Effect of Metakaolin on the Microstructural and Chloride Ion Transport Properties of Concrete in Ocean Wave Splashing Zones
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Specimen Preparation
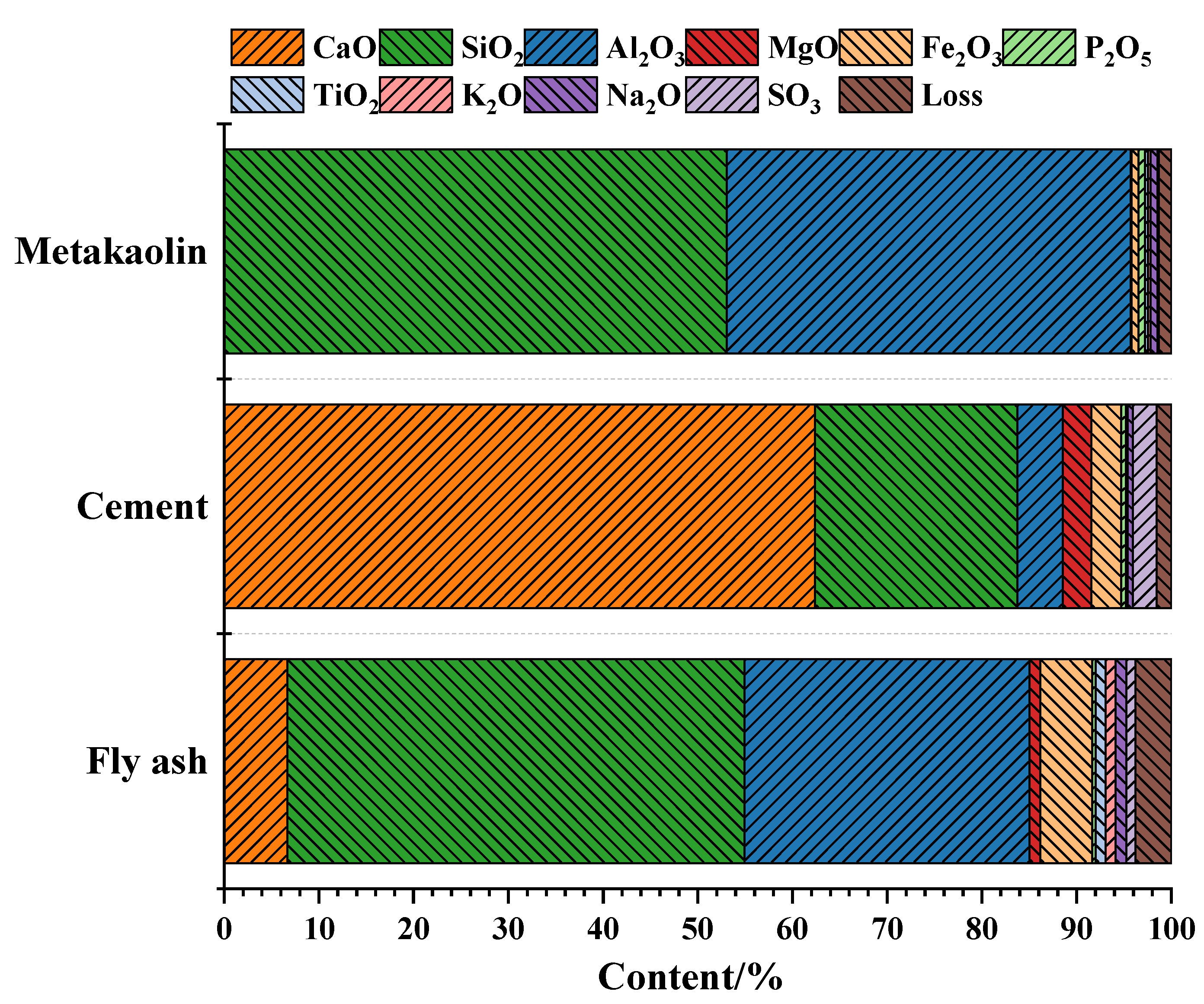
2.1.1. Raw Materials
2.1.2. Specimen Preparation
2.1.3. Corrosion Regime
2.2. Test Methods
2.2.1. Compressive Strength Test
2.2.2. Capillary Water Absorption Test
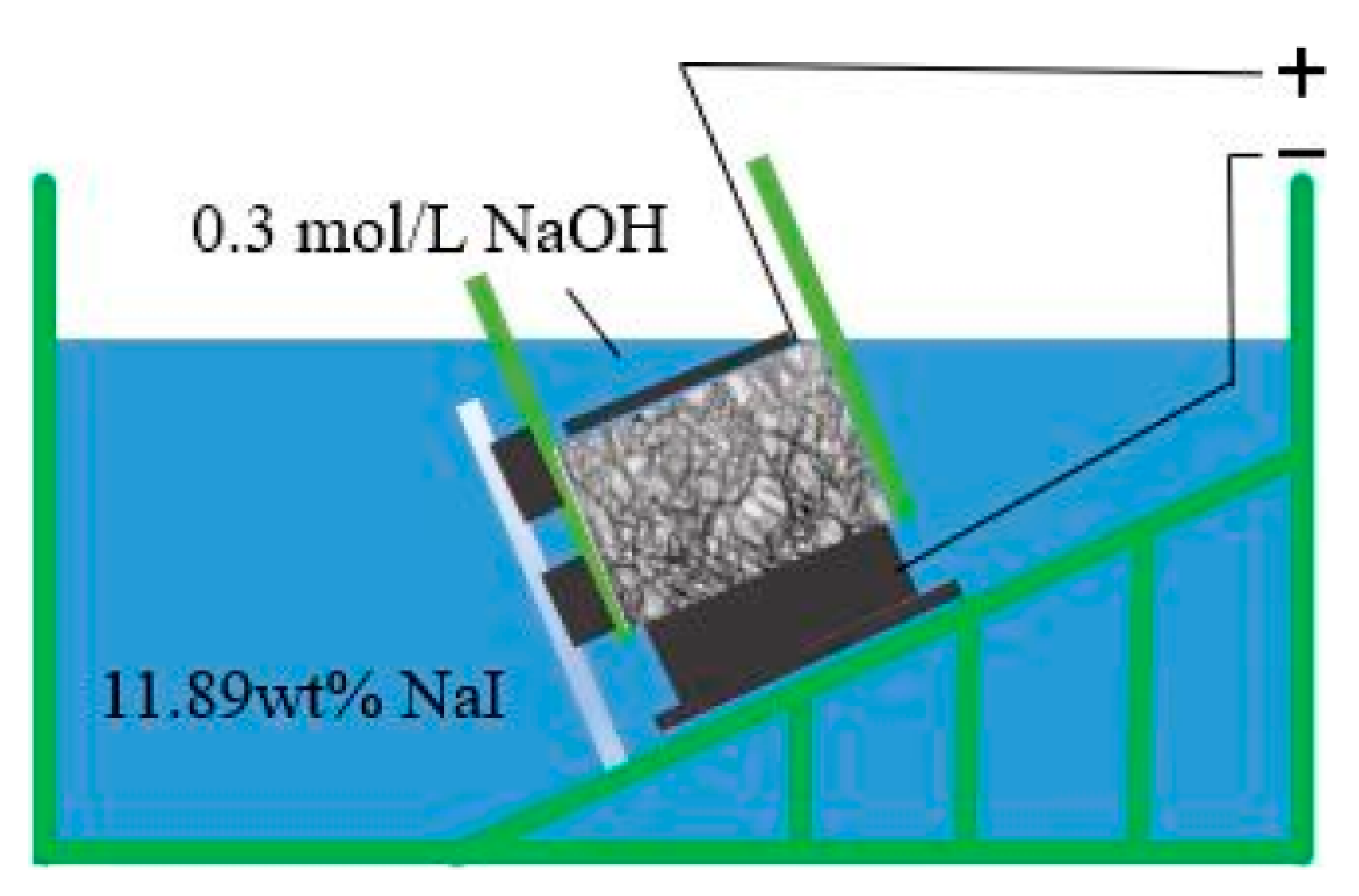
2.2.3. Rapid Iodine Ion Migration Test
2.2.4. Mercury Injection Test
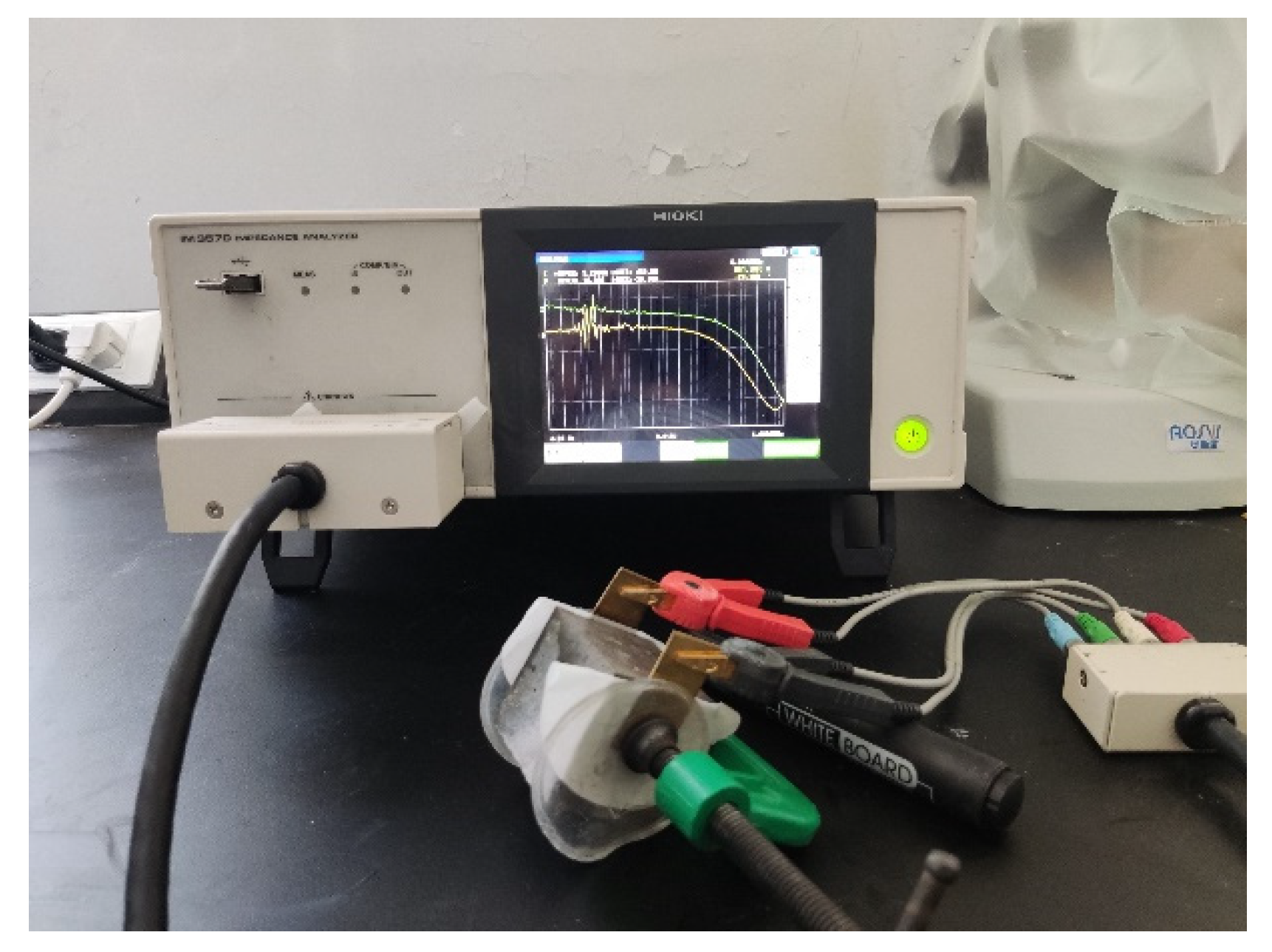
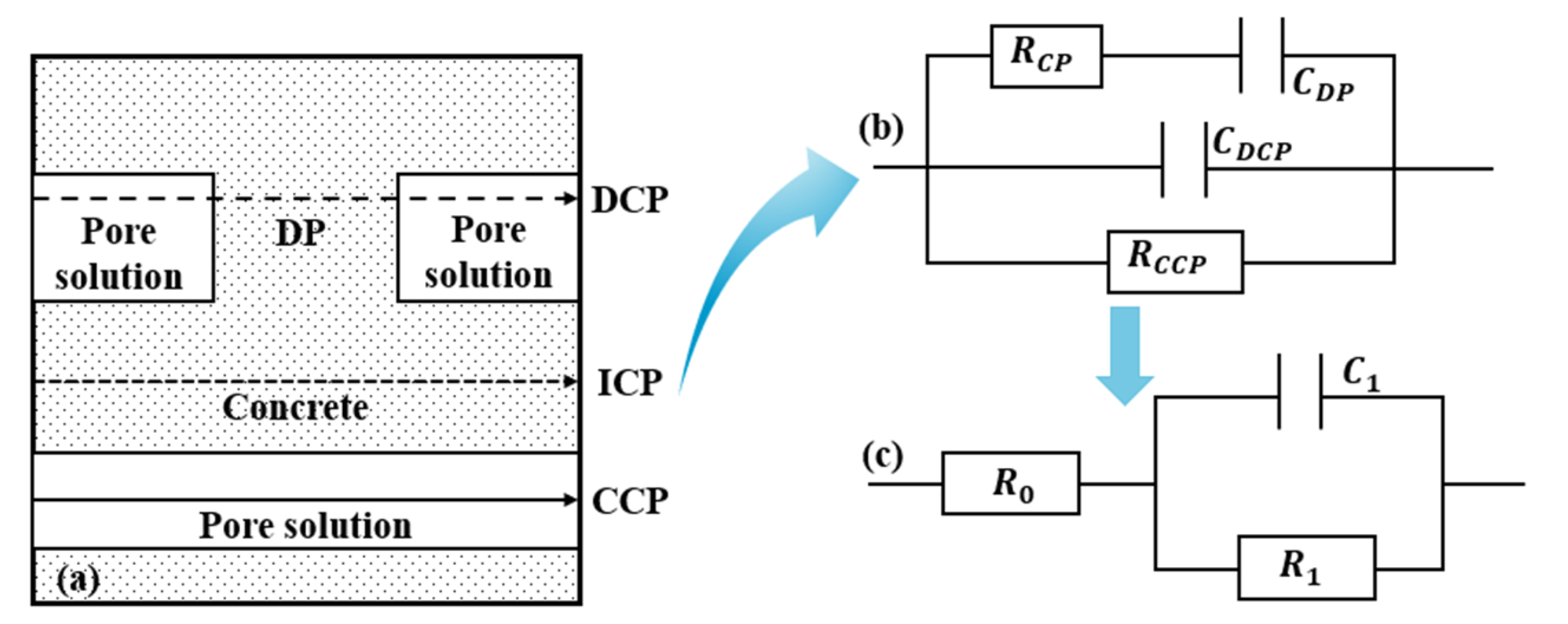
2.2.5. Alternating-Current Impedance
3. Results and Discussion
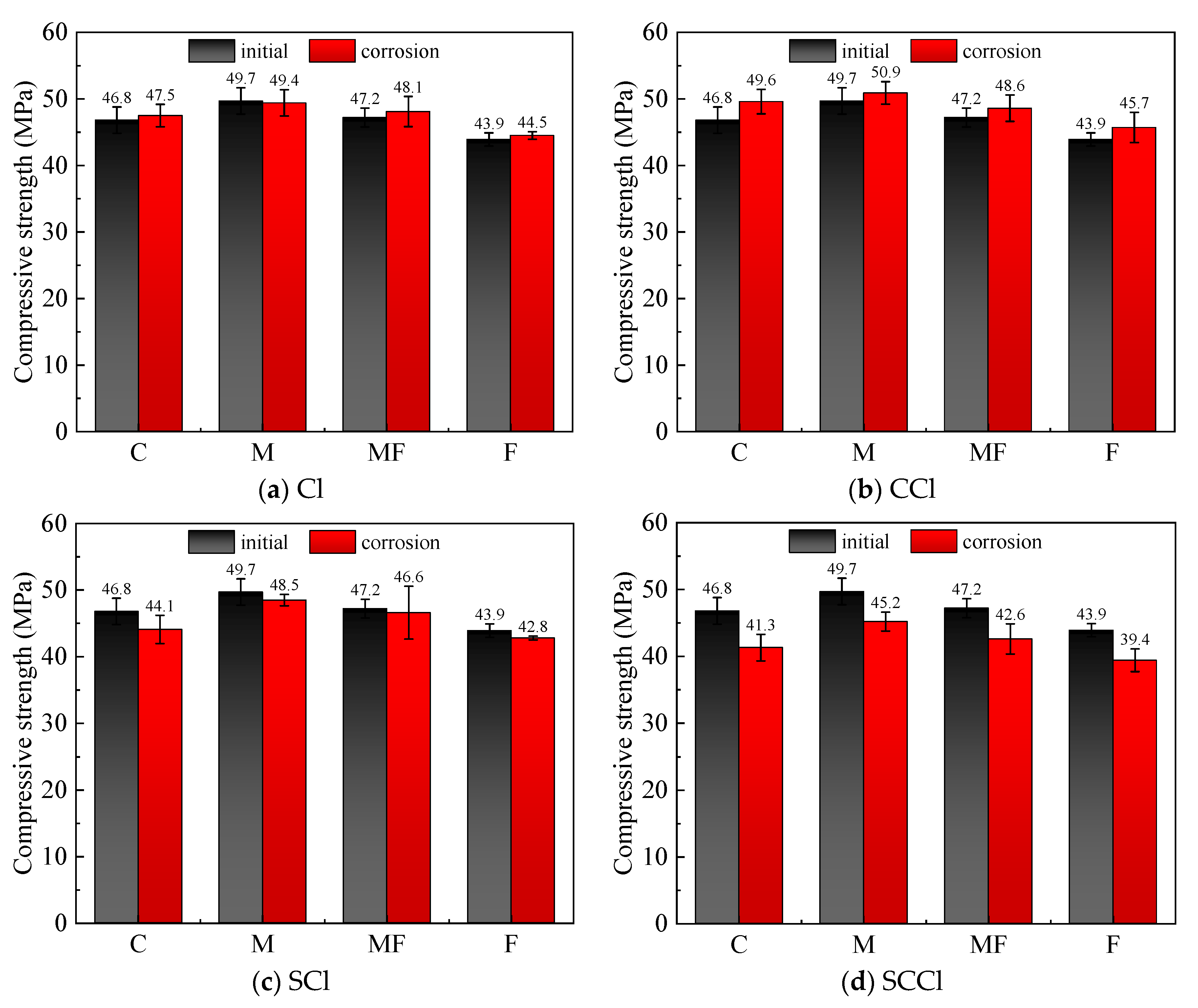
3.1. Compressive Strength
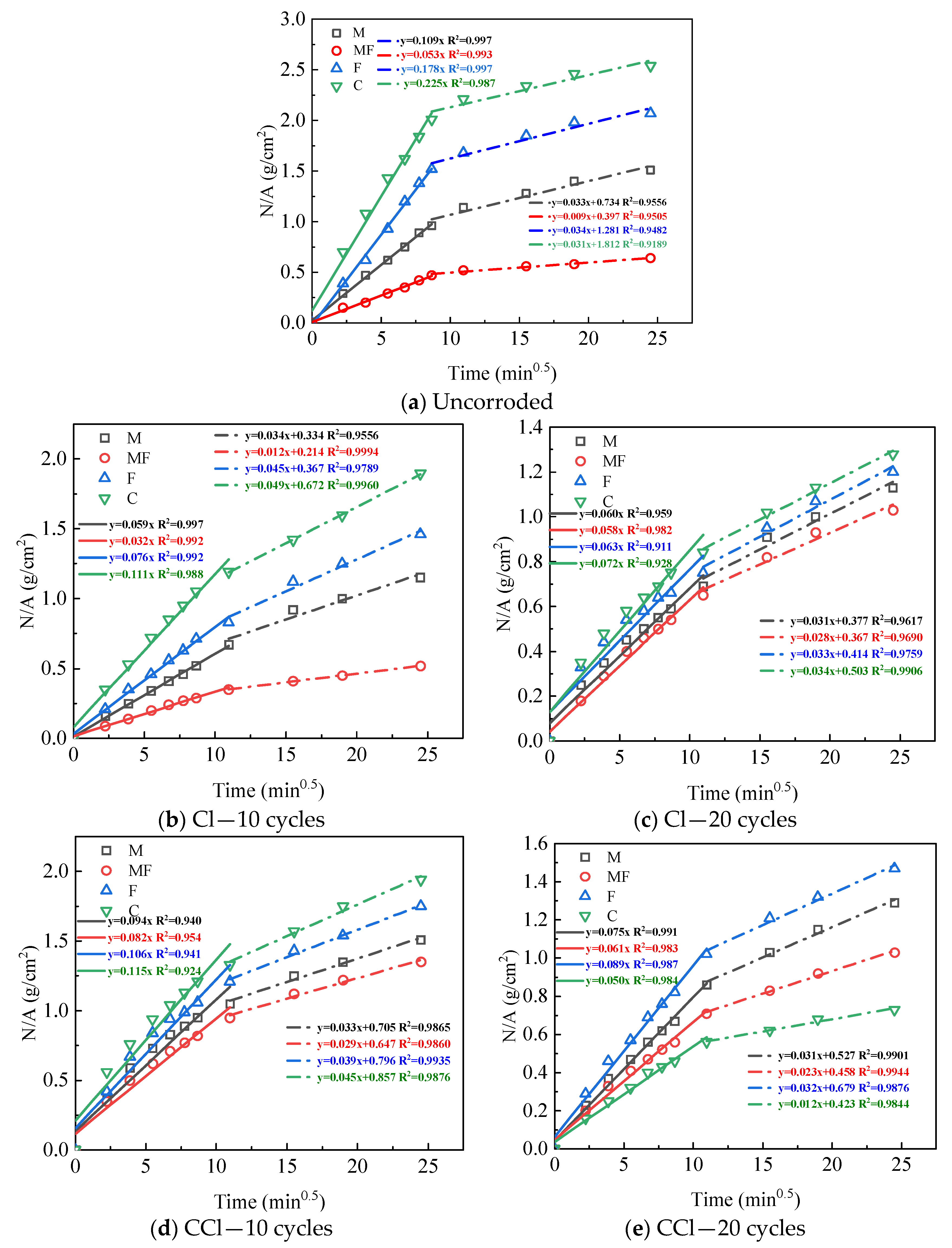
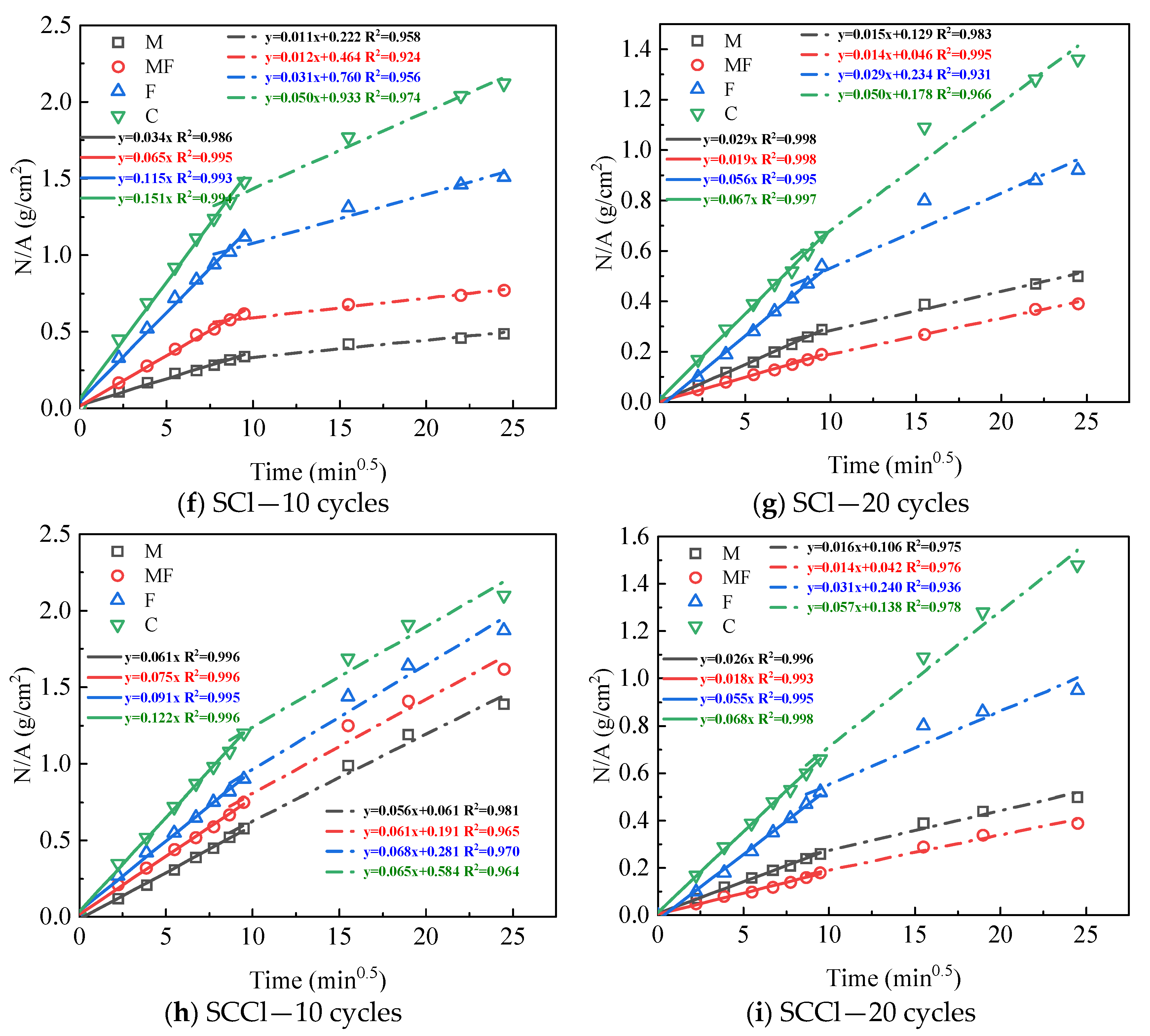
3.2. Capillary Water Absorption Performance
3.3. Ion Migration Performance
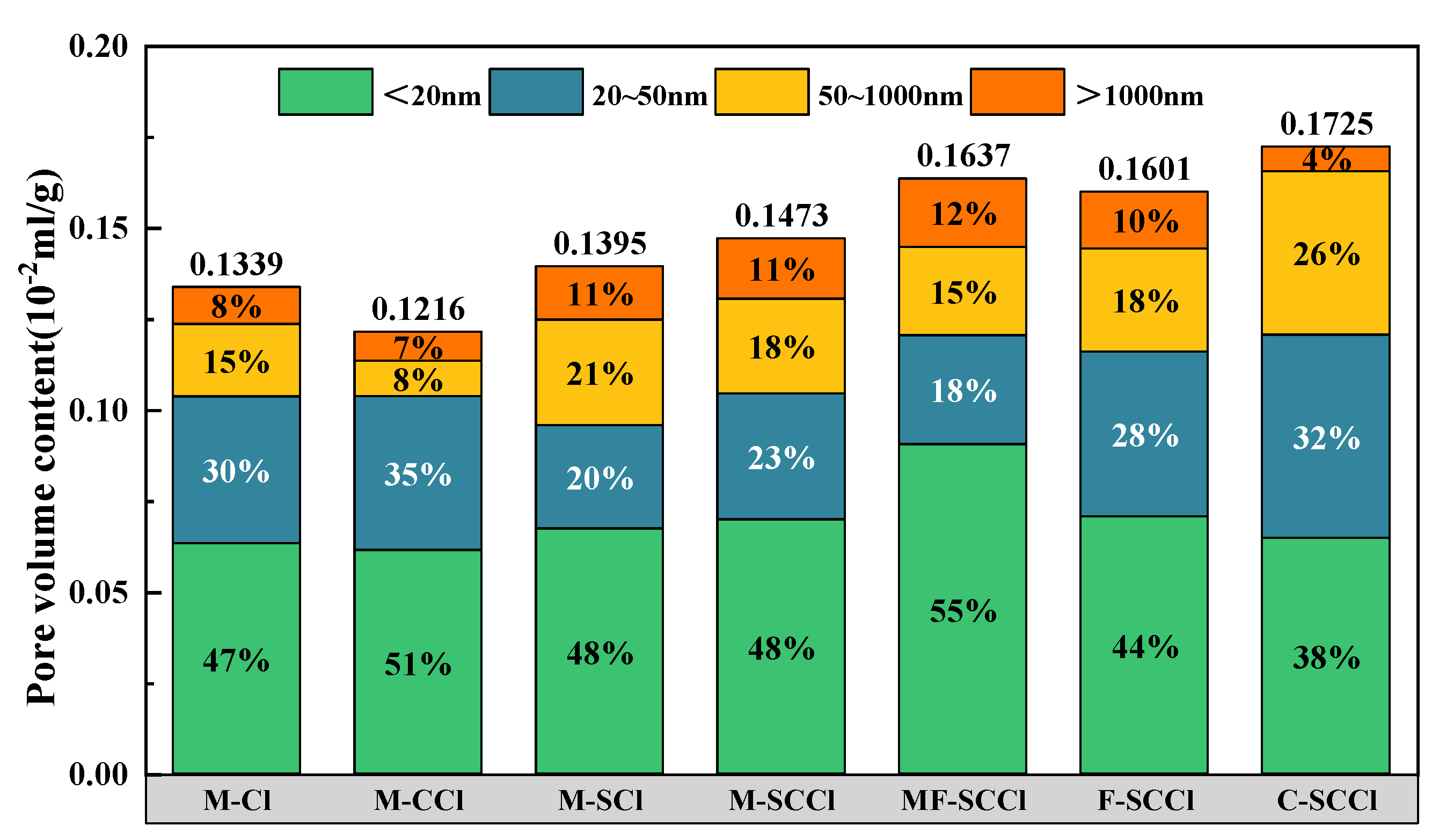
3.4. Analysis of the Pore Structure
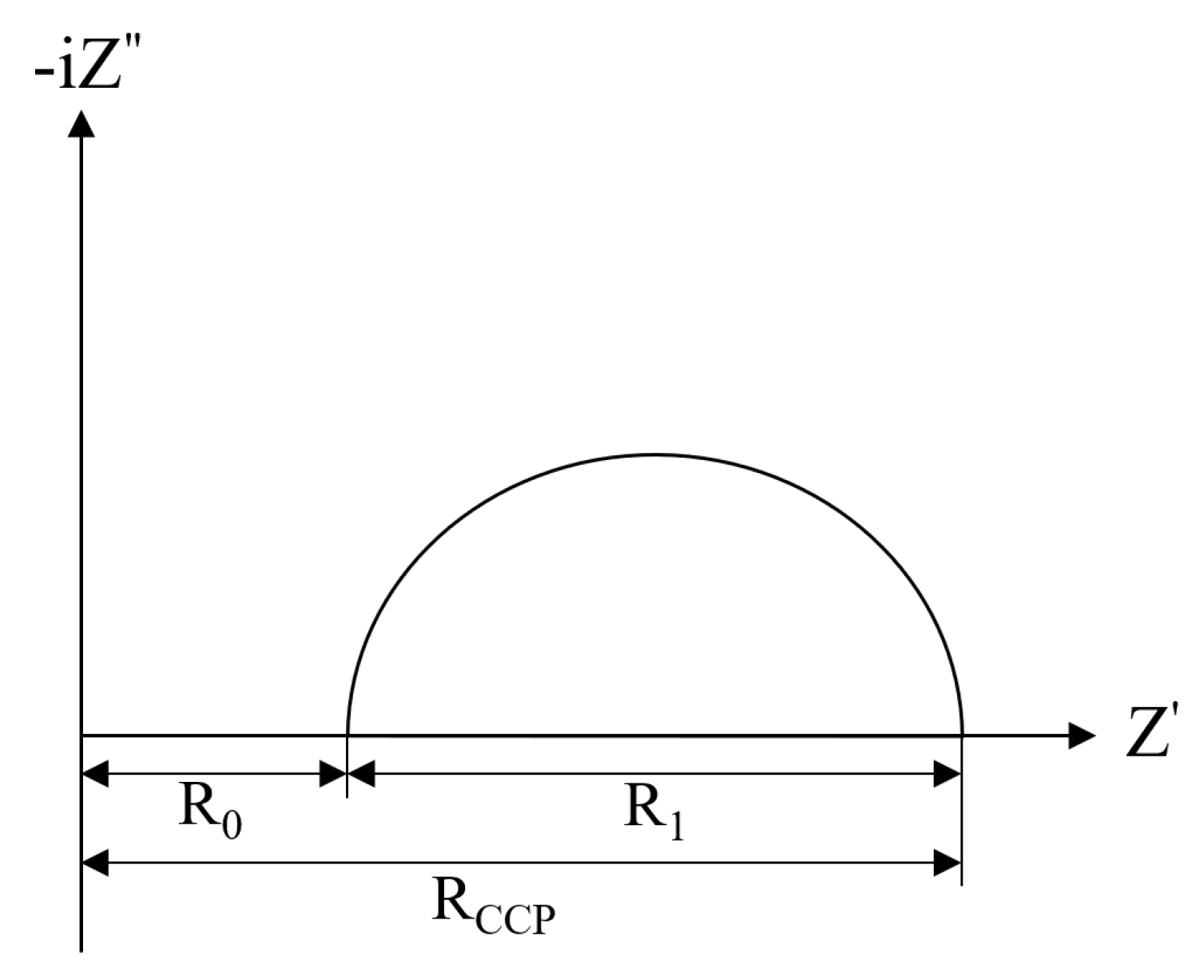
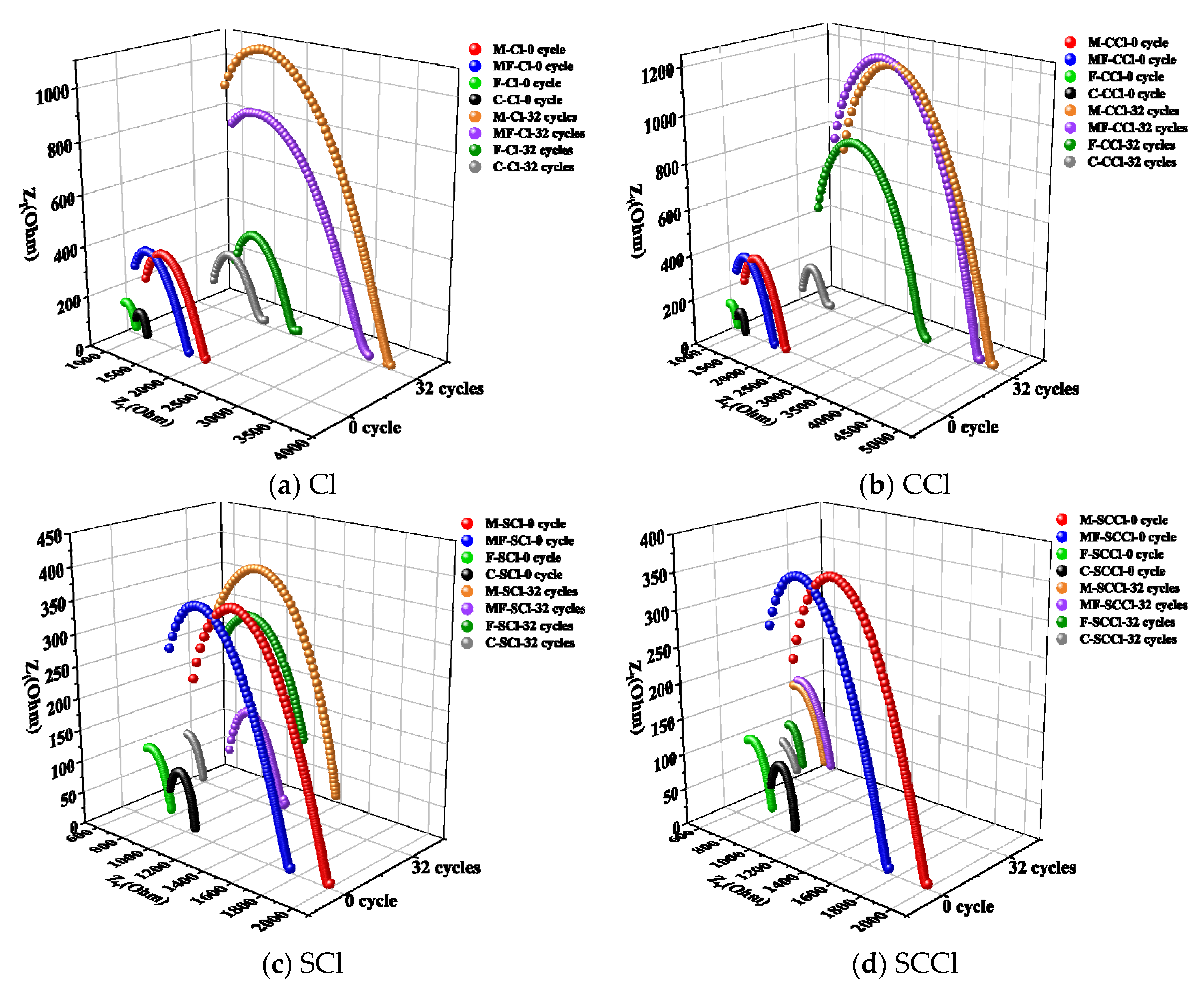
3.5. Electrochemical Impedance Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuosa, H.; Ferreira, R.M.; Holt, E.; Leivo, M.; Vesikari, E. Effect of coupleddeterioration by freeze–thaw, carbonation and chlorides on concrete service life. Cem. Concr. Compos. 2014, 47, 32–40. [Google Scholar] [CrossRef]
- Zheng, M. The sources, hazards, mechanism of action and detection methods of chloride ions in concrete. Constr. Mater. Decor. 2019, 4, 54–55. [Google Scholar]
- Wang, A.; Zheng, Y.; Zhang, Z.; Liu, K.; Yan, L.; Liang, S.; Sun, D. The durability of alkali-activated materials in comparison with ordinary Portland cements and concretes: A review. Engineering 2020, 6, 695–706. [Google Scholar] [CrossRef]
- Wang, B. Study on Chloride Ion Transportion in Unsaturated Concrete; Qingdao Technological University: Qingdao, China, 2013. [Google Scholar]
- Jin, L.; Yu, H.; Wang, Z.; Wang, Z.; Tai, F. Developing a model for chloride transport through concrete considering the key factors. Case Stud. Constr. Mater. 2022, 17, e01168. [Google Scholar]
- Shi, H.S.; Wang, Q. Research on the factors influencing on the chloride ingression in concrete. J. Build. Mater. 2004, 7, 286–290. (In Chinese) [Google Scholar]
- Yang, L.F.; Cai, R.; Yu, B. Formation mechanism and multi-factor model for surface chloride concentration of concrete in marine atmosphere zone. Chin. Civil. Eng. J. 2017, 50, 46–55. (In Chinese) [Google Scholar]
- Wang, J.G.; Zhang, J.X.; Guo, Y.Y.; Zhou, T.J. Influence mechanism of different factors on chloride ion penetration of concrete. Concrete 2018, 8, 49–53. (In Chinese) [Google Scholar]
- Wang, S.D.; Huang, Y.B.; Wang, Z. Concrete resistance to chloride ingress: Effect of cement composition. J. Chin. Ceram. Soc. 2000, 28, 570–574. (In Chinese) [Google Scholar]
- Tian, H.; Cui, Z.; Ma, H.; Zhao, P.; Yan, M.; Xin, W.; Cui, H. Corrosion evolution and stress corrosion cracking behavior of a low carbon bainite steel in the marine environments: Effect of the marine zones. Corros. Sci. 2022, 206, 110490. [Google Scholar] [CrossRef]
- Nie, L.; Xu, J.; Bai, E. Dynamic stress-strain relationship of concrete subjected to chloride and sulfate attack. Constr. Build. Mater. 2018, 165, 232–240. [Google Scholar] [CrossRef]
- Sirivivatnanon, R.V. Effect of carbon dioxide on chloride penetration and chloride ion diffusion coefficient of blended Portland cement mortar. Constr. Build. Mater. 2008, 22, 1701–1707. [Google Scholar]
- Boddy, A.; Bentz, E.; Thomas, M.D.A.; Hootonb, R.D. An overview and sensitivity study of a multimechanistic chloride transport model. Cem. Concr. Res. 1999, 29, 827–837. [Google Scholar] [CrossRef]
- Yuan, Q.; Shi, C.; De Schutter, G.; Audenaert, K.; Dehua, D. Chloride binding of cement-based materials subjected to external chloride environment–a review. Constr. Build. Mater. 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Neville, A. The confused world of sulfate attack on concrete. Cem. Concr. Res. 2004, 34, 1275–1296. [Google Scholar] [CrossRef]
- Glasser, F.P.; Marchand, J.; Samson, E. Durability of concrete—Degradation phenomena involving detrimental chemical reactions. Cem. Concr. Res. 2008, 38, 226–246. [Google Scholar] [CrossRef]
- Castellote, M.; Fernandez, L.; Andrade, C.; Alonso, C. Chemical changes and phase analysis of OPC pastes carbonated at different CO2 concentrations. Mater. Struct. 2009, 42, 515–525. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, Q.; Chen, X.; Wang, X.; Feng, X.; Han, N.; He, Y. Degradation of fly ash concrete under the coupled effect of carbonation and chloride aerosol ingress. Corros. Sci. 2016, 112, 364–372. [Google Scholar] [CrossRef]
- Cao, Y.; Guo, L.; Chen, B. Influence of sulfate on the chloride diffusion mechanism in mortar. Constr. Build. Mater. 2019, 197, 398–405. [Google Scholar] [CrossRef]
- Geng, J.; Easterbrook, D.; Li, L.Y.; Mo, L. The stability of bound chlorides in cement paste with sulfate attack. Cem. Concr. Res. 2015, 68, 211–222. [Google Scholar] [CrossRef]
- Amran, M.; Al-Fakih, A.; Chu, S.H.; Roman, F.; Sani, H.; Afonso, A.; Nikolai, V. Long-term durability properties of geopolymer concrete: An in-depth review. Case Stud. Constr. Mater. 2021, 15, e00661. [Google Scholar] [CrossRef]
- Gao, S.; Guo, J.; Gong, Y.; Ban, S.; Liu, A. Study on the penetration and diffusion of chloride ions in interface transition zone of recycled concrete prepared by modified recycled coarse aggregates. Case Stud. Constr. Mater. 2022, 16, e01034. [Google Scholar] [CrossRef]
- Elahi, M.M.A.; Shearer, C.R.; Reza, A.N.R.; Naser, A. Improving the sulfate attack resistance of concrete by using supplementary cementitious materials (SCMs): A review. Constr. Build. Mater. 2021, 281, 122628. [Google Scholar] [CrossRef]
- Tafraoui, A.; Escadeillas, G.; Vidal, T. Durability of the ultra high performances concrete containing metakaolin. Constr. Build. Mater. 2016, 112, 980–987. [Google Scholar] [CrossRef]
- Li, C.; Xiao, K. Chloride threshold, modelling of corrosion rate and pore structure of concrete with metakaolin addition. Constr. Build. Mater. 2021, 305, 124666. [Google Scholar] [CrossRef]
- Sujjavanich, S.; Suwanvitaya, P.; Chaysuwan, D.; Heness, G. Synergistic effect of metakaolin and fly ash on properties of concrete. Constr. Build. Mater. 2017, 155, 830–837. [Google Scholar] [CrossRef]
- Al-alaily, H.S.; Hassan, A.A.A. Time-dependence of chloride diffusion for concrete containing metakaolin. J. Build. Eng. 2016, 7, 159–169. [Google Scholar] [CrossRef]
- Lay, S.; Liebl, S.; Hilbig, H.; Schießl, P. New method to measure the rapid chloride migration coefficient of chloride-contaminated concrete. Cem. Concr. Res. 2004, 34, 421–427. [Google Scholar] [CrossRef]
- Noushini, A.; Nguyen, Q.D.; Castel, A. Assessing alkali-activated concrete performance in chloride environments using NT Build 492. Mater. Struct. 2021, 54, 57. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Wang, D.; Liu, Y.; Liu, L. Study on the influence of sample size and test conditions on the capillary water absorption coefficient of porous building materials. J. Build. Eng. 2021, 43, 103120. [Google Scholar] [CrossRef]
- Lü, Q.; Qiu, Q.; Zheng, J.; Wang, J.; Zeng, Q. Fractal dimension of concrete incorporating silica fume and its correlations to pore structure, strength and permeability. Constr. Build. Mater. 2019, 228, 116986. [Google Scholar] [CrossRef]
- Song, G. Equivalent circuit model for AC electrochemical impedance spectroscopy of concrete. Cem. Concr. Res. 2000, 30, 1723–1730. [Google Scholar] [CrossRef]



| Factors | Results | Refs. | |
|---|---|---|---|
| Internal factors | Adsorption of cementitious materials | C-S-H gel has an adsorption effect on chloride ions. | [6] |
| w/b | The chloride ion diffusion coefficient increases with an increase in the w/b. | [7] | |
| Pore structure | With the increase in hole connectivity and aperture, impermeability worsens. | [8] | |
| C3A content | There is higher impermeability with an increase in C3A content. | [9] | |
| External factors | Corrosion time | The apparent diffusion coefficient decreases with an increase in corrosion time. | [7] |
| Ambient temperature | The chloride ion diffusion coefficient increases with an increase in temperature. | [6] | |
| Stress | Under a stretching condition, the diffusion coefficient increases. | [6] | |
| Cracking | Crack zone promotes chloride ion diffusion. | [6] |
| Number | Cement | Metakaolin | FLY ASH | Fine Aggregate | Coarse Aggregate | Water | Water Reducing Agent |
|---|---|---|---|---|---|---|---|
| C | 450 | — | — | 1250 | 2840 | 176 | 9 |
| M | 315 | 135 | — | 1250 | 2840 | 176 | 9 |
| MF | 315 | 67.5 | 67.5 | 1250 | 2840 | 176 | 9 |
| F | 315 | — | 135 | 1250 | 2840 | 176 | 9 |
| M-Cl | M-CCl | M-SCl | M-SCCl | MF-Cl | MF-CCl | MF-SCl | MF-SCCl | |
| 0 cycle | 0.109 | 0.109 | 0.109 | 0.109 | 0.053 | 0.053 | 0.053 | 0.053 |
| 10 cycles | 0.059 | 0.094 | 0.034 | 0.061 | 0.032 | 0.082 | 0.065 | 0.075 |
| 20 cycles | 0.060 | 0.075 | 0.029 | 0.026 | 0.058 | 0.061 | 0.019 | 0.018 |
| F-Cl | F-CCl | F-SCl | F-SCCl | C-Cl | C-CCl | C-SCl | C-SCCl | |
| 0 cycle | 0.178 | 0.178 | 0.178 | 0.178 | 0.225 | 0.225 | 0.225 | 0.225 |
| 10 cycles | 0.076 | 0.106 | 0.115 | 0.091 | 0.111 | 0.115 | 0.151 | 0.122 |
| 20 cycles | 0.063 | 0.089 | 0.056 | 0.055 | 0.072 | 0.050 | 0.067 | 0.068 |
| No. | M | MF | F | C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Impedance Parameter | R0 | R1 | RCCP | R0 | R1 | RCCP | R0 | R1 | RCCP | R0 | R1 | RCCP |
| 0 cycle | 987 | 822 | 1809 | 713 | 884 | 1597 | 586 | 319 | 905 | 854 | 221 | 1075 |
| Cl—32 cycles | 1186 | 6466 | 7652 | 963 | 4181 | 5147 | 1147 | 3725 | 4872 | 880 | 583 | 1463 |
| CCl—32 cycles | 1161 | 4358 | 5519 | 1136 | 4169 | 5305 | 1829 | 2802 | 4631 | 963 | 856 | 1819 |
| SCl—32 cycles | 739 | 3291 | 4030 | 784 | 379 | 1163 | 521 | 913 | 1434 | 342 | 248 | 590 |
| SCCl—32 cycles | 834 | 1197 | 2031 | 236 | 2740 | 2976 | 723 | 835 | 1558 | 808 | 448 | 1256 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Niu, K.; Tian, B.; Li, L.; Ji, J.; Feng, Y. Effect of Metakaolin on the Microstructural and Chloride Ion Transport Properties of Concrete in Ocean Wave Splashing Zones. Materials 2023, 16, 7. https://doi.org/10.3390/ma16010007
Yuan Y, Niu K, Tian B, Li L, Ji J, Feng Y. Effect of Metakaolin on the Microstructural and Chloride Ion Transport Properties of Concrete in Ocean Wave Splashing Zones. Materials. 2023; 16(1):7. https://doi.org/10.3390/ma16010007
Chicago/Turabian StyleYuan, Yezhen, Kaimin Niu, Bo Tian, Lihui Li, Jianrui Ji, and Yunxia Feng. 2023. "Effect of Metakaolin on the Microstructural and Chloride Ion Transport Properties of Concrete in Ocean Wave Splashing Zones" Materials 16, no. 1: 7. https://doi.org/10.3390/ma16010007
APA StyleYuan, Y., Niu, K., Tian, B., Li, L., Ji, J., & Feng, Y. (2023). Effect of Metakaolin on the Microstructural and Chloride Ion Transport Properties of Concrete in Ocean Wave Splashing Zones. Materials, 16(1), 7. https://doi.org/10.3390/ma16010007





