M.; Lazneva, E.F.; Aleshin, A.N.; Pshenichnyuk, S.A.; Gerasimova, N.B.; Panina, Y.A.; Zashikhin, G.D.; Baramygin, A.V. Thermally induced modification of the graphene oxide film on the tantalum surface. Mater. Des. 2017, 113, 319–325. [Google Scholar] [CrossRef]
- Komolov, A.S.; Lazneva, E.F.; Gerasimova, N.B.; Panina, Y.A.; Sobolev, V.S.; Koroleva, A.V.; Pshenichnyuk, S.A.; Asfandiarov, N.L.; Modelli, A.; Handke, B.; et al. Conduction band electronic states of ultrathin layers of thiophene/phenylene co-oligomers on an oxidized silicon surface. J. Electron Spectrosc. Relat. Phenom. 2019, 235, 40–45. [Google Scholar] [CrossRef]
- Luo, Z.; Lin, N.; Sun, M.; Wang, Y.; Zhu, X. Synthesis of 3D-interconnected hierarchical porous carbon from heavy fraction of bio-oil using crayfish shell as the biological template for high-performance supercapacitors. Carbon 2021, 173, 910–917. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, M.; Zhang, Y.; Zhao, W.; Wang, C. A biomass-derived nitrogen-doped porous carbon for high-energy supercapacitor. Carbon 2018, 140, 404–412. [Google Scholar] [CrossRef]
- Tyagi, A.; Sinha, P.; Kar, K.K.; Yokoi, H. Acid-directed preparation of micro/mesoporous heteroatom doped defective graphitic carbon as bifunctional electroactive material: Evaluation of trace metal impurity. J. Colloid Interface Sci. 2021, 604, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Cao, H.; Zhu, S.; Hou, L.; Yuan, C. Hierarchical micro-/mesoporous N- and O-enriched carbon derived from disposable cashmere: A competitive cost-effective material for high-performance electrochemical capacitors. Green Chem. 2015, 17, 2373–2382. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.Q.; Bandosz, T.J. Combined effect of nitrogen- and oxygen-containing functional groups of microporous activated carbon on its electrochemical performance in supercapacitors. Adv. Funct. Mater. 2009, 19, 438–447. [Google Scholar] [CrossRef]
- Costentin, C.; Porter, T.R.; Savéant, J.-M. How do pseudocapacitors store energy? Theoretical analysis and experimental illustration. ACS Appl. Mater. Interfaces 2017, 9, 8649–8658. [Google Scholar] [CrossRef]
- Sheng, Z.; Lin, X.; Wei, H.; Zhang, Y.; Tian, Z.; Wang, C.; Xu, D.; Wang, Y. Green synthesis of nitrogen-doped hierarchical porous carbon nanosheets derived from polyvinyl chloride towards high-performance supercapacitor. J. Power Sources 2021, 515, 230629. [Google Scholar] [CrossRef]
- Hou, L.; Yang, W.; Li, Y.; Wang, P.; Jiang, B.; Xu, C.; Zhang, C.; Huang, G.; Yang, F.; Li, Y. Dual-template endowing N, O co-doped hierarchically porous carbon from potassium citrate with high capacitance and rate capability for supercapacitors. Chem. Eng. J. 2021, 417, 129289. [Google Scholar] [CrossRef]
- Qu, W.-H.; Xu, Y.-Y.; Lu, A.-H.; Zhang, X.-Q.; Li, W.-C. Converting biowaste corncob residue into high value added porous carbon for supercapacitor electrodes. Bioresour. Technol. 2015, 189, 285–291. [Google Scholar] [CrossRef]
- Zhai, S.; Jin, K.; Zhou, M.; Fan, Z.; Zhao, H.; Zhao, Y.; Li, X.; Cai, Z. In-situ growth of flower-like CuS microsphere on carbonized cotton for high-performance flexible supercapacitor. Colloids Surf. A 2019, 575, 75–83. [Google Scholar] [CrossRef]
- Shi, S.; Wan, G.; Wu, L.; He, Z.; Wang, K.; Tang, Y.; Xu, X.; Wang, G. Ultrathin manganese oxide nanosheets uniformly coating on carbon nanocoils as high-performance asymmetric supercapacitor electrodes. J. Colloid Interface Sci. 2019, 537, 142–150. [Google Scholar] [CrossRef]
- Zhu, L.; Peh, C.K.N.; Zhu, T.; Lim, Y.-F.; Ho, G.W. Bifunctional 2D-on-2D MoO3 nanobelt/Ni (OH)2 nanosheets for supercapacitor-driven electrochromic energy storage. J. Mater. Chem. A 2017, 5, 8343–8351. [Google Scholar] [CrossRef]
- Huang, H.; Xu, R.; Feng, Y.; Zeng, S.; Jiang, Y.; Wang, H.; Luo, W.; Yu, Y. Sodium/potassium-ion batteries: Boosting the rate capability and cycle life by combining morphology, defect and structure engineering. Adv. Mater. 2020, 32, 1904320. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Feng, J.; Yan, R.; Wang, G.-C.; Antonietti, M.; Oschatz, M. Breaking the limits of ionic liquid-based supercapacitors: Mesoporous carbon electrodes functionalized with manganese oxide nanosplotches for dense, stable, and wide-temperature energy storage. Adv. Funct. Mater. 2018, 28, 1801298. [Google Scholar] [CrossRef]
- Selvaraj, A.R.; Muthusamy, A.; Inho, C.; Kim, H.-J.; Senthil, K.; Prabakar, K. Ultrahigh surface area biomass derived 3D hierarchical porous carbon nanosheet electrodes for high energy density supercapacitors. Carbon 2021, 174, 463–474. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, K.; Si, M.; Wang, H.; Chai, L.; Shi, Y. Three-dimensional carbon nanosheets derived from micro-morphologically regulated biomass for ultrahigh-performance supercapacitors. Carbon 2019, 153, 707–716. [Google Scholar] [CrossRef]
- Gong, C.; Wang, X.; Ma, D.; Chen, H.; Zhang, S.; Liao, Z. Microporous carbon from a biological waste-stiff silkworm for capacitive energy storage. Electrochim. Acta 2016, 220, 331–339. [Google Scholar] [CrossRef]
- Raj, C.J.; Rajesh, M.; Manikandan, R.; Yu, K.H.; Anusha, J.R.; Ahn, J.H.; Kim, D.-W.; Park, S.Y.; Kim, B.C. High electrochemical capacitor performance of oxygen and nitrogen enriched activated carbon derived from the pyrolysis and activation of squid gladius chitin. J. Power Sources 2018, 386, 66–76. [Google Scholar] [CrossRef]
- Guan, T.; Li, K.; Zhao, J.; Zhao, R.; Zhang, G.; Zhang, D.; Wang, J. Template-free preparation of layer-stacked hierarchical porous carbons from coal tar pitch for high performance all-solid-state supercapacitors. J. Mater. Chem. A 2017, 5, 15869–15878. [Google Scholar] [CrossRef]
- Díez, N.; Mysyk, R.; Zhang, W.; Goikolea, E.; Carriazo, D. One-pot synthesis of highly activated carbons from melamine and terephthalaldehyde as electrodes for high energy aqueous supercapacitors. J. Mater. Chem. A 2017, 5, 14619–14629. [Google Scholar] [CrossRef]
- Wu, J.; Xia, M.; Zhang, X.; Chen, Y.; Sun, F.; Wang, X.; Yang, H.; Chen, H. Hierarchical porous carbon derived from wood tar using crab as the template: Performance on supercapacitor. J. Power Sources 2020, 455, 227982. [Google Scholar] [CrossRef]
- Shao, J.; Ma, F.; Wu, G.; Dai, C.; Geng, W.; Song, S.; Wan, J. In-situ MgO (CaCO3) templating coupled with KOH activation strategy for high yield preparation of various porous carbons as supercapacitor electrode materials. Chem. Eng. J. 2017, 321, 301–313. [Google Scholar] [CrossRef]
- Niu, Q.; Gao, K.; Tang, Q.; Wang, L.; Han, L.; Fang, H.; Zhang, Y.; Wang, S.; Wang, L. Large-size graphene-like porous carbon nanosheets with controllable N-doped surface derived from sugarcane bagasse pith/chitosan for high performance supercapacitors. Carbon 2017, 123, 290–298. [Google Scholar] [CrossRef]
- Liu, B.; Yang, M.; Chen, H.; Liu, Y.; Yang, D.; Li, H. Graphene-like porous carbon nanosheets derived from salvia splendens for high-rate performance supercapacitors. J. Power Sources 2018, 397, 1–10. [Google Scholar] [CrossRef]
- He, X.; Li, R.; Qiu, J.; Xie, K.; Ling, P.; Yu, M.; Zhang, X.; Zheng, M. Synthesis of mesoporous carbons for supercapacitors from coal tar pitch by coupling microwave-assisted KOH activation with a MgO template. Carbon 2012, 50, 4911–4921. [Google Scholar] [CrossRef]
- Li, Z.; Wu, D.; Liang, Y.; Fu, R.; Matyjaszewski, K. Synthesis of well-defined microporous carbons by molecular-scale templating with polyhedral oligomeric silsesquioxane moieties. J. Am. Chem. Soc. 2014, 136, 4805–4808. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, K.; Zhang, W.; Ren, K.; Zhu, E.; Lu, J.; Chen, J.; Yin, P.; Yang, L.; Guan, X.; Wang, G. Electrochemical Performance of Corn Waste Derived Carbon Electrodes Based on the Intrinsic Biomass Properties. Materials 2023, 16, 5022. https://doi.org/10.3390/ma16145022
Xie K, Zhang W, Ren K, Zhu E, Lu J, Chen J, Yin P, Yang L, Guan X, Wang G. Electrochemical Performance of Corn Waste Derived Carbon Electrodes Based on the Intrinsic Biomass Properties. Materials. 2023; 16(14):5022. https://doi.org/10.3390/ma16145022
Chicago/Turabian StyleXie, Kunhan, Wen Zhang, Kai Ren, Enze Zhu, Jianyi Lu, Jingyang Chen, Penggang Yin, Liu Yang, Xiaohui Guan, and Guangsheng Wang. 2023. "Electrochemical Performance of Corn Waste Derived Carbon Electrodes Based on the Intrinsic Biomass Properties" Materials 16, no. 14: 5022. https://doi.org/10.3390/ma16145022
APA StyleXie, K., Zhang, W., Ren, K., Zhu, E., Lu, J., Chen, J., Yin, P., Yang, L., Guan, X., & Wang, G. (2023). Electrochemical Performance of Corn Waste Derived Carbon Electrodes Based on the Intrinsic Biomass Properties. Materials, 16(14), 5022. https://doi.org/10.3390/ma16145022





