Comparison of (K0.5Na0.5)NbO3 Single Crystals Grown by Seed-Free and Seeded Solid-State Single Crystal Growth
Abstract
1. Introduction
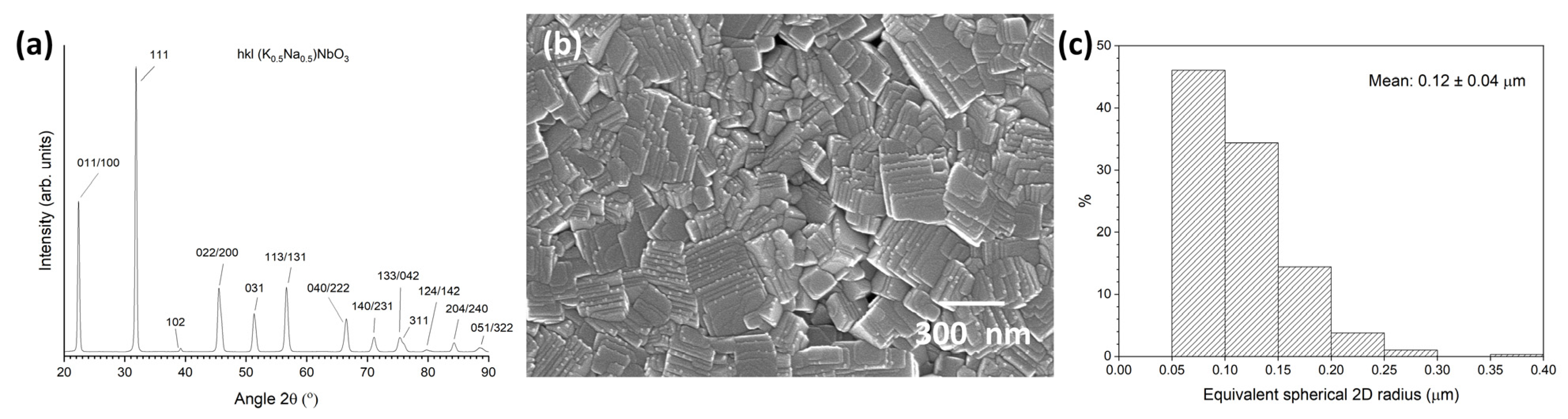
2. Materials and Methods
3. Results
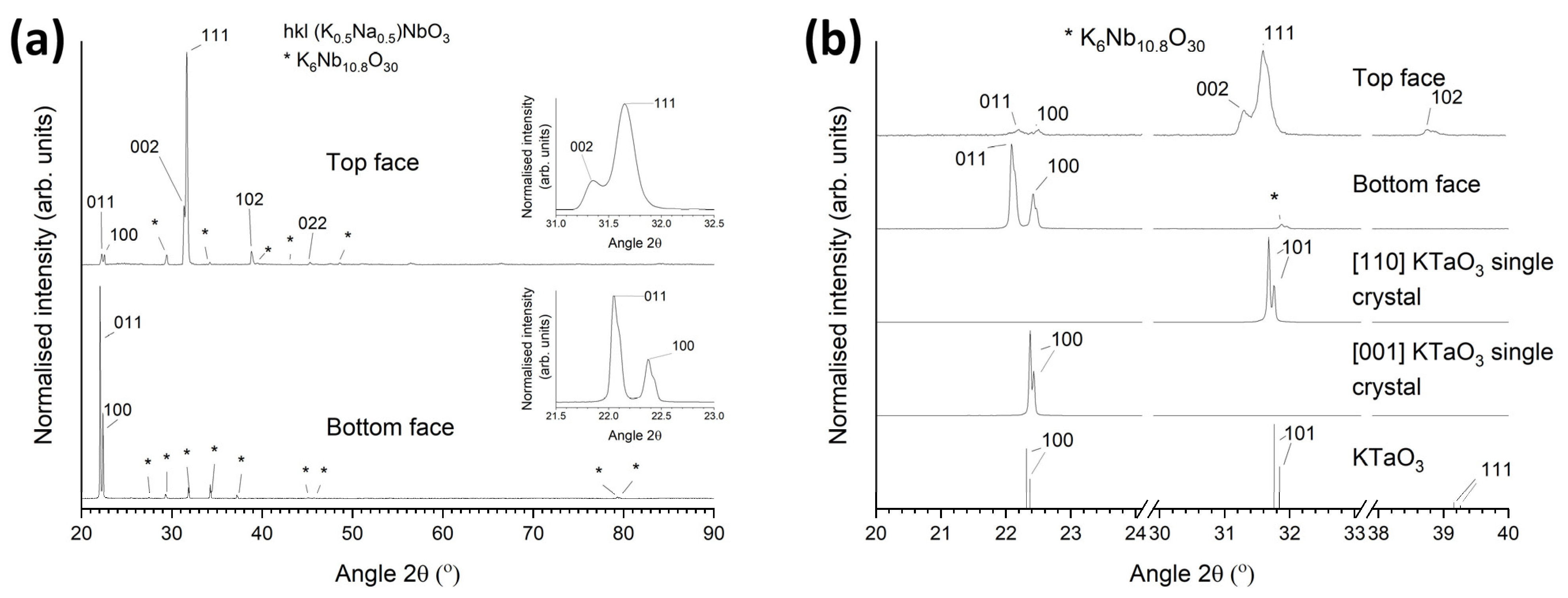
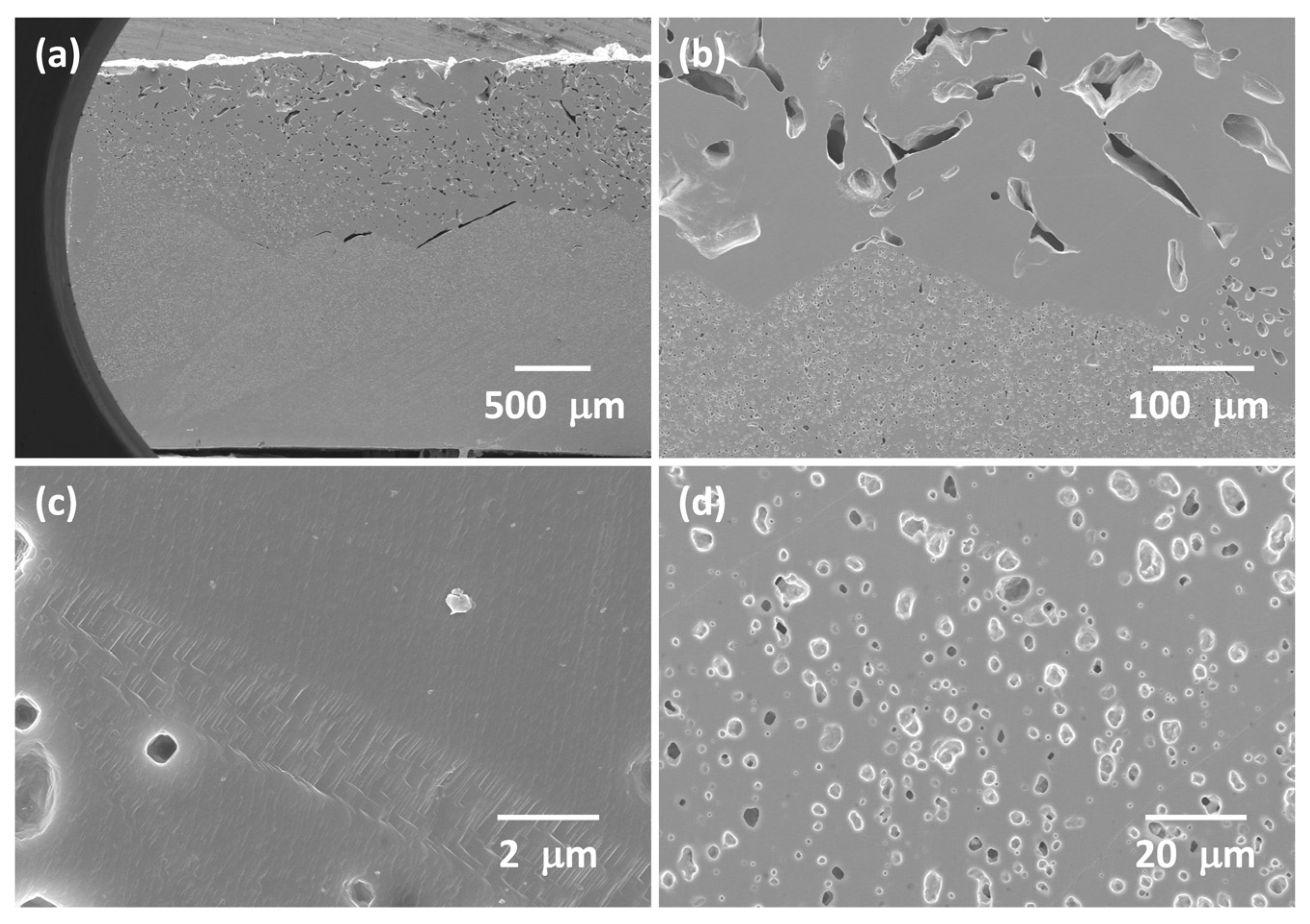
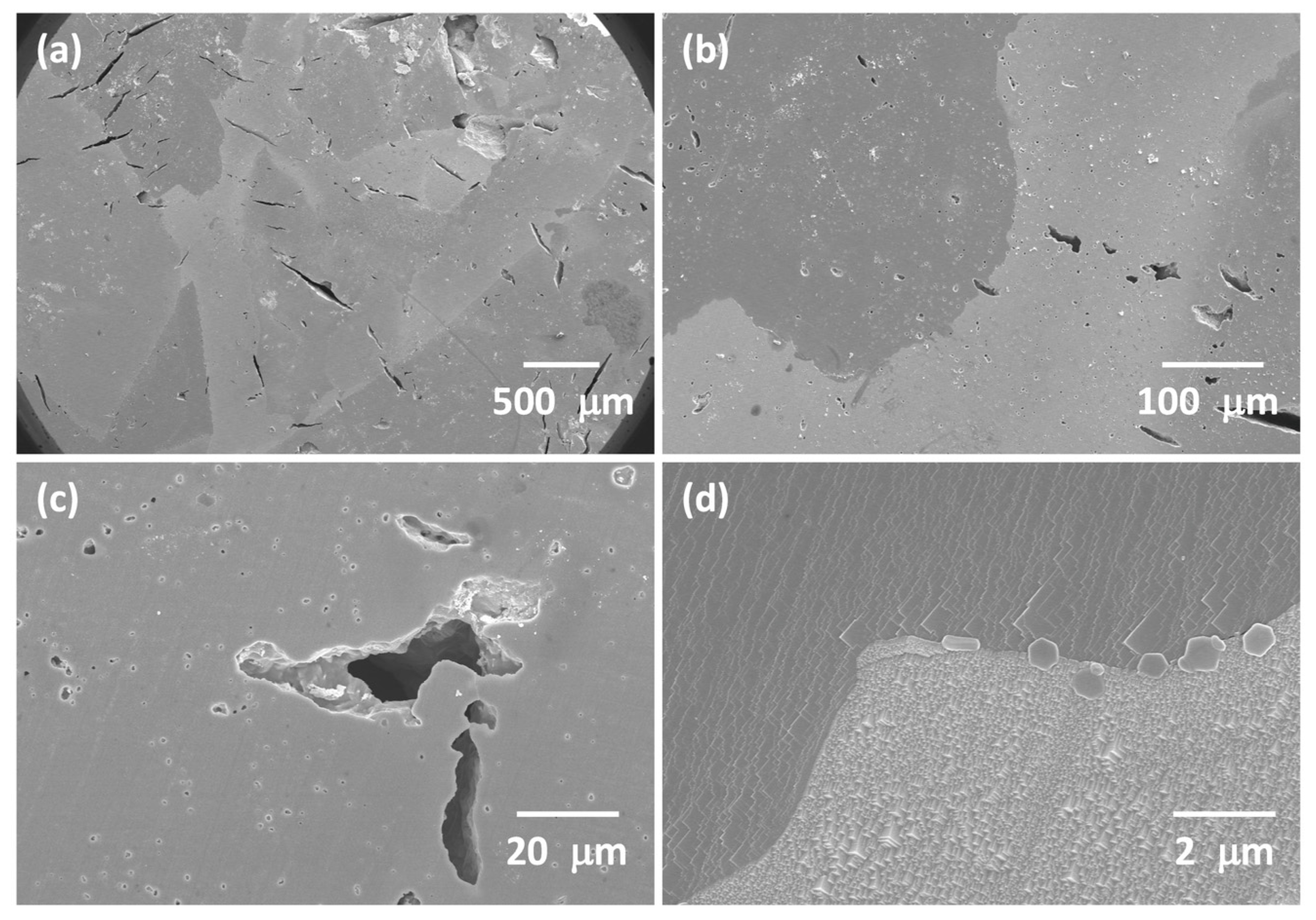
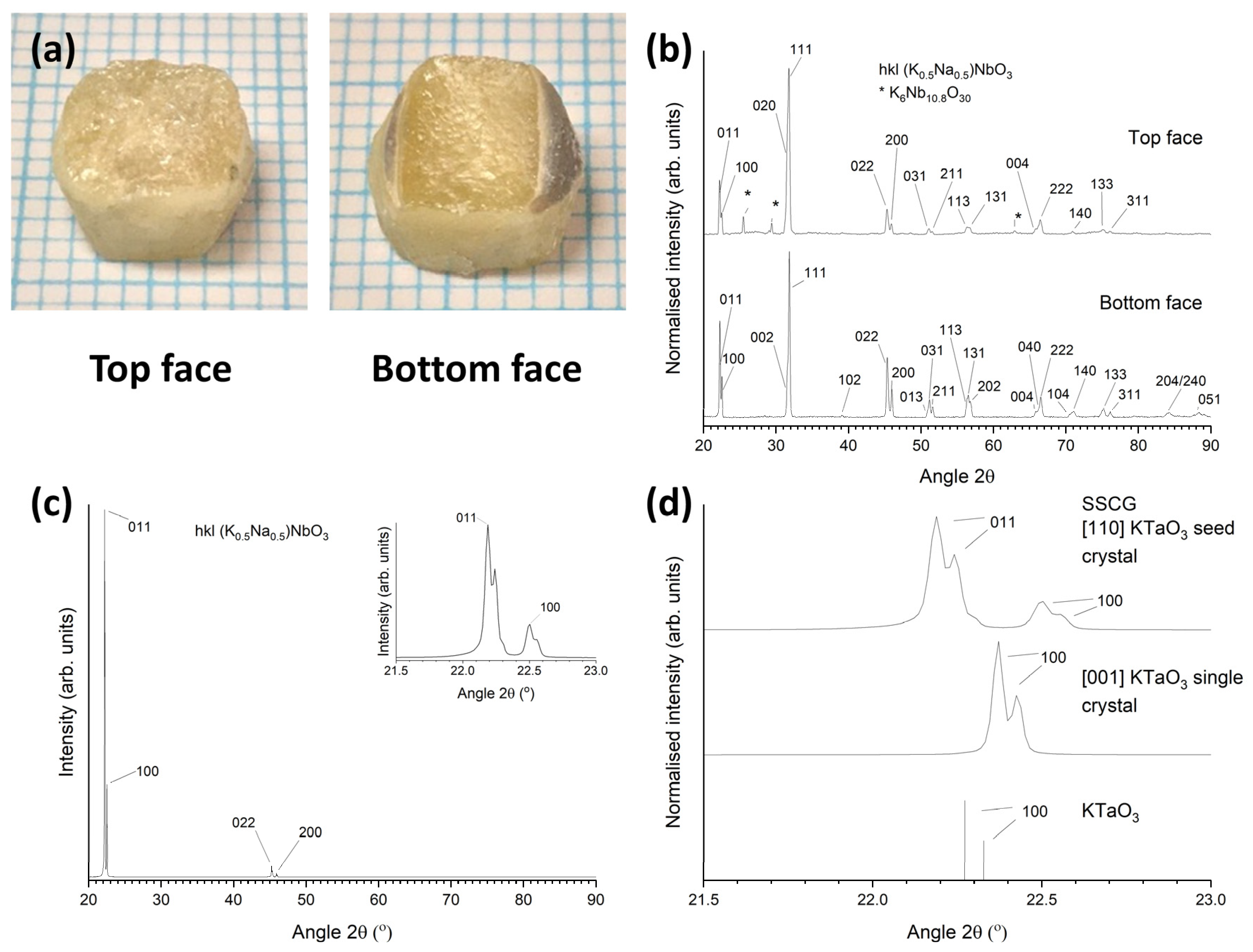
3.1. 98.5 mol% (K0.5Na0.5)NbO3-1.5 mol% Ba1.05Nb0.77O3 Seed-Free Solid-State Crystal Growth
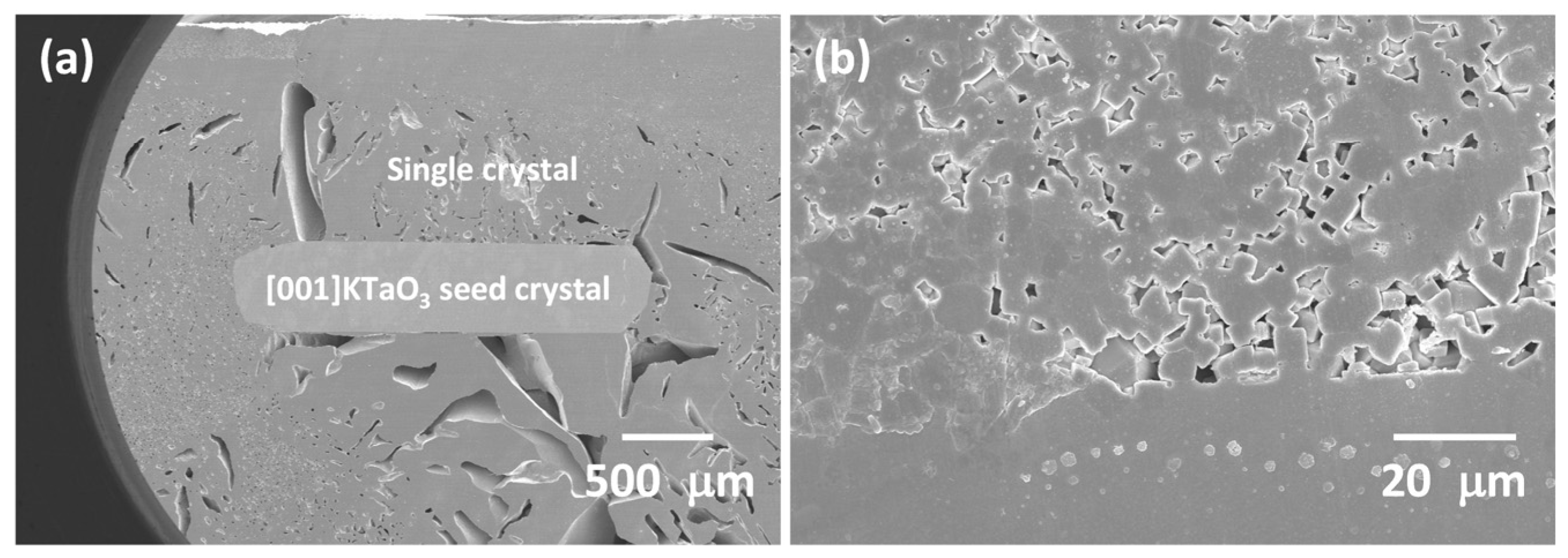
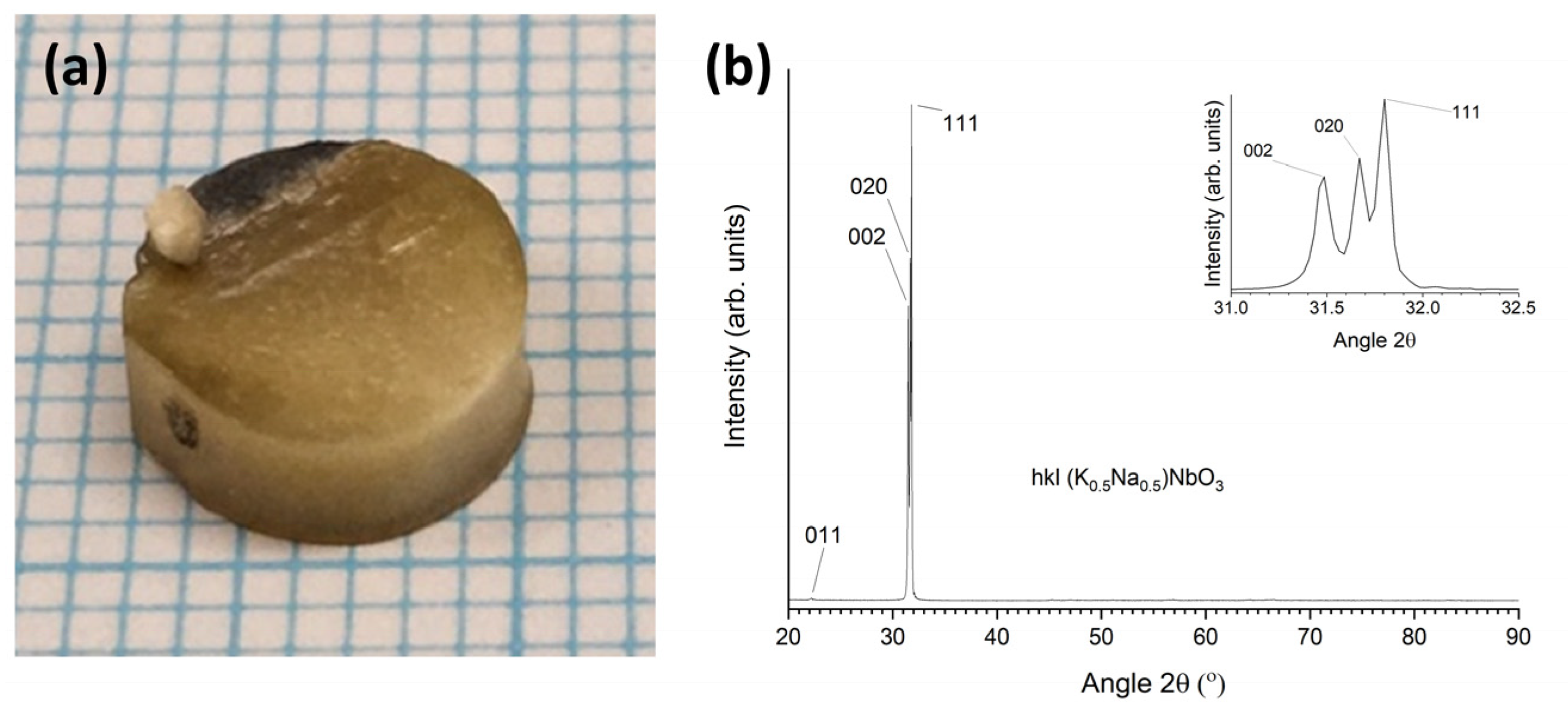
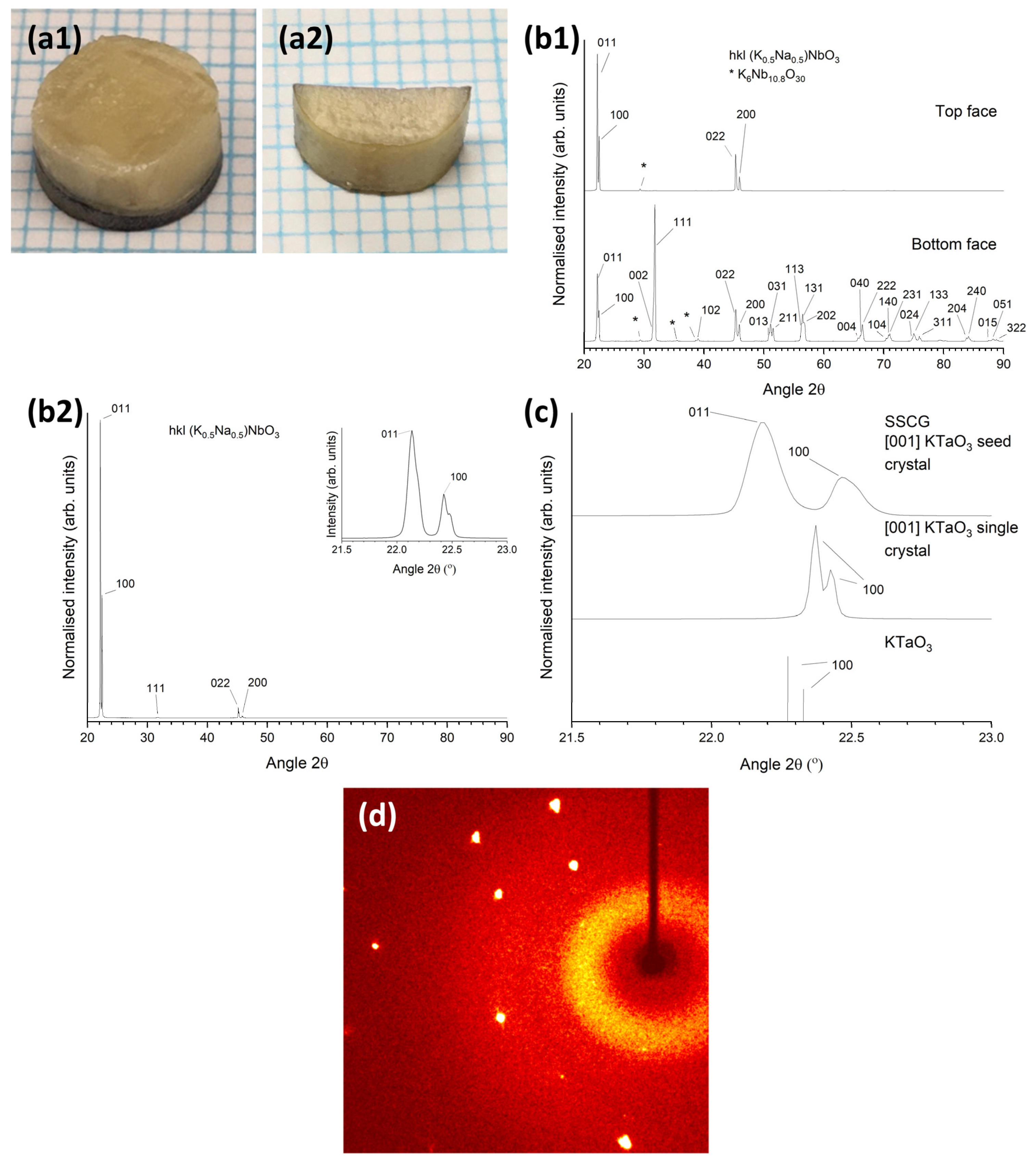
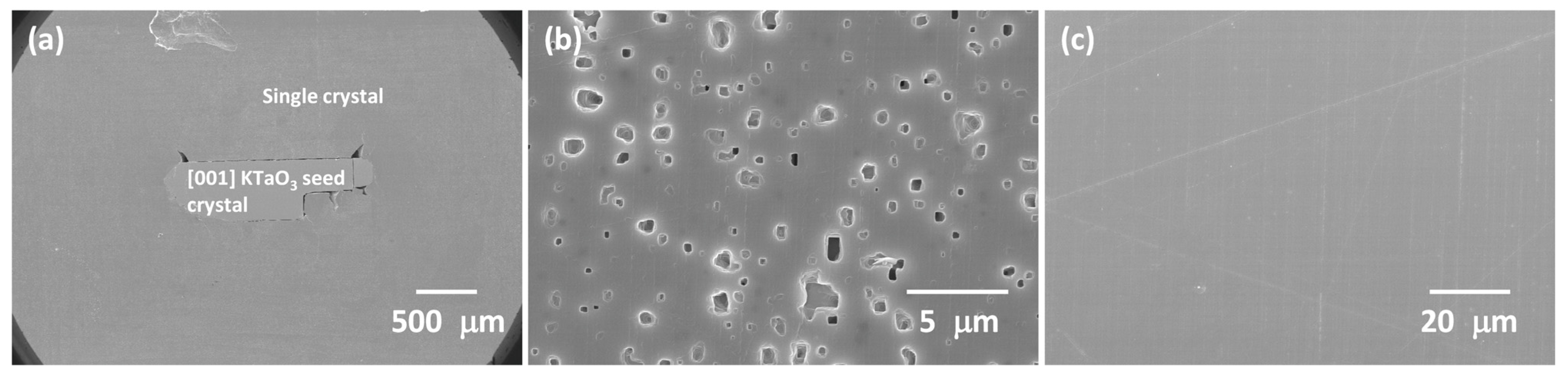
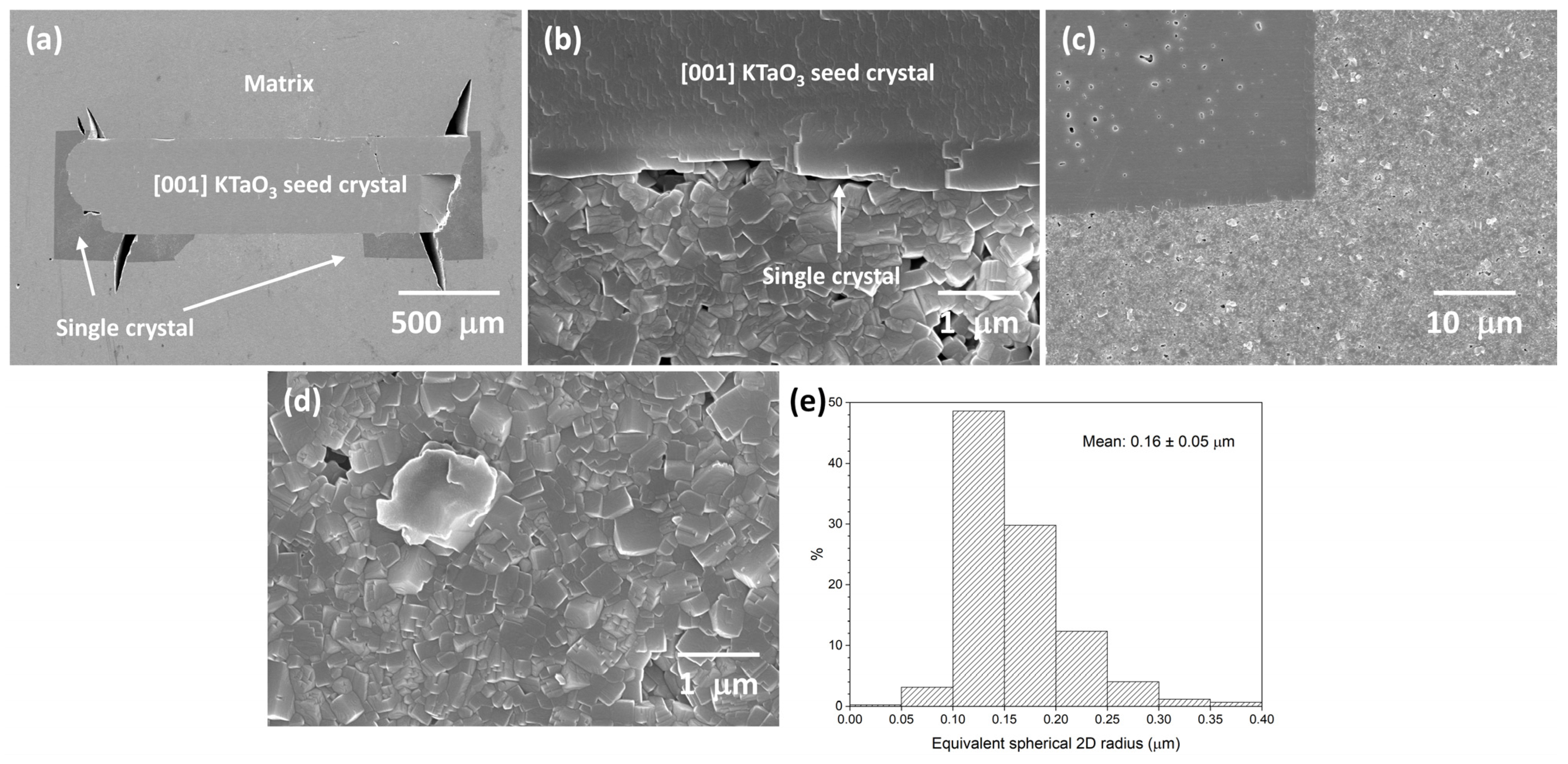
3.2. 98.5 mol% (K0.5Na0.5)NbO3-1.5 mol% Ba1.05Nb0.77O3 Seeded Solid-State Crystal Growth: [001] KTaO3 Seed
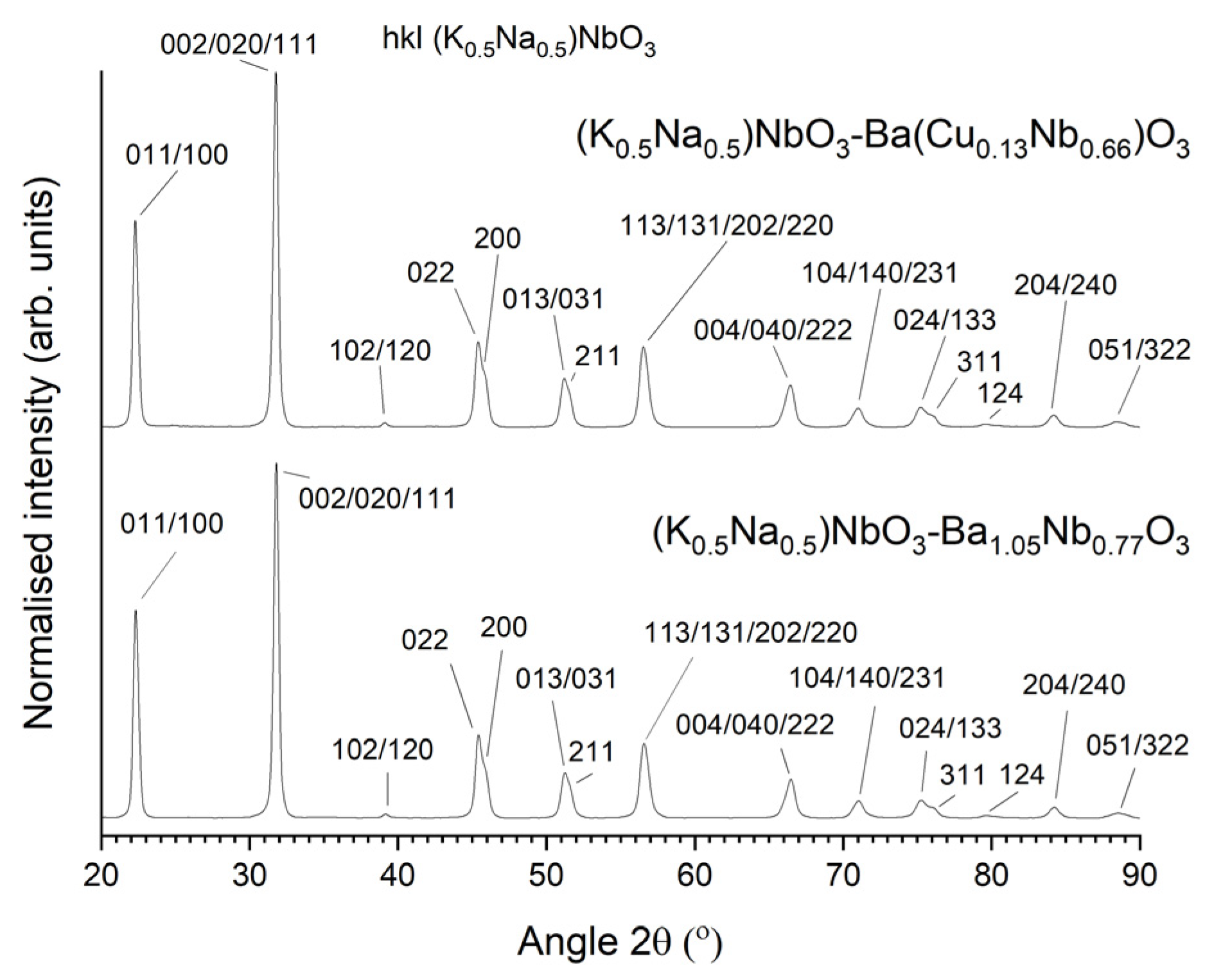
3.3. 98.5 mol% (K0.5Na0.5)NbO3-1.5 mol% Ba(Cu0.13Nb0.66)O3 Seed-Free Solid-State Crystal Growth
3.4. 98.5 mol% (K0.5Na0.5)NbO3-1.5 mol% Ba(Cu0.13Nb0.66)O3 Seeded Solid-State Crystal Growth: [001] KTaO3 Seed
3.5. 98.5 mol% (K0.5Na0.5)NbO3-1.5 mol% Ba(Cu0.13Nb0.66)O3 Seeded Solid-State Crystal Growth: [110] KTaO3 Seed
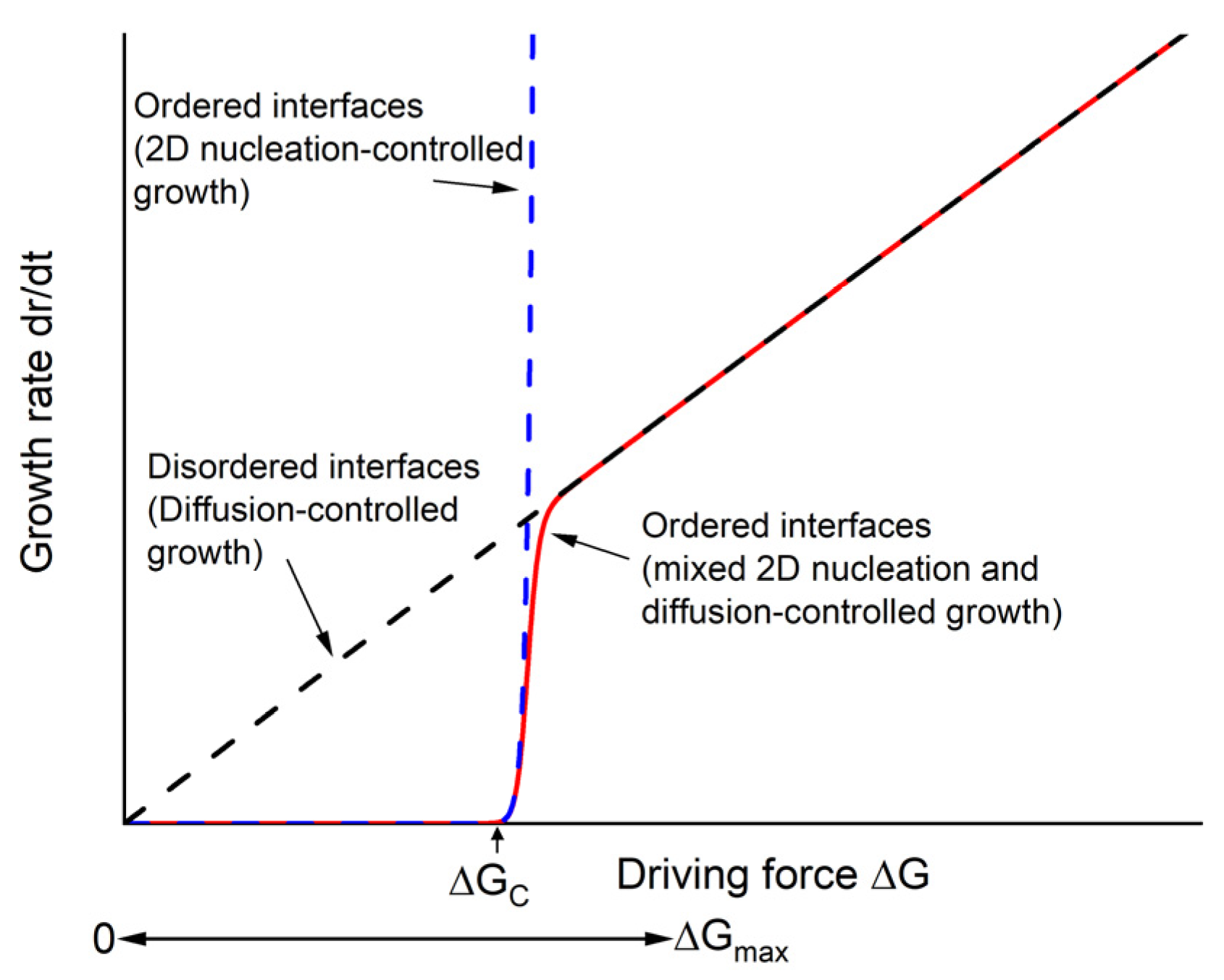
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, T.; Wu, J.; Xiao, D.; Zhu, J. Recent development in lead-free perovskite piezoelectric bulk materials. Prog. Mater. Sci. 2018, 98, 552–624. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, D.; Zhu, J. Potassium–Sodium Niobate Lead-Free Piezoelectric Materials: Past, Present, and Future of Phase Boundaries. Chem. Rev. 2015, 115, 2559–2595. [Google Scholar] [CrossRef] [PubMed]
- Ringgaard, E.; Wurlitzer, T.; Wolny, W.W. Properties of Lead-Free Piezoceramics Based on Alkali Niobates. Ferroelectrics 2005, 319, 97–107. [Google Scholar] [CrossRef]
- Jaeger, R.E.; Egerton, L. Hot Pressing of Potassium-Sodium Niobates. J. Am. Ceram. Soc. 1962, 45, 209–213. [Google Scholar] [CrossRef]
- Lv, X.; Wu, J.; Zhao, C.; Xiao, D.; Zhu, J.; Zhang, Z.; Zhang, C.; Zhang, X.-x. Enhancing temperature stability in potassium-sodium niobate ceramics through phase boundary and composition design. J. Eur. Ceram. Soc. 2019, 39, 305–315. [Google Scholar] [CrossRef]
- Go, S.-H.; Eum, J.-M.; Kim, D.-S.; Chae, S.-J.; Kim, S.-W.; Kim, E.-J.; Chae, Y.-G.; Woo, J.-U.; Nahm, S. Piezoelectricity of (K,Na)(Nb,Sb)O3–SrZrO3(Bi,Ag)ZrO3 piezoceramics and their application in planar-type actuators. J. Mater. Chem. C 2021, 9, 16741–16750. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Xiao, D.; Cheng, X.; Zheng, T.; Zhang, B.; Lou, X.; Zhu, J. Large d33 in (K,Na)(Nb,Ta,Sb)O3-(Bi,Na,K)ZrO3 lead-free ceramics. J. Mater. Chem. A 2014, 2, 4122–4126. [Google Scholar] [CrossRef]
- Lv, X.; Wu, J.; Zhu, J.; Xiao, D. Enhanced temperature stability in the R–T phase boundary with dominating intrinsic contribution. Phys. Chem. Chem. Phys. 2018, 20, 20149–20159. [Google Scholar] [CrossRef]
- Liu, H.; Veber, P.; Rödel, J.; Rytz, D.; Fabritchnyi, P.B.; Afanasov, M.I.; Patterson, E.A.; Frömling, T.; Maglione, M.; Koruza, J. High-performance piezoelectric (K,Na,Li)(Nb,Ta,Sb)O3 single crystals by oxygen annealing. Acta Mater. 2018, 148, 499–507. [Google Scholar] [CrossRef]
- Hu, C.; Tian, H.; Meng, X.; Shi, G.; Cao, W.; Zhou, Z. High-quality K0.47Na0.53NbO3 single crystal toward high performance transducer. RSC Adv. 2017, 7, 7003–7007. [Google Scholar] [CrossRef]
- Ge, W.; Liu, H.; Zhao, X.; Li, X.; Pan, X.; Lin, D.; Xu, H.; Jiang, X.; Luo, H. Orientation dependence of electrical properties of 0.96Na0.5Bi0.5TiO3-0.04BaTiO3 lead-free piezoelectric single crystal. Appl. Phys. A 2009, 95, 761–767. [Google Scholar] [CrossRef]
- Fujii, I.; Ueno, S.; Wada, S. Fabrication of <111>c-oriented (K0.5Na0.5)NbO3 Single Crystal by Solid-State Cyrstal Growth Method. In Proceedings of the 2020 Joint Conference of the IEEE International Frequency Control Symposium and International Symposium on Applications of Ferroelectrics (IFCS-ISAF), Keystone, CO, USA, 19–23 July 2020; pp. 1–2. [Google Scholar]
- Wada, S.; Seike, A.; Tsurumi, T. Poling Treatment and Piezoelectric Properties of Potassium Niobate Ferroelectric Single Crystals. Jpn. J. Appl. Phy. 2001, 40, 5690–5697. [Google Scholar] [CrossRef]
- Liu, H.; Koruza, J.; Veber, P.; Rytz, D.; Maglione, M.; Rödel, J. Orientation-dependent electromechanical properties of Mn-doped (Li,Na,K)(Nb,Ta)O3 single crystals. Appl. Phys. Lett. 2016, 109, 152902. [Google Scholar] [CrossRef]
- Deng, H.; Zhao, X.; Zhang, H.; Chen, C.; Li, X.; Lin, D.; Ren, B.; Jiao, J.; Luo, H. Orientation dependence of electrical properties of large-sized sodium potassium niobate lead-free single crystals. CrystEngComm 2014, 16, 2760–2765. [Google Scholar] [CrossRef]
- Wada, S.; Muraoka, K.; Kakemoto, H.; Tsurumi, T.; Kumagai, H. Enhanced Piezoelectric Properties of Potassium Niobate Single Crystals by Domain Engineering. Jpn. J. Appl. Phys. 2004, 43, 6692–6700. [Google Scholar] [CrossRef]
- Sun, E.; Cao, W. Relaxor-based ferroelectric single crystals: Growth, domain engineering, characterization and applications. Prog. Mater. Sci. 2014, 65, 124–210. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, C.; Qiao, L.; Song, K.; Guo, H.; Xu, Z.; Li, F. Impact of alternating current electric field poling on piezoelectric and dielectric properties of Pb(In1/2Nb1/2)O3–Pb(Mg1/3Nb2/3)O3–PbTiO3 ferroelectric crystals. J. Appl. Phys. 2020, 128, 094104. [Google Scholar] [CrossRef]
- Fisher, J.G.; Benčan, A.; Holc, J.; Kosec, M.; Vernay, S.; Rytz, D. Growth of potassium sodium niobate single crystals by solid state crystal growth. J. Cryst. Growth 2007, 303, 487–492. [Google Scholar] [CrossRef]
- Chen, K.; Xu, G.; Yang, D.; Wang, X.; Li, J. Dielectric and piezoelectric properties of lead-free 0.95(Na0.5K0.5)NbO3–0.05LiNbO3 crystals grown by the Bridgman method. J. Appl. Phys. 2007, 101, 044103. [Google Scholar] [CrossRef]
- Tian, H.; Hu, C.; Meng, X.; Tan, P.; Zhou, Z.; Li, J.; Yang, B. Top-Seeded Solution Growth and Properties of K1–xNaxNbO3 Crystals. Cryst. Growth Des. 2015, 15, 1180–1185. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Q.; Li, Y.; Liu, Y. Growth mechanism and enhanced electrical properties of K0.5Na0.5NbO3-based lead-free piezoelectric single crystals grown by a solid-state crystal growth method. J. Eur. Ceram. Soc. 2016, 36, 541–550. [Google Scholar] [CrossRef]
- Benčan, A.; Tchernychova, E.; Uršič, H.; Kosec, M.; Fisher, J. Growth and Characterization of Single Crystals of Potassium Sodium Niobate by Solid State Crystal Growth. In Ferroelectrics-Material Aspects; Lallart, M., Ed.; InTech: Rijeka, Croatia, 2011; pp. 87–108. [Google Scholar]
- Kang, S.J.L.; Park, J.H.; Ko, S.Y.; Lee, H.Y. Solid-State Conversion of Single Crystals: The Principle and the State-of-the-Art. J. Am. Ceram. Soc. 2015, 98, 347–360. [Google Scholar] [CrossRef]
- Milisavljevic, I.; Wu, Y. Current status of solid-state single crystal growth. BMC Mater. 2020, 2, 2. [Google Scholar] [CrossRef]
- Fujii, I.; Ueno, S.; Wada, S. Effect of sintering temperature on the growth of (K0.5Na0.5)NbO3 single crystals fabricated by the solid-state crystal growth method. Jpn. J. Appl. Phys. 2019, 58, SLLD01. [Google Scholar] [CrossRef]
- Fujii, I.; Ueno, S.; Wada, S. Effects of sintering aid and atmosphere powder on the growth of (K0.5Na0.5)NbO3 single crystals fabricated by solid-state crystal growth method. J. Eur. Ceram. Soc. 2020, 40, 2970–2976. [Google Scholar] [CrossRef]
- Bencan, A.; Tchernychova, E.; Godec, M.; Fisher, J.; Kosec, M. Compositional and structural study of a (K0.5Na0.5)NbO3 single crystal prepared by solid state crystal growth. Microsc. Microanal. 2009, 15, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Ursic, H.; Bencan, A.; Skarabot, M.; Godec, M.; Kosec, M. Dielectric, ferroelectric, piezoelectric, and electrostrictive properties of K0.5Na0.5NbO3 single crystals. J. Appl. Phys. 2010, 107, 033705. [Google Scholar] [CrossRef]
- Fisher, J.G.; Benčan, A.; Bernard, J.; Holc, J.; Kosec, M.; Vernay, S.; Rytz, D. Growth of (Na, K, Li)(Nb, Ta)O3 single crystals by solid state crystal growth. J. Eur. Ceram. Soc. 2007, 27, 4103–4106. [Google Scholar] [CrossRef]
- Uwiragiye, E.; Farooq, M.U.; Moon, S.-H.; Pham, T.L.; Nguyen, D.T.; Lee, J.-S.; Fisher, J.G. High performance lead-free piezoelectric 0.96(K0.48Na0.52)NbO3-0.03[Bi0.5(Na0.7K0.2Li0.1)0.5]ZrO3-0.01(Bi0.5Na0.5)TiO3 single crystals grown by solid state crystal growth and their phase relations. J. Eur. Ceram. Soc. 2017, 37, 4597–4607. [Google Scholar] [CrossRef]
- Uwiragiye, E.; Pham, T.L.; Fisher, J.G.; Lee, J.S.; Lee, B.W.; Ko, J.H.; Kim, H.P.; Jo, W. Sintering Aid-Assisted Growth of 0.95(K0.5Na0.5)NbO3–0.05(Bi0.5Na0.5)(Zr0.85Sn0.15)O3 Single Crystals by the Solid-State Crystal Growth Method and Their Characterization. Phys. Status Solidi 2022, 219, 2100875. [Google Scholar] [CrossRef]
- Yao, X.; Jiang, M.; Li, W.; Lu, H.; Li, L.; Rao, G. Effects of the calcined temperature of ingredients on the growth, structure and electrical properties of (K0.5Na0.5)NbO3-based single crystals. J. Mater. Sci. Mater. Electron. 2020, 31, 21971–21980. [Google Scholar] [CrossRef]
- Yang, J.; Fu, Z.; Yang, Q.; Li, Y.; Liu, Y. Effect of seeds and sintering additives on (K,Na,Li)NbO3 lead-free single crystals grown by a solid-state crystal growth method. J. Ceram. Soc. Jpn. 2016, 124, 365–369. [Google Scholar] [CrossRef]
- Gupta, S.; Belianinov, A.; Okatan, M.B.; Jesse, S.; Kalinin, S.V.; Priya, S. Fundamental limitation to the magnitude of piezoelectric response of <001>(pc) textured K0.5Na0.5NbO3 ceramic. Appl. Phys. Lett. 2014, 104, 5. [Google Scholar] [CrossRef]
- Yu, Q.; Li, J.F.; Sun, W.; Zhou, Z.; Xu, Y.; Xie, Z.K.; Lai, F.P.; Wang, Q.M. Electrical properties of K0.5Na0.5NbO3 thin films grown on Nb:SrTiO3 single-crystalline substrates with different crystallographic orientations. J. Appl. Phys. 2013, 113, 5. [Google Scholar] [CrossRef]
- Fisher, J.G.; Bencan, A.; Kosec, M.; Vernay, S.; Rytz, D. Growth of dense single crystals of potassium sodium niobate by a combination of solid-state crystal growth and hot pressing. J. Am. Ceram. Soc. 2008, 91, 1503–1507. [Google Scholar] [CrossRef]
- Koruza, J.; Liu, H.; Höfling, M.; Zhang, M.-H.; Veber, P. (K,Na)NbO3-based piezoelectric single crystals: Growth methods, properties, and applications. J. Mater. Res. 2020, 35, 990–1016. [Google Scholar] [CrossRef]
- Ahn, C.-W.; Rahman, A.; Ryu, J.; Choi, J.-J.; Kim, J.-W.; Yoon, W.-H.; Choi, J.-H.; Park, D.-S.; Hahn, B.-D. Composition Design for Growth of Single Crystal by Abnormal Grain Growth in Modified Potassium Sodium Niobate Ceramics. Cryst. Growth Des. 2016, 16, 6586–6592. [Google Scholar] [CrossRef]
- Jiang, M.; Randall, C.A.; Guo, H.; Rao, G.; Tu, R.; Gu, Z.; Cheng, G.; Liu, X.; Zhang, J.; Li, Y. Seed-Free Solid-State Growth of Large Lead-Free Piezoelectric Single Crystals: (Na1/2K1/2)NbO3. J. Am. Ceram. Soc. 2015, 98, 2988–2996. [Google Scholar] [CrossRef]
- Rahman, A.; Cho, K.-H.; Ahn, C.-W.; Ryu, J.; Choi, J.-J.; Kim, J.-W.; Yoon, W.-H.; Choi, J.-H.; Park, D.-S.; Hahn, B.-D. A composition design rule for crystal growth of centimeter scale by normal sintering process in modified potassium sodium niobate ceramics. J. Eur. Ceram. Soc. 2018, 38, 1416–1420. [Google Scholar] [CrossRef]
- Ahn, C.-W.; Lee, H.-Y.; Han, G.; Zhang, S.; Choi, S.-Y.; Choi, J.-J.; Kim, J.-W.; Yoon, W.-H.; Choi, J.-H.; Park, D.-S.; et al. Self-Growth of Centimeter-Scale Single Crystals by Normal Sintering Process in Modified Potassium Sodium Niobate Ceramics. Sci. Rep. 2015, 5, 17656. [Google Scholar] [CrossRef]
- Morimoto, T.; Shimono, S.; Yoshiichi, Y.; Kishimura, H.; Ishii, K. Conditions of large unitary (K, Na)NbO3 system single crystals for rapid solid-state crystal growth method. Jpn. J. Appl. Phy. 2023, 62, 035501. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, M.; Cheng, G.; Gu, Z.; Liu, X.; Song, J.; Li, L.; Du, Y. Microstructure, piezoelectric, ferroelectric and dielectric properties of Na0.5K0.5NbO3 single crystals prepared by seed-free solid-state crystal growth. Ferroelectrics 2016, 502, 210–220. [Google Scholar] [CrossRef]
- Song, J.; Hao, C.; Yan, Y.; Zhang, J.; Li, L.; Jiang, M. Enhanced piezoelectric property and microstructure of large CaZrO3-doped Na0.5K0.5NbO3-based single crystal with 20 mm over. Mater. Lett. 2017, 204, 19–22. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J.; Rao, G.; Li, D.; Randall, C.A.; Li, T.; Peng, B.; Li, L.; Gu, Z.; Liu, X.; et al. Ultrahigh piezoelectric coefficient of a lead-free K0.5Na0.5NbO3-based single crystal fabricated by a simple seed-free solid-state growth method. J. Mater. Chem. C 2019, 7, 14845–14854. [Google Scholar] [CrossRef]
- Li, D.; Jiang, M.; Han, S.; Jin, Q.; Xu, Y.; Yao, X.; Zhang, K.; Li, L.; Miao, L.; Zhou, C.; et al. Influence of trace lithium addition on the structure and properties of K0.5Na0.5NbO3-based single crystals. J. Mater. Sci. Mater. Electron. 2020, 31, 4857–4866. [Google Scholar] [CrossRef]
- Yao, X.; Jiang, M.; Han, S.; Li, D.; Xu, Y.; Li, L.; Rao, G. Microstructure and electrical properties of CuO-doped K0.5Na0.5NbO3-based single crystals with low dielectric loss. J. Mater. Res. 2021, 36, 1182–1194. [Google Scholar] [CrossRef]
- Lu, H.; Jiang, M.; Yao, X.; Zhang, Z.; Wang, W.; Li, L.; Rao, G. The effect of the annealing temperature on the structure and electrical properties of Li/Ta-modified (K0.5Na0.5)NbO3-based piezoelectric crystals. J. Mater. Sci. Mater. Electron. 2022, 33, 2816–2828. [Google Scholar] [CrossRef]
- Ren, P.; Jiang, M.; Lu, H.; Li, L.; Cheng, S.; Wang, T.; Zhao, Y.; Rao, G. Optimized microstructure and electrical properties in 0.997(0.996K0.5Na0.5NbO3–0.004MnBiO3)–0.003B2O3 single crystals via Li-doping. J. Mater. Sci. Mater. Electron. 2023, 34, 465. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, M.; Li, L.; Cheng, S.; Lu, H.; Ren, P.; Zhao, Y.; Rao, G. Effects of MnO2-doping on growth, structure and electrical properties of lead-free piezoelectric K0.5Na0.5NbO3-BiAlO3 single crystals. J. Alloys Compd. 2023, 935, 168126. [Google Scholar] [CrossRef]
- Zhu, B.P.; Zhu, Y.H.; Yang, J.; Ou-Yang, J.; Yang, X.F.; Li, Y.X.; Wei, W. New Potassium Sodium Niobate Single Crystal with Thickness-independent High-performance for Photoacoustic Angiography of Atherosclerotic Lesion. Sci. Rep. 2016, 6, 8. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, F.; Yang, Q.; Liu, Z.; Li, Y.; Liu, Y.; Zhang, Q. Large piezoelectric properties in KNN-based lead-free single crystals grown by a seed-free solid-state crystal growth method. Appl. Phys. Lett. 2016, 108, 182904. [Google Scholar] [CrossRef]
- Morimoto, T.; Shimono, S.; Ishii, K. Growth of large unitary (K,Na)NbO3 single crystals using a seed-free solid-state crystal growth method by adjusting the B-site excess ratios and additional Bi2O3. Jpn. J. Appl. Phy. 2020, 59, SP1001. [Google Scholar] [CrossRef]
- Hao, C.Y.; Gu, Z.F.; Cheng, G.; Li, L.; Zhang, J.W.; Song, J.G.; Yan, Y.F.; Jiang, M.H. Composition design and electrical property of a pure KxNa1−xNbO3 single crystal fabricated by the seed-free solid-state crystal growth. J. Mater. Sci. Mater. Electron. 2017, 28, 18357–18365. [Google Scholar] [CrossRef]
- Ha, S.-J.; Park, J.-H.; Cho, K.-H.; Cha, H.-A.; Choi, J.-J.; Han, B.-D.; Ahn, C.-W. Research on eco-friendly piezoelectric seed material manufacturing using abnormal grain growth. In 2022 Fall Meeting of the Korean Ceramic Society; The Korean Ceramic Society: Seoul, Republic of Korea, 2022; p. 45. [Google Scholar]
- Bruker-AXS. APEX2. Version 2014.11-0; Bruker-AXS: Madison, WI, USA, 2014. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Chapter 5 Diffraction III: Real Samples. In Elements of X-ray Diffraction; Prentice Hall: Upper Saddle River, NJ, USA, 2001; pp. 167–184. [Google Scholar]
- Lee, M. Characterization of Thin Films by X-Ray Diffraction. In X-ray Diffraction for Materials Research: From Fundamentals to Applications; Apple Academic Press Inc.: Oakville, ON, Canada, 2016; pp. 182–223. [Google Scholar]
- Iamsasri, T.; Tutuncu, G.; Uthaisar, C.; Pojprapai, S.; Jones, J.L. Analysis methods for characterizing ferroelectric/ferroelastic domain reorientation in orthorhombic perovskite materials and application to Li-doped Na0.5K0.5NbO3. J. Mater. Sci. 2013, 48, 6905–6910. [Google Scholar] [CrossRef]
- Ochoa, D.A.; Esteves, G.; Iamsasri, T.; Rubio-Marcos, F.; Fernández, J.F.; García, J.E.; Jones, J.L. Extensive domain wall contribution to strain in a (K,Na)NbO3-based lead-free piezoceramics quantified from high energy X-ray diffraction. J. Eur. Ceram. Soc. 2016, 36, 2489–2494. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, H.; Zhao, X.; Chen, C.; Wang, X.A.; Li, X.; Lin, D.; Ren, B.; Jiao, J.; Luo, H. Direct observation of monoclinic ferroelectric phase and domain switching process in (K0.25Na0.75)NbO3 single crystals. CrystEngComm 2015, 17, 2872–2877. [Google Scholar] [CrossRef]
- Malic, B.; Jenko, D.; Bernard, J.; Cilensek, J.; Kosec, M. Synthesis and Sintering of (K,Na)NbO3 Based Ceramics. Mat. Res. Soc. Proc. 2002, 755, 83–88. [Google Scholar] [CrossRef]
- Pecharsky, V.K.; Zavalij, P.Y. Chapter 8 The Powder Diffraction Pattern. In Fundamentals of Powder Diffraction and Structural Characterization of Materials, 2nd ed.; Springer Science + Business Media: New York, NY, USA, 2009; pp. 151–202. [Google Scholar]
- Daniels, J.E.; Jones, J.L.; Finlayson, T.R. Characterization of domain structures from diffraction profiles in tetragonal ferroelastic ceramics. J. Phys. D Appl. Phys. 2006, 39, 5294. [Google Scholar] [CrossRef]
- Peelen, J.G.J.; Metselaar, R. Light scattering by pores in polycrystalline materials: Transmission properties of alumina. J. Appl. Phys. 1974, 45, 216–220. [Google Scholar] [CrossRef]
- Apetz, R.; Bruggen, M.P.B. Transparent Alumina: A Light-Scattering Model. J. Am. Ceram. Soc. 2003, 86, 480–486. [Google Scholar] [CrossRef]
- Chiang, Y.M.; Birnie, D.; Kingery, W.D. Chapter 2 Defects in Ceramics. In Physical Ceramics: Principles for Ceramic Science and Engineering; John Wiley and Sons: New York, NY, USA, 1997; pp. 101–184. [Google Scholar]
- Pecharsky, V.K.; Zavalij, P.Y. Chapter 7 Geometry of Diffraction by Lattices. In Fundamentals of Powder Diffraction and Structural Characterization of Materials, 2nd ed.; Springer Science + Business Media: New York, NY, USA, 2009; pp. 133–149. [Google Scholar]
- Tellier, J.; Malic, B.; Dkhil, B.; Jenko, D.; Cilensek, J.; Kosec, M. Crystal structure and phase transitions of sodium potassium niobate perovskites. Solid State Sci. 2009, 11, 320–324. [Google Scholar] [CrossRef]
- Kosec, M.; Kolar, D. On activated sintering and electrical properties of NaKNbO3. Mater. Res. Bull. 1975, 10, 335–340. [Google Scholar] [CrossRef]
- Williams, E.D.; Bartelt, N.C. Thermodynamics of Surface Morphology. Science 1991, 251, 393–400. [Google Scholar] [CrossRef]
- Jeong, H.-C.; Williams, E.D. Steps on surfaces: Experiment and theory. Surf. Sci. Rep. 1999, 34, 171–294. [Google Scholar] [CrossRef]
- Ke, S.M.; Huang, H.T.; Fan, H.Q.; Lee, H.K.; Zhou, L.M.; Mai, Y.W. Antiferroelectric-like properties and enhanced polarization of Cu-doped K0.5Na0.5NbO3 piezoelectric ceramics. Appl. Phys. Lett. 2012, 101, 082901. [Google Scholar] [CrossRef]
- Eichel, R.-A.; Erünal, E.; Drahus, M.D.; Smyth, D.M.; Van Tol, J.; Acker, J.; Kungl, H.; Hoffmann, M.J. Local variations in defect polarization and covalent bonding in ferroelectric Cu2+-doped PZT and KNN functional ceramics at the morphotropic phase boundary. Phys. Chem. Chem. Phys. 2009, 11, 8698. [Google Scholar] [CrossRef]
- Chung, S.Y.; Yoon, D.Y.; Kang, S.J.L. Effects of donor concentration and oxygen partial pressure on interface morphology and grain growth behavior in SrTiO3. Acta Mater. 2002, 50, 3361–3371. [Google Scholar] [CrossRef]
- An, S.M.; Kang, S.J.L. Boundary structural transition and grain growth behavior in BaTiO3 with Nd2O3 doping and oxygen partial pressure change. Acta Mater. 2011, 59, 1964–1973. [Google Scholar] [CrossRef]
- Jung, Y.I.; Yoon, D.Y.; Kang, S.J.L. Coarsening of polyhedral grains in a liquid matrix. J. Mater. Res. 2009, 24, 2949–2959. [Google Scholar] [CrossRef]
- Kang, S.J.L.; Lee, M.G.; An, S.M. Microstructural Evolution During Sintering with Control of the Interface Structure. J. Am. Ceram. Soc. 2009, 92, 1464–1471. [Google Scholar] [CrossRef]
- Park, Y.J.; Hwang, N.M.; Yoon, D.Y. Abnormal growth of faceted (WC) grains in a (Co) liquid matrix. Metall. Mater. Trans. A 1996, 27, 2809–2819. [Google Scholar] [CrossRef]
- Fisher, J.G.; Kang, S.J.L. Microstructural changes in (Na0.5K0.5)NbO3 ceramics sintered in various atmospheres. J. Eur. Ceram. Soc. 2009, 29, 2581–2588. [Google Scholar] [CrossRef]
- Fisher, J.G.; Choi, S.Y.; Kang, S.J.L. Influence of Sintering Atmosphere on Abnormal Grain Growth Behaviour in Potassium Sodium Niobate Ceramics Sintered at Low Temperature. J. Korean Ceram. Soc. 2011, 48, 641–647. [Google Scholar] [CrossRef]
- Lee, B.K.; Chung, S.Y.; Kang, S.J.L. Grain boundary faceting and abnormal grain growth in BaTiO3. Acta Mater. 2000, 48, 1575–1580. [Google Scholar] [CrossRef]
- Yoon, B.K.; Lee, B.A.; Kang, S.J.L. Growth behavior of rounded (Ti,W)C and faceted WC grains in a Co matrix during liquid phase sintering. Acta Mater. 2005, 53, 4677–4685. [Google Scholar] [CrossRef]
- Jang, C.-W.; Kim, J.; Kang, S.-J.L. Effect of Sintering Atmosphere on Grain Shape and Grain Growth in Liquid-Phase-Sintered Silicon Carbide. J. Am. Ceram. Soc. 2002, 85, 1281–1284. [Google Scholar] [CrossRef]
- Lee, S.B.; Yoon, D.Y.; Hwang, N.M.; Henry, M.F. Grain boundary faceting and abnormal grain growth in nickel. Metall. Mater. Trans. A 2000, 31, 985–994. [Google Scholar] [CrossRef]
- Kwon, S.K.; Hong, S.H.; Kim, D.Y.; Hwang, N.M. Coarsening Behavior of Tricalcium Silicate (C3S) and Dicalcium Silicate (C2S) Grains Dispersed in a Clinker Melt. J. Am. Ceram. Soc. 2000, 83, 1247–1252. [Google Scholar] [CrossRef]
- Jung, S.H.; Yoon, D.Y.; Kang, S.J.L. Mechanism of abnormal grain growth in ultrafine-grained nickel. Acta Mater. 2013, 61, 5685–5693. [Google Scholar] [CrossRef]
- Choi, K.; Hwang, N.M.; Kim, D.Y. Effect of Grain Shape on Abnormal Grain Growth in Liquid-Phase-Sintered Nb1−xTixC–Co Alloys. J. Am. Ceram. Soc. 2002, 85, 2313–2318. [Google Scholar] [CrossRef]
- Le, P.G.; Tran, H.T.; Lee, J.-S.; Fisher, J.G.; Kim, H.-P.; Jo, W.; Moon, W.-J. Growth of single crystals in the (Na1/2Bi1/2)TiO3–(Sr1–xCax)TiO3 system by solid state crystal growth. J. Adv. Ceram. 2021, 10, 973–990. [Google Scholar] [CrossRef]
- Fisher, J.G.; Kang, S.-J.L. Strategies and practices for suppressing abnormal grain growth during liquid phase sintering. J. Am. Ceram. Soc. 2019, 102, 717–735. [Google Scholar] [CrossRef]
- Kang, S.-J.L.; Jung, Y.-I.; Jung, S.-H.; Fisher, J.G. Interface Structure-Dependent Grain Growth Behavior in Polycrystals. In Microstructural Design of Advanced Engineering Materials; Molodov, D.A., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA.: Weinheim, Germany, 2013; pp. 299–322. [Google Scholar]
- Yoon, D.Y.; Cho, Y.K. Roughening transition of grain boundaries in metals and oxides. J. Mater. Sci. 2005, 40, 861–870. [Google Scholar] [CrossRef]
- An, S.M.; Yoon, B.K.; Chung, S.Y.; Kang, S.J.L. Nonlinear driving force-velocity relationship for the migration of faceted boundaries. Acta Mater. 2012, 60, 4531–4539. [Google Scholar] [CrossRef]
- Jung, S.H.; Kang, S.J.L. Repetitive grain growth behavior with increasing temperature and grain boundary roughening in a model nickel system. Acta Mater. 2014, 69, 283–291. [Google Scholar] [CrossRef]
- Kang, S.J.L.; Ko, S.Y.; Moon, S.Y. Mixed control of boundary migration and the principle of microstructural evolution. J. Ceram. Soc. Jpn. 2016, 124, 259–267. [Google Scholar] [CrossRef]
- Burton, W.K.; Cabrera, N.; Frank, F.C. The Growth of Crystals and the Equilibrium Structure of Their Surfaces. Philos. Trans. R. Soc. A 1951, 243, 299–358. [Google Scholar]
- Hirth, J.P.; Pound, G.M. Growth and Evaporation of Liquids and Dislocation-Free Crystals. In Condensation and Evaporation: Nucleation and Growth Kinetics; Pergamon Press: Oxford, UK, 1963; pp. 77–106. [Google Scholar]
- Markov, I.V. Crystal-Ambient Phase Equilibrium. In Crystal Growth for Beginners: Fundamentals of Nucleation, Crystal Growth and Epitaxy, 2nd ed.; World Scientific: Singapore, 2003; pp. 1–76. [Google Scholar]
- Cho, Y.K.; Yoon, D.Y.; Kim, B.K. Surface Roughening Transition and Coarsening of NbC Grains in Liquid Cobalt-Rich Matrix. J. Am. Ceram. Soc. 2004, 87, 443–448. [Google Scholar] [CrossRef]
- Heo, Y.H.; Jeon, S.C.; Fisher, J.G.; Choi, S.Y.; Hur, K.H.; Kang, S.J.L. Effect of step free energy on delayed abnormal grain growth in a liquid phase-sintered BaTiO3 model system. J. Eur. Ceram. Soc. 2011, 31, 755–762. [Google Scholar] [CrossRef]
- Trung, D.T.; Fisher, J.G. Controlled-Atmosphere Sintering of KNbO3. Appl. Sci. 2020, 10, 2131. [Google Scholar] [CrossRef]
- Jo, W.; Kim, D.Y.; Hwang, N.M. Effect of Interface Structure on the Microstructural Evolution of Ceramics. J. Am. Ceram. Soc. 2006, 89, 2369–2380. [Google Scholar] [CrossRef]
- Effect of Crystal Shape on the Grain Growth during Liquid Phase Sintering of Ceramics. J. Korean Ceram. Soc. 2006, 43, 728–733. [CrossRef]
- Moon, K.S.; Kang, S.J.L. Coarsening Behavior of Round-Edged Cubic Grains in the Na1/2Bi1/2TiO3-BaTiO3 System. J. Am. Ceram. Soc. 2008, 91, 3191–3196. [Google Scholar] [CrossRef]
- Fisher, J.G.; Benčan, A.; Godnjavec, J.; Kosec, M. Growth behaviour of potassium sodium niobate single crystals grown by solid-state crystal growth using K4CuNb8O23 as a sintering aid. J. Eur. Ceram. Soc. 2008, 28, 1657–1663. [Google Scholar] [CrossRef]
- Farooq, M.U.; Ko, S.Y.; Fisher, J.G. Effect of SrTiO3 content on the growth of (100-x)(K0.5Na0.5)NbO3-xSrTiO3 lead-free piezoelectric single crystals grown by the solid-state crystal growth method. Adv. Appl. Ceram. 2016, 115, 81–88. [Google Scholar] [CrossRef]
- Rafiq, M.A.; Costa, V.M.E.; Vilarinho, P.M. Establishing the Domain Structure of (K0.5Na0.5)NbO3 (KNN) Single Crystals by Piezoforce-Response Microscopy. Sci. Adv. Mater. 2014, 6, 426–433. [Google Scholar] [CrossRef]
- Bah, M.; Alyabyeva, N.; Retoux, R.; Giovannelli, F.; Zaghrioui, M.; Ruyter, A.; Delorme, F.; Monot-Laffez, I. Investigation of the domain structure and hierarchy in potassium-sodium niobate lead-free piezoelectric single crystals. RSC Adv. 2016, 6, 49060–49067. [Google Scholar] [CrossRef]
- Hu, C.; Meng, X.; Zhang, M.-H.; Tian, H.; Daniels, J.E.; Tan, P.; Huang, F.; Li, L.; Wang, K.; Li, J.-F.; et al. Ultra-large electric field–induced strain in potassium sodium niobate crystals. Sci. Adv. 2020, 6, eaay5979. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, M.A.; Tkach, A.; Costa, M.E.; Vilarinho, P.M. Defects and charge transport in Mn-doped K0.5Na0.5NbO3 ceramics. Phys. Chem. Chem. Phys. 2015, 17, 24403–24411. [Google Scholar] [CrossRef] [PubMed]
- Popovič, A.; Bencze, L.; Koruza, J.; Malič, B. Vapour pressure and mixing thermodynamic properties of the KNbO3–NaNbO3 system. RSC Adv. 2015, 5, 76249–76256. [Google Scholar] [CrossRef]
- Samardzžija, Z.; Bernik, S.; Marinenko, R.B.; Malič, B.; Čeh, M. An EPMA Study on KNbO3 and NaNbO3 Single Crystals–Potential Reference Materials for Quantitative Microanalysis. Microchim. Acta 2004, 145, 203–208. [Google Scholar] [CrossRef]
- Chiang, Y.M.; Birnie, D.; Kingery, W.D. Chapter 5 Microstructure. In Physical Ceramics: Principles for Ceramic Science and Engineering; John Wiley and Sons: New York, NY, USA, 1997; pp. 351–513. [Google Scholar]
- Kang, S.J.L. Grain Growth and Densification in Porous Materials. In Sintering: Densification, Grain Growth and Microstructure; Elsevier Butterworth Heineman: Oxford, UK, 2005; pp. 145–170. [Google Scholar]
- German, R.M. Solid-State Sintering Fundamentals. In Sintering Theory and Practice; John Wiley and Sons: New York, NY, USA, 1996; pp. 67–141. [Google Scholar]
- Oh, U.C.; Chung, Y.S.; Kim, D.Y.; Yoon, D.N. Effect of Grain Growth on Pore Coalescence During the Liquid-Phase Sintering of MgO-CaMgSiO4 Systems. J. Am. Ceram. Soc. 1988, 71, 854–857. [Google Scholar] [CrossRef]
- German, R.M. Liquid-Phase Sintering. In Sintering Theory and Practice; John Wiley and Sons: New York, NY, USA, 1996; pp. 225–312. [Google Scholar]
- Xiong, Y.; Hu, J.; Shen, Z. Dynamic pore coalescence in nanoceramic consolidated by two-step sintering procedure. J. Eur. Ceram. Soc. 2013, 33, 2087–2092. [Google Scholar] [CrossRef]
- Liu, Y.; Chu, R.; Xu, Z.; Lv, H.; Wu, L.; Yang, Y.; Li, G. Effects of K4CuNb8O23 on phase structure and electrical properties of K0.5Na0.5NbO3–LiSbO3 lead-free piezoceramics. Phys. B Condens. Matter 2012, 407, 2573–2577. [Google Scholar] [CrossRef]
- Ahmadi Moghadam, H.; Barzegar, A. Low-temperature sintering of K0.5Na0.5NbO3 lead free ceramics using nano CuO sintering aid. J. Mater. Sci. Mater. Electron. 2017, 28, 13161–13167. [Google Scholar] [CrossRef]
- Rheinheimer, W.; Bäurer, M.; Chien, H.; Rohrer, G.S.; Handwerker, C.A.; Blendell, J.E.; Hoffmann, M.J. The equilibrium crystal shape of strontium titanate and its relationship to the grain boundary plane distribution. Acta Mater. 2015, 82, 32–40. [Google Scholar] [CrossRef]



| Peak | d-Spacing (Å) | FWHM (° 2θ) |
|---|---|---|
| KNBaN seed-free SSCG (top face) 111 | 2.8264 | 0.1541 |
| KNBaN seed-free SSCG (top face) 002 | 2.8523 | 0.1327 |
| KNBaN seed-free SSCG (bottom face) 011 | 4.0283 | 0.0517 |
| KNBaN seed-free SSCG (bottom face) 100 | 3.9710 | 0.0657 |
| KNBaN SSCG [100] KTaO3 seed crystal 011 | 3.9992 | 0.1687 |
| KNBaN SSCG [100] KTaO3 seed crystal 100 | 3.945 | 0.1701 |
| KNBaCuN seed-free SSCG 111 | 2.8096 | 0.0678 |
| KNBaCuN seed-free SSCG 020 | 2.8203 | 0.0721 |
| KNBaCuN seed-free SSCG 002 | 2.8368 | 0.1031 |
| KNBaCuN SSCG [100] KTaO3 seed crystal 011 | 4.0067 | 0.0942 |
| KNBaCuN SSCG [100] KTaO3 seed crystal 100 | 3.9547 | 0.0763 |
| KNBaCuN SSCG [110] KTaO3 seed crystal 011 | 3.9945 | 0.0444 |
| KNBaCuN SSCG [110] KTaO3 seed crystal 100 | 3.9404 | 0.0477 |
| KTaO3 substrate 100 | 3.9711 | 0.0348 |
| KTaO3 substrate 110 | 2.8227 | 0.0520 |
| Sample | Mean Porosity (%) | Standard Deviation (%) | Number of Micrographs Measured |
|---|---|---|---|
| KNBaN seed-free SSCG (top face) | 12.91 | 9.73 | 5 |
| KNBaN seed-free SSCG (bottom face) | 7.41 | 2.24 | 3 |
| KNBaN seeded SSCG [100] KTaO3 seed crystal | 10.15 | 5.25 | 3 |
| KNBaCuN seed-free SSCG | 2.98 | 3.85 | 4 |
| KNBaCuN seeded SSCG [100] KTaO3 seed crystal (region next to seed crystal) | 8.32 | 1.17 | 2 |
| KNBaCuN seeded SSCG [110] KTaO3 seed crystal | 12.10 | 5.80 | 2 |
| Element | Na | K | Ba | Nb |
|---|---|---|---|---|
| Top half | 0.477 ± 0.007 | 0.472 ± 0.006 | 0.015 ± 0.000 | 0.997 |
| Bottom half | 0.483 ± 0.003 | 0.464 ± 0.005 | 0.016 ± 0.001 | 0.997 |
| Nominal | 0.493 | 0.493 | 0.016 | 0.997 |
| Sample | Crystal System | Space Group | Unit Cell Parameters (Å) | Unit Cell Angles (°) | Unit Cell Volume (Å3) | Formula Units per Unit Cell | Theoretical Density (g⋅ cm−3) | R1 | wR2 |
|---|---|---|---|---|---|---|---|---|---|
| KNBaN seeded SSCG [100] KTaO3 seed crystal | monoclinic | P2 | a = 3.9761(4) b = 3.9637(5) c = 3.9918(4) | α = 90 β = 90.072(6) γ = 90 | 62.911(12) | 1 | 4.58 | 0.0212 | 0.0575 |
| KNBaCuN seeded SSCG [100] KTaO3 seed crystal | monoclinic | P2 | a = 3.9714(6) b = 3.9683(5) c = 3.9996(6) | α = 90 β = 89.999(9) γ = 90 | 63.033(16) | 1 | 4.56 | 0.0265 | 0.0715 |
| Element | Na | K | Ba | Cu | Sb | Nb |
|---|---|---|---|---|---|---|
| 0.434 ± 0.014 | 0.416 ± 0.004 | 0.017 ± 0.001 | 0.001 ± 0.001 | 0.004 ± 0.000 | 0.992 ± 0.001 | |
| Nominal | 0.493 | 0.493 | 0.015 | 0.002 | 0 | 0.995 |
| Element | Na | K | Ba | Cu | Sb | Nb |
|---|---|---|---|---|---|---|
| 0.413 ± 0.012 | 0.464 ± 0.004 | 0.015 ± 0.001 | 0.002 ± 0.001 | 0.004 ± 0.000 | 0.991 ± 0.001 | |
| Nominal | 0.493 | 0.493 | 0.015 | 0.002 | 0 | 0.995 |
| Sample Name | Nominal Composition | Composition | Sintering Temperature (°C) | Sintering Time (h) | Size (mm) | Output Ratio (Area%) | Reference |
|---|---|---|---|---|---|---|---|
| KNBaN seed-free SSCG | (K0.493Na0.493Ba0.016) Nb0.997O3 | (K0.472Na0.477Ba0.015) Nb0.997O3/ (K0.464Na0.483Ba0.016) Nb0.997O3 | 1135 | 20–21 | ~5 | ~100 | This work |
| KNBaN SSCG [100] KTaO3 seed crystal | (K0.493Na0.493Ba0.016) Nb0.997O3 | Not analysed | 1135 | 5–20 h | ~5 | ~67 | This work |
| KNBaCuN seed-free SSCG | (K0.493Na0.493Ba0.015) (Nb0.995Cu0.002)O3 | (K0.416Na0.434Ba0.017) (Nb0.992Cu0.001Sb0.004)O3 | 1125 | 20–21 | ~7 | ~91 | This work |
| KNBaCuN SSCG [100] KTaO3 seed crystal | (K0.493Na0.493Ba0.015) (Nb0.995Cu0.002)O3 | (K0.464Na0.413Ba0.015) (Nb0.991Cu0.002Sb0.004)O3 | 1125 | 10 | ~7 | ~100 | This work |
| KNBaCuN SSCG [110] KTaO3 seed crystal | (K0.493Na0.493Ba0.015) (Nb0.995Cu0.002)O3 | Not analysed | 1125 | 10 | ~8 | ~78 | This work |
| KNBaCuN seed-free SSCG | (K0.493Na0.493Ba0.015) (Nb0.995Cu0.005)O3 | (K0.454Na0.426Ba0.014) (Nb1.106Cu0.000)O3 | 1120 | 2 | ~23 | ~62–89 | [42] |
| KNN-LiBiO3 | 99.5(99.6K0.5Na0.5NbO3– 0.4LiBiO3)–0.5MnO2 | Na0.525K0.443NbO3 | 1105 | 21 | ~18 | ~93–99 | [33] |
| KNN-LiBiO3 | (1-x)Na0.5K0.5NbO3-xLiBiO3 (x = 0.001–0.006) | (Na0.51K0.47Bi0.005)Nb1.125O3 (1110 °C, 12 h) | 1080–1120 | 3–24 | ~12 | ~90–100 | [40] |
| KNN-CuO-Bi2O3 | Na0.495K0.495NbO3-1.5 wt% CuO-0.5 wt% Bi2O3 | (Na0.494K0.468Bi0.004) (Nb1.032Cu0.002)O3 | 1060 | 15 | ~15 | ~68 | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fisher, J.G.; Sim, S.-H.; Ðoàn, T.T.; Uwiragiye, E.; Mok, J.; Lee, J. Comparison of (K0.5Na0.5)NbO3 Single Crystals Grown by Seed-Free and Seeded Solid-State Single Crystal Growth. Materials 2023, 16, 3638. https://doi.org/10.3390/ma16103638
Fisher JG, Sim S-H, Ðoàn TT, Uwiragiye E, Mok J, Lee J. Comparison of (K0.5Na0.5)NbO3 Single Crystals Grown by Seed-Free and Seeded Solid-State Single Crystal Growth. Materials. 2023; 16(10):3638. https://doi.org/10.3390/ma16103638
Chicago/Turabian StyleFisher, John G., Su-Hyeon Sim, Trung Thành Ðoàn, Eugenie Uwiragiye, Jungwi Mok, and Junseong Lee. 2023. "Comparison of (K0.5Na0.5)NbO3 Single Crystals Grown by Seed-Free and Seeded Solid-State Single Crystal Growth" Materials 16, no. 10: 3638. https://doi.org/10.3390/ma16103638
APA StyleFisher, J. G., Sim, S.-H., Ðoàn, T. T., Uwiragiye, E., Mok, J., & Lee, J. (2023). Comparison of (K0.5Na0.5)NbO3 Single Crystals Grown by Seed-Free and Seeded Solid-State Single Crystal Growth. Materials, 16(10), 3638. https://doi.org/10.3390/ma16103638








