Development and Characterization of Thermoformed Bilayer Trays of Paper and Renewable Succinic Acid Derived Biopolyester Blends and Their Application to Preserve Fresh Pasta
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
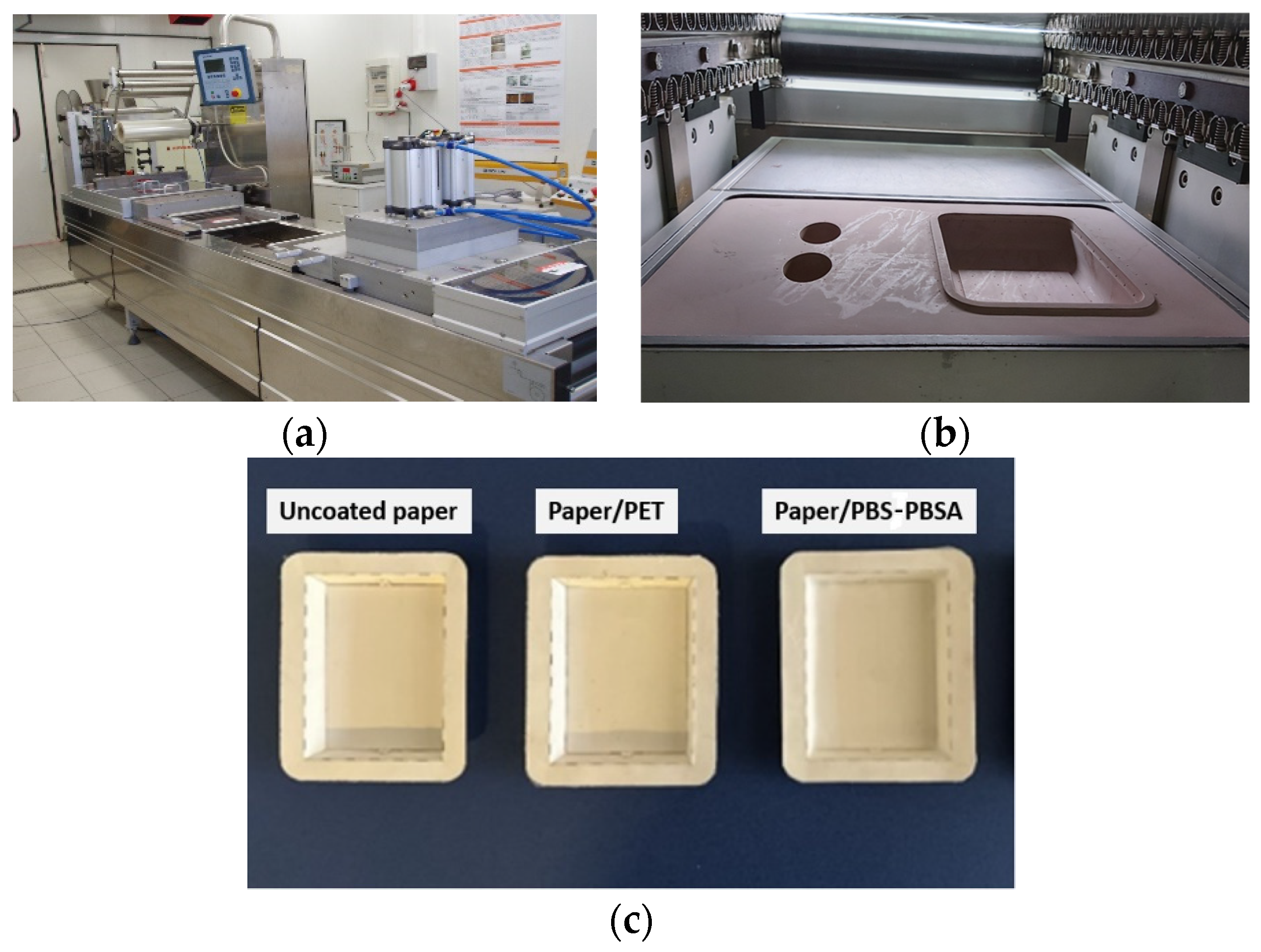
2.2. Development of Paper-Based Trays
2.3. Characterization of Paper-Based Trays
2.3.1. Film Thickness and Conditioning
2.3.2. Optical Evaluation
2.3.3. Microstructural Analysis
2.3.4. Thermal Characterization
2.3.5. Mechanical Analysis
2.3.6. Permeance Measurements
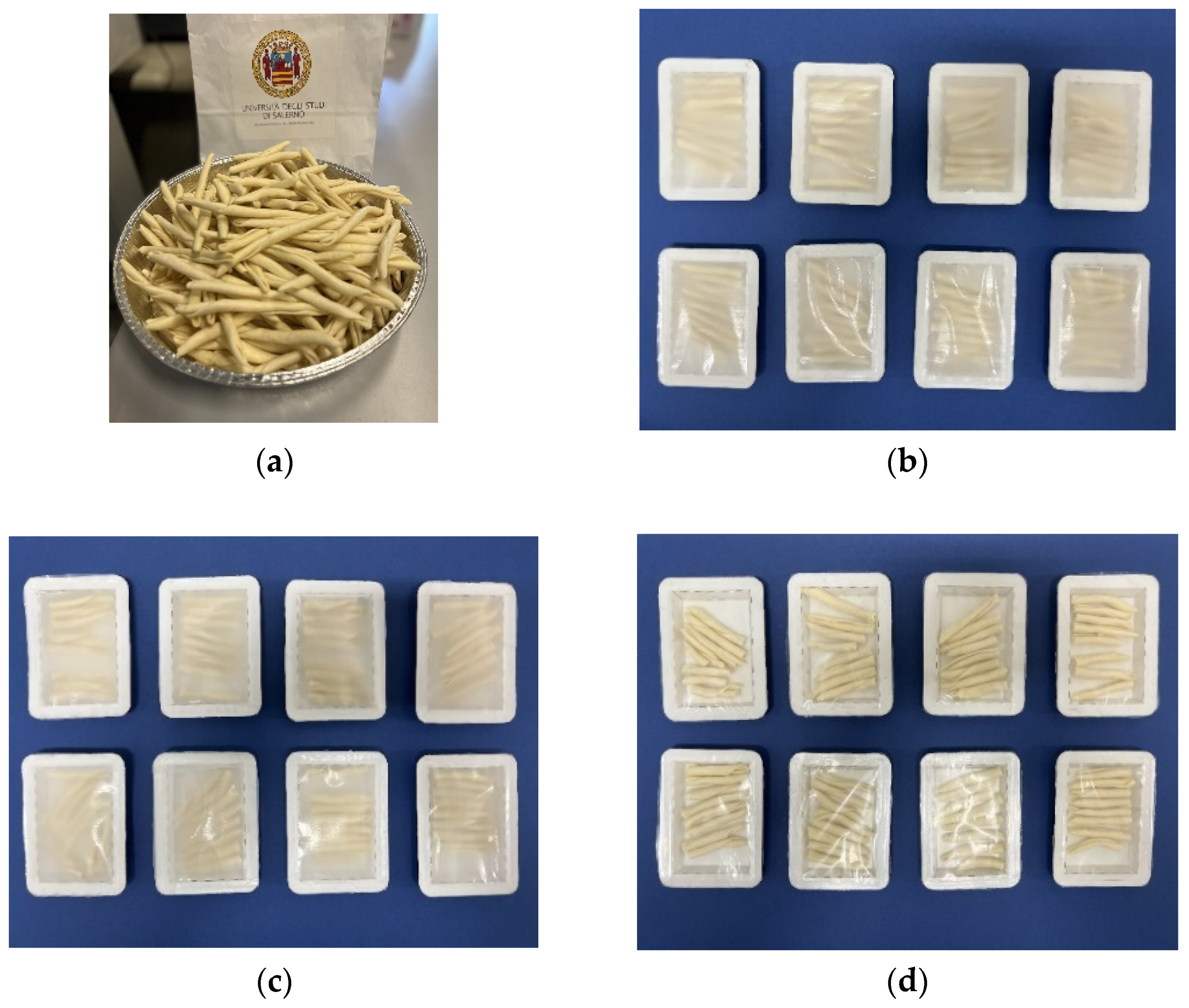
2.4. Shelf-Life Evaluation of Fresh Pasta
2.4.1. Packaging and Storage
2.4.2. Evaluation of Shelf-Life
2.5. Overall Migration Tests
2.6. Statistical Analysis
3. Results
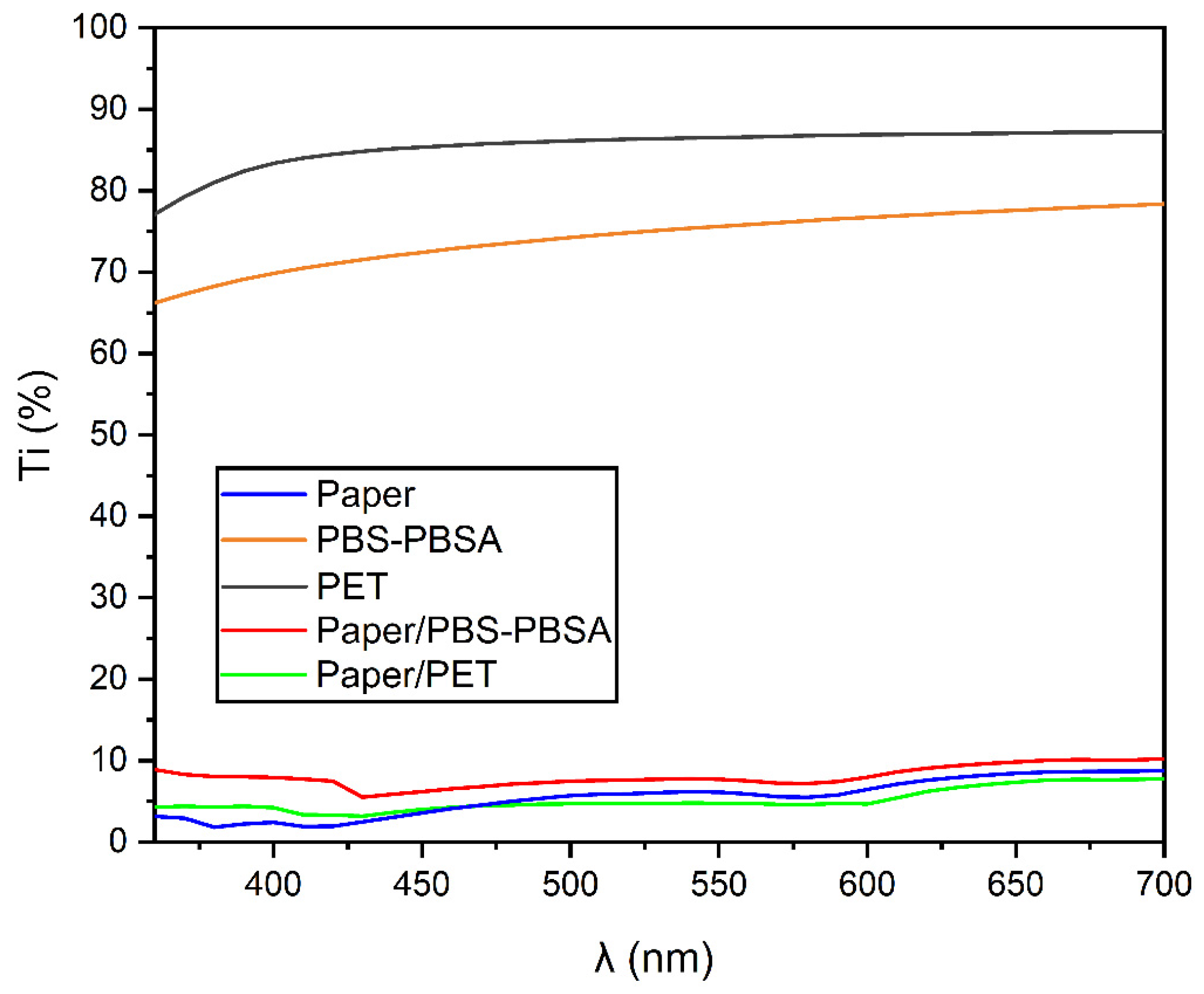
3.1. Optical Properties
3.2. Thermal Properties
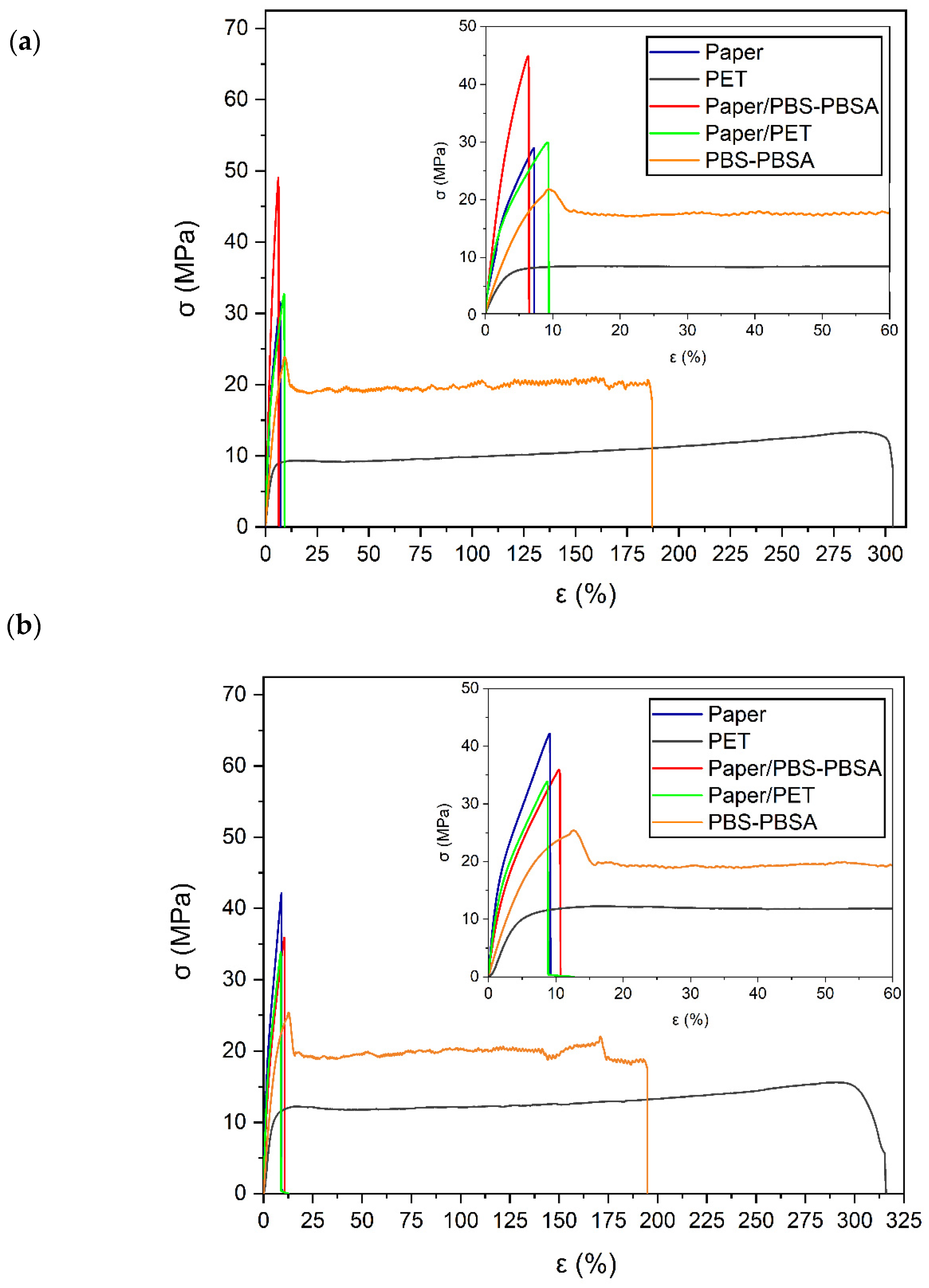
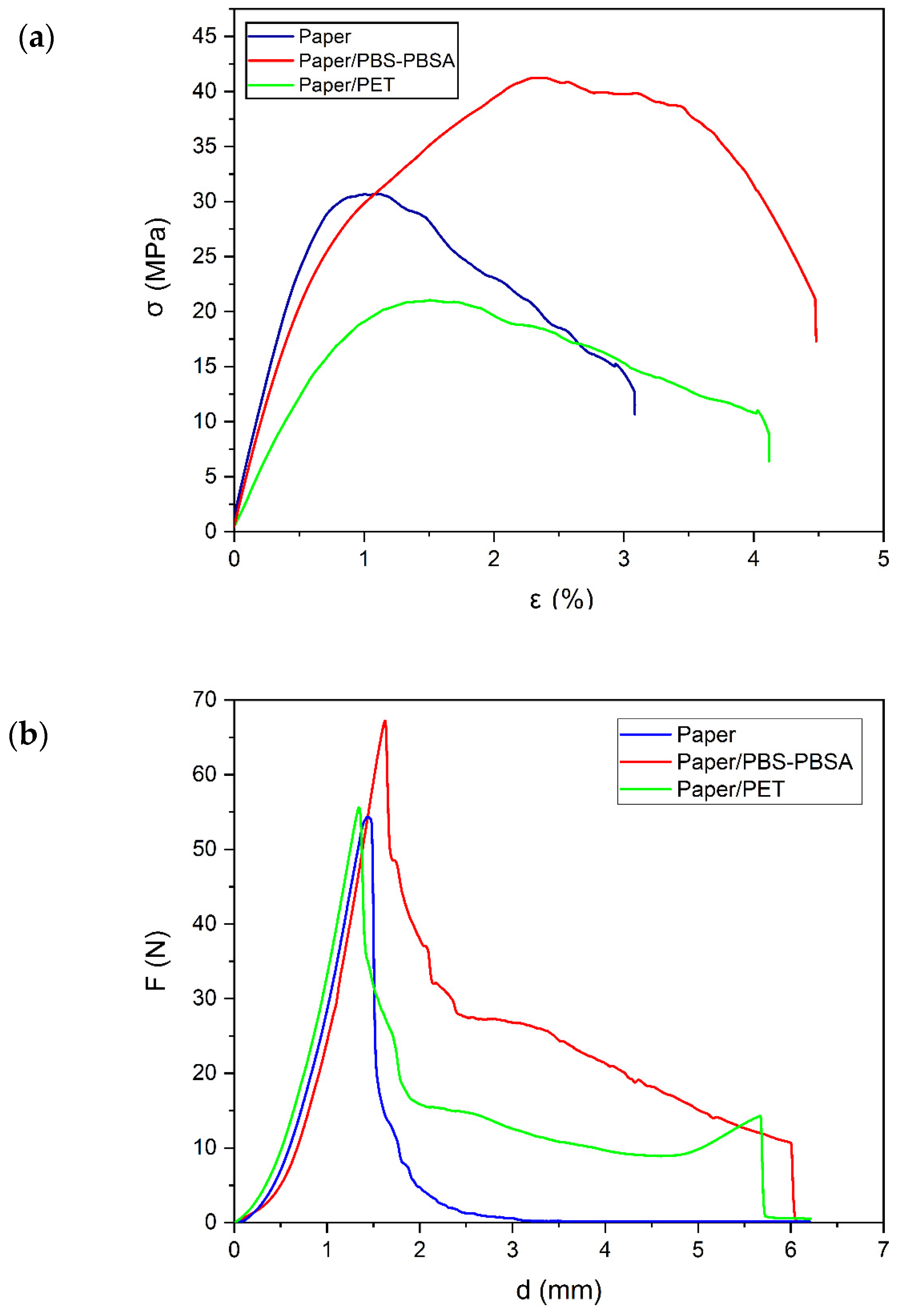
3.3. Mechanical Properties
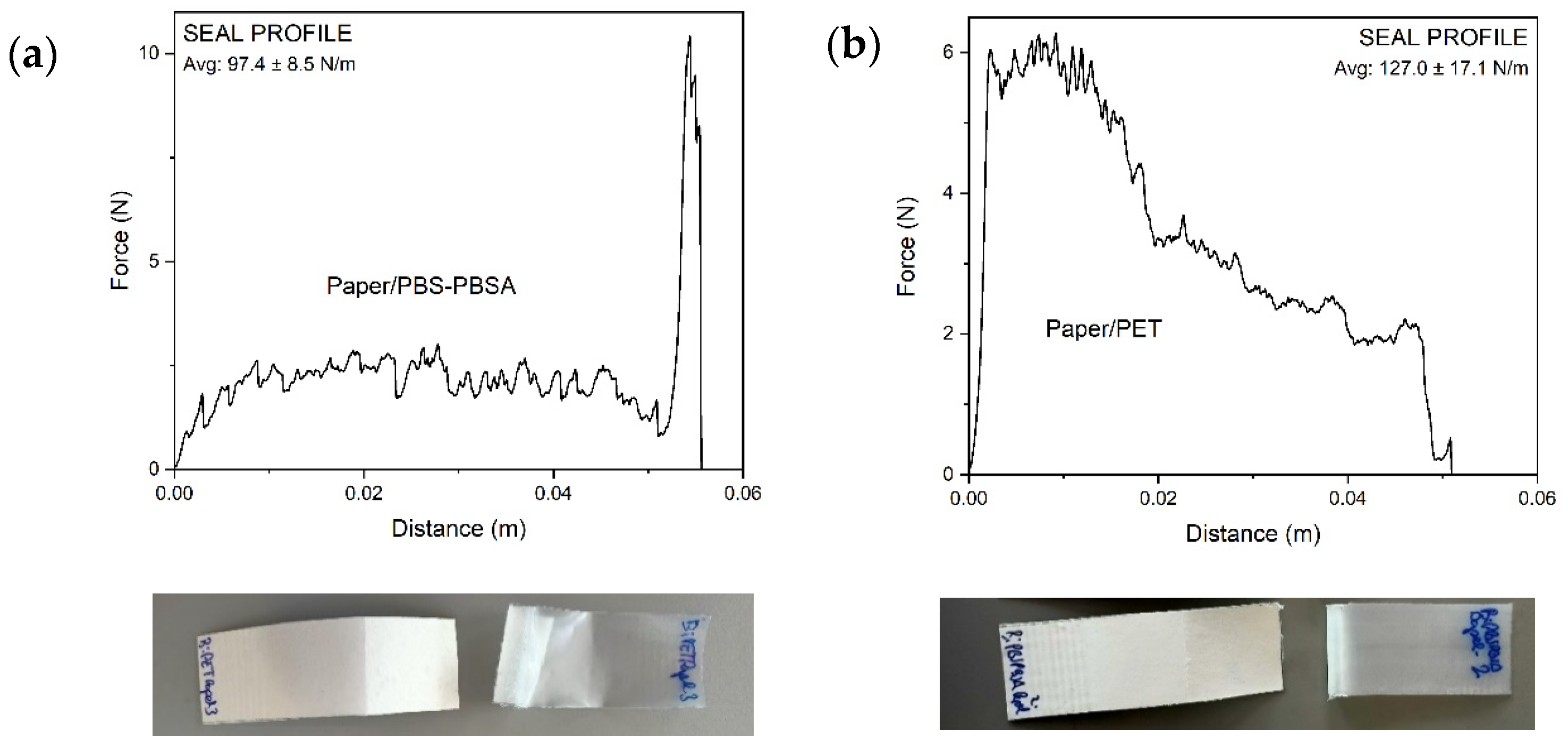
3.4. Barrier Properties
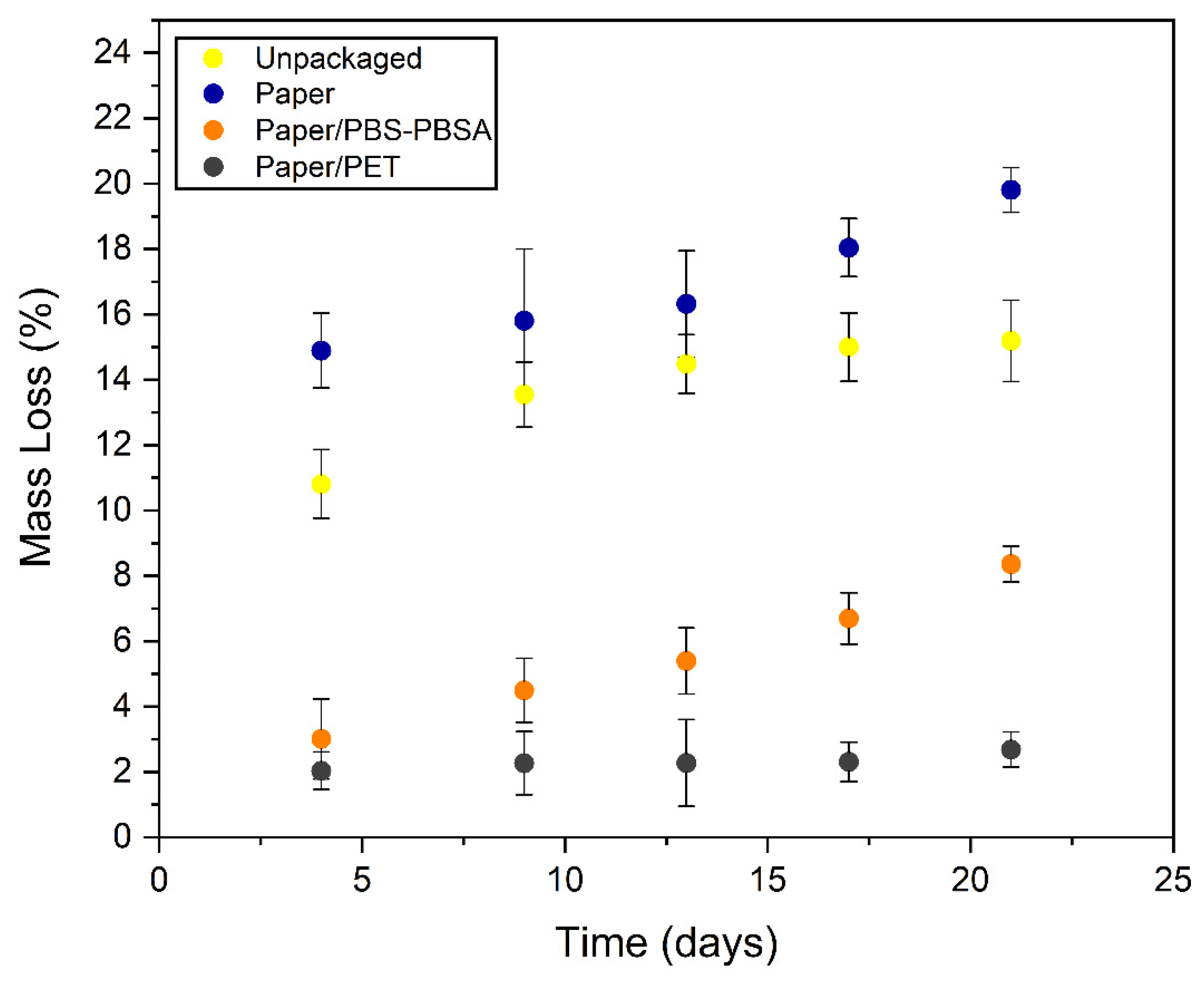
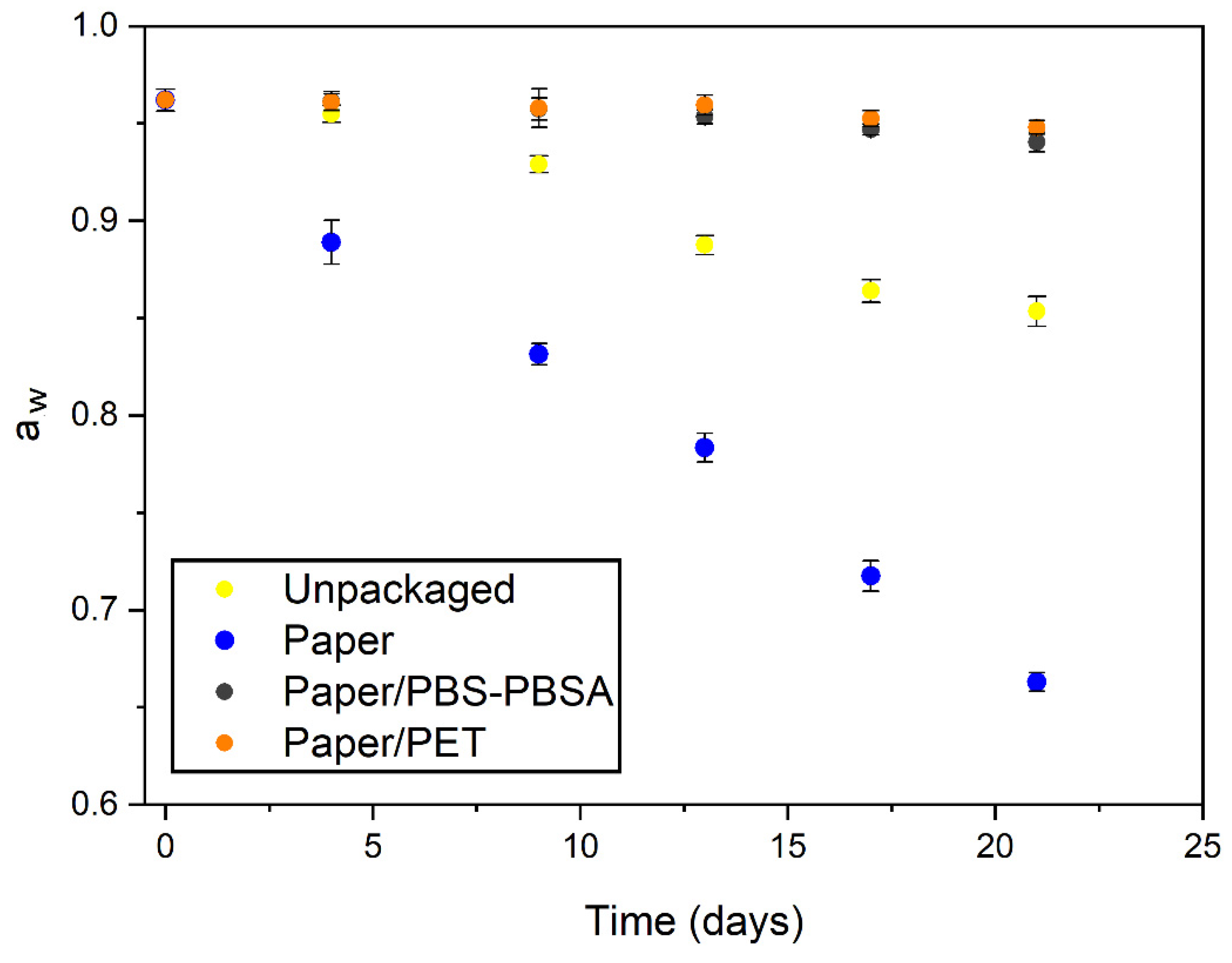
3.5. Preservation of Fresh Pasta
3.6. Overall Migration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bugatti, V.; Viscusi, G.; Gorrasi, G. Formulation of a Bio-Packaging Based on Pure Cellulose Coupled with Cellulose Acetate Treated with Active Coating: Evaluation of Shelf Life of Pasta Ready to Eat. Foods 2020, 9, 1414. [Google Scholar] [CrossRef] [PubMed]
- Lagarón, J.M.; Lopez-Rubio, A. Nanotechnology for bioplastics: Opportunities, challenges and strategies. Trends Food Sci. Technol. 2011, 22, 611–617. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; González-Martínez, C.; Chiralt, A. Biodegradable Antimicrobial Films for Food Packaging: Effect of Antimicrobials on Degradation. Foods 2021, 10, 1256. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Panjagari, N.R.; Alam, T. An overview of paper and paper based food packaging materials: Health safety and environmental concerns. J. Food Sci. Technol. 2019, 56, 4391–4403. [Google Scholar] [CrossRef]
- Muthu, S.S. (Ed.) Environmental Footprints of Packaging; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Bajpai, P. Environmental Aspects of Recycling. In Recycling and Deinking of Recovered Paper; Elsevier: Amsterdam, The Netherlands, 2014; pp. 271–282. [Google Scholar] [CrossRef]
- Merrild, H.; Damgaard, A.; Christensen, T.H. Recycling of paper: Accounting of greenhouse gases and global warming contributions. Waste Manag. Res. 2009, 27, 746–753. [Google Scholar] [CrossRef]
- Tiggelman, I.; Pasch, H.; Hartmann, P.C. Rapid Comparison of Mineral Oils Vapor Transmission Rate through Paper and Board PACKAGING Materials; Stellenbosch University: Stellenbosch, South Africa, 2012; Volume 11, pp. 41–47. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, Y. Fabrication of thermally and mechanically stable superhydrophobic coatings for cellulose-based substrates with natural and edible ingredients for food applications. Food Hydrocoll. 2021, 120, 106877. [Google Scholar] [CrossRef]
- Khwaldia, K.; Arab-Tehrany, E.; Desobry, S. Biopolymer coatings on paper packaging materials. Compr. Rev. Food Sci. Food Saf. 2010, 9, 82–91. [Google Scholar] [CrossRef]
- Kansal, D.; Hamdani, S.S.; Ping, R.; Rabnawaz, M. Starch and zein biopolymers as a sustainable replacement for PFAS, silicone oil and plastic-coated paper. Ind. Eng. Chem. Res. 2020, 59, 12075–12084. [Google Scholar] [CrossRef]
- Torres-Giner, S. Sustainable Polymer Technologies for a Circular Economy. Appl. Sci. 2023, 13, 5864. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Chiralt, A.; González-Martínez, C. Biodegradation of PLA-PHBV Blend Films as Affected by the Incorporation of Different Phenolic Acids. Foods 2022, 11, 243. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Figueroa-Lopez, K.J.; Melendez-Rodriguez, B.; Prieto, C.; Pardo-Figuerez, M.; Lagaron, J.M. Emerging Trends in Biopolymers for Food Packaging. In Sustainable Food Packaging Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 1–33. [Google Scholar] [CrossRef]
- Hernández-García, E.; Freitas, P.A.V.; Zomeño, P.; González-Martínez, C.; Torres-Giner, S. Multilayer Sheets Based on Double Coatings of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) on Paper Substrate for Sustainable Food Packaging Applications. Appl. Sci. 2023, 13, 179. [Google Scholar] [CrossRef]
- Kasmi, N.; Majdoub, M.; Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis and crystallization of new fully renewable resources-based copolyesters: Poly(1,4-cyclohexanedimethanol-co-isosorbide 2,5-furandicarboxylate). Polym. Degrad. Stab. 2018, 152, 177–190. [Google Scholar] [CrossRef]
- Borodina, I.; Nielsen, J. Advances in metabolic engineering of yeast Saccharomyces cerevisiae for production of chemicals. Biotechnol. J. 2014, 9, 609–620. [Google Scholar] [CrossRef]
- Hwang, S.; Yoo, E.; Im, S. The synthesis of copolymers, blends and composites based on poly(butylene succinate). Polym. J. 2012, 44, 1179–1190. [Google Scholar] [CrossRef]
- De Matos Costa, A.R.; Crocitti, A.; Hecker de Carvalho, L.; Carroccio, S.C.; Cerruti, P.; Santagata, G. Properties of Biodegradable Films Based on Poly(butylene Succinate) (PBS) and Poly(butylene Adipate-co-Terephthalate) (PBAT) Blends. Polymers 2020, 12, 2317. [Google Scholar] [CrossRef] [PubMed]
- Feijoo, P.; Samaniego-Aguilar, K.; Sánchez-Safont, E.; Torres-Giner, S.; Lagaron, J.M.; Gamez-Perez, J.; Cabedo, L. Development and Characterization of Fully Renewable and Biodegradable Polyhydroxyalkanoate Blends with Improved Thermoformability. Polymers 2022, 14, 2527. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union. 2011, 12, 1–89. [Google Scholar]
- UNE-EN 13432; Packaging-Requirements for Packaging Recoverable through Composting and Biodegradation-Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. European Standard: Brussels, Belgium, 2015.
- UNE-EN 17427; Packaging—Requirements and Test Scheme for Carrier Bags Suitable for Treatment in Well-Managed Home Composting Installations. European Standard: Brussels, Belgium, 2022.
- Quiles-Carrillo, L.; Montanes, N.; Lagarón, J.M.; Balart, R.; Torres-Giner, S. In Situ Compatibilization of Biopolymer Ternary Blends by Reactive Extrusion with Low-Functionality Epoxy-Based Styrene–Acrylic Oligomer. J. Polym. Environ. 2019, 27, 84–96. [Google Scholar] [CrossRef]
- Hernández-García, E.; Pacheco-Romeralo, M.; Pascual-Ramírez, L.; Vargas, M.; Torres-Giner, S. Synthesis of Polyamide 1010 and Evaluation of its Cast-Extruded Films for Meat Preservation. Food Packag. Shelf Life 2023, 36, 101058. [Google Scholar] [CrossRef]
- Mikhail, S.S.; Azer, S.S.; Johnston, W.M. Accuracy of Kubelka-Munk reflectance theory for dental resin composite material. Dent. Mater. 2012, 28, 729–735. [Google Scholar] [CrossRef]
- Schabbach, L.M.; Dos Santos, B.C.; De Bortoli, L.S.; Fredel, M.C.; Henriques, B. Application of Kubelka-Munk model on the optical characterization of translucent dental zirconia. Mater. Chem. Phys. 2021, 258, 123994. [Google Scholar] [CrossRef]
- ASTM D882-12; Standard Test Method for Tensile Properties of Thin Plastic Sheeting; American Society for Testing and Materials: Philadelphia, PA, USA, 2012.
- UNE-EN ISO 178; Plastics—Determination of Flexural Properties (ISO 178:2019). ISO: Geneva, Switzerland, 2020.
- Hernández-García, E.; Vargas, M.; Chiralt, A. Active Starch-Polyester Bilayer Films with Surface-Incorporated Ferulic Acid. Membranes 2022, 12, 976. [Google Scholar] [CrossRef] [PubMed]
- F88/F88M-15; Standard Test Methods for Seal Strength of Flexible Barrier Materials. ASTM, Annual Book of ASTM Standards. American Society for Testing and Materials: Philadelphia, PA, USA, 2015.
- ASTM F1306; Standard Test Method for Slow Rate Penetration Resistance of Flexible Barrier Films and Laminates. American Society for Testing and Materials: Philadelphia, PA, USA, 2020.
- ASTM E96–96M-16; Standard Test Methods for Water Vapor Transmission of Materials. American Society for Testing and Materials: Philadelphia, PA, USA, 2021.
- Hernández-García, E.; Vargas, M.; Torres-Giner, S. Quality and Shelf-Life Stability of Pork Meat Fillets Packaged in Multilayer Polylactide Films. Foods 2022, 11, 426. [Google Scholar] [CrossRef] [PubMed]
- ASTM D3985-05; Standard Test Method for Oxygen Gas Transmission Rate through Plastic Film and Sheeting Using a Coulometric Sensor. American Society for Testing and Materials: Philadelphia, PA, USA, 2010; pp. 1–7.
- UNE-EN 1186-5; Materials and Articles in Contact with Foodstuffs. Plastics. Part 5: Test Methods for Overall Migration into Aqueous Food Simulants by Cell. Asociación Española de Normalización: Madrid, Spain, 2002.
- UNE-EN 14338; Paper and Board Intended to Come in Contact with Foodstuffs. Conditions for Determination of Migration from Paper and Board Using Modified Polyphenylene Oxide (MPPO) as a Simulant. Asociación Española de Normalización: Madrid, Spain, 2004.
- Zhu, H.; Fang, Z.; Preston, C.; Li, Y.; Hu, L. Transparent paper: Fabrications, properties, and device applications. Energy Environ. Sci. 2014, 7, 269–287. [Google Scholar] [CrossRef]
- Wazir, H.; Chay, S.Y.; Ibadullah, W.Z.W.; Zarei, M.; Mustapha, N.A.; Saari, N. Lipid oxidation and protein co-oxidation in ready-to-eat meat products as affected by temperature, antioxidant, and packaging material during 6 months of storage. RSC Adv. 2021, 11, 38565–38577. [Google Scholar] [CrossRef]
- Zhu, Q.; Tan, J.; Li, D.; Zhang, T.; Liu, Z.; Cao, Y. Cross-linked chitosan/tannin extract as a biodegradable and repulpable coating for paper with excellent oil-resistance, gas barrier and UV-shielding. Prog. Org. Coat. 2023, 176, 107399. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Fenollar, O.; Balart, R.; Torres-Giner, S.; Rallini, M.; Dominici, F.; Torre, L. A comparative study on the reactive compatibilization of melt-processed polyamide 1010/polylactide blends by multi-functionalized additives derived from linseed oil and petroleum. Express Polym. Lett. 2020, 14, 583–604. [Google Scholar] [CrossRef]
- Mokrzycki, W.S.; Tatol, M. Colour difference ∆E—A survey. Mach. Graph. Vis. Int. J. 2011, 20, 383–411. [Google Scholar]
- Maruhashi, Y.; Lida, S. Transparency of polymer blends. Polym. Eng. Sci. 2004, 41, 1987–1995. [Google Scholar] [CrossRef]
- Seoane, I.T.; Luzi, F.; Puglia, D.; Cyras, V.P.; Manfredi, L.B. Enhancement of paperboard performance as packaging material by layering with plasticized polyhydroxybutyrate/nanocellulose coatings. J. Appl. Sci. 2018, 135, 46872. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Torres-Giner, S.; Aldureid, A.; Cabedo, L.; Lagaron, J.M. Reactive Melt Mixing of Poly(3-Hydroxybutyrate)/Rice Husk Flour Composites with Purified Biosustainably Produced Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate). Materials 2019, 12, 2152. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Ndiaye, M.; Myler, P.; Kandola, B.K. Thermoplastic composites: Modelling melting, decomposition and combustion of matrix polymers. J. Compos. Sci. 2022, 6, 27. [Google Scholar] [CrossRef]
- Rhim, J.W.; Lee, J.H.; Hong, S.I. Increase in water resistance of paperboard by coating with poly(lactide). Packag. Technol. Sci. 2007, 20, 393–402. [Google Scholar] [CrossRef]
- Bordenave, N.; Grelier, J.; Pichavant, F.; Coma, V. Water and moisture susceptibility of chitosan and paper-based materials: Structure property reationships. Agric. Food Chem. 2007, 55, 9479–9488. [Google Scholar] [CrossRef] [PubMed]
- Kjellgren, H.; Gällstedt, M.; Engström, G.; Järnström, L. Barrier and surface properties of chitosan-coated greaseproof paper. Carbohydr. Polym. 2006, 65, 453–460. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W. Effects of poly(butylene adipate-co-terephtalate) coating on the water resistant, mechanical, and antibacterial properties of Kraft paper. Prog. Org. Coat. 2018, 123, 153–159. [Google Scholar] [CrossRef]
- Reis, A.B.; Yoshida, C.M.P.; Reis, A.P.C.; Franco, T.T. Application of chitosan emulsion as a coating on Kraft paper. Polym. Int. 2011, 60, 963–969. [Google Scholar] [CrossRef]
- Liu, L.; Yu, J.; Cheng, L.; Qu, W. Mechanical properties of poly(butylene succinate) (PBS) biocomposites reinforced with surface modified jute fibre. Compos. Part. A Appl. Sci. Manuf. 2009, 40, 669–674. [Google Scholar] [CrossRef]
- Ahmad Thirmizir, M.Z.; Mohd Ishak, Z.A.; Mat Taib, R.; Rahim, S. Effect of Maleated Compatibiliser (PBS-g-MA) Addition on the Flexural Properties and Water Absorption of Poly(butylene succinate)/Kenaf Bast Fibre Composites. Sains Malays. 2013, 42, 435–441. [Google Scholar]
- Harder, N.; Rodriguez-Uribe, A.; Snowdon, M.R.; Misra, M.; Mohanty, A.K. Hop natural reinforced poly(butylene succinate-co-butylene adipate) (PBSA) biodegradable plastic: Effect of fiber length on performance of biocomposites. Adv. Mater. 2023, 4, 1502–1514. [Google Scholar] [CrossRef]
- Qi, Z.; Ye, H.; Xu, J.; Chen, J.; Guo, B. Improved the thermal and mechanical properties of poly(butylene succinate-co-butylene adipate) by forming nanocomposites with attapulgite. Colloids Surf. A Physicochem. Eng. Asp. 2013, 421, 109–117. [Google Scholar] [CrossRef]
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.S.; Tawakkal, I.A.; Zaman, K.; Asim, M.; Nurrazi, M.N.; Lee, C.H. A Review on Properties and Application of Bio-Based Poly(Butylene Succinate). Polymers 2021, 13, 1436. [Google Scholar] [CrossRef] [PubMed]
- Bamps, B.; Guimaraes, R.M.M.; Duijsters, G.; Hermans, D.; Vanminsel, J.; Vervoort, E.; Buntinx, M.; Peeters, R. Characterizing Mechanical, Heat Seal, and Gas Barrier Performance of Biodegradable Films to Determine Food Packaging Applications. Polymers 2022, 14, 2569. [Google Scholar] [CrossRef]
- Shahmardani, M.; Mohanmmadi, R. The role of interfacial adhesion on the mechanical behavior of thin metal/polymer laminate with surface roughness. Polymers 2022, 14, 3131. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Gil, L.; Pascual-Ramírez, L.; Garde-Belza, J.A. Packaging: Food waste reduction. In Encyclopedia of Polymer Applications; Mishra, M., Ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Lagarón, J.M. 1—Multifunctional and nanoreinforced polymers for food packaging. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Lagarón, J.-M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 1–28. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, H.; Qian, L. Enhanced water vapour barrier and grease resistance of paper bilayer-coated with chitosan and beeswax. Carbohydr. Polym. 2014, 101, 401–406. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Torres-Giner, S.; Zavagna, L.; Sammon, C.; Cabedo, L.; Prieto, C.; Lagaron, J.M. Development and Characterization of Electrospun Fiber-Based Poly(ethylene-co-vinyl Alcohol) Films of Application Interest as High-Gas-Barrier Interlayers in Food Packaging. Polymers 2021, 13, 2061. [Google Scholar] [CrossRef]
- Costa, C.; Lucera, A.; Mastromatteo, M.; Conte, A.; Del Nobile, M.A. Shelf life extension of durum semolina-based fresh pasta: Shelf life extension of durum semolina-based fresh pasta. Int. J. Food Sci. Technol. 2010, 45, 1545–1551. [Google Scholar] [CrossRef]
- Angiolillo, L.; Conte, A.; del Nobile, M.A. Biotechnological Approach to preserve fresh pasta quality. J. Food Prot. 2017, 80, 2006–2013. [Google Scholar] [CrossRef]
- Cruz, R.S.; Ferreira Soares, N.F.; Andrade, N.J. Evaluation of oxygen absorber on antimicrobial preservation of lasagna-type fresh pasta under vacuum packed. Sci. Agrotec. Lavras 2006, 30, 1135–1138. [Google Scholar] [CrossRef]
- Marzano, M.; Calasso, M.; Caponio, G.R.; Celano, G.; Fosso, B.; De Palma, D.; Vacca, M.; Notario, E.; Pesole, G.; De Leo, F.; et al. Extension of the shelf-life of fresh pasta using modified atmosphere packaging and bioprotective cultures. Front. Microbiol. 2022, 13, 1003437. [Google Scholar] [CrossRef] [PubMed]
- Lomonaco, S.; Grassi, M.A.; Vallone, L.; Pistone, V.; Civiera, T. Presence of fungal contamination in the production chain of fresh filled pasta with particular regard to the penicillium genus. Ital. J. Food Saf. 2011, 2, 33–37. [Google Scholar] [CrossRef]
- Zardetto, S.; Fregonese, M.; Pasini, G. Effects of modified atmospheric packaging configuration on spoilage mould growth in damaged packages of fresh pasta. J. Food Eng. 2022, 314, 110760. [Google Scholar] [CrossRef]
- Carini, E.; Vittadini, E.; Curti, E.; Antoniazzi, F. Effects of different shaping modes on physico-chemical properties and water status of fresh pasta. J. Food Eng. 2009, 93, 400–406. [Google Scholar] [CrossRef]
- Sousa, G.M.; Yamashita, F.; Soares Júnior, M.S. Application of biodegradable films made from rice flour, poly(butylene adipate-co-terphthalate), glycerol and potassium sorbate in the preservation of fresh food pastas. LWT 2016, 65, 39–45. [Google Scholar] [CrossRef]
- Zardetto, S.; Dalla Rosa, M. Study of the effect of lamination process on pasta by physical chemical determination and near infrared spectroscopy analysis. J. Food Eng. 2006, 74, 402–409. [Google Scholar] [CrossRef]
- Mathlouthi, M. Water content, water activity, water structure and the stability of foodstuffs. Food Control. 2001, 12, 409–417. [Google Scholar] [CrossRef]
- Li, M.; Zhu, K.; Guo, X.; Peng, W.; Zhou, H. Effect of water activity (aw) and irradiation on the shelf-life of fresh noodles. Innov. Food Sci. Emerg. Technol. 2011, 12, 526–530. [Google Scholar] [CrossRef]
- Roberts, T.A.; Cordier, J.-L.; Gram, L.; Tompkin, R.B.; Pitt, J.I.; Gorris, L.G.M.; Swanson, K.M.J. (Eds.) International Commission on Microbiological Specifications for Foods. In Microbiology of Foods 6: Microbial Ecology of Food Commodities, 2nd ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2005. [Google Scholar]
- DPR, 2001. Decreto del Presidente della Repubblica 9 Febbraio 2001, n.187. Regolamento per la Revisione Della Normativa Sulla Produzione e Commercializzazione di Sfarinati e Paste Alimentari, a Norma dell’articolo 50 Della Legge 22 Febbraio 1994, n. 146. Available online: https://www.politicheagricole.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/2772 (accessed on 10 February 2023).
- Tabanelli, G.; Barbieri, F.; Campedelli, I.; Venturini, M.C.; Gardini, F.; Montanari, C. Effects of bioprotective cultures on the microbial community during storage of Italian fresh filled pasta. Food Control 2020, 115, 107304. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, J.; Liu, C.; Zheng, X.; Bian, K.; Guan, E. Quality changes in fresh noodles prepared by different heat treatments during storage. J. Food Process. Preserv. 2021, 45, e15506. [Google Scholar] [CrossRef]
- Sanguinetti, A.M.; Del Caro, A.; Scanu, A.; Fadda, C.; Milella, G.; Catzeddu, P.; Piga, A. Extending the shelf life of gluten free fresh filled pasta by modified armosphere packaging. LWT 2016, 71, 96–101. [Google Scholar] [CrossRef]
- Hernández-García, E.; Chiralt, A.; Vargas, M.; Torres-Giner, S. Lid Films of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Microfibrillated Cellulose Composites for Fatty Food Preservation. Foods 2023, 12, 375. [Google Scholar] [CrossRef] [PubMed]



| Sample | L* | a* | b* | Cab* | hab* | ΔEab* |
|---|---|---|---|---|---|---|
| Paper | 94.77 ± 0.01 a | 1.56 ± 0.02 a | −5.83 ± 0.06 a | 6.03 ± 0.07 a | 284.94 ± 0.04 a | - |
| PBS–PBSA | 89.41 ± 0.88 b | −0.44 ± 0.04 b | 1.44 ± 0.48 b | 1.52 ± 0.44 b | 108.44 ± 8.17 b | - |
| PET | 88.03 ± 0.36 c | −1.54 ± 0.06 c | 4.25 ± 0.11 c | 4.52 ± 0.12 c | 108.89 ± 0.45 b | - |
| Paper/PBS–PBSA | 94.45 ± 0.15 d | 0.22 ± 0.05 d | −0.59 ± 0.06 d | 0.63 ± 0.07 d | 290.43 ± 2.07 c | 5.42 ± 0.10 a |
| Paper/PET | 95.13 ± 0.21 e | 0.84 ± 0.01 e | −3.48 ± 0.05 e | 3.58 ± 0.05 e | 283.57 ± 1.81 a | 2.48 ± 0.09 b |
| Sample | Tonset (°C) | Tdeg (°C) | Remaining Mass (%) | ||
|---|---|---|---|---|---|
| Tdeg 1 | Tdeg 2 | Tdeg 3 | |||
| Paper | 279.6 ± 5.3 a | 335.1 ± 0.1 a | 472.5 ± 4.7 a | - | 16.3 ± 0.12 a |
| PBS–PBSA | 297.5 ± 7.8 b | 392.5 ± 0.5 b | - | - | 0.3 ± 0.4 b |
| PET | 298.0 ± 2.8 b | 353.5 ± 0.7 c | 434.3 ± 4.1 b | 544.0 ± 1.4 a | 0.5 ± 0.5 b |
| Paper/PBS–PBSA | 284.5 ± 3.5 a | 381.5 ± 3.1 d | 479.2 ± 1.9 c | - | 6.0 ± 1.6 c |
| Paper/PET | 287.5 ± 3.5 ab | 336.2 ± 0.5 e | 429.0 ± 4.2 b | 491.5 ± 14.8 b | 6.5 ± 1.2 c |
| Sample | Initial Day | 21 Days | ||||
|---|---|---|---|---|---|---|
| Etensile (MPa) | σy (MPa) | εb (%) | E (MPa) | σy (MPa) | εb (%) | |
| Paper | 1787 ± 41 a,1 | 31.9 ± 1.0 a,1 | 6.9 ± 0.9 a,1 | 1124 ± 78 a,2 | 41.7 ± 3.3 a,2 | 9.2 ± 0.0 a,2 |
| PBS–PBSA | 367 ± 15 b,1 | 21.6 ± 1.1 b,1 | 158.4 ± 26.5 b,1 | 359 ± 14 b,1 | 23.2 ± 1.3 b,1 | 156.3 ± 10.9 b,1 |
| PET | 263 ± 31 c,1 | 8.8 ± 0.2 c,1 | 303.9 ± 82.4 c,1 | 267 ± 21 c,1 | 12.9 ± 0.6 c,2 | 331.2 ± 28.5 c,1 |
| Paper/PBS–PBSA | 1081 ± 31 d,1 | 49.6 ± 2.2 d,1 | 6.6 ± 0.5 a,1 | 859 ± 34 d,2 | 36.5 ± 0.9 d,2 | 11.2 ± 0.7 d,2 |
| Paper/PET | 946 ± 22 e,1 | 32.3 ± 1.4 a,1 | 8.9 ± 0.4 d,1 | 924 ± 38 d,1 | 32.6 ± 2.8 e,1 | 8.4 ± 0.7 e,1 |
| Sample | Flexural Test | Puncture Test | ||||
|---|---|---|---|---|---|---|
| Eflexural (MPa) | σy flexural (MPa) | εy flexural (%) | Fmax (N) | dtotal (mm) | Epuncture (mJ) | |
| Paper | 1498 ± 34 a | 30.4 ± 2.1 a | 0.69 ± 0.1 a | 53 ± 4 a | 1.4 ± 0.1 a | 25 ± 2 a |
| Paper/PBS–PBSA | 1235 ± 23 b | 35.9 ± 1.3 b | 1.14 ± 0.2 b | 64 ± 5 b | 5.4 ± 0.5 b | 130 ± 7 b |
| Paper/PET | 912 ± 32 c | 20.3 ± 0.9 c | 1.36 ± 0.2 b | 56 ± 3 ab | 5.3 ± 0.6 b | 85 ± 4 c |
| Sample | Thickness | Water Vapor | Limonene Vapor | Oxygen Gas | |||
|---|---|---|---|---|---|---|---|
| (µm) | Permeance (kg/Pa·s·m2) | Permeability (kg·m/Pa·s·m2) | Permeance (kg/Pa·s·m2) | Permeability (kg·m/Pa·s·m2) | Permeance (m3/m·s·Pa) | Permeability (m3·m/m2·s·Pa) | |
| ×1010 | ×1015 | ×1010 | ×1015 | ×1015 | ×1019 | ||
| Paper | 291 ± 6 a | 110.16 ± 8.89 a | 3205.65 ± 87.11 a | 22.34 ± 1.34 a | 650.15 ± 9.42 a | >D.L. | >D.L. |
| PBS–PBSA | 212 ± 5 b | 1.47 ± 0.07 b | 31.56 ± 0.96 b | 2.63 ± 0.08 b | 55.76 ± 2.66 b | 6.17 ± 0.14 a | 12.74 ± 0.30 a |
| PET * | 101 ± 1 c | 0.55 ± 0.07 c | 5.58 ± 0.71 c | 0.51 ± 0.02 c | 5.15 ± 0.19 c | 2.15 ± 0.01 b | 2.17 ± 0.02 b |
| Paper/PBS–PBSA | 461 ± 19 d | 1.77 ± 0.77 b | - | 1.70 ± 0.14 d | - | 5.15 ± 0.02 c | - |
| Paper/PET | 350 ± 3 e | 0.19 ± 0.01 d | - | 1.20 ± 0.22 e | - | 2.34 ± 0.01 d | - |
| Time (Days) | Sample | L* | a* | b* | Cab* | hab* | ΔEab* |
|---|---|---|---|---|---|---|---|
| 0 | Initial | 81.33 ± 1.24 1 | −2.17 ± 0.36 1 | 16.64 ± 1.78 1 | 16.78 ± 1.58 1 | 82.57 ± 2.85 1 | - |
| 4 | Unpackaged | 75.47 ± 1.74 a,2 | −2.01 ± 0.76 a,1 | 19.36 ± 2.02 a,12 | 19.46 ± 1.56 a,2 | 84.07 ± 2.18 a,1 | 6.46 ± 1.08 c,1 |
| Paper | 78.98 ± 1.10 b,2 | −2.10 ± 0.55 a,1 | 18.36 ± 1.87 a,12 | 18.48 ± 1.24 a,2 | 83.47 ± 2.78 a,1 | 2.91 ± 0.47 b,1 | |
| Paper/PBS–PBSA | 79.89 ± 1.83 b,12 | −2.36 ± 0.56 a,1 | 17.30 ± 1.16 a,1 | 17.46 ± 1.23 a,1 | 82.23 ± 1.98 a,1 | 1.60 ± 0.28 a,1 | |
| Paper/PET | 79.93 ± 1.02 b,12 | −2.72 ± 0.98 a,1 | 16.81 ± 1.34 a,1 | 17.03 ± 1.39 a,1 | 80.81 ± 2.13 ab,1 | 1.51 ± 0.34 a,1 | |
| 9 | Unpackaged | 74.07 ± 1.45 a,2 | −1.98 ± 0.89 a,1 | 20.96 ± 1.23 a,2 | 21.05 ± 1.45 a,3 | 84.60 ± 2.45 a,2 | 8.45 ± 1.35 c,2 |
| Paper | 77.52 ± 1.52 b,2 | −2.65 ± 0.72 a,1 | 20.06 ± 1.63 a,2 | 20.23 ± 1.03 a,3 | 82.47 ± 2.12 b,2 | 5.14 ± 1.06 b,2 | |
| Paper/PBS–PBSA | 78.08 ± 1.10 b,23 | −1.97 ± 0.89 a,1 | 18.24 ± 1.68 a,1 | 18.35 ± 1.06 b,2 | 83.84 ± 2.05 b,2 | 3.63 ± 0.57 a,2 | |
| Paper/PET | 78.55 ± 1.23 b,23 | −2.41 ± 0.45 a,1 | 18.32 ± 1.56 a,1 | 18.48 ± 1.16 b,2 | 82.51 ± 1.89 b,2 | 3.26 ± 0.48 a,2 | |
| 13 | Unpackaged | 73.87 ± 1.69 a,2 | −2.18 ± 0.34 a,1 | 21.34 ± 1.26 a,2 | 21.45 ± 1.24 a,3 | 84.17 ± 1.98 a,2 | 8.82 ± 1.59 d,2 |
| Paper | 75.63 ± 2.45 ab,23 | −2.98 ± 0.65 a,1 | 21.72 ± 2.09 a,2 | 21.92 ± 1.20 a,3 | 82.19 ± 2.38 b,2 | 7.68 ± 1.32 c,3 | |
| Paper/PBS–PBSA | 76.64 ± 2.39 ab,2345 | −2.12 ± 0.87 a,1 | 19.58 ± 1.34 a,1 | 19.69 ± 1.38 b,2 | 83.82 ± 2.57 b,2 | 5.54 ± 1.02 b,3 | |
| Paper/PET | 78.42 ± 1.45 b,23 | −2.57 ± 0.69 a,1 | 18.93 ± 1.07 b,1 | 19.10 ± 1.56 b,2 | 82.27 ± 3.04 b,2 | 3.72 ± 0.67 a,3 | |
| 17 | Unpackaged | 73.02 ± 1.57 a,2 | −1.98 ± 0.49 a,1 | 21.16 ± 2.46 a,2 | 21.25 ± 1.86 a,3 | 84.65 ± 2.08 a,2 | 9.46 ± 1.77 c,3 |
| Paper | 71.04 ± 3.21 a,34 | −3.01 ± 0.89 a,1 | 21.02 ± 3.11 a,2 | 21.23 ± 1.32 a,3 | 81.85 ± 3.19 b,2 | 11.21 ± 1.65 c,4 | |
| Paper/PBS–PBSA | 75.34 ± 1.70 a,345 | −2.56 ± 0.65 a,1 | 18.91 ± 1.78 a,1 | 19.08 ± 1.68 b,2 | 82.29 ± 2.75 b,2 | 6.42 ± 1.21 b,4 | |
| Paper/PET | 78.19 ± 0.78 b,23 | −2.29 ± 0.85 a,1 | 18.89 ± 1.63 a,1 | 19.03 ± 1.92 b,2 | 83.09 ± 2.63 b,2 | 3.86 ± 0.46 a,4 | |
| 21 | Unpackaged | 72.27 ± 2.86 a,2 | −1.34 ± 0.90 a,1 | 21.32 ± 2.36 a,2 | 21.36 ± 1.65 a,3 | 86.40 ± 1.88 a,3 | 10.23 ± 1.98 c,4 |
| Paper | 69.07 ± 2.86 a,4 | −1.58 ± 0.87 a,1 | 21.26 ± 2.65 a,2 | 21.32± 1.76 a,3 | 85.75 ± 2.14 a,3 | 13.11 ± 1.87 c,4 | |
| Paper/PBS–PBSA | 73.04 ± 1.66 a,5 | −2.77 ± 0.56 a,1 | 20.91 ± 1.12 a,1 | 21.09± 1.37 a,3 | 82.45 ± 2.67 b,3 | 9.34 ± 1.78 b,5 | |
| Paper/PET | 76.89 ± 0.92 b,3 | −2.68 ± 0.71 a,1 | 19.97 ± 1.85 a,1 | 20.15± 1.62 a,3 | 82.36 ± 2.73 b,3 | 5.57 ± 1.02 a,5 |
| Sample | Ethanol 10% v/v (mg/dm2) | Tenax (mg/dm2) |
|---|---|---|
| Paper | - | 1.6 ± 0.2 a |
| PBS–PBSA | 3.6 ± 0.3 a | - |
| Paper/PBS–PBSA | 1.9 ± 0.2 b | 1.5 ± 0.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-García, E.; Pacheco-Romeralo, M.; Zomeño, P.; Viscusi, G.; Malvano, F.; Gorrasi, G.; Torres-Giner, S. Development and Characterization of Thermoformed Bilayer Trays of Paper and Renewable Succinic Acid Derived Biopolyester Blends and Their Application to Preserve Fresh Pasta. Materials 2023, 16, 3872. https://doi.org/10.3390/ma16103872
Hernández-García E, Pacheco-Romeralo M, Zomeño P, Viscusi G, Malvano F, Gorrasi G, Torres-Giner S. Development and Characterization of Thermoformed Bilayer Trays of Paper and Renewable Succinic Acid Derived Biopolyester Blends and Their Application to Preserve Fresh Pasta. Materials. 2023; 16(10):3872. https://doi.org/10.3390/ma16103872
Chicago/Turabian StyleHernández-García, Eva, Marta Pacheco-Romeralo, Pedro Zomeño, Gianluca Viscusi, Francesca Malvano, Giuliana Gorrasi, and Sergio Torres-Giner. 2023. "Development and Characterization of Thermoformed Bilayer Trays of Paper and Renewable Succinic Acid Derived Biopolyester Blends and Their Application to Preserve Fresh Pasta" Materials 16, no. 10: 3872. https://doi.org/10.3390/ma16103872
APA StyleHernández-García, E., Pacheco-Romeralo, M., Zomeño, P., Viscusi, G., Malvano, F., Gorrasi, G., & Torres-Giner, S. (2023). Development and Characterization of Thermoformed Bilayer Trays of Paper and Renewable Succinic Acid Derived Biopolyester Blends and Their Application to Preserve Fresh Pasta. Materials, 16(10), 3872. https://doi.org/10.3390/ma16103872











