Investigating the Supercapacitive Performance of Cobalt Sulfide Nanostructures Prepared Using a Hydrothermal Method
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis
2.3. Physical Characterization
2.4. Electrochemical Characterization
3. Results and Discussion
3.1. XRD Analysis of CoS Nanostructures
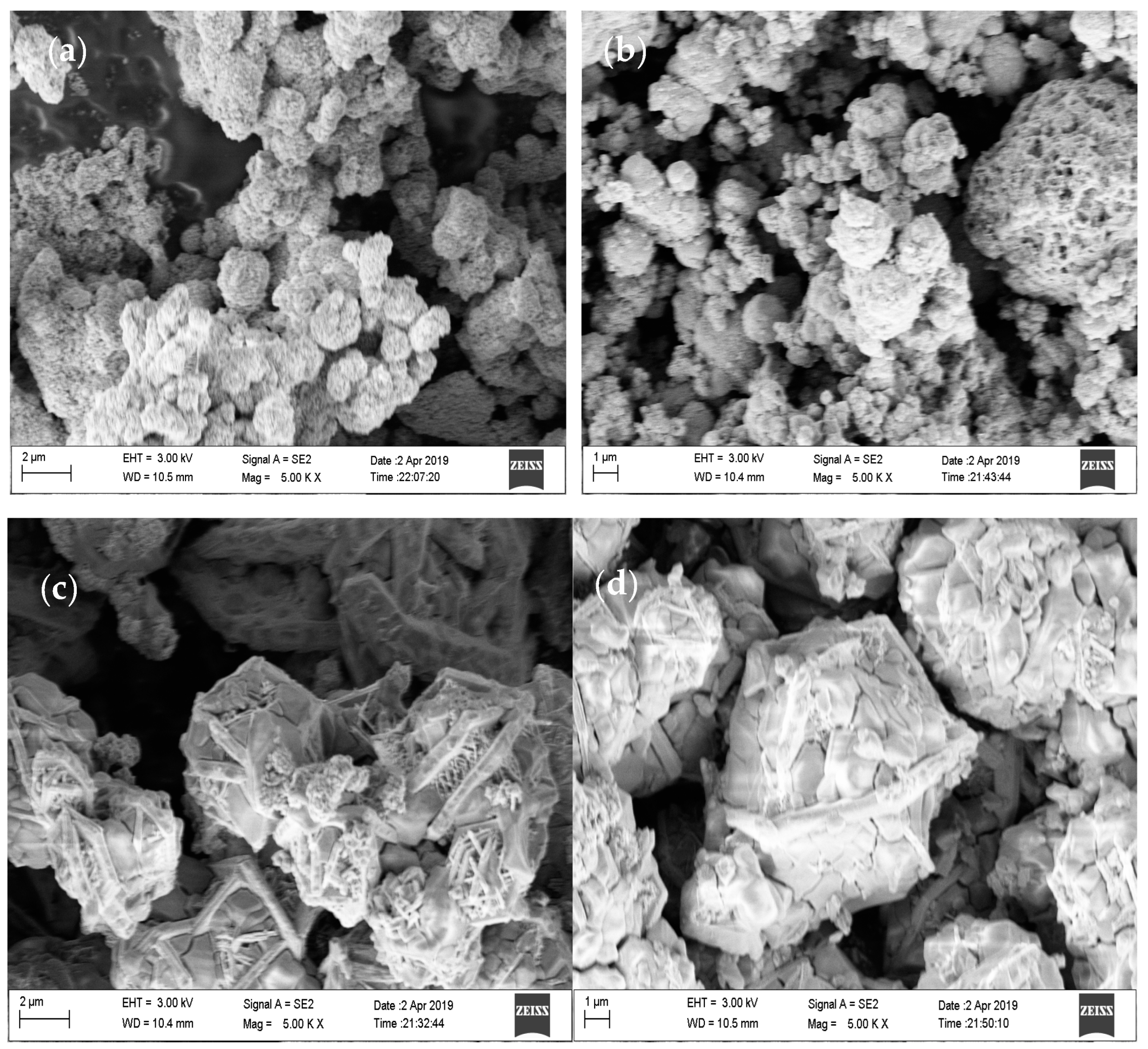
3.2. SEM Analysis of CoS Nanostructures
3.3. EDX Analysis
3.4. Electrochemical Analysis
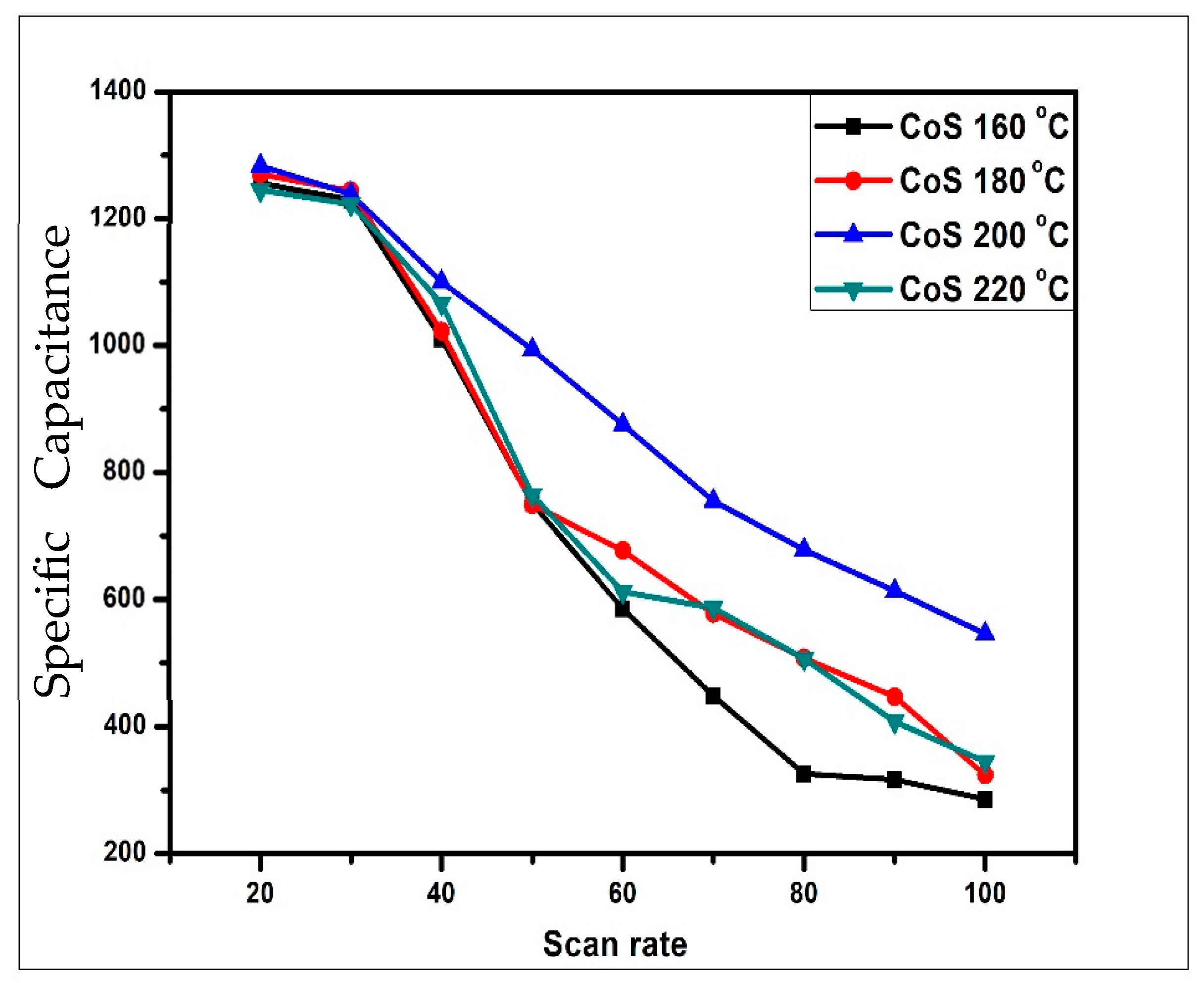
3.4.1. Cyclic Voltammetry Analysis
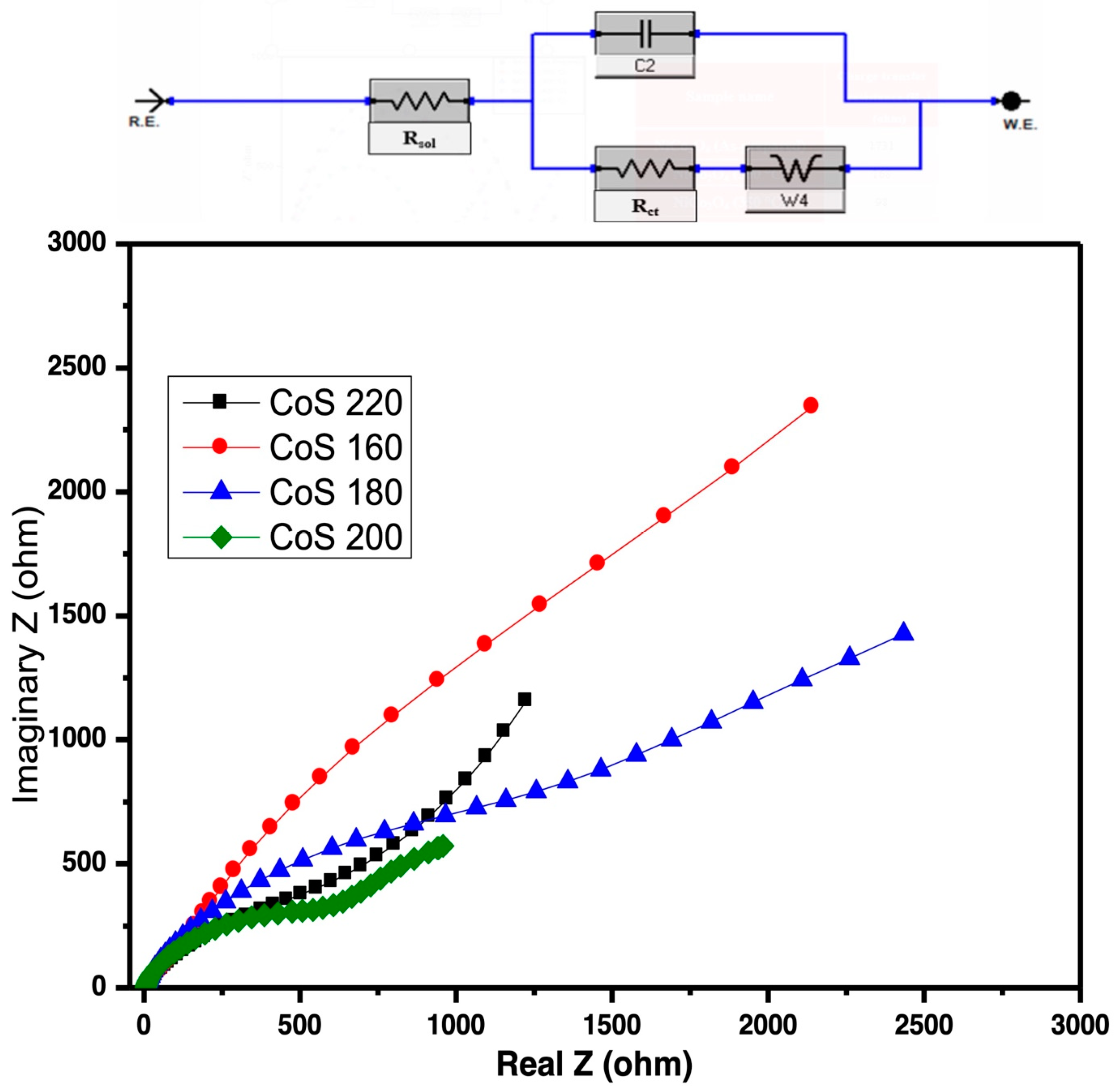
3.4.2. Electrochemical Impedance Spectroscopy (EIS) Analysis
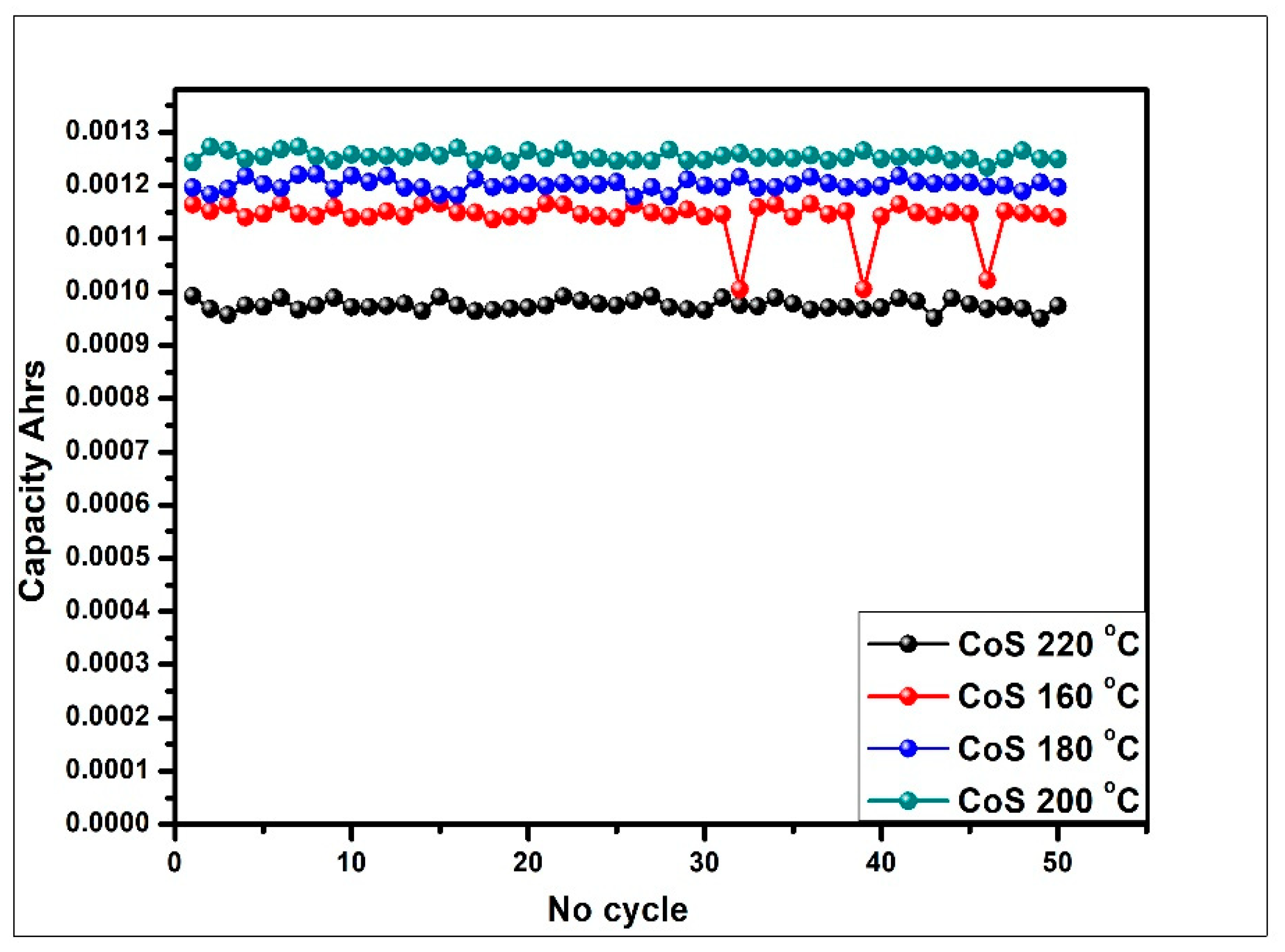
3.4.3. Galvanostatic Charge Discharge (GCD) Analysis
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asif, M.; Muneer, T. Energy supply, its demand and security issues for developed and emerging economies. Renew. Sustain. Energy Rev. 2007, 11, 1388–1413. [Google Scholar] [CrossRef]
- Barbir, F.; Veziroǧlu, T.N.; Plass, H.J. Environmental damage due to fossil fuels use. Int. J. Hydrog. Energy 1990, 15, 739–749. [Google Scholar] [CrossRef]
- Güney, T. Renewable energy, non-renewable energy, and sustainable development. Int. J. Sustain. Dev. World Ecol. 2019, 26, 389–397. [Google Scholar] [CrossRef]
- Dincer, I.; Rosen, M.A. Energy, environment and sustainable development. Appl. Energy 1999, 64, 427–440. [Google Scholar] [CrossRef]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Al-Shetwi, A.Q.; Hannan, M.A.; Jern, K.P.; Mansur, M.; Mahlia, T.M.I. Grid-connected renewable energy sources: Review of the recent integration requirements and control methods. J. Clean. Prod. 2020, 253, 119831. [Google Scholar] [CrossRef]
- Zheng, L.; Cheng, G.; Chen, J.; Lin, L.; Wang, J.; Liu, Y.; Li, H.; Wang, Z.L. A Hybridized Power Panel to Simultaneously Generate Electricity from Sunlight, Raindrops, and Wind around the Clock. Adv. Energy Mater. 2015, 5, 1501152. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Verma, R.U. Generalized relaxed proximal point algorithms involving relative maximal accretive models with applications in Banach spaces. Commun. Korean Math. Soc. 2010, 25, 313–325. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Li, B.; Dai, F.; Xiao, Q.; Yang, L.; Shen, J.; Zhang, C.; Cai, M. Activated Carbon from Biomass Transfer for High-Energy Density Lithium-Ion Supercapacitors. Adv. Energy Mater. 2016, 6, 1600802. [Google Scholar] [CrossRef]
- Choi, C.; Ashby, D.S.; Butts, D.M.; DeBlock, R.H.; Wei, Q.; Lau, J.; Dunn, B. Achieving high energy density and high power density with pseudocapacitive materials. Nat. Rev. Mater. 2020, 5, 5–19. [Google Scholar] [CrossRef]
- Cabrane, Z.; Ouassaid, M.; Maaroufi, M. Analysis and evaluation of battery-supercapacitor hybrid energy storage system for photovoltaic installation. Int. J. Hydrog. Energy 2016, 41, 20897–20907. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization, and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef]
- Lin, Y.P.; Wu, N.L. Characterization of MnFe2O4/LiMn2O 4 aqueous asymmetric supercapacitor. J. Power Sources 2011, 196, 851–854. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, P.; Jiang, Y.; Pan, D.; Tao, H.; Song, J.; Fang, T.; Xu, W. Supercapacitor performances of thermally reduced graphene oxide. J. Power Sources 2012, 198, 423–427. [Google Scholar] [CrossRef]
- Vatamanu, J.; Vatamanu, M.; Bedrov, D. Non-Faradaic Energy Storage by Room Temperature Ionic Liquids in Nanoporous Electrodes. ACS Nano 2015, 9, 5999–6017. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Strong, V.; Dubin, S.; Kaner, R.B. Laser scribing of high-performance and flexible graphene-based electrochemical capacitors. Science 2012, 335, 1326–1330. [Google Scholar] [CrossRef]
- Chu, A.; Braatz, P. Comparison of commercial supercapacitors and high-power lithium-ion batteries for power-assist applications in hybrid electric vehicles: I. Initial characterization. J. Power Sources 2002, 112, 236–246. [Google Scholar] [CrossRef]
- Khaligh, A.; Li, Z. Battery, ultracapacitor, fuel cell, and hybrid energy storage systems for electric, hybrid electric, fuel cell, and plug-in hybrid electric vehicles: State of the art. IEEE Trans. Veh. Technol. 2010, 59, 2806–2814. [Google Scholar] [CrossRef]
- Mothkuri, S.; Chakrabarti, S.; Gupta, H.; Padya, B.; Rao, T.; Jain, P. Synthesis of MnO2 nano-flakes for high-performance supercapacitor application. Mater. Today Proc. 2018, 26, 142–147. [Google Scholar] [CrossRef]
- Kim, B.K.; Sy, S.; Yu, A.; Zhang, J. Electrochemical Supercapacitors for Energy Storage and Conversion. In Handbook of Clean Energy Systems; John Whiley and Sons: Hoboken, NJ, USA, 2015; pp. 1–25. [Google Scholar] [CrossRef]
- Samson, G.T.; Undeland, T.M.; Ulleberg, O.; Vie, P.J.S. Optimal load sharing strategy in a hybrid power system based on PV/fuel cell/battery/supercapacitor. In Proceedings of the 2009 International Conference on Clean Electrical Power, Capri, Italy, 9–11 June 2009; pp. 141–146. [Google Scholar] [CrossRef]
- Inal, I.I.G.; Holmes, S.M.; Banford, A.; Aktas, Z. The performance of supercapacitor electrodes developed from chemically activated carbon produced from waste tea. Appl. Surf. Sci. 2015, 357, 696–703. [Google Scholar] [CrossRef]
- Frackowiak, E.; Metenier, K.; Bertagna, V.; Beguin, F. Supercapacitor electrodes from multiwalled carbon nanotubes. Appl. Phys. Lett. 2000, 77, 2421–2423. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Huang, Q.; Gamboa, S.; Sebastian, P.J. Studies on preparation and performances of carbon aerogel electrodes for the application of supercapacitor. J. Power Sources 2006, 158, 784–788. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, S.; Tian, X.N.; Zhao, X.S. Layered graphene oxide nanostructures with sandwiched conducting polymers as supercapacitor electrodes. Langmuir 2010, 26, 17624–17628. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, S.; Guan, L.; Tao, J. Prospect of Ni-related metal oxides for high-performance supercapacitor electrodes. J. Mater. Sci. 2021, 56, 1897–1918. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, Y.; Ji, X.; Banks, C.E. Transition Metal Oxides as Supercapacitor Materials. In Nanomaterials in Advanced Batteries and Supercapacitors; Springer: Berlin/Heidelberg, Germany, 2016; pp. 317–344. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, D.; Hu, P.; Wu, X. Ternary core-shell structured transition metal chalcogenide for hybrid electrochemical capacitor. Chinese Chem. Lett. 2018, 29, 1799–1803. [Google Scholar] [CrossRef]
- Elsiddig, Z.A.; Xu, H.; Wang, D.; Zhang, W.; Guo, X.; Zhang, Y.; Sun, Z.; Chen, J. Modulating Mn4+ Ions and Oxygen Vacancies in Nonstoichiometric LaMnO3 Perovskite by a Facile Sol-Gel Method as High-Performance Supercapacitor Electrodes. Electrochim. Acta 2017, 253, 422–429. [Google Scholar] [CrossRef]
- Zhu, X.; Tao, H.; Li, M. Co-precipitation synthesis of nickel cobalt hexacyanoferrate for binder-free high-performance supercapacitor electrodes. Int. J. Hydrog. Energy 2020, 45, 14452–14460. [Google Scholar] [CrossRef]
- Lu, M.; Yuan, X.P.; Guan, X.H.; Wang, G.S. Synthesis of nickel chalcogenide hollow spheres using an l-cysteine-assisted hydrothermal process for efficient supercapacitor electrodes. J. Mater. Chem. A 2017, 5, 3621–3627. [Google Scholar] [CrossRef]
- Pumera, M.; Sofer, Z.; Ambrosi, A. Layered transition metal dichalcogenides for electrochemical energy generation and storage. J. Mater. Chem. A 2014, 2, 8981–8987. [Google Scholar] [CrossRef]
- Jung, Y.; Zhou, Y.; Cha, J.J. Intercalation in two-dimensional transition metal chalcogenides. Inorg. Chem. Front. 2016, 3, 452–463. [Google Scholar] [CrossRef]
- Chen, Q.; Ding, Q.; Wang, Y.; Xu, Y.; Wang, J. Electronic and Magnetic Properties of a Two-Dimensional Transition Metal Phosphorous Chalcogenide TMPS4. J. Phys. Chem. C 2020, 124, 12075–12080. [Google Scholar] [CrossRef]
- Yuan, H.; Kong, L.; Li, T.; Zhang, Q. A review of transition metal chalcogenide/graphene nanocomposites for energy storage and conversion. Chin. Chem. Lett. 2017, 28, 2180–2194. [Google Scholar] [CrossRef]
- Heine, T. Transition metal chalcogenides: Ultrathin inorganic materials with tunable electronic properties. Acc. Chem. Res. 2015, 48, 65–72. [Google Scholar] [CrossRef]
- Voiry, D.; Yang, J.; Chhowalla, M. Recent Strategies for Improving the Catalytic Activity of 2D TMD Nanosheets Toward the Hydrogen Evolution Reaction. Adv. Mater. 2016, 28, 6197–6206. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, L.; Du, H.; Yang, J.; Huan, Q.; Peng, W.; Si, Y.; Wang, Y.; Yuan, H. Facile synthesis and superior supercapacitor performances of three-dimensional cobalt sulfide hierarchitectures. CrystEngComm 2011, 13, 6960–6963. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, X.; Liu, W.; Wang, Y.; Zhang, H.; Yang, M.; Wang, X.; Zhao, X.; Feng, S. A facile template-free approach for the solid-phase synthesis of CoS2 nanocrystals and their enhanced storage energy in supercapacitors. RSC Adv. 2014, 4, 50220–50225. [Google Scholar] [CrossRef]
- Liu, S.; Mao, C.; Niu, Y.; Yi, F.; Hou, J.; Lu, S.; Jiang, J.; Xu, M.; Li, C. Facile Synthesis of Novel Networked Ultralong Cobalt Sulfide Nanotubes and Its Application in Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 25568–25573. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Sun, C.; Guo, G.; Sun, W.; Huang, W.; Yan, Q.; Dong, X. Controlled synthesis of zinc cobalt sulfide nanostructures in oil phase and their potential applications in electrochemical energy storage. J. Mater. Chem. A 2015, 3, 11462–11470. [Google Scholar] [CrossRef]
- Tao, F.; Zhao, Y.Q.; Zhang, G.Q.; Li, H.L. Electrochemical characterization on cobalt sulfide for electrochemical supercapacitors. Electrochem. Commun. 2007, 9, 1282–1287. [Google Scholar] [CrossRef]
- Wan, H.; Ji, X.; Jiang, J.; Yu, J.; Miao, L.; Zhang, L.; Bie, S.; Chen, H.; Ruan, Y. Hydrothermal synthesis of cobalt sulfide nanotubes: The size control and its application in supercapacitors. J. Power Sources 2013, 243, 396–402. [Google Scholar] [CrossRef]
- Ali, S.; Saleem, S.; Salman, M.; Khan, M. Synthesis, structural and optical properties of ZnS–ZnO nanocomposites. Mater. Chem. Phys. 2020, 248, 122900. [Google Scholar] [CrossRef]
- Bulakhe, R.N.; Arote, S.A.; Kwon, B.; Park, S.; In, I. Facile synthesis of nickel cobalt sulfide nano flowers for high performance supercapacitor applications. Mater. Today Chem. 2020, 15, 100210. [Google Scholar] [CrossRef]
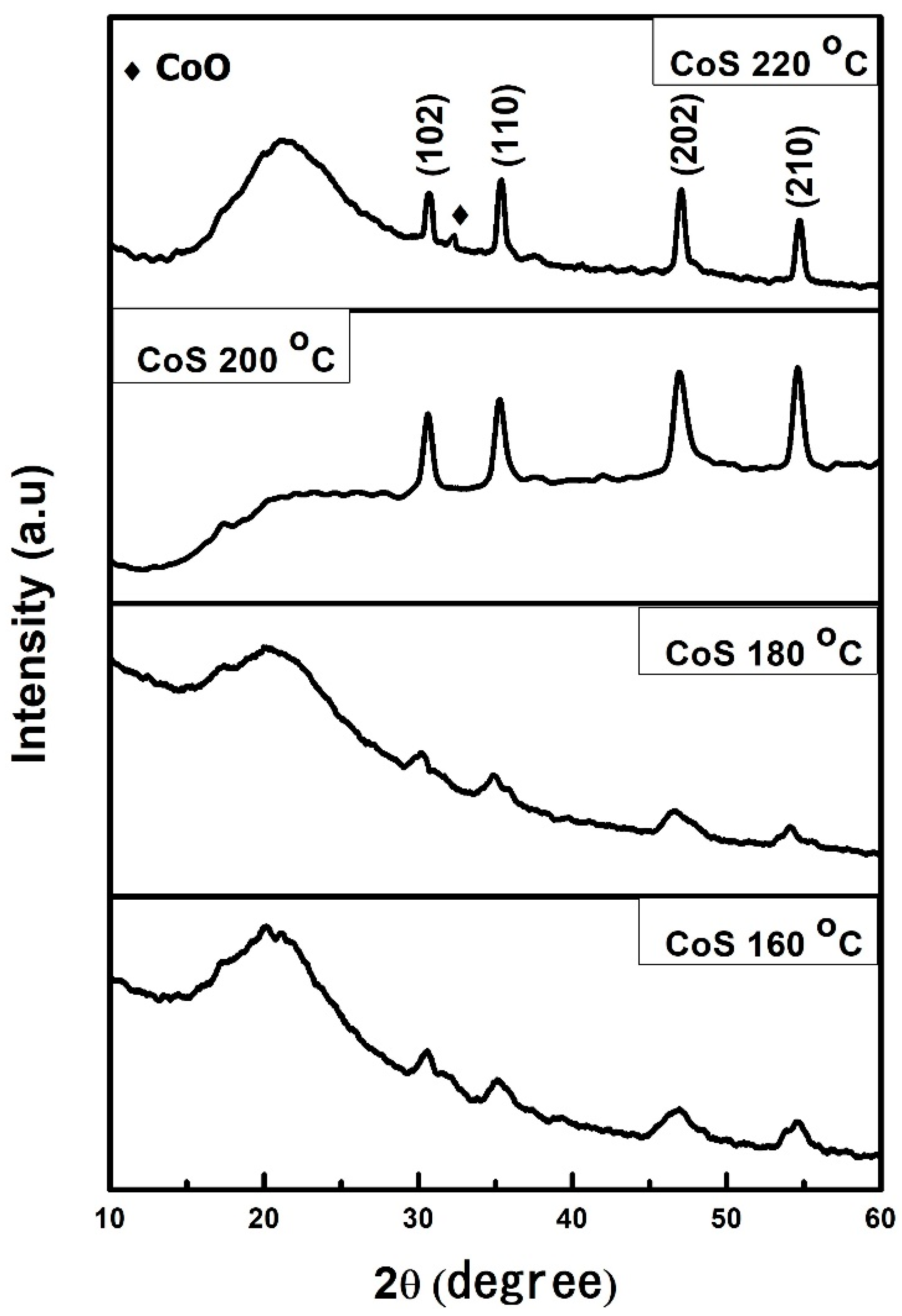



| S.No: | Sample | Average Crystallite Size |
|---|---|---|
| 1 | CoS 160 °C | 8 nm |
| 2 | CoS 180 °C | 14 nm |
| 3 | CoS 200 °C | 18 nm |
| 4 | CoS 220 °C | 24 nm |
| Sample | Csp (F/g) 20 mV/s | Csp (F/g) 30 mV/s | Csp (F/g) 40 mV/s | Csp (F/g) 50 mV/s | Csp (F/g) 60 mV/s | Csp (F/g) 70 mV/s | Csp (F/g) 80 mV/s | Csp (F/g) 90 mV/s | Csp (F/g) 100 mV/s | Retention % |
|---|---|---|---|---|---|---|---|---|---|---|
| CoS 160 °C | 1256 | 1230 | 1011 | 752 | 586 | 449 | 326 | 317 | 286 | 21.3 |
| CoS 180 °C | 1270 | 1244 | 1023 | 750 | 677 | 578 | 508 | 447 | 324 | 25.5 |
| CoS 200 °C | 1480 | 1239 | 1101 | 994 | 876 | 756 | 678 | 614 | 546 | 42.55 |
| CoS 220 °C | 1245 | 1223 | 1067 | 765 | 612 | 587 | 507 | 408 | 345 | 27.7 |
| Sample Name | CoS 160 | CoS 180 | CoS 200 | CoS 220 |
|---|---|---|---|---|
| Rct (ohms) | 58 | 43 | 10 | 23 |
| High frequency |Z| (ohms) | 87 | 71 | 21 | 22 |
| Low frequency |Z| (ohms) | 3173 | 3224 | 2479 | 2993 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshoaibi, A. Investigating the Supercapacitive Performance of Cobalt Sulfide Nanostructures Prepared Using a Hydrothermal Method. Materials 2023, 16, 4512. https://doi.org/10.3390/ma16134512
Alshoaibi A. Investigating the Supercapacitive Performance of Cobalt Sulfide Nanostructures Prepared Using a Hydrothermal Method. Materials. 2023; 16(13):4512. https://doi.org/10.3390/ma16134512
Chicago/Turabian StyleAlshoaibi, Adil. 2023. "Investigating the Supercapacitive Performance of Cobalt Sulfide Nanostructures Prepared Using a Hydrothermal Method" Materials 16, no. 13: 4512. https://doi.org/10.3390/ma16134512
APA StyleAlshoaibi, A. (2023). Investigating the Supercapacitive Performance of Cobalt Sulfide Nanostructures Prepared Using a Hydrothermal Method. Materials, 16(13), 4512. https://doi.org/10.3390/ma16134512






