Iron Oxide Nanoparticles: A Review on the Province of Its Compounds, Properties and Biological Applications
Abstract
1. Introduction
2. Formation of Iron Oxide Nanoparticles
2.1. Coprecipitation Synthesis
2.2. Hydrothermal Synthesis
2.3. Sol-Gel Synthesis
2.4. Microemulsion Synthesis
2.5. Sonochemical Synthesis
2.6. Electrochemical Synthesis
2.7. Green Synthesis
3. Structure of Iron Oxide Nanoparticles
3.1. Magnetite
3.2. Hematite
3.3. Maghemite
3.4. β-Fe2O3
3.5. ϵ-Fe2O3
3.6. ζ-Fe2O3
3.7. Goethite
3.8. Ferrihydrite
3.9. Wüstite
3.10. Akaganeite
3.11. Lepidocrocite
4. Properties of Iron Oxide Nanoparticles
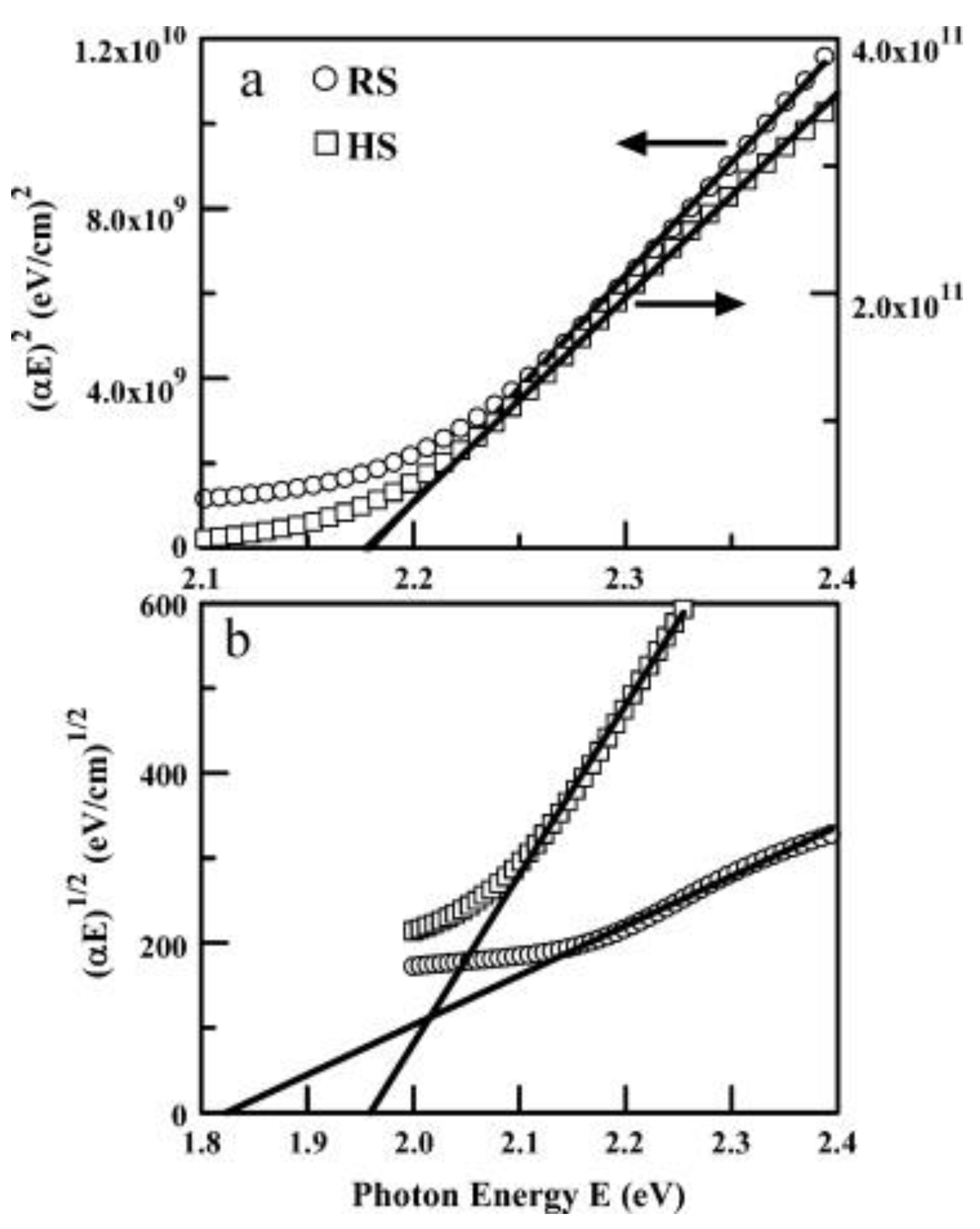
4.1. Optical Properties
4.2. Magnetic Properties
4.3. Rheological Properties
5. Influence on Oxidation States and Phase Transformations
6. Biological Applications of Iron Oxide Nanoparticles
6.1. Iron Oxide Nanoparticles as Contrast Agents
6.2. Iron Oxide Nanoparticles in Immuno-Toxicity and Cell Toxicity
6.3. Iron Oxide Nanoparticles in Therapeutic Applications
6.4. Iron Oxide Nanoparticles in Biosensing Applications
6.5. Iron Oxide Nanoparticles as Anti-Bacterial Agents
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lodhia, J.; Mandarano, G.; Ferris, N.; Eu, P.; Cowell, S. Development and use of iron oxide nanoparticles (Part 1): Synthesis of iron oxide nanoparticles for MRI. Biomed. Imaging Interv. J. 2010, 6, e12. [Google Scholar] [CrossRef] [PubMed]
- Dhak, P.; Kim, M.-K.; Lee, J.H.; Kim, M.; Kim, S.-K. Linear-chain assemblies of iron oxide nanoparticles. J. Magn. Magn. Mater. 2017, 433, 47–52. [Google Scholar] [CrossRef]
- Sivula, K.; Le Formal, F.; Grätzel, M. Solar Water Splitting: Progress Using Hematite (α-Fe2O3) Photoelectrodes. ChemSusChem 2011, 4, 432–449. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurences and Uses, 2nd ed.; VCH: Weinheim, Germany, 2003. [Google Scholar]
- Tuček, J.; Machala, L.; Ono, S.; Namai, A.; Yoshikiyo, M.; Imoto, K.; Tokoro, H.; Ohkoshi, S.-I.; Zbořil, R. Zeta-Fe2O3—A new stable polymorph in iron (III) oxide family. Sci. Rep. 2015, 5, 15091. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Bagheri, S.; Hamid, S.B.A. Progress in electrochemical synthesis of magnetic iron oxide nanoparticles. J. Magn. Magn. Mater. 2014, 368, 207–229. [Google Scholar] [CrossRef]
- Bhavani, P.; Rajababu, C.; Arif, M.; Reddy, I.V.S.; Reddy, N.R. Synthesis of high saturation magnetic iron oxide nanomaterials via low temperature hydrothermal method. J. Magn. Magn. Mater. 2017, 426, 459–466. [Google Scholar] [CrossRef]
- De Tercero, M.D.; Bruns, M.; Martínez, I.G.; Türk, M.; Fehrenbacher, U.; Jennewein, S.; Barner, L. Continuous Hydrothermal Synthesis of In Situ Functionalized Iron Oxide Nanoparticles: A General Strategy to Produce Metal Oxide Nanoparticles with Clickable Anchors. Part. Part. Syst. Charact. 2013, 30, 229–234. [Google Scholar] [CrossRef]
- Takami, S.; Sato, T.; Mousavand, T.; Ohara, S.; Umetsu, M.; Adschiri, T. Hydrothermal synthesis of surface-modified iron oxide nanoparticles. Mater. Lett. 2007, 61, 4769–4772. [Google Scholar] [CrossRef]
- Ge, S.; Shi, X.; Sun, K.; Li, C.; Uher, C.; Baker, J.R., Jr.; Banaszak Holl, M.M.; Orr, B.G. Facile Hydrothermal Synthesis of Iron Oxide Nanoparticles with Tunable Magnetic Properties. J. Phys. Chem. C 2009, 113, 13593–13599. [Google Scholar] [CrossRef]
- Li, J.; Shi, X.; Shen, M. Hydrothermal Synthesis and Functionalization of Iron Oxide Nanoparticles for MR Imaging Applications. Part. Part. Syst. Charact. 2014, 31, 1223–1237. [Google Scholar] [CrossRef]
- Ravikumar, C.; Bandyopadhyaya, R. Mechanistic Study on Magnetite Nanoparticle Formation by Thermal Decomposition and Coprecipitation Routes. ACS Publ. 2011, 115, 1380–1387. [Google Scholar] [CrossRef]
- LaGrow, A.P.; Besenhard, M.O.; Hodzic, A.; Sergides, A.; Bogart, L.K.; Gavriilidis, A.; Thanh, N.T.K. Unravelling the growth mechanism of the co-precipitation of iron oxide nanoparticles with the aid of synchrotron X-Ray diffraction in solution. Nanoscale 2019, 11, 6620–6628. [Google Scholar] [CrossRef] [PubMed]
- Zarnegar, Z.; Safari, J. Modified chemical coprecipitation of magnetic magnetite nanoparticles using linear–dendritic copolymers. Green Chem. Lett. Rev. 2017, 10, 235–240. [Google Scholar] [CrossRef]
- Okoli, C.; Sanchez-Dominguez, M.; Boutonnet, M.; Järås, S.; Civera, C.; Solans, C.; Kuttuva, G.R. Comparison and Functionalization Study of Microemulsion-Prepared Magnetic Iron Oxide Nanoparticles. Langmuir 2012, 28, 8479–8485. [Google Scholar] [CrossRef]
- Lopez-Perez, J.; Lopez-Quintela, M.; Mira, J.; Rivas, J. Preparation of magnetic fluids with particles obtained in microemulsions. IEEE Trans. Magn. 1997, 33, 4359–4362. [Google Scholar] [CrossRef]
- Basak, S.; Chen, D.-R.; Biswas, P. Electrospray of ionic precursor solutions to synthesize iron oxide nanoparticles: Modified scaling law. Chem. Eng. Sci. 2007, 62, 1263–1268. [Google Scholar] [CrossRef]
- Vijayakumar, R.; Koltypin, Y.; Felner, I.; Gedanken, A. Sonochemical synthesis and characterization of pure nanometer-sized Fe3O4 particles. Mater. Sci. Eng. A 2000, 286, 101–105. [Google Scholar] [CrossRef]
- Hassanjani-Roshan, A.; Vaezi, M.R.; Shokuhfar, A.; Rajabali, Z. Synthesis of iron oxide nanoparticles via sonochemical method and their characterization. Particuology 2011, 9, 95–99. [Google Scholar] [CrossRef]
- Zeng, P.; Zhao, Y.; Lin, Y.; Wang, X.; Li, J.; Wang, W.; Fang, Z. Enhancement of Electrochemical Performance by the Oxygen Vacancies in Hematite as Anode Material for Lithium-Ion Batteries. Nanoscale Res. Lett. 2017, 12, 13. [Google Scholar] [CrossRef]
- Cabrera, L.; Gutierrez, S.; Menendez, N.; Morales, M.; Herrasti, P. Magnetite nanoparticles: Electrochemical synthesis and characterization. Electrochimica Acta 2007, 53, 3436–3441. [Google Scholar] [CrossRef]
- Gonzalez-Moragas, L.; Yu, S.-M.; Murillo-Cremaes, N.; Laromaine, A.; Roig, A. Scale-up synthesis of iron oxide nanoparticles by microwave-assisted thermal decomposition. Chem. Eng. J. 2015, 281, 87–95. [Google Scholar] [CrossRef]
- Lastovina, T.A.; Budnyk, A.P.; Soldatov, M.A.; Rusalev, Y.V.; Guda, A.A.; Bogdan, A.S.; Soldatov, A.V. Microwave-assisted synthesis of magnetic iron oxide nanoparticles in oleylamine–oleic acid solutions. Mendeleev Commun. 2017, 27, 487–489. [Google Scholar] [CrossRef]
- Bonfim, L.; Passos, P.D.Q.S.; Gonçalves, K.D.O.; Courrol, L.C.; Silva, F.R.D.O.; Vieira, D.P. Microwave-mediated synthesis of iron-oxide nanoparticles for use in magnetic levitation cell cultures. Appl. Nanosci. 2019, 9, 1707–1717. [Google Scholar] [CrossRef]
- Singh, M.; Ulbrich, P.; Prokopec, V.; Svoboda, P.; Šantavá, E.; Štěpánek, F. Vapour phase approach for iron oxide nanoparticle synthesis from solid precursors. J. Solid State Chem. 2013, 200, 150–156. [Google Scholar] [CrossRef]
- Lin, L.; Starostin, S.A.; Hessel, V.; Wang, Q. Synthesis of iron oxide nanoparticles in microplasma under atmospheric pressure. Chem. Eng. Sci. 2017, 168, 360–371. [Google Scholar] [CrossRef]
- Lakshminarayanan, S.; Shereen, M.F.; Niraimathi, K.L.; Brindha, P.; Arumugam, A. One-pot green synthesis of iron oxide nanoparticles from Bauhinia tomentosa: Characterization and application towards synthesis of 1, 3 diolein. Sci. Rep. 2021, 11, 8643. [Google Scholar] [CrossRef] [PubMed]
- Priya; Naveen; Kaur, K.; Sidhu, A.K. Green Synthesis: An Eco-friendly Route for the Synthesis of Iron Oxide Nanoparticles. Front. Nanotechnol. 2021, 3, 655062. [Google Scholar] [CrossRef]
- Bibi, I.; Nazar, N.; Ata, S.; Sultan, M.; Ali, A.; Abbas, A.; Jilani, K.; Kamal, S.; Sarim, F.M.; Khan, M.I.; et al. Green synthesis of iron oxide nanoparticles using pomegranate seeds extract and photocatalytic activity evaluation for the degradation of textile dye. J. Mater. Res. Technol. 2019, 8, 6115–6124. [Google Scholar] [CrossRef]
- Devi, H.S.; Boda, M.A.; Shah, M.A.; Parveen, S.; Wani, A.H. Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity. Green Process. Synth. 2019, 8, 38–45. [Google Scholar] [CrossRef]
- Bhuiyan, S.H.; Miah, M.Y.; Paul, S.C.; Das Aka, T.; Saha, O.; Rahaman, M.; Sharif, J.I.; Habiba, O. Ashaduzzaman Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: Application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon 2020, 6, e04603. [Google Scholar] [CrossRef]
- Glasgow, W.; Fellows, B.; Qi, B.; Darroudi, T.; Kitchens, C.; Ye, L.; Crawford, T.M.; Mefford, O.T. Continuous synthesis of iron oxide (Fe3O4) nanoparticles via thermal decomposition. Particuology 2016, 26, 47–53. [Google Scholar] [CrossRef]
- Babes, L.; Denizot, B.; Tanguy, G.; Le Jeune, J.J.; Jallet, P. Synthesis of Iron Oxide Nanoparticles Used as MRI Contrast Agents: A Parametric Study. J. Colloid Interface Sci. 1999, 212, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Sodipo, B.K.; Aziz, A.A. Recent advances in synthesis and surface modification of superparamagnetic iron oxide nanoparticles with silica. J. Magn. Magn. Mater. 2016, 416, 275–291. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2010, 110, 2574. [Google Scholar] [CrossRef]
- Solinas, S.; Piccaluga, G.; Morales, M.; Serna, C. Sol-gel formation of γ-Fe2O3/SiO2 nanocomposites. Acta Mater. 2001, 49, 2805–2811. [Google Scholar] [CrossRef]
- Chin, A.B.; Yaacob, I.I. Synthesis and characterization of magnetic iron oxide nanoparticles via w/o microemulsion and Massart’s procedure. J. Mater. Process. Technol. 2007, 191, 235–237. [Google Scholar] [CrossRef]
- Vidal-Vidal, J.; Rivas, J.; López-Quintela, M. Synthesis of monodisperse maghemite nanoparticles by the microemulsion method. Colloids Surfaces A Physicochem. Eng. Asp. 2006, 288, 44–51. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mahanty, S.; Das, P.; Chaudhuri, P.; Das, S. Biofabrication of iron oxide nanoparticles using manglicolous fungus Aspergillus niger BSC-1 and removal of Cr (VI) from aqueous solution. Chem. Eng. J. 2020, 385, 123790. [Google Scholar] [CrossRef]
- Fatemi, M.; Mollania, N.; Momeni-Moghaddam, M.; Sadeghifar, F. Extracellular biosynthesis of magnetic iron oxide nanoparticles by Bacillus cereus strain HMH1: Characterization and in vitro cytotoxicity analysis on MCF-7 and 3T3 cell lines. J. Biotechnol. 2018, 270, 1–11. [Google Scholar] [CrossRef]
- Jubran, A.; Al-Zamely, O.M.; Al-Ammar, M.H. A Study of Iron Oxide Nanoparticles Synthesis by Using Bacteria. Int. J. Pharm. Qual. Assur. 2020, 11, 88–92. [Google Scholar] [CrossRef]
- Salem, D.M.; Ismail, M.M.; Aly-Eldeen, M.A. Biogenic synthesis and antimicrobial potency of iron oxide (Fe3O4) nanoparticles using algae harvested from the Mediterranean Sea, Egypt. Egypt. J. Aquat. Res. 2019, 45, 197–204. [Google Scholar] [CrossRef]
- Verwey, E.J.W. The Crystal Structure of γ-Fe2O3 and γ-Al2O3. Z. Krist. Cryst. Mater. 1938, 91, 65–69. [Google Scholar] [CrossRef]
- Tipsawat, P.; Wongpratat, U.; Phumying, S.; Chanlek, N.; Chokprasombat, K.; Maensiri, S. Magnetite (Fe3O4) nanoparticles: Synthesis, characterization and electrochemical properties. Appl. Surf. Sci. 2018, 446, 287–292. [Google Scholar] [CrossRef]
- Klencsár, Z.; Ábrahám, A.; Szabó, L.; Szabó, E.G.; Stichleutner, S.; Kuzmann, E.; Homonnay, Z.; Tolnai, G. The effect of preparation conditions on magnetite nanoparticles obtained via chemical co-precipitation. Mater. Chem. Phys. 2018, 223, 122–132. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef]
- Di Iorio, E.; Colombo, C.; Cheng, Z.; Capitani, G.; Mele, D.; Ventruti, G.; Angelico, R. Characterization of magnetite nanoparticles synthetized from Fe (II)/nitrate solutions for arsenic removal from water. J. Environ. Chem. Eng. 2019, 7, 102986. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Xing, Y.; Song, S.; Yu, S.; Shi, W.; Guo, X.; Yang, J.; Lei, Y.; Cao, F. Morphology-Controlled Synthesis of Magnetites with Nanoporous Structures and Excellent Magnetic Properties. Chem. Mater. 2008, 20, 198–204. [Google Scholar] [CrossRef]
- Zboril, R.; Mashlan, M.; Petridis, D. ChemInform Abstract: Iron (III) Oxides from Thermal Processes—Synthesis, Structural and Magnetic Properties, Moessbauer Spectroscopy Characterization, and Applications. Chem Mater. 2002, 14, 969–982. [Google Scholar] [CrossRef]
- Weiss, B.P.; Kim, S.S.; Kirschvink, J.L.; Kopp, R.E.; Sankaran, M.; Kobayashi, A.; Komeili, A. Ferromagnetic resonance and low-temperature magnetic tests for biogenic magnetite. Earth Planet. Sci. Lett. 2004, 224, 73–89. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, Z.J. Correlation between Spin−Orbital Coupling and the Superparamagnetic Properties in Magnetite and Cobalt Ferrite Spinel Nanocrystals. J. Phys. Chem. B 2006, 110, 11205–11209. [Google Scholar] [CrossRef]
- De Biasi, R.S.; Devezas, T.C. Anisotropy field of small magnetic particles as measured by resonance. J. Appl. Phys. 1978, 49, 2466. [Google Scholar] [CrossRef]
- Radu, T.; Iacovita, C.; Benea, D.; Turcu, R. X-Ray Photoelectron Spectroscopic Characterization of Iron Oxide Nanoparticles. Appl. Surf. Sci. 2017, 405, 337–343. [Google Scholar] [CrossRef]
- Kuhn, L.T.; Bojesen, A.; Timmermann, L.; Nielsen, M.M.; Rup, S.M. Structural and magnetic properties of core shell iron iron oxide nanoparticles. J. Physics Condens. Matte. 2002, 14, 13551–13567. [Google Scholar] [CrossRef]
- Tadic, M.; Trpkov, D.; Kopanja, L.; Vojnovic, S.; Panjan, M. Hydrothermal synthesis of hematite (α-Fe2O3) nanoparticle forms: Synthesis conditions, structure, particle shape analysis, cytotoxicity and magnetic properties. J. Alloy. Compd. 2019, 792, 599–609. [Google Scholar] [CrossRef]
- Herrera-Navarro, A.M.; Jiménez-Hernández, H.; Terol-Villalobos, I.R. Framework for characterizing circularity based on a probability distribution. Measurement 2013, 46, 4232–4243. [Google Scholar] [CrossRef]
- Kopanja, L.; Žunić, D.; Lončar, B.; Gyergyek, S.; Tadić, M. Quantifying shapes of nanoparticles using modified circularity and ellipticity measures. Measurement 2016, 92, 252–263. [Google Scholar] [CrossRef]
- Souza, D.; Menegalli, F. Image analysis: Statistical study of particle size distribution and shape characterization. Powder Technol. 2011, 214, 57–63. [Google Scholar] [CrossRef]
- Kopanja, L.; Lončar, B.; Tadić, M. Nanoparticle shapes: Quantification by elongation, convexity and circularity measures. J. Electr. Eng. 2019, 70, 44–50. [Google Scholar] [CrossRef]
- Suzuki, H.; Fujiwara, M.; Iwatsuki, K.; Hirata, A.; Nagatsuma, T. Photonic millimetre-wave generator using intensity and phase modulators for 10 Gbit/s wireless link. Electron. Lett. 2005, 41, 355–356. [Google Scholar] [CrossRef]
- Trpkov, D.; Panjan, M.; Kopanja, L.; Tadić, M. Hydrothermal synthesis, morphology, magnetic properties and self-assembly of hierarchical α-Fe2O3 (hematite) mushroom-, cube- and sphere-like superstructures. Appl. Surf. Sci. 2018, 457, 427–438. [Google Scholar] [CrossRef]
- Bødker, F.; Hansen, M.F.; Koch, C.B.; Lefmann, K.; Mørup, S. Magnetic properties of hematite nanoparticles. Phys. Rev. B 2000, 61, 6826–6838. [Google Scholar] [CrossRef]
- El Aal, S.A.; Abdelhady, A.; Mansour, N.; Hassan, N.M.; Elbaz, F.; Elmaghraby, E.K. Physical and chemical characteristics of hematite nanoparticles prepared using microwave-assisted synthesis and its application as adsorbent for Cu, Ni, Co, Cd and Pb from aqueous solution. Mater. Chem. Phys. 2019, 235, 121771. [Google Scholar] [CrossRef]
- Jubb, A.; Allen, H.C. Vibrational Spectroscopic Characterization of Hematite, Maghemite, and Magnetite Thin Films Produced by Vapor Deposition. ACS Appl. Mater. Interfaces 2010, 2, 2804–2812. [Google Scholar] [CrossRef]
- Show, B.; Mukherjee, N.; Mondal, A. α-Fe2O3 nanospheres: Facile synthesis and highly efficient photo-degradation of organic dyes and surface activation by nano-Pt for enhanced methanol sensing. RSC Adv. 2016, 6, 75347–75358. [Google Scholar] [CrossRef]
- Can, M.M.; Coşkun, M.; Fırat, T. A comparative study of nanosized iron oxide particles; magnetite (Fe3O4), maghemite (γ-Fe2O3) and hematite (α-Fe2O3), using ferromagnetic resonance. J. Alloy. Compd. 2012, 542, 241–247. [Google Scholar] [CrossRef]
- Sastry, M.D.; Nagar, Y.C.; Bhushan, B.; Mishra, K.P.; Balaram, V.; Singhvi, A.K. An unusual radiation dose dependent EPR line at geff = 2.54 in feldspars: Possible evidence of Fe3+O2−↔ Fe2+O−and exchange coupled Fe3+–Fe2+–nO−. J. Physics: Condens. Matter 2007, 20, 025224. [Google Scholar] [CrossRef]
- Murphy, B.J.; Hidalgo, R.; Roessler, M.M.; Evans, R.M.; Ash, P.A.; Myers, W.K.; Vincent, K.A.; Armstrong, F.A. Discovery of Dark pH-Dependent H+ Migration in a [NiFe]-Hydrogenase and Its Mechanistic Relevance: Mobilizing the Hydrido Ligand of the Ni-C Intermediate. J. Am. Chem. Soc. 2015, 137, 8484–8489. [Google Scholar] [CrossRef]
- Chiesa, M.; Paganini, M.C.; Giamello, E.; Di Valentin, C.; Pacchioni, G. Electron Traps on Oxide Surfaces: (H+) (e−) Pairs Stabilized on the Surface of 17O Enriched CaO. ChemPhysChem 2006, 7, 728–734. [Google Scholar] [CrossRef]
- Machala, L.; Tuček, J.; Zbořil, R. Polymorphous Transformations of Nanometric Iron (III) Oxide: A Review. Chem. Mater. 2011, 23, 3255–3272. [Google Scholar] [CrossRef]
- Daou, T.J.; Greneche, J.-M.; Lee, S.-J.; Lee, S.; Lefevre, C.; Bégin-Colin, S.; Pourroy, G. Spin Canting of Maghemite Studied by NMR and In-Field Mössbauer Spectrometry. J. Phys. Chem. C 2010, 114, 8794–8799. [Google Scholar] [CrossRef]
- Rahman, M.M.; Jamal, A.; Khan, S.B.; Faisal, M. Fabrication of chloroform sensor based on hydrothermally prepared low-dimensional β-Fe2O3 nanoparticles. Superlattices Microstruct. 2011, 50, 369–376. [Google Scholar] [CrossRef]
- Carraro, G.; Barreca, D.; Yusta, M.C.; Gasparotto, A.; Maccato, C.; Morales, J.; Sada, C.; Sánchez, L. Vapor-Phase Fabrication of β-Iron Oxide Nanopyramids for Lithium-Ion Battery Anodes. ChemPhysChem 2012, 13, 3798–3801. [Google Scholar] [CrossRef]
- Rahman, M.M.; Asiri, A.M. Development of ionic-sensor based on sono-chemically prepared low-dimensional β-Fe2O3 nanoparticles onto flat-gold electrodes by an electrochemical approach. Sens. Bio-Sens. Res. 2015, 4, 109–117. [Google Scholar] [CrossRef]
- Rahman, M.M.; Jamal, A.; Khan, S.B.; Faisal, M. Characterization and applications of as-grown β-Fe2O3 nanoparticles prepared by hydrothermal method. J. Nanoparticle Res. 2011, 13, 3789–3799. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, S.B.; Faisal, M.; Rub, M.A.; Al-Youbi, A.O.; Asiri, A.M. Electrochemical determination of olmesartan medoxomil using hydrothermally prepared nanoparticles composed SnO2–Co3O4 nanocubes in tablet dosage forms. Talanta 2012, 99, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Tuček, J.; Zbořil, R.; Namai, A.; Ohkoshi, S.-I. ε-Fe2O3: An Advanced Nanomaterial Exhibiting Giant Coercive Field, Millimeter-Wave Ferromagnetic Resonance, and Magnetoelectric Coupling. Chem. Mater. 2010, 22, 6483–6505. [Google Scholar] [CrossRef]
- López-Sánchez, J.; Serrano, A.; Del Campo, A.; Abuín, M.; de la Fuente, O.R.; Carmona, N. Sol–Gel Synthesis and Micro-Raman Characterization of ε-Fe2O3 Micro- and Nanoparticles. Chem. Mater. 2016, 28, 511–518. [Google Scholar] [CrossRef]
- Schmeißer, D.; Haeberle, J.; Richter, M.; Brazda, P. Spin state and satellite structures of ε-Fe2O3 as determined by resonant photoelectron spectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. Beam Interact. Mater. Atoms 2015, 364, 127–131. [Google Scholar] [CrossRef]
- Yakushkin, S.; Balaev, D.; Dubrovskiy, A.; Semenov, S.; Knyazev, Y.; Bayukov, O.; Kirillov, V.; Ivantsov, R.; Edelman, I.; Martyanov, O. ε-Fe2O3 nanoparticles embedded in silica xerogel—Magnetic metamaterial. Ceram. Int. 2018, 44, 17852–17857. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Su, Z.; Li, G.; Jiang, T. Phase Transformation of MnO2 and Fe2O3 Briquettes Roasted Under CO–CO2 Atmospheres. In Characterization of Minerals, Metals, and Materials 2017; Springer: Berlin/Heidelberg, Germany, 2017; pp. 311–319. [Google Scholar] [CrossRef]
- Ohkoshi, S.-I.; Sakurai, S.; Jin, J.; Hashimoto, K. The addition effects of alkaline earth ions in the chemical synthesis of ε-Fe2O3 nanocrystals that exhibit a huge coercive field. J. Appl. Phys. 2005, 97, 10K312. [Google Scholar] [CrossRef]
- Nikolic, V.N.; Spasojevic, V.; Panjan, M.; Kopanja, L.; Mrakovic, A.; Tadic, M. Re-formation of metastable ε-Fe2O3 in post-annealing of Fe2O3/SiO2 nanostructure: Synthesis, computational particle shape analysis in micrographs and magnetic properties. Ceram. Int. 2017, 43, 7497–7507. [Google Scholar] [CrossRef]
- Nikolić, V.N.; Tadić, M.; Panjan, M.; Kopanja, L.; Cvjetićanin, N.; Spasojević, V. Influence of annealing treatment on magnetic properties of Fe2O3/SiO2 and formation of ε-Fe2O3 phase. Ceram. Int. 2017, 43, 3147–3155. [Google Scholar] [CrossRef]
- Benitez, M.J.; Mishra, D.; Szary, P.; Confalonieri, G.A.B.; Feyen, M.; Lu, A.H.; Agudo, L.; Eggeler, G.; Petracic, O.; Zabel, H. Structural and magnetic characterization of self-assembled iron oxide nanoparticle arrays. J. Phys. Condens. Matter 2011, 23, 126003. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kurmoo, M.; Rehspringer, J.-L.; Hutlova, A.; D’Orléans, C.; Vilminot, S.; Estournès, C.; Niznansky, D. Formation of Nanoparticles of ε-Fe2O3 from Yttrium Iron Garnet in a Silica Matrix: An Unusually Hard Magnet with a Morin-Like Transition below 150 K. Chem. Mater. 2005, 17, 1106–1114. [Google Scholar] [CrossRef]
- de Faria, D.L.A.; Venâncio Silva, S.; de Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Owens, F.J.; Orosz, J. Effect of nanosizing on lattice and magnon modes of hematite. Solid State Commun. 2006, 138, 95–98. [Google Scholar] [CrossRef]
- Martin, T.P.; Merlin, R.; Huffman, D.R.; Cardona, M. Resonant two magnon scaterring in a-Fe2O. Solid State Commun. 1977, 22, 565–567. [Google Scholar] [CrossRef]
- Dejoie, C.; Sciau, P.; Li, W.; Noé, L.; Mehta, A.; Chen, K.; Luo, H.; Kunz, M.; Tamura, N.; Liu, Z. Learning from the past: Rare ε-Fe2O3 in the ancient black-glazed Jian (Tenmoku) wares. Sci. Rep. 2014, 4, 4941. [Google Scholar] [CrossRef]
- Dubrovskiy, A.A.; Balaev, D.A.; Shaykhutdinov, K.A.; Bayukov, O.A.; Pletnev, O.N.; Yakushkin, S.S.; Bukhtiyarova, G.A.; Martyanov, O.N. Size effects in the magnetic properties of ε-Fe2O3 nanoparticles. J. Appl. Phys. 2015, 118, 213901. [Google Scholar] [CrossRef]
- Gich, M.; Frontera, C.; Roig, A.; Taboada, E.; Molins, E.; Rechenberg, H.R.; Ardisson, J.D.; Macedo, W.A.A.; Ritter, C.; Hardy, V.; et al. High- and Low-Temperature Crystal and Magnetic Structures of ε-Fe2O3 and Their Correlation to Its Magnetic Properties. Chem. Mater. 2006, 18, 3889–3897. [Google Scholar] [CrossRef]
- Kotani, A.; Shin, S. Resonant inelastic x-ray scattering spectra for electrons in solids. Rev. Mod. Phys. 2001, 73, 203–246. [Google Scholar] [CrossRef]
- Stemig, A.M.; Do, T.A.; Yuwono, V.M.; Arnold, W.A.; Penn, R.L. Goethite nanoparticle aggregation: Effects of buffers, metal ions, and 4-chloronitrobenzene reduction. Environ. Sci. Nano 2014, 1, 478–487. [Google Scholar] [CrossRef]
- Tiraferri, A.; Saldarriaga Hernandez, L.A.; Bianco, C.; Tosco, T.; Sethi, R. Colloidal behavior of goethite nanoparticles modified with humic acid and implications for aquifer reclamation. J. Nanoparticle Res. 2017, 19, 107. [Google Scholar] [CrossRef]
- Carta, D.; Casula, M.; Corrias, A.; Falqui, A.; Navarra, G.; Pinna, G. Structural and magnetic characterization of synthetic ferrihydrite nanoparticles. Mater. Chem. Phys. 2009, 113, 349–355. [Google Scholar] [CrossRef]
- Koralewski, M.; Pochylski, M.; Gierszewski, J. Magnetic properties of ferritin and akaganeite nanoparticles in aqueous suspension. J. Nanoparticle Res. 2013, 15, 1920. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.-Y.; Su, B.-L. Surfactant-assisted nanoparticle assembly of mesoporous β-FeOOH (akaganeite). Chem. Phys. Lett. 2003, 381, 710–714. [Google Scholar] [CrossRef]
- Agarwal, A.; Joshi, H.; Kumar, A. Synthesis, characterization and application of nano lepidocrocite and magnetite in the deg-radation of carbon tetrachloride. South Afr. J. Chem. 2011, 64, 218–224. [Google Scholar]
- Martinez, A.; Garcia-Lobato, M.A.; Perry, D.L. Study of the properties of iron oxide nanostructures. Res Nanotechnol Dev. 2009, 19, 184–193. [Google Scholar]
- He, Y.P.; Miao, Y.M.; Li, C.R.; Wang, S.Q.; Cao, L.; Xie, S.S.; Yang, G.Z.; Zou, B.S.; Burda, C. Size and structure effect on optical transitions of iron oxide nanocrystals. Phys. Rev. B Condens. Matter. Mater. Phys. 2005, 71, 125411. [Google Scholar] [CrossRef]
- Nair, S.S.; Mathews, M.; Anantharaman, M. Evidence for blueshift by weak exciton confinement and tuning of bandgap in superparamagnetic nanocomposites. Chem. Phys. Lett. 2005, 406, 398–403. [Google Scholar] [CrossRef]
- Kumar, P.; Malik, H.K.; Ghosh, A.; Thangavel, R.; Asokan, K. Bandgap tuning in highly c-axis oriented Zn1−xMgxO thin films. Appl. Phys. Lett. 2013, 102, 221903. [Google Scholar] [CrossRef]
- Deotale, A.J.; Nandedkar, R. Correlation between Particle Size, Strain and Band Gap of Iron Oxide Nanoparticles. Mater. Today Proc. 2016, 3, 2069–2076. [Google Scholar] [CrossRef]
- Pérez, N.; Ruiz-Rubio, L.; Vilas, J.L.; Rodríguez, M.; Martinez-Martinez, V.; León, L.M. Synthesis and characterization of near-infrared fluorescent and magnetic iron zero-valent nanoparticles. J. Photochem. Photobiol. A Chem. 2016, 315, 1–7. [Google Scholar] [CrossRef]
- Chen, C.-J.; Chiang, R.-K.; Lai, H.-Y.; Lin, C.-R. Characterization of Monodisperse Wüstite Nanoparticles following Partial Oxidation. J. Phys. Chem. C 2010, 114, 4258–4263. [Google Scholar] [CrossRef]
- Al-Kuhaili, M.; Saleem, M.; Durrani, S. Optical properties of iron oxide (α-Fe2O3) thin films deposited by the reactive evaporation of iron. J. Alloy. Compd. 2012, 521, 178–182. [Google Scholar] [CrossRef]
- Patsula, V.; Moskvin, M.; Dutz, S.; Horák, D. Size-dependent magnetic properties of iron oxide nanoparticles. J. Phys. Chem. Solids 2016, 88, 24–30. [Google Scholar] [CrossRef]
- Milivojević, D.; Babić-Stojić, B.; Jokanović, V.; Jagličić, Z.; Makovec, D.; Jović, N. Magnetic properties of ultrasmall iron-oxide nanoparticles. J. Alloy. Compd. 2014, 595, 153–157. [Google Scholar] [CrossRef]
- Rafi, M.M.; Ahmed, K.S.Z.; Nazeer, K.P.; Kumar, D.S.; Thamilselvan, M. Synthesis, characterization and magnetic properties of hematite (α-Fe2O3) nanoparticles on polysaccharide templates and their antibacterial activity. Appl. Nanosci. 2014, 5, 515–520. [Google Scholar] [CrossRef]
- Brok, E.; Frandsen, C.; Madsen, D.E.; Jacobsen, H.; Birk, J.O.; Lefmann, K.; Bendix, J.; Pedersen, K.S.; Boothroyd, C.B.; Berhe, A.A.; et al. Magnetic properties of ultra-small goethite nanoparticles. J. Phys. D Appl. Phys. 2014, 47, 365003. [Google Scholar] [CrossRef]
- Leong, S.A.N.; Samin, P.M.; Idris, A.; Mazlan, S.A.; Rahman, A.H.A. Synthesis, characterization and magnetorheological properties of carbonyl iron suspension with superparamagnetic nanoparticles as an additive. Smart Mater. Struct. 2016, 25, 025025. [Google Scholar] [CrossRef]
- Mohammed, A.S. Effect of temperature on the rheological properties with shear stress limit of iron oxide nanoparticle modified bentonite drilling muds. Egypt. J. Pet. 2017, 26, 791–802. [Google Scholar] [CrossRef]
- Vshivkov, S.A.; Soliman, T.S. Effect of a magnetic field on the rheological properties of the systems hydroxypropyl cellulose–ethanol and hydroxypropyl cellulose–dimethyl sulfoxide. Polym. Sci. Ser. A 2016, 58, 307–314. [Google Scholar] [CrossRef]
- Hernández, R.; Zamora-Mora, V.; Sibaja-Ballestero, M.; Vega-Baudrit, J.; López, D.; Mijangos, C. Influence of iron oxide nanoparticles on the rheological properties of hybrid chitosan ferrogels. J. Colloid Interface Sci. 2009, 339, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Felicia, L.J.; Vinod, S.; Philip, J. Recent Advances in Magnetorheology of Ferrofluids (Magnetic Nanofluids)—A Critical Review. J. Nanofluids 2016, 5, 1–22. [Google Scholar] [CrossRef]
- Devi, M.; Mohanta, D. Rheological Properties of Iron Oxide Based Ferrofluids. AIP Conf. Proc. 2009, 1147, 495–501. [Google Scholar] [CrossRef]
- Carvalho, M.; Henriques, F.; Ferreira, L.; Godinho, M.; Cruz, M. Iron oxide nanoparticles: The Influence of synthesis method and size on composition and magnetic properties. J. Solid State Chem. 2013, 201, 144–152. [Google Scholar] [CrossRef]
- Sakurai, S.; Namai, A.; Hashimoto, K.; Ohkoshi, S.-I. First Observation of Phase Transformation of All Four Fe2O3 Phases (γ → ε → β → α-Phase). J. Am. Chem. Soc. 2009, 131, 18299–18303. [Google Scholar] [CrossRef]
- Deka, S.; Joy, P.A. Enhancement of the phase transformation temperature of γ-Fe2O3by Zn2+doping. J. Mater. Chem. 2006, 17, 453–456. [Google Scholar] [CrossRef]
- Tianshu, Z.; Hongmei, L.; Huanxing, Z.; Ruifang, Z.; Yusheng, S. Synthesis and gas-sensing characteristics of high thermostability γ-Fe2O3 powder. Sens. Actuators B Chem. 1996, 32, 181–184. [Google Scholar] [CrossRef]
- Ennas, G.; Musinu, A.; Piccaluga, A.G.; Zedda, D.; Gatteschi, D.; Sangregorio, A.C.; Stanger, J.L.; Concas, G.; Spano, G. Characterization of Iron Oxide Nanoparticles in an Fe2O3−SiO2 Composite Prepared by a Sol−Gel Method. Chem. Mater. 1998, 10, 495–502. [Google Scholar] [CrossRef]
- Jiang, F.; Li, X.; Zhu, Y.; Tang, Z. Synthesis and magnetic characterizations of uniform iron oxide nanoparticles. Phys. B Condens. Matter 2014, 443, 1–5. [Google Scholar] [CrossRef]
- Justus, J.S.; Roy, S.D.D.; Raj, M.E. Synthesis and characterization of hematite nanopowders. Mater. Res. Express 2016, 3, 105037. [Google Scholar] [CrossRef]
- Tanimoto, A.; Kuribayashi, S. Application of superparamagnetic iron oxide to imaging of hepatocellular carcinoma. Eur. J. Radiol. 2006, 58, 200–216. [Google Scholar] [CrossRef]
- Reimer, P.; Jahnke, N.; Fiebich, M.; Schima, W.; Deckers, F.; Marx, C.; Holzknecht, N.; Saini, S. Hepatic Lesion Detection and Characterization: Value of Nonenhanced MR Imaging, Superparamagnetic Iron Oxide-enhanced MR Imaging, and Spiral CT—ROC Analysis. Radiology 2000, 217, 152–158. [Google Scholar] [CrossRef]
- Naganawa, S.; Sato, C.; Nakamura, T.; Kumada, H.; Ishigaki, T.; Miura, S.; Maruyama, K.; Takizawa, O. Diffusion-weighted images of the liver: Comparison of tumor detection before and after contrast enhancement with superparamagnetic iron oxide. J. Magn. Reson. Imaging 2005, 21, 836–840. [Google Scholar] [CrossRef]
- Pauleit, D.; Textor, J.; Bachmann, R.; Conrad, R.; Flacke, S.; Layer, G.; Kreft, B.; Schild, H. Hepatocellular Carcinoma: Detection with Gadolinium- and Ferumoxides-enhanced MR Imaging of the Liver. Radiology 2002, 222, 73–80. [Google Scholar] [CrossRef]
- Maurea, S.; Mainenti, P.P.; Tambasco, A.; Imbriaco, M.; Mollica, C.; Laccetti, E.; Camera, L.; Liuzzi, R.; Salvatore, M. Diagnostic accuracy of MR imaging to identify and characterize focal liver lesions: Comparison between gadolinium and superparamagnetic iron oxide contrast media. Quant. Imaging Med. Surg. 2014, 4, 181–189. [Google Scholar] [CrossRef]
- Ward, J.; Guthrie, J.A.; Scott, D.J.; Atchley, J.; Wilson, D.; Davies, M.H.; Wyatt, J.I.; Robinson, P.J. Hepatocellular Carcinoma in the Cirrhotic Liver: Double-Contrast MR Imaging for Diagnosis. Radiology 2000, 216, 154–162. [Google Scholar] [CrossRef]
- Yilmaz, A.; Dengler, M.A.; Van Der Kuip, H.; Yildiz, H.; Rösch, S.; Klumpp, S.; Klingel, K.; Kandolf, R.; Helluy, X.; Hiller, K.-H.; et al. Imaging of myocardial infarction using ultrasmall superparamagnetic iron oxide nanoparticles: A human study using a multi-parametric cardiovascular magnetic resonance imaging approach. Eur. Heart J. 2012, 34, 462–475. [Google Scholar] [CrossRef]
- Kim, S.J.; Lewis, B.; Steiner, M.S.; Bissa, U.V.; Dose, C.; Frank, J.A. Superparamagnetic iron oxide nanoparticles for direct labeling of stem cells and in vivo MRI tracking. Contrast Media Mol. Imaging 2015, 11, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.J.; De Cuyper, M. Assessing iron oxide nanoparticle toxicity in vitro: Current status and future prospects. Nanomedicine 2010, 5, 1261–1275. [Google Scholar] [CrossRef]
- Zhou, J.; Qiao, X.; Binks, B.P.; Sun, K.; Bai, M.; Li, Y.; Liu, Y. Magnetic Pickering Emulsions Stabilized by Fe3O4 Nanoparticles. Langmuir 2011, 27, 3308–3316. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.E.; Lee, J.; Seo, Y.; Clay, N.; Park, J.; Shkumatov, A.; Ernenwein, D.; Lai, M.-H.; Misra, S.; Sing, C.E.; et al. Worm-Like Superparamagnetic Nanoparticle Clusters for Enhanced Adhesion and Magnetic Resonance Relaxivity. ACS Appl. Mater. Interfaces 2017, 9, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Peng, E.; Wang, F.; Xue, J.M. Nanostructured magnetic nanocomposites as MRI contrast agents. J. Mater. Chem. B 2015, 3, 2241–2276. [Google Scholar] [CrossRef]
- Zanganeh, S.Q.J.; Jafari, T.; Khakpash, N.; Erfanzadeh, M.; Spitler, R. The Evolution of Iron Oxide Nanoparticles for use in Bio-medical MRI Applications. SM J. Clin. Med. Imaging 2016, 2, 1–11. [Google Scholar]
- Lee, M.K.; Clay, N.E.; Ko, E.; Smith, C.E.; Chen, L.; Cho, N.; Sung, H.-J.; DiPietro, L.; Lee, J.; Kong, H. Spatial Organization of Superparamagnetic Iron Oxide Nanoparticles in/on Nano/Microsized Carriers Modulates the Magnetic Resonance Signal. Langmuir 2018, 34, 15276–15282. [Google Scholar] [CrossRef]
- Yu, J.; Chu, X.; Hou, Y. Stimuli-responsive cancer therapy based on nanoparticles. Chem. Commun. 2014, 50, 11614–11630. [Google Scholar] [CrossRef]
- Jie, L.; Lang, D.; Kang, X.; Yang, Z.; Du, Y.; Ying, X. Superparamagnetic Iron Oxide Nanoparticles/Doxorubicin-Loaded Starch-Octanoic Micelles for Targeted Tumor Therapy. J. Nanosci. Nanotechnol. 2019, 19, 5456–5462. [Google Scholar] [CrossRef]
- Bai, F.; Wang, D.; Huo, Z.; Chen, W.; Liu, L.; Liang, X.; Chen, C.; Wang, X.; Peng, Q.; Li, Y. A Versatile Bottom-up Assembly Approach to Colloidal Spheres from Nanocrystals. Angew. Chem. Int. Ed. 2007, 46, 6650–6653. [Google Scholar] [CrossRef]
- Zhou, Z.; Tian, R.; Wang, Z.; Yang, Z.; Liu, Y.; Liu, G.; Wang, R.; Gao, J.; Song, J.; Nie, L.; et al. Artificial local magnetic field inhomogeneity enhances T2 relaxivity. Nat. Commun. 2017, 8, 15468. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Bae, K.H.; Kim, C.; Park, T.G. Clustered Magnetite Nanocrystals Cross-Linked with PEI for Efficient siRNA Delivery. Biomacromolecules 2010, 12, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, X.; Wang, X. Fabrication of Hybrid Nanostructures Based on Fe3O4 Nanoclusters as Theranostic Agents for Magnetic Resonance Imaging and Drug Delivery. Nanoscale Res. Lett. 2019, 14, 200. [Google Scholar] [CrossRef]
- Campbell, E.; Hasan, M.T.; Pho, C.; Callaghan, K.; Akkaraju, G.R.; Naumov, A.V. Graphene Oxide as a Multifunctional Platform for Intracellular Delivery, Imaging, and Cancer Sensing. Sci. Rep. 2019, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rodriguez, R.; Campbell, E.; Naumov, A. Multifunctional graphene oxide/iron oxide nanoparticles for magnetic targeted drug delivery dual magnetic resonance/fluorescence imaging and cancer sensing. PLoS ONE 2019, 14, e0217072. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhang, A.; Kong, Y.; Yu, N.; Xie, T.; Dou, B.; Li, K.; Wang, Y.; Li, J.; Xu, K. Multifunctional iron oxide-carbon hybrid nanoparticles for targeted fluorescent/MR dual-modal imaging and detection of breast cancer cells. Anal. Chim. Acta 2019, 1067, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, L.; Napolitano, A.; Visconti, E.; Longo, D.; Romano, A.; Tomà, P.; Espagnet, M.C.R. Gadolinium-Based Contrast Agent-Related Toxicities. CNS Drugs 2018, 32, 229–240. [Google Scholar] [CrossRef]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals 2016, 29, 365–376. [Google Scholar] [CrossRef]
- Kucheryavy, P.; He, J.; John, V.T.; Maharjan, P.; Spinu, L.; Goloverda, G.Z.; Kolesnichenko, V.L. Superparamagnetic Iron Oxide Nanoparticles with Variable Size and an Iron Oxidation State as Prospective Imaging Agents. Langmuir 2012, 29, 710–716. [Google Scholar] [CrossRef]
- Rodriguez-Enriquez, S.; He, L.; Lemasters, J.J. Role of mitochondrial permeability transition pores in mitochondrial autophagy. Int. J. Biochem. Cell Biol. 2004, 36, 2463–2472. [Google Scholar] [CrossRef]
- Wu, Y.-N.; Yang, L.-X.; Shi, X.-Y.; Li, I.-C.; Biazik, J.M.; Ratinac, K.R.; Chen, D.-H.; Thordarson, P.; Shieh, D.-B.; Braet, F. The selective growth inhibition of oral cancer by iron core-gold shell nanoparticles through mitochondria-mediated autophagy. Biomaterials 2011, 32, 4565–4573. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Oh, S.Y.; Kim, Y.; Yoon, C.; Lee, B.-S.; Kim, S.D.; Kim, J.S. Distribution and immunotoxicity by intravenous injection of iron nanoparticles in a murine model. J. Appl. Toxicol. 2015, 36, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]
- Thorat, N.D.; Otari, S.V.; Patil, R.M.; Bohara, R.A.; Yadav, H.M.; Koli, V.B.; Chaurasia, A.K.; Ningthoujam, R.S. Synthesis, characterization and biocompatibility of chitosan functionalized superparamagnetic nanoparticles for heat activated curing of cancer cells. Dalton Trans. 2014, 43, 17343–17351. [Google Scholar] [CrossRef]
- Decker, T.; Lohmann-Matthes, M.-L. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J. Immunol. Methods 1988, 115, 61–69. [Google Scholar] [CrossRef]
- Thorat, N.; Khot, V.; Salunkhe, A.; Ningthoujam, R.; Pawar, S. Functionalization of La0.7Sr0.3MnO3 nanoparticles with polymer: Studies on enhanced hyperthermia and biocompatibility properties for biomedical applications. Colloids Surf. B Biointerfaces 2012, 104, 40–47. [Google Scholar] [CrossRef]
- Końca, K.; Lankoff, A.; Banasik, A.; Lisowska, H.; Kuszewski, T.; Góźdź, S.; Koza, Z.; Wojcik, A. A cross-platform public domain PC image-analysis program for the comet assay. Mutat. Res. Toxicol. Environ. Mutagen. 2002, 534, 15–20. [Google Scholar] [CrossRef]
- Amara, N.; Bachoual, R.; Desmard, M.; Golda, S.; Guichard, C.; Lanone, S.; Aubier, M.; Ogier-Denis, E.; Boczkowski, J. Diesel exhaust particles induce matrix metalloprotease-1 in human lung epithelial cells via a NADP(H) oxidase/NOX4 redox-dependent mechanism. Am. J. Physiol. Cell Mol. Physiol. 2007, 293, L170–L181. [Google Scholar] [CrossRef]
- Patil, R.M.; Thorat, N.D.; Shete, P.B.; Bedge, P.A.; Gavde, S.; Joshi, M.G.; Tofail, S.A.; Bohara, R.A. Comprehensive cytotoxicity studies of superparamagnetic iron oxide nanoparticles. Biochem. Biophys. Rep. 2018, 13, 63–72. [Google Scholar] [CrossRef]
- Hilger, I.; Frühauf, S.; Linß, W.; Hiergeist, R.; Andrä, W.; Hergt, R.; Kaiser, W.A. Cytotoxicity of selected magnetic fluids on human adenocarcinoma cells. J. Magn. Magn. Mater. 2003, 261, 7–12. [Google Scholar] [CrossRef]
- Baccile, N.; Noiville, R.; Stievano, L.; Van Bogaert, I. Sophorolipids-functionalized iron oxide nanoparticles. Phys. Chem. Chem. Phys. 2012, 15, 1606–1620. [Google Scholar] [CrossRef] [PubMed]
- Caizer, C. Computational study on superparamagnetic hyperthermia with biocompatible SPIONs to destroy the cancer cells. J. Phys. Conf. Ser. 2014, 521, 012015. [Google Scholar] [CrossRef]
- Kalber, T.L.; Ordidge, K.L.; Southern, P.; Loebinger, M.R.; Kyrtatos, P.G.; Pankhurst, Q.A.; Lythgoe, M.F.; Janes, S. Hyperthermia treatment of tumors by mesenchymal stem cell-delivered superparamagnetic iron oxide nanoparticles. Int. J. Nanomed. 2016, 11, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Hervault, A.; Thanh, N.T.K. Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale 2014, 6, 11553–11573. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Rahman, A.U.; Husen, A. Tajuddin Biogenic Fabrication of Iron/Iron Oxide Nanoparticles and Their Application. Nanoscale Res. Lett. 2016, 11, 498. [Google Scholar] [CrossRef] [PubMed]
- Easo, S.L.; Mohanan, P. In vitro hematological and in vivo immunotoxicity assessment of dextran stabilized iron oxide nanoparticles. Colloids Surf. B Biointerfaces 2015, 134, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, S.M.; Singh, N.; Jenkins, G.J.S.; Williams, P.M.; Orbaek, A.W.; Barron, A.R.; Wright, C.J.; Doak, S.H. Dextran Coated Ultrafine Superparamagnetic Iron Oxide Nanoparticles: Compatibility with Common Fluorometric and Colorimetric Dyes. Anal. Chem. 2011, 83, 3778–3785. [Google Scholar] [CrossRef]
- Siffert, R.S. The Role of Alkaline Phosphatase In Osteogenesis. J. Exp. Med. 1951, 93, 415–426. [Google Scholar] [CrossRef]
- Kulterer, B.; Friedl, G.; Jandrositz, A.; Sanchez-Cabo, F.; Prokesch, A.; Paar, C.; Scheideler, M.; Windhager, R.; Preisegger, K.-H.; Trajanoski, Z. Gene expression profiling of human mesenchymal stem cells derived from bone marrow during expansion and osteoblast differentiation. BMC Genom. 2007, 8, 70. [Google Scholar] [CrossRef]
- Birmingham, E.; Niebur, G.; McHugh, P.; Shaw, G.; Barry, F.; McNamara, L. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur. Cells Mater. 2012, 23, 13–27. [Google Scholar] [CrossRef] [PubMed]
- 222. Rached, M.; Kode, A.; Lili, X.; Yoshikawa, Y.; Paik, J.; DePinho, R.A.; Kousteni, S. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metabolism. 2010, 11, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, J.; Li, C.; Yu, Y. Role of bone morphogenetic protein-2 in osteogenic differentiation of mesenchymal stem cells. Mol. Med. Rep. 2015, 12, 4230–4237. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cao, L.; Liu, Y.; Zheng, A.; Wu, J.; Jiang, X.; Ji, P. Evaluation of synergistic osteogenesis between icariin and BMP2 through a micro/meso hierarchical porous delivery system. Int. J. Nanomed 2017, 12, 7721–7735. [Google Scholar] [CrossRef] [PubMed]
- Um, S.; Kim, H.Y.; Seo, B.-M. Effects of BMP-2 on the osteogenic differentiation of bone marrow stem cells in fibrous dysplasia. Oral Dis. 2018, 24, 1057–1067. [Google Scholar] [CrossRef]
- Jang, W.-G.; Kim, E.-J.; Kim, D.-K.; Ryoo, H.-M.; Lee, K.-B.; Kim, S.-H.; Choi, H.-S.; Koh, J.-T. BMP2 Protein Regulates Osteocalcin Expression via Runx2-mediated Atf6 Gene Transcription. J. Biol. Chem. 2012, 287, 905–915. [Google Scholar] [CrossRef]
- Xiao, G.; Jiang, D.; Gopalakrishnan, R.; Franceschi, R.T. Fibroblast Growth Factor 2 Induction of the Osteocalcin Gene Requires MAPK Activity and Phosphorylation of the Osteoblast Transcription Factor, Cbfa1/Runx2. J. Biol. Chem. 2002, 277, 36181–36187. [Google Scholar] [CrossRef]
- Gersbach, C.A.; Le Doux, J.M.; Guldberg, R.E.; García, A.J. Inducible regulation of Runx2-stimulated osteogenesis. Gene Ther. 2006, 13, 873–882. [Google Scholar] [CrossRef]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cell Mater. 2014, 28, 269–286. [Google Scholar] [CrossRef]
- Zhao, H.; Ito, Y.; Chappel, J.; Andrews, N.; Ross, F.P.; Teitelbaum, S.L. How Do Bone Cells Secrete Proteins? Osteoimmunology 2009, 658, 105–109. [Google Scholar] [CrossRef]
- Yi, C.; Liu, D.; Fong, C.-C.; Zhang, J.; Yang, M. Gold Nanoparticles Promote Osteogenic. ACS Nano 2010, 4, 6439–6448. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, B.; Cao, M.; Sun, J.; Wu, H.; Zhao, P.; Xing, J.; Yang, Y.; Zhang, X.; Ji, M.; et al. Response of MAPK pathway to iron oxide nanoparticles in vitro treatment promotes osteogenic differentiation of hBMSCs. Biomaterials 2016, 86, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Alcantar, N.A.; Aydil, E.S.; Israelachvili, J.N. Polyethylene glycol-coated biocompatible surfaces. J. Biomed. Mater. Res. 2000, 51, 343–351. [Google Scholar] [CrossRef]
- Prodan, E.; Radloff, C.; Halas, N.J.; Nordlander, P. A Hybridization Model for the Plasmon Response of Complex Nanostructures. Science 2003, 302, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, C.; Min, Y.; Gueorguieva, M.; McDougall, C.; Volovick, A.; Prentice, P.; Wang, Z.; Melzer, A.; Cuschieri, A.; Wang, L. Hybrid gold-iron oxide nanoparticles as a multifunctional platform for biomedical application. J. Nanobiotechnol. 2012, 10, 27. [Google Scholar] [CrossRef]
- Shah, R.R.; Dombrowsky, A.R.; Paulson, A.L.; Johnson, M.P.; Nikles, D.E.; Brazel, C.S. Determining iron oxide nanoparticle heating efficiency and elucidating local nanoparticle temperature for application in agarose gel-based tumor model. Mater. Sci. Eng. C 2016, 68, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Khan, R.; Solanki, P.R.; Pandey, P.; Alam, J.; Ahmad, S.; Malhotra, B. Iron oxide nanoparticles–chitosan composite based glucose biosensor. Biosens. Bioelectron. 2008, 24, 676–683. [Google Scholar] [CrossRef]
- Singh, A.K. Synthesis and characterization of bulk and nanomaterials using different techniques. Ph.D. Thesis, Department of Physics, University of Allahabad, Allahabad, India, 2010. [Google Scholar]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin Receptor Signaling in Normal. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef]
- Samovski, D.; Dhule, P.; Pietka, T.; Jacome-Sosa, M.; Penrose, E.; Son, N.-H.; Flynn, C.R.; Shoghi, K.I.; Hyrc, K.L.; Goldberg, I.J.; et al. Regulation of Insulin Receptor Pathway and Glucose Metabolism by CD36 Signaling. Diabetes 2018, 67, 1272–1284. [Google Scholar] [CrossRef]
- Joshi, H.; Shirude, P.S.; Bansal, V.; Ganesh, K.N.; Sastry, M. Isothermal Titration Calorimetry Studies on the Binding of Amino Acids to Gold Nanoparticles. J. Phys. Chem. B 2004, 108, 11535–11540. [Google Scholar] [CrossRef]
- Kebede, A.; Singh, A.K.; Rai, P.K.; Giri, N.K.; Rai, A.K.; Watal, G.; Gholap, A.V. Controlled synthesis, characterization, and application of iron oxide nanoparticles for oral delivery of insulin. Lasers Med. Sci. 2012, 28, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Augustin, E.; Czubek, B.; Nowicka, A.M.; Kowalczyk, A.; Stojek, Z.; Mazerska, Z. Improved cytotoxicity and preserved level of cell death induced in colon cancer cells by doxorubicin after its conjugation with iron-oxide magnetic nanoparticles. Toxicol. Vitr. 2016, 33, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xu, Y.; Chen, L.; Yin, X.; Lin, F.; Weng, S.; Lin, X. Dual-Modal Biosensor for the Determination of Femtomolar miRNA-126 Based on Electrochemical Impedance Spectroscopy and Electrochemiluminescence with Hybridization Chain Reaction Amplification. J. Electrochem. Soc. 2020, 167, 167502. [Google Scholar] [CrossRef]
- Kumar, S.; Umar, M.; Saifi, A.; Kumar, S.; Augustine, S.; Srivastava, S.; Malhotra, B.D. Electrochemical paper based cancer biosensor using iron oxide nanoparticles decorated PEDOT:PSS. Anal. Chim. Acta 2019, 1056, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Lin, Y.; Liu, X.; Xin, Y.; Tian, Q.; Zhang, J. Ultrasensitive and preprocessing-free electrochemical biosensing platform for the detection of cancer-derived exosomes based on spiky-shaped aptamer-magnetic beads. Biosens. Bioelectron. 2022, 217, 114705. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kou, B.; Yuan, Y.; Chai, Y.; Yuan, R. Porous Fe3O4@COF-Immobilized gold nanoparticles with excellent catalytic performance for sensitive electrochemical detection of ATP. Biosens. Bioelectron. 2021, 197, 113758. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Ganesh, V. Bio-assisted preparation of efficiently architectured nanostructures of γ-Fe2O3 as a molecular recognition platform for simultaneous detection of biomarkers. Sci. Rep. 2020, 10, 15071. [Google Scholar]
- Fang, B.; Wang, G.; Zhang, W.; Li, M.; Kan, X. Fabrication of Fe3O4 Nanoparticles Modified Electrode and Its Application for Voltammetric Sensing of Dopamine. Electroanalysis 2005, 17, 744–748. [Google Scholar] [CrossRef]
- Tomé, L.I.N.; Brett, C.M.A. Polymer/Iron Oxide Nanoparticle Modified Glassy Carbon Electrodes for the Enhanced Detection of Epinephrine. Electroanalysis 2019, 31, 704–710. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, H.; Wang, X.; Yang, X. Sensitive electrochemical sensor for hydrogen peroxide using Fe3O4 magnetic nanoparticles as a mimic for peroxidase. Mikrochim. Acta 2011, 174, 183–189. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Shadjou, N.; de la Guardia, M. Iron and iron-oxide magnetic nanoparticles as signal-amplification elements in electrochemical biosensing. TrAC Trends Anal. Chem. 2015, 72, 1–9. [Google Scholar] [CrossRef]
- Wang, D.; Gan, N.; Zhang, H.; Li, T.; Qiao, L.; Cao, Y.; Su, X.; Jiang, S. Simultaneous electrochemical immunoassay using graphene–Au grafted recombinant apoferritin-encoded metallic labels as signal tags and dual-template magnetic molecular imprinted polymer as capture probes. Biosens. Bioelectron. 2015, 65, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Gupta, P.K.; Solanki, P.R. Electrochemical immunosensor based on magnetite nanoparticles incorporated electrospun polyacrylonitrile nanofibers for Vitamin-D3 detection. Mater. Sci. Eng. C 2018, 93, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Agrawal, V.V.; Srivastava, A.K.; Govind, G.; Nain, L.; Imran, M.; Kabi, S.R.; Sinha, R.K.; Malhotra, B.D. Phase control of nanostructured iron oxide for application to biosensor. J. Mater. Chem. B 2012, 1, 464–474. [Google Scholar] [CrossRef]
- Nor, N.M.; Lockman, Z.; Razak, K.A. Study of ITO Glass Electrode Modified with Iron Oxide Nanoparticles and Nafion for Glucose Biosensor Application. Procedia Chem. 2016, 19, 50–56. [Google Scholar] [CrossRef][Green Version]
- Thandavan, K.; Gandhi, S.; Nesakumar, N.; Sethuraman, S.; Rayappan, J.B.B.; Krishnan, U.M. Hydrogen peroxide biosensor utilizing a hybrid nano-interface of iron oxide nanoparticles and carbon nanotubes to assess the quality of milk. Sens. Actuators B Chem. 2015, 215, 166–173. [Google Scholar] [CrossRef]
- Baião, V.; Tomé, L.I.; Brett, C.M. Iron Oxide Nanoparticle and Multiwalled Carbon Nanotube Modified Glassy Carbon Electrodes. Application to Levodopa Detection. Electroanalysis 2018, 30, 1342–1348. [Google Scholar] [CrossRef]
- Islam, N.; Gorgannezhad, L.; Masud, M.K.; Tanaka, S.; Hossain, S.A.; Yamauchi, Y.; Nguyen, N.-T.; Shiddiky, M.J.A. Graphene-Oxide-Loaded Superparamagnetic Iron Oxide Nanoparticles for Ultrasensitive Electrocatalytic Detection of MicroRNA. Chemelectrochem 2018, 5, 2488–2495. [Google Scholar] [CrossRef]
- Pardini, B.; De Maria, D.; Francavilla, A.; Di Gaetano, C.; Ronco, G.; Naccarati, A. MicroRNAs as markers of progression in cervical cancer: A systematic review. BMC Cancer 2018, 18, 696. [Google Scholar] [CrossRef]
- Baabu, P.R.S.; Srinivasan, S.; Nagarajan, S.; Muthamilselvan, S.; Selvi, T.; Suresh, R.R.; Palaniappan, A. End-to-end computational approach to the design of RNA biosensors for detecting miRNA biomarkers of cervical cancer. Synth. Syst. Biotechnol. 2022, 7, 802–814. [Google Scholar] [CrossRef]
- Kim, H.Y.; Song, J.; Park, H.G.; Kang, T. Electrochemical detection of zeptomolar miRNA using an RNA-triggered Cu2+ reduction method. Sens. Actuators B Chem. 2022, 360, 131666. [Google Scholar] [CrossRef]
- Xue, T.; Liang, W.; Li, Y.; Sun, Y.; Xiang, Y.; Zhang, Y.; Dai, Z.; Duo, Y.; Wu, L.; Qi, K.; et al. Ultrasensitive detection of miRNA with an antimonene-based surface plasmon resonance sensor. Nat. Commun. 2019, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, K.M.; Kanak, M.A.; Harrell, J.C.; Dhakal, S. Single-Molecule Sensor for High-Confidence Detection of miRNA. ACS Sens. 2022, 7, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Mir, A.; Mallik, D.; Sinha, A.; Nayar, S.; Webster, T.J. Bactericidal effect of iron oxide nanoparticles on Staphylococcus aureus. Int. J. Nanomed. 2010, 5, 277–283. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Saravanan, M.; Prakash, N.U.; Arasu, M.V.; Vijayakumar, B.; Vincent, S. Enhanced antibacterial activity of iron oxide magnetic nanoparticles treated with Argemone mexicana L. leaf extract: An in vitro study. Mater. Res. Bull. 2013, 48, 3323–3327. [Google Scholar] [CrossRef]
- Thukkaram, M.; Sitaram, S.; Kannaiyan, S.K.; Subbiahdoss, G. Antibacterial Efficacy of Iron-Oxide Nanoparticles against Biofilms on Different Biomaterial Surfaces. Int. J. Biomater. 2014, 2014, 716080. [Google Scholar] [CrossRef]














| Iron Oxide Phases | Chemical Formula | Oxidation State | Crystal Structure | Magnetic Properties | Influence on Oxidation States and Phase Transformations | References |
|---|---|---|---|---|---|---|
| Magnetite | Fe3O4 | +2 and +3 | Face centered Cubic (a = b = c = 0.8396 nm) | Ferrimagnetic | Higher oxidation rate to maghemite when left at room temperature as size of nanoparticles decrease | [6,70,101] |
| Hematite | α-Fe2O3 | +3 | Rhombohedral with hexagonal closed packing (a = 0.5035 nm, c = 1.3752 nm) | Antiferromagnetic below 955 K and paramagnetic above 955 K | Transformed to have increased oxygen vacancies to have enhanced electrochemical performance | [6,70,120,125] |
| Maghemite | γ-Fe2O3 | +3 | Allotropic form of magnetite Cubic (a = 0.8346 nm) | Ultra-small nanoparticles exhibiting super-paramagnetism | Transformed into hematite directly or indirectly with ϵ-Fe2O3 as intermediate | [6,70,71,120] |
| Iron Oxide beta phase | β-Fe2O3 | +3 | Body centered Cubic (a = 0.9393 nm) | Paramagnetic between 100–119 K and antiferromagnetic below 100 K | Directly transforms to hematite/magnetite or even ζ-Fe2O3 under high-pressure transformation but hollow nanoparticles transform into maghemite | [6,70,120] |
| Iron Oxide eta phase | ϵ-Fe2O3 | +3 | Orthorhombic (a = 0.5072 nm, b = 0.8736 nm, c = 0.9418 nm) | Two magnetic transitions
| Directly transforms to hematite | [70,78] |
| Iron Oxide zeta phase | ζ-Fe2O3 | +3 | Monoclinic (a = 0.9683 nm, b = 1.0000 nm, c = 0.8949 nm) | Transition from paramagnetic to antiferromagnetic state at 69 K | N.A. | [5] |
| Goethite | α-FeO(OH) | - | Orthorhombic (a = 0.995 nm, b = 0.301 nm, c = 0.462 nm) | Superparamagnetic | N.A. | [6,112] |
| Ferrihydrite | Fe5HO8.4H2O | - | Hexagonal (a = 0.5958 nm, c = 0.8965 nm) | Ferromagnetic—increase in property with increase in surface to volume ratio | N.A. | [6,97] |
| Wüstite | FeO | +2 | Cubic (a = 0.4239 nm) | Antiferromagnetic | N.A. | [6,107] |
| Akaganeite | β-FeO(OH) | - | Monoclinic (a = 1.0561 nm, b = 3.031 nm, c = 1.0483 nm) | Exhibits magnetic birefringence when coupled with polysaccharide solution Particle size brings anomalies | N.A. | [6,98,99] |
| Lepidocrocite | γ-FeO(OH) | - | Orthorhombic (a = 0.388 nm, b = 1.254 nm, c = 0.307 nm) | Antiferromagnetic | N.A. | [6,100] |
| Biosensor Components | Detection Method | Electrochemical Method Employed | Biomarker Detected | Linear Range | Detection Limit (LOD) | References |
|---|---|---|---|---|---|---|
| Recombinant apoferritin-encoded Fe3O4 nanoparticles + dual-template magnetic molecularly imprinted polymers | Immuno-sensing | Square wave voltammetry | Simultaneous detection of AFP and CEA | 0.001–5 ng/mL | AFP—0.3 pg/mL CEA—0.35 pg/mL | [205] |
| Magnetite nanoparticles + polyacrylonitrile nanofibers | Immuno-sensing | Differential Pulse Voltammetry | Vitamin-D3 | 10–100 ng/mL | 0.12 ng/mL | [206] |
| Magnetite Nanoparticles passivated with carbon shell | Enzymatic | Cyclic Voltammetry and Impedance Spectroscopy | Cholesterol | 25–400 mg/dL | Not reported | [207] |
| Citric acid capped maghemite nanoparticles + Nafion | Enzymatic | Cyclic Voltammetry | Glucose | 1–8 mM | Not reported | [208] |
| Nafion-Magnetite-CNT hybrid nanocomposite | Enzymatic | Amperometry | Hydrogen Peroxide | 1.2–21.6 μM | 3.7 nM | [209] |
| Fe2O3 nanoparticles + MWCNTs + Chitosan | Non-enzymatic | Differential Pulse Voltammetry | Levodopa | 0.3–8 μM | 0.24 μM | [210] |
| Graphene oxide loaded maghemite superparamagnetic nanoparticles | Non-enzymatic | Cyclic Voltammetry | miRNA-21 | Not reported | 1 fM | [211] |
| Magnetite@SiO2@Au core-shell nanoparticles coated with cDNA | Non-enzymatic (HCR) | Impedance Spectroscopy | miRNA-126 | 5–5000 fM | 2 fM | [196] |
| Nanostructured Fe2O3 + PEDOT: PSS | Immuno-sensing | Amperometry | CEA | 4–25 ng/mL | Not reported | [197] |
| Prussian blue/Graphene oxide + spiky Au@magnetite nanoparticles | Non-enzymatic | Cyclic Voltammetry | MCF-7 exosomes | 200—5 × 105 particles/μL | 80 particles/μL | [198] |
| Magnetite + covalent organic framework + Au nanoparticles | Aptameric | Cyclic Voltammetry and Impedance Spectroscopy | ATP | 5 pM–50 μM | 1.6 pM | [199] |
| Maghemite nanoparticles (different morphologies) | Non-enzymatic | Amperometry | Dopamine Uric Acid | Dopamine: 0.15–75 μM Uric Acid: 5 μM–0.15 mM | Dopamine: 0.15 μM Uric Acid: 5 μM | [200] |
| Fe2O3 nanoparticles | Non-enzymatic | Differential Pulse Voltammetry | Epinephrine | 0.05–15 μM | 1.6 μM | [202] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baabu, P.R.S.; Kumar, H.K.; Gumpu, M.B.; Babu K, J.; Kulandaisamy, A.J.; Rayappan, J.B.B. Iron Oxide Nanoparticles: A Review on the Province of Its Compounds, Properties and Biological Applications. Materials 2023, 16, 59. https://doi.org/10.3390/ma16010059
Baabu PRS, Kumar HK, Gumpu MB, Babu K J, Kulandaisamy AJ, Rayappan JBB. Iron Oxide Nanoparticles: A Review on the Province of Its Compounds, Properties and Biological Applications. Materials. 2023; 16(1):59. https://doi.org/10.3390/ma16010059
Chicago/Turabian StyleBaabu, Priyannth Ramasami Sundhar, Hariprasad Krishna Kumar, Manju Bhargavi Gumpu, Jayanth Babu K, Arockia Jayalatha Kulandaisamy, and John Bosco Balaguru Rayappan. 2023. "Iron Oxide Nanoparticles: A Review on the Province of Its Compounds, Properties and Biological Applications" Materials 16, no. 1: 59. https://doi.org/10.3390/ma16010059
APA StyleBaabu, P. R. S., Kumar, H. K., Gumpu, M. B., Babu K, J., Kulandaisamy, A. J., & Rayappan, J. B. B. (2023). Iron Oxide Nanoparticles: A Review on the Province of Its Compounds, Properties and Biological Applications. Materials, 16(1), 59. https://doi.org/10.3390/ma16010059





