Abstract
Tin oxide (SnO2) is a versatile n-type semiconductor with a wide bandgap of 3.6 eV that varies as a function of its polymorph, i.e., rutile, cubic or orthorhombic. In this review, we survey the crystal and electronic structures, bandgap and defect states of SnO2. Subsequently, the significance of the defect states on the optical properties of SnO2 is overviewed. Furthermore, we examine the influence of growth methods on the morphology and phase stabilization of SnO2 for both thin-film deposition and nanoparticle synthesis. In general, thin-film growth techniques allow the stabilization of high-pressure SnO2 phases via substrate-induced strain or doping. On the other hand, sol–gel synthesis allows precipitating rutile-SnO2 nanostructures with high specific surfaces. These nanostructures display interesting electrochemical properties that are systematically examined in terms of their applicability to Li-ion battery anodes. Finally, the outlook provides the perspectives of SnO2 as a candidate material for Li-ion batteries, while addressing its sustainability.
Keywords:
SnO2; nanomaterials; synthesis; polymorphism; band gap; defect states; electrochemical properties 1. Introduction
The transition to carbon-neutral energy production, storage and use is at the forefront of the EU’s decarbonized energy transition. To that end, lithium-ion batteries (LiBs) are currently at the forefront of energy storage devices because of their high energy density, high specific energy, high output voltage, low self-discharge, wide operational temperature range and rechargeability [1,2,3,4,5]. Even though LiBs serve as an energy source for smaller electronic devices, their performance is still insufficient for certain applications, such as electric vehicles (EV), which require good cyclic performances and low self-discharge. In fact, the choice of the anode material in LIBs is essential in determining the storage capacity or energy density of the battery. Currently, transition metal oxides, e.g., Fe2O3, Co3O4, NiO and TiO2, and composites of graphite with Sn, Sb and Al have raised interest as new-generation electrode materials [6,7,8]. Among them, Sn-based materials (e.g., SnO2, SnS2, SnSO4, Cu6Sn5 and Ni3Sn4) possess a higher specific capacity and a lower potential hysteresis than other transition metal oxides [9,10]. Due to their high conductivity and transparency, SnO2 materials are used in solar cells, catalytic supports, solid-state sensors and electrode materials for battery applications. SnO2 is also an important n-type wide-bandgap (3.6 eV) semiconductor, and its most stable polymorph is the natural and abundant cassiterite ore that crystalizes in the tetragonal rutile structure (P42/mnm). Additionally, compared to the commercialized graphite anode, SnO2 anode materials have demonstrated a much higher theoretical specific capacity of 1494 mAhg−1 against the 372 mAhg−1 of graphite [2,4,6,7,8,9], proving their applicability in commercial anode materials. In addition, the theoretical specific capacity of SnO2 is higher for reversible electrochemical half-cell reactions. Even though the alloying and dealloying of Li with metallic Sn are completely reversible, reduction of SnO2 into Sn during lithiation is considered as mainly irreversible. Nevertheless, the theoretical specific capacity still remains high at ~780 mAhg−1 owing to the formation of various LixSn compounds after the first cycle [11,12,13,14]. Both the theoretical calculations [15] and cyclic voltammetry studies [16,17] confirm the presence of these intermediate phases resulting from SnO2 lithiation. In addition, the ternary phase diagram of Sn-O-Li [18] also indicates that both the Li2SnO3 and Li8SnO6 intermediate phases are likely. Lithiation of metallic Sn presents certain disadvantages, such as a large volume expansion (~200–300%) that creates internal stress at the anode and consequently leads to its degradation [9,13,19].
One way to overcome these irreversible reactions that reduce the specific capacitance of the electrode is by using nanomaterials. In fact, nanomaterials are applied to a wide range of fields, e.g., medicine [20], food [21], the environment [22], textiles [23], cosmetics [24], electronics [25] and energy [26]. As the particle size decreases, the surface-to-volume ratio increases, leading to a high specific surface. Recent studies [5,12,27] have shown that shrinking the SnO2 nanoparticle size to less than 11 nm [28] enables the reversibility of the lithiation–delithiation processes. In fact, the large surface area in very small nanoparticles generates a large number of reactive sites for Li2O nucleation on the surface of SnO2 that improves inter-diffusion kinetics. On the other hand, Sn-metal nanoparticles tend to enlarge in order to reduce their surface-to-interface energy, i.e., Gibbs free energy, leading to the decrease in the number of active sites for Li2O nucleation and impeding the full particle conversion of Sn to SnO2. In fact, Sn coarsening is likely to be the major reason for the decay in energy capacity [5,28,29]. In addition, Li2O coarsens simultaneously and acts as an electron insulator because of its poor electronic conductivity and, thus, obstructs electron shuttling between electrodes. Therefore, several strategies have been adopted in order to hinder Sn-nanoparticle coarsening [5]. These methods include combining SnO2 with carbonaceous materials such as graphite [5,30], graphene [5,31,32] or carbon nanotubes [5,33]. In turn, these strategies promote reversible reactions owing to a better dispersion of nanoparticles in the matrix. While the carbon matrix usually enhances electrical conductivity, incorporating grain boundaries with a hybrid interface improves Li+ insertion that then deters the coarsening of Sn nanoparticles. Another strategy consists of alloying with Co metal that generates intermetallic phases [34,35,36] with high theoretical capacities, i.e., up to 851 mAhg−1 for CoSn3, 796 mAhg−1 for CoSn2, 663 mAhg−1 for CoSn and 569 mAhg−1 for Co3Sn2 [37].
SnO2 can also be grown as hierarchical structures, such as nanorods, nanowires and nanoflowers, with a high surface-to-volume ratio. These morphologies tend to reduce the strain and coarsening of SnO2 caused by repeated lithiation–delithiation processes owing to their high-aspect ratio and specific surface that provide ample sites for Li2O lithiation. Even though there are reports on their syntheses [1,2,38,39,40,41,42], controlling the final morphology via synthesis parameters still remains a challenge. Furthermore, SnO2 bandgap varies as a function of the crystal structure and defects, and both depend upon the synthesis conditions [2,3]. Several high-pressure polymorphs of SnO2 possess interesting electronic properties; however, stabilizing them as single-phase free-standing nanostructures has not yet been achieved. Nevertheless, thin-film deposition methods have introduced novel ways to achieve the stabilization of these high-pressure polymorphs owing to substrate-induced strain and dopants [4,5,7,8,43]. For instance, the pure orthorhombic phase is mainly stabilized in SnO2 thin films via chemical deposition methods resulting in an epitaxial growth induced by substrate strain. On the other hand, the pure cubic phase was only achieved by direct-current sputtering using nitrogen and antimony dopants [44].
Therefore, this review compiles SnO2 synthesis methods and correlates them to the structural, morphological, electronic and optical properties. In fact, the bandgap, crystal structure, morphology and defects can be controlled by the synthesis conditions, such as synthesis temperature, duration, precursor and solvent. These properties are then further correlated to the electrochemical properties, such as energy capacity, redox mechanisms and cyclability of the anode materials, that are compared to commercial batteries. This paper aims at providing a reliable survey of the state of the art on the control of SnO2 properties.
2. Stabilization of SnO2 Polymorphs
The tetragonal rutile structure (P42/mnm) is the most common polymorph of SnO2. Other polymorphs of SnO2 have been stabilized by various growth techniques and through the use of dopants. Furthermore, the insertion of dopants not only modifies its crystal structure but also its physical and chemical properties. However, to date, the electrochemical performance for each SnO2 phase has not been systematically quantified for battery applications. Polymorphs of SnO2 vary in their polyhedral stacking and are a result of pressure-induced phase transitions of SnO2, shown in Figure 1, starting from the rutile structure, studied by the density functional theory (DFT) [45,46]. In fact, SnO2 undergoes a phase transition from the rutile-type tetragonal P42/mnm to the CaCl2-type orthorhombic Pnnm phase (Figure 1). The Pnnm polymorph of SnO2 usually stabilizes at a pressure of 12 GPa but can also exist in its metastable form of α-PbO2-type orthorhombic or the scrutinyite structure belonging to the space group of Pbcn [47]. The scrutinyite phase is under-stoichiometric in oxygen and is produced by an oxygen-vacancy-mediated transformation that not only increases the unit cell volume but also provides them with interesting physical properties, such as enhanced gas sensing. Oxygen vacancies can also be created by introducing trivalent dopants that substitute Sn in the structure, whereupon generating oxygen vacancies in the structure. For example, both Co and Mn are capable of stabilizing the scrutinyite phase, as they possess several oxidation states [43,48]. However, single-phase scrutinyite SnO2 is difficult to stabilize and often exists as a mixture of the rutile and orthorhombic Pnnm phases. Subsequently, the transformation of scrutinyite into the pyrite-type cubic Pa occurs at 17 GPa. Several works claim that high-pressure cubic phases were in fact stabilized from a pressure of 21 GPa onward. Similarly, pressures as high as 48 GPa were used to stabilize the Pa phase with a lattice constant of a = 4.87 Å. However, theoretically, a phase transition from Pa to fluorite-type cubic Fmm was obtained at a lower pressure of 24 GPa. The differences in the pyrite- and fluorite-type cubic phases lie mainly in the oxygen co-ordination. In the pyrite cubic structure, the Sn atoms are coordinated to six oxygen atoms and two more situated further away. On the other hand, in the fluorite structure, eight oxygen atoms are coordinated equidistantly from Sn. The release of pressure reverses the phase transformation to orthorhombic and then to tetragonal [49]. However, at a lower pressure of ~28 GPa, a ZrO2-type I orthorhombic phase transformation at 18 GPa from the pyrite structure belonging to the Pbca space group is stabilized, along with a 2% increase in the volume of the unit cell. The volume expansion is due to the presence of a higher number of Sn+4-coordinated oxygen, which increases to 7. Similarly, the fluorite-type cubic Fmm has a Sn+4 cation coordinated to eight oxygen anions. Finally, the cotunnite-type orthorhombic phase II with a Pnam space group appears at 33 GPa with the Sn cation being coordinated to nine oxygen anions [45,49,50].
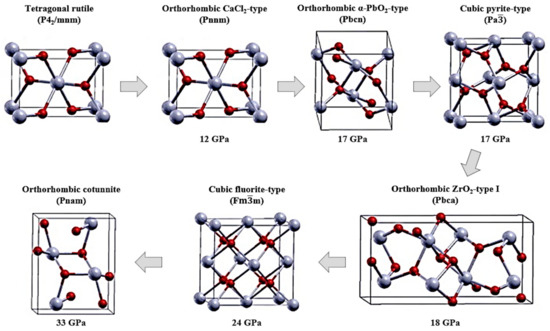
Figure 1.
Crystal structures of pressure-induced SnO2 polymorphs. Adapted with permission from [51], 2007 American Chemical Society.
Although the formation of the orthorhombic phase usually requires high pressure, i.e., extremely energetic conditions, orthorhombic SnO2 can nevertheless be stabilized as thin films by controlling the deposition conditions, such as temperature and pressure, or through doping. Several physical deposition techniques are reported in the literature, such as sputtering [52] and pulsed laser deposition (PLD) [53,54], in addition to chemical techniques, such as plasma-enhanced atomic layer deposition (PE-ALD) [55,56] or mist chemical vapor deposition (CVD) [57]. The addition of transition-metal ions or rare-earth-ion dopants has also shown promising results in the stabilization of the orthorhombic phase. In general, comparable-sized or smaller-radius metal ions usually substitute the Sn4+ cation in the lattice. For instance, manganese (Mn3+ radius: 0.65 Å and Mn4+ radius: 0.54 Å) [43], zinc (Zn2+ radius: 0.74 Å) [58] or cobalt (Co2+ radius: 0.58 Å) [59] ions, depending on their oxidation number, possess very similar atomic radii to Sn4+ ions (0.69 Å). Nevertheless, larger-radius ions can also stabilize the orthorhombic phase. In fact, ionic radii of Ce4+ (0.87 Å) and Ce3+ (1.01 Å) are much larger than the Sn4+ ionic radius; however, via the generation of lattice disorders and structural defects, the orthorhombic phase with a molar content of 41% can be stabilized in the solid solution of Sn0.7Ce0.3O2 [60]. Other than lattice disorders, orthorhombic phase stabilization can be obtained by doping SnO2 with Zn2+, Mn3+, Co2+ or Ce3+, they having lower oxidation states that trigger the formation of oxygen vacancies in the structure and, in turn, trigger the stabilization of other high temperature phases. For smaller cations such as Mn3+, the lattice distortions induced by changes in bond length are responsible for stabilization of the lower symmetry orthorhombic phase of SnO2 [43]. However, in the case of Sb doping, since Sn and Sb have similar atomic radii, Sb substitutes Sn without modifying the crystal structure even though they have different valences [33]. The presence of Sb3+ creates oxygen vacancies in the structure; nevertheless, a critical number of these oxygen vacancies is needed to stabilize the cubic phase.
The SnO2 cubic structure, i.e., pyrite or fluorite types, is a higher-pressure phase than the orthorhombic ones. In order to stabilize these phases, the substitution of oxygen by nitrogen atoms in epitaxially grown SnO2 films has been carried out [44,61]. The mechanism, once again, is based on oxygen-vacancy creation, where a N3− anion substitutes an O2− anion, creating an oxygen-deficient structure. Meanwhile, the co-doping with Sb3+ cations further exacerbates the formation of oxygen vacancies [44].
Even though many studies describe the stabilization of other SnO2 metastable phases via thin-film deposition techniques, the synthesis of orthorhombic or cubic SnO2 phases remains a challenge. In addition, other metal oxides also show a similar behavior. For example, the stable polymorph of HfO2 at room temperature is the monoclinic phase, but the tetragonal and cubic phases can be stabilized by varying certain synthesis parameters, e.g., doping and defects [62]. On the other hand, a reductive atmosphere at a synthesis temperature of 300 °C tends to induce oxygen vacancies, enabling the synthesis of the HfO2 cubic phase without dopants [63]. To the best of our knowledge, there are no reports available describing the stabilization of high-pressure single-phase SnO2, i.e., cubic or orthorhombic at atmospheric pressure, as these usually exist as mixed phases.
3. Electronic Structure of SnO2
Bulk SnO2 has a bandgap of 3.6 eV; however, experimental bandgaps range from 1.7 eV to 4 eV, thereupon widening its range of applications to photovoltaics and photocatalysis [42,64,65]. Bandgap engineering is widely studied in SnO2, as it belongs to the family of transparent conducting oxides (TCO). Additionally, bandgaps can be controlled via parameters, such as synthesis routes and the application of a substrate-induced strain [66] for thin-film growth that simultaneously produce intrinsic defects and structural changes. First-principles calculations indicate that the bandgap can be narrowed by increasing the distortion in the SnO6 octahedra, provoking changes in the bond length and bond angles in the unit cell [67]. The molecular-orbital bonding diagram of Figure 2a illustrates the hybridization of B2g, A1g and Eg with π2py and σ2pz between Sn-O. The density of states in Figure 2b,c provides the available states that the electrons occupy in the valence and conduction bands. The bottom of the conduction band is mostly the result of the contribution of Sn-4d orbitals with a hybridization of O-2p orbitals. Furthermore, the valence band can be divided into three parts, where the lower part of the valence band corresponds to O-2s and Sn-5s hybridization, the middle part consists of O-O interactions of O-2p, and hybridization of O-2p orbitals with Sn-5p and Sn-4f and the upper part is formed by O-2p and Sn-3d and Sn-4f orbitals [68,69]. Since six atoms of oxygen are at the apices of the octahedra, there are also O-O interactions in the molecular-orbital bonding diagram, in addition to Sn-O interactions. The completely filled Sn-4d and the partially filled O-2p orbitals define the antibonding character of the upper part of the valence band. On the other hand, the middle part has Sn-5p states with fewer than one electron; therefore, all the hybridization interactions with O-2p orbitals possess the bonding character. The presence of O or Sn vacancies affects the bonding character, creating distortion of the unit cell, which in turn modifies the bandgap as explained below.
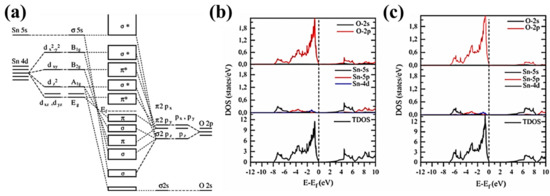
Figure 2.
(a) Molecular-orbital bonding structure, 2010 Elsevier [70], partial and total density of states calculated by (b) generalized gradient approximation by Perdrew et al. [71] (GGAP–PBE) and (c) Tran and Blata [72] modified Becke and Johnson [73] potential (Tb–mBJ), 2017 Springer [74] for rutile SnO2.
Experimental and theoretical results have demonstrated that SnO2 possesses both a direct and indirect band gap [42,75,76]. The allowed direct transition corresponds to a 3.68 eV direct bandgap, while the two forbidden direct transitions correspond to 3.03 eV and 3.50 eV [42]. In addition to direct bandgap transitions, there exist also indirect bandgaps corresponding to indirect transition of 2.62 eV and 2.90 eV, respectively [42]. In fact, the fundamental band gap of SnO2 is estimated to be much lower at ~3 eV [77], but certain band-to-band optical transitions are dipole forbidden, which lead to a higher optical bandgap of 3.8 eV [78]. In summary, the bandgaps, i.e., direct and indirect, are affected by the presence of defects in the volume of the SnO2 structures and the distortion of the oxygen polyhedron owing to polymorphism, as well as surface defects generated as a result of size reduction. This implies that certain transitions that are forbidden in bulk SnO2 may be allowed in defective or nano SnO2 because of the breaking of the long-range ordering of the crystal lattice at the surface of the nanoparticle [79], which in turn favors the generation of bandgap states.
3.1. Bandgap Engineering in SnO2
Direct bandgaps are systematically located at the high-symmetry Γ point. Besides, the crystalline quality of thin films related to defects and impurities has been shown to influence the bandgap [80]. The phase transition of SnO2 to higher-pressure-induced phases also encourages a decrease in the direct bandgap, which is the result of a more compact lattice, ensuing higher orbital overlapping [81]. In agreement with these observations, Table 1 compiles the structural and electronic properties of all the SnO2 polymorphs. However, experimentally, the synthesis and stabilization of higher-symmetry SnO2 polymorphs is complicated. Few studies report the presence of other SnO2 phases for Fe-doped SnO2, owing to the substitution of Sn by Fe ions [82], where the rutile phase co-exists with a small amount of α-PbO2 (Pbcn) secondary phase. After annealing at 800 °C, they observed that only traces of the SnO2 orthorhombic phase remained, which confirms the low stability of the orthorhombic phase at high temperatures compared to the SnO2 rutile structure. In addition, the possible presence of iron oxide lowers the SnO2 band gap to 2–3 eV [83]. In fact, according to the crystal field theory, Fe3+ ions are placed in an octahedral configuration in the presence of a weak field ligand (oxide) in a high-spin configuration. In addition, Fe3+ ions possess an ionic radius (0.645 Å) that is slightly smaller than Sn4+ ions (0.69 Å), which decreases the lattice parameters and, consequently, increases orbital overlapping between Sn4+ and O2− ions, leading to a lower bandgap. Radaf et al. succeeded in stabilizing the orthorhombic SnO2 structure by adding Cr3+ dopant [84]. The crystallite size decreased with the increase in Cr concentration, and the bandgap consequently decreased from 3.6 eV for the undoped SnO2 thin film to 3.28 eV with 5% of Cr. While the addition of those metals leads to the stabilization of the orthorhombic phase, Keskenler et al. [85] have demonstrated that W incorporation also stabilizes the Pbca cubic phase until a doping threshold of 2.0 at. %. Here, W6+ is likely to substitute Sn4+, which shrinks the lattice owing to the lower ionic radius. When the W concentration exceeds 2.0 at. %, lower oxidation states of tungsten could also substitute Sn4+ sites, which counteract the unit-cell shrinkage [85]. This can be explained by the Moss–Burstein effect, where materials with high carrier concentration, such as W, fill unoccupied states deep within the conduction band. Consequently, the Fermi level of the n-type SnO2 shifts into the conduction band. The increase in the optical bandgap is due to the excited electrons transitioning from the valence band to empty states in the conduction band localized at higher energy levels [85,86].

Table 1.
Crystal structure of SnO2 polymorphs, volume of the unit cell (Å3), its density (in g.cm−3) and direct bandgaps (eV).
3.2. Point-Defect Engineering in SnO2
The n-type conductivity of undoped rutile SnO2 materials can be explained by the defects present in the structure. Among the four different intrinsic defects, i.e., oxygen vacancy VO, tin interstitial Sni, tin antisite SnO and oxygen interstitial Oi, the predominant and combined occurrence of VO and Sni leads to electron donor properties [50,89]. SnO2 nanostructures exist in diverse morphologies (e.g., nanorods, nanocubes, nanosheets, nanowire and nanospheres) as a result of the synthesis route. Interstitial and vacancy-mediated defects are specific to each morphology, as the shape and size of the nanoparticle influence the surface and volume defects generated [90,91]. Hence, engineering SnO2 nanoparticles via controlled synthesis conditions allows the tailoring of their size, shape, morphology, intrinsic and surface defects. These properties play an important role in their electrochemical properties and redox mechanisms, especially for LiB applications. Defect engineering in semiconductors, more particularly in nanomaterials, is important for several applications. In fact, surface defects in nanomaterials are capital for surface-related phenomena in catalysis. Surface-defect engineering of SnO2 has already been studied for photocatalytic [92] and gas sensing [93,94] applications. Furthermore, oxygen-related defects generated in an oxygen-poor environment create exposed Sn4+ cations, as well as oxygen vacancies at the surface leading to abundant reactive sites [95]. In general, point defects such as surface-oxygen vacancies are common in nanomaterials owing to the high surface-to-volume ratio [96,97]. These defects are tailored via synthesis conditions, i.e., oxygen-rich or oxygen-poor conditions, synthesis temperature and annealing atmospheres. In addition, synthesizing faceted nanoparticles and exposing certain crystal facets to enhance catalytic activity are important topics in catalysis [98]. Furthermore, doping with foreign atoms to create VO and VSn, as explained before, stabilizes higher-symmetry polymorphs through the production of oxygen vacancies in the structure. On the other hand, in optoelectronic and electronic applications, passivating surface defects is necessary for enhancing the conductivity of SnO2, as in the case of F-doped SnO2 [99]. The principle is to eliminate defect states within the bandgap of the material by reducing these surface traps and, consequently, increasing the charge mobility and conductivity of SnO2. Since an oxygen anion is doubly ionized, the depletion of oxygen leads to a general enhancement of the charge-carrier concentration. These oxygen vacancies, i.e., VO, can have three different charge states, commonly termed as neutral VOx, singly ionized VO● and doubly ionized oxygen vacancy VO●● in Kröger–Vink notation. Two types of stoichiometric defects can occur inside the SnO2 lattice, i.e., Frenkel and Schottky defects, which do not influence the conductivity of the material, as the stoichiometry remains the same and charges remain in equilibrium. Schottky defects involve the simultaneous presence of charge-equivalent metal vacancies VSn⁄⁄⁄⁄ (quadruply negatively charged Sn vacancy) and oxygen vacancies VO●●. The mechanism consists of one Sn4+ ion leaving its lattice site (SnSnx), along with two oxygen atoms leaving their lattice sites (OOx) simultaneously and diffusing within the crystal in order to create charged vacancies, VSn⁄⁄⁄⁄ and two VO●●, respectively, whereas Frenkel defects are a type of point defect, where SnSnx or OOx leaves its original lattice site and occupies an interstitial site.
Stoichiometric defect mechanisms do not interfere with electronic properties of SnO2 nanoparticles, unlike nonstoichiometric defects. Surface defects in bulk materials have an insignificant influence on their physical and chemical properties because of their low proportion. However, surface defects in nanomaterials can drastically change catalytic and electronic properties, as a result of their high surface-to-volume ratio. Formation of VSn⁄⁄⁄⁄/VSn⁄⁄, VO●●/VO●/VOx, Sni and Oi depends on the oxygen environment during synthesis [100]. In the case of oxygen-deficient SnO2 nanomaterials, oxygen vacancies are formed by the transfer of oxygen atoms from their site (OOx) to the ambient because of an oxygen-poor environment during synthesis. This leads to a metastable state where oxygen vacancies are filled with the remaining two electrons of O2− (VO×) that then maintain the charge neutrality of the structure. However, this intermediate state is still unstable and leads to the subsequent release of electrons into the surroundings. The electrons released during the formation of the ionized oxygen vacancies are transferred to the Sn-5s state of the conduction band and ionize the Sn cation. These vacancies play a critical role as acceptors, whereupon they form new energy levels deep within the bandgap of the material. However, VO, as usual, couples with Sni in oxygen-deficient conditions, whereupon complex defects are formed, i.e., VO●● + Sni⁄⁄, giving rise to the n-type conductivity of SnO2 with electron mobility from the SnSn⁄⁄ to SnSn⁄⁄⁄⁄ sites. The mechanism for n-type conductivity involves the hybridization of the Sn-5s and O-2p states near such vacancies, facilitating electron transfer from the valence to the conduction band [101]. The only possibility to obtain p-type conductivity is via the introduction of VSn⁄⁄⁄⁄ + 4 h. In the case of Sn-deficient SnO2, VSn are the predominant defects that are created. An opposite reaction occurs where four electrons are taken from the valence band to form holes in order to generate interstitial site SnSn⁄⁄⁄⁄. The production of Sn vacancies can be mediated in Sn-poor conditions or by doping SnO2 with tri-valent elements substituting the SnSn× that create VSn [102,103]. Simultaneous doping with elements, such as N, creates acceptor states that then facilitate p-type conductivity. The computational and experimental studies on co-doping suggest the replacement of approximately four Sn atoms by four Al atoms and one O atom by one N atom [104]. In the case of metal excess, the defect equation governed by this mechanism involves the formation of Sn interstitial atoms, Snix, which further act as electron donors and can be successively doubly ionized to Sni●● or quadruply ionized to Sni●●●●. These doubly ionized Sn2+ states can act as traps that restrict the possibility of transition from the conduction band minimum to holes just above the valence band maximum. Therefore, passivation of the Sni●●●● on the surface of the SnO2 nanoparticles tends to enhance the oxygen-vacancy-related transitions. Lastly, in oxygen-rich conditions, Oi⁄⁄ have the lowest formation energy and are therefore abundant.
Among all these point defects described above, oxygen vacancies caused by oxygen-poor conditions are the most abundant intrinsic defects occurring in SnO2 nanomaterials because of the lowest formation enthalpy [100]. Moreover, many studies [105,106,107,108,109,110] have probed these new energy levels via photoluminescence (PL) spectroscopy. As previously mentioned, unstable VO× vacancy acts as a donor level and is located at 0.03 eV, just under the conduction band. In addition, ionized VO● is also considered a shallow donor, as it is located 0.15 eV below the conduction band, while VO●● is an acceptor level located at 1.4 eV above the valence band [109,110]. In SnO2 nanomaterials, the surface-oxygen vacancy is doubly ionized (or VO●●) and is the most dominant emission [111]. Wang et al. have investigated the photoluminescence mechanisms under a 255 nm excitation wavelength, resulting in band-to-band and defect excitations. Each peak was successfully identified and energy levels in the band diagram of SnO2 also corroborate them. For example, the transition between the VOx donor level to the VO●● acceptor level is attributed to the 467 nm (2.65 eV) emission peak, whereas the electron transition from VO● to the valence band can be assigned to the 439 nm (2.83 eV) emission peak. In addition, Snix is a shallow donor, as Sn interstitials tend to occur exclusively in the +4 state located very near the conduction band, contributing to the n-type semiconductor properties of SnO2, even though Sn interstitials are not abundant. The band-to-band transition is identified by the 328 nm emission, corresponding to an energy of 3.78 eV. Habte et al. [112] have demonstrated that the addition of Zn2+ cations to the SnO2 lattice leads to PL emission peak shifts, shown in Figure 3d,e, toward lower energies. There could be two reasons for the optical bandgap reduction. Since Zn2+ cations are smaller, their insertion should promote orbital overlapping because of a reduction in the lattice parameter. The other reason could be the shift toward longer wavelengths corresponding to the ZnO bandgap (3.37 eV). However, they highlighted that the lattice structure remains unchanged with Zn2+; therefore, the decrease in the optical bandgap can be attributed to the presence of Zn-O complexes. Salem et al. [113] have observed similar changes in the bandgap with Ni-doped ZnO. Nevertheless, the addition of Zn2+ should also enhance emission peak intensities, since the substitution of a smaller and lower valency cation encourages the formation of oxygen vacancies.
Since SnO2 is an n-type intrinsic semiconductor, the most prominent defects are, therefore, VO and Sni because of the lowest formation enthalpy. They are present in the volume of the material and contribute to the electronic conductivity. In nanomaterials, these defects are present on the surface and are instrumental in several catalytic reactions, including oxygen evolution reaction, hydrogen evolution reaction, gas sensing or electrocatalytic CO2 reduction [114]. On the other hand, for applications in electronic devices, these surface states are detrimental to the device’s functional properties. Photoluminescence spectroscopy is commonly used to identify these defects by providing information on optical transition between defect levels and band edges [115]. Depending on the application, these surface defects need to be either passivated or exacerbated. The importance of doping SnO2 with acceptors lies in the possibility of obtaining a p-type semiconductor that would eventually lead to a SnO2 homojunction diode. In general, surface defects act as trap states that enhance defect-level emission from the bandgap states. Furthermore, these defect states also extend the photo absorption of the materials to the visible region. Consequently, several new applications in LED, visible light detectors and photocatalysis are likely. In addition, defect-induced structures lead to the stabilization of higher-pressure polymorphs of SnO2, further leading to bandgap variations and other interesting properties, as explained in the section below.
4. Synthesis of SnO2 Nanostructures: Thin Films, Nanoparticles and Nanocomposites
4.1. Thin-Film Growth of SnO2: Role of Substrate-Induced Strain in the Stabilization of High-Pressure Phases of SnO2
Several studies report the stabilization of different phases of SnO2 as thin films through the optimization of growth conditions. In fact, epitaxial growth can promote the stabilization of high-pressure phases of SnO2 through substrate-induced strain [66]. Table 2 resumes SnO2 thin-film growth parameters by physical and chemical vapor deposition techniques along with the phase stabilized. Physical vapor deposition (PVD) techniques demonstrate some advantages over chemical deposition techniques as different polymorphs of SnO2 as thin films can be grown through PVD more easily. For PLD, the most important parameter is the deposition temperature [53,54,116]. At low temperatures (~150–300 °C), thin films are mainly amorphous because of low atomic mobility [54,116]. At a higher temperature (700 °C), orthorhombic and tetragonal phases coexist; however, at very high temperatures (1150 °C), the atomic diffusion is extremely high and SnO2 therefore tends to rearrange itself in its most stable structure, i.e., rutile [54]. Similarly, for DC and RF sputtering, authors demonstrated that it is possible to stabilize the rutile, orthorhombic or cubic phases depending on the synthesis parameters. Ham et al. [117] succeeded in growing a polycrystalline thin film with a co-existence of the orthorhombic and tetragonal phases. A small amount of rutile SnO2 at the interface diminishes the mismatch strain to 0.42%, and in turn, the remaining substrate-induced strain enables a heteroepitaxial growth of orthorhombic SnO2 on the c-plane of sapphire. The high-resolution TEM image in Figure 3a reveals a smooth interface between the substrate and the film oriented [11,12,13,14,15,16,17,18,19,20] and [001], respectively, implying that the growth direction of tetragonal-phase SnO2 is <100>. Whereas the orthorhombic phase can be obtained by sputtering at a high temperature [117,118], the cubic phase is mainly obtained by substituting O atoms by N atoms with N2 gas [44,61,119]. Similar to other metal oxides, such as CeO2 [120], ZrO2 [121] and HfO2 [122], SnO2 can be doped with nitrogen, where the substitution of O by N atoms involves an increase in ordered oxygen vacancies within the crystal structure. By virtue of the epitaxial strain at the interface of SnO2 and the substrate, crystallization of the orthorhombic or cubic phase can occur. However, at exceedingly high temperatures, the stabilization of the orthorhombic or cubic phase is hindered and the tetragonal rutile phase, where the Gibbs free energy is the lowest, is promoted [54].
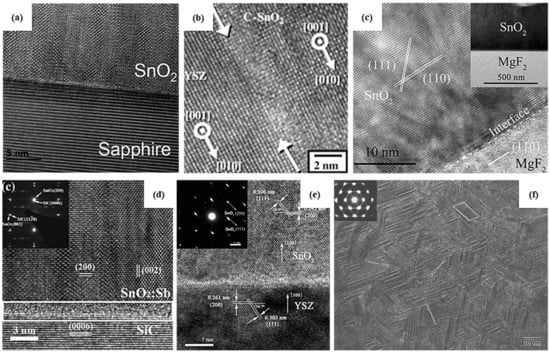
Figure 3.
HRTEM images of thin films of orthorhombic SnO2 (a) on c-plane sapphire by RF sputtering, 2022 Elsevier [117], (b) on yttrium-stabilized zirconia (YSZ) substrate by PE-ALD, 2012 Elsevier [55], (c) of rutile SnO2 on MgF2, 2018 Elsevier [123], orthorhombic SnO2 on (d) 6H-SiC substrate, 2021 Springer Nature [124], and (e) YSZ substrate, 2010 Elsevier [125], both by MOCVD; (f) Plane-view TEM image of SnO2 thin film deposited by MOCVD, 2011 Springer Nature [126].
Only a few reports of chemical deposition techniques describing the stabilization of the orthorhombic SnO2 phase are available. Bae et al. [57] have successfully grown the tetragonal and orthorhombic structures via mist-CVD, using two different solvents, i.e., methanol and acetone. The difference in the boiling point of these two solvents favors the stabilization of one phase over the other. As acetone has a lower boiling point, it would supply oxygen atoms more readily than methanol, leading to oxygen-rich conditions. Their results from DFT calculations were also consistent with the experimental results [57], and they concluded that the SnO2 orthorhombic phase is thermodynamically more favorable under Sn- and O-rich conditions. Another deposition using plasma-enhanced atomic-layer deposition for epitaxial growth of the orthorhombic phase by Kim et al. [55] on an yttrium-stabilized zirconia (YSZ) substrate (Figure 3b) with a lattice mismatch of 2% or less has been realized. Furthermore, the deposition of SnO2 thin films using metal–organic chemical vapor deposition techniques has also been investigated on different substrates. Deposition on both sapphire and MgF2 (Figure 3c) substrates leads to the tetragonal rutile SnO2 structure [55,123,127]. On the other hand, the orthorhombic phase is the result of the deposition at similar temperatures (around 500 °C) but on different substrates, i.e., 6H-SiC (Figure 3d) or yttrium-stabilized zirconia (YSZ) (Figure 3e) [124,125]. Furthermore, in that work, Sb doping was responsible for the stabilization of the orthorhombic structure. However, phase stabilization is unlikely because of Sb doping alone, which creates oxygen vacancies, but is also the result of substrate orientation, enabling the epitaxial growth of SnO2, owing to the equivalent crystallographic properties of the substrates. Liu et al. have demonstrated that the grain size of epitaxially grown SnO2 via MOCVD (Figure 3f) can be controlled by varying the lattice mismatch between the substrate and the film through substrate orientation. Furthermore, Kong et al. report that the [100] interplanar spacing of orthorhombic SnO2 film and YSZ substrate are comparable, with a lattice mismatch between c and a lattice parameter of SnO2 and YSZ being equal to 1.3% [125], which allows epitaxial thin-film growth.

Table 2.
SnO2 thin-films growth by physical and chemical deposition techniques and their structural properties.
Table 2.
SnO2 thin-films growth by physical and chemical deposition techniques and their structural properties.
| Deposition Technique | Target/Precursor | Oxygen Supply | Substrate | Conditions | Structure | Ref. |
|---|---|---|---|---|---|---|
| Physical deposition methods | ||||||
| Pulsed laser deposition (PLD) | Rutile SnO2 | O2 | Si [100] | 308 nm 10 Hz 20–400 °C <10−5 Pa | Amorphous + Tetragonal rutile | [116] |
| PLD | Sintered rutile SnO2 | Target | Si [001] | 532 nm 5 pulses/s 20–1150 °C 4 h | Tetragonal rutile + Orthorhombic | [54] |
| PLD | Rutile SnO2 | Target | Si [100] | 248 nm 10 Hz 320 °C 3 × 10−2 Pa | Tetragonal rutile + Orthorhombic | [53] |
| Direct-current (DC) sputtering | Tin metal plate | O2 | Si [100] | Ar gas 40–60 W 5 × 10−5 Pa 550 °C | Tetragonal rutile | [128] |
| DC sputtering | Rutile SnO2 | Target | SiO2 | N2-Ar gas 15 W 5 × 10−1 Pa 20–500 °C | Tetragonal rutile + cubic | [119] |
| DC sputtering | Tin metallic disk | O2 | Si [100] | Ar gas 60 W 40 min 1 × 10−1 Pa 148–243 °C | Tetragonal rutile + Orthorhombic | [118] |
| DC sputtering | Sb2O3 doped rutile SnO2 | Target | Si [100] | N2-Ag gas 60 W 4 × 10−1 Pa 300 °C | Cubic | [44] |
| Radio-frequency (RF) sputtering | Pure metallic Sn | O2 | SiO2 | Ar gas 25 W 2 h | Tetragonal rutile | [129] |
| RF sputtering | Rutile SnO2 | Target | Sapphire (0001) | 50 W 6.67 × 10−4 Pa 600 °C | Tetragonal rutile + Orthorhombic | [117] |
| RF sputtering | Rutile SnO2 | Target | Si/SiO2 + MgO [001] | N2-NH3 gas 25 to 75 W 0.67 Pa 400 °C | Cubic | [61] |
| Chemical deposition methods | ||||||
| Plasma-enhanced atomic-layer deposition (PEALD) | SnCl4 | O2 | Si [100] | Ar gas 100 to 400 W 400 Pa 150–350 °C | Tetragonal rutile | [130] |
| PEALD | Dibutyl tin acetate | O2 | yttria-stabilized zirconia | Ar gas 100 W 2.67 Pa 300 °C | Orthorhombic | [55] |
| PEALD | dimethylamino-2-methyl-2-propoxy-tin(II) | O2 or H2O | Si | N2 gas 100–300 °C | Tetragonal rutile + orthorhombic | [56] |
| Combustion vapor deposition | tin(II) 2-ethylhexanoate in absolute ethanol | Precursor | SiO2 | 20 min 850 °C | Tetragonal rutile | [131] |
| Aerosol-assisted chemical vapor deposition | MgxSn1−xO2 in ethanol | Precursor | Glass | Ar gas 30 min 400 °C | Tetragonal rutile | [132] |
| Metal–organic chemical vapor deposition (MOCVD) | Dibutyl tin acetate | O2 | Sapphire (0001) | N2 gas 2667 Pa 600–700 °C 30–100 sccm | Tetragonal rutile | [126] |
| MOCVD | Tetraethyl tin | O2 | MgF2 (001) | N2 gas 50 sccm 2 h 540–660 °C | Tetragonal rutile | [127] |
| MOCVD | Tetraethyl tin and trimethylstibine | O2 | 6H-SiC (0001) | N2 gas 40 sccm 2 h 600 °C | Orthorhombic | [124] |
| MOCVD | Dibutyl tin acetate | O2 | yttria-stabilized zirconia (100) | N2 gas 30 sccm 500–600 °C | Orthorhombic | [125] |
| Mist chemical vapor deposition | SnCl2·2H2O in methanol | Precursor | Si | 1.5 MHz N2 gas 1000 sccm 250–300 °C | Tetragonal rutile | [57] |
| MCVD | SnCl2·2H2O in acetone | Precursor | Si | 1.5 MHz N2 gas 1000 sccm 350–400 °C | Orthorhombic | [57] |
4.2. Hydrothermal Synthesis of SnO2 Nanomaterials
The structure–property relationship in SnO2 nanomaterials is controlled by the synthesis conditions. This signifies that the bandgap, size of the nanoparticle, morphology and chemical properties can be tuned through the synthesis conditions. Gavaskar et al. [133] have synthesized nanoparticles of SnO2 with the rutile structure using a SnCl4·5H2O precursor in ethanol at 200 °C for 20 h. They obtained quasi-spherical nanoparticles with diameters ranging from 50 to 90 nm and with a direct bandgap (3.35 eV) narrower than most SnO2 bandgaps. NaOH is also often used as an oxygen or hydroxyl source, since SnO2 crystals grow from stannate Sn(OH)62− that then condense to SnO2 and water [134,135,136]. Vuong et al. [134] added an aqueous solution of NaOH to SnCl4·5H2O dissolved in ethanol, which led to the growth of nanoflowerlike structures in Figure 4a under equivalent synthesis conditions. In addition, SnO2 anisotropic crystal growth depends on the diffusion of Sn(OH)62− at the surface of preferred orientations of the rutile crystal structure, which occurs after a sufficient amount of time (24 h) and temperature (190 °C). Guan et al. [135] realized complementary studies, in which they investigated the influence of the ratio of the SnCl4·5H2O precursor to NaOH on the morphology and structure. At a high ratio of NaOH to SnCl4, the nanoparticles are larger compared to low NaOH concentrations. They also explained that a high NaOH concentration promotes the growth of SnO2 along preferential crystallographic directions, leading to the flowerlike SnO2 nanorod bundles in Figure 4b [135]. Despite having a completely different shape and crystallite size, direct bandgap energies still range from 3.68 eV to 3.72 eV at both low and high NaOH:SnCl4 ratios, respectively. The addition of a surfactant, such as PEG [137], has been shown to further improve the previous synthesis protocol by precipitating the uniform flowerlike SnO2 nanorod bundles in Figure 4c.

Figure 4.
FESEM image of the SnO2 nanostructure synthesized with a (a) SnCl4·5H2O:NaOH molar ratio of 1:7.8, 2011 Elsevier [134], (b) SnCl4:NaOH molar ratio of 1:8 (with PEG), AIP publishing [135] and (c) 10:1 molar ratio of SnCl4:NaOH, 2010 Elsevier [137]. (d) HRTEM image of SnO2 nanocubes synthesized with SnCl4, urea and fuming urea with a synthesis time of 15 h, 2010 American Chemical Society [138].
Cao et al. confirmed the production of nanoflowers under a high NaOH:SnCl4 ratio and reduced the synthesis time from 24 h to 12 h [139]. By using sodium dodecyl sulfate catalyst and Na2SnO3 precursor, they also succeeded in synthesizing SnO2 nanocubes by shortening the reaction time to only 4 h, whereupon limiting the growth to preferential crystallographic directions. Runa et al. [140] also managed to synthesize SnO2 nanocubes using SnCl4 precursor with urea as a surfactant and absolute ethanol as a solvent in an acidic solution. Similar temperatures and time were employed, but an acidic medium and short synthesis time led to a cube morphology. These conditions limit the growth along the thermodynamically preferential [001] direction of SnO2 nanorods and lead to a pseudo-cubic shape. Similarly, Xi et al. [138] also synthesized SnO2 nanocubes, as shown in Figure 4d, from a SnCl4 precursor with urea and HCl. They suggest that hydroxyl ions from the ammonia–water reaction are in equilibrium with ammonium and hydroxide, thus favoring uniform growth morphologies.
5. Prospects of SnO2 Nanomaterials as Anode Materials in LiB: Correlating Their Morphology Obtained from Synthesis Routes to Their Electrochemical Performance
Nanocomposites of SnO2 have gained attention as anode materials for LiB applications because of their high theoretical specific capacity. It represents one of the most promising anode materials because of its high energy capacity, low cost and high energy density. Among common metal oxide compounds used as anodes in LiB [141], SnO2 exhibits the highest theoretical energy capacity (1494 mAhg−1), against 890 mAhg−1 for Co3O4 [142], 1007 mAhg−1 for Fe2O3 [143] and 1230 mAhg−1 for MnO2 [144]. In addition to higher energy capacity and density, SnO2 possesses a low overall potential, i.e., charge and discharge voltages of 0.3 V and 0.5 V vs. Li/Li+, respectively [145], against 1.0 and 2.2 for Co3O4 [146] and 1.3 and 1.6 for MnO2 [147]. SnO2 nanomaterials could solve issues faced by other metal oxides related to lithium alloying, which leads to irreversible capacity reduction and volume changes [17]. A commercial battery is assessed by different criteria, such as energy density (amount of energy that can be stored per unit mass of the battery), battery power (rate at which the electrical current can be moved through the battery), cycle life (number of charge–discharge cycles until its performance drops), watt hours (amount of power deliverable in an hour), charge speed and resistance or impedance. To solve challenges concerning energy storage systems and their sustainability, measurable quantities are crucial. While energy capacity is a direct indicator of the energy storage performance of the anode, the coulombic efficiency quantifies the reversibility and the efficiency of electron transition from the anode to the cathode over a cycle. Capacity retention evaluates the cycle-life performances of a battery, as it provides the ratio between the discharge energy capacity of successive cycles to the initial one [148].
Hu et al. demonstrated that a spherical nanoparticle with a size under 11 nm is required for a completely reversible lithiation–delithiation reaction [28]. Table 3 summarizes different synthesis routes of SnO2 nanostructures and nanocomposites with their electrochemical performances. Hydrothermal methods are a common way to synthesize SnO2 nanoparticles because of their low cost and simple synthesis protocols that can be easily scaled up. Yin et al. [149] developed a hydrothermal method based on the utilization of HCl, SnCl4∙5H2O and ammonia in an aqueous solution heated at 160 °C for 30 min that produced SnO2 nanoparticles with sizes ranging from 9 to 21 nm. These nanoparticles display an irreversible discharge capacity of 22.8% after 50 cycles (from 1196.6 mAhg−1 to 217.0 mAhg−1) with a current density of 100 mAg−1 (Figure 5a,d). Although the short synthesis time enables the growth of small nanoparticles, aqueous synthesis does not allow proper control of growth kinetics. On the other hand, nonaqueous synthesis methods are usually preferred for the production of small-sized metal–oxide nanoparticles, as they offer better control of the synthesis and tailor particle shape and size. Etacheri et al. [150] also prepared ultrathin SnO2 nanoparticles by reflux in an aqueous solution. High-capacity retention is obtained after calcination of the SnO2 nanopowder post-synthesis, referred to as “ordered interconnected SnO2 nanoparticles” in Figure 6a vs. pristine materials called “disordered SnO2 nanoparticles”. Whereas heat-treated SnO2 nanoparticles demonstrate a high-capacity retention (81.9%) and specific capacity (500 mAhg−1) after 100 cycles, pristine SnO2 nanoparticles exhibit poor electrochemical properties, i.e., 35.2% of capacity retention and a specific capacity of about 92 mAhg−1. Both SnO2 nanostructures are agglomerated, but the reason behind the enhancement of their electrochemical properties dwells in the nature of the agglomerates. In fact, aqueous synthesis tends to produce uncontrolled agglomerates with numerous and large electro-inactive clusters, while calcination rearranges the structure into a porous network because of the release of hydroxyl groups. Porous networks are commonly tested for battery applications, such as sub-microtube [151], hollow microspheres [152] and porous nanotube [153] structures (Figure 6b–d), because of their potentially high electrochemical properties. Therefore, reaction reversibility is possible by reducing the lithium-ion diffusion pathway by creating a highly porous structure made of nanorods, nanoflakes, nanobelts and nanosheets [154]. Narsimulu et al. [155] prepared ~10 nm thick SnO2 nanosheets by a microwave-assisted synthesis method using a stannic chloride precursor and citric acid in an aqueous medium. In theory, the thin SnO2 nanosheets, along with their porous nature, should enable high electrochemical reversibility; however, at a current density of 100 mAg−1, the discharge energy density decreases from 1350 mAhg−1 to 257.8 mAhg−1 after 50 cycles (Figure 6b,e), representing a capacity retention of only 19.1%. In fact, the ordered interconnected SnO2 network mentioned before by Etacheri et al. [150] possesses a four-times larger active surface area (204 m2g−1) than the nanosheets (59.28 m2g−1). Nano–Köhler theory [156] refers to the activation of inorganic cluster growth by spontaneous condensation [157], but in the case of a prolonged hydrothermal synthesis, it tends to promote a longer coagulation time and, thus, leads to larger nanostructures, such as nanoflower bundles [158] or nanorod arrays [159]. Wen et al. [158] have synthesized flowerlike structures similar to Guan et al. [135] and Cao et al. [139] using NaOH and SnCl4∙5H2O precursors in aqueous media. However, since cycling performances are carried out at a 10-times lower current density, i.e., 78 mAg−1 instead of 782 mAg−1, the initial and final energy capacities are not directly comparable. Liu et al. [159] have grown organized nanorods on an Fe plate with similar conditions, using stannic chloride and NaOH precursors heated at 200 °C (vs. 180 °C) for 24 h in an autoclave. In this case, long nanorods with dimensions of 60 nm × 670 nm were produced that exhibited greater initial energy charge and discharge capacities of 1128 mAhg−1 and 1918 mAhg−1, respectively. On the other hand, for the same current density, nanoflower bundles [158] display charge and discharge energy capacity of 815 mAhg−1 and 1673 mAhg−1, respectively. Higher electrochemical performances are again due to the nanoarrays presenting a larger organized network facilitating Li+ ion diffusion.
Therefore, reaction reversibility is possible on reducing the lithium-ion diffusion pathway, such as in highly porous structures made of nanorods, nanoflakes, nanobelts, nanosheets and hallow nanospheres (Figure 5d–f) [144]. While the synthesis of 0D nanostructures generally requires a SnCl4 precursor, hierarchical nanostructures are synthesized by using lower oxidation states, Sn(II), of the SnCl2 precursor. Sharma et al. [160] and Ding et al. [161] have, respectively, synthesized 1D SnO2 nanowires (Figure 5d) and 3D hollow nanospheres (Figure 5f) using the template-assisted synthesis route. These nanostructures tend to retain their energy capacity for a higher number of cycles compared to free-standing SnO2 nanoparticles. Wu et al. [162] and Narsimulu et al. [155] have both, respectively, prepared ~35 nm (Figure 6e) and ~10 nm thick SnO2 nanosheets by a hydrothermal method. Both types of nanosheets exhibited good cycling capacity and charge retention.
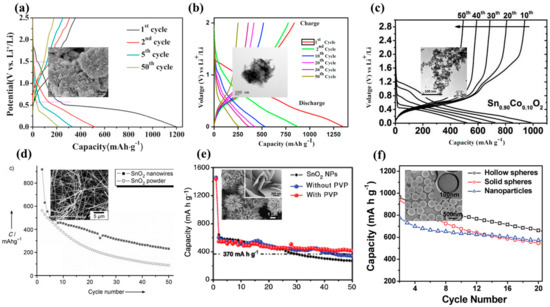
Figure 5.
Charge–discharge profiles at 100 mAhg−1 current density of (a) SnO2 nanoparticles, 2016 Elsevier [139], (b) SnO2 nanosheets, 2018 Elsevier [145] and (c) Co-doped SnO2 nanoparticles 2018 Elsevier. Cycling performance at 100 mAhg−1 current density of (d) 1D SnO2 nanowire, 2007 Wiley [163], (e) 2D SnO2 nanosheet, 2012 Elsevier [164] and (f) 3D SnO2 hollow nanospheres, 2010 American Chemical Society [161]. Insets correspond to their respective SEM or TEM images.
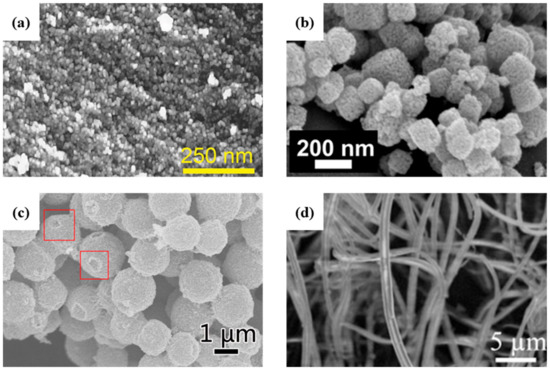
Figure 6.
SEM images of SnO2 (a) porous ordered interconnected, 2014 Wiley [140], (b) porous submicrotubes, 2016 Wiley [141], (c) porous microspheres, 2018 Elsevier [142] and (d) nanotubes, 2010 Elsevier [143].
The coupling of carbonaceous materials with SnO2 nanoparticles has been widely studied because of the possible enhancement of cycling capacity and conductivity through their combination. Liu et al. [165] recently illustrated the efficiency of carbon nanotubes and carbon nanotube hairballs coupled with SnO2 nanoparticles. These carbonaceous materials and a tin precursor were mixed and stirred in an absolute ethanol (nonaqueous) medium before heating at 150 °C for 10 h. Once the product was washed and dried, the resulting powder was calcinated at 360 °C for 10 min. The initial specific discharge capacity tripled (from 768.1 mAhg−1 to 2255.2 mAhg−1) when coupled with carbon nanotube hairballs. In addition to higher initial charge and discharge capacities, capacity retention also increases after 100 cycles at 200 mAhg−1 from 29.9% (244.8 mAhg−1) to 74.2% (809.2 mAhg−1), highlighting the high contribution of carbon nanotubes to anode performance. Deng et al. [166] have compiled a large number of syntheses involving SnO2 nanostructures coupled with graphene and have evaluated their respective electrochemical performances. The study concludes that SnO2–graphene nanocomposites are promising as they could significantly improve electrochemical and electrical properties, even though some issues persist, such as the high cost of graphene fabrication and the large irreversible capacity loss of the initial cycle.
Another solution to improve SnO2 properties is through doping with transition metals to enhance the chemical, defect and structural properties of SnO2. Mueller et al. [167] measured the electrochemical properties of Fe-doped SnO2 nanoparticles in a carbon matrix. Fe-doped SnO2 nanoparticles were synthesized via hydrothermal synthesis from tin acetate, sucrose, acetic acid and iron (II) gluconate at 150 °C for 10 h. The doping of SnO2 nanoparticles with smaller Fe cations decreases the cassiterite lattice structure through the incorporation of oxygen vacancies, which results in the production of smaller nanoparticles (7 to 8 nm vs. 15 nm). Mueller et al. [167] also illustrate that the Fe-ion doping creates smaller nanoparticles with surface defects. The utilization of a carbon matrix coupled with Fe doping doubles the energy capacity, i.e., from 764 mAhg−1 to 1519 mAhg−1 after 10 cycles at a current density of 50 mAhg−1. This is likely due to the enhanced conductivity of the carbon matrix allowing a better ionic diffusion. In addition, Ma et al. [168] have distinctly quantified the contribution of Co doping on SnO2 nanoparticles and the carbon matrix to the system, separately. This synthesis differs from the usual hydrothermal synthesis carried out inside an autoclave. They applied a one-pot synthesis route at 180 °C until complete evaporation occurred, followed by heat treatment at 450 °C for 3 h, which lead to the production of ultra-small nanoparticles doped with Co less than 10 nm in diameter. After 50 cycles at 100 mAg−1, Co-doped SnO2 nanoparticles show a reversible capacity of 493 mAhg−1 (Figure 5c,f), which is higher than undoped SnO2 nanoparticles having a reversible capacity of 242 mAhg−1. The reversible capacity was then significantly enhanced with the addition of the carbon matrix, where the specific energy capacity remains above 1000 mAhg−1. Ou et al. [169] investigated the properties of Ni-doped SnO2 nanoparticles synthesized using urea as a surfactant. The protocol requires a SiO2 nanosphere template, on the surface of which a thin layer of SnO2 nanoparticles is deposited using urea as a surfactant, hindering the excess growth of SnO2 despite the long synthesis time of 36 h at 170 °C. The SiO2 template is then removed via HCl etching before being calcinated at 400 °C for 4 h under an Ar atmosphere. Although the initial capacity loss is still prevalent after 300 cycles, they showed that Ni doping enhances the specific discharge capacity by more than 600%. SnO2 metal–oxide nanocomposites demonstrate significant improvement in the overall electrochemical properties. W. Zhou et al. [170] synthesized SnO2-Fe2O3 nanowire composites that possess a two-times higher energy density than SnO2 nanowires. The high-aspect ratio and the compatibility in the electronic structure are responsible for the enhancement. Wang et al. successfully synthesized SnO2-graphene-oxide-Co3O4 nanocomposites that exhibit long cycling stability (641 mAhg−1 at 1000 mAg−1 after 900 cycles) with complete reversibility (CR = 100%, 1038 mAhg−1) after 100 cycles at a lower energy density (100 mAg−1) [171]. Both SnO2-graphene and SnO2-graphene-metal–oxide nanocomposites tend to significantly improve the electrochemical and electrical properties of SnO2.
The synthesis of SnO2-based nanoparticles is mainly carried out by hydrothermal synthesis routes because of the ability of operating at low synthesis temperatures, including room temperature. Nevertheless, in order to eliminate reaction by-products and improve the properties of the as-synthesized SnO2, post-synthesis annealing is usually required in the range of 350–500 °C. Annealing can also induce the formation of porous structures and networks with higher specific surfaces. Other methods such as template-assisted syntheses are also applied to create SnO2 porous network nanostructures. In fact, porous connected networks such as SnO2 nanotubes provide pathways for Li diffusion, which increases the overall charge retention. In addition, the coupling of SnO2 nanostructures with carbonaceous materials, especially graphene, which is a well-known strategy to enhance the electrochemical properties of SnO2 nanomaterials, is still limited by the fabrication cost of graphene. For other hierarchical structures, such as nanorods, nanoflowers and nanosheets, an overall enhancement in the electrochemical properties is also observed. Additionally, using dopants in these syntheses, such as Fe, Co and Ni, clearly enhance charge retention and coulombic efficiency. Furthermore, when doped SnO2 is combined with carbon-based nanomaterials, its charge capacity is at least doubled. All these strategies starting from simple hydrothermal SnO2 synthesis followed by doping and combining with nanocarbons are important technological steps toward increasing the electrochemical properties of SnO2. Morphologies exhibiting high surface areas, such as nanoflowers, 3D porous nanostructures or any three-dimensional nanomaterials can promote lithium diffusion within the network owing to the significantly increased surface area. However, 1D and 2D nanomaterials are generally preferred because of their isotropic configuration that enables the stacking of nanomaterials while delivering free active sites.

Table 3.
Synthesis of SnO2 nanocomposites and their electrochemical properties.
Table 3.
Synthesis of SnO2 nanocomposites and their electrochemical properties.
| Label | Chemicals | Sample Preparation | Shape and Size | Potential Window vs. Li/Li+ (V) | Initial Energy Density (mAhg−1) | CE/CR | Energy Capacity (mAhg−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| SnO2 | SnCl4·5H2O, NH3, HCl in water | Autoclave at 160 °C for 30 min | Nanospheres 6–21 | 0.01–2.0 | Discharge: 1196.6 Charge: 520 (100 mAg−1) | CE = 42% first cycle CE > 98% after 10 cycles CR = 22.8% 50th cycle | 217.0 mAhg−1 at 100 mAg−1 after 50 cycles | [149] |
| SnO2 | SnCl4·5H2O, citric acid in water | Microwave at 2.4 GHz under 160 °C for 30 min | Nanosheets <10 nm thick | 0.005–2.0 | Discharge: 1350 Charge: 840 (100 mAg−1) | CE = 62% first cycle and >97% from the 10th cycle CR = 19.1% 50th cycle | 257.8 mAhg−1 at 100 mAg−1 after 50 cycles | [155] |
| SnO2 | Tin(IV) isopropoxide in water | Hydrothermal in air at reflux for 30 min then calcination at 400 °C for 1 h | Nanospheres 9 nm agglomerated in um blocks | 0.01–1.5 | Discharge: 674.5 (782 mAg−1) | CR = 81.9% 100th (782 mAg−1) | 500 mAhg−1 at 782 mAg−1 100th cycle | [150] |
| SnO2 | SnCl4·5H2O, NaOH, oleic acid in water | Hydrothermal, 180 °C for 24 h then annealed for 24 h at 500 °C | Flowerlike nanorod bundles 30 nm | 0–2.5 | Discharge: 1673 Charge: 815 (78 mAg−1) | CE = 49% 1st cycle CR = 41.5% 40th cycle | 694 mAhg−1 at 78.2 mAg−1 after 40 cycles | [158] |
| SnO2 | SnCl4·5H2O, NaOH in water | Hydrothermal, 200 °C for 24 h | Nanorods of 60 by 670 nm | 0.005–2.5 | Discharge: 1918 Charge: 1128 (78.1 mAg−1) | CE = 59% 1st cycle CR = 57.5% after 100th cycle | 645 mAhg−1 at 78.2 mAg−1 after 100 cycles | [159] |
| SnO2 | SnCl2·2H2O in ethanol | Autoclave 150 °C, 10 h then heat treated at 360 °C for 10 min | Micrometric aggregates | 0.01–3.0 | Discharge: 768.1 Charge: 414.8 (100 mAg−1) | CE = 54.0% 1st cycle CE = 93.2% 3rd cycle CR = 29.9% 100th cycle at 200 mAg−1 | 244.8 mAhg−1 at 200 mAg−1 after 100 cycles | [165] |
| SnO2-CNTH | SnCl2·2H2O, CNTH in ethanol | Autoclave 150 °C 10 h then heat treated at 360 °C for 10 min | Micrometric hairball shape containing SnO2 nanospheres (5–10 nm) and CNTH (30 nm diameter) | 0.01–3.0 | Discharge: 2255.2 Charge: 1098.3 (100 mAg−1) | CE = 48.7% 1st cycle CE = 88.8% 3rd cycle CR = 74.2% 100th cycle at 200 mAg−1 | 809.2 mAhg−1 at 200 mAg−1 after 100 cycles | [165] |
| SnO2 | Sucrose, acetic acid, tin acetate in water | 180 °C to evaporate then 300 °C to completely dry then calcinated at 450 °C for 3 h | Nanospheres of 15 nm | 0.01–3.0 | Discharge: 1139 Charge: 679 (50 mAg−1) | CE = 40.4% 1st cycle | 764 mAhg−1 at 50 mAg−1 after 10 cycles | [167] |
| Fe-doped SnO2 | Iron(II) gluconate·2H2O sucrose, acetic acid, tin acetate in water | 180 °C to evaporate then 300 °C to completely dry then calcinated at 450 °C for 3 h | Nanospheres of 7–8 nm | 0.01–3.0 | Discharge: 1726 Charge: 1241 (50 mAg−1) | CE = 28.1% 1st cycle | 1519 mAhg−1 at 50 mAg−1 after 10 cycles | [167] |
| SnO2 | Tin acetate and sucrose in water | 180 °C to evaporate then 450 °C for 3 h | Nanospheres of 12.5 nm | 0.01–3.0 | Discharge: ~850 Charge: ~750 | CE = 92.7% 50th cycle | 242 mAhg−1 at 50 mAg−1 after 50 cycles | [168] |
| Co-doped SnO2 | Cobalt(II) gluconate, tin acetate and sucrose in water | 180 °C to evaporate then 450 °C for 3 h | Nanospheres of 6.7–7.7–10.1 nm | 0.01–3.0 | Discharge: ~1200 Charge: ~1100 | CE = 70.4% 1st cycle CE = 94.6–94.9% 50th cycle | 493 mAhg−1 at 100 mAg−1 after 50 cycles 435.8 mAhg−1 at 50 mAg−1 after 50 cycles | [168] |
| Co-doped SnO2 with C coating | Cobalt(II) gluconate, tin acetate, sucrose and glucose in water | 180 °C to evaporate then 450 °C for 3 h. Heat up again at 180 °C for 13 h | Nanospheres of ~10 nm embedded in carbon matrix | 0.01–3.0 | Discharge: ~1900 Charge: ~1700 | CE = 74.2–74.4% 1st cycle CE = 96.0–97.2% 50th cycle | 1000–1200 mAhg−1 at 50 mAg−1 after 50 cycles | [168] |
| SnO2 | Na2SnO3·3H2O, urea in water | 170 °C for 36 h then calcinated at 500 °C for 4 h | Shallow nanospheres of 500 nm and 38 nm thick | 0.01–3.0 | Discharge: 1203 | CE = 61.6% 1st cycle | 87 mAhg−1 at 100 mAg−1 after 300 cycles | [169] |
| Ni-doped SnO2 | Na2SnO3·3H2O, urea, NiNO3 in ethanol/water | 170 °C for 36 h then calcinated at 500 °C for 4 h | Shallow nanospheres of 500 nm and 20 nm thick | 0.01–3.0 | Discharge: 1463–1581 | CE = 58.8–62.1% 1st cycle | 542 mAhg−1 at 100 mAg−1 after 300 cycles | [169] |
| SnO2 | SnO2 nanopowder | vapor deposition process at 1050 °C for 1 h 15 mbar | Nanowires of lengths 50 nm and 500 nm | 0.005–2.5 | Discharge: 612 Charge: 267 (1000 mAg−1) | CE = 43.6% first cycle | 148 mAhg−1 at 1000 mAg−1 after 30 cycles | [170] |
| SnO2-Fe2O3 | SnO2 nanopowder and FeCl3·6H2O | vapor deposition process at 1050 °C for 1 h 15 mbar | Nanowires with Fe2O3 nanoarrays | 0.005–2.5 | Discharge: 1167 Charge: 809 (1000 mAg−1) | CE = 69.4% first cycle | 207 mAhg−1 at 1000 mAg−1 after 30 cycles | [170] |
| SnO2-graphene oxide- | SnCl2 and graphene oxide | Autoclave 220 °C 24 h | 5–10 nm SnO2 nanoparticles | 0.01–3.0 | Discharge: 810 (100 mAg−1) | CR = 73.8% after 100 cycles | 597 mAhg−1 at 100 mAg−1 after 100 cycles | [171] |
| SnO2-graphene oxide- Co3O4 | SnCl2 and graphene oxide and Co(CH3COO)2 | Autoclave 220 °C 24 h then 80 °C for 8 h | 5–10 nm SnO2 and Co3O4 nanoparticles | 0.01–3.0 | Discharge: 1038 (1000 mAg−1) | CR = 100% after 100 cycles | 1038 mAhg−1 at 100 mAg−1 after 100 cycles | [171] |
6. Conclusions and Outlook
SnO2 is a versatile material whose bandgap can be modified by several strategies, including higher symmetry polymorph stabilization, doping and formation of defects in the structure through the synthesis process. Since the SnO2 compound is a potential candidate for applications in energy storage, studying SnO2 phase stabilization is, therefore, of interest. Amongst the available strategies, it has been shown that metal dopants can affect the morphology, decrease lattice parameters and simultaneously create oxygen-related defects. The use of metal dopants also tends to enhance the energy capacity, and when combined with a carbon matrix, e.g., graphene, graphite or carbon nanotubes, the reversibility of the reaction can be improved. In order to significantly improve the electrochemical properties of SnO2 nanostructures, the stabilization of higher symmetry polymorphs, i.e., high-pressure-induced phases, such as orthorhombic or cubic, is being intensively investigated. However, due to the difficulties encountered in stabilizing these phases, the electrochemical properties cannot be systematically probed. Nevertheless, the use of dopants such as nitrogen or metals to produce oxygen vacancies appears to be the most promising solution to stabilize the orthorhombic or cubic structures. However, the doping of SnO2 nanoparticles is still unable to stabilize single-phase SnO2, and several polymorphs tend to co-precipitate. However, in epitaxial thin films, the substrate-induced strain is capable of surpassing the activation free-energy barrier and leading to the stabilization of single-phase SnO2.
Today, there are several challenges related to introducing new nanomaterials for LiB electrode application. In particular, for the SnO2 compound, the main drawbacks, such as the huge initial capacity loss and extensive capacity fading after prolonged cycling, make their commercial breakthrough challenging compared to the well-established and omnipresent graphite. These drawbacks are mainly due to the volume expansion during charge–discharge processes that are still unaccounted for, despite consistent progress in the field. These stresses induced by the volume expansion have been alleviated to some extent by the addition of carbonaceous materials or by creating hollow structures that allow slightly higher flexibility for volume expansion. Therefore, hollow and hierarchical nanostructures are at the forefront of SnO2 research. Tailoring SnO2 nanostructures into desired morphologies is also a strategy to increase the active surface area, which in turn enhances the Li+ activity. In that regard, it has been shown that optimal electrochemical performances are obtained using ultra-small nanoparticles with a highly active specific surface. However, the scaling up of SnO2 synthesis processes in order to meet LiB demands needs to be cost effective and is one of the technical challenges to solve. Even if these aforementioned issues are surmountable, the technology transfer of nanomaterials to battery technologies involves many logistic issues. In fact, the weight of the active anode materials can attain 16% of the total battery weight in an electric vehicle, representing over 70 kg only for the anode material. Although SnO2 nanomaterials appear promising for increasing the general lifespan of a battery, their environmental impact is only succinctly addressed in the literature. The end-of-life recycling of materials has been shown to release free-standing ultrafine nanomaterials into the environment during the shredding process. Therefore, combining SnO2 nanoparticles with carbonaceous materials, including the presently employed graphite, could curb their release into the environment, in addition to improving the electrochemical properties of batteries. However, for a successful technology transfer, the sustainability of SnO2 is an important issue. In 2021, the production of Sn exceeded 370,000 tons. Being the 49th most-abundant element on Earth implies that Sn is quite scarce, and this would eventually lead to massive environmental and sustainability issues. To that end, growing nanostructures of SnO2 with large surface-to-volume ratios will be an advantage through the reduction in the volume of the material. This increases the sustainability of the SnO2 compound in LiB owing to the incorporation of lower quantities of Sn in the nanostructures.
Battery energy storage systems (BESS) are an important part of the net-zero energy transition. They have a wide range of power and storage capacities for both small-scale devices, including mobile phones, and large-scale devices in industrial utilities. LiBs have powered up to 90% of BESS globally. Present-day anodes employ cost-effective and light-weight carbon-based materials that tend to disintegrate after a finite number of charge–discharge cycles. In addition, nanomaterials have been proposed as anode materials to first overcome issues of volume expansion, which leads to the terminal degradation of the anode. Second, the high surface-to-volume ratio provided by nanostructured morphologies offers a higher number of active sites for Li storage that also facilitate reversible reactions. Therefore, integrating the SnO2 compound into the existing graphite-based anodes for LiB would offer a quick adaptation of this nanomaterial in battery applications. In fact, graphite-based anodes currently being employed already exhibit a strong network for SnO2–nanomaterial integration with a potential for a better battery cycle life and higher capacity, while making related processes more sustainable and cost effective.
Author Contributions
Conceptualization: R.P. and P.R.; methodology: R.P., P.R. and E.R.; validation, P.R. and E.R.; formal analysis, R.P.; investigation, R.P., P.R. and E.R.; resources, P.R. and E.R.; data curation, R.P.; writing—original draft preparation, R.P.; writing—review and editing, P.R. and E.R.; supervision, P.R. and E.R.; project administration, P.R.; funding acquisition, P.R. and E.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been supported by the European Regional Development Fund project grant number TK134 “EQUiTANT”, Eesti Maaülikool (EMÜ Bridge Funding (P200030TIBT)).
Informed Consent Statement
Not applicable.
Data Availability Statement
All figure were reproduced with permission or via creative commons attribution.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bruno Scrosati, J.G. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Nishi, Y. Lithium ion secondary batteries; past 10 years and the future. J. Power Sources 2001, 100, 101–106. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, 1–11. [Google Scholar] [CrossRef]
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2016, 164, A5019–A5025. [Google Scholar] [CrossRef]
- Zhao, S.; Sewell, C.D.; Liu, R.; Jia, S.; Wang, Z.; He, Y.; Yuan, K.; Jin, H.; Wang, S.; Liu, X.; et al. SnO2 as Advanced Anode of Alkali-Ion Batteries: Inhibiting Sn Coarsening by Crafting Robust Physical Barriers, Void Boundaries, and Heterophase Interfaces for Superior Electrochemical Reaction Reversibility. Adv. Energy Mater. 2019, 10, 1902657. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Yan, B.; Li, D.; Lawes, S.; Sun, X. Significant impact of 2D graphene nanosheets on large volume change tin-based anodes in lithium-ion batteries: A review. J. Power Sources 2015, 274, 869–884. [Google Scholar] [CrossRef]
- Zhang, W.-J. A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J. Power Sources 2011, 196, 13–24. [Google Scholar] [CrossRef]
- Peng, B.; Chen, J. Functional materials with high-efficiency energy storage and conversion for batteries and fuel cells. Coord. Chem. Rev. 2009, 253, 2805–2813. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Tin-based materials as versatile anodes for alkali (earth)-ion batteries. J. Power Sources 2018, 3955, 41–59. [Google Scholar] [CrossRef]
- Ovejas, V.J.; Cuadras, A. Effects of cycling on lithium-ion battery hysteresis and overvoltage. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, J.; Wang, N.; Wu, C.; Dong, G.; Guan, L. Fully Reversible Conversion between SnO2 and Sn in SWNTs@SnO2@PPy Coaxial Nanocable As High Performance Anode Material for Lithium Ion Batteries. J. Phys. Chem. 2012, 116, 18612–18617. [Google Scholar] [CrossRef]
- Hu, R.; Chen, D.; Waller, G.; Ouyang, Y.; Chen, Y.; Zhao, B.; Rainwater, B.; Yang, C.; Zhu, M.; Liu, M. Dramatically enhanced reversibility of Li2O in SnO2-based electrodes: The effect of nanostructure on high initial reversible capacity. Energy Environ. Sci. 2016, 9, 595–603. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.-C.; Kim, S.-B.; Kim, Y.-S.; Choi, J.-H.; Park, K.-W. Porous SnO2 nanostructure with a high specific surface area for improved electrochemical performance. RSC Adv. 2020, 10, 10519–10525. [Google Scholar] [CrossRef] [PubMed]
- Zoller, F.; Böhm, D.; Bein, T.; Fattakhova-Rohlling, D. Tin Oxide Based Nanomaterials and Their Application as Anodes in Lithium-Ion Batteries and Beyond. ChemSusChem 2019, 12, 4140–4159. [Google Scholar] [CrossRef]
- Cheng, Y.; Nie, A.; Gan, L.-Y.; Zhang, Q.; Schwingenschlögl, U. A global view of the phase transitions of SnO2 in rechargeable batteries based on results of high throughput calculations. J. Phys. Chem. A 2015, 3, 19486–19489. [Google Scholar] [CrossRef]
- Ferraresi, G.; Villevieille, C.; Czekaj, I.; Horisberger, M.; Novák, P.; El Kazzi, M. SnO2 Model Electrode Cycled in Li-Ion Battery Reveals the Formation of Li2SnO3 and Li8SnO6 Phases through Conversion Reactions. ACS Appl. Mater. Interfaces 2018, 10, 8712–8720. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Dhara, A.; Dendooven, J.; Detavernier, C. Atomic Layer Deposition of SnO2-Based Composite Anodes for Thin-Film Lithium-Ion Batteries. Front. Energy Res. 2020, 8, 609417. [Google Scholar] [CrossRef]
- Scott Kriklin, J.E.S.; Meredig, B.; Thomson, A.; Doak, J.W.; Aykol, M.; Rühl, S.; Wolverton, C. The Open Quantum Materials Database (OQMD): Assessing the accuracy of DFT formation energies. NPJ Comput. Mater. 2015, 1, 1–15. [Google Scholar] [CrossRef]
- Ahn, H.-J.; Choi, H.-C.; Park, K.-W.; Kim, S.-B.; Sung, Y.-E. Investigation of the Structural and Electrochemical Properties of Size-Controlled SnO2 Nanoparticles. J. Phys. Chem. B 2004, 108, 9815–9820. [Google Scholar] [CrossRef]
- Emerich, D.F.; Thanos, C.G. Nanotechnology and medicine. Expert Opin. Biol. Ther. 2003, 3, 655–663. [Google Scholar] [CrossRef]
- Sekhon, B.S. Food nanotechnology—An overview. Nanotechnol. Sci. Appl. 2010, 3, 1–15. [Google Scholar]
- Ali Mansoori, G.; Bastami, T.R.; Ahmadpour, A.; Eshaghi, Z. Environmental application of nanotechnology. In Annual Review of Nano Research; World Scientific: Singapore, 2008; Volume 2, pp. 439–493. [Google Scholar]
- Patra, J.K.; Gouda, S. Application of nano technology in textile engineering: An overview. J. Eng. Technol. Res. 2013, 5, 104–111. [Google Scholar] [CrossRef]
- Raj, S.; Jose, S.; Sumod, U.S.; Sabitha, M. Nanotechnology in cosmetics: Opportunities and challenges. J. Pharm. Bioallied Sci. 2012, 4, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Payal; Pandey, P. Role of Nanotechnology in Electronics: A Review of Recent Developments and Patents. Recent Pat. Nanotechnol. 2022, 16, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Serrano, E.; Rus, G.; García-Martínez, J. Nanotechnology for sustainable energy. Renew. Sustain. Energy Rev. 2009, 13, 2373–2384. [Google Scholar] [CrossRef]
- Wen, H.; Kang, W.; Liu, X.; Li, W.; Zhang, L.; Zhang, C. Two-phase interface hydrothermal synthesis of binder-free SnS2/graphene flexible paper electrodes for high-performance Li-ion batteries. RSC Adv. 2019, 9, 23607–23613. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zhang, H.; Lu, Z.; Liu, J.; Zeng, M.; Yang, L.; Yuan, B.; Zhu, M. Unveiling critical size of coarsened Sn nanograins for achieving high round-trip efficiency of reversible conversion reaction in lithiated SnO2 nanocrystals. Nano Energy 2018, 45, 255–265. [Google Scholar] [CrossRef]
- Liang, Z.; Li, S.; Yuan, B.; Hu, R.; Liu, J.; Zhu, M. Reversible formation of metastable Sn-rich solid solution in SnO2-based anode for high-performance lithium storage. Appl. Mater. Today 2021, 25, 101242. [Google Scholar] [CrossRef]
- Wei, L.; Yu, Q.; Yang, X.; Li, J.-H.; Shen, W.; Kang, F.; Lv, R.; Ma, L.; Huang, Z.-H. A facile assembly of SnO2 nanoparticles and moderately exfoliated graphite for advanced lithium-ion battery anode. Electrochim. Acta 2022, 432, 141210. [Google Scholar] [CrossRef]
- Xu, X.; Xu, F.; Qu, C.; Jiang, G.; Yu, H.; Repich, H.; Han, H.; Cao, F.; Li, L.; Wang, H. Laser-Manufactured Metastable Supranano SnOx for Efficient Electron/Ion Bridging in SnO2-Graphene Heterostructure Boosting Lithium Storage. Adv. Funct. Mater. 2021, 31, 2101059. [Google Scholar] [CrossRef]
- Dai, Y.; Li, F.; Fu, Y.-X.; Mo, D.-C.; Lyu, S.-S. Carbon-coated SnO2 riveted on a reduced graphene oxide composite (C@SnO2/RGO) as an anode material for lithium-ion batteries. RSC Adv. 2021, 11, 8521–8529. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, Y.; Li, L.; Wang, N.; Chai, Y. Nano SnO2 and Sb2O3 combined with CNTs as a high-capacity lithium storage material. Appl. Surf. Sci. 2021, 543, 148870. [Google Scholar] [CrossRef]
- Kim, W.-S.; Hwa, Y.; Kim, H.-C.; Choi, J.-H.; Sohn, H.-J.; Hong, S.-H. SnO2@Co3O4 hollow nano-spheres for a Li-ion battery anode with extraordinary performance. Nano Res. 2014, 7, 1128–1136. [Google Scholar] [CrossRef]
- Liang, T.; Hu, R.; Zhang, H.; Zhang, H.; Wang, H.; Ouyang, Y.; Liu, J.; Yang, L.; Zhu, M. A scalable ternary SnO2–Co–C composite as a high initial coulombic efficiency, large capacity and long lifetime anode for lithium ion batteries. J. Mater. Chem. A 2018, 6, 7206–7220. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Luo, Z.; Zhang, C.; Zuo, Y.; Zhang, T.; Tang, P.; Infante-Carrió, M.F.; Arbiol, J.; Llorca, J.; et al. Co–Sn Nanocrystalline Solid Solutions as Anode Materials in Lithium-Ion Batteries with High Pseudocapacitive Contribution. ChemSusChem 2019, 12, 1451–1458. [Google Scholar] [CrossRef]
- Yi, Z.; Wang, Z.; Cheng, Y.; Wang, L. Sn-based Intermetallic Compounds for Li-ion Batteries: Structures, Lithiation Mechanism, and Electrochemical Performances. Energy Environ. Mater. 2018, 1, 132–147. [Google Scholar] [CrossRef]
- Christian Julien, A.M.; Vijh, A.; Zaghib, K. Lithium Batteries; Springer: Cham, Switzerland, 2016; p. 619. [Google Scholar]
- Chen, J.S.; Lou, X.W. SnO2-Based Nanomaterials: Synthesis and Application in Lithium-Ion Batteries. Small 2013, 9, 1877–1893. [Google Scholar] [CrossRef]
- Goodenough, J.B. Evolution of Strategies for Modern Rechargeable Batteries. Acc. Chem. Res. 2012, 45, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Arlinghaus, F.J. Energy bands in stannic oxide (SnO2). J. Phys. Chem. Solids 1974, 35, 931–935. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, P.; Miao, C.; Chen, Z.; Lawrence Wu, C.M.; Shek, C.-H. Formation of orthorhombic SnO2 originated from lattice distortion by Mn-doped tetragonal SnO2. RSC Adv. 2015, 5, 39285–39290. [Google Scholar] [CrossRef]
- Le, T.; Dang, H.P. Effect of the Sb content in the SnO2 lattice and N2 percentage in a mixed sputtering gas on the N solubility in SnO2 films. Sens. Actuators A Phys. 2020, 316, 112421. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, C.-M.; Lv, M.-B.; Chen, X.-R.; Zhu, J.; Ji, G.-F. Structures, phase transition, elastic properties of SnO2 from first-principles analysis. Phys. B Condens. Matter 2011, 406, 3508–3513. [Google Scholar] [CrossRef]
- Shieh, S.R.; Kubo, A.; Duffy, T.S.; Prakapenka, V.B.; Shen, G. High-pressure phases in SnO2 to 117 GPa. Phys. Rev. B 2006, 73, 014105. [Google Scholar] [CrossRef]
- Lian, Y.; Huang, X.; Yu, J.; Tang, T.B.; Zhang, W.; Gu, M. Characterization of scrutinyite SnO2 and investigation of the transformation with 119Sn NMR and complex impedance method. AIP Adv. 2018, 8, 125226. [Google Scholar] [CrossRef]
- Hays, J.; Punnoose, A.; Baldner, R.; Engelhard, M.H.; Peloquin, J.; Reddy, K.M. Relationship between the structural and magnetic properties of Co-doped SnO2 nanoparticles. Phys. Rev. B 2005, 72, 075203. [Google Scholar] [CrossRef]
- Haines, J.; Léger, J.M.; Schulte, O. Pa3 Modified Fluorite-Type Structures in Metal Dioxides at High Pressure. Science 1996, 271, 629–631. [Google Scholar] [CrossRef]
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Gracia, L.; Beltrán, A.; Andrés, J. Characterization of the High-Pressure Structures and Phase Transformations in SnO2. A Density Functional Theory Study. J. Phys. Chem. B 2007, 111, 6479–6485. [Google Scholar] [CrossRef] [PubMed]
- Saldaña-Ramírez, A.; Cruz, M.R.A.; Juárez-Ramírez, I.; Torres-Martínez, L.M. Influence of the power density and working pressure in the magnetron co-sputtering deposition of ZnO–SnO2 thin films and their effect in photocatalytic hydrogen production. Opt. Mater. 2020, 110, 110501. [Google Scholar] [CrossRef]
- Chen, Z.; Lai, J.K.L.; Shek, C.-H. Facile strategy and mechanism for orthorhombic SnO2 thin films. Appl. Phys. Lett. 2006, 89, 231902. [Google Scholar] [CrossRef]
- Lamelas, F.J.; Reid, S.A. Thin-film synthesis of the orthorhombic phase of SnO2. Phys. Rev. B 1999, 60, 9347–9352. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.-H.; Hong, S.-H. Epitaxial growth of orthorhombic SnO2 films on various YSZ substrates by plasma enhanced atomic layer deposition. J. Cryst. Growth 2012, 348, 15–19. [Google Scholar] [CrossRef]
- Won, J.H.; Han, S.H.; Park, B.K.; Chung, T.-M.; Han, J.H. Effect of Oxygen Source on the Various Properties of SnO2 Thin Films Deposited by Plasma-Enhanced Atomic Layer Deposition. Coatings 2020, 10, 692. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Park, J.; Kim, H.Y.; Kim, H.-S.; Park, J.-S. Facile Route to the Controlled Synthesis of Tetragonal and Orthorhombic SnO2 Films by Mist Chemical Vapor Deposition. ACS Appl. Mater. Interfaces 2015, 7, 12074–12079. [Google Scholar] [CrossRef]
- Tian, S.; Gao, Y.; Zeng, D.; Xie, C. Effect of Zinc Doping on Microstructures and Gas-Sensing Properties of SnO2 Nanocrystals. J. Am. Ceram. Soc. 2012, 95, 436–442. [Google Scholar] [CrossRef]
- Parthibavarman, M.; Renganathan, B.; Sastikumar, D. Development of high sensitivity ethanol gas sensor based on Co-doped SnO2 nanoparticles by microwave irradiation technique. Curr. Appl. Phys. 2013, 13, 1537–1544. [Google Scholar] [CrossRef]
- Carvalho, M.H.; Pereira, E.C.; de Oliveira, A.J.A. Orthorhombic SnO2 phase observed composite (Sn1−xCex)O2 synthesized by sol–gel route. RSC Adv. 2018, 8, 3958–3963. [Google Scholar] [CrossRef]
- Gwon, H.J.; Kang, N.-R.; Lee, Y.; Won, S.O.; Chang, H.J.; Choi, J.-W.; Kang, C.-Y.; Kim, S.K.; Kwon, B.; Nahm, S.; et al. Enhancement of Mechanical Hardness in SnOxNy with a Dense High-Pressure Cubic Phase of SnO2. Chem. Mater. 2016, 28, 7051–7057. [Google Scholar] [CrossRef]
- Dubourdieu, C.; Rauwel, E.; Roussel, H.; Ducroquet, F.; Holländer, B.; Rossell, M.; Van Tendeloo, G.; Lhostis, S.; Rushworth, S. Addition of yttrium into HfO2 films: Microstructure and electrical properties. J. Vac. Sci. Technol. A 2009, 27, 503–514. [Google Scholar] [CrossRef]
- Rauwel, P.; Rauwel, E.; Persson, C.; Sunding, M.F.; Galeckas, A. One step synthesis of pure cubic and monoclinic HfO2 nanoparticles: Correlating the structure to the electronic properties of the two polymorphs. J. Appl. Phys. 2012, 112, 104107. [Google Scholar] [CrossRef]
- Barbarat, P.; Matar, S.F. First-principles investigations of the electronic, optical and chemical bonding properties of SnO2. Comput. Mater. Sci. 1998, 10, 368–372. [Google Scholar] [CrossRef]
- Kucheyev, S.O.; Baumann, T.F.; Sterne, P.A.; Wang, Y.M.; van Buuren, T.; Hamza, A.V.; Terminello, L.J.; Willey, T.M. Surface electronic states in three-dimensional SnO2 nanostructures. Phys. Rev. B 2005, 72, 035404. [Google Scholar] [CrossRef]
- Buzin, E.R.; Prellier, W.; Mercey, B.; Ch, S.; Raveau, B. Relations between structural distortions and transport properties in Nd0.5Ca0.5MnO3 strained thin films. J. Phys. Condens. Matter 2002, 14, 3951. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, Y.; Yang, Y.; Wu, P. Band Gap Engineering of SnO2 by Epitaxial Strain: Experimental and Theoretical Investigations. J. Phys. Chem. C 2014, 118, 6448–6453. [Google Scholar] [CrossRef]
- Xiao, W.-Z.; Xiao, G.; Wang, L.-L. A first-principles study of the SnO2 monolayer with hexagonal structure. J. Chem. Phys. 2016, 145, 174702. [Google Scholar] [CrossRef]
- Bouamra, F.; Boumeddiene, A.; Rérat, M.; Belkhir, H. First principles calculations of magnetic properties of Rh-doped SnO2 (110) surfaces. Appl. Surf. Sci. 2013, 269, 41–44. [Google Scholar] [CrossRef]
- Liu, Q.-J.; Liu, Z.-T.; Feng, L.-P. First-principles calculations of structural, electronic and optical properties of tetragonal SnO2 and SnO. Comput. Mater. Sci. 2010, 47, 1016–1022. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Tran, F.; Blaha, P. Accurate Band Gaps of Semiconductors and Insulators with a Semilocal Exchange-Correlation Potential. Phys. Rev. Lett. 2009, 102, 226401. [Google Scholar] [CrossRef]
- Becke, A.D.; Johnson, E.R. A simple effective potential for exchange. J. Chem. Phys. 2006, 124, 221101. [Google Scholar] [CrossRef]
- Slassi, A.; Hammi, M.; Oumekloul, Z.; Nid-bahami, A.; Arejdal, M.; Ziat, Y.; El Rhazouani, O. Effect of halogens doping on transparent conducting properties of SnO2 rutile: An ab initio investigation. Opt. Quantum Electron. 2017, 50, 8. [Google Scholar] [CrossRef]
- Ikhmayies, S.J.; Ahmad-Bitar, R.N. An investigation of the bandgap and Urbach tail of vacuum-evaporated SnO2 thin films. Renew. Energy 2013, 49, 143–146. [Google Scholar] [CrossRef]
- Abass, A.K.; Mohammad, M.T. Optical properties of fluorine-doped SnO2 films. J. Appl. Phys. 1986, 59, 1641–1643. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, P.; Wei, S.-H. Revisit of the band gaps of rutile SnO2 and TiO2: A first-principles study. J. Semicond. 2019, 40, 092101. [Google Scholar] [CrossRef]
- Han, X.; Deng, R.; Sun, B.; Jiang, D.; Zhao, M.; Yao, B.; Li, Y. Tuning optical and electrical properties of TixSn1−xO2 alloy thin films with dipole-forbidden transition via band gap and defect engineering. J. Alloys Compd. 2021, 885, 160974. [Google Scholar] [CrossRef]
- Li, Y.; Yin, W.; Deng, R.; Chen, R.; Chen, J.; Yan, Q.; Yao, B.; Sun, H.; Wei, S.-H.; Wu, T. Realizing a SnO2-based ultraviolet light-emitting diode via breaking the dipole-forbidden rule. NPG Asia Mater. 2012, 4, e30. [Google Scholar] [CrossRef]
- Wang, Y.; Su, J.; Lin, Z.; Zhang, J.; Chang, J.; Hao, Y. Recent progress on the effects of impurities and defects on the properties of Ga2O3. J. Mater. Chem. C 2022, 10, 13395–13436. [Google Scholar] [CrossRef]
- Hu, Z.; Lin, Z.; Su, J.; Zhang, J.; Chang, J.; Hao, Y. A Review on Energy Band-Gap Engineering for Perovskite Photovoltaics. Sol. RRL 2019, 3, 1900304. [Google Scholar] [CrossRef]
- Jithin, P.V.; Sudheendran, K.; Sankaran, K.J.; Kurian, J. Influence of Fe-doping on the structural and photoluminescence properties and on the band-gap narrowing of SnO2 nanoparticles. Opt. Mater. 2021, 120, 111367. [Google Scholar] [CrossRef]
- Munetoshi, S. Bandgap-Engineered Iron Oxides for Solar Energy Harvesting. In Iron Ores and Iron Oxide Materials; Volodymyr, S., Ed.; IntechOpen: Rijeka, Croatia, 2018; Chapter 13. [Google Scholar]
- El Radaf, I.M.; Hameed, T.A.; El Komy, G.M.; Dahy, T.M. Synthesis, structural, linear and nonlinear optical properties of chromium doped SnO2 thin films. Ceram. Int. 2019, 45, 3072–3080. [Google Scholar] [CrossRef]
- Keskenler, E.F.; Turgut, G.; Aydın, S.; Doğan, S. W doped SnO2 growth via sol–gel routes and characterization: Nanocubes. Optik 2013, 124, 4827–4831. [Google Scholar] [CrossRef]
- Butcher, K.S.A.; Hirshy, H.; Perks, R.M.; Wintrebert-Fouquet, M.; Chen, P.P.T. Stoichiometry effects and the Moss–Burstein effect for InN. Phys. Status Solidi A 2006, 203, 66–74. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Erdem, I.; Kart, H.H.; Cagin, T. High pressure phase transitions in SnO2 polymorphs by first-principles calculations. J. Alloys Compd. 2014, 587, 638–645. [Google Scholar] [CrossRef]
- Jarzebski, Z.M.; Marton, J.P. Physical Properties of SnO2 Materials: I. Preparation and Defect Structure. J. Electrochem. Soc. 1976, 123, 199C. [Google Scholar] [CrossRef]
- Crapanzano, R.; Villa, I.; Mostoni, S.; D’Arienzo, M.; Di Credico, B.; Fasoli, M.; Scotti, R.; Vedda, A. Morphology Related Defectiveness in ZnO Luminescence: From Bulk to Nano-Size. Nanomaterials 2020, 10, 1983. [Google Scholar] [CrossRef]
- Najib, S.; Bakan, F.; Abdullayeva, N.; Bahariqushchi, R.; Kasap, S.; Franzò, G.; Sankir, M.; Demirci Sankir, N.; Mirabella, S.; Erdem, E. Tailoring morphology to control defect structures in ZnO electrodes for high-performance supercapacitor devices. Nanoscale 2020, 12, 16162–16172. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.; Olszówka, J.; Sain, S.; Sloman, S.-R.I.; Montes, O.; Fernández, A.; Pradhan, S.K.; Wheatley, A.E.H. Morphological effects on the photocatalytic properties of SnO2 nanostructures. J. Alloys Compd. 2019, 810, 151718. [Google Scholar] [CrossRef]
- Xiong, Y.; Lin, Y.; Wang, X.; Zhao, Y.; Tian, J. Defect engineering on SnO2 nanomaterials for enhanced gas sensing performances. Adv. Powder Mater. 2022, 1, 100033. [Google Scholar] [CrossRef]
- Wang, X.; Ren, P.; Tian, H.; Fan, H.; Cai, C.; Liu, W. Enhanced gas sensing properties of SnO2: The role of the oxygen defects induced by quenching. J. Alloys Compd. 2016, 669, 29–37. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Guo, Y.; Xu, J.; Xu, X.; Fang, X.; Liu, J.; Chen, W.; Arandiyan, H.; Wang, X. Tuning SnO2 Surface Area for Catalytic Toluene Deep Oxidation: On the Inherent Factors Determining the Reactivity. Ind. Eng. Chem. Res. 2018, 57, 14052–14063. [Google Scholar] [CrossRef]
- Rauwel, E.; Galeckas, A.; Rauwel, P. Photoluminescent cubic and monoclinic HfO2 nanoparticles: Effects of temperature and ambient. Mater. Res. Express 2014, 1, 015035. [Google Scholar] [CrossRef]
- Rauwel, E.; Galeckas, A.; Soares, M.R.; Rauwel, P. Influence of the Interface on the Photoluminescence Properties in ZnO Carbon-Based Nanohybrids. J. Phys. Chem. C 2017, 121, 14879–14887. [Google Scholar] [CrossRef]
- Peng, Y.-K.; Tsang, S.C.E. Facet-dependent photocatalysis of nanosize semiconductive metal oxides and progress of their characterization. Nano Today 2018, 18, 15–34. [Google Scholar] [CrossRef]
- Bahuguna, G.; Verma, M.; Gupta, R. Chemical insights into electrophilic fluorination of SnO2 for photoelectrochemical applications. J. Mater. Chem. A 2021, 9, 19965–19974. [Google Scholar] [CrossRef]
- Godinho, K.G.; Walsh, A.; Watson, G.W. Energetic and Electronic Structure Analysis of Intrinsic Defects in SnO2. J. Phys. Chem. C 2009, 113, 439–448. [Google Scholar] [CrossRef]
- Sun, X.; Long, R.; Cheng, X.; Zhao, X.; Dai, Y.; Huang, B. Structural, Electronic, and Optical Properties of N-doped SnO2. J. Phys. Chem. C 2008, 112, 9861–9864. [Google Scholar] [CrossRef]
- Dai, G.; Zhou, W.; Ma, X.; Yuan, J.; Wu, P. Structure and magnetic properties of Cr-Doped tin monoxide prepared by hydrothermal method. Ceram. Int. 2020, 46, 13350–13355. [Google Scholar] [CrossRef]
- Ricciarelli, D.; Meggiolaro, D.; Ambrosio, F.; De Angelis, F. Instability of Tin Iodide Perovskites: Bulk p-Doping versus Surface Tin Oxidation. ACS Energy Lett. 2020, 5, 2787–2795. [Google Scholar] [CrossRef]
- Villamagua, L.; Stashans, A.; Lee, P.-M.; Liu, Y.-S.; Liu, C.-Y.; Carini, M. Change in the electrical conductivity of SnO2 crystal from n-type to p-type conductivity. Chem. Phys. 2015, 452, 71–77. [Google Scholar] [CrossRef]
- Ahmed, A.; Naseem Siddique, M.; Alam, U.; Ali, T.; Tripathi, P. Improved photocatalytic activity of Sr doped SnO2 nanoparticles: A role of oxygen vacancy. Appl. Surf. Sci. 2019, 463, 976–985. [Google Scholar] [CrossRef]
- Zeng, Q.; Cui, Y.; Zhu, L.; Yao, Y. Increasing oxygen vacancies at room temperature in SnO2 for enhancing ethanol gas sensing. Mater. Sci. Semicond. Process. 2020, 111, 104962. [Google Scholar] [CrossRef]
- Toloman, D.; Popa, A.; Stefan, M.; Silipas, T.D.; Suciu, R.C.; Barbu-Tudoran, L.; Pana, O. Enhanced photocatalytic activity of Co doped SnO2 nanoparticles by controlling the oxygen vacancy states. Opt. Mater. 2020, 110, 110472. [Google Scholar] [CrossRef]
- Mehraj, S.; Ansari, M.S.; Al-Ghamdi, A.A.; Alimuddin. Annealing dependent oxygen vacancies in SnO2 nanoparticles: Structural, electrical and their ferromagnetic behavior. Mater. Chem. Phys. 2016, 171, 109–118. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Di, Q.; Zhao, H.; Liang, B.; Yang, J. Mutual Effects of Fluorine Dopant and Oxygen Vacancies on Structural and Luminescence Characteristics of F Doped SnO2 Nanoparticles. Materials 2017, 10, 1398. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Ruan, X.; Yan, J.; Zhang, Z.; Yun, J.; Zhao, W.; Li, T.; Shi, Y. Synthesis, growth mechanism, and photoluminescence property of hierarchical SnO2 nanoflower-rod arrays: An experimental and first principles study. J. Mater. Sci. 2016, 51, 9613–9624. [Google Scholar] [CrossRef]
- Gu, F.; Wang, S.F.; Lü, M.K.; Zhou, G.J.; Xu, D.; Yuan, D.R. Photoluminescence Properties of SnO2 Nanoparticles Synthesized by Sol–Gel Method. J. Phys. Chem. B 2004, 108, 8119–8123. [Google Scholar] [CrossRef]
- Habte, A.G.; Hone, F.G.; Dejene, F.B. Zn doping effect on the properties of SnO2 nanostructure by co-precipitation technique. Appl. Phys. A 2019, 125, 402. [Google Scholar] [CrossRef]
- Salem, J.K.; Hammad, T.M.; Harrison, R.R. Synthesis, structural and optical properties of Ni-doped ZnO micro-spheres. J. Mater. Sci. Mater. Electron. 2013, 24, 1670–1676. [Google Scholar] [CrossRef]
- Mishra, R.K.; Sahay, P.P. Synthesis, characterization and alcohol sensing property of Zn-doped SnO2 nanoparticles. Ceram. Int. 2012, 38, 2295–2304. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kushwaha, A.; Sahay, P.P. Cr-induced modifications in the structural, photoluminescence and acetone-sensing behaviour of hydrothermally synthesised SnO2 nanoparticles. J. Exp. Nanosci. 2015, 10, 1042–1056. [Google Scholar] [CrossRef]
- Khandelwal, R.; Singh, A.P.; Kapoor, A.; Grigorescu, S.; Miglietta, P.; Stankova, N.E.; Perrone, A. Effects of deposition temperature on the structural and morphological properties of SnO2 films fabricated by pulsed laser deposition. Opt. Laser Technol. 2009, 41, 89–93. [Google Scholar] [CrossRef]
- Ham, D.; Oh, S.; Kang, H.C. Competing phases in epitaxial SnO2 thin films deposited on sapphire (0001) substrates using radio-frequency powder sputtering. Ceram. Int. 2022, 48, 28396–28403. [Google Scholar] [CrossRef]
- Camacho-López, M.A.; Galeana-Camacho, J.R.; Esparza-García, A.; Sánchez-Pérez, C.; Julien, C.M. Characterization of nanostructured SnO2 films deposited by reactive DC-magnetron sputtering. Superf. Vacio 2013, 26, 95–99. [Google Scholar]
- Nguyen, T.T.; Dang, H.P.; Luc, Q.H.; Le, T. Studying the influence of deposition temperature and nitrogen contents on the structural, optical, and electrical properties of N-doped SnO2 films prepared by direct current magnetron sputtering. Ceram. Int. 2019, 45, 9147–9156. [Google Scholar] [CrossRef]
- Xu, Y.; Li, R. Wet-chemical synthesis and characterization of nitrogen-doped CeO2 powders for oxygen storage capacity. Appl. Surf. Sci. 2018, 455, 997–1004. [Google Scholar] [CrossRef]
- Sharma, R.; Naedele, D.; Schweda, E. In Situ Studies of Nitridation of Zirconia (ZrO2). Chem. Mater. 2001, 13, 4014–4018. [Google Scholar] [CrossRef]
- Xu, L.; Nishimura, T.; Shibayama, S.; Yajima, T.; Migita, S.; Toriumi, A. Ferroelectric phase stabilization of HfO2 by nitrogen doping. Appl. Phys. Express 2016, 9, 091501. [Google Scholar] [CrossRef]
- He, L.; Luan, C.; Feng, X.; Xiao, H.; Ma, J. Effect of niobium doping on the structural, electrical and optical properties of epitaxial SnO2 films on MgF2 (110) substrates by MOCVD. J. Alloys Compd. 2018, 741, 677–681. [Google Scholar] [CrossRef]
- Luan, C.; Zhu, Z. Structural and electrical properties of epitaxial SnO2: Sb films deposited on 6 H-SiC by MOCVD. J. Mater. Sci. Mater. Electron. 2021, 32, 21798–21803. [Google Scholar] [CrossRef]
- Kong, L.; Ma, J.; Zhu, Z.; Luan, C.; Yu, X.; Yu, Q. Synthesis of orthorhombic structure epitaxial tin oxide film. Mater. Lett. 2010, 64, 1350–1353. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Q.; Chang, H.L.M.; Chen, H. Variant structure in metal-organic-chemical-vapor-deposition-derived SnO2 thin films on sapphire (0001). J. Mater. Res. 1995, 10, 1516–1522. [Google Scholar] [CrossRef]
- He, L.; Luan, C.; Cao, Q.; Feng, X.; Zhao, W.; Ma, J. Characterization of Rutile SnO2 Epitaxial Films Grown on MgF2 (001) Substrates by MOCVD. Cryst. Res. Technol. 2018, 53, 1700168. [Google Scholar] [CrossRef]
- Sheng Pan, S.; Fung Yu, S.; Xia Zhang, Y.; Yuan Luo, Y.; Wang, S.; Min Xu, J.; Hai Li, G. Crystallite size-modulated exciton emission in SnO2 nanocrystalline films grown by sputtering. J. Appl. Phys. 2013, 113, 143104. [Google Scholar] [CrossRef]
- Saipriya, S.; Sultan, M.; Singh, R. Effect of environment and heat treatment on the optical properties of RF-sputtered SnO2 thin films. Phys. B Condens. Matter 2011, 406, 812–817. [Google Scholar] [CrossRef]
- Lee, D.-K.; Wan, Z.; Bae, J.-S.; Lee, H.-B.-R.; Ahn, J.-H.; Kim, S.-D.; Kim, J.; Kwon, S.-H. Plasma-enhanced atomic layer deposition of SnO2 thin films using SnCl4 and O2 plasma. Mater. Lett. 2016, 166, 163–166. [Google Scholar] [CrossRef]
- Liu, Y.; Koep, E.; Liu, M. A Highly Sensitive and Fast-Responding SnO2 Sensor Fabricated by Combustion Chemical Vapor Deposition. Chem. Mater. 2005, 17, 3997–4000. [Google Scholar] [CrossRef]
- Ali, S.M.; Hussain, S.T.; Bakar, S.A.; Muhammad, J.; ur Rehman, N. Effect of doping on the Structural and Optical Properties of SnO2 Thin Films fabricated by Aerosol Assisted Chemical Vapor Deposition. J. Phys. Conf. Ser. 2013, 439, 012013. [Google Scholar] [CrossRef]
- Gavaskar, D.S.; Nagaraju, P.; Vijayakumar, Y.; Reddy, P.S.; Ramana Reddy, M.V. Low-cost ultra-sensitive SnO2-based ammonia sensor synthesized by hydrothermal method. J. Asian Ceram. Soc. 2020, 8, 605–614. [Google Scholar] [CrossRef]
- Vuong, D.D.; Hien, V.X.; Trung, K.Q.; Chien, N.D. Synthesis of SnO2 micro-spheres, nano-rods and nano-flowers via simple hydrothermal route. Phys. E Low-Dimens. Syst. Nanostructures 2011, 44, 345–349. [Google Scholar] [CrossRef]
- Guan, M.; Zhao, X.; Duan, L.; Cao, M.; Guo, W.; Liu, J.; Zhang, W. Controlled synthesis of SnO2 nanostructures with different morphologies and the influence on photocatalysis properties. J. Appl. Phys. 2013, 114, 114302. [Google Scholar] [CrossRef]
- Mishra, R.K.; Upadhyay, S.B.; Sahay, P.P. Volatile Organic Compounds (VOCs) Response Characteristics of the Hydrothermally Synthesized SnO2 Nanocapsules. Sens. Lett. 2013, 11, 1611–1616. [Google Scholar] [CrossRef]
- Zhou, X.; Fu, W.; Yang, H.; Ma, D.; Cao, J.; Leng, Y.; Guo, J.; Zhang, Y.; Sui, Y.; Zhao, W.; et al. Synthesis and ethanol-sensing properties of flowerlike SnO2 nanorods bundles by poly(ethylene glycol)-assisted hydrothermal process. Mater. Chem. Phys. 2010, 124, 614–618. [Google Scholar] [CrossRef]
- Xi, G.; Ye, J. Ultrathin SnO2 Nanorods: Template- and Surfactant-Free Solution Phase Synthesis, Growth Mechanism, Optical, Gas-Sensing, and Surface Adsorption Properties. Inorg. Chem. 2010, 49, 2302–2309. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zeng, W.; Zhang, H.; Li, Y. Hydrothermal synthesis of SnO2 nanocubes and nanospheres and their gas sensing properties. J. Mater. Sci. Mater. Electron. 2015, 26, 2871–2878. [Google Scholar] [CrossRef]
- Runa, A.; Bala, H.; Wang, Y.; Chen, J.; Zhang, B.; Li, H.; Fu, W.; Wang, X.; Sun, G.; Cao, J.; et al. Synthesis, characterization, and gas-sensing properties of monodispersed SnO2 nanocubes. Appl. Phys. Lett. 2014, 105, 053102. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, S.; Chen, Y.; Liu, C.; Li, Q. Brief Introduction on Manufacturing and Characterization of Metallic Electrode and Corresponding Modified Materials. Metals 2023, 13, 703. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, P.; Xie, Q.; Zhang, C.; Wang, L.; Peng, D.-L. Intrinsic performance regulation in hierarchically porous Co3O4 microrods towards high-rate lithium ion battery anode. Mater. Today Energy 2020, 16, 100383. [Google Scholar] [CrossRef]
- Noerochim, L.; Indra, M.A.T.; Purwaningsih, H.; Subhan, A. Porous Fe2O3 Microspheres as Anode for Lithium-Ion Batteries. IOP Conf. Ser. Mater. Sci. Eng. 2018, 367, 012038. [Google Scholar] [CrossRef]
- Zhang, L.; Song, J.; Liu, Y.; Yuan, X.; Guo, S. Tailoring nanostructured MnO2 as anodes for lithium ion batteries with high reversible capacity and initial Coulombic efficiency. J. Power Sources 2018, 379, 68–73. [Google Scholar] [CrossRef]
- Shi, S.; Deng, T.; Zhang, M.; Yang, G. Fast facile synthesis of SnO2/Graphene composite assisted by microwave as anode material for lithium-ion batteries. Electrochim. Acta 2017, 246, 1104–1111. [Google Scholar] [CrossRef]
- Kim, A.-R.; Nahm, K.S.; Yoo, D.J. Lithium Insertion Behavior of Nanoscopic Co3O4 Prepared with Avian Egg Membrane as a Template. Bull. Korean Chem. Soc. 2011, 32, 1204–1208. [Google Scholar] [CrossRef]
- Khamsanga, S.; Pornprasertsuk, R.; Yonezawa, T.; Mohamad, A.A.; Kheawhom, S. δ-MnO2 nanoflower/graphite cathode for rechargeable aqueous zinc ion batteries. Sci. Rep. 2019, 9, 8441. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Ojanguren, A.; Niederberger, M.; Lizundia, E. Degradation Behavior, Biocompatibility, Electrochemical Performance, and Circularity Potential of Transient Batteries. Adv. Sci. 2021, 8, 2004814. [Google Scholar] [CrossRef]
- Yin, L.; Chai, S.; Wang, F.; Huang, J.; Li, J.; Liu, C.; Kong, X. Ultrafine SnO2 nanoparticles as a high performance anode material for lithium ion battery. Ceram. Int. 2016, 42, 9433–9437. [Google Scholar] [CrossRef]
- Etacheri, V.; Seisenbaeva, G.A.; Caruthers, J.; Daniel, G.; Nedelec, J.-M.; Kessler, V.G.; Pol, V.G. Ordered Network of Interconnected SnO2 Nanoparticles for Excellent Lithium-Ion Storage. Adv. Energy Mater. 2015, 5, 1401289. [Google Scholar] [CrossRef]
- Huang, B.; Li, X.; Pei, Y.; Li, S.; Cao, X.; Massé, R.C.; Cao, G. Novel Carbon-Encapsulated Porous SnO2 Anode for Lithium-Ion Batteries with Much Improved Cyclic Stability. Small 2016, 12, 1945–1955. [Google Scholar] [CrossRef]
- Li, H.; Su, Q.; Kang, J.; Huang, M.; Feng, M.; Feng, H.; Huang, P.; Du, G. Porous SnO2 hollow microspheres as anodes for high-performance lithium ion battery. Mater. Lett. 2018, 217, 276–280. [Google Scholar] [CrossRef]
- Li, L.; Yin, X.; Liu, S.; Wang, Y.; Chen, L.; Wang, T. Electrospun porous SnO2 nanotubes as high capacity anode materials for lithium ion batteries. Electrochem. Commun. 2010, 12, 1383–1386. [Google Scholar] [CrossRef]
- Tan, L.; Wang, M.S.; Liu, Y.J.; Xiao, X.C.; Fan, L.Z.; Wang, Y.D. Synthesis of SnO2 nanorods and hollow spheres and their electrochemical properties as anode materials for lithium ion batteries. Mater. Technol. 2012, 27, 191–195. [Google Scholar] [CrossRef]
- Narsimulu, D.; Vinoth, S.; Srinadhu, E.S.; Satyanarayana, N. Surfactant-free microwave hydrothermal synthesis of SnO2 nanosheets as an anode material for lithium battery applications. Ceram. Int. 2018, 44, 201–207. [Google Scholar] [CrossRef]
- Kulmala, M.; Kerminen, V.-M. On the formation and growth of atmospheric nanoparticles. Atmos. Res. 2008, 90, 132–150. [Google Scholar] [CrossRef]
- Kulmala, M.; Kerminen, V.-M.; Anttila, T.; Laaksonen, A.; O’Dowd, C.D. Organic aerosol formation via sulphate cluster activation. J. Geophys. Res. Atmos. 2004, 109, D04205. [Google Scholar] [CrossRef]
- Wen, Z.; Zheng, F.; Yu, H.; Jiang, Z.; Liu, K. Hydrothermal synthesis of flowerlike SnO2 nanorod bundles and their application for lithium ion battery. Mater. Charact. 2013, 76, 1–5. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Huang, X.; Ding, R.; Hu, Y.; Jiang, J.; Liao, L. Direct growth of SnO2 nanorod array electrodes for lithium-ion batteries. J. Mater. Chem. 2009, 19, 1859–1864. [Google Scholar] [CrossRef]
- Sharma, A.; Khosla, A.; Arya, S. Synthesis of SnO2 nanowires as a reusable and flexible electrode for electrochemical detection of riboflavin. Microchem. J. 2020, 156, 104858. [Google Scholar] [CrossRef]
- Ding, S.; Chen, J.S.; Qi, G.; Duan, X.; Wang, Z.; Giannelis, E.P.; Archer, L.A.; Lou, X.W. Formation of SnO2 Hollow Nanospheres inside Mesoporous Silica Nanoreactors. J. Am. Chem. Soc. 2011, 133, 21–23. [Google Scholar] [CrossRef]
- Wu, H.B.; Chen, J.S.; Lou, X.W.; Hng, H.H. Synthesis of SnO2 Hierarchical Structures Assembled from Nanosheets and Their Lithium Storage Properties. J. Phys. Chem. C 2011, 115, 24605–24610. [Google Scholar] [CrossRef]
- Park, M.-S.; Wang, G.-X.; Kang, Y.-M.; Wexler, D.; Dou, S.-X.; Liu, H.-K. Preparation and Electrochemical Properties of SnO2 Nanowires for Application in Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2007, 46, 750–753. [Google Scholar] [CrossRef]
- Chen, J.S.; Lou, X.W. SnO2 and TiO2 nanosheets for lithium-ion batteries. Mater. Today 2012, 15, 246–254. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, S.; Dong, H.; Chen, X.; Gao, S.; Sun, Y.; Li, W.; Xu, J.; Chen, L.; Yuan, A.; et al. Nano-SnO2/Carbon Nanotube Hairball Composite as a High-Capacity Anode Material for Lithium Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 4195–4203. [Google Scholar] [CrossRef]
- Deng, Y.; Fang, C.; Chen, G. The developments of SnO2/graphene nanocomposites as anode materials for high performance lithium ion batteries: A review. J. Power Sources 2016, 304, 81–101. [Google Scholar] [CrossRef]
- Mueller, F.; Bresser, D.; Chakravadhanula, V.S.K.; Passerini, S. Fe-doped SnO2 nanoparticles as new high capacity anode material for secondary lithium-ion batteries. J. Power Sources 2015, 299, 398–402. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Ulissi, U.; Ji, Y.; Streb, C.; Bresser, D.; Passerini, S. Influence of the doping ratio and the carbon coating content on the electrochemical performance of Co-doped SnO2 for lithium-ion anodes. Electrochim. Acta 2018, 277, 100–109. [Google Scholar] [CrossRef]
- Ou, P.; Cao, Z.; Rong, J. Multi-scale Design and Synthesis Strategy of Ni-doped SnO2 Hollow Spheres as Anode Material for Lithium-ion Batteries. Int. J. Electrochem. Sci. 2022, 17, 2. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, C.; Liu, J.; Tay, Y.Y.; Jiang, J.; Jia, X.; Zhang, J.; Gong, H.; Hng, H.H.; Yu, T.; et al. Epitaxial Growth of Branched α-Fe2O3/SnO2 Nano-Heterostructures with Improved Lithium-Ion Battery Performance. Adv. Funct. Mater. 2011, 21, 2439–2445. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Z.X.; Shi, Y.; Wong, J.I.; Ding, M.; Yang, H.Y. Designed hybrid nanostructure with catalytic effect: Beyond the theoretical capacity of SnO2 anode material for lithium ion batteries. Sci. Rep. 2015, 5, 9164. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).