Bioleaching Modeling—A Review
Abstract
:1. Introduction
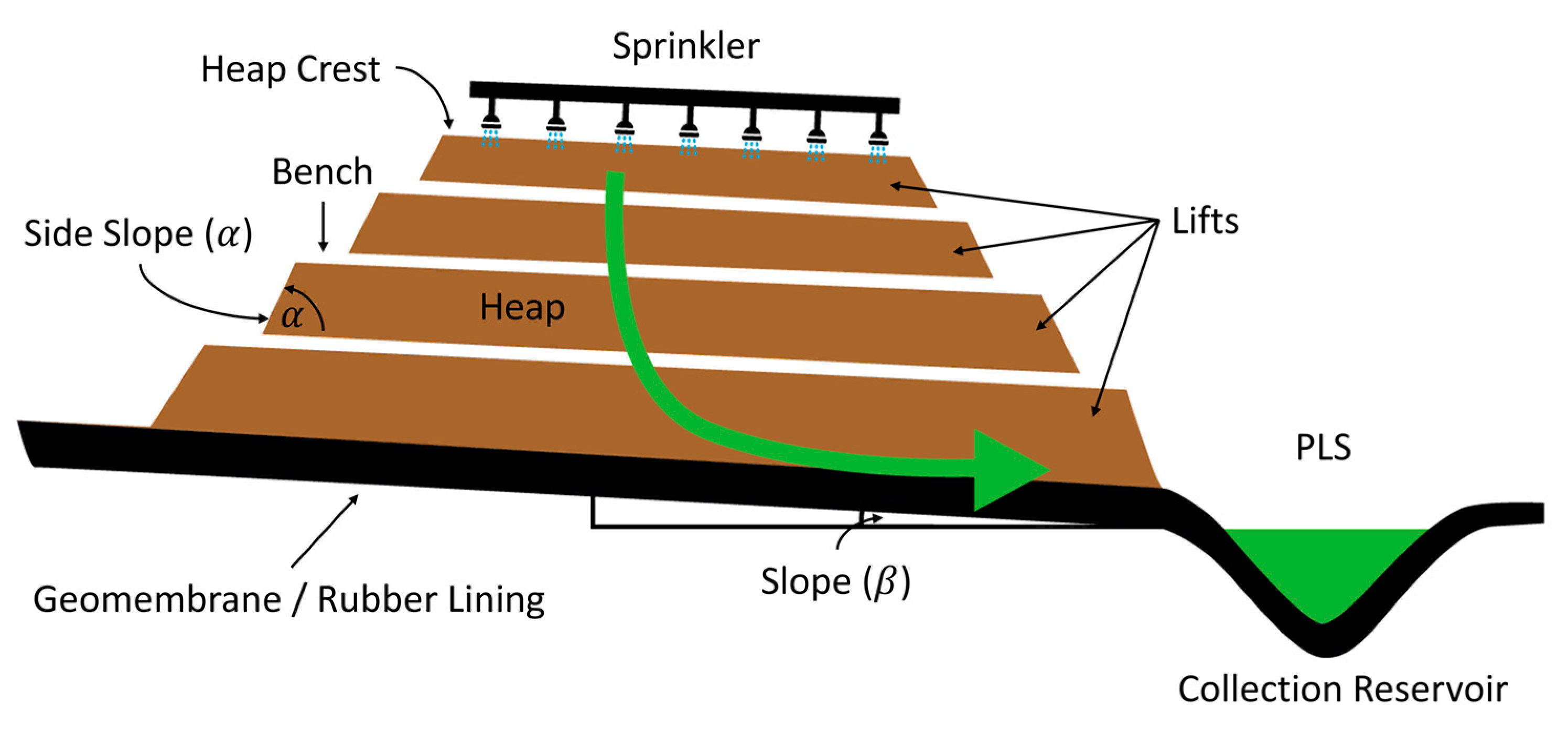
2. Bioleaching Process
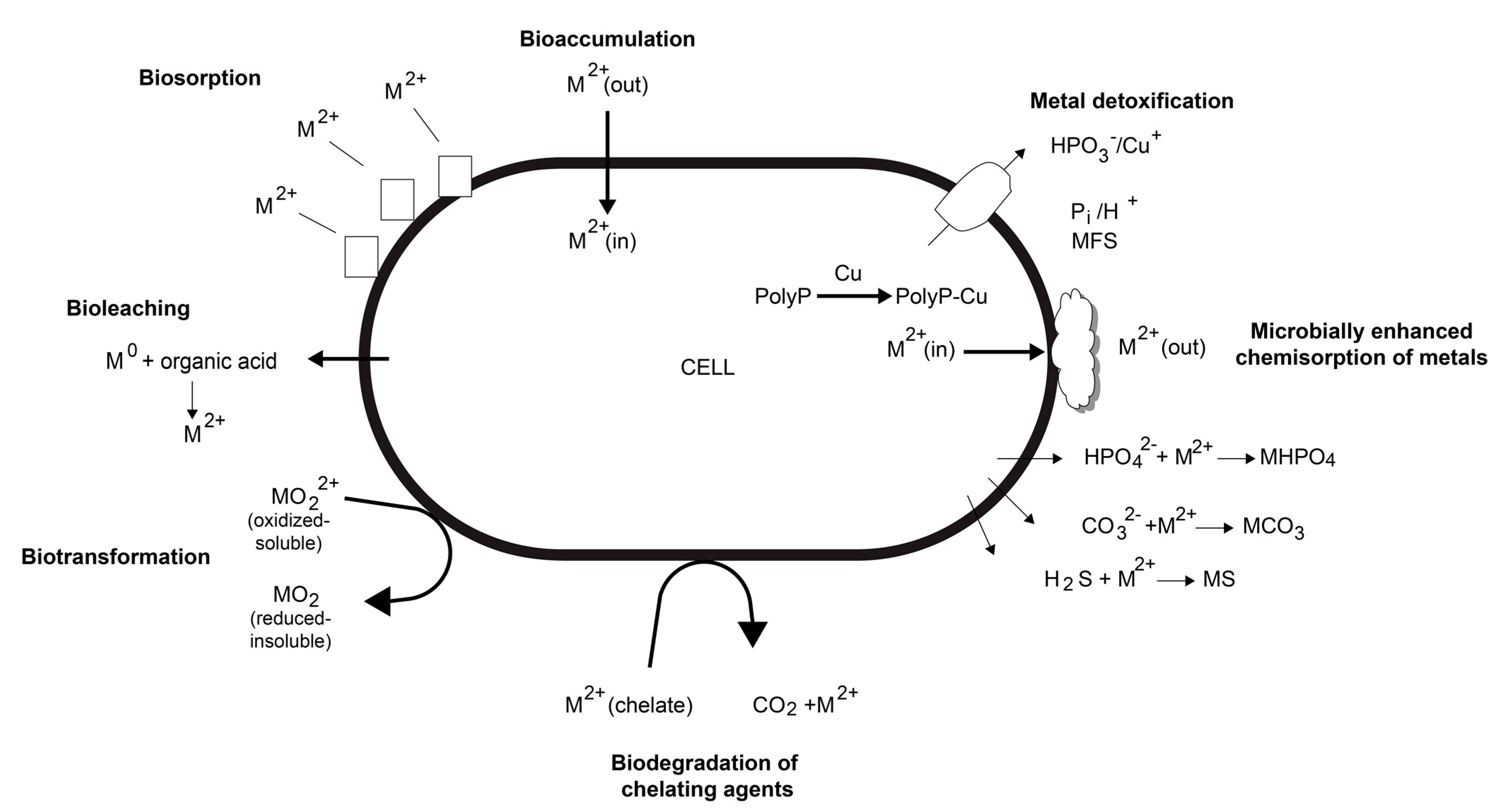
3. Microorganisms in Bioleaching
4. Modeling of Mineral Bioleaching
4.1. Process Modeling and Bibliometric Analysis
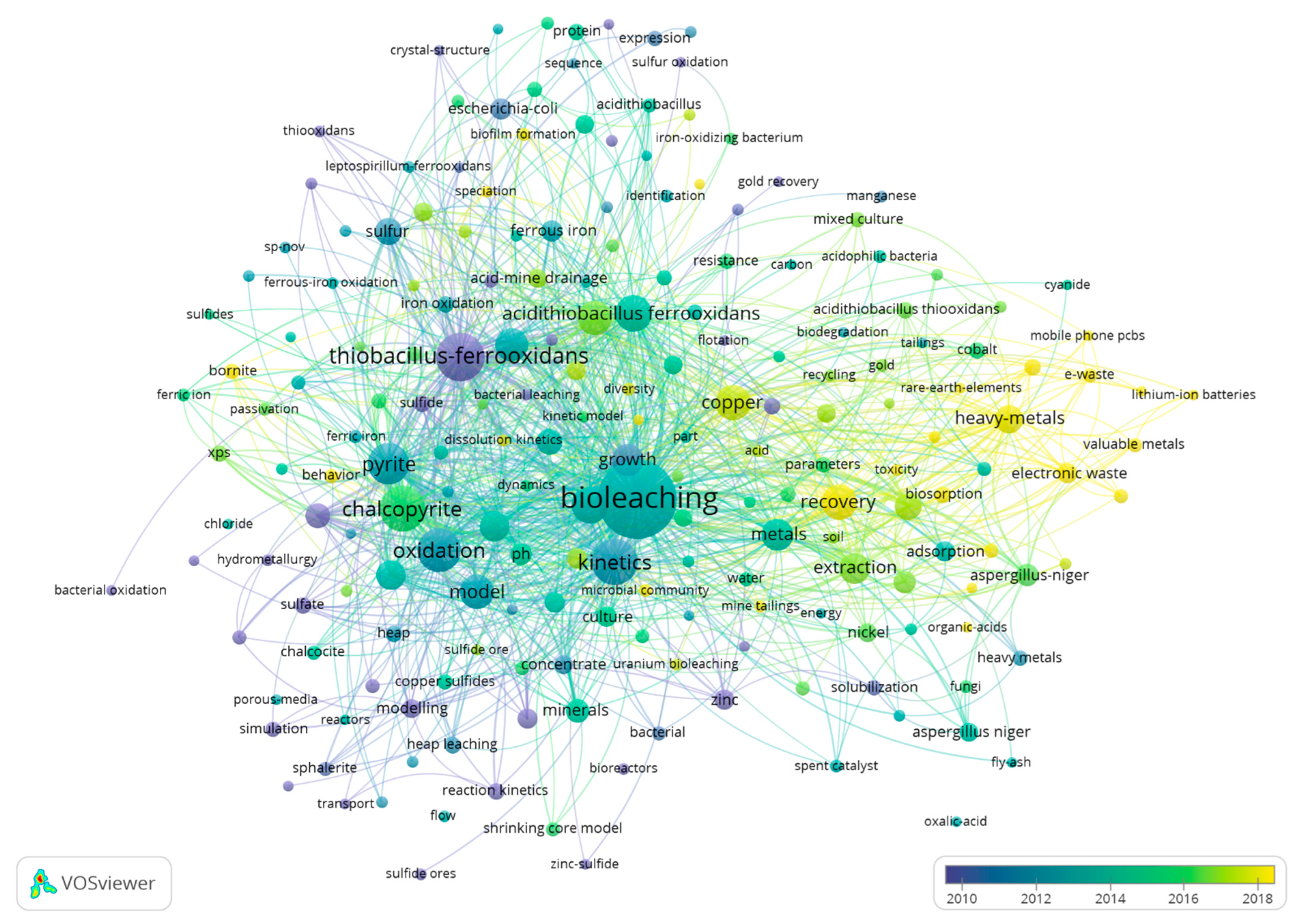
4.2. Leach Modeling
- Diffusion-controlled process through the product layer: The creation of a layer of product around the material can impede the diffusion of the oxidant to the material’s surface, resulting in a deceleration of the leaching process.
- Chemically controlled process: The product layer is absent or its presence does not affect the free movement of the oxidant. The reaction between the surface and the reactant is slower than the diffusion of the oxidant.
- Film diffusion process: The oxidant movement to the surface can be hindered by the bulk leach solution, resulting in slower leaching kinetics.
4.3. Bioleaching Modeling
4.4. Modeling of the Bioleaching Process Using Machine Learning
5. Conclusions and Future Perspectives
- Use of deeper deposits, lower grades, and more complexity;
- Exploration of the use of space resources in situ;
- Mining of strategic metals and unconventional minerals;
- Waste mining and industrial ecology;
- Saline water processing;
- Microbe engineering;
- Removal of impurities and integrated processes;
- Development of alternative leaching;
- Use of artificial intelligence and digital twins.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flanagan, D.M. Copper. In Mineral Commodity Summaries 2021; U.S. Geological Survey: Reston, VA, USA, 2021; pp. 52–53. Available online: https://pubs.usgs.gov/periodicals/mcs2021/mcs2021.pdf (accessed on 13 March 2023).
- CED. “Leaching|Meaning in the Cambridge English Dictionary”, Cambridge English Dictionary. 2021. Available online: https://dictionary.cambridge.org/dictionary/english/leaching (accessed on 12 May 2021).
- Moguillansky, G. Chile: Las inversiones en el sector minero, 1980–2000. Ser. Históricas (Comisión Económica para América Latina y el Caribe, CEPAL) 1998, 3, 1–61. [Google Scholar]
- Habashi, F. A short history of hydrometallurgy. Hydrometallurgy 2005, 79, 15–22. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Lawson, F. The kinetics of leaching chalcocite in acidic oxygenated sulphate-chloride solutions. Hydrometallurgy 1991, 27, 249–268. [Google Scholar] [CrossRef]
- Senanayake, G. Chloride assisted leaching of chalcocite by oxygenated sulphuric acid via Cu(II)-OH-Cl. Miner. Eng. 2007, 20, 1075–1088. [Google Scholar] [CrossRef]
- Havlik, T. Hydrometallurgy: Principles and Application, 1st ed.; Woodhead Publishing: Cambridge, UK, 2008. [Google Scholar]
- Watling, H.R.; Shiers, D.W.; Li, J.; Chapman, N.M.; Douglas, G.B. Effect of water quality on the leaching of a low-grade copper sulfide ore. Miner. Eng. 2014, 58, 39–51. [Google Scholar] [CrossRef]
- Nicol, M.; Basson, P. The anodic behaviour of covellite in chloride solutions. Hydrometallurgy 2017, 172, 60–68. [Google Scholar] [CrossRef]
- Pérez, K.; Toro, N.; Saldaña, M.; Salinas-Rodríguez, E.; Robles, P.; Torres, D.; Jeldres, R.I. Statistical Study for Leaching of Covellite in a Chloride Media. Metals 2020, 10, 477. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Nicol, M.; Miki, H. The dissolution of chalcopyrite in chloride solutions Part 1. The effect of solution potential. Hydrometallurgy 2010, 103, 108–113. [Google Scholar] [CrossRef]
- Yévenes, L.V.; Miki, H.; Nicol, M. The dissolution of chalcopyrite in chloride solutions: Part 2: Effect of various parameters on the rate. Hydrometallurgy 2010, 103, 80–85. [Google Scholar] [CrossRef]
- Nicol, M.; Miki, H.; Velásquez-Yévenes, L. The dissolution of chalcopyrite in chloride solutions Part 3. Mechanisms. Hydrometallurgy 2010, 103, 86–95. [Google Scholar] [CrossRef]
- Valencia, J.A.; Méndez, D.A.; Cueto, J.Y.; Cisternas, L.A. Saltpeter extraction and modelling of caliche mineral heap leaching. Hydrometallurgy 2008, 90, 103–114. [Google Scholar] [CrossRef]
- Ordóñez, J.; Condori, A.; Moreno, L.; Cisternas, L. Heap Leaching of Caliche Ore. Modeling of a Multicomponent System with Particle Size Distribution. Minerals 2017, 10, 180. [Google Scholar] [CrossRef]
- Bogdanović, G.D.; Petrović, S.; Sokić, M.; Antonijević, M.M. Chalcopyrite leaching in acid media: A review. Metall. Mater. Eng. 2020, 26, 177–198. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Franzidis, J.P.; Petersen, J. Heap leaching technology-Current State, innovations, and future directions: A review. Miner. Process. Extr. Metall. Rev. 2016, 37, 73–119. [Google Scholar] [CrossRef]
- Senanayake, G. Acid leaching of metals from deep-sea manganese nodules–A critical review of fundamentals and applications. Miner. Eng. 2011, 24, 1379–1396. [Google Scholar] [CrossRef]
- Watling, H.R. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Dopson, M.; Okibe, N. Biomining Microorganisms: Diversity and Modus Operandi. In Biomining Technologies; Springer: Cham, Switzerland, 2023; pp. 89–110. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Won, S.; Ha, M.G.; Nguyen, D.D.; Kang, H.Y. Bioleaching for environmental remediation of toxic metals and metalloids: A review on soils, sediments, and mine tailings. Chemosphere 2021, 282, 131108. [Google Scholar] [CrossRef] [PubMed]
- Medina-Díaz, H.L.; Acosta, I.; Muñoz, M.; Bellido, F.J.L.; Villaseñor, J.; Llanos, J.; Rodríguez, L.; Fernández-Morales, F.J. A classical modelling of abandoned mine tailings’ bioleaching by an autochthonous microbial culture. J. Environ. Manag. 2022, 323, 116251. [Google Scholar] [CrossRef]
- Monachon, M.; Albelda-Berenguer, M.; Joseph, E. Biological oxidation of iron sulfides. Adv. Appl. Microbiol. 2019, 107, 1–27. [Google Scholar] [CrossRef]
- Pradhan, N.; Nathsarma, K.C.; Rao, K.S.; Sukla, L.B.; Mishra, B.K. Heap bioleaching of chalcopyrite: A review. Miner. Eng. 2008, 21, 355–365. [Google Scholar] [CrossRef]
- Rohwerder, T.; Gehrke, T.; Kinzler, K.; Sand, W. Bioleaching review part A: Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation. Appl. Microbiol. Biotechnol. 2003, 63, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Olson, G.J.; Brierley, J.A.; Brierley, C.L. Bioleaching review part B: Progress in bioleaching: Applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2003, 63, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Gentina, J.C.; Acevedo, F. Copper Bioleaching in Chile. Minerals 2016, 6, 23. [Google Scholar] [CrossRef]
- Yin, S.; Wang, L.; Kabwe, E.; Chen, X.; Yan, R.; An, K.; Zhang, L.; Wu, A. Copper Bioleaching in China: Review and Prospect. Minerals 2018, 8, 32. [Google Scholar] [CrossRef]
- Abdollahi, H.; Saneie, R.; Shafaei, Z.; Mirmohammadi, M.; Mohammadzadeh, A.; Tuovinen, O.H. Bioleaching of cobalt from magnetite-rich cobaltite-bearing ore. Hydrometallurgy 2021, 204, 105727. [Google Scholar] [CrossRef]
- Cameron, R.A.; Lastra, R.; Thibault, Y.; Morin, L.; Gould, W.D. Stirred-tank bioleaching of nickel and cobalt from pyrrhotite-rich tailings from Sudbury, Ontario. Hydrometallurgy 2021, 204, 105592. [Google Scholar] [CrossRef]
- Petersen, J.; Dixon, D.G. Modelling zinc heap bioleaching. Hydrometallurgy 2007, 85, 127–143. [Google Scholar] [CrossRef]
- Mäkinen, J.; Wendling, L.; Lavonen, T.; Kinnunen, P. Sequential Bioleaching of Phosphorus and Uranium. Minerals 2019, 9, 331. [Google Scholar] [CrossRef]
- Abdulla, H.M.; Taher, H.S.; Ibrahim, H.A. Bioleaching of Uranium from Egyptian Rocks Using Native Actinomycete Strains. Geomicrobiol. J. 2018, 35, 91–99. [Google Scholar] [CrossRef]
- Mukaba, J.L.; Eze, C.P.; Pereao, O.; Petrik, L.F. Rare Earths’ Recovery from Phosphogypsum: An Overview on Direct and Indirect Leaching Techniques. Minerals 2021, 11, 1051. [Google Scholar] [CrossRef]
- Brisson, V.L.; Zhuang, W.Q.; Alvarez-Cohen, L. Bioleaching of rare earth elements from monazite sand. Biotechnol. Bioeng. 2016, 113, 339–348. [Google Scholar] [CrossRef]
- Brisson, V.L.; Zhuang, W.Q.; Alvarez-Cohen, L. Metabolomic Analysis Reveals Contributions of Citric and Citramalic Acids to Rare Earth Bioleaching by a Paecilomyces Fungus. Front. Microbiol. 2020, 10, 3008. [Google Scholar] [CrossRef]
- Tian, Y.; Hu, X.; Song, X.; Yang, A.J. Bioleaching of rare-earth elements from phosphate rock using Acidithiobacillus ferrooxidans. Lett. Appl. Microbiol. 2022, 75, 1111–1121. [Google Scholar] [CrossRef]
- Tayar, S.P.; Palmieri, M.C.; Bevilaqua, D. Sulfuric acid bioproduction and its application in rare earth extraction from phosphogypsum. Miner. Eng. 2022, 185, 107662. [Google Scholar] [CrossRef]
- Zhang, D.-R.; Chen, H.-R.; Nie, Z.-Y.; Xia, J.-L.; Li, E.-P.; Fan, X.-L.; Zheng, L. Extraction of Al and rare earths (Ce, Gd, Sc, Y) from red mud by aerobic and anaerobic bi-stage bioleaching. Chem. Eng. J. 2020, 401, 125914. [Google Scholar] [CrossRef]
- Auerbach, R.; Bokelmann, K.; Stauber, R.; Gutfleisch, O.; Schnell, S.; Ratering, S. Critical raw materials–Advanced recycling technologies and processes: Recycling of rare earth metals out of end of life magnets by bioleaching with various bacteria as an example of an intelligent recycling strategy. Miner. Eng. 2019, 134, 104–117. [Google Scholar] [CrossRef]
- Marra, A.; Cesaro, A.; Rene, E.R.; Belgiorno, V.; Lens, P.N.L. Bioleaching of metals from WEEE shredding dust. J. Environ. Manag. 2018, 210, 180–190. [Google Scholar] [CrossRef]
- CODELCO. Biolixiviación, Tecnología Para la Nueva Minería. 17 February 2011. Available online: https://www.codelco.com/biolixiviacion-tecnologia-para-la-nueva-mineria/prontus_codelco/2011-02-17/092512.html (accessed on 31 October 2021).
- Hoque, M.E.; Philip, O.J. Biotechnological recovery of heavy metals from secondary sources—An overview. Mater. Sci. Eng. C 2011, 31, 57–66. [Google Scholar] [CrossRef]
- Petersen, J.; Dixon, D.G. Modeling and Optimization of Heap Bioleach Processes. In Biomining; Springer: Berlin/Heidelberg, Germany, 2007; pp. 153–176. [Google Scholar] [CrossRef]
- CODELCO. Proceso de Biolixiviación del Cobre|Codelco Educa. 2018. Available online: https://www.codelcoeduca.cl/codelcoeduca/site/edic/base/port/biolixiviacion.html (accessed on 31 October 2021).
- Srichandan, H.; Mohapatra, R.K.; Singh, P.K.; Mishra, S.; Parhi, P.K.; Naik, K. Column bioleaching applications, process development, mechanism, parametric effect and modelling: A review. J. Ind. Eng. Chem. 2020, 90, 1–16. [Google Scholar] [CrossRef]
- Crundwell, F.K. How do bacteria interact with minerals? Hydrometallurgy 2003, 71, 75–81. [Google Scholar] [CrossRef]
- Rodríguez, Y.; Ballester, A.; Blázquez, M.L.; González, F.; Munoz, J.A. Study of Bacterial Attachment during the Bioleaching of Pyrite, Chalcopyrite, and Sphalerite. Geomicrobiol. J. 2010, 20, 131–141. [Google Scholar] [CrossRef]
- Jerez, C.A. Metal Extraction and Biomining. In Encyclopedia of Microbiology, 4th ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 39–52. [Google Scholar] [CrossRef]
- Huang, T.; Wei, X.; Zhang, S. Bioleaching of copper sulfide minerals assisted by microbial fuel cells. Bioresour. Technol. 2019, 288, 121561. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, Z.; Sun, Z.; Liu, Y.; Chen, G.; Liao, Q.; Xu, L.; Wang, X.; Li, J.; Zhou, Y. Enhanced uranium bioleaching high-fluorine and low-sulfur uranium ore by a mesophilic acidophilic bacterial consortium with pyrite. J. Radioanal. Nucl. Chem. 2019, 321, 711–722. [Google Scholar] [CrossRef]
- Pal, S.; Pradhan, D.; Das, T.; Sukla, L.B.; Chaudhury, G.R. Bioleaching of low-grade uranium ore using Acidithiobacillus ferrooxidans. Indian J. Microbiol. 2010, 50, 70–75. [Google Scholar] [CrossRef]
- Olson, G.J. Microbial oxidation of gold ores and gold bioleaching. FEMS Microbiol. Lett. 1994, 119, 1–6. [Google Scholar] [CrossRef]
- Li, J.; Wen, J.; Guo, Y.; An, N.; Liang, C.; Ge, Z. Bioleaching of gold from waste printed circuit boards by alkali-tolerant Pseudomonas fluorescens. Hydrometallurgy 2020, 194, 105260. [Google Scholar] [CrossRef]
- Siezen, R.J.; Wilson, G. Bioleaching genomics. Microb. Biotechnol. 2009, 2, 297. [Google Scholar] [CrossRef]
- Rawlings, D.E.; Johnson, D.B. The microbiology of biomining: Development and optimization of mineral-oxidizing microbial consortia. Microbiology 2007, 153, 315–324. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1999. [Google Scholar]
- Yang, C.; Sun, B. Kinetic modeling of the competitive-consecutive reaction system. In Modeling, Optimization, and Control of Zinc Hydrometallurgical Purification Process; Academic Press: Cambridge, MA, USA, 2021; pp. 39–62. [Google Scholar] [CrossRef]
- Herrera, M.N.; Wiertz, J.V.; Ruiz, P.; Neuburg, H.J.; Badilla-Ohlbaum, R. A phenomenological model of the bioleaching of complex sulfide ores. Hydrometallurgy 1989, 22, 193–206. [Google Scholar] [CrossRef]
- Asai, S.; Konishi, Y.; Yoshida, K. Kinetic model for batch bacterial dissolution of pyrite particles by Thiobacillus ferrooxidans. Chem. Eng. Sci. 1992, 47, 133–139. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Deng, T.; Wang, K. Kinetic modeling for the bacterial leaching of chalcopyrite catalyzed by silver ions. Miner. Eng. 2004, 17, 943–947. [Google Scholar] [CrossRef]
- Kargi, F.; Weissman, J.G. A dynamic mathematical model for microbial removal of pyritic sulfur from coal. Biotechnol. Bioeng. 1984, 26, 604–612. [Google Scholar] [CrossRef]
- Braun, R.L.; Lewis, A.E.; Wadsworth, M.E. In-place leaching of primary sulfide ores: Laboratory leaching data and kinetics model. Metall. Trans. 1974, 5, 1717–1726. [Google Scholar] [CrossRef]
- Madsen, B.W.; Wadsworth, M.E.; Groves, R.D. Application of a mixed kinetics model to the leaching of low grade copper sulfide ores. Trans. Soc. Min. Eng. AIME 1975, 258, 69–74. [Google Scholar]
- Bhattacharya, P.; Sarkar, P.; Mukherjea, R.N. Reaction kinetics model for chalcopyrite bioleaching using Thiobacillus ferrooxidans. Enzyme. Microb. Technol. 1990, 12, 873–876. [Google Scholar] [CrossRef]
- Neuburg, H.J.; Castillo, J.A.; Herrera, M.N.; Wiertz, J.V.; Vargas, T.; Badilla-Ohlbaum, R. A model for the bacterial leaching of copper sulfide ores in pilot-scale columns. Int. J. Miner. Process 1991, 31, 247–264. [Google Scholar] [CrossRef]
- Konishi, Y.; Asai, S.; Katoh, H. Bacterial dissolution of pyrite by Thiobacillus ferrooxidans. Bioprocess Eng. 1990, 5, 231–237. [Google Scholar] [CrossRef]
- Casas, J.M.; Martinez, J.; Moreno, L.; Vargas, T. Bioleaching model of a copper-sulfide ore bed in heap and dump configurations. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 1998, 29, 899–909. [Google Scholar] [CrossRef]
- Mehta, K.D.; Pandey, B.D.; Mankhand, T.R. Studies on kinetics of biodissolution of metals from Indian Ocean nodules. Miner. Eng. 2003, 16, 523–527. [Google Scholar] [CrossRef]
- Sohn, H.Y. Rate Processes of Extractive Metallurgy, 1st ed.; Plenum Press: New York, NY, USA, 1979. [Google Scholar]
- Sidborn, M.; Casas, J.; Martínez, J.; Moreno, L. Two-dimensional dynamic model of a copper sulphide ore bed. Hydrometallurgy 2003, 71, 67–74. [Google Scholar] [CrossRef]
- Leahy, M.J.; Davidson, M.R.; Schwarz, M.P. A model for heap bioleaching of chalcocite with heat balance: Bacterial temperature dependence. Miner. Eng. 2005, 18, 1239–1252. [Google Scholar] [CrossRef]
- Dixon, D.G.; Petersen, J. Comprehensive Modelling Study of Chalcocite Column and Heap Bioleaching. In Hydrometallurgy of Copper (Book 2); Canadian Institute of Mining, Metallurgy and Petroleum/Chilean Institute of Mining Engineers: Santiago, Chile, 2003; pp. 493–515. [Google Scholar]
- Leahy, M.J.; Schwarz, M.P. Modelling jarosite precipitation in isothermal chalcopyrite bioleaching columns. Hydrometallurgy 2009, 98, 181–191. [Google Scholar] [CrossRef]
- Lizama, H.M. A kinetic description of percolation bioleaching. Miner. Eng. 2004, 17, 23–32. [Google Scholar] [CrossRef]
- Miller, D.M.; Hansford, G.S. Batch biooxidation of a gold-bearing pyrite-arsenopyrite concentrate. Miner. Eng. 1992, 5, 613–629. [Google Scholar] [CrossRef]
- Lizama, H.M.; Harlamovs, J.R.; McKay, D.J.; Dai, Z. Heap leaching kinetics are proportional to the irrigation rate divided by heap height. Miner. Eng. 2005, 18, 623–630. [Google Scholar] [CrossRef]
- Petersen, J. Modelling of bioleach processes: Connection between science and engineering. Hydrometallurgy 2010, 104, 404–409. [Google Scholar] [CrossRef]
- Vilcáez, J.; Suto, K.; Inoue, C. Modeling the auto-thermal performance of a thermophilic bioleaching heap employing mesophilic and thermophilic microbes. Hydrometallurgy 2008, 94, 82–92. [Google Scholar] [CrossRef]
- Bouffard, S.C.; Dixon, D.G. Modeling pyrite bioleaching in isothermal test columns with the HeapSim model. Hydrometallurgy 2009, 95, 215–226. [Google Scholar] [CrossRef]
- Bouffard, S.C. Application of the HeapSim model to the heap bioleaching of the Pueblo Viejo ore deposit. Hydrometallurgy 2008, 93, 116–123. [Google Scholar] [CrossRef]
- Bouffard, S.C.; Dixon, D.G. Investigative study into the hydrodynamics of heap leaching processes. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2001, 32, 763–776. [Google Scholar] [CrossRef]
- Yin, S.; Wu, A.; Li, X.; Wang, Y. Mathematical model for coupled reactive flow and solute transport during heap bioleaching of copper sulfide. J. Cent. South Univ. 2011, 18, 1434–1440. [Google Scholar] [CrossRef]
- Paul, B.C.; Sohn, H.Y.; McCarter, M.K. Model for ferric sulfate leaching of copper ores containing a variety of sulfide minerals: Part I. Modeling uniform size ore fragments. Metall. Trans. B 1992, 23, 537–548. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Jafari, A.; Yaghmaei, S.; Vossoughi, M.; Sarkomaa, P. Computer simulation of fluid motion in a porous bed using a volume of fluid method: Application in heap leaching. Miner. Eng. 2006, 19, 1077–1083. [Google Scholar] [CrossRef]
- Sheikhzadeh, G.A.; Mehrabian, M.A.; Mansouri, S.H.; Sarrafi, A. Computational modelling of unsaturated flow of liquid in heap leaching-Using the results of column tests to calibrate the model. Int. J. Heat Mass. Transf. 2005, 48, 279–292. [Google Scholar] [CrossRef]
- Dixon, D.G. Analysis of heat conservation during copper sulphide heap leaching. Hydrometallurgy 2000, 58, 27–41. [Google Scholar] [CrossRef]
- Ahmadi, A.; Ranjbar, M.; Schaffie, M.; Petersen, J. Kinetic modeling of bioleaching of copper sulfide concentrates in conventional and electrochemically controlled systems. Hydrometallurgy 2012, 127-128, 16–23. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Z.; Wang, Z.; Hu, K. A coupled model of partial differential equations for uranium ores heap leaching and its parameters identification. J. Inverse Ill Posed Probl. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Gongsheng, L.; Jin, C.; De, Y.; Hongliang, L.; Jijun, L. One-dimensional equilibrium model and source parameter determination for soil-column experiment. Appl. Math Comput. 2007, 190, 1365–1374. [Google Scholar] [CrossRef]
- Schlögl, F. On thermodynamics near a steady state. Z. Für Phys. A Hadron. Nucl. 1971, 248, 446–458. [Google Scholar] [CrossRef]
- Kampen, V.N.G. Stochastic Processes in Physics and Chemistry, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Moghaddam, M.Y.; Tonkaboni, S.Z.S.; Noaparast, M.; Ardejani, F.D. A mathematical model to simulate Heap (bio)-leaching process: An exact conceptual model, Homotopy theory and comparative insights with conventional methods. Int. J. Model. Simul. Sci. Comput. 2017, 8, 1750018. [Google Scholar] [CrossRef]
- Thorstenson, D.C.; Pollock, D.W. Gas transport in unsaturated porous media: The adequacy of Fick’s law. Rev. Geophys. 1989, 27, 61. [Google Scholar] [CrossRef]
- Paster, A.; Bolster, D.; Benson, D.A. Particle tracking and the diffusion-reaction equation. Water Resour. Res. 2013, 49, 1–6. [Google Scholar] [CrossRef]
- Clairambault, J. Reaction-Diffusion-Advection Equation. In Encyclopedia of Systems Biology; Springer: New York, NY, USA, 2013; p. 1817. [Google Scholar] [CrossRef]
- Govender-Opitz, E.; Kotsiopoulos, A.; Bryan, C.G.; Harrison, S.T.L. Modelling microbial transport in simulated low-grade heap bioleaching systems: The hydrodynamic dispersion model. Chem. Eng. Sci. 2017, 172, 545–558. [Google Scholar] [CrossRef]
- Govender, E.; Kotsiopoulos, A.; Bryan, C.G.; Harrison, S.T.L. Modelling microbial transport in simulated low-grade heap bioleaching systems: The biomass transport model. Hydrometallurgy 2014, 150, 299–307. [Google Scholar] [CrossRef]
- Noei, S.B.; Sheibani, S.; Rashchi, F.; Mirazimi, S.M.J. Kinetic modeling of copper bioleaching from low-grade ore from the Shahrbabak Copper Complex. Int. J. Miner. Metall. Mater. 2017, 24, 611–620. [Google Scholar] [CrossRef]
- da Silva, G. Relative importance of diffusion and reaction control during the bacterial and ferric sulphate leaching of zinc sulphide. Hydrometallurgy 2004, 73, 313–324. [Google Scholar] [CrossRef]
- Nazemi, M.K.; Rashchi, F.; Mostoufi, N. A new approach for identifying the rate controlling step applied to the leaching of nickel from spent catalyst. Int. J. Miner. Process. 2011, 100, 21–26. [Google Scholar] [CrossRef]
- Li, J.F.; Zhong, S.P.; Tong, L.L.; Zhang, D.C.; Bao, D.B.; Yang, H.Y. Modeling heap biooxidation of arsenic-bearing gold ore. J. Cent. South Univ. 2020, 27, 1424–1431. [Google Scholar] [CrossRef]
- Laurent, G.; Izart, C.; Lechenard, B.; Golfier, F.; Marion, P.; Collon, P.; Truche, L.; Royer, J.-J.; Filippov, L. Numerical modelling of column experiments to investigate in-situ bioleaching as an alternative mining technology. Hydrometallurgy 2019, 188, 272–290. [Google Scholar] [CrossRef]
- Jalali, F.; Fakhari, J.; Zolfaghari, A. Response surface modeling for lab-scale column bioleaching of low-grade uranium ore using a new isolated strain of Acidithiobacillus Ferridurans. Hydrometallurgy 2019, 185, 194–203. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, Z.; Sun, Z.; Chen, G.; Xu, L.; Liao, Q. Optimization of bioleaching high-fluorine and low-sulfur uranium ore by response surface method. J. Radioanal. Nucl. Chem. 2019, 322, 781–790. [Google Scholar] [CrossRef]
- Sun, J.Z.; Wu, B.; Chen, B.W.; Wen, J.K. Application of response surface methodology in optimization of bioleaching parameters for high-magnesium nickel sulfide ore. J. Cent. South Univ. 2022, 29, 1488–1499. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Y.; Ma, J.; Sun, J.; Li, G.; Zhang, R.; Cui, Z.; Li, T.; Liu, X. Sulfur enhancement effects for uranium bioleaching in column reactors from a refractory uranium ore. Front. Microbiol. 2023, 14, 118. [Google Scholar] [CrossRef]
- Shang, H.; Gao, W.C.; Wu, B.; Wen, J.K. Bioleaching and dissolution kinetics of pyrite, chalcocite and covellite. J. Cent. South Univ. 2021, 28, 2037–2051. [Google Scholar] [CrossRef]
- Sundramurthy, V.P.; Rajoo, B.; Srinivasan, N.R.; Kavitha, R. Bioleaching of Zn from sphalerite using Leptospirillum ferriphilum isolate: Effect of temperature and kinetic aspects. Appl. Biol. Chem. 2020, 63, 44. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, W.; Liu, Y.; Jia, H.; Zhou, J.; Wei, P.; Zhou, H. Bioleaching of dewatered electroplating sludge for the extraction of base metals using an adapted microbial consortium: Process optimization and kinetics. Hydrometallurgy 2020, 191, 105227. [Google Scholar] [CrossRef]
- Pathak, A.; Srichandan, H.; Kim, D.J. Column bioleaching of metals from refinery spent catalyst by Acidithiobacillus thiooxidans: Effect of operational modifications on metal extraction, metal precipitation, and bacterial attachment. J. Environ. Manag. 2019, 242, 372–383. [Google Scholar] [CrossRef]
- Becci, A.; Amato, A.; Rodríguez-Maroto, J.M.; Beolchini, F. Bioleaching of End-of-Life Printed Circuit Boards: Mathematical Modeling and Kinetic Analysis. Ind. Eng. Chem. Res. 2021, 60, 4261–4268. [Google Scholar] [CrossRef]
- Saldaña, M.; Neira, P.; Flores, V.; Robles, P.; Moraga, C. A decision support system for changes in operation modes of the copper heap leaching process. Metals 2021, 11, 1025. [Google Scholar] [CrossRef]
- Saldaña, M.; González, J.; Jeldres, R.I.; Villegas; Castillo, J.; Quezada, G.; Toro, N. A Stochastic Model Approach for Copper Heap Leaching through Bayesian Networks. Metals 2019, 9, 1198. [Google Scholar] [CrossRef]
- Agarwal, S.; Darbar, S.; Saha, S.; Choudhury, M.; Singh, R.P. E-waste management using different cost-effective, eco-friendly biological techniques: An overview. In Waste Management and Resource Recycling in the Developing World; Elsevier: Amsterdam, The Netherlands, 2023; pp. 205–235. [Google Scholar] [CrossRef]
- Demergasso, C.; Véliz, R.; Galleguillos, P.; Marín, S.; Acosta, M.; Zepeda, V.; Zeballos, J.; Henríquez, F.; Pizarro, R.; Bekios-Calfa, J. Decision support system for bioleaching processes. Hydrometallurgy 2018, 181, 113–122. [Google Scholar] [CrossRef]
- Mokarian, P.; Bakhshayeshi, I.; Taghikhah, F.; Boroumand, Y.; Erfani, E.; Razmjou, A. The advanced design of bioleaching process for metal recovery: A machine learning approach. Sep. Purif. Technol. 2022, 291, 120919. [Google Scholar] [CrossRef]
- Kang, J.K.; Cho, K.H.; Kim, S.B.; Choi, N.C. Artificial Neural Network Modeling for Prediction of Dynamic Changes in Solution from Bioleaching by Indigenous Acidophilic Bacteria. Appl. Sci. 2020, 10, 7569. [Google Scholar] [CrossRef]
- Priyadarshini, J.; Elangovan, M.; Mahdal, M.; Jayasudha, M. Machine-Learning-Assisted Prediction of Maximum Metal Recovery from Spent Zinc-Manganese Batteries. Processes 2022, 10, 1034. [Google Scholar] [CrossRef]
- Zhang, L.; Zhong, Y.; Geng, Y. A bibliometric and visual study on urban mining. J. Clean. Prod. 2019, 239, 118067. [Google Scholar] [CrossRef]
- Trivedi, A.; Hait, S. Metal bioleaching from printed circuit boards by bio-Fenton process: Optimization and prediction by response surface methodology and artificial intelligence models. J. Environ. Manag. 2023, 326, 116797. [Google Scholar] [CrossRef]
- Vyas, S.; Das, S.; Ting, Y.P. Predictive modeling and response analysis of spent catalyst bioleaching using artificial neural network. Bioresour. Technol. Rep. 2020, 9, 100389. [Google Scholar] [CrossRef]
- Ruhatiya, C.; Gandra, R.; Kondaiah, P.; Manivas, K.; Samhith, A.; Gao, L.; Lam, J.S.L.; Garg, A. Intelligent optimization of bioleaching process for waste lithium-ion batteries: An application of support vector regression approach. Int. J. Energy Res. 2021, 45, 6152–6162. [Google Scholar] [CrossRef]
- Annamalai, M.; Gurumurthy, K. Neural network prediction of bioleaching of metals from waste computer printed circuit boards using Levenberg-Marquardt algorithm. Comput. Intell. 2020, 36, 1548–1568. [Google Scholar] [CrossRef]
| Expression | Broadcast on Film | Product Layer Diffusion | Chemical Reaction | |
|---|---|---|---|---|
| constant size particles | ||||
| Particles decrease in size with solid being dislodged | Sphere Small particles Stokes regime | natural convection | Does not apply | |
| Sphere large particles | forced convection | Does not apply | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saldaña, M.; Jeldres, M.; Galleguillos Madrid, F.M.; Gallegos, S.; Salazar, I.; Robles, P.; Toro, N. Bioleaching Modeling—A Review. Materials 2023, 16, 3812. https://doi.org/10.3390/ma16103812
Saldaña M, Jeldres M, Galleguillos Madrid FM, Gallegos S, Salazar I, Robles P, Toro N. Bioleaching Modeling—A Review. Materials. 2023; 16(10):3812. https://doi.org/10.3390/ma16103812
Chicago/Turabian StyleSaldaña, Manuel, Matías Jeldres, Felipe M. Galleguillos Madrid, Sandra Gallegos, Iván Salazar, Pedro Robles, and Norman Toro. 2023. "Bioleaching Modeling—A Review" Materials 16, no. 10: 3812. https://doi.org/10.3390/ma16103812
APA StyleSaldaña, M., Jeldres, M., Galleguillos Madrid, F. M., Gallegos, S., Salazar, I., Robles, P., & Toro, N. (2023). Bioleaching Modeling—A Review. Materials, 16(10), 3812. https://doi.org/10.3390/ma16103812







