The Effect of Hydrothermal, Microwave, and Mechanochemical Treatments of Tin Phosphate on Sorption of Some Cations
Abstract
1. Introduction
2. Materials and Methods
2.1. Adsorbents
2.2. Physicochemical Methods of Investigations
2.3. Testing the Sorption Properties
3. Results and Discussion
3.1. Composition and Crystal Structure of Adsorbents
3.2. Porous Structure of Adsorbents
3.3. Sorption Properties
3.4. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clearfield, A. Inorganic ion-exchangers, past, present and future. Solvent Extr. Ion Exch. 2000, 18, 655–678. [Google Scholar] [CrossRef]
- Coker, E.N. Inorganic ion exchangers. In Encyclopedia of Separation Science; Academic Press: Cambridge, UK, 2000. [Google Scholar]
- Naushad, M. Inorganic and composite ion exchange materials and their applications (Review). Ion Exch. Lett. 2009, 2, 1–14. [Google Scholar]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef]
- Alby, D.; Charnay, C.; Heran, M.; Prelot, B.; Zajac, J. Recent developments in nanostructured inorganic materials for sorption of cesium and strontium: Synthesis and shaping, sorption capacity, mechanisms, and selectivity—A review. J. Hazard. Mater. 2018, 344, 511–530. [Google Scholar] [CrossRef]
- Krynitsyn, A.P.; Simanovskaya, I.Y.; Strykhar, O.L. Investigation of the interaction of water with structural and fuel-containing materials in the premises of the “Ukrytie” facility. Radiochemistry 1998, 40, 279–288. (In Russian) [Google Scholar]
- Inoue, Y. Studies on the synthetic inorganic ion exchangers—I. J. Inorg. Nucl. Chem. 1964, 26, 2241–2253. [Google Scholar] [CrossRef]
- Bhattacharyya, D.K.; De, A. Role of stannic phosphate in the uptake of cations. Separation of zirconium from thorium and cadmium from indium at tracer concentrations. J. Radioanal. Nucl. Chem. 1986, 100, 277–285. [Google Scholar] [CrossRef]
- Constantino, U.; Gaspizroni, A. Crystalline insoluble acid salts of tetravalent metals XI. Synthesis and ion-exchange properties of tin (IV) phosphate and tin(IV) arsenate. J. Chromatogr. 1970, 51, 289–296. [Google Scholar] [CrossRef]
- Zakutevskyy, O.; Sydorchuk, V.; Kovtun, M.; Khalameida, S.; Skwarek, E. Sorption of some cations on ammonium molybdophosphate embedded into structure of silica and titania. Res. Chem. Intermed. 2023, 49, 2233–2255. [Google Scholar] [CrossRef]
- Fuller, M. On exchange properties of tin (IV) materials -IV crystalline tin(IV) phosphate. J. Inorg. Nucl. Chern. 1971, 33, 559–566. [Google Scholar] [CrossRef]
- Sahu, B.B.; Mishra, H.K.; Parida, K. Cation Exchange and Sorption Properties of Tin (IV) Phosphate. J. Colloid Interface Sci. 2000, 225, 511–519. [Google Scholar] [CrossRef]
- Huang, W.; Komarneni, S.; Aref, A.R.; Noh, Y.D.; Ma, J.; Chen, K.; Xue, D.; Jiang, B. Nanolayered tin phosphate: A remarkably selective Cs ion sieve for acidic waste solutions. Chem. Comm. 2015, 51, 15661–15664. [Google Scholar] [CrossRef] [PubMed]
- Parida, K.; Sahu, B.; Das, D. A comparative study on textural characterization: Cation-exchange and sorption properties of crystalline α-zirconium (IV), tin(IV), and titanium(IV) phosphates. J. Colloid Interface Sci. 2004, 270, 436–445. [Google Scholar] [CrossRef]
- Ahn, B.-S. Physicochemical Characteristics of Selective Adsorption of Tin Phosphate on the Transition metal ions. J. Korea Appl. Sci. Technol. 2020, 37, 1222–1228. [Google Scholar]
- Stenina, I. Cation mobility and ion exchange in acid tin phosphate. Solid State Ion 2003, 162–163, 191–195. [Google Scholar] [CrossRef]
- Datkova, E.A.; Dimova, L.M.; Ratovskii, G.V.; Tyukalova, O.V.; Belonogova, L.N.; Smirnov, G.I. Study of ion-exchange properties of tin(IV) phosphate modified with sodium hexanitrocobaltate. Russ. J. Appl. Chem. 2012, 85, 1157–1162. [Google Scholar] [CrossRef]
- Zhu, C.; Dong, X.; Chen, Z.; Naidu, R. Adsorption of aqueous Pb(II), Cu(II), Zn(II) ions by amorphous tin(VI) hydrogen phosphate: An excellent inorganic adsorbent. Int. J. Environ. Sci. Technol. 2016, 13, 1257–1268. [Google Scholar] [CrossRef]
- Li, W.-Q.; Liu, D.; Qu, J.-Y.; Luo, J.-H. Hydrothermal synthesis of a novel nanolayered tin phosphate for removing Cr(III). RSC Adv. 2021, 11, 3202–3208. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, W.; Yang, L.; Luo, J. Synthesis and characterization of a reusable layered tin titanium phosphate for removing Cu(II). J. Solid State Chem. 2022, 314, 123362. [Google Scholar] [CrossRef]
- Abass, M.R.; Ragab Maree, R.M.; Sami, N.M. Adsorptive features of cesium and strontium ions on zirconium tin(IV) phosphate nanocomposite from aqueous solutions. Int. J. Environ. Anal. Chem. 2022. [Google Scholar] [CrossRef]
- Samsonenko, M.; Zakutevskyy, O.; Khalameida, S.; Charmas, B.; Skubiszewska-Zieba, J. Influence of mechanochemical and microwave modification on ion-exchange properties of tin dioxide with respect to uranyl ions. Adsorption 2019, 25, 451–457. [Google Scholar] [CrossRef]
- Samsonenko, M.; Zakutevskyy, O.; Khalameida, S.; Skubiszewska-Zieba, J.; Kovtun, M. Study of the physical-chemical and sorption properties of SnO2 prepared by mechanochemical and microwave routes. Chem. Phys. Technol. Surf. 2018, 9, 383–392. [Google Scholar] [CrossRef]
- Zakutevskyy, O.I.; Khalameida, S.V.; Sydorchuk, V.V.; Shaposhnikova, T.A. Effect of Modification of Titanium Phosphate by Hydrothermal, Microwave, and Mechanochemical Treatment on the Sorption of Cs, Sr, and U(VI) Ions. Theor. Exp. Chem. 2019, 55, 280–286. [Google Scholar] [CrossRef]
- Janusz, W.; Khalameida, S.; Skwarek, E.; Sydorchuk, V.; Charmas, B. The effect of hydrothermal modification of titanium phosphate on the adsorption affinity towards cadmium ions. Physicochem. Probl. Miner. Process. 2019, 55, 1568–1576. [Google Scholar]
- Khalameida, S.; Skwarek, E.; Janusz, W.; Sydorchuk, V.; Leboda, R.; Skubiszewska-Zięba, J. Electokinetic and adsorption properties of different titanium dioxides at the solid/solution interface. Cent. Eur. J. Chem. 2014, 12, 1194–1205. [Google Scholar] [CrossRef]
- Janusz, W.; Sydorchuk, V.; Skwarek, E.; Khalameida, S. Effect of hydrothermal treatment of zirconium phosphate xerogel on its surface groups properties and affinity adsorption to Cd (II) ions from acidic solutions. Mater. Res. Bull. 2022, 148, 111674. [Google Scholar] [CrossRef]
- Sydorchuk, V.; Khalameida, S.; Charmas, B.; Ivasiv, V.; Nebesnyi, R.; Shcherban, N. Influence of hydrothermal, microwave and mechanochemical treatment of tin phosphate on porous structure and catalytic properties. J. Sol-Gel Sci. Technol. 2021, 100, 252–270. [Google Scholar] [CrossRef]
- Zhuravlev, I.; Zakutevsky, O.; Psareva, T.; Kanibolotsky, V.; Strelko, V.; Taffet, M.; Gallios, G.J. Uranium sorption on amorphous titanium and zirconium phosphates modified by Al3+ or Fe3+ ions. J. Radioanal. Nucl. Chem. 2002, 254, 85–89. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, M.; Chen, D. Electrosorption of uranium(VI) by highly porous phosphate-functionalized graphene hydrogel. Appl. Surf. Sci. 2019, 484, 83–96. [Google Scholar] [CrossRef]
- Janusz, W.; Khalameida, S.; Sydorchuk, V.; Skwarek, E.; Zazhigalov, V.; Skubiszewska-Zięba, J.; Leboda, R. Some properties of milled vanadium phosphates. Adsorption 2010, 16, 333–341. [Google Scholar] [CrossRef]
- Khalameida, S.; Sydorchuk, V.; Skubiszewska-Zięba, J.; Charmas, B.; Skwarek, E.; Janusz, W. Hydrothermal, microwave and mechanochemical modification of amorphous zirconium phosphate structure. J. Therm. Anal. Calorim. 2017, 128, 795–806. [Google Scholar] [CrossRef]
- Janusz, W.; Skwarek, E.; Sydorchuk, V.; Khalameida, S. Adsorption of Ag (I) Ions at the Zirconium Phosphate/KNO3 Aqueous Solution Interface. Materials 2022, 15, 5050. [Google Scholar] [CrossRef]
- Serre, C.; Auroux, A.; Hervieu, M.; Gervasini, A.; Hervieu, M.; Férey, G. Hexagonal and Cubic Thermally Stable Mesoporous Tin(IV) Phosphates with Acidic and Catalytic Properties. Angew. Chem. Int. Ed. 2002, 114, 1664–1667. [Google Scholar] [CrossRef]
- Marcu, I.; Sandulescu, I.; Millet, J. Oxidehydrogenation of n-butane over tetravalent metal phosphates based catalysts. Appl. Catal. A Gen. 2002, 227, 309–320. [Google Scholar] [CrossRef]
- Dutta, A.; Gupta, D.; Patra, A.; Saha, B.; Bhaumik, A. Synthesis of 5-Hydroxymethylfurural from Carbohydrates using Large-Pore Mesoporous Tin Phosphate. ChemSusChem 2014, 7, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, F.; Huang, C.; Li, Y.; Chen, B. Highly active tin (IV) phosphate phase transfer catalysts for the production of lactic acid from triose sugars. Catal. Sci. Technol. 2015, 5, 4410–4421. [Google Scholar] [CrossRef]
- Lee, J.; Son, D.; Kim, C.; Park, B. Electrochemical properties of tin phosphates with various mesopore ratios. J. Power Sources 2007, 172, 908–912. [Google Scholar] [CrossRef]
- Pramanik, M.; Lee, J.; Tominaka, S.; Ide, Y.; Kim, J.H.; Yamauchi, Y. Unique nanocrystalline frameworks in mesoporous tin phosphate prepared through a hydrofluoric acid assisted chemical reaction. J. Mater. Chem. A 2016, 4, 18091–18099. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.-X.; Wei, Y.-Z.; Mimura, H. Development of adsorption and solidification process for decontamination of Cs-contaminated radioactive water in Fukushima through silica-based AMP hybrid adsorbent. Sep. Purif. Technol. 2017, 181, 76–84. [Google Scholar] [CrossRef]
- Sachse, A.; Merceille, A.; Barré, Y.; Grandjean, A.; Fajula, F.; Galarneau, A. Macroporous LTA-monoliths for in-flow removal of radioactive strontium from aqueous effluents: Application to the case of Fukushima. Microporous Mesoporous Mater. 2012, 164, 251–258. [Google Scholar] [CrossRef]
- Marczenko, Z.; Balcerzak, M. Separation, Preconcentration and Spectrophotometry in Inorganic Analysis; Elsevier Science B.V: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Rao, K.T.V.; Souzanchi, S.; Yuan, Z.; Ray, M.B.; Xu, C.C. Simple and green route for preparation of tin phosphate catalysts by solid-state grinding for dehydration of glucose to 5-hydroxymethylfurfural (HMF). RSC Adv. 2017, 7, 48501–48511. [Google Scholar] [CrossRef]
- Sinhamahapatra, A.; Sutradhar, N.; Roy, B.; Tarafdar, A.; Bajaj, H.C.; Panda, A.B. Mesoporous zirconium phosphate catalyzed reactions: Synthesis of industrially important chemicals in solvent-free conditions. Appl. Catal. A Gen. 2010, 385, 22–30. [Google Scholar] [CrossRef]
- Mal, N.K.; Ichikawa, S.; Fujiwara, M. Synthesis of a novel mesoporous tin phosphate, SnPO4. Chem. Commun. 2002, 2, 112–113. [Google Scholar] [CrossRef]
- Iqbal, N.; Mobin, M.; Rafiquee, M.Z.A.; Al-Lohedan, H.A. Removal of Cu2+, Cd2+, Pb2+ and Hg2+ Ions by the synthesized sodium dodecyl benzene sulphonate-based tin (IV) phosphate (SDBS-SnP) cation exchanger. Desalination Water Treat. 2013, 51, 6688–6697. [Google Scholar] [CrossRef]
- Velásquez, C.; Rojas, F.; Lara, V.H.; Campero, A. On the textural and morphological properties of crystalline and amorphous α-tin phosphate. Phys. Chem. Chem. Phys. 2004, 6, 4714–4721. [Google Scholar] [CrossRef]
- Nzihou, A.; Sharrock, P. Role of Phosphate in the Remediation and Reuse of Heavy Metal Polluted Wastes and Sites. Waste Biomass Valorization 2010, 1, 163–174. [Google Scholar] [CrossRef]
- Zakutevskyy, O.; Psareva, T.; Strelko, V. Sorption of U(VI) Ions on Sol-Gel-Synthesized Amorphous Spherically Granulated Titanium Phosphates. Russ. J. Appl. Chem. 2012, 85, 1366–1370. [Google Scholar] [CrossRef]
- Mehta, V.S.; Maillot, F.; Wang, Z.; Catalano, J.G.; Giammar, D.E. Effect of Reaction Pathway on the Extent and Mechanism of Uranium(VI) Immobilization with Calcium and Phosphate. Environ. Sci. Technol. 2016, 50, 3128–3136. [Google Scholar] [CrossRef] [PubMed]
- Kapnisti, M.; Noli, F.; Misaelides, P.; Vourlias, G.; Karfaridis, D.; Hatzidimitriou, A. Enhanced sorption capacities for lead and uranium using titanium phosphates; sorption, kinetics, equilibrium studies and mechanism implication. Chem. Eng. J. 2018, 342, 184–195. [Google Scholar] [CrossRef]
- Skwarek, E.; Gładysz-Płaska, A.; Choromańska, J.B.; Broda, E. Adsorption of uranium ions on nano-hydroxyapatite and modified by Ca and Ag ions. Adsorption 2019, 25, 639–647. [Google Scholar] [CrossRef]
- Hayashi, H.; Ebina, T.; Onodera, Y.; Iwasaki, T. Strontium Immobilization by Fibrous Cerium(IV) bis(monohydrogenphosphate) under Hydrothermal Conditions. Bull. Chem. Soc. Jpn. 1997, 70, 1701–1708. [Google Scholar] [CrossRef]
- Fanizza, M.F.; Yoon, H.; Zhang, C.; Oostrom, M.; Wietsma, T.W.; Hess, N.J.; Bowden, M.E.; Strathmann, T.J.; Finneran, K.T.; Werth, C.J. Pore-scale evaluation of uranyl phosphate precipitation in a model groundwater system. Water Resour. Res. 2013, 49, 874–890. [Google Scholar] [CrossRef]
- Munasinghe, P.S.; Elwood Madden, M.E.; Brooks, S.C.; Elwood Madden, A.S. Dynamic interplay between uranyl phosphate precipitation, sorption, and phase evolution. Appl. Geochem. 2015, 58, 147–160. [Google Scholar] [CrossRef]
- Comarmond, M.J.; Steudtner, R.; Stockmann, M.; Heim, K.; Müller, K.; Brendler, V.; Payne, T.E.; Foerstendorf, H. The Sorption Processes of U(VI) onto SiO2 in the Presence of Phosphate: From Binary Surface Species to Precipitation. Environ. Sci. Technol. 2016, 50, 11610–11618. [Google Scholar] [CrossRef]
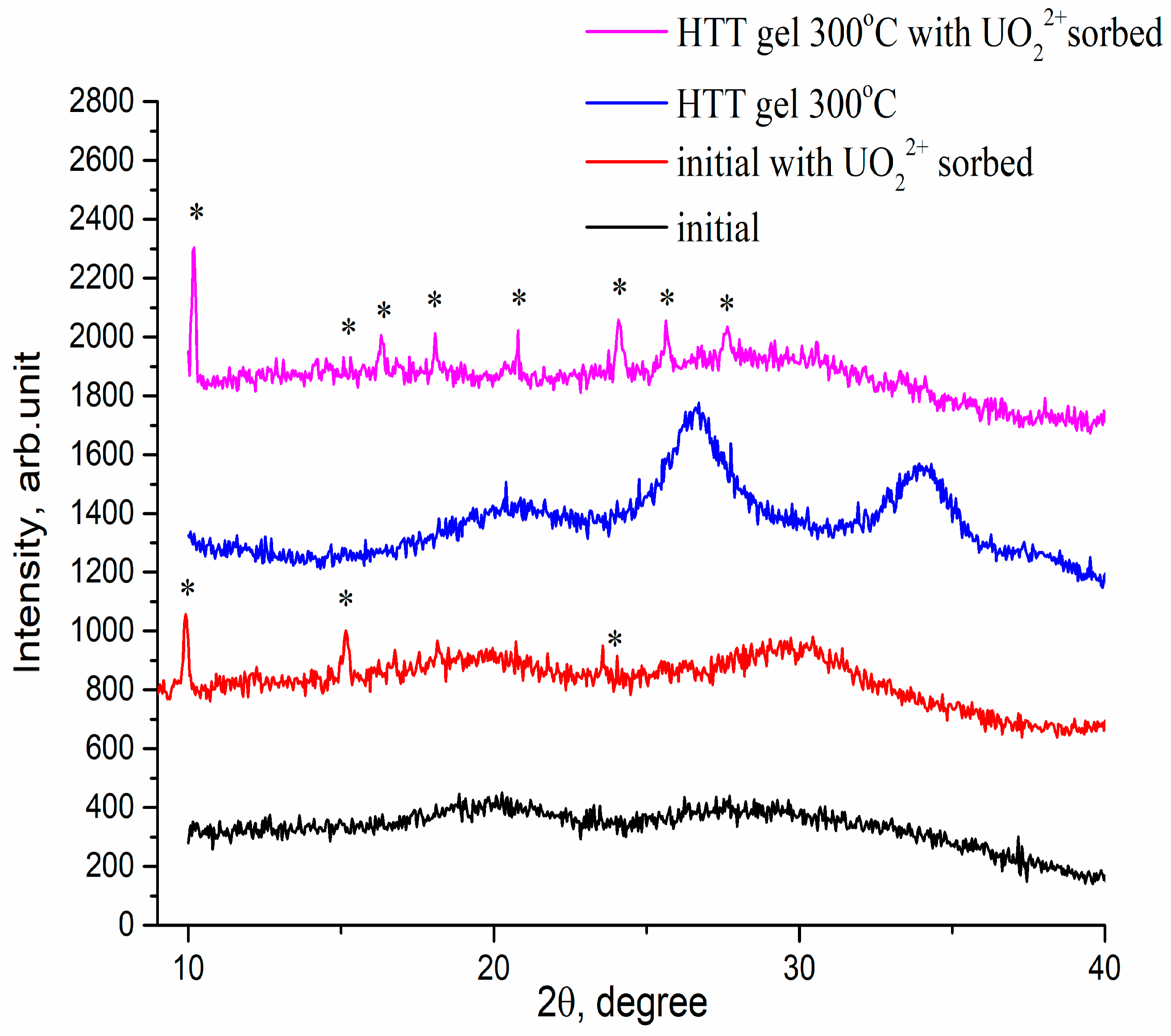
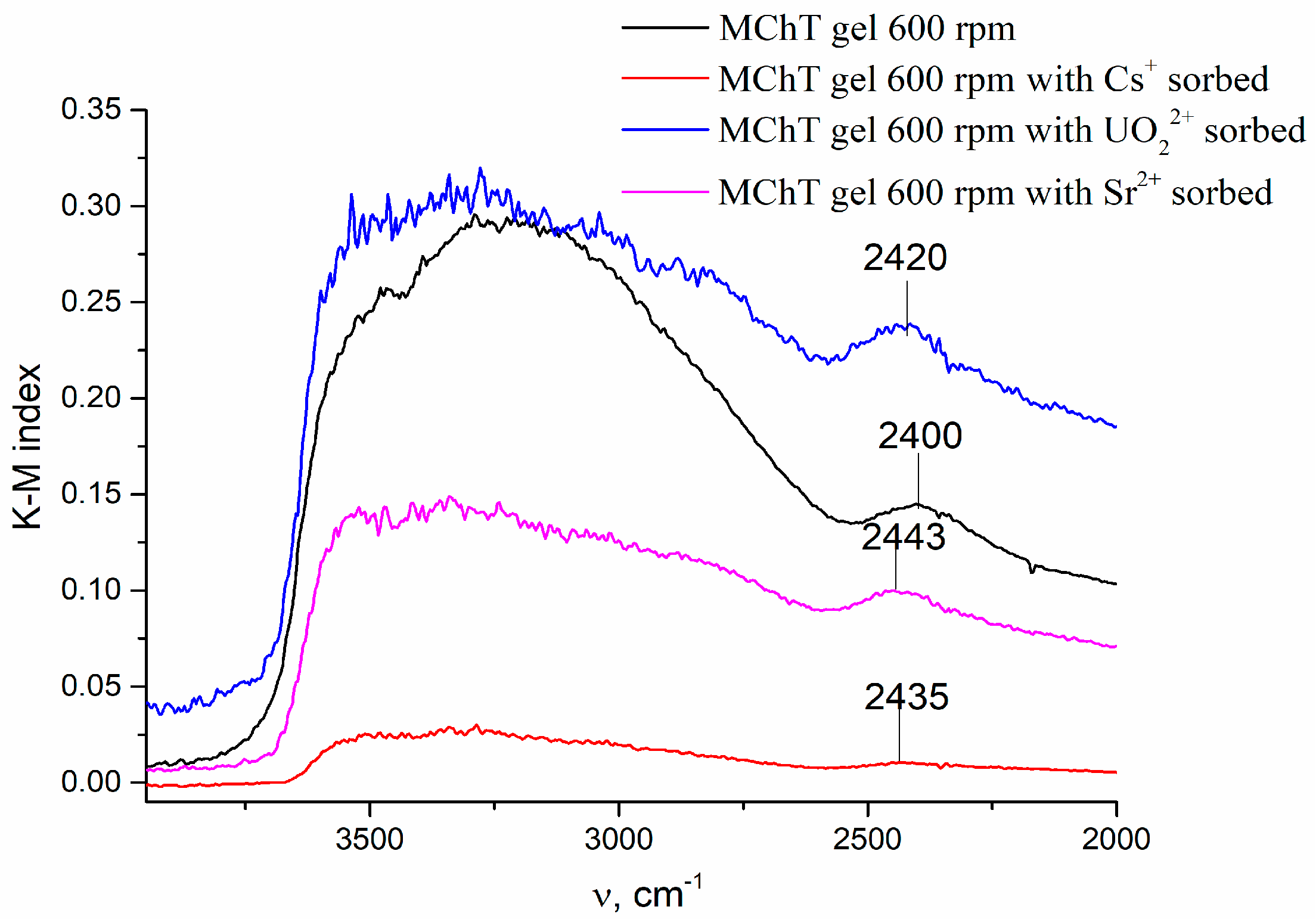
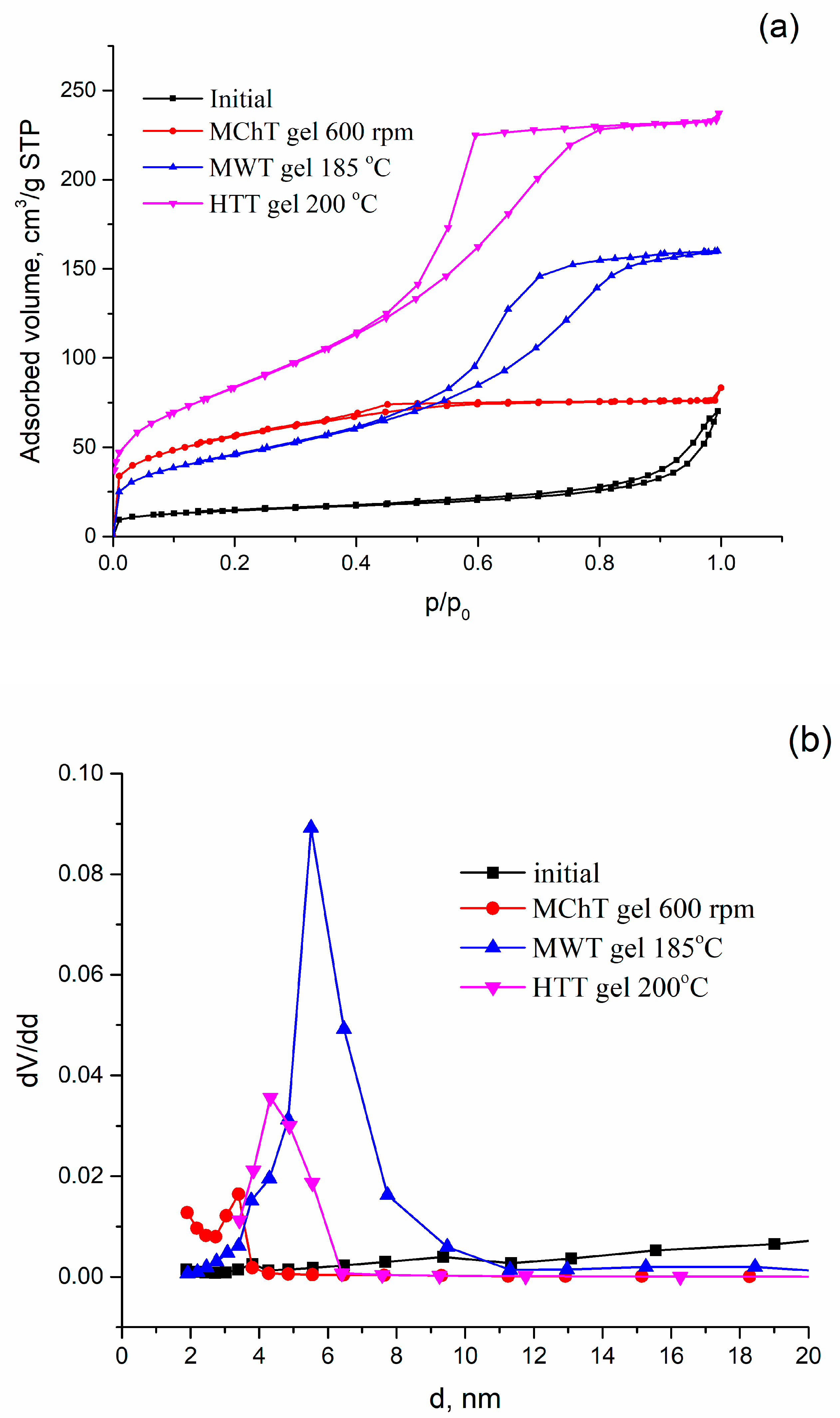
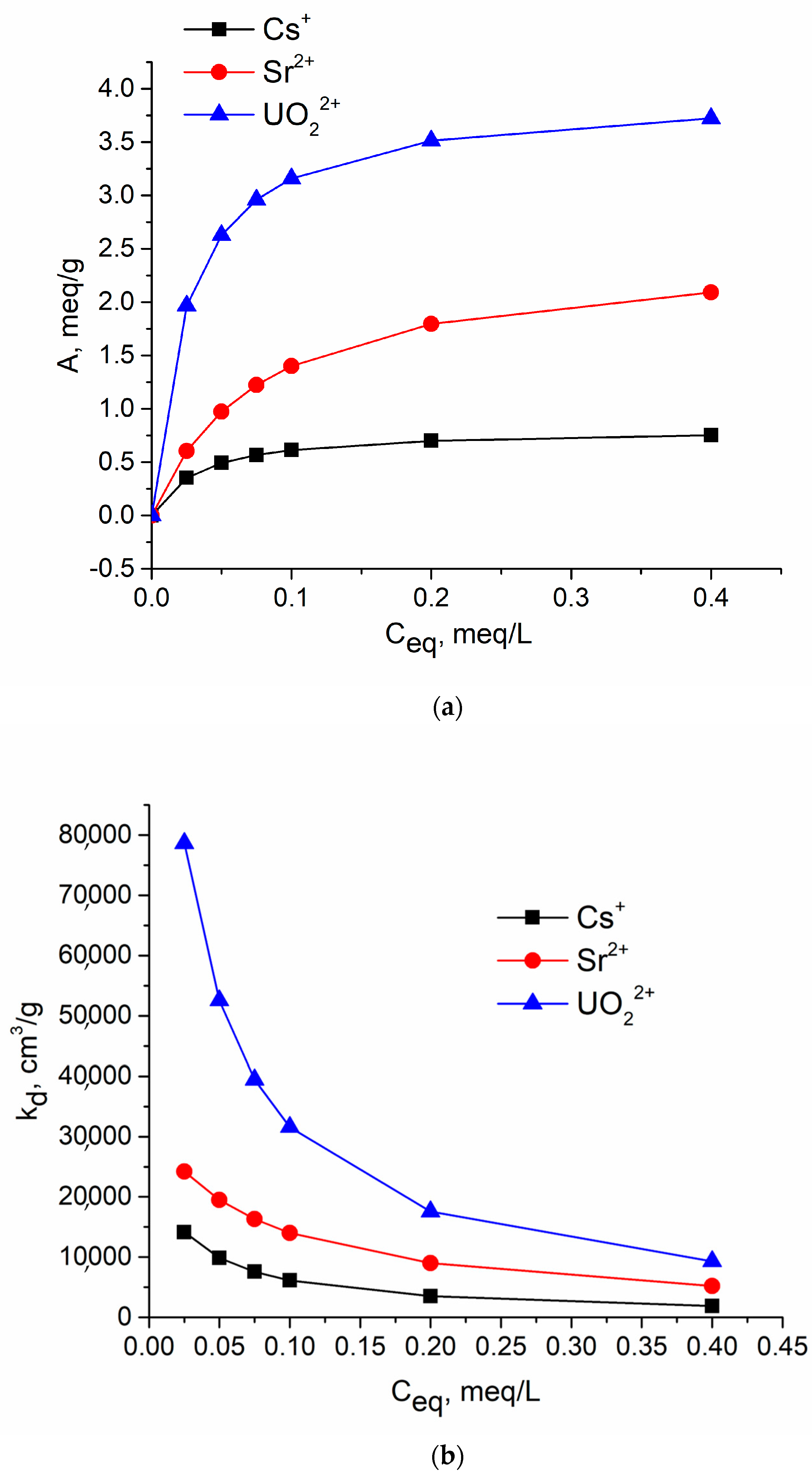
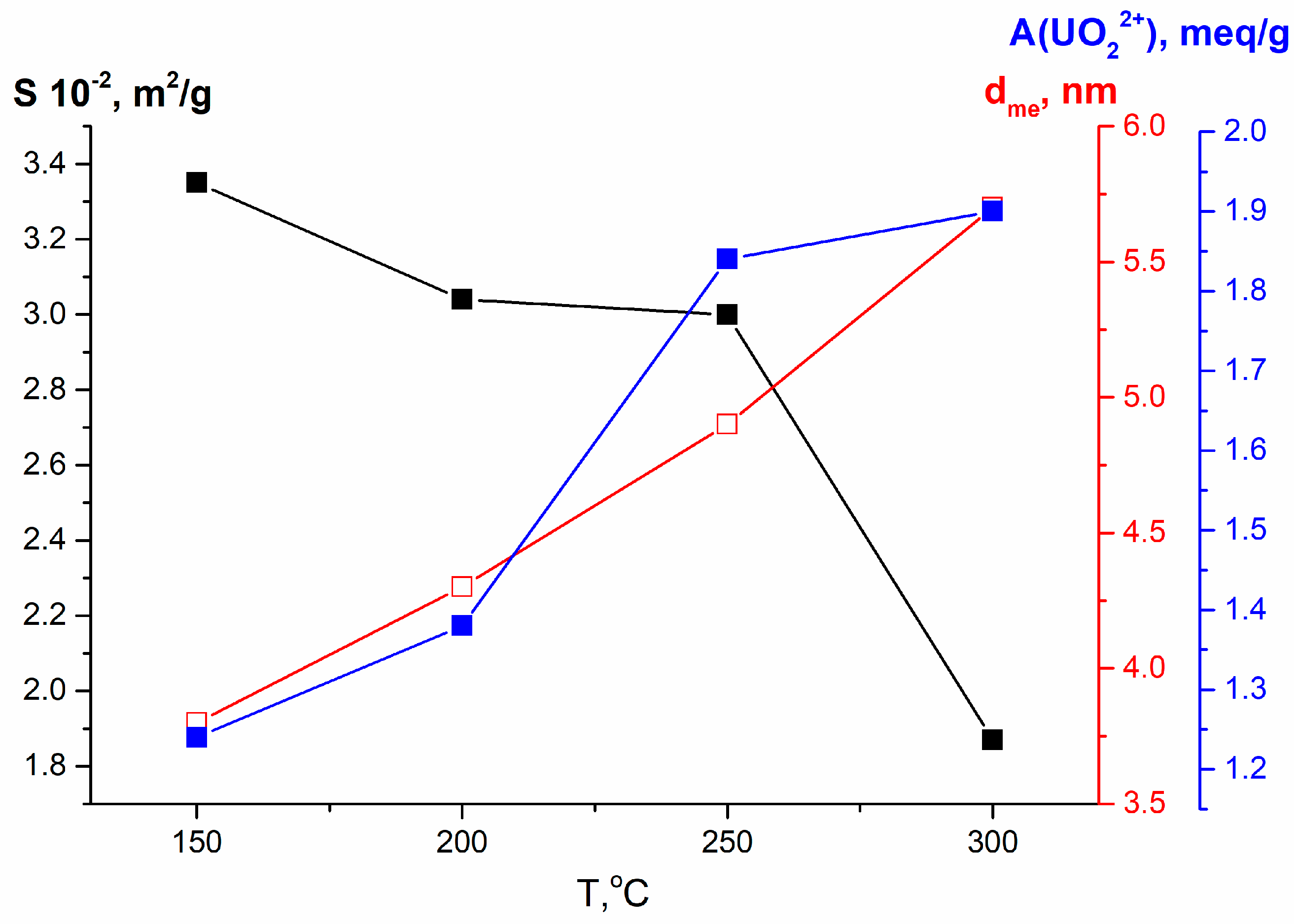
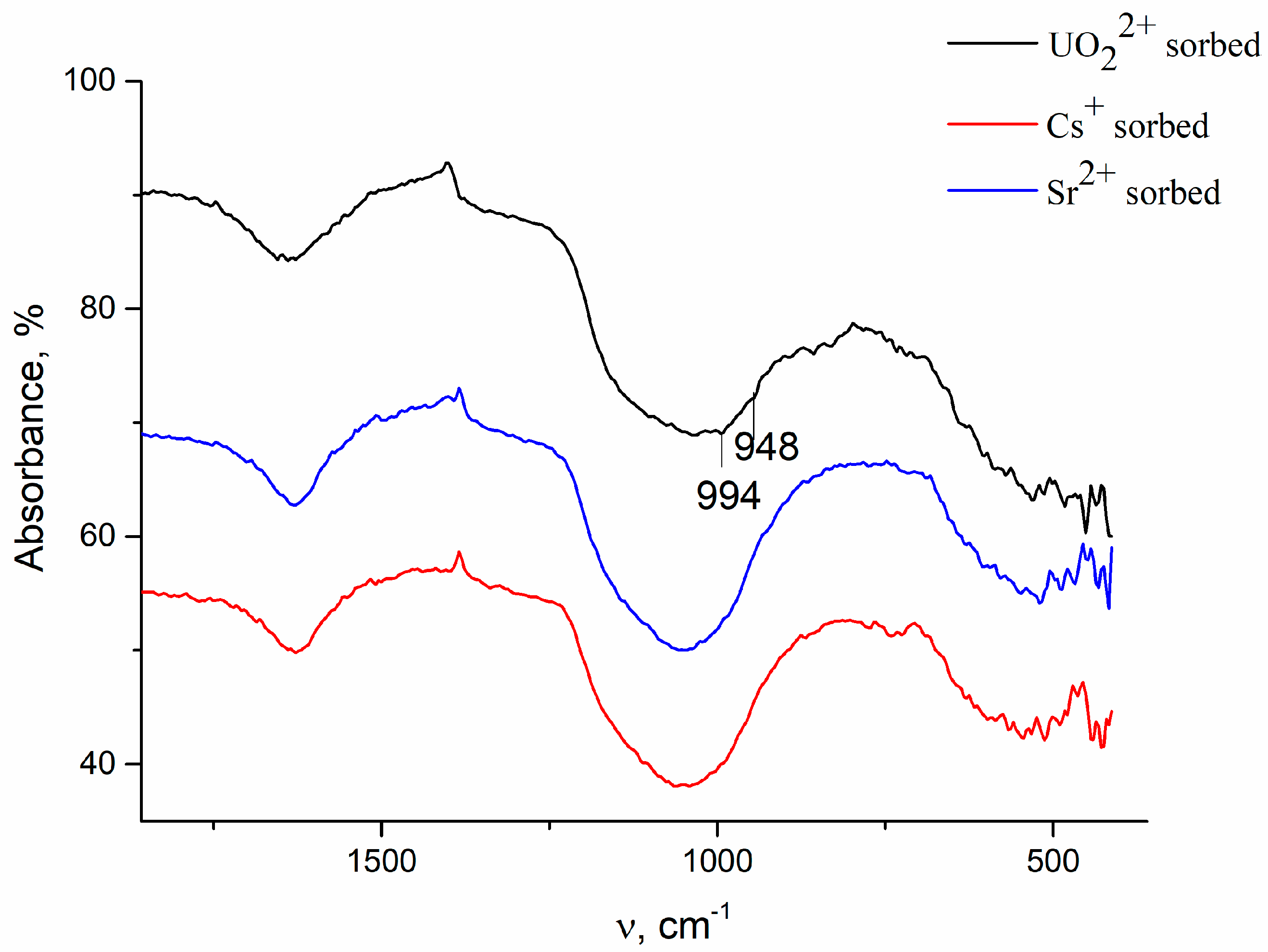
| N | Samples | S, m2/g | VΣ | Vme | Vmi | Vma | d, nm |
|---|---|---|---|---|---|---|---|
| cm3/g | |||||||
| 1 | Initial | 50 | 0.11 | 0.10 | 0.01 | - | 3.0 |
| 2 | MChT gel 600 rpm | 208 | 0.23 | 0.05 | 0.08 | 0.10 | 3.3 |
| 3 | MChT xerogel H2O 600 rpm | 102 | 0.47 | 0.15 | 0.03 | 0.28 | 3.5; 40 |
| 4 | MWT gel 185 °C | 125 | 0.50 | 0.50 | - | - | 6.6 |
| 5 | MWT xerogel 250 °C | 62 | 0.31 | 0.16 | - | 0.15 | 4.3 |
| 6 | HTT xerogel 200 °C | 86 | 0.2 | 0.18 | 0.005 | 0.015 | 4.0 |
| 7 | HTT xerogel 300 °C | 19 | 0.32 | 0.075 | - | 0.245 | 7.7 |
| 8 | HTT gel 150 °C | 335 | 0.70 | 0.23 | 0.09 | 0.38 | 3.8 |
| 9 | HTT gel 200 °C | 304 | 0.53 | 0.34 | 0.02 | 0.16 | 4.3 |
| 10 | HTT gel 250 °C | 300 | 0.47 | 0.38 | 0.01 | 0.08 | 4.9 |
| 11 | HTT gel 300 °C | 187 | 0.46 | 0.27 | - | 0.18 | 5.7 |
| N | Samples | Cs | Sr | U | |||
|---|---|---|---|---|---|---|---|
| A, meq/g (% of Sorption) | Kd, cm3/g | A, meq/g (% of Sorption) | Kd, cm3/g | A, meq/g (% of Sorption) | Kd, cm3/g | ||
| 1 | Initial | 0 | 0 | 0.052 (14.5) | 323 | 0.44 (22.4) | 586 |
| 2 | MChT gel 600 rpm | 0.20 (57.2) | 2706 | 0.31 (80.7) | 8477 | 1.64 (87.9) | 13,838 |
| 3 | MChT xerogel H2O 600 rpm | 0.19 (55.5) | 2446 | 0.28 (74.7) | 5800 | 1.87 (98.9) | 170,661 |
| 4 | MWT gel 185 °C | 0.12 (34.9) | 1036 | 0.25 (67.5) | 4004 | 1.67 (88.8) | 15,261 |
| 5 | MWT xerogel 250 °C | 0 | 0 | 0.018 (4.8) | 101 | 0.37 (19.1) | 472 |
| 6 | HTT xerogel 200 °C | 0.094 (26.8) | 783 | 0.17 (44.7) | 1619 | 0.56 (29.5) | 778 |
| 7 | HTT xerogel 300 °C | 0.049 (14.0) | 350 | 0.080 (21.0) | 533 | 0.26 (13.7) | 299 |
| 8 | HTT gel 150 °C | 0.20 (60.7) | 3011 | 0.31 (84.3) | 10,496 | 1.24 (65.3) | 3673 |
| 9 | HTT gel 200 °C | 0.21 (63.8) | 3313 | 0.30 (83.1) | 9282 | 1.38 (75.0) | 5661 |
| 10 | HTT gel 250 °C | 0.20 (59.4) | 2828 | 0.27 (74.7) | 5711 | 1.84 (97.7) | 83,043 |
| 11 | HTT gel 300 °C | 0.20 (59.8) | 2847 | 0.32 (89.2) | 15,721 | 1.90 (100) | — |
| Ions | Q0, meq/g | b, L/meq | A = f(Ceq) | Kd = f(Ceq) |
|---|---|---|---|---|
| Cs | 0.81 | 30.86 | A = (1.23 + (0.040/Ceq))−1 | Kd = ((1.23 + (0.040/Ceq))∙Ceq)−1∙1000 |
| Sr | 2.50 | 12.90 | A = (0.40 + (0.031/Ceq))−1 | Kd = ((0.40 + (0.031/Ceq))∙Ceq)−1∙1000 |
| U | 4.00 | 39.06 | A = (0.25 + (0.0064/Ceq))−1 | Kd = ((0.25 + (0.0064/Ceq))∙Ceq)−1∙1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakutevskyy, O.; Khalameida, S.; Sydorchuk, V.; Kovtun, M. The Effect of Hydrothermal, Microwave, and Mechanochemical Treatments of Tin Phosphate on Sorption of Some Cations. Materials 2023, 16, 4788. https://doi.org/10.3390/ma16134788
Zakutevskyy O, Khalameida S, Sydorchuk V, Kovtun M. The Effect of Hydrothermal, Microwave, and Mechanochemical Treatments of Tin Phosphate on Sorption of Some Cations. Materials. 2023; 16(13):4788. https://doi.org/10.3390/ma16134788
Chicago/Turabian StyleZakutevskyy, Oleg, Svitlana Khalameida, Volodymyr Sydorchuk, and Mariia Kovtun. 2023. "The Effect of Hydrothermal, Microwave, and Mechanochemical Treatments of Tin Phosphate on Sorption of Some Cations" Materials 16, no. 13: 4788. https://doi.org/10.3390/ma16134788
APA StyleZakutevskyy, O., Khalameida, S., Sydorchuk, V., & Kovtun, M. (2023). The Effect of Hydrothermal, Microwave, and Mechanochemical Treatments of Tin Phosphate on Sorption of Some Cations. Materials, 16(13), 4788. https://doi.org/10.3390/ma16134788






