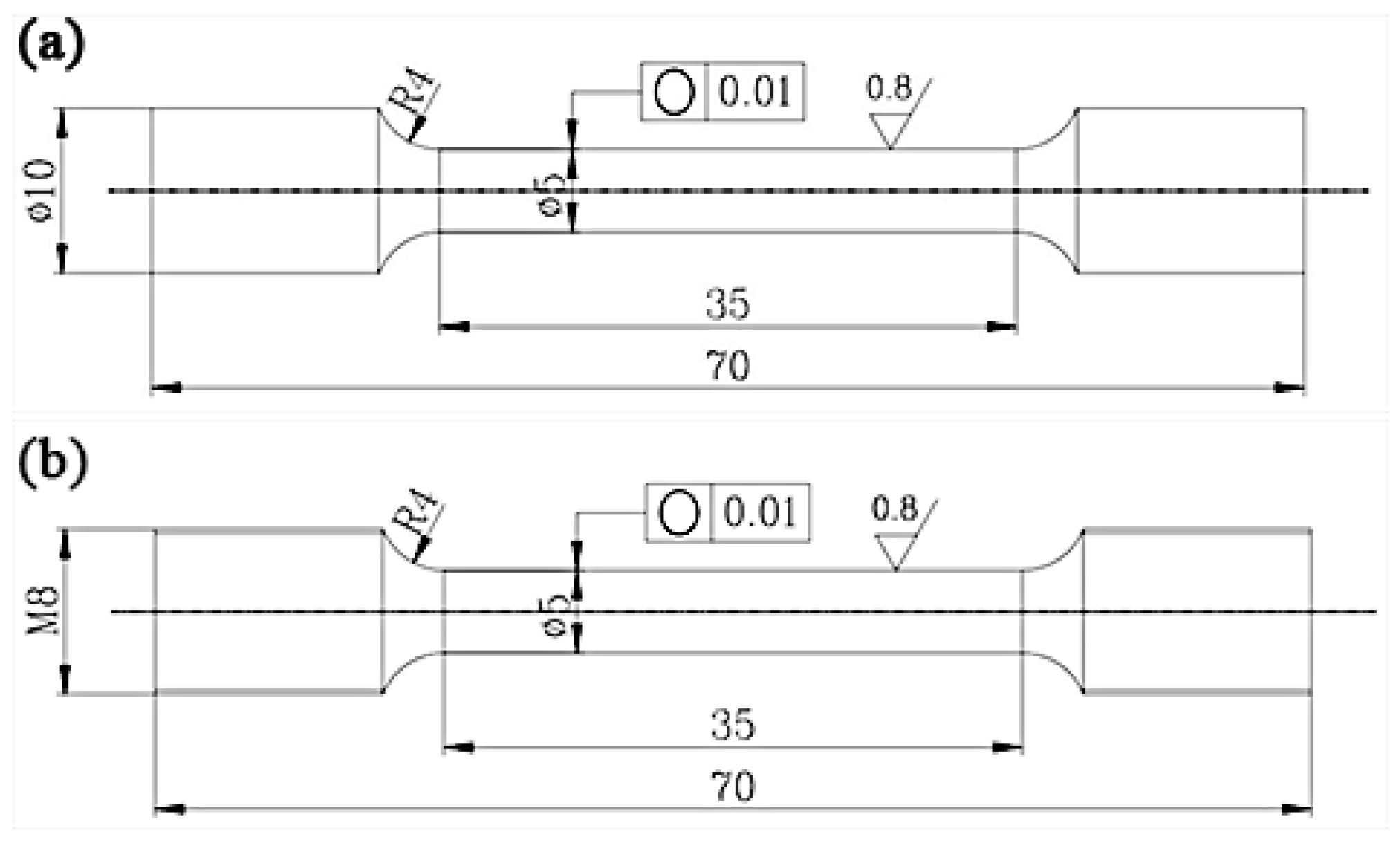
3.1. As-Cast Microstructure
One characteristic of the Mg alloy is its low density. In order to confirm whether the density of the studied alloy still meets the requirements of the light alloy, the density test was conducted on the as-cast ZTM641-0.2Ca-xAl (x = 0, 0.5, 1, 2) alloy, and the test results are shown in
Table 3. It can be seen from the table that the density of the as-cast ZTM641-0.2Ca is 1.7666 g/cm
3, the density of ZTM641-0.2Ca-0.5Al is 1.8240 g/cm
3, and the density of ZTM641-0.2Ca-1Al is 1.8284 g/cm
3. The density of ZTM641-0.2Ca-2Al is 1.8359 g/cm
3. Generally speaking, the alloy density increases with the increase in Al content.
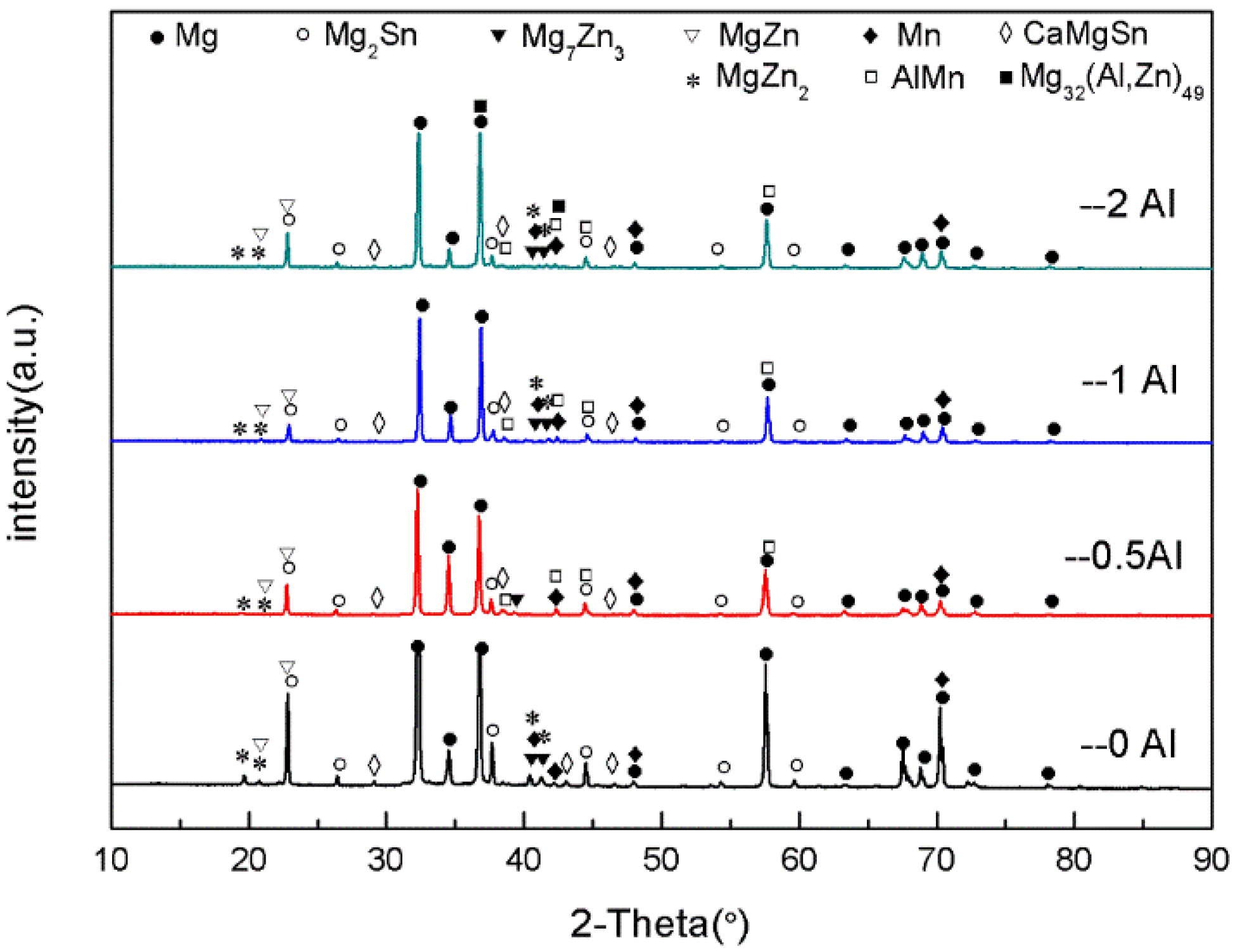
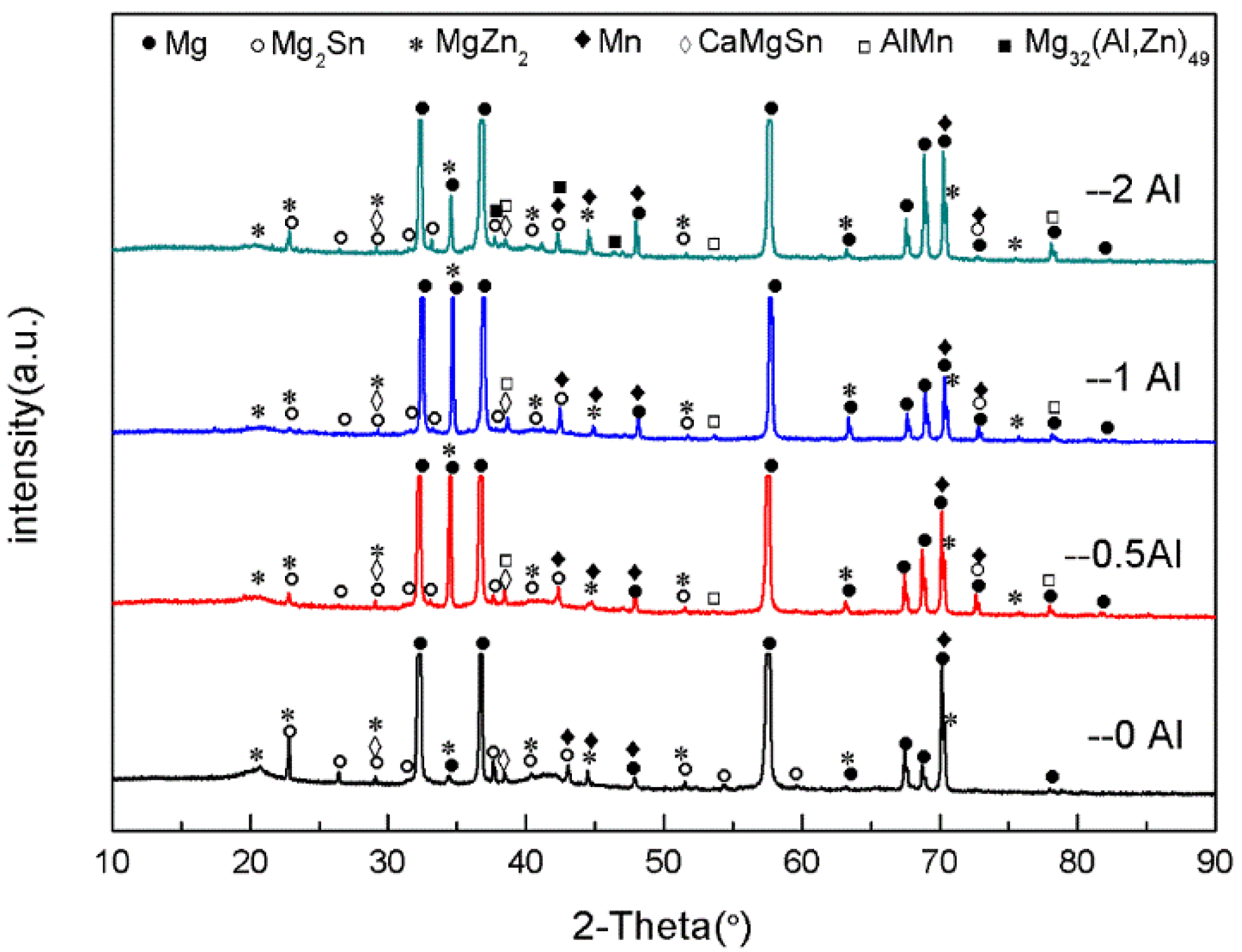
Figure 2 is the XRD graph of the as-cast ZTM641-0.2Ca-xAl (x = 0, 0.5, 1, 2) alloy. From the figure, we can see that the ZTM641-0.2Ca alloy mainly has α-Mg, Mg
2Sn, Mg
7Zn
3, MgZn, α-Mn and CaMgSn phases. As 0.5%Al is added to the alloy, the new diffraction peak of the AlMn phase appears. As the Al element continues to increase to 1%, the diffraction peak of the AlMn phase is significantly enhanced, which indicates that the number of AlMn phases in the ZTM641-0.2Ca-1Al alloy increases significantly. When the Al element continues to increase, a new diffraction peak of Mg
32(Al,Zn)
49 phases is found in the ZTM641-0.2Ca-2Al alloy, but the diffraction peak intensity of the AlMn phases is slightly weakened.
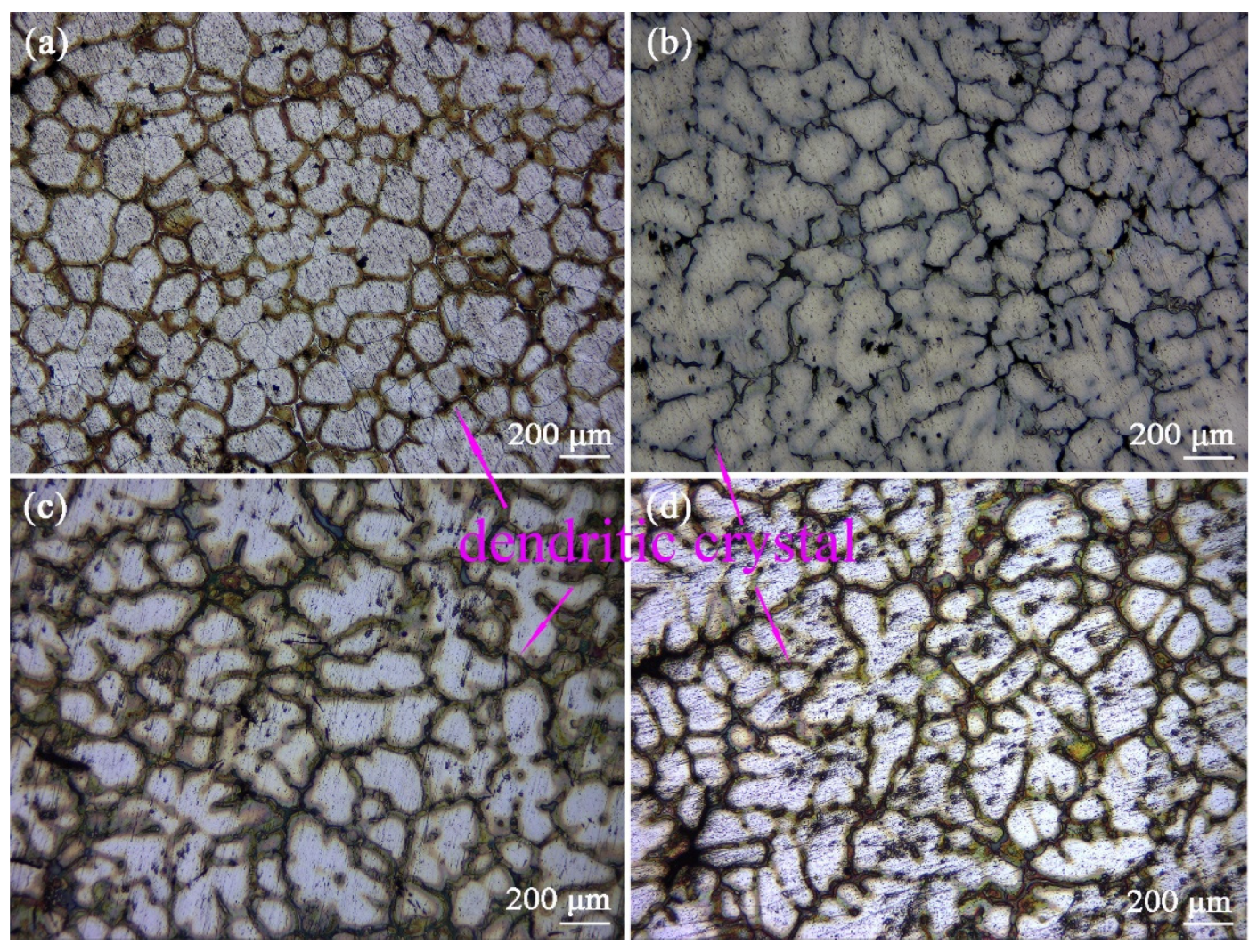
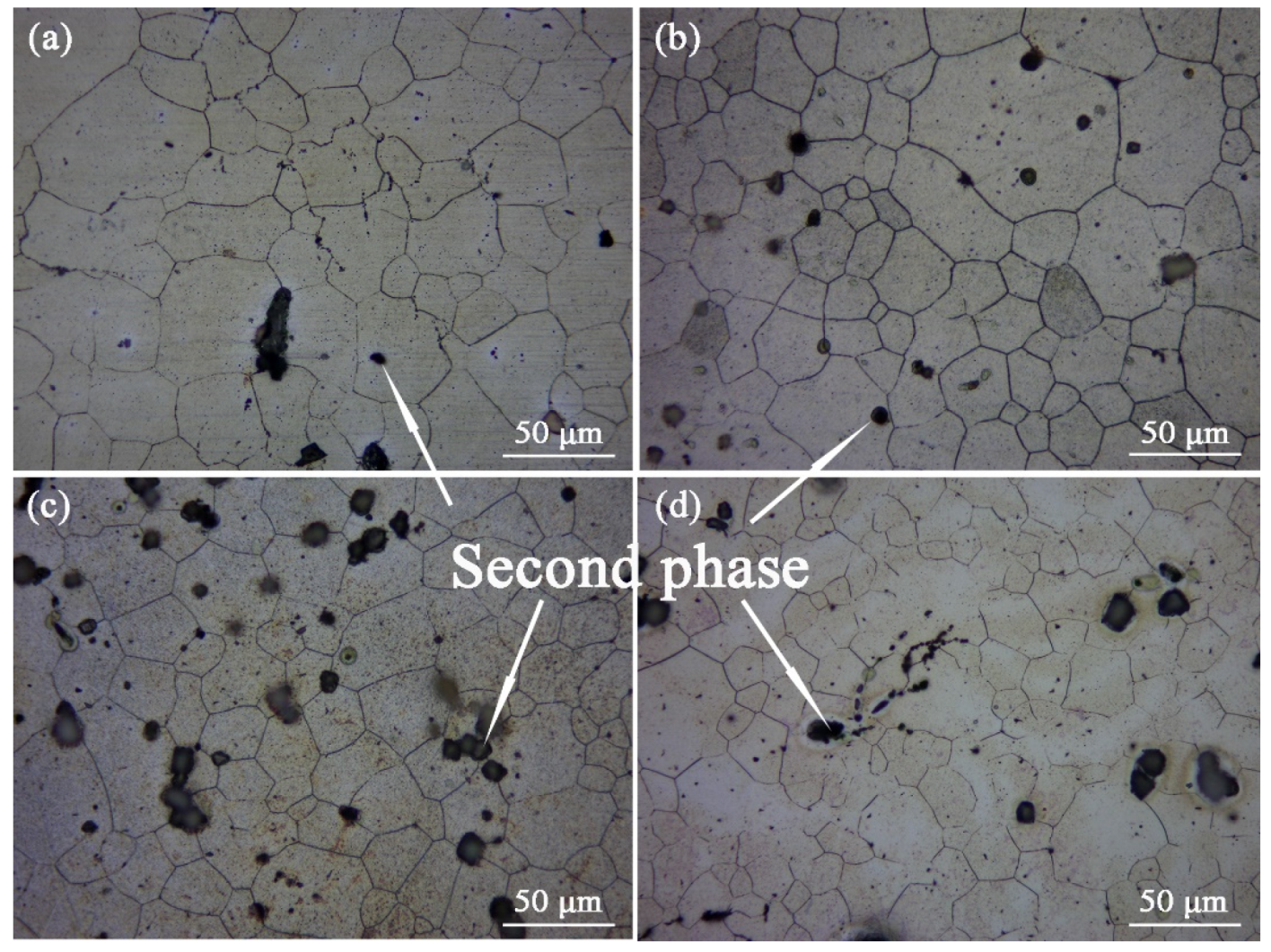
Figure 3 is the Optical graphs of as-cast ZTM641-0.2Ca-xAl (x = 0, 0.5, 1, 2) alloys. From the figure, we can see that the as-cast microstructure mainly consists of an Mg matrix, dendrites, and a dispersed-distributed second phase. Compared with
Figure 3a–d, the secondary growth of the dendrite is more sufficient, and the dendrite distribution is more dense with the addition of Al from 0 to 2%. This is due to the formation of the component supercooling of the Al element at the liquid-solid interface. When the amount of the Al element increases, the composition undercooling is obvious, which accelerates the growth rate of the dendrite tip, and then leads to the spacing decrease in the secondary dendrite.
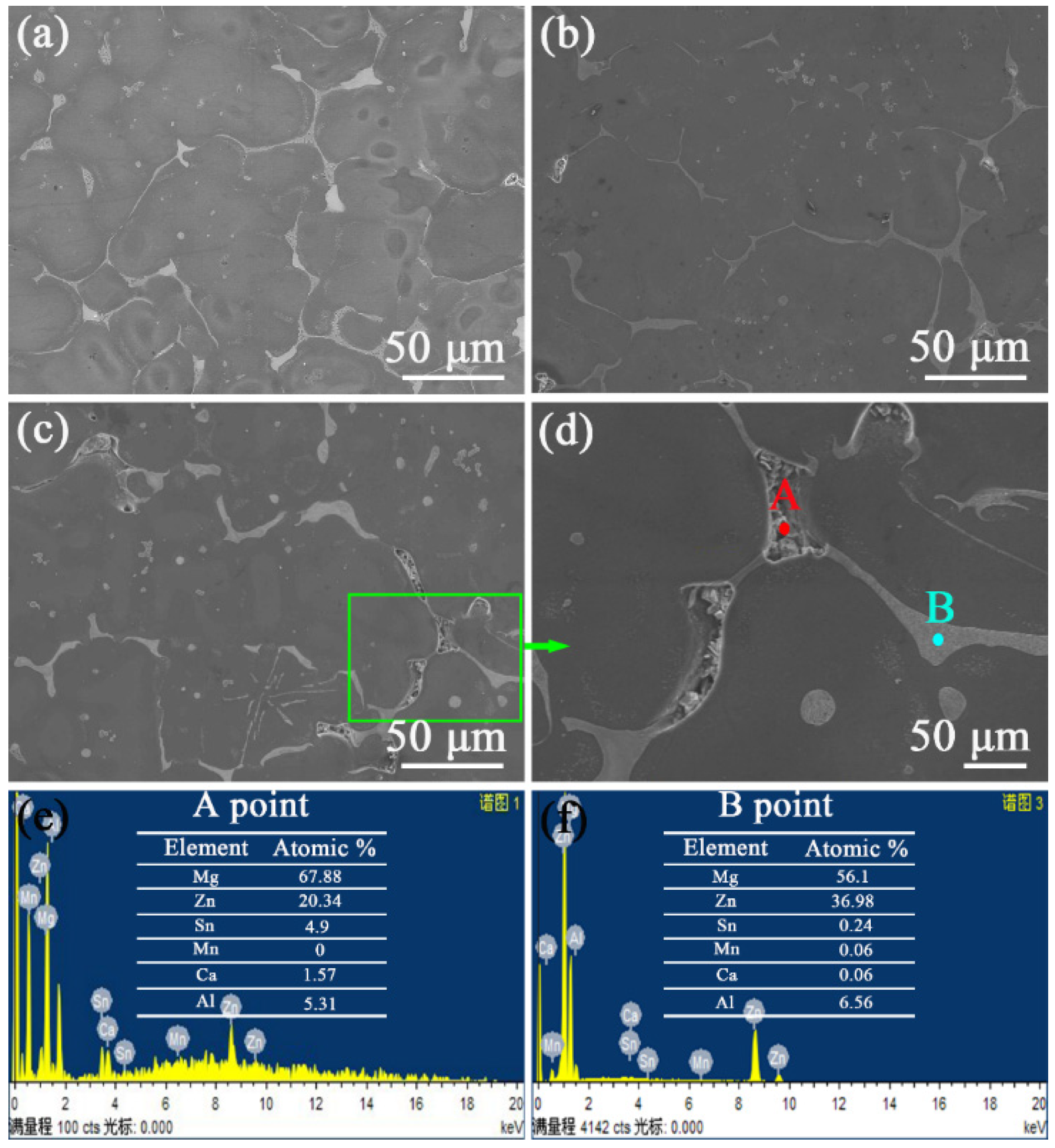
Figure 4 shows the SEM graphs of the dendrites of the as-cast ZTM641-0.2Ca-xAl (x = 0.5, 1, 2) alloy. It can be seen that the dendrite of the ZTM641-0.2Ca-0.5Al alloy mainly has two phases, one being the Mg
2Sn phase and the other the Mg
7Zn
3 phase, in which the Mg
7Zn
3 phase shows a network structure. As the Al element increases to 1%, the type for the dendrite phases does not change, but the morphology of the Mg
7Zn
3 phase changes significantly, and its network structure is obviously refined, as depicted in
Figure 4b. When the Al content continues to increase, the morphology and type of dendrite phase are changed for the ZTM641-0.2Ca-2Al alloy. It mainly has an Mg
2Sn phase, an Mg
7Zn
3 phase, and an Mg
32(Al,Zn)
49 phase. Meanwhile, the network structure of the Mg
7Zn
3 phase is further refined, as shown in
Figure 4c. The gradual refinement of the network structure of the Mg
7Zn
3 phase may result from the aliquation of the Al element on the Mg-Zn eutectic compound. According to the EDS data for point A, B and C, The Mg
7Zn
3 phase contains different Al content, and the greater the Al element, the finer the network structure.
3.3. As-Extruded Microstructure
Table 4 shows the density of the as-extruded ZTM641-0.2Ca-xAl (x = 0, 0.5, 1, 2) alloy. It can be seen from the table that the density of as-extruded ZTM641-0.2Ca-0.5Al is 1.8318 g/cm
3. The density of as-extruded ZTM641-0.2Ca-1Al and ZTM641-0.2Ca-2Al is 1.8469 g/cm
3 and 1.8641 g/cm
3, respectively. The density increases with the increase of the Al content, which is the same as that of the as-cast alloy. However, the density of the as-extruded alloy is slightly higher. This is because after the homogenization and extrusion treatment, the as-extruded composition is more uniform and the microstructure is more closely distributed, which can be confirmed in the subsequent microstructure analysis.
Figure 6 shows the XRD patterns for the as-extruded ZTM641-0.2Ca-xAl (x = 0, 0.5, 1, 2) alloy. According to the results, the as-extruded ZTM641-0.2Ca-xAl alloys mainly consist of the α-Mg, Mg
2Sn, Mg
7Zn
3, MgZn, α-Mn, CaMgSn, MgZn
2, AlMn, and Mg
32(Al,Zn)
49 phases. Unlike the as-cast alloys, these fine diffraction impurity peaks in the as-extruded alloys are significantly reduced after homogenization treatment and extrusion plastic deformation. At the same time, a new diffraction peak of the MgZn
2 phase appears in the as-extruded alloy, and the diffraction peak intensity of the MgZn
2 phases is significantly weakened after the addition of the Al element, which may be due to the combination of Al with the Mg and Zn elements to generate the Mg
32(Al,Zn)
49 phase, which consumes part of Zn element. The variation of diffraction peak intensity of the other phases is similar to that of an as-cast alloy.
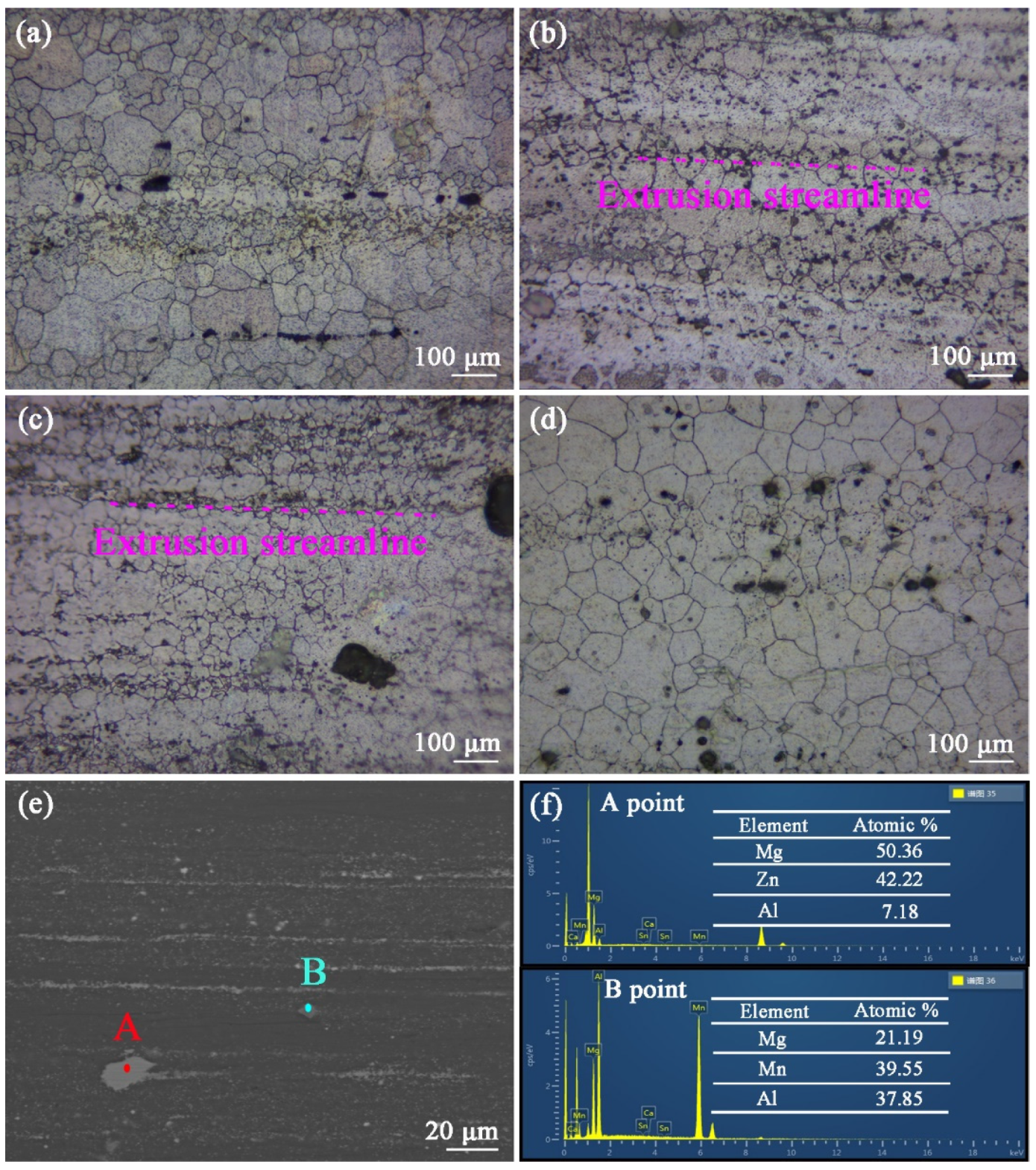
Figure 7a–d are the longitudinal metallographic for the as-extruded ZTM641-0.2Ca-xAl (x = 0, 0.5, 1, 2) alloys. It shows that dynamic recrystallization occurs after the hot extrusion treatment, and these bulk second phases are broken into small particles; these small particles are streamlined distribution. Compared with figure (a), (b), (c) and (d), it was determined that the recrystallized grain size of the ZTM641-0.2Ca-1Al alloy was the smallest. According to the research of Humphreys F J [
19], the large second phase will cause lattice distortion, produce large-angle grain boundaries, promote the crystal grains nucleation, increase the number of crystal grains, and significantly reduce the crystal grain diameter. For studying these second phases of the as-extruded alloys, the as-extruded ZTM641-0.2Ca-2Al alloy was chosen for SEM observation. The observation results are shown in
Figure 7e. It can be seen that the white second phase particles are distributed into the matrix with a streamlined distribution, and some large second phases are also observed. Through the EDS results in
Figure 7f, as we can see that point A mainly contains three elements: Mg, Zn and Al. According to the XRD pattern in
Figure 6, the second phase can be determined as the Mg
32(Al,Zn)
49 phase. Point B mainly contains the three elements of Mg, Al and Mn, and Al:Mn = 1:1. Combined with the XRD pattern, the second phase can be determined as the AlMn phase.
Figure 8 shows the TEM micrographs of the as-extruded ZTM641-0.2Ca-2Al alloy. As shown in
Figure 8a, the microstructure of the as-extruded ZTM641-0.2Ca-2Al alloy contains some rod-like phases of about 1 μm in diameter, and the distribution is relatively concentrated. The aggregation of rod-like phases may be due to the large number of boundary surfaces of the rod-like phases, and the aggregation growth can reduce part of the interface. According to the minimum energy criterion, the decrease of interface energy can make the system tend to a more stable state. The EDS results and XRD patterns confirm that these rod-like phases are MgZn phases. The rod-like MgZn phase is also observed in
Figure 8b. Through the EDS results, the CaMgSn phase is observed in the dislocation pile-up. This indicates that the Ca element and Sn element are enriched here. This is due to the lower energy required for the movement of atoms in dislocations, which makes it easier for Ca to combine with the Sn and Mg atoms. Therefore, the CaMgSn phase is nucleated and precipitated with dislocation and dislocation entanglement.
3.4. Solution-Treated and Aged Microstructures
The phases of the solid solution ZTM641-0.2Ca-xAl (x = 0, 0.5, 1, 2) alloy are analyzed by XRD patterns. The results are shown in
Figure 9. Among them, the solid solution ZTM641-0.2Ca alloy mainly consists of α-Mg, Mg
2Sn, MgZn
2, α-Mn and CaMgSn phases. As 0.5%Al is added to the alloy, the ZTM641-0.2Ca-0.5Al alloy is α-Mg, Mg
2Sn, MgZn
2, α-Mn, CaMgSn and AlMn phases, and the Al element is mainly used to form the AlMn phase. As the Al content increases to 1%, the phase type does not change. As the Al element continues to increase to 2%, the phase of ZTM641-0.2Ca-2Al changes into the α-Mg, Mg
2Sn, MgZn
2, α-Mn, CaMgSn, AlMn and Mg
32(Al,Zn)
49 phases. Due to the further increase of the Al content, the Al element is mainly used to form the AlMn phase and the Mg
32(Al,Zn)
49 ternary phase. Compared with the as-extruded alloys, the diffraction peaks of the Mg
7Zn
3 and MgZn phases are not detected. This indicates that these two phases all melt into the matrix, and the Zn element is mainly combined with Mg and Al to form the MgZn
2 phase and the Mg
32(Al,Zn)
49 phase. Comparing the several curves, it is found that when the Al element increases, the diffraction peak intensity of the AlMn phase increases significantly.
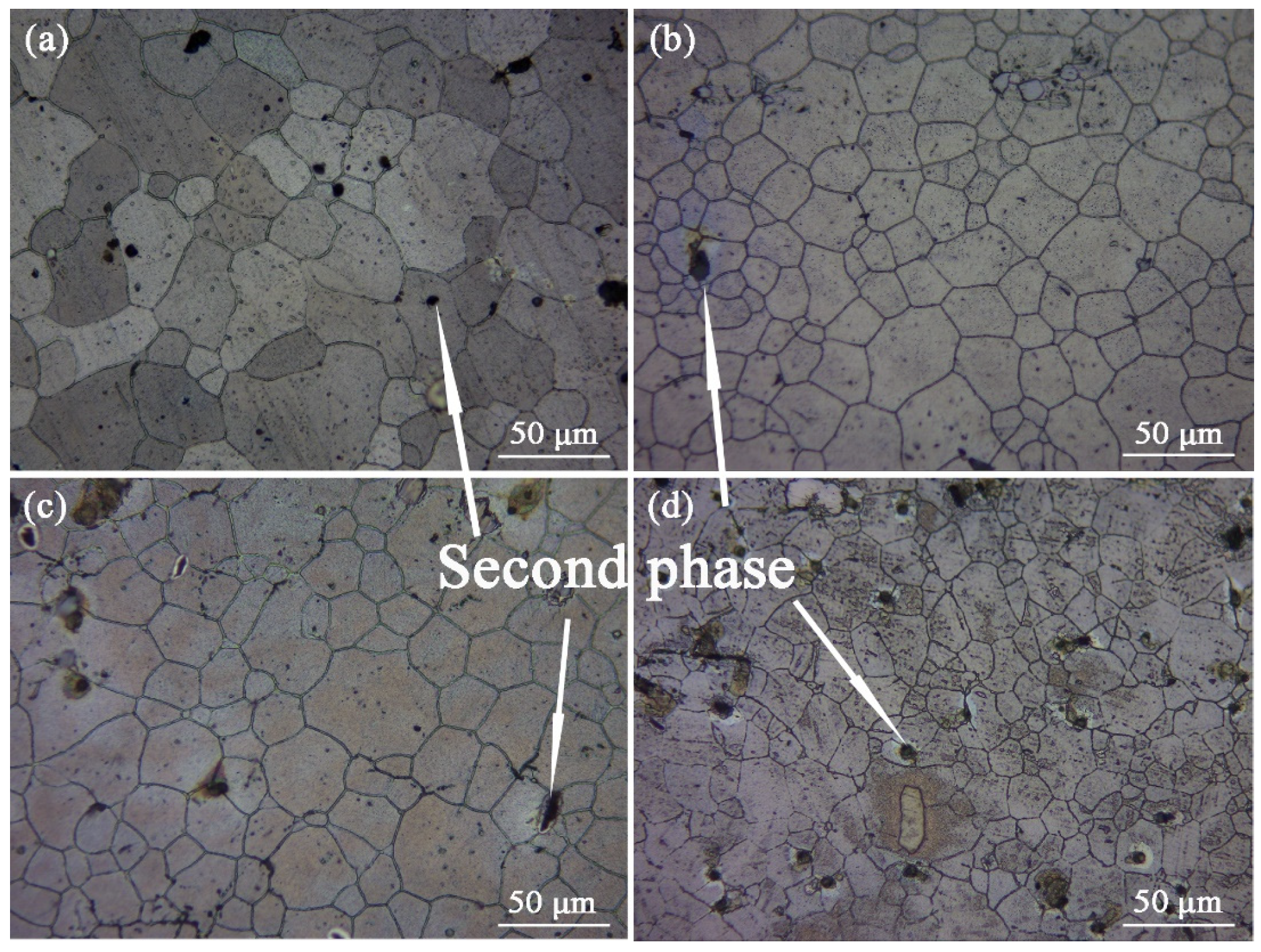
Figure 10 shows the optical micrographs of the solid solution ZTM641-0.2Ca-xAl (x = 0, 0.5, 1, 2) alloy. According to the figure, the microstructure for the solid solution alloy includes the Mg matrix, grain, and second phases. After this solution treatment, the recrystallized grain grows further, and the average grain size decline with the Al element rises, although the change is not very obvious, which indicates that the Al element plays a certain role in grain refinement for the alloys. The grain refinement may be due to the combination of the Al element and the Mn element to form the AlMn phase. The AlMn phase preferably precipitates and concentrates at the front-end α-Mg phase, which pins the grain boundary and hinders the grain growth. For the ZTM641-0.2Ca alloy, the majority of second phases have been dissolved into the matrix, as shown in
Figure 10a. When 0.5%Al is being added, some black second phase with a diameter of about 5 μm is observed in the microstructure, a large number of them are distributed on the grain boundary, and a few are distributed in the grain. As the Al element increases to 1%, the number of second phase particles rise, and the diameters of these particles rise to about 10 μm. All of them are distributed on the grain boundaries, and some of them are of an aggregated distribution. When the Al content continues to increase to 2%, the second phase particle continues to grow.
To observe the microstructure and morphology for solid solution alloys from a clearer perspective, the solid solution ZTM641-0.2Ca-0.5Al alloy is selected for scanning electron microscope observation. The observation results are shown in
Figure 11a. We can see that the solid solution structure has substrate and second phase particles, and the particle sizes are not unique, among which the particles with a small diameter are point-like. They are mainly located in the grain boundary, although a small portion of them are located in the grain interior. The second phase, which is larger in size, is angular and massive, mainly distributed on the grain boundary.
Figure 10b is the red box’s magnification in
Figure 10a. We can clearly observe that these second phases of the bright white angular block are located at the intersection of several grain boundaries. The grain diameter is about 5 μm. In order to determine the specific composition of the second phase particles in the figure, the region shown in
Figure 10b was scanned by the energy spectrum, as shown in
Figure 11. It can be observed that Mg mainly exists in the matrix. Zn appears in the matrix and second phases. Sn elements are also segregated in the matrix and second phases. The Mn element appears to be segregated in the second phase and disperses in the matrix. The Ca elements are uniformly distributed in the matrix and second phases. This distribution of Al elements in the matrix is uniform, and there is segregation in the second phase particles. In general, these second phases of the angular block are AlMn phases.
After solid solution treatment, the ZTM641-0.2Ca-xAl (x = 0, 0.5, 1, 2) alloy is subjected to artificial aging treatment. Because there is no obvious difference in the microstructure between one-stage and two-stage aged alloys, we selected the two-stage aged alloy as that representing the microstructure analysis in this paper.
Figure 12 is the optical micrograph of the two-stage aged ZTM641-0.2Ca-xAl (x = 0, 0.5, 1, 2) alloy. We can see that these second phase particles at the microstructure are less for the ZTM641-0.2Ca-0.5Al alloy. The second phase particles increased significantly for the ZTM641-0.2Ca-1Al alloy, most of which are located on the grain boundary, as shown in
Figure 12c. When the Al element increases to 2%, the more second phases particles that appear in the microstructure, the more diffuse is the distribution, the majority of them are located in grain boundaries, and a few of them are distributed in grains. Compared with
Figure 12a–d, it was found that the grain size declines when the Al element increases, and the grain size of the ZTM641-0.2Ca-2Al alloy is the smallest.
Figure 13 shows the XRD patterns of the two-stage aged ZTM641-0.2Ca-xAl (x = 0, 0.5, 1, 2) alloy. From this figure, we can see that the phase composition of the two-stage aged alloy are mainly the α-Mg, Mg
2Sn, MgZn
2, α-Mn, CaMgSn, AlMn and Mg
32(Al,Zn)
49 phases. The addition of Al mainly forms the AlMn phase and the Mg
32(Al,Zn)
49 phase with the other elements in the alloy. The variation of other precipitated phases is similar to that of the solid solution alloy, but these diffraction peaks of Mg
2Sn and MgZn
2 phases for the two-stage aged alloy are obviously wide, which indicates that the particle size for these two precipitated phases is small.
To clearly observe the microstructure of the aged alloy, the two-stage aged ZTM641-0.2Ca-xAl (x = 0.5, 1, 2) alloy is observed in these SEM images. As shown in
Figure 14, the microstructure of the alloys mainly consists of the Mg matrix and second phases. Among them, the ZTM641-0.2Ca-0.5Al alloy has a large second phase with a diameter of about 10 μm and a blocky morphology. In addition, most of these second phases are located on grain boundaries, while a few are located at the grain. For the ZTM641-0.2Ca-1Al alloy, the number of large second phase particles of these alloys increases, while the particles’ morphology and distribution are similar to the ZTM641-0.2Ca-0.5Al alloy. For the ZTM641-0.2Ca-2Al alloy, the number of second phase particles of these alloys increases further, and the morphology is still angular and blocky. It can be seen in Figure (c) that these second phase particles are mainly located on the grain boundary.
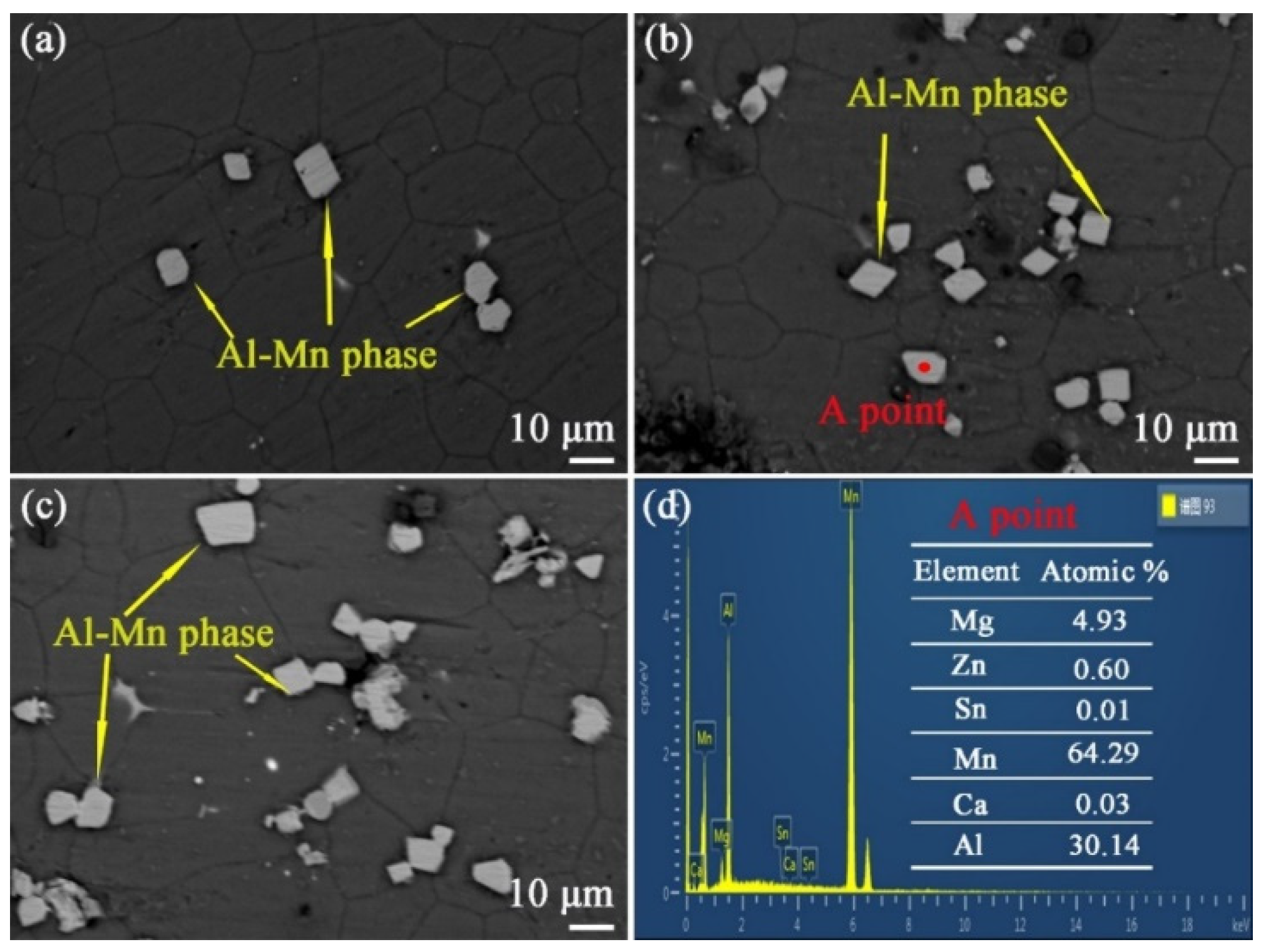
In order to determine the composition of these second phases, EDS testing was chosen, and these results are shown at
Figure 14d. As shown in the graph, the elemental composition of the second phase in point A is mainly Mg, Al and Mn. Due to the second phase being located on the matrix, the Mg element will inevitably exist in the EDS results. Therefore, this second phase is the Al-Mn phase. Compared with
Figure 10 and
Figure 12, the aged alloy has more second phase particles. This is due to most of the Al and Mn atoms being dissolved into the matrix during the solid solution process. When the alloy is subjected to an aging treatment, the Al and Mn atoms will continue to precipitate with the form of Al-Mn phase. It is worthy of note that in the EDS result in
Figure 14d, the atomic ratio of the precipitated phase is Al:Mn = 1:2, but the XRD graph in
Figure 13 shows that the Al-Mn phase is the AlMn phase. These results conflict with each other. According to the previous literature [
20], some researchers think that the Al-Mn phase is the Al
8Mn
5 phase. In this paper, this phase was tentatively named the Al-Mn phase after thorough consideration.
3.5. Mechanical Properties
Figure 15 shows the mechanical properties of the as-extruded ZTM641-0.2Ca-xAl (x = 0, 0.5, 1, 2) alloy at room temperature. As can be seen, the as-extruded ZTM641-0.2Ca alloy has the highest comprehensive mechanical property; that is, the UTS, YS and elongation are 336 MPa, 230 MPa and 14.5%. According to our previous research, the UTS, YS and the elongation of the Mg-6Sn-4Zn-1Mn alloy are 328 MPa, 255 MPa and 10.76%, respectively. When 0.5% Al was added, the properties of the alloy decreased slightly, with the UTS, YS and elongation at 329 MPa, 224 MPa and 11.9%, respectively. When the Al content increases to 1%, the UTS and YS decrease to 327 MPa and 221 MPa, but the elongation increases to 13%. When the Al content is 2%, the UTS is 324 MPa, the YS is 218 MPa, and the elongation is 14%. In general, the properties of the as-extruded ZTM641-0.2Ca-xAl (x = 0, 0.5, 1, 2) alloy decreased slightly with the increase of Al content, but the overall difference was not significant. The reason for this variation trend is that the Al element was added to the alloy and combined with the Mn element in the alloy to form the AlMn phase. According to the previous microstructure analysis, the higher Al content, the larger the second phase, and the worse binding force with the matrix. When the alloy is deformed, the coordinating deformation ability of the alloy is poor, resulting in a decrease in the strength of the alloy.
Figure 16 shows the strength and plasticity of the aged ZTM641-0.2Ca-xAl (x = 0, 0.5, 1, 2) alloy at room temperature, and Figure (a) and Figure (b) correspond to the one-stage aging and two-stage aging, respectively. From Figure (a), we can see that the one-stage aged ZTM641-0.2Ca alloy has the highest strength, with the UTS and YS being 392 MPa and 365 MPa, respectively. According to our previous research [
21], the UTS and YS of the one-stage aged ZTM641-0.2Al alloy are 382 MPa and 352 MPa, and the UTS and YS of one-stage aged ZTM641-0.5Al alloy are 350 MPa and 299 MPa. When 0.5% Al is added to the alloy, the strength and plasticity show a decreasing trend, while the UTS and YS are 365 MPa and 325 MPa, respectively. When the Al content increases to 1%, the UTS and YS continually decrease to 350 MPa and 304 MPa, respectively. In addition, the UTS and YS of the ZTM641-0.2Ca-2Al alloy are further reduced to 333 MPa and 269 MPa, respectively. On the other hand, with the gradual increase of the Al content, the elongation of the alloy gradually increases. When the Al content increases to 2%, the elongation increases significantly, to 13.2%. For the two-stage aged alloy, the variation of properties is consistent with that of the one-stage aged alloy. As shown in
Figure 16b, the two-stage aged ZTM641-0.2Ca alloy also has the highest strength, with UTS and YS at 407 MPa and 392 MPa, respectively. According to our previous research, the UTS and YS of the two-stage aged ZTM641-0.2Al alloy are 384 MPa and 360 MPa. When 0.5%Al is added to the alloy, the alloy strength decreases slightly, and the UTS and YS are 369 MPa and 328 MPa, respectively. When the Al content continues to increase, the strength of the alloy also continues to decrease. When the Al content is 2%, the UTS and YS of the alloy decreases to 350 MPa and 305 MPa. When the Al atom content is 2%, the elongation of the alloy is the best, at 12%. The gradual improvement of the plasticity may be due to the more Al atoms that are dissolved into the matrix with the increase of Al content, which effectively reduces the stacking fault energy of the alloy. Due to the low stacking fault energy of the alloy, dislocation cells do not form easily, which effectively prevents the nucleation and growth of cracks, and improves the plasticity of the alloy [
22]. As for the alloy strength gradually decreasing, this may be due to the large size of the Al-Mn phase in the alloy. On the one hand, the large size of the second phase reduces the coordinated deformation ability of the matrix. On the other hand, it can be clearly observed from
Figure 14 that the large size of the Al-Mn phase is mainly distributed on the grain boundary. With the increase of Al content, the amount of the Al-Mn phase on the grain boundary also increased. Due to the size of the second phase particles on the grain boundary affecting the plastic deformation ability, when the size and number of the second phase particles on the grain boundary are larger, the cracks are more likely to appear in the alloy, and the corresponding strength is lower. Therefore, the strength of the ZTM641-0.2Ca-2Al alloy is the lowest.
Figure 17a,b show the strength and plasticity of the as-extruded ZTM641-0.2Ca-xAl (x = 0, 0.5, 1, 2) alloy at 150 °C and 200 °C, respectively. It can be seen from Figure (a) that the UTS and YS of the ZTM641-0.2Ca-xAl alloy gradually increases with the addition of the Al element. Among them, the strength of the ZTM641-0.2Ca-2Al alloy i the highest, and the UTS and YS are 159 MPa and 132 MPa, respectively. However, the elongation of the ZTM641-0.2Ca-xAl alloy decreases gradually with the addition of Al; the elongation of ZTM641-0.2Ca-2Al alloy is only 5%. According to Figure (b), the variation trend of the mechanical properties at 200 °C is consistent with that at 150 °C. The UTS and YS of the ZTM641-0.2Ca-2Al alloy are the highest, at 103 MPa and 90 MPa. However, the elongation is lower, at 59.8%.
According to the previous as-extruded microstructure, it can be seen that the Al element is added to the alloy and combines with the Mn element to generate the AlMn phase. The AlMn phase is a high-melting phase with a melting point of 670 °C. When the alloy is deformed under high temperature, the AlMn phase can pin the grain boundaries, hindering dislocation slip. According to metal theory [
23], when the alloy is subjected to stress and deformation at high temperatures, if the size of second phase is large, the dislocation cannot cut through the second phase, but can only bypass the second phase. In this process, the dislocation must overcome the resistance caused by the bending dislocation tension. Specifically, the shear stress required by the dislocation bypass phase is shown in the following formula:
where G is the tangent modulus of elasticity,
b is the Berkovian vector of dislocations, and λ is the distance between the dislocation and the precipitate. Based on the previous microstructure analysis, when the Al content increases from 0.5% to 1% and 2%, the number and size of the second phase also increases correspondingly, and the required shear stress also increases, which improves the strength. However, due to the AlMn phase being a brittle phase, the higher content of the AlMn phase will inevitably lead to the deterioration of the plasticity.