Effect of a 3.5% NaCl−10% HCl Corrosive Environment on the Fatigue Behavior of Hot Rolled Aluminum 5083-H111
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
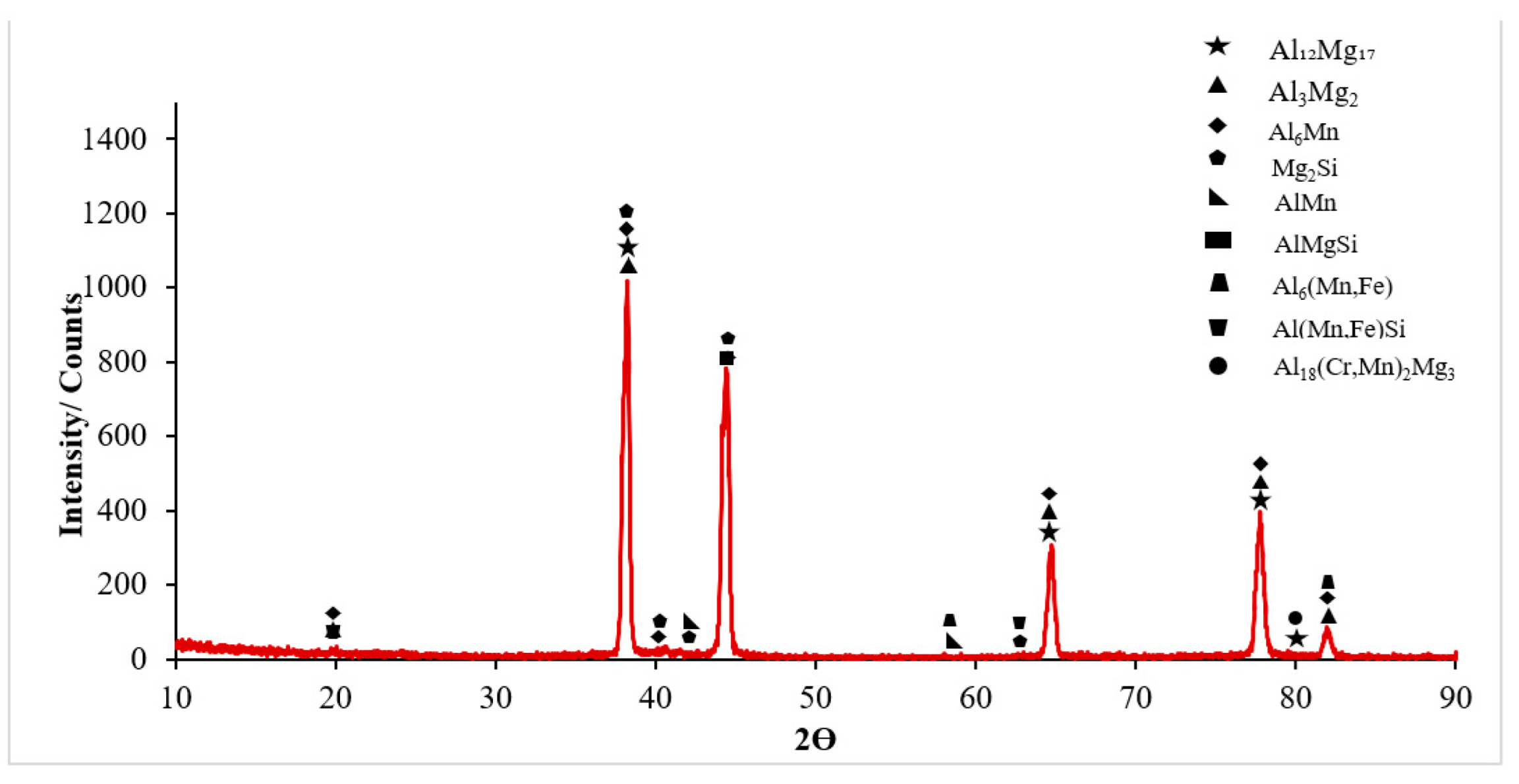
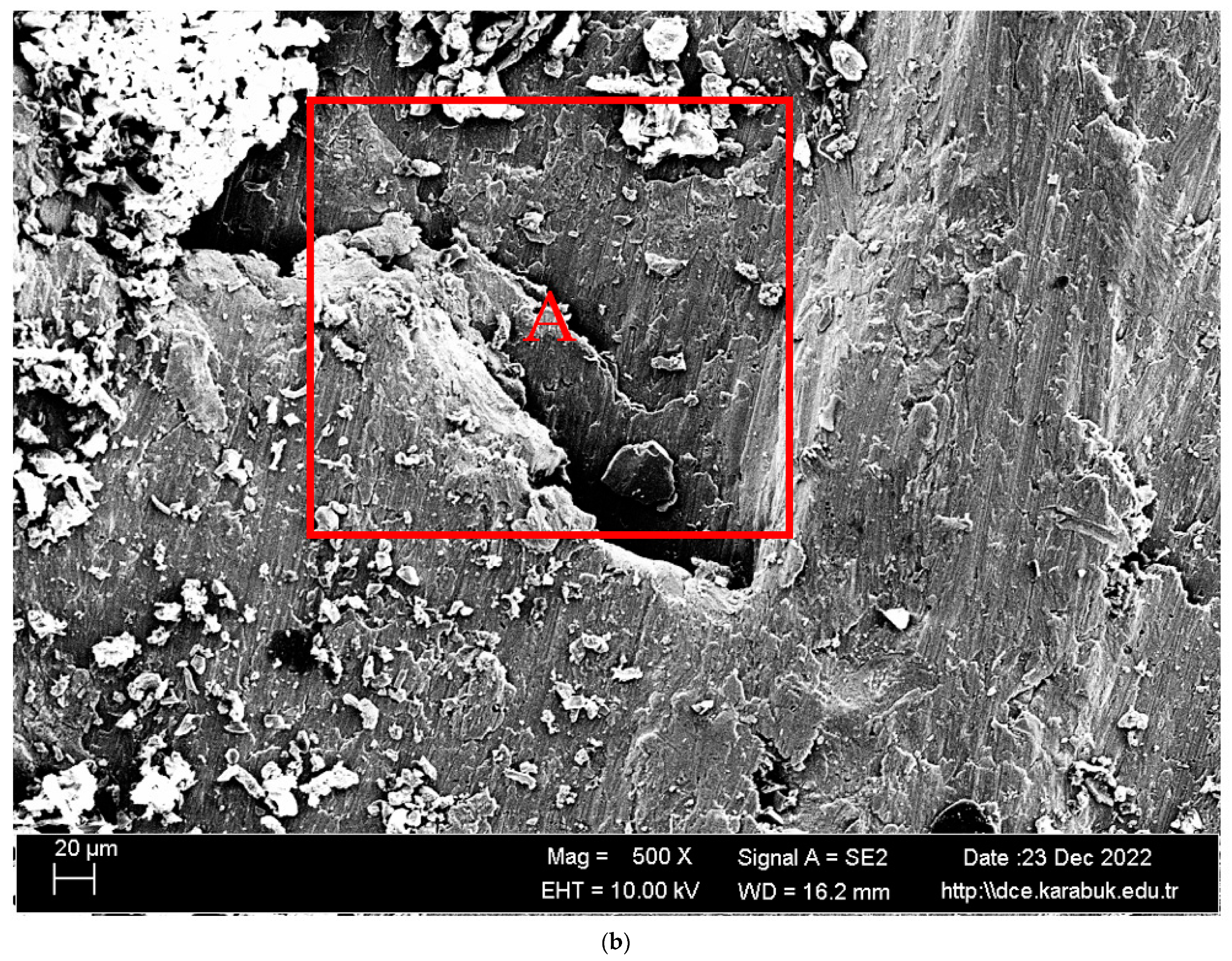
3.1. Microstructural Characterisation
3.2. Hardness Test Results
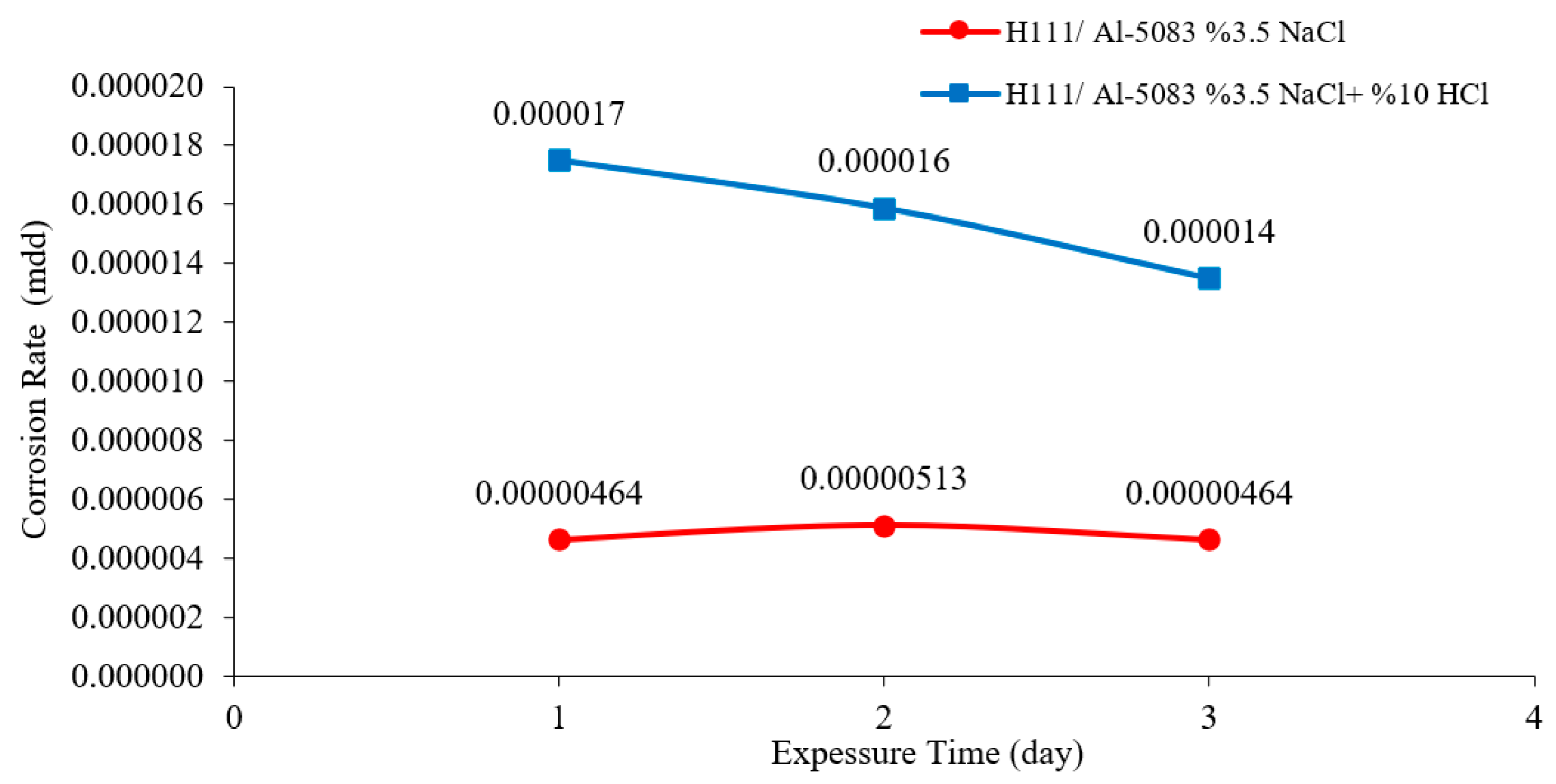
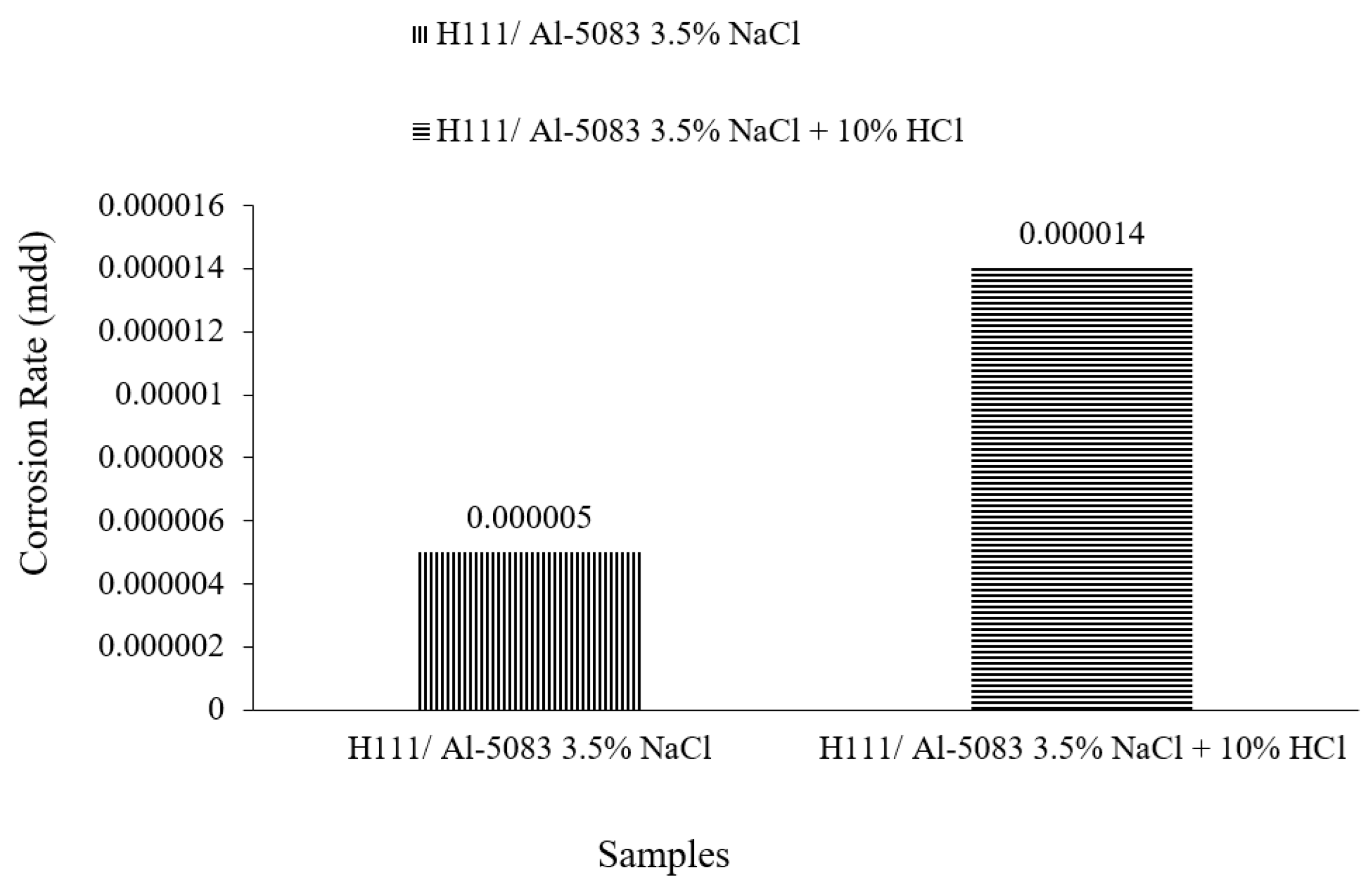
3.3. Immersion Corrosion Test Results
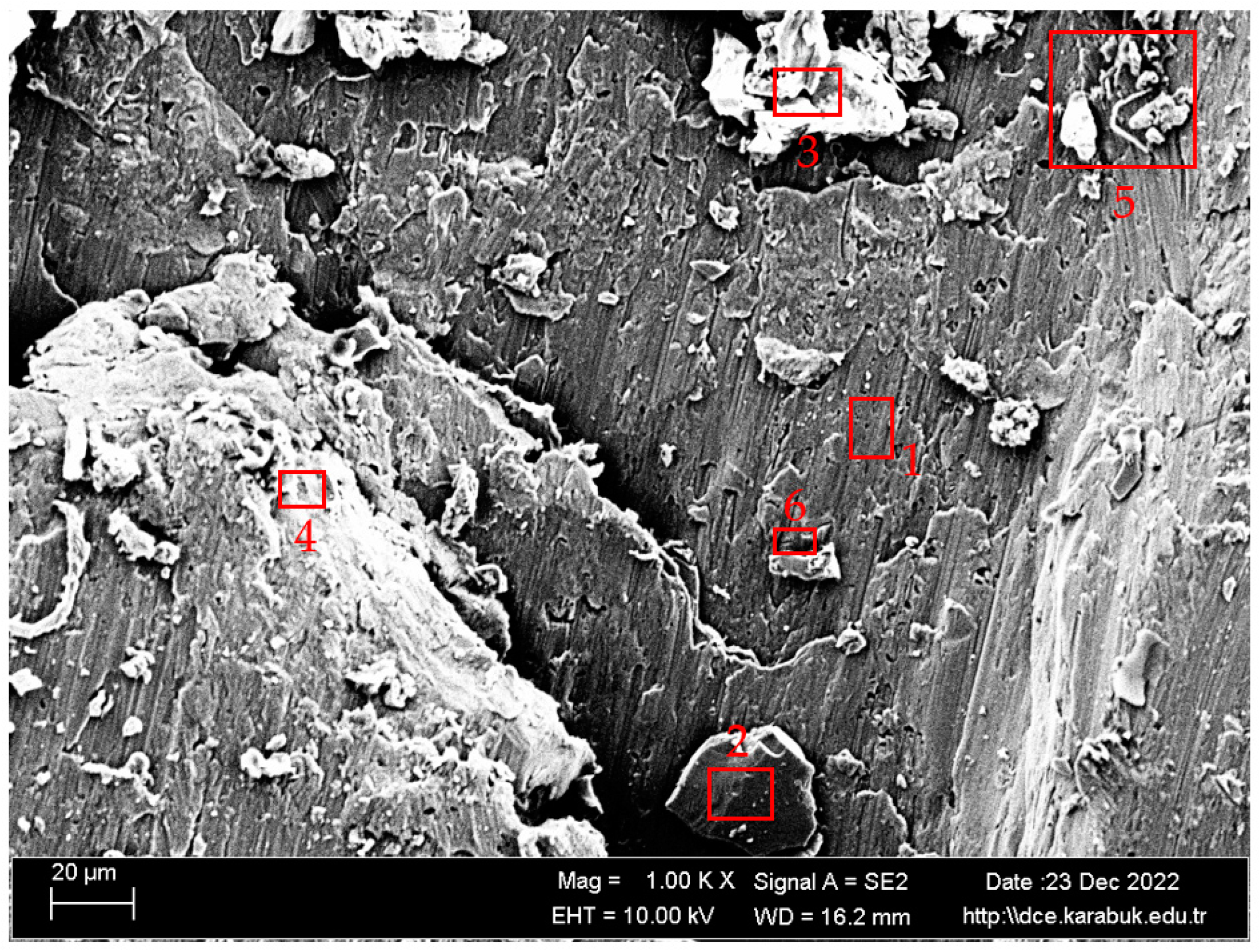
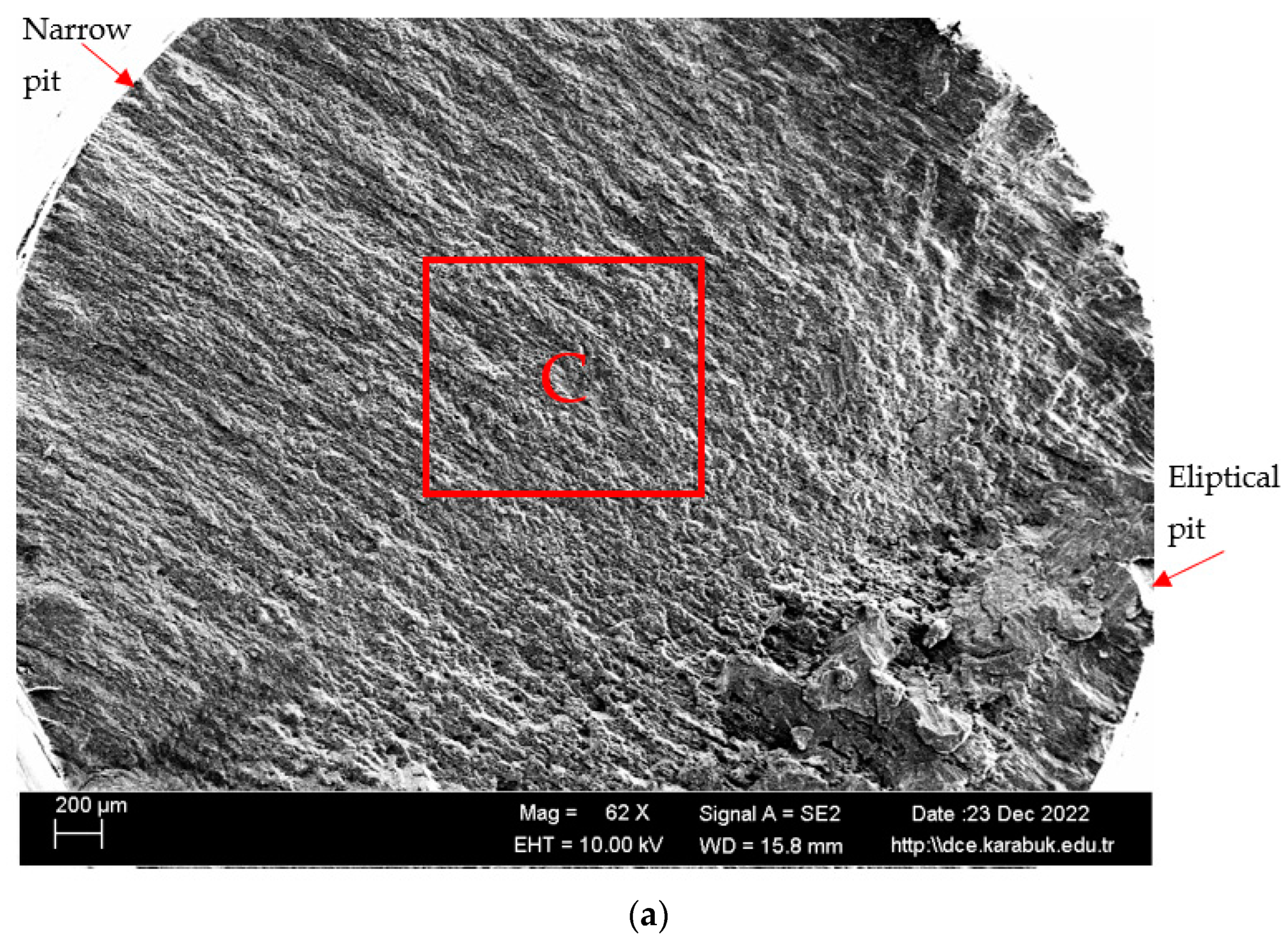
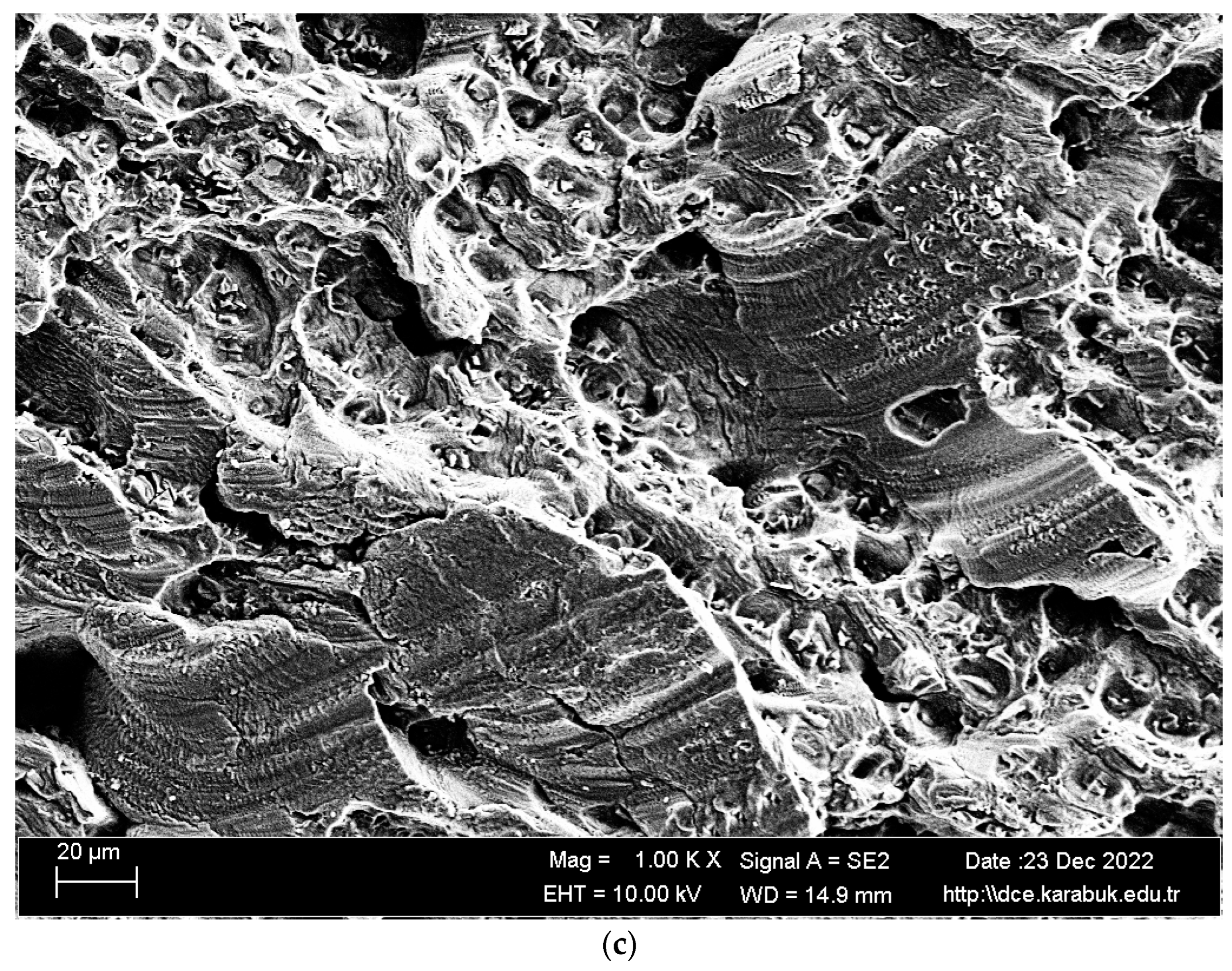
3.4. Rotary Bending Fatigue Test Results
4. Conclusions
- It was seen that the microstructure of the Al5083-H111 material consisted of grains oriented towards the rolling direction. We noticed that intermetallic phases precipitated at the grain boundary and were not evenly distributed.
- The hardness result of the Al5083-H111 material was measured as 68.67 ± 1.84 HB.
- According to the results of the immersion corrosion, while the Al5083 sample was more resistant to corrosion in a 3.5% NaCl environment, it showed less resistant behavior in a 3.5% NaCl + 10% HCl environment. The reason for this is thought to be intergranular corrosion in the material in the 3.5% NaCl + 10% HCl corrosion environment. The sample showed a more stable corrosion behavior after 24 h in a 3.5% NaCl environment. However, the same situation was not valid for a 3.5% NaCl + 10% HCl environment. According to the corrosion rates at the end of 72 h, the presence of the 10% HCl solution increased the corrosion rate by 180%.
- According to the fatigue results, it was observed that the non-corrosive sample showed a better fatigue life than the samples exposed to corrosion. The 3.5% NaCl corrosion fatigue sample, on the other hand, provided better results in the fatigue tests compared with the 3.5% NaCl + 10%HCl corrosion fatigue sample. The stable difference deteriorated after 48 h and the fatigue life of the sample exposed to corrosion in a 3.5% NaCl + 10% HCl environment decreased the most in the following hours compared with the other samples. According to the fatigue rate results, the presence of 10% HCl solution in the corrosion electrolyte reduced the fatigue life by 257%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Demirci, A.H.; Yiğit, K.; Aydın, H. MgCu2 Partikülleri Içeren Al-Cu Alaşımının Verilen Bir Tribosistem ve Yaşlandırma Şartlarında Aşınma Davranışlarının Belirlenmesi. In Proceedings of the 10. Denizli Malzeme Sempozyumu ve Sergisi Konferansı, Denizli, Türkiye, 14–16 April 2004; pp. 187–193. [Google Scholar]
- Brown, K.R.; Venie, M.S.; Woods, R.A. The Increasing Use of Aluminum in Automotive Applications. JOM 1995, 47, 20–23. [Google Scholar] [CrossRef]
- Stojanovic, B.; Bukvic, M.; Epler, I. Application of Aluminum and Aluminum Alloys in Engineering. Appl. Eng. Lett. 2018, 3, 52–62. [Google Scholar] [CrossRef]
- Santos, M.C.; Machado, A.R.; Sales, W.F.; Barrozo, M.A.S.; Ezugwu, E.O. Machining of Aluminum Alloys: A Review. Int. J. Adv. Manuf. Technol. 2016, 86, 3067–3080. [Google Scholar] [CrossRef]
- Campbell, F.C. Elements of Metallurgy and Engineering Alloys; Campbell, F.C., Ed.; ASM International: Almere, The Netherlands, 2008; ISBN 978-1-62708-251-8. [Google Scholar]
- Kalyoncu, S. Alüminyum Alaşımlarında Alçak Basınçlı Döküm Simülasyonu; Manisa Celal Bayar Üniversitesi: Manisa, Türkiye, 2020. [Google Scholar]
- Mutombo, K.; Du, M. Corrosion Fatigue Behaviour of Aluminium 5083-H111 Welded Using Gas Metal Arc Welding Method. In Arc Welding; InTech Open Access Publishing: London, UK, 2011; pp. 177–218. [Google Scholar]
- Mısırlı, C. 5083 Kalite Alüminyum Alaşımının Homojenize Edilerek Sertlik Değerlerinin Ve Mikroyapılarının İncelenmesi; Trakya Üniversitesi: Edirne, Turkey, 2011. [Google Scholar]
- Sielski, R. Research Needs in Aluminum Structure. Ships Offshore Struct. 2008, 3, 57–65. [Google Scholar] [CrossRef]
- Huskins, E.L.; Cao, B.; Ramesh, K.T. Strengthening Mechanisms in an Al–Mg Alloy. Mater. Sci. Eng. A 2010, 527, 1292–1298. [Google Scholar] [CrossRef]
- Yi, G.; Free, M.L.; Zhu, Y.; Derrick, A.T. Capillarity Effect Controlled Precipitate Growth at the Grain Boundary of Long-Term Aging Al 5083 Alloy. In Proceedings of the Metallurgical and Materials Transactions A: Physical Metallurgy and Materials Science; Springer: Boston, FL, USA, 2014; Volume 45, pp. 4851–4862. [Google Scholar]
- Lean, P.; Gil, L.; Ureña, A. Dissimilar Welds between Unreinforced AA6082 and AA6092/SiC/25p Composite by Pulsed-MIG Arc Welding Using Unreinforced Filler Alloys (Al–5Mg and Al–5Si). J. Mater. Process. Technol. 2003, 143–144, 846–850. [Google Scholar] [CrossRef]
- Czechowski, M. Corrosion Fatigue of Gma Welded Al—Mg Alloy. Adv. Mater. Sci. 2004, 4, 16–24. [Google Scholar]
- Praveen, P.; Yarlagadda, P.K.D.V. Meeting Challenges in Welding of Aluminum Alloys through Pulse Gas Metal Arc Welding. J. Mater. Process. Technol. 2005, 164–165, 1106–1112. [Google Scholar] [CrossRef]
- Olson, D.L.; Siewert, T.A.; Liu, S.; Edwards, G.R. Welding, Brazing, and Soldering; ASM International: Detroit, MI, USA, 2007. [Google Scholar]
- Ralston, K.D.; Birbilis, N.; Weyland, M.; Hutchinson, C.R. The Effect of Precipitate Size on the Yield Strength-Pitting Corrosion Correlation in Al–Cu–Mg Alloys. Acta Mater. 2010, 58, 5941–5948. [Google Scholar] [CrossRef]
- Ishıhara, S.; Saka, S.; Nan, Z.Y.; Goshıma, T.; Sunada, S. Prediction of Corrosion Fatigue Lives of Aluminium Alloy on the Basis of Corrosion Pit Growth Law. Fatigue Fract. Eng. Mater. Struct. 2006, 29, 472–480. [Google Scholar] [CrossRef]
- Rokhlin, S.I.; Kim, J.-Y.; Nagy, H.; Zoofan, B. Effect of Pitting Corrosion on Fatigue Crack Initiation and Fatigue Life. Eng. Fract. Mech. 1999, 62, 425–444. [Google Scholar] [CrossRef]
- Chlıstovsky, R.; Heffernan, P.; Duquesnay, D. Corrosion-Fatigue Behaviour of 7075-T651 Aluminum Alloy Subjected to Periodic Overloads. Int. J. Fatigue 2007, 29, 1941–1949. [Google Scholar] [CrossRef]
- Sharma, M.M.; Tomedi, J.D.; Parks, J.M. A Microscopic Study on the Corrosion Fatigue of Ultra-Fine Grained and Conventional Al-Mg Alloy. Corros. Sci. 2015, 93, 180–190. [Google Scholar] [CrossRef] [Green Version]
- Holtz, R.L.; Pao, P.S.; Bayles, R.A.; Longazel, T.M.; Goswami, R. Corrosion-Fatigue Behavior of Aluminum Alloy 5083-H131 Sensitized at 448 K (175 C). Proc. Metall. Mater. Trans. Phys. Metall. Mater. Sci. 2012, 43, 2839–2849. [Google Scholar] [CrossRef]
- Rudra, A.; Ashiq, M.; Tiwari, J.K.; Das, S.; Dasgupta, R. Study of Processing Map and Effect of Hot Rolling on Mechanical Properties of Aluminum 5083 Alloy. Trans. Indian Inst. Met. 2020, 73, 1809–1826. [Google Scholar] [CrossRef]
- Aballe, A.; Bethencourt, M.; Botana, F.; Cano, M.; Marcos, M. Localized Alkaline Corrosion of Alloy AA5083 in Neutral 3.5% NaCl Solution. Corros. Sci. 2001, 43, 1657–1674. [Google Scholar] [CrossRef]
- Engler, O.; Miller-Jupp, S. Control of Second-Phase Particles in the Al-Mg-Mn Alloy AA 5083. J. Alloys Compd. 2016, 689, 998–1010. [Google Scholar] [CrossRef]
- Zhu, Y.; Cullen, D.A.; Kar, S.; Free, M.L.; Allard, L.F. Evaluation of Al3Mg2 Precipitates and Mn-Rich Phase in Aluminum-Magnesium Alloy Based on Scanning Transmission Electron Microscopy Imaging. Metall. Mater. Trans. A 2012, 43, 4933–4939. [Google Scholar] [CrossRef]
- Goswami, R.; Holtz, R.L. Transmission Electron Microscopic Investigations of Grain Boundary Beta Phase Precipitation in Al 5083 Aged at 373 K (100 °C). Metall. Mater. Trans. A 2013, 44, 1279–1289. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhang, W.Z.; Marthinsen, K. Precipitation Crystallography of Plate-Shaped Al6(Mn,Fe) Dispersoids in AA5182 Alloy. Acta Mater. 2012, 60, 5963–5974. [Google Scholar] [CrossRef]
- Yi, G.; Sun, B.; Poplawsky, J.D.; Zhu, Y.; Free, M.L. Investigation of Pre-Existing Particles in Al 5083 Alloys. J. Alloys Compd. 2018, 740, 461–469. [Google Scholar] [CrossRef]
- Engler, O.; Liu, Z.; Kuhnke, K. Impact of Homogenization on Particles in the Al–Mg–Mn Alloy AA 5454—Experiment and Simulation. J. Alloys Compd. 2013, 560, 111–122. [Google Scholar] [CrossRef]
- Zajac, S.; Hutchinson, B.; Johansson, A.; Gullman, L.-O. Microstructure Control and Extrudability of Al–Mg–Si Alloys Microalloyed with Manganese. Mater. Sci. Technol. 1994, 10, 323–333. [Google Scholar] [CrossRef]
- Flores-V, A.; Sukiennik, M.; Castillejos-E, A.H.; Acosta-G, F.A.; Escobedo-B, J.C. A Kinetic Study on the Nucleation and Growth of the Al8FeMnSi2 Intermetallic Compound for Aluminum Scrap Purification. Intermetallics 1998, 6, 217–227. [Google Scholar] [CrossRef]
- Kılınç, M. 5083 Kalite Al Alaşımının Mekanik Özelliklerine Termomekanik Isıl İşleminin Etkisi; Karabük Üniversitesi: Karabuk, Türkiye, 2021. [Google Scholar]
- Tan, L.; Allen, T.R. Effect of Thermomechanical Treatment on the Corrosion of AA5083. Corros. Sci. 2010, 52, 548–554. [Google Scholar] [CrossRef]
- Yi, G.; Zeng, W.; Poplawsky, J.D.; Cullen, D.A.; Wang, Z.; Free, M.L. Characterizing and Modeling the Precipitation of Mg-Rich Phases in Al 5xxx Alloys Aged at Low Temperatures. J. Mater. Sci. Technol. 2017, 33, 991–1003. [Google Scholar] [CrossRef]
- Yan, J.; Hodge, A.M. Study of β Precipitation and Layer Structure Formation in Al 5083: The Role of Dispersoids and Grain Boundaries. J. Alloys Compd. 2017, 703, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Bethencourt, M.; Botana, F.J.; Calvino, J.J.; Marcos Bárcena, M.; Pérez, J.; Rodríguez, M.A. The Influence of the Surface Distribution of Al6(MnFe) Intermetallic on the Electrochemical Response of AA5083 Aluminium Alloy in NaCl Solutions. Mater. Sci. Forum 1998, 289–292, 567–574. [Google Scholar] [CrossRef]
- Arenas, M.A.; Bethencourt, M.; Botana, F.J.; De Damborenea, J.; Marcos, M. Inhibition of 5083 Aluminium Alloy and Galvanised Steel by Lanthanide Salts. Corros. Sci. 2001, 43, 157–170. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Pitting Corrosion of Aluminum. Corros. Sci. 1999, 41, 1743–1767. [Google Scholar] [CrossRef]
- Lucas, K.A.; Clarke, H. Corrosion of Aluminum-Based Metal Matrix Composites; Research Studies Press Ltd.: Baldock, UK, 1993. [Google Scholar]
- Speidel, M.O.; Hyatt, M.V.; Fontana, M.G.; Staettle, R.W. Advances in Corrosion Science and Technology; Plenum Press: New York, NY, USA, 1972. [Google Scholar]
- Pidaparti, R.M.; Rao, A.S. Analysis of Pits Induced Stresses Due to Metal Corrosion. Corros. Sci. 2008, 50, 1932–1938. [Google Scholar] [CrossRef]
- Aliravci, C.A.; Pekgüleryüz, M.Ö. Calculation of Phase Diagrams for the Metastable Al-Fe Phases Forming in Direct-Chill (DC)-Cast Aluminum Alloy Ingots. Calphad 1998, 22, 147–155. [Google Scholar] [CrossRef]
- Sharma, M.M.; Ziemian, C.W. Pitting and Stress Corrosion Cracking Susceptibility of Nanostructured Al-Mg Alloys in Natural and Artificial Environments. J. Mater. Eng. Perform. 2008, 17, 870–878. [Google Scholar] [CrossRef]
- Sharma, M.M.; Tomedi, J.D.; Weigley, T.J. Slow Strain Rate Testing and Stress Corrosion Cracking of Ultra-Fine Grained and Conventional Al–Mg Alloy. Mater. Sci. Eng. A 2014, 619, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Sikora, E.; Wei, X.J.; Shaw, B.A. Corrosion Behavior of Nanocrystalline Bulk Al-Mg-Based Alloys. Corrosion 2004, 60, 387–398. [Google Scholar] [CrossRef]
















| Alloy | Fe | Si | Cu | Mn | Mg | Zn | Cr | Ti | Other | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| Al5083 | 0.4 | 0.4 | 0.1 | 0.4–1.0 | 4.0–4.9 | 0.25 | 0.50–0.25 | 0.15 | 0.15 | Balance |
| Mg | Si | S | Cl | Cr | Mn | Fe | Ni | Cu | Zn | Al | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample 5083 | 3.6500 | 0.3486 | 0.0108 | 0.0376 | 0.1218 | 0.5259 | 0.3483 | 0.0126 | 0.069 | 0.0345 | 94.8408 |
| Spectrum | Mg | Al | Si | Cr | Mn | Fe |
|---|---|---|---|---|---|---|
| 1 | 5.19 | 94.81 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2 | 2.06 | 33.29 | 63.76 | 0.28 | 0.61 | 0.00 |
| 3 | 4.62 | 93.34 | 0.92 | 0.16 | 0.81 | 1.06 |
| 4 | 5.16 | 93.68 | 0.50 | 0.22 | 0.45 | 0.00 |
| Spectrum | O | Na | Mg | Al | Si | Cl | Cr | Mn | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.48 | 0.53 | 5.16 | 92.42 | 0.33 | 0.00 | 0.08 | 0.00 | 0.00 |
| 2 | 1.75 | 0.46 | 0.29 | 2.21 | 94.98 | 0.17 | 0.15 | 0.00 | 0.00 |
| 3 | 6.57 | 0.30 | 4.41 | 86.48 | 0.70 | 0.00 | 0.40 | 0.47 | 0.68 |
| 4 | 7.43 | 0.44 | 4.51 | 86.87 | 0.41 | 0.04 | 0.30 | 0.00 | 0.00 |
| 5 | 6.71 | 0.33 | 3.91 | 75.45 | 12.55 | 0.01 | 0.33 | 0.35 | 0.34 |
| 6 | 3.96 | 0.36 | 0.83 | 10.37 | 81.76 | 0.00 | 0.26 | 1.26 | 1.19 |
| Spectrum | H | O | Na | Mg | Al | Si | Cl | Cr | Mn | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.04 | 10.77 | 2.05 | 4.38 | 82.13 | 0.11 | 0.00 | 0.00 | 0.00 | 0.51 |
| 2 | 76.00 | 11.48 | 0.05 | 0.46 | 6.16 | 0.45 | 0.00 | 3.67 | 1.73 | 0.00 |
| 3 | 3.96 | 19.09 | 4.62 | 3.43 | 66.13 | 0.59 | 0.69 | 0.58 | 0.00 | 0.91 |
| 4 | 0.20 | 2.20 | 0.38 | 4.84 | 91.97 | 0.29 | 0.12 | 0.00 | 0.00 | 0.00 |
| 5 | 2.29 | 1.79 | 0.30 | 4.46 | 90.02 | 0.00 | 0.13 | 0.00 | 0.75 | 0.27 |
| 6 | 1.42 | 3.84 | 0.98 | 4.05 | 80.41 | 0.11 | 8.54 | 0.12 | 0.17 | 0.35 |
| 7 | 16.77 | 3.43 | 0.54 | 1.49 | 29.39 | 0.00 | 47.68 | 0.00 | 0.42 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhasslouk, M.M.M.; Esen, İ.; Ahlatcı, H.; Akın, B. Effect of a 3.5% NaCl−10% HCl Corrosive Environment on the Fatigue Behavior of Hot Rolled Aluminum 5083-H111. Materials 2023, 16, 4996. https://doi.org/10.3390/ma16144996
Elhasslouk MMM, Esen İ, Ahlatcı H, Akın B. Effect of a 3.5% NaCl−10% HCl Corrosive Environment on the Fatigue Behavior of Hot Rolled Aluminum 5083-H111. Materials. 2023; 16(14):4996. https://doi.org/10.3390/ma16144996
Chicago/Turabian StyleElhasslouk, Masoud M. M., İsmail Esen, Hayrettin Ahlatcı, and Bengu Akın. 2023. "Effect of a 3.5% NaCl−10% HCl Corrosive Environment on the Fatigue Behavior of Hot Rolled Aluminum 5083-H111" Materials 16, no. 14: 4996. https://doi.org/10.3390/ma16144996
APA StyleElhasslouk, M. M. M., Esen, İ., Ahlatcı, H., & Akın, B. (2023). Effect of a 3.5% NaCl−10% HCl Corrosive Environment on the Fatigue Behavior of Hot Rolled Aluminum 5083-H111. Materials, 16(14), 4996. https://doi.org/10.3390/ma16144996






