Porous Mullite Ceramic Modification with Nano-WO3
Abstract
1. Introduction
2. Raw Materials and Testing Methods
2.1. Raw Materials
2.2. Material Proportions
2.3. Sample Preparation Method
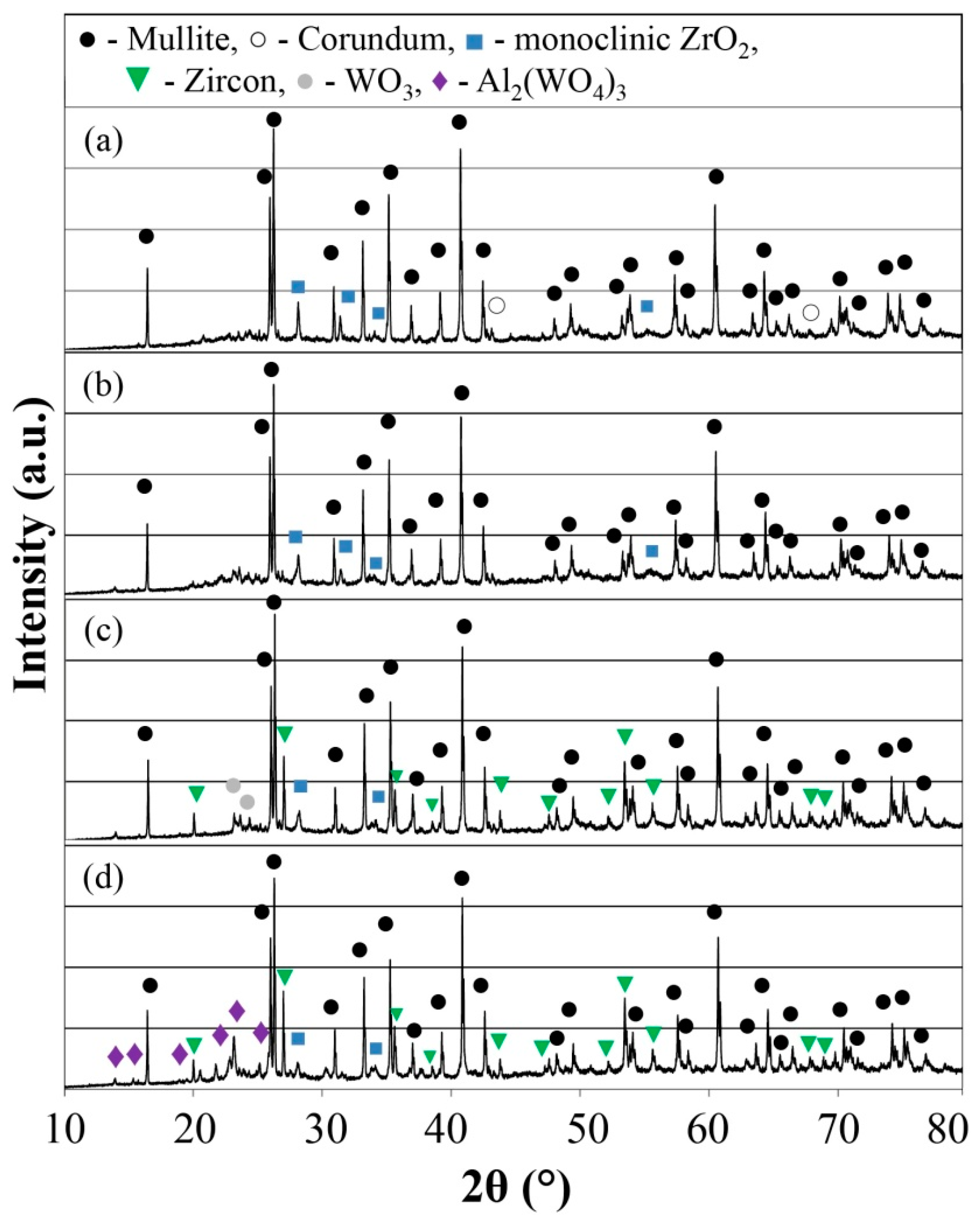
2.4. X-ray Diffraction Analysis
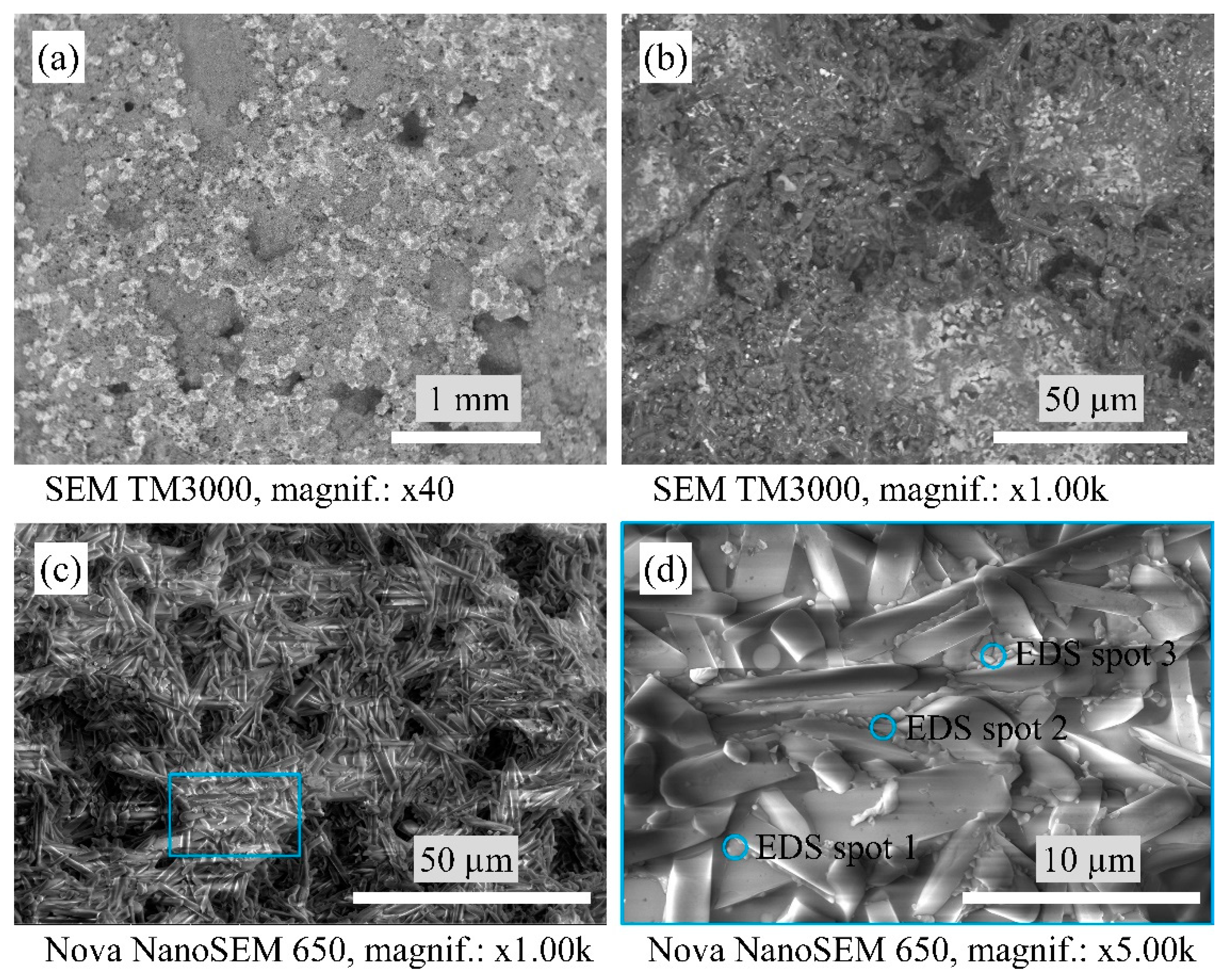
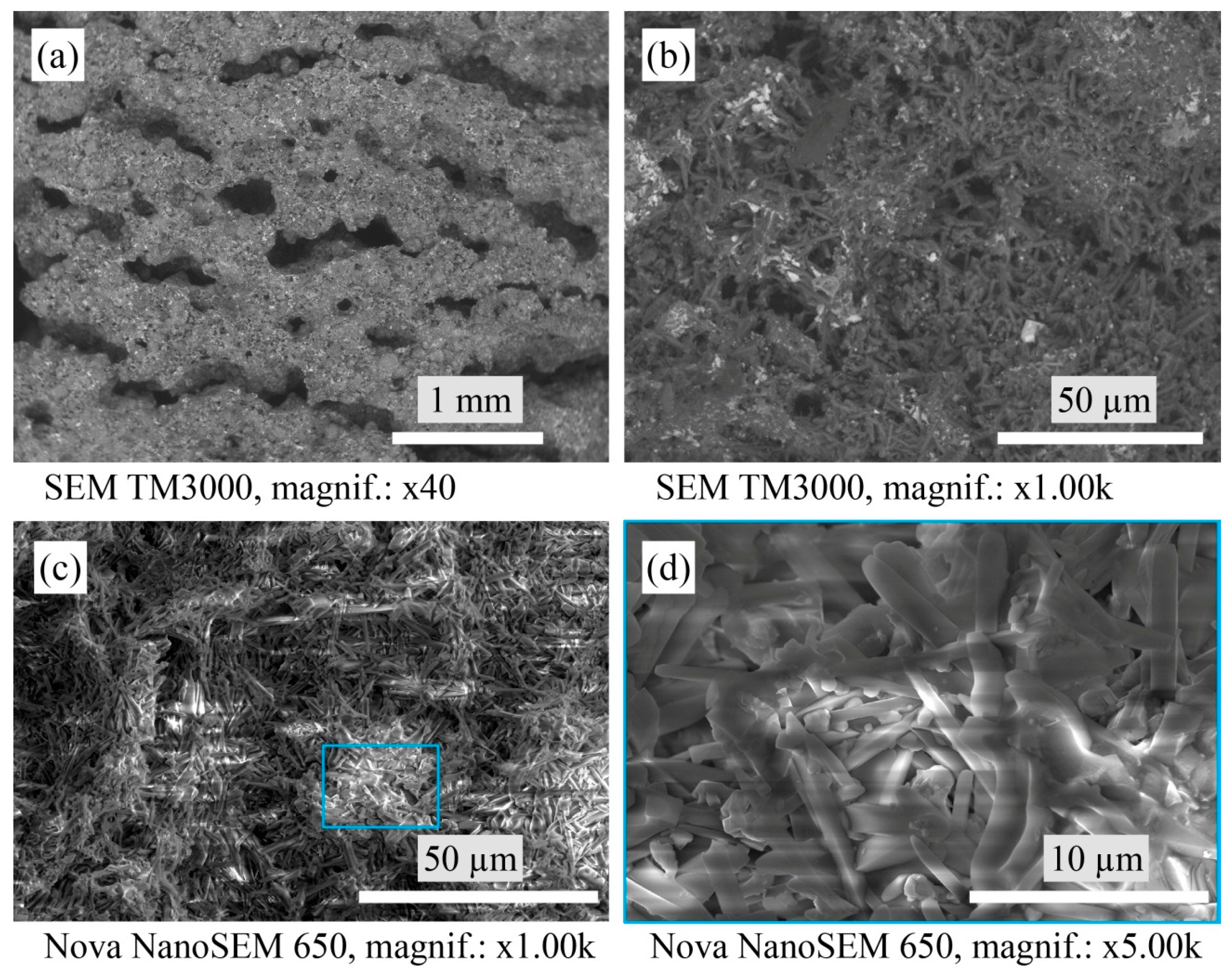
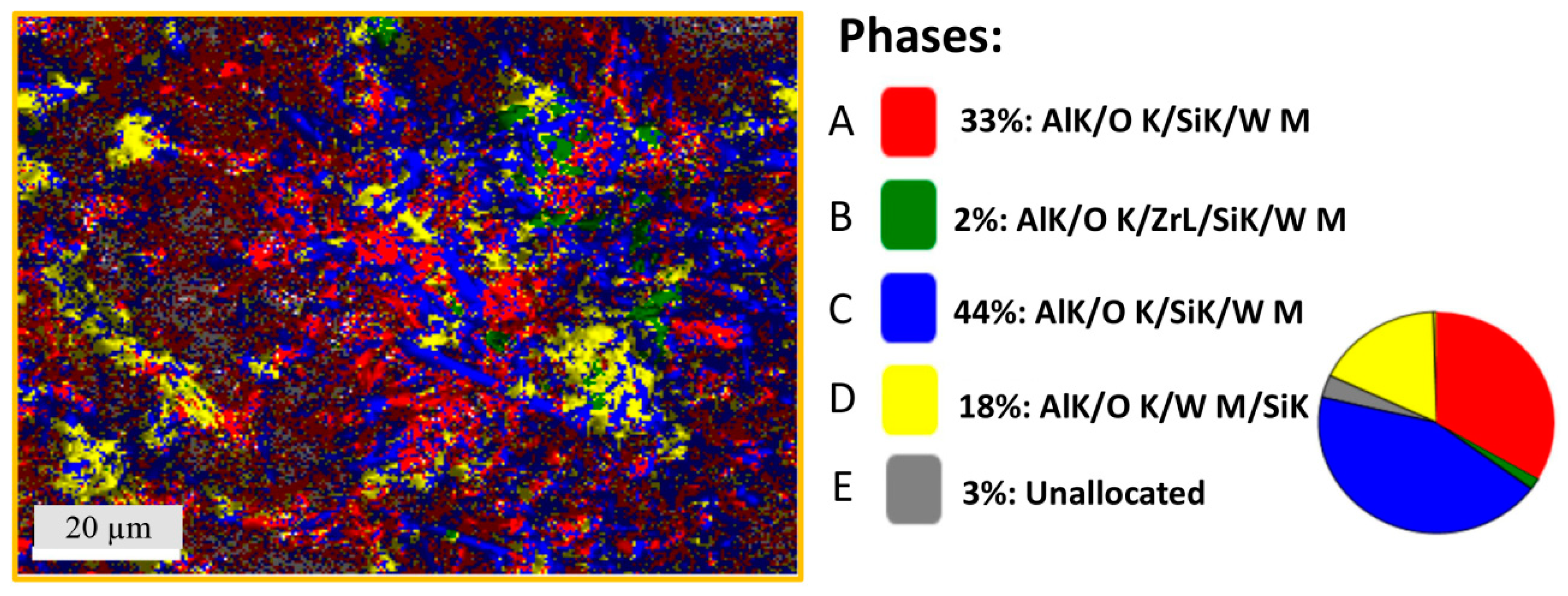
2.5. Scanning Electron Microscopy
2.6. Apparent Porosity
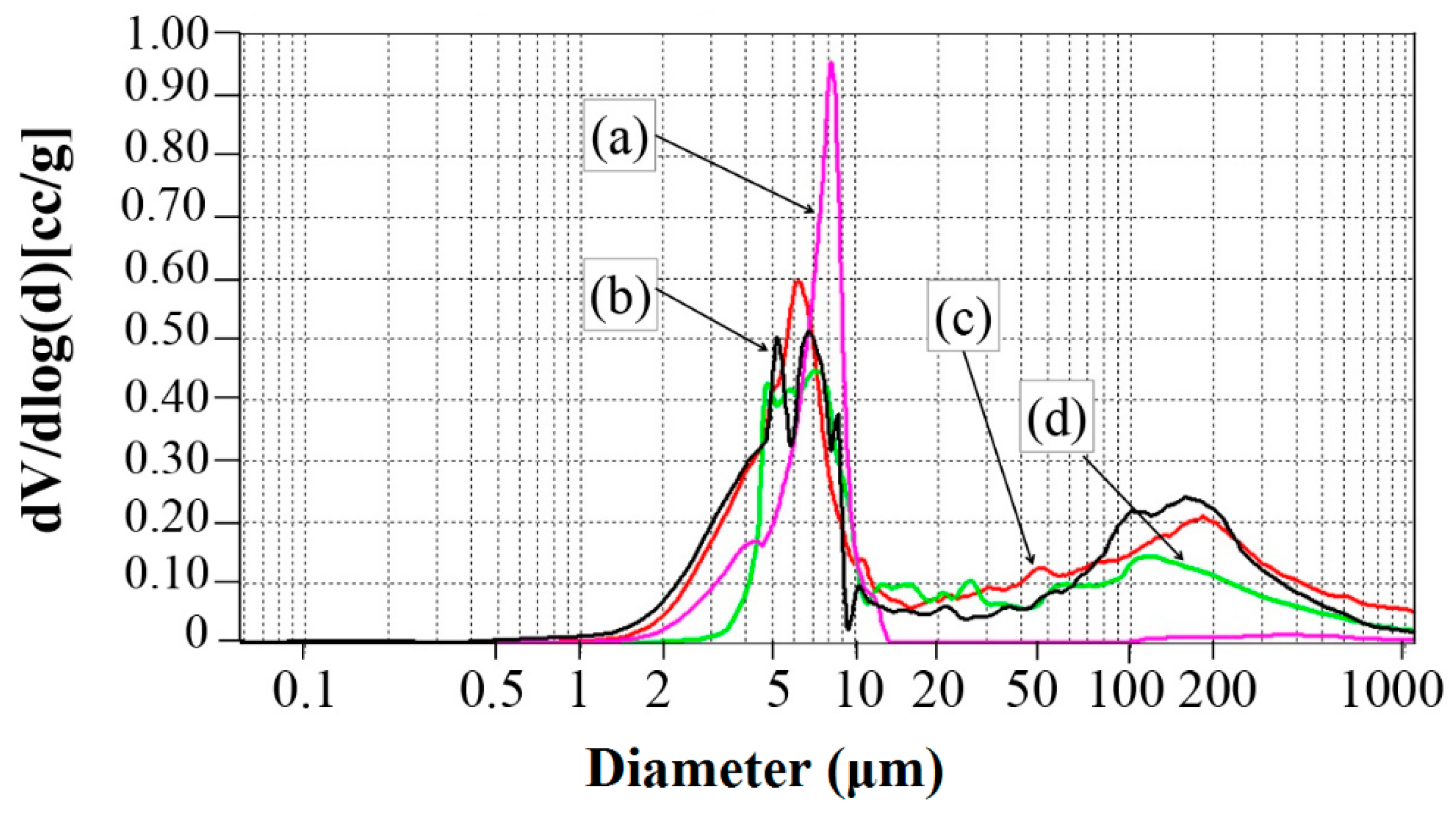
2.7. Mercury Porosimetry
- rpore—corresponding pore radius, µm;
- γ—surface tension of mercury, N/m;
- θ—wetting angle between the solid surface of the material and mercury.
2.8. Thermal Shock Resistance Testing
2.9. E Modulus Measurement
3. Results and Discussion
3.1. Crystalline Phase Compositions
3.2. Macrostructure and Microstructure
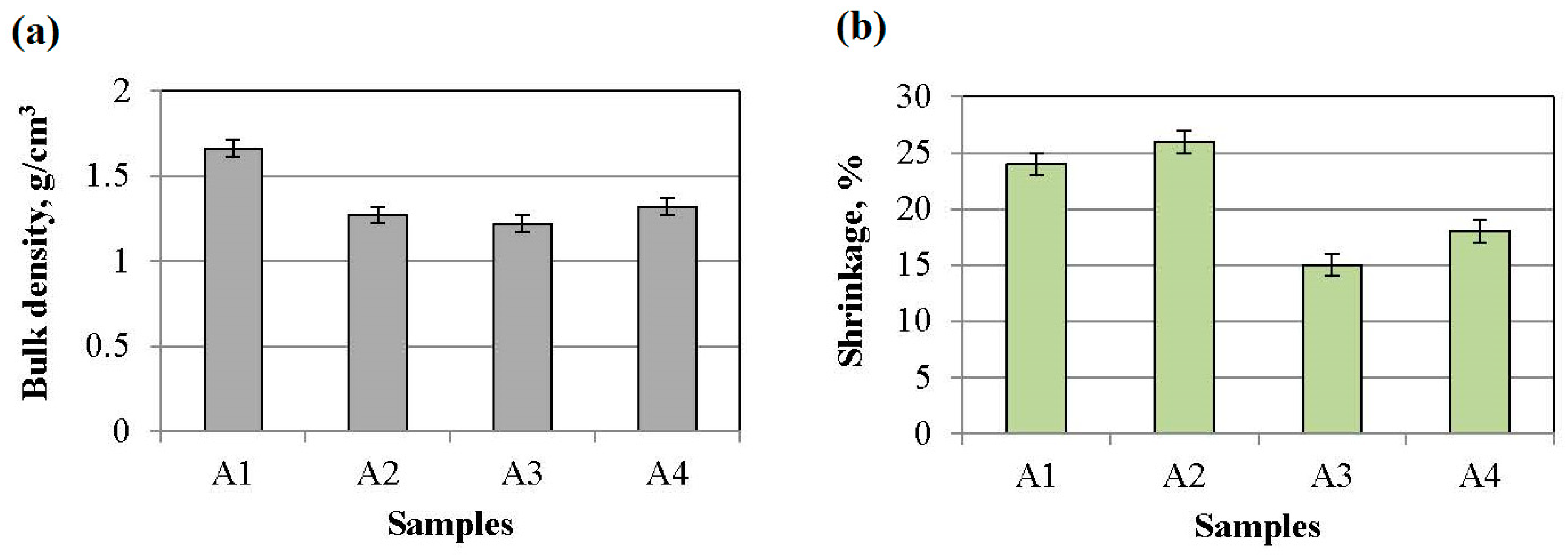
3.3. Bulk Density and Change in Samples’ Dimensions
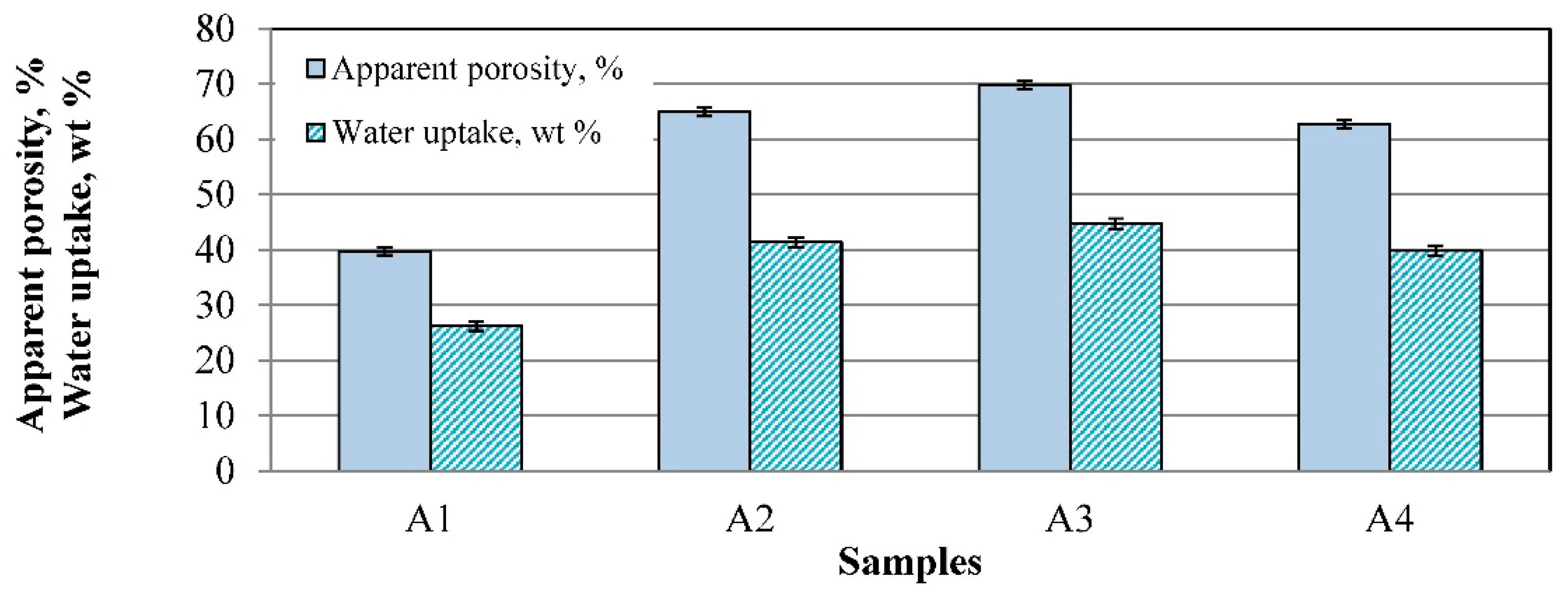
3.4. Apparent Porosity, Water Uptake, and Pore Sizes
3.5. Resistance to Thermal Shock
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, F.; Hao, S.; Dong, B.; Ke, N.; Khan, N.Z.; Hao, L.; Yin, L.; Xu, X.; Agathopoulos, S. Porous-foam mullite-bonded SiC-ceramic membranes for high-efficiency high-temperature particulate matter capture. J. Alloys Compd. 2022, 893, 162231. [Google Scholar] [CrossRef]
- Aryal, S.; Rulis, P.; Ching, W.-Y. Mechanical properties and electronic structure of mullite phases using first-principles modeling. J. Eur. Ceram. Soc. 2012, 95, 2075–2088. [Google Scholar] [CrossRef]
- Hammel, E.C.; Ighodaro, O.L.-R.; Okoli, O.I. Processing and properties of advanced porous ceramics: An application based review. Ceram. Int. 2014, 140, 15351–15370. [Google Scholar] [CrossRef]
- Hemra, K.; Aungkavattana, P. Effect of zirconia content on mechanical and thermal properties of mullite–zirconia composite. Adv. Appl. Ceram. 2014, 113, 323–327. [Google Scholar] [CrossRef]
- Hsiung, C.-H.H.; Pyzik, A.J.; Gulsoy, E.B.; Carlo, F.; Xiao, X.; Faber, K.T. Impact of doping on the mechanical properties of acicular mullite. J. Eur. Ceram. Soc. 2013, 33, 1955–1965. [Google Scholar] [CrossRef]
- Ouali, A.; Heraiz, M.; Sahnoune, F.; Belhouchet, H.; Fatmi, M.; Saheb, N. Effect of MgO addition on the mechanical and thermal properties of mullite synthesised through reaction sintering of Al2O3 and Algerian kaolin. Am. J. Mod. Phys. 2013, 5, 270–275. [Google Scholar] [CrossRef]
- Salomão, R.; Oliveira, K.; Fernandes, L.; Tiba, P.; Prado, U. Porous refractory ceramics for high-temperature thermal insulation Part 1: The Science Behind Energy Saving. Interceram Int. Ceram. Rev. 2021, 70, 38–45. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, D.; Chen, Y.; Liang, B.; Zhou, J. Preparation of high open porosity ceramic foams via direct foaming molded and dried at room temperature. J. Eur. Ceram. Soc. 2014, 34, 2443–2452. [Google Scholar] [CrossRef]
- Pirzada, T.J.; Liu, D.; Ell, J.; Barnard, H.; Šulak, I.; Galano, M.; Marrow, T.J.; Ritchie, R.O. In situ observation of the deformation and fracture of an alumina-alumina ceramic-matrix composite at elevated temperature using X-ray computed tomography. J. Eur. Ceram. Soc. 2021, 41, 4217–4230. [Google Scholar] [CrossRef]
- Zhua, Z.; Wei, Z.; Shen, J.; Zhuc, L.; Xu, L.; Zhang, Y.; Wang, S.; Liu, T. Fabrication and catalytic growth mechanism of mullite ceramic whiskers using molybdenum oxide as catalyst. Ceram. Int. 2017, 43, 2871–2875. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X.; Ji, H.; Yu, H.; Yu, B.; Qi, T. Constructing secondary-pore structure by in-situ synthesized mullite whiskers to prepare whiskers aerogels with ultralow thermal conductivity. J. Eur. Ceram. Soc. 2019, 39, 1344–1351. [Google Scholar] [CrossRef]
- Yang, P.; Liu, S.; Mao, Z.; Wang, D. Preparation and properties of hierarchical structural alumina/mullite composites. J. Eur. Ceram. Soc. 2023, 43, 468–477. [Google Scholar] [CrossRef]
- Qin, Z.; Xu, X.; Xu, T.; Cao, Y.; Wu, J.; Yan, L.; Hou, F.; Liu, J.; Guo, A. High-strength thermal insulating porous mullite fiber-based ceramics. J. Eur. Ceram. Soc. 2022, 42, 7209–7218. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, F.; Zhao, S.; Li, K.; Chen, J.; Fei, Z.; Chen, G. In-situ growth of mullite whiskers and their effect on the microstructure and properties of porous mullite ceramics with an open/closed pore structure. J. Eur. Ceram. Soc. 2021, 41, 299–308. [Google Scholar] [CrossRef]
- Qiao, M.; Li, X.; Chen, P.; Zhu, Y.; Zhu, B. Pore evolution and slag resistance of corundum castables with nano zirconia addition. Ceram. Int. 2021, 47, 6947–6954. [Google Scholar] [CrossRef]
- Dudczig, S.; Veres, D.; Aneziris, C.G.; Skiera, E.; Steinbrech, R.W. Nano- and micrometre additions of SiO2, ZrO2 and TiO2 in fine grained alumina refractory ceramics for improved thermal shock performance. Ceram. Int. 2012, 38, 2011–2019. [Google Scholar] [CrossRef]
- Badiee, S.H.; Otroj, S. The effect of nano-titania addition on the properties of high-alumina low-cement self-flowing refractory castables. Ceram. Silik. 2011, 55, 319–325. [Google Scholar]
- Qian, L.; Yang, L.; Li, G.; Jiang, W.; Fan, Z. Effect of nano-TiO2 on properties of 3 mol% yttria-stabilized zirconia ceramic via layered extrusion forming. J. Eur. Ceram. Soc. 2020, 40, 4539–4546. [Google Scholar] [CrossRef]
- Khaskhoussi, A.; Calabrese, L.; Bouaziz, J.; Proverbio, E. Effect of TiO2 addition on microstructure of zirconia/alumina sintered ceramics. Ceram. Int. 2017, 48, 10392–10402. [Google Scholar] [CrossRef]
- Esmaili, M.; Nilforoushan, M.R.; Tayebi, M.; Aghaie, E. Effect of micro/nano TiO2 addition on the densification behavior and mechanical properties of multifunctional resistant porcelains. Ceram. Int. 2021, 47, 17435–17444. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Sedeh, M.B.; Dehsheikh, H.G.; Shahraki, A.; Farooghi, M. Densification and properties of ZrO2 nanoparticles added magnesia–doloma refractories. Ceram. Int. 2016, 42, 15658–15663. [Google Scholar] [CrossRef]
- Gogtas, C.; Lopez, H.F.; Sobolev, K. Effect of nano-YSZ and nano-ZrO2 additions on the strength and toughness behavior of self-flowing alumina castables. Ceram. Int. 2016, 42, 1847–1855. [Google Scholar] [CrossRef]
- Wang, F.; Dong, B.; Wang, J.; Ke, N.; Tan, C.; Huang, A.; Wu, Y.; Hao, L.; Yin, L.; Xu, X.; et al. Self-supported porous heterostructure WC/WO3−x ceramic electrode for hydrogen evolution reaction in acidic and alkaline media. J. Adv. Ceram. 2022, 11, 1208–1221. [Google Scholar] [CrossRef]
- Dutta, V.; Sharma, S.; Raizada, P.; Thakur, V.K.; Khan, A.A.P.; Saini, V.; Asiri, A.M.; Singh, P. An overview on WO3 based photocatalyst for environmental remediation. J. Environ. Chem. Eng. 2021, 9, 105018. [Google Scholar] [CrossRef]
- Anandaraj, C.; Bharath Sabarish, V.C.; Durairajan, A.; Graca, M.P.F.; Valente, M.A.; Gajendiran, J.; Raj, S.G.; Kumar, G.R. Influence of tungsten trioxide (WO3) on the topographical, structural, optical absorption and electrochemical characteristics of BFO-MWCNT and BFO-Graphene nanocomposite ceramics. Chem. Phys. 2022, 563, 111699. [Google Scholar] [CrossRef]
- Ji, C.; Wang, J.; Bai, X.; Hao, M.; Chen, G.; He, J.; Fan, T.; Zhang, Z.; Chen, C.; Fu, C. Effects of MnO2 and WO3 co-doping and sintering temperature on microstructure, microwave dielectric properties of Ba2Ti9O20 microwave ceramics. Ceram. Int. 2022, 48, 10713–10720. [Google Scholar] [CrossRef]
- Vadivel, S.; Saravanakumar, B.; Kumaravel, M.; Maruthamani, D.; Balasubramanian, N.; Manikand, A.; Ramadoss, G.; Paul, B.; Hariganesh, S. Facile solvothermal synthesis of BiOI microsquares as a novel electrode material for supercapacitor applications. Mater. Lett. 2018, 210, 109–112. [Google Scholar] [CrossRef]
- Paul, B.; Manwar, N.; Bhanja, P.; Sellaiyan, S.; Sharma, S.K.; Khatun, R.; Jain, S.; Bal, R. Morphology controlled synthesis of 2D heterostructure Ag/WO3 nanocomposites for enhanced photoelectrochemical CO2 reduction performance. J. CO2 Util. 2020, 41, 101284. [Google Scholar] [CrossRef]
- Paul, B.; Sharma, S.; Purkayasthab, D.D.; Dharb, S.S.; Bala, R. Facile synthesis of size-controlled Ag supported on WO3 nanorods and their application as novel and active catalyst in oxidant-free dehydrogenation of benzyl alcohols. Catal. Commun. 2019, 132, 105804. [Google Scholar] [CrossRef]
- Mahnicka-Goremikina, L.; Svinka, R.; Svinka, V. Influence of ZrO2 and WO3 doping additives on the thermal properties of porous mullite ceramics. Ceram. Int. 2018, 44, 16873–16879. [Google Scholar] [CrossRef]
- DIN EN 623-2:1993; Advanced Technical Ceramics; MONOLITHIC ceramics; General and Textural Properties; Determination of Density and Porosity. European standards, 1993.
- Salmas, C.; Androutsopoulos, G. Mercury Porosimetry: Contact Angle Hysteresis of Materials with Controlled Pore Structure. J. Coll. Interf. Sci. 2001, 239, 178–189. [Google Scholar] [CrossRef]
- Kutz, M. Handbook of Materials Selection; John Wiley & Sons: New York, NY, USA, 2002; 1497p. [Google Scholar]
- Itoh, T. Formation of polycrystalline zircon (ZrSiO4) from amorphous silica and amorphous zirconia. J. Cryst. Growth 1992, 125, 223–228. [Google Scholar] [CrossRef]
- Mori, T.; Yamamura, H.; Kobayashi, H.; Mitamura, T. Formation mechanism of ZrSiO4 powders. J. Mater. Sci. 1993, 28, 4970–4973. [Google Scholar] [CrossRef]
- Awaad, M.; Zawrah, M.F.; Khalil, N.M. In situ formation of zirconia–alumina–spinel–mullite ceramic composites. Ceram. Int. 2008, 34, 429–434. [Google Scholar] [CrossRef]
- Nozahic, F.; Carabat, A.L.; Mao, W.; Monceau, D.; Estournes, C.; Kwakernaak, C.; Zwaag, S.; Sloof, W.G. Kinetics of zircon formation in yttria partially stabilized zirconia as a result of oxidation of embedded molybdenum disilicide. Acta Mater. 2019, 174, 206–2016. [Google Scholar] [CrossRef]
- Wang, L.; Hu, H.; Xu, J.; Zhu, S.; Ding, A.; Deng, C. WO3 nanocubes: Hydrothermal synthesis, growth mechanism, and photocatalytic performance. J. Mater. Res. 2019, 34, 2955–2963. [Google Scholar] [CrossRef]
- Ponnusamy, R.; Gangan, A.; Chakraborty, B.; Sekhar Rout, C. Tuning the pure monoclinic phase of WO3 and WO3-Ag nanostructures for non-enzymatic glucose sensing application with theoretical insight from electronic structure simulations. J. Appl. Phys. 2018, 123, 024701. [Google Scholar] [CrossRef]
- Chacon, C.; Rodriguez-Perez, M.; Oskam, G.; Rodrıguez-Gattorno, G. Synthesis and characterization of WO3 polymorphs: Monoclinic, orthorhombic and hexagonal structures. J. Mater. Sci. Mater. Electron. 2015, 26, 5526–5531. [Google Scholar] [CrossRef]
- Koseva, I.I.; Nikolov, V.S. Preparation of aluminum tungstate Al2(WO4)3 using sol-gel modified Pechini method. Bul. Chem. Com. 2010, 42, 300–304. [Google Scholar]
- Batista, F.M.C.; La Porta, F.A.; Gracia, L.; Cerdeiras, E.; Mestres, L.; Siu Li, M.; Batista, N.C.; Andrés, J.; Longo, E.; Cavalcante, L.S. A joint experimental and theoretical study on the electronic structure and photoluminescence properties of Al2(WO4)3 powders. J. Mol. Struc. 2015, 1081, 381–388. [Google Scholar] [CrossRef]
- Foo, C.T.; Salleh, M.A.M.; Ying, K.K.; Matori, K.A. Mineralogy and thermal expansion study of mullite-based ceramics synthesized from coal fly ash and aluminum dross industrial wastes. Ceram. Int. 2019, 45, 7488–7494. [Google Scholar] [CrossRef]
- Rendtorff, N.M.; Garrido, L.B.; Aglietti, E.F. Thermal shock resistance and fatigue of Zircon–Mullite composite materials. Ceram. Int. 2011, 37, 1427–1434. [Google Scholar] [CrossRef]
- Achary, S.N.; Mukherjee, G.D.; Tyagi, A.K.; Vaidya, S.N. Preparation, thermal expansion, high pressure and high temperature behaviour of Al2(WO4)3. J. Mater. Sci. 2002, 37, 2501–2509. [Google Scholar] [CrossRef]



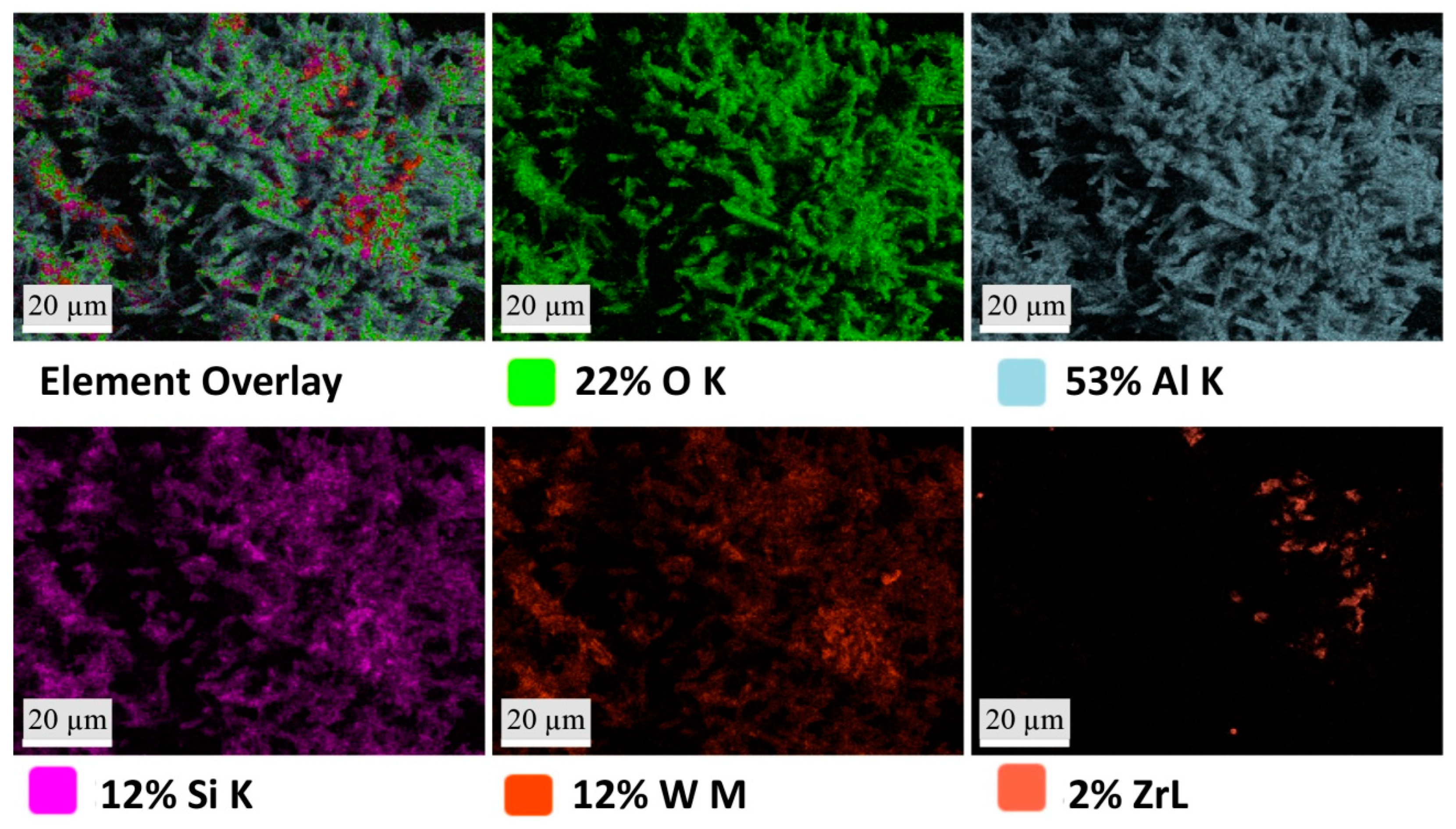

| EDS Spot 1 | EDS Spot 2 | EDS Spot 3 | ||||
|---|---|---|---|---|---|---|
| Elements | wt% | Atomic% | wt% | Atomic% | wt% | Atomic% |
| O K | 42.4 | 65.9 | 38.5 | 70.4 | 47.3 | 70.7 |
| AlK | 23.5 | 21.7 | 18.8 | 20.4 | 20.6 | 18.3 |
| SiK | 9.5 | 8.4 | 3.9 | 1.3 | 8.4 | 7.2 |
| W M | 19.7 | 2.7 | 34.0 | 5.4 | 19.4 | 2.5 |
| Y L | 3.3 | 0.9 | 3.3 | 1.1 | 2.7 | 0.7 |
| ZrL | 1.6 | 0.4 | 1.5 | 1.4 | 1.6 | 0.6 |
| Phase A | Phase B | Phase C | Phase D | |||||
|---|---|---|---|---|---|---|---|---|
| Elements | wt% | Atomic% | wt% | Atomic% | wt% | Atomic% | wt% | Atomic% |
| O K | 33.6 | 46.9 | 43.3 | 67.9 | 48.5 | 62.8 | 44.0 | 61.9 |
| AlK | 49.0 | 40.5 | 13.0 | 12.2 | 34.8 | 26.7 | 24.6 | 20.4 |
| SiK | 15.6 | 12.4 | 13.7 | 12.2 | 13.6 | 10.0 | 20.3 | 16.2 |
| W M | 1.8 | 0.2 | 3.8 | 0.5 | 2.6 | 0.3 | 10.3 | 1.3 |
| ZrL | 0 | 0 | 26.2 | 7.2 | 0.5 | 0.2 | 0.8 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahnicka-Goremikina, L.; Svinka, R.; Svinka, V.; Goremikins, V.; Ilic, S.; Grase, L.; Juhnevica, I.; Rundans, M.; Eiduks, T.V.; Pludons, A. Porous Mullite Ceramic Modification with Nano-WO3. Materials 2023, 16, 4631. https://doi.org/10.3390/ma16134631
Mahnicka-Goremikina L, Svinka R, Svinka V, Goremikins V, Ilic S, Grase L, Juhnevica I, Rundans M, Eiduks TV, Pludons A. Porous Mullite Ceramic Modification with Nano-WO3. Materials. 2023; 16(13):4631. https://doi.org/10.3390/ma16134631
Chicago/Turabian StyleMahnicka-Goremikina, Ludmila, Ruta Svinka, Visvaldis Svinka, Vadims Goremikins, Svetlana Ilic, Liga Grase, Inna Juhnevica, Maris Rundans, Toms Valdemars Eiduks, and Arturs Pludons. 2023. "Porous Mullite Ceramic Modification with Nano-WO3" Materials 16, no. 13: 4631. https://doi.org/10.3390/ma16134631
APA StyleMahnicka-Goremikina, L., Svinka, R., Svinka, V., Goremikins, V., Ilic, S., Grase, L., Juhnevica, I., Rundans, M., Eiduks, T. V., & Pludons, A. (2023). Porous Mullite Ceramic Modification with Nano-WO3. Materials, 16(13), 4631. https://doi.org/10.3390/ma16134631






