Advances in the Removal of Cr(III) from Spent Industrial Effluents—A Review
Abstract
1. Introduction
2. Origin and Composition of Spent Industrial Effluents
3. Hydrometallurgical Methods for the Removal of Cr(III) from Waste Effluents
3.1. Precipitation
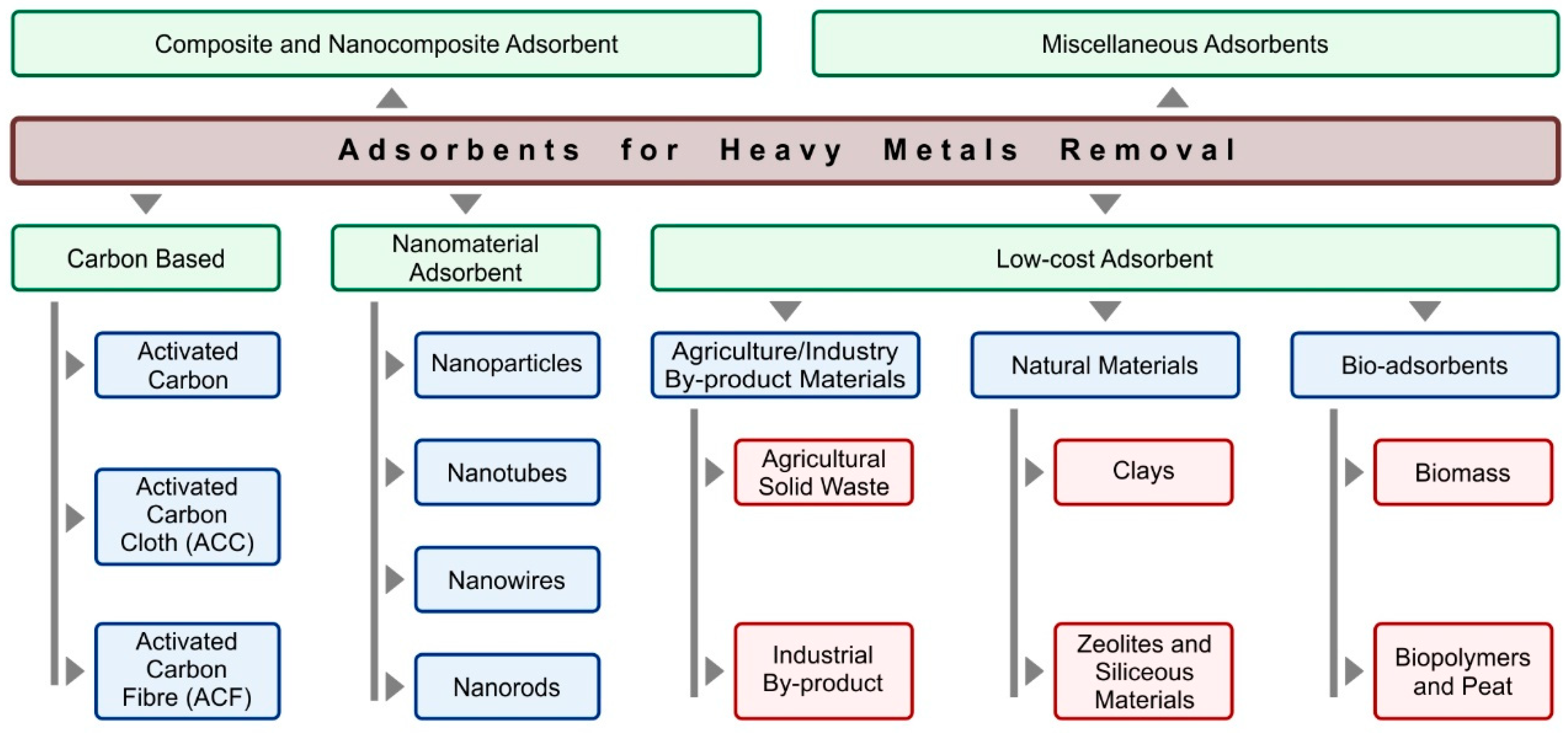
3.2. Adsorption
| Materials | Adsorption Capacity (mg/g) |
|---|---|
| Rice husk | 0.79 |
| Raw rice bran | 0.8 |
| Coconut shell charcoal | 3.65 |
| Modified rice hull | 23.4 |
| Activated alumina | 1.6 |
| Activated charcoal | 0.9614 |
| Wheat bran | 0.942 |
| Activated rice husk carbon | 0.8 |
| Pine leaves | 0.277 |
| Modified oak sawdust | 1.7 |
| CETYL-amended zeolite | 0.65 |
| Cornelian cherry | 59.4 |
| Apricot stone | 59.64 |
| Sodium carboxy methyl cellulose stabilized iron nanoparticles | 255.0 |
| Scrap iron | 19.0 |
| Fe@SiO2 | 467.0 |
| Wool | 41.2 |
| Olive cake | 33.4 |
| Magnetic calcite | 24.2 |
3.3. Ion Exchange
3.4. Conventional and Unconventional Extraction
3.5. Membrane Techniques
3.6. Microbial-Based Techniques
3.7. Electrochemical Techniques
4. Summary and Future Perspectives
| Technique | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Precipitation | Simple design Low operating cost | Lack of chromium recycling Landfilling Secondary pollution by chromium ions | [1,18,111,112] |
| Adsorption/ion exchange | Simple design Low investment cost High adsorption capacity Broad availability of various adsorbents | Low efficiency Weak selectivity Large volumes of diluted eluents | [1,111,112] |
| Liquid–liquid extraction | Operational flexibility Broad selection of extractants High intensity of mass transport Mature conventional operation | Use of VOC diluents (fire hazard) Loss of the organic phase (solubility with water) Large volumes of A and O phases Problems with separation of the phases | [18,70] |
| Membrane techniques | Compact, modular construction Easy to combine with other techniques Easy scaling-up Large contact area | High operating cost Undesirable fouling, scaling, etc. Auxiliary operations required (cleaning, prefiltering) | [1,113,114] |
| Microbial-based | Sustainability of the bioprocess Low operating cost No need to separate biomass cultivation nor harvesting biomass from the environment | Limited by metal concentration tolerated by microorganisms Highly sensitive to operational conditions Necessity for external source of energy for cell growing | [27,90] |
| Electrochemical | High process efficiency Relatively low cost of the equipment | High operating cost due to high energy consumption | [100,101] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Li, S.; Wang, W. Review: Research Progress on Liquid–Liquid Extraction of Chromium. JOM 2021, 73, 1371–1385. [Google Scholar] [CrossRef]
- Statista Mine Production of Chromium Worldwide from 2010 to 2021. Available online: https://www.statista.com/statistics/587342/mine-production-of-mercury-worldwide/ (accessed on 20 November 2022).
- Insider_Strategy&Stats, S. Report Id: SNS/C&M/2557: Chrome Plating Market Research Report. Global Forecast from 2022 to 2030. Available online: https://dataintelo.com/report/chrome-plating-market-report/ (accessed on 20 November 2022).
- Royal Society of Chemistry Periodic Table. Chromium. Available online: https://www.rsc.org/periodic-table/element/24/chromium (accessed on 20 November 2022).
- Nur-E-Alam, M.; Mia, M.A.S.; Ahmad, F.; Rahman, M.M. An Overview of Chromium Removal Techniques from Tannery Effluent. Appl. Water Sci. 2020, 10, 1–22. [Google Scholar] [CrossRef]
- World Health Organization. Chromium in Drinking-Water: A Background Document for Development of World Health Organisation Guidelines for Drinking Water (WHO/HEP/ECH/WSH/2020.3); World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Mani Tripathi, S.; Chaurasia, S.R. Detection of Chromium in Surface and Groundwater and Its Bio-Absorption Using Bio-Wastes and Vermiculite. Eng. Sci. Technol. Int. J. 2020, 23, 1153–1161. [Google Scholar] [CrossRef]
- Singh, D.; Singh, C.K.; Singh, D.; Sarkar, S.K.; Prasad, S.K.; Sharma, N.L.; Singh, I. Glycine Betaine Modulates Chromium (VI)-Induced Morpho-Physiological and Biochemical Responses to Mitigate Chromium Toxicity in Chickpea (Cicer arietinum, L.) Cultivars. Sci. Rep. 2022, 12, 1–17. [Google Scholar] [CrossRef]
- Kocaoba, S.; Cetin, G.; Akcin, G. Chromium Removal from Tannery Wastewaters with a Strong Cation Exchange Resin and Species Analysis of Chromium by MINEQL+. Sci. Rep. 2022, 12, 9618. [Google Scholar] [CrossRef]
- Prasad, S.; Yadav, K.K.; Kumar, S.; Gupta, N.; Cabral-Pinto, M.M.S.; Rezania, S.; Radwan, N.; Alam, J. Chromium Contamination and Effect on Environmental Health and Its Remediation: A Sustainable Approaches. J. Environ. Manag. 2021, 285, 112174. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, M.; Kortenkamp, A. Chromium and Cancer. In Heavy Metals in The Environment; CRC Press: Boca Raton, FL, USA, 2002; pp. 250–283. ISBN 9780429221767. [Google Scholar]
- Khan, A.; Michelsen, N.; Marandi, A.; Hossain, R.; Hossain, M.A.; Roehl, K.E.; Zahid, A.; Hassan, M.Q.; Schüth, C. Processes Controlling the Extent of Groundwater Pollution with Chromium from Tanneries in the Hazaribagh Area, Dhaka, Bangladesh. Sci. Total Environ. 2020, 710, 136213. [Google Scholar] [CrossRef]
- Wionczyk, B.; Apostoluk, W.; Charewicz, W.A. Solvent Extraction of Chromium (III) from Spent Tanning Liquors with Aliquat 336. Hydrometallurgy 2006, 82, 83–92. [Google Scholar] [CrossRef]
- Vaiopoulou, E.; Gikas, P. Regulations for Chromium Emissions to the Aquatic Environment in Europe and Elsewhere. Chemosphere 2020, 254, 126876. [Google Scholar] [CrossRef]
- Tumolo, M.; Ancona, V.; De Paola, D.; Losacco, D.; Campanale, C.; Massarelli, C.; Uricchio, V.F. Chromium Pollution in European Water, Sources, Health Risk, and Remediation Strategies: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 5438. [Google Scholar] [CrossRef]
- Kadłubowicz, A.; Janiszewska, M.; Baraniak, M.; Lota, G.; Staszak, K.; Regel-Rosocka, M. Diffusion Dialysis and Extraction Integrated System for Recovery of Cobalt(II) from Industrial Effluent. J. Water Process Eng. 2021, 39, 101754. [Google Scholar] [CrossRef]
- Kostrzewa, M.; Staszak, K.; Ginter-Kramarczyk, D.; Kruszelnicka, I.; Góra, W.; Baraniak, M.; Lota, G.; Regel-Rosocka, M. Chromium(III) Removal from Nickel(II)-Containing Waste Solutions as a Pretreatment Step in a Hydrometallurgical Process. Materials 2022, 15, 6217. [Google Scholar] [CrossRef] [PubMed]
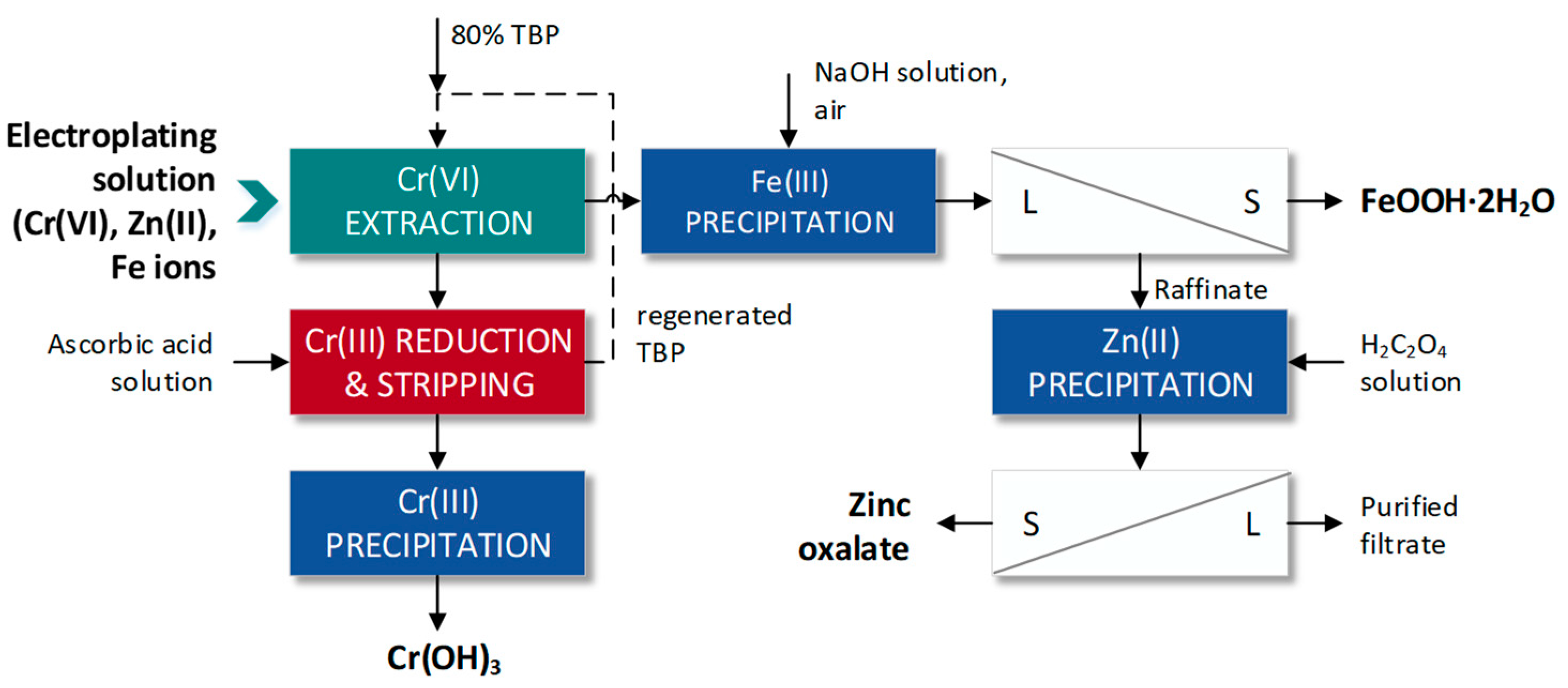
- Ishfaq, A.; Ilyas, S.; Yaseen, A.; Farhan, M. Hydrometallurgical Valorization of Chromium, Iron, and Zinc from an Electroplating Effluent. Sep. Purif. Technol. 2019, 209, 964–971. [Google Scholar] [CrossRef]
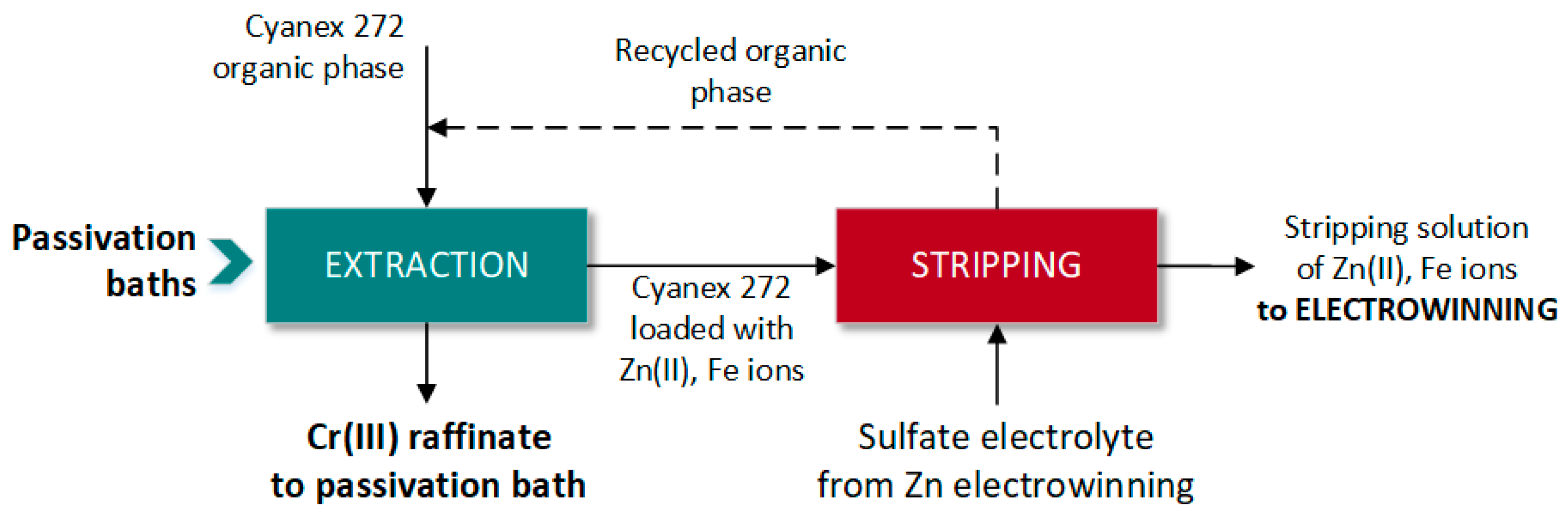
- Alguacil, F.J.; Diban, N.; Urtiaga, A. Zinc and Iron Removal from Chromium(III) Passivation Baths by Solvent Extraction with Cyanex 272. Desalin. Water Treat. 2018, 133, 252–256. [Google Scholar] [CrossRef]
- Nayl, A.A.; Aly, H.F. Solvent Extraction of V(V) and Cr(III) from Acidic Leach Liquors of Ilmenite Using Aliquat 336. Trans. Nonferrous Met. Soc. China 2015, 25, 4183–4191. [Google Scholar] [CrossRef]
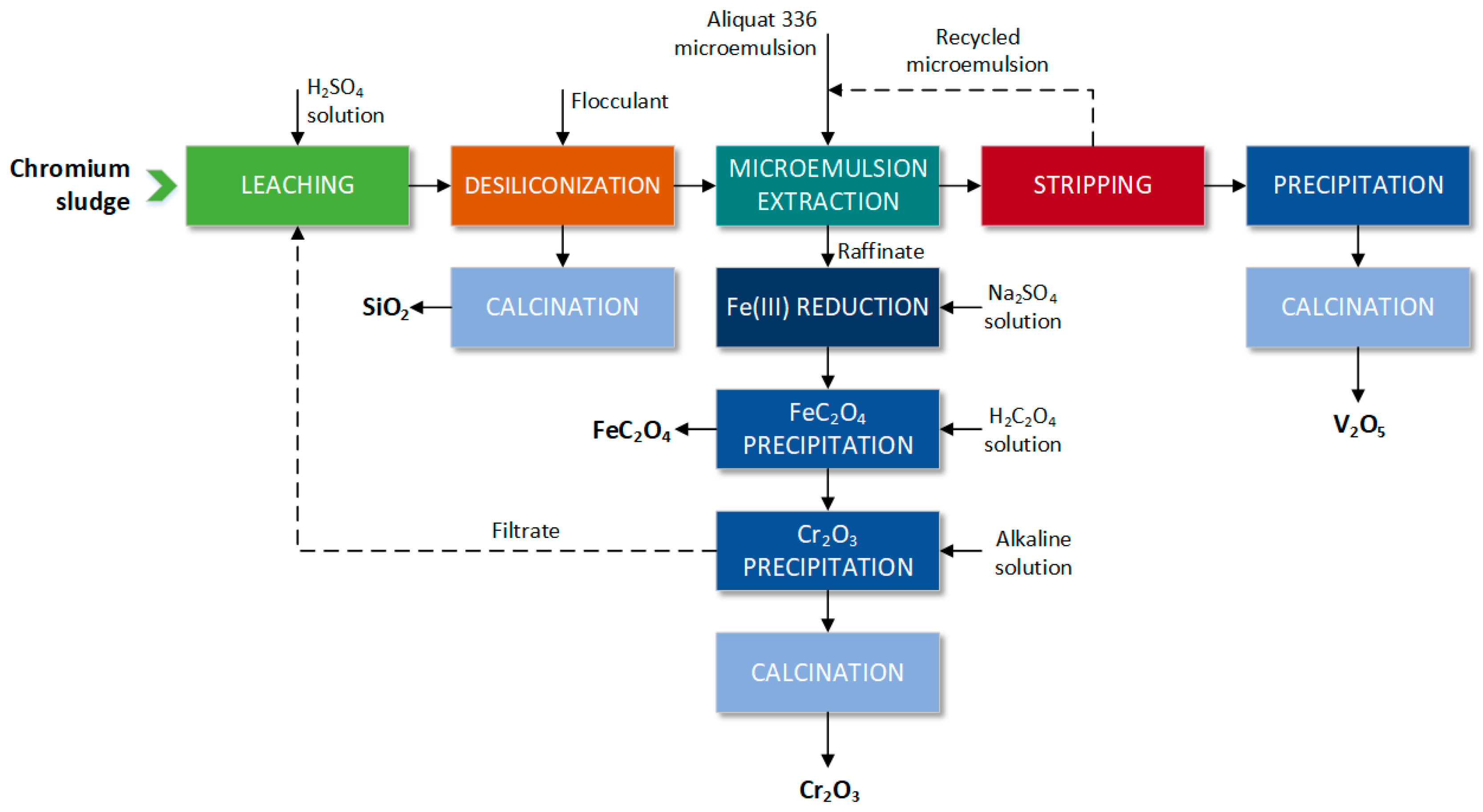
- Guo, Y.; Li, H.Y.; Shen, S.; Cheng, J.; Diao, J.; Xie, B. A Novel Process for Comprehensive Resource Utilization of Hazardous Chromium Sludge: Progressive Recovery of Si, V, Fe and Cr. J. Hazard. Mater. 2021, 405, 124669. [Google Scholar] [CrossRef]
- García, V.; Steeghs, W.; Bouten, M.; Ortiz, I.; Urtiaga, A. Implementation of an Eco-Innovative Separation Process for a Cleaner Chromium Passivation in the Galvanic Industry. J. Clean. Prod. 2013, 59, 274–283. [Google Scholar] [CrossRef]
- García-Antón, J.; Fernández-Domene, R.M.; Sánchez-Tovar, R.; Escrivà-Cerdán, C.; Leiva-García, R.; García, V.; Urtiaga, A. Improvement of the Electrochemical Behaviour of Zn-Electroplated Steel Using Regenerated Cr (III) Passivation Baths. Chem. Eng. Sci. 2014, 111, 402–409. [Google Scholar] [CrossRef]
- Selvaraj, R.; Santhanam, M.; Selvamani, V.; Sundaramoorthy, S.; Sundaram, M. A Membrane Electroflotation Process for Recovery of Recyclable Chromium(III) from Tannery Spent Liquor Effluent. J. Hazard. Mater. 2018, 346, 133–139. [Google Scholar] [CrossRef]
- Reyes-Serrano, A.; López-Alejo, J.E.; Hernández-Cortázar, M.A.; Elizalde, I. Removing Contaminants from Tannery Wastewater by Chemical Precipitation Using CaO and Ca(OH)2. Chin. J. Chem. Eng. 2020, 28, 1107–1111. [Google Scholar] [CrossRef]
- Mohammed, K.; Sahu, O. Recovery of Chromium from Tannery Industry Waste Water by Membrane Separation Technology: Health and Engineering Aspects. Sci. Afr. 2019, 4, e00096. [Google Scholar] [CrossRef]
- Reyes-Romero, B.; Gutiérrez-López, A.N.; Hernández-Altamirano, R.; Mena-Cervantes, V.Y.; Ruiz-Baca, E.; Neri-Torres, E.E.; Chairez, I.; García-Solares, S.M.; Vazquez-Arenas, J. Removal of Concentrated Cr(III) from Real Tannery Wastewater Using Abiotic and Anaerobic Processes with Native Microbial Consortia. J. Environ. Chem. Eng. 2021, 9, 104626. [Google Scholar] [CrossRef]
- Moreno-García, A.F.; Neri-Torres, E.E.; Mena-Cervantes, V.Y.; Altamirano, R.H.; Pineda-Flores, G.; Luna-Sánchez, R.; García-Solares, M.; Vazquez-Arenas, J.; Suastes-Rivas, J.K. Sustainable Biorefinery Associated with Wastewater Treatment of Cr (III) Using a Native Microalgae Consortium. Fuel 2021, 290, 119040. [Google Scholar] [CrossRef]
- Ahmed, E.; Abdulla, H.M.; Mohamed, A.H.; El-Bassuony, A.D. Remediation and Recycling of Chromium from Tannery Wastewater Using Combined Chemical–Biological Treatment System. Process Saf. Environ. Prot. 2016, 104, 1–10. [Google Scholar] [CrossRef]
- Becker, D.S.; Long, E.R.; Proctor, D.M.; Ginn, T.C. Evaluation of Potential Toxicity and Bioavailability of Chromium in Sediments Associated with Chromite Ore Processing Residue. Environ. Toxicol. Chem. 2006, 25, 2576–2583. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, V.; Kumar, P.; Singhal, G. Toxicity of Heavy Metals Pollutants in Textile Mills Effluents. Int. J. Sci. Eng. Res. 2014, 5, 664–666. [Google Scholar]
- Karegar, S.; Bhargavi, M.; Divekar, S.V. Treatmet of Wastewater from Chrome Plating Industry by Ion Exchange Method. Int. J. Res. Eng. Technol. 2015, 4, 393–401. [Google Scholar] [CrossRef]
- Sas, W.; Głuchowski, A.; Radziemska, M.; Dzięcioł, J.; Szymański, A. Environmental and Geotechnical Assessment of the Steel Slags as a Material for Road Structure. Materials 2015, 8, 4857–4875. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, J.; Zhu, H.; Wu, X.; Dai, L.; Chen, R.; Van der Bruggen, B. Recovery of Trivalent and Hexavalent Chromium from Chromium Slag Using a Bipolar Membrane System Combined with Oxidation. J. Colloid Interface Sci. 2022, 619, 280–288. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J. Removal of Chromium from Wastewater by Membrane Filtration, Chemical Precipitation, Ion Exchange, Adsorption Electrocoagulation, Electrochemical Reduction, Electrodialysis, Electrodeionization, Photocatalysis and Nanotechnology: A Review. Environ. Chem. Lett. 2020, 18, 2055–2068. [Google Scholar] [CrossRef]
- Yatim, S.R.M.; Zainuddin, N.A.; Mokhtar, N.S.; Syahjidan, H.N.; Kamsuri, S.N.H. Competitiveness in Removing Copper, Zinc and Chromium Trivalent in Plating Industrial Effluent by Using Hydroxide Precipitation versus Sulphide Precipitation. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1053, 12084. [Google Scholar] [CrossRef]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Ain Zainuddin, N.; Azwan Raja Mamat, T.; Imam Maarof, H.; Wahidah Puasa, S.; Rohana Mohd Yatim, S. Removal of Nickel, Zinc and Copper from Plating Process Industrial Raw Effluent Via Hydroxide Precipitation Versus Sulphide Precipitation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 551, 12122. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. NPJ Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient Techniques for the Removal of Toxic Heavy Metals from Aquatic Environment: A Review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Ye, M.; Li, G.; Yan, P.; Ren, J.; Zheng, L.; Han, D.; Sun, S.; Huang, S.; Zhong, Y. Removal of Metals from Lead-Zinc Mine Tailings Using Bioleaching and Followed by Sulfide Precipitation. Chemosphere 2017, 185, 1189–1196. [Google Scholar] [CrossRef]
- Uddin, M.M.; Hasan, J.; Islam, M.D.; Rahaman, A.; Shamsuddin, S.M. Removal of Chromium(III) and Other Physical Parameters from Chrome Tan Wastewater and Recovery of Chromium from the Precipitating Sludge. Text. Leather Rev. 2020, 3, 64–77. [Google Scholar] [CrossRef]
- Kokkinos, E.; Zouboulis, A. Hydrometallurgical Recovery of CR(III) from Tannery Waste: Optimization and Selectivity Investigation. Water 2020, 12, 719. [Google Scholar] [CrossRef]
- Bilal, M.; Ihsanullah, I.; Younas, M.; Ul Hassan Shah, M. Recent Advances in Applications of Low-Cost Adsorbents for the Removal of Heavy Metals from Water: A Critical Review. Sep. Purif. Technol. 2021, 278, 119510. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A Review on Conventional and Novel Materials towards Heavy Metal Adsorption in Wastewater Treatment Application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Isotherm Models: Classification, Physical Meaning, Application and Solving Method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.B.; Nagpal, G.; Agrawal, S. Rachna Water Purification by Using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Zaimee, M.Z.A.; Sarjadi, M.S.; Rahman, M.L. Heavy Metals Removal from Water by Efficient Adsorbents. Water 2021, 13, 2659. [Google Scholar] [CrossRef]
- Afzaal, M.; Hameed, S.; Abbasi, N.A.; Liaqat, I.; Rasheed, R.; Khan, A.A.; Manan, H.A. Removal of Cr(III) from Wastewater by Using Raw and Chemically Modified Sawdust and Corn Husk. Water Pract. Technol. 2022, 17, 1937–1958. [Google Scholar] [CrossRef]
- Samaraweera, A.P.G.M.V.; Gunathilake, N.P.W.S.S.; Kulasooriya, P.A.K.T.P.K. Biosorption of Cr(III) and Cr(VI) Species on NaOH—Modified Peel of Artocarpus Nobilis Fruit. 1. Investigation of Kinetics. Appl. Water Sci. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Jacob, J.J.; Varalakshmi, R.; Gargi, S.; Jayasri, M.A.; Suthindhiran, K. Removal of Cr (III) and Ni (II) from Tannery Effluent Using Calcium Carbonate Coated Bacterial Magnetosomes. NPJ Clean Water 2018, 1, 1. [Google Scholar] [CrossRef]
- Wang, R.; Zhong, M.; Li, W.; Chen, Y.; Tan, Z.; Li, X.; Zhang, J. Isothermal and Kinetic Studies of Biosorption of Low Concentration Cr(III) from Aqueous Solution by 4 Microbial Biosorbents. Pol. J. Environ. Stud. 2022, 31, 1363–1376. [Google Scholar] [CrossRef]
- Šehović, E.; Memić, M.; Sulejmanović, J.; Hameed, M.; Begić, S.; Ljubijankić, N.; Selović, A.; Ghfar, A.A.; Sher, F. Thermodynamic Valorisation of Lignocellulosic Biomass Green Sorbents for Toxic Pollutants Removal. Chemosphere 2022, 307, 135737. [Google Scholar] [CrossRef]
- Zhang, H.; Carrillo, F.; López-Mesas, M.; Palet, C. Valorization of Keratin Biofibers for Removing Heavy Metals from Aqueous Solutions. Text. Res. J. 2019, 89, 1153–1165. [Google Scholar] [CrossRef]
- Zhang, H.; Carrillo-navarrete, F.; Palet-ballús, C.; Zhang, H.; Carrillo-navarrete, F.; Palet-ballús, C. Human Hair Biogenic Fiber as a Biosorbent of Multiple Heavy Metals from Aqueous Solutions Human Hair Biogenic Fiber as a Biosorbent of Multiple Heavy Metals from Aqueous Solutions. J. Nat. Fibers 2020, 19, 1–16. [Google Scholar] [CrossRef]
- Saha, S.; Zubair, M.; Sandra, M.A.K.; Aman, S. Keratin and Chitosan Biosorbents for Wastewater Treatment: A Review. J. Polym. Environ. 2019, 27, 1389–1403. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Jiang, S.; Huang, J.; Lv, Y.; Liu, Y.; Liu, M. Rapid Removal of Cr(III) from High-Salinity Wastewater by Cellulose-g-Poly-(Acrylamide-Co-Sulfonic Acid) Polymeric Bio-Adsorbent. Carbohydr. Polym. 2021, 270, 118356. [Google Scholar] [CrossRef] [PubMed]
- Godiya, C.B.; Martins Ruotolo, L.A.; Cai, W. Functional Biobased Hydrogels for the Removal of Aqueous Hazardous Pollutants: Current Status, Challenges, and Future Perspectives. J. Mater. Chem. A 2020, 8, 21585–21612. [Google Scholar] [CrossRef]
- Godiya, C.B.; Kumar, S.; Xiao, Y. Amine Functionalized Egg Albumin Hydrogel with Enhanced Adsorption Potential for Diclofenac Sodium in Water. J. Hazard. Mater. 2020, 393, 122417. [Google Scholar] [CrossRef] [PubMed]
- Godiya, C.B.; Revadekar, C.; Kim, J.; Park, B.J. Amine-Bilayer-Functionalized Cellulose-Chitosan Composite Hydrogel for the Efficient Uptake of Hazardous Metal Cations and Catalysis in Polluted Water. J. Hazard. Mater. 2022, 436, 129112. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.; Wabaidur, S.M.; Khan, M.R.; Islam, M.S.; Al-Resayes, S.I. Removal of Chromium(III) and Cadmium(II) Heavy Metal Ions from Aqueous Solutions Using Treated Date Seeds: An Eco-Friendly Method. Molecules 2021, 26, 3718. [Google Scholar] [CrossRef]
- Aravindhan, R.; Fathima, A.; Selvamurugan, M.; Rao, J.R.; Balachandran, U.N. Adsorption, Desorption, and Kinetic Study on Cr(III) Removal from Aqueous Solution Using Bacillus Subtilis Biomass. Clean Technol. Environ. Policy 2012, 14, 727–735. [Google Scholar] [CrossRef]
- Singare, P.U.; Lokhande, R.S. Studies on Ion-Isotopic Exchange Reactions Using Nuclear Grade Ion Exchange Resins. Ionics 2012, 18, 351–357. [Google Scholar] [CrossRef]
- Meng, S.; Wen, S.; Han, G.; Wang, X.; Feng, Q. Wastewater Treatment in Mineral Processing of Non-Ferrous Metal Resources: A Review. Water 2022, 14, 726. [Google Scholar] [CrossRef]
- Salinas, G.; Frontana-Uribe, B.A. Electrochemical Analysis of Heavy Metal Ions Using Conducting Polymer Interfaces. Electrochem 2022, 3, 492–506. [Google Scholar] [CrossRef]
- Naushad, M.; ALOthman, Z.A.; Sharma, G.; Inamuddin. Kinetics, Isotherm and Thermodynamic Investigations for the Adsorption of Co(II) Ion onto Crystal Violet Modified Amberlite IR-120 Resin. Ionics 2015, 21, 1453–1459. [Google Scholar] [CrossRef]
- Hamida, T.N.C. Heavy Metal Sequestration from Contaminated Water: A Review. J. Mater. Environ. Sci. 2018, 9, 2345–2355. [Google Scholar]
- Velusamy, S.; Roy, A.; Sundaram, S.; Kumar Mallick, T. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide-Based Adsorption Strategies for Textile Wastewater Treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef] [PubMed]
- Rivas, B.L.; Morales, D.V.; Kabay, N.; Bryjak, M. Cr(III)Removal from Aqueous Solution Byion Exchange Resins Containing Carboxylic Acid and Sulphonic Acid Groups. J. Chil. Chem. Soc. 2018, 63, 4012–4018. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Alguacil, F.J. Recent Trends in Metals Extraction. Rev. Metal. 2013, 49, 292–316. [Google Scholar] [CrossRef]
- Tasker, P.A.; Tong, C.C.; Westra, A.N. Co-Extraction of Cations and Anions in Base Metal Recovery. Coord. Chem. Rev. 2007, 251, 1868–1877. [Google Scholar] [CrossRef]
- Larsen, K.K.; Wielandt, D.; Schiller, M.; Bizzarro, M. Chromatographic Speciation of Cr(III)-Species, Inter-Species Equilibrium Isotope Fractionation and Improved Chemical Purification Strategies for High-Precision Isotope Analysis. J. Chromatogr. A 2016, 1443, 162–174. [Google Scholar] [CrossRef]
- Wionczyk, B.; Apostoluk, W.S. Solvent Extraction of Chromium(III) from Alkaline Media with Quaternary Ammonium Compounds. Part I. Hydrometallurgy 2004, 72, 185–193. [Google Scholar] [CrossRef]
- Wionczyk, B.; Apostoluk, W. Equilibria of Extraction of Chromium(III) from Alkaline Solutions with Trioctylmethylammonium Chloride (Aliquat 336). Hydrometallurgy 2005, 78, 116–128. [Google Scholar] [CrossRef]
- Khwaja, A.R.; Singh, R.; Tandon, S.N. Recovery of Cr(III) from Tannery Spent Chrome Liquor for Reuse. J. Environ. Eng. 2000, 126, 307–312. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Staszak, K. 8 Polymers in Separation Processes. In Polymer Engineering; Tylkowski, B., Wieszczycka, K., Jastrząb, R., Montane, X., Eds.; De Gruyter: Berlin, Germany, 2022; pp. 249–308. ISBN 9783110733822. [Google Scholar]
- Verma, B.; Balomajumder, C.; Sabapathy, M.; Gumfekar, S.P. Pressure-Driven Membrane Process: A Review of Advanced Technique for Heavy Metals Remediation. Processes 2021, 9, 752. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Yue, C.; Song, L.; Lv, Y.; Liu, F.; Li, A. Insight into the Efficient Co-Removal of Cr(VI) and Cr(III) by Positively Charged UiO-66-NH2 Decorated Ultrafiltration Membrane. Chem. Eng. J. 2021, 404, 126546. [Google Scholar] [CrossRef]
- Islam, J.B.; Furukawa, M.; Tateishi, I.; Katsumata, H.; Kaneco, S. Formic Acid Motivated Photocatalytic Reduction of Cr(VI) to Cr(III) with ZnFe2O4 Nanoparticles under UV Irradiation. Environ. Technol. 2021, 42, 2740–2748. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.S.; Ramzan, N.; Najam, T.; Abbas, G.; Gu, X.; Arif, M.; Qasim, M.; Bashir, H.; Shah, S.S.A.; Sillanpää, M. Metallic Nanoparticles for Catalytic Reduction of Toxic Hexavalent Chromium from Aqueous Medium: A State-of-the-Art Review. Sci. Total Environ. 2022, 829, 154475. [Google Scholar] [CrossRef] [PubMed]
- Njoya, O.; Zhao, S.; Kong, X.; Shen, J.; Kang, J.; Wang, B.; Chen, Z. Efficiency and Potential Mechanism of Complete Cr(VI) Removal in the Presence of Oxalate by Catalytic Reduction Coupled with Membrane Filtration. Sep. Purif. Technol. 2021, 275, 118915. [Google Scholar] [CrossRef]
- Noah, N.F.M.; Sulaiman, R.N.R.; Othman, N.; Jusoh, N.; Rosly, M.B. Extractive Continuous Extractor for Chromium Recovery: Chromium (VI) Reduction to Chromium (III) in Sustainable Emulsion Liquid Membrane Process. J. Clean. Prod. 2020, 247, 119167. [Google Scholar] [CrossRef]
- Noah, N.F.M.; Jusoh, N.; Othman, N.; Sulaiman, R.N.R.; Parker, N.A.M.K. Development of Stable Green Emulsion Liquid Membrane Process via Liquid–Liquid Extraction to Treat Real Chromium from Rinse Electroplating Wastewater. J. Ind. Eng. Chem. 2018, 66, 231–241. [Google Scholar] [CrossRef]
- Rajewski, J.; Dobrzyńska-Inger, A. Application of Response Surface Methodology (RSM) for the Optimization of Chromium(III) Synergistic Extraction by Supported Liquid Membrane. Membranes 2021, 11, 854. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, J.; Zhu, H.; Wu, X.; Dai, L.; Chen, R.; Jin, Y.; Van der Bruggen, B. Retrieval of Trivalent Chromium by Converting It to Its Dichromate State from Soil Using a Bipolar Membrane Electrodialysis System Combined with H2O2 Oxidation. Sep. Purif. Technol. 2022, 300, 121882. [Google Scholar] [CrossRef]
- Alvarado Montalvo, L.G.; Álvarez Cisneros, J.M.; Vizguerra Morales, P.; Salazar Hernández, M.M.; Rodriguez, G.; Ruiz Camacho, B.; Tirado Torres, D.; Baltazar Vera, J.C. Validation of the CFD Simulation of the Diffusion of Cr(III) in a Cationic Membrane of an Electrodialysis System. ECS Trans. 2021, 101, 139–146. [Google Scholar] [CrossRef]
- Feijoo, G.C.; Barros, K.S.; Scarazzato, T.; Espinosa, D.C.R. Electrodialysis for Concentrating Cobalt, Chromium, Manganese, and Magnesium from a Synthetic Solution Based on a Nickel Laterite Processing Route. Sep. Purif. Technol. 2021, 275, 119192. [Google Scholar] [CrossRef]
- Baysak, F.K. A Novel Approach to Chromium Rejection from Sewage Wastewater by Pervaporation. J. Mol. Struct. 2021, 1233, 130082. [Google Scholar] [CrossRef]
- Shao, M.; Li, Y.; Meng, L.; Guo, J.; Gao, Y.; Liu, Y.; Huang, M. Simultaneous Removal of Antimony, Chromium and Aniline by Forward Osmosis Membrane: Preparation, Performance and Mechanism. Desalination 2021, 520, 115363. [Google Scholar] [CrossRef]
- Chojnacka, K. Biosorption and Bioaccumulation—The Prospects for Practical Applications. Environ. Int. 2010, 36, 299–307. [Google Scholar] [CrossRef]
- Aharchaou, I.; Rosabal, M.; Liu, F.; Battaglia, E.; Vignati, D.A.L.; Fortin, C. Bioaccumulation and Subcellular Partitioning of Cr(III) and Cr(VI) in the Freshwater Green Alga Chlamydomonas Reinhardtii. Aquat. Toxicol. 2017, 182, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.D.G.; Hansdah, K.; Dhal, B.; Mehta, K.D.; Pandey, B.D. Bioremoval of Chromium (III) from Model Tanning Effluent by Novel Microbial Isolate. Int. J. Metall. Eng. 2012, 1, 12–16. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, S.; Ling, Z.; Zhou, T.; Liu, P.; Li, X. Enhanced Removal of Trivalent Chromium from Leather Wastewater Using Engineered Bacteria Immobilized on Magnetic Pellets. Sci. Total Environ. 2021, 775, 145647. [Google Scholar] [CrossRef] [PubMed]
- Sundar, K.; Mukherjee, A.; Sadiq, M.; Chandrasekaran, N. Cr (III) Bioremoval Capacities of Indigenous and Adapted Bacterial Strains from Palar River Basin. J. Hazard. Mater. 2011, 187, 553–561. [Google Scholar] [CrossRef]
- Aravindhan, R.; Madhan, B.; Rao, J.R.; Nair, B.U.; Ramasami, T. Bioaccumulation of Chromium from Tannery Wastewater: An Approach for Chrome Recovery and Reuse. Environ. Sci. Technol. 2004, 38, 300–306. [Google Scholar] [CrossRef]
- Pereira, M.; Bartolomé, M.C.; Sánchez-Fortún, S. Bioadsorption and Bioaccumulation of Chromium Trivalent in Cr(III)-Tolerant Microalgae: A Mechanisms for Chromium Resistance. Chemosphere 2013, 93, 1057–1063. [Google Scholar] [CrossRef]
- Tattibayeva, Z.; Tazhibayeva, S.; Kujawski, W.; Zayadan, B.; Musabekov, K.; Adilbekova, A. Analysis of Cr(III) Ions Adsorption on the Surface of Algae: Implications for the Removal of Heavy Metal Ions From Water. Eastern-Eur. J. Enterp. Technol. 2021, 4, 14–23. [Google Scholar] [CrossRef]
- Plestenjak, E.; Kraigher, B.; Leskovec, S.; Mandic Mulec, I.; Marković, S.; Ščančar, J.; Milačič, R. Reduction of Hexavalent Chromium Using Bacterial Isolates and a Microbial Community Enriched from Tannery Effluent. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Estrada, A.; Mena-Cervantes, V.Y.; Fuentes-García, J.; Vazquez-Arenas, J.; Palma-Goyes, R.; Flores-Vela, A.I.; Vazquez-Medina, R.; Altamirano, R.H. Cr(III) Removal from Synthetic and Real Tanning Effluents Using an Electro-Precipitation Method. J. Environ. Chem. Eng. 2018, 6, 1219–1225. [Google Scholar] [CrossRef]
- Selvabharathi, G.; Adishkumar, S.; Banu, J.R. Removal of Chromium(III) from Tannery Wastewater by Electrochemical Peroxidation Process in a Bench Scale Reactor. Desalin. Water Treat. 2019, 156, 340–348. [Google Scholar] [CrossRef]
- Bonola, B.; Sosa-Rodríguez, F.S.; García-Pérez, U.M.; Romero-Ibarra, I.; Henquin, E.R.; Vazquez-Arenas, J. The Influence of Cathode Material, Current Density and PH on the Rapid Cr(III) Removal from Concentrated Tanning Effluents via Electro-Precipitation. J. Hazard. Mater. Adv. 2021, 2, 100008. [Google Scholar] [CrossRef]
- Elabbas, S.; Adjeroud, N.; Mandi, L.; Berrekhis, F.; Pons, M.N.; Leclerc, J.P.; Ouazzani, N. Eggshell Adsorption Process Coupled with Electrocoagulation for Improvement of Chromium Removal from Tanning Wastewater. Int. J. Environ. Anal. Chem. 2022, 102, 2966–2978. [Google Scholar] [CrossRef]
- Zhao, S.; Liao, Z.; Fane, A.; Li, J.; Tang, C.; Zheng, C.; Lin, J.; Kong, L. Engineering Antifouling Reverse Osmosis Membranes: A Review. Desalination 2021, 499, 114857. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Yang, B.; Xiao, K.; Zhao, H. Separation, Anti-Fouling, and Chlorine Resistance of the Polyamide Reverse Osmosis Membrane: From Mechanisms to Mitigation Strategies. Water Res. 2021, 195, 116976. [Google Scholar] [CrossRef]
- Long, M.; Yang, C.; You, X.; Zhang, R.; Yuan, J.; Guan, J.; Zhang, S.; Wu, H.; Khan, N.A.; Kasher, R.; et al. Electrostatic Enhanced Surface Segregation Approach to Self-Cleaning and Antifouling Membranes for Efficient Molecular Separation. J. Memb. Sci. 2021, 638, 119689. [Google Scholar] [CrossRef]
- Dong, D.; Zhu, Y.; Fang, W.; Ji, M.; Wang, A.; Gao, S.; Lin, H.; Huang, R.; Jin, J. Double-Defense Design of Super-Anti-Fouling Membranes for Oil/Water Emulsion Separation. Adv. Funct. Mater. 2022, 32, 2113247. [Google Scholar] [CrossRef]
- Dehghankar, M.; Mohammadi, T.; Moghadam, M.T.; Tofighy, M.A. Metal-Organic Framework/Zeolite Nanocrystal/Polyvinylidene Fluoride Composite Ultrafiltration Membranes with Flux/Antifouling Advantages. Mater. Chem. Phys. 2021, 260, 124128. [Google Scholar] [CrossRef]
- Bagheri, M.; Akbari, A.; Mirbagheri, S.A. Advanced Control of Membrane Fouling in Filtration Systems Using Artificial Intelligence and Machine Learning Techniques: A Critical Review. Process Saf. Environ. Prot. 2019, 123, 229–252. [Google Scholar] [CrossRef]
- Niu, C.; Li, X.; Dai, R.; Wang, Z. Artificial Intelligence-Incorporated Membrane Fouling Prediction for Membrane-Based Processes in the Past 20 Years: A Critical Review. Water Res. 2022, 216, 118299. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, D.J.; Li, Z.; Baetz, B.W.; Hong, Y.; Donnaz, S.; Zhao, X.; Zhou, P.; Ding, H.; Dong, Q. Membrane Fouling Prediction and Uncertainty Analysis Using Machine Learning: A Wastewater Treatment Plant Case Study. J. Memb. Sci. 2022, 660, 120817. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Kumar, P.S.; Rozbu, M.R.; Chowdhury, A.T.; Nuzhat, S.; Rafa, N.; Mahlia, T.M.I.; Ong, H.C.; Mofijur, M. Heavy Metal Toxicity, Sources, and Remediation Techniques for Contaminated Water and Soil. Environ. Technol. Innov. 2022, 25, 102114. [Google Scholar] [CrossRef]
- Razzak, S.A.; Faruque, M.O.; Alsheikh, Z.; Alsheikhmohamad, L.; Alkuroud, D.; Alfayez, A.; Hossain, S.M.Z.; Hossain, M.M. A Comprehensive Review on Conventional and Biological-Driven Heavy Metals Removal from Industrial Wastewater. Environ. Adv. 2022, 7, 100168. [Google Scholar] [CrossRef]
- Kerur, S.S.; Bandekar, S.; Hanagadakar, M.S.; Nandi, S.S.; Ratnamala, G.M.; Hegde, P.G. Removal of Hexavalent Chromium-Industry Treated Water and Wastewater: A Review. Mater. Today Proc. 2021, 42, 1112–1121. [Google Scholar] [CrossRef]
- Staszak, K.; Wieszczycka, K.; Tylkowski, B. Membrane Technologies; Staszak, K., Wieszczycka, K., Tylkowski, B., Eds.; De Gruyter: Berlin, Germany, 2022; ISBN 9783110688269. [Google Scholar]


| Origin | Composition | Ref. |
|---|---|---|
| Steel leaching | in g/dm3: 20.4–37.2 Ni(II); 11.4–21.4 Co(II); 13.4–24.5 Cr(III); 7.20–8.78 Al(III); 0.02–0.53 Cu(II); 0.04–0.138 Fe(III); 0.11 Na(I); 0.023 Mg(II); 0.023 Zn(II) in mol/dm3: 3.46–4.98 H+; 2.28–3.22 SO42−; 0.16–0.34 Cl− | [16,17] |
| Ilmenite leaching | in mol/dm3: 2.1×10−3 V(V); 5.41×10−3 Cr(III); 0.627 Ti(IV); 0.39 Fetotal; 2.73×10−2 Mg(II); 1.74×10−2 Al(III); 6.4×10−4 Ln(III); 6 H2SO4 | [20] |
| Chromium sludge leaching | in g/dm3: 20.64 Cr(III); 2.87 V(V); 5.84 Fe(III); 2.01 Si(IV); 0.83 Ca(II); 0.70 Mn ions; 0.54 Mg(II) in H2SO4 | [21] |
| Passivation bath | in g/dm3: 11–20.5 Zn(II); 3–7 Crtot, in mg/dm3: 15–100 Fetot; acidic pH | [19,22,23] |
| Spent tanning liquor 1 | in mol/dm3: 0.042 Cr(III); 0.201 SO42−; 0.35 Cl−; pH 4.35 | [13] |
| Spent tanning liquor 2 | in mol/dm3: 0.102 Cr(III); 0.324 SO42−; 0.752 Cl−; pH 3.70 | [13] |
| Tannery effluents (six different leather industries in Bara and Parsa districts (Nepal)) | in mg/dm3: Cr 0.7–345 | [10] |
| Tannery spent effluent collected from CSIR-CLRI (Central Leather Research Institute), Chennai | in mg/dm3: total Cr 2481; Cl− 36,000; SO42− 28,480; protein 570; lipid 981; pH 4.4 | [24] |
| Tannery wastewater after chemical treatment | in mg/dm3: total Cr 2007.08; Ca 755.3; Fe 1.998; Na 31,030; Ni 0.3054; Zn 20.69; SO42− 60,414.61; CN− 2; pH 4.13 | [25] |
| Tannery wastewater from Kombolcha Tannery Share Company, Ethiopia | in mg/dm3: total Cr 200; dissolved solid 3000; suspended solid 2100; pH 5.3 | [26] |
| Tannery effluent from Mexico | in mg/dm3: 2760 Cr(III); 0.023 Cr(VI); 19,080 Na(I); 832.7 Ca(II); 0.14 Cu(II); 0.029 Pb ions; 0.014 Ni(II), pH 4 | [27] |
| Tannery effluent from Mexico | in mg/dm3: 5061 Cr(III); 0.023 Cr(VI); pH 5.23 | [28] |
| Tannery effluent from Old Cairo, Egypt | in mg/dm3: 2131 Cr(III); 821 Cr(VI); 249 SO42−, pH 3.6 | [29] |
| Chromite ore processing waste (Hackensack River (NJ, USA) | in mg/kg: Cr total 199–3970; Cr(VI) 0.3–19; As 8.9–59.6; Cd 0.7–9.6; Fe 11,100–47,500; Pb 44.7–281; Mn 232–585; Hg 0.08–2.45; Zn 95.3–597 | [30] |
| Textile mill effluents (Eight textile industries in Delhi NCR, India) | in mg/dm3: Cr 0.11–0.21; Cu 0.17–0.28; Fe 0.39–0.90; Pb 0.02–0.10; Ni 0.11–0.22; Zn 0.11–0.51; Cd 0.01 | [31] |
| Chrome plating industry wastewater | in mg/dm3: Cr(VI) 5721.95; Fe 79.5; Pb 1.095; Cu 28.3, pH 2.09 | [32] |
| Steel industry slags | in mg/kg: Cr 2915; Zn 1084; Ba 380; Sr 266; Cu 175; Zr 109; V 92; Nb 62; Pb 59; Ni 26; Sn 15; Mo 11; Rb 11; As 10; Cd 8; U 4; Br 5; Ce, Co, La < 5; Y, Th, Bi, Ga < 3 | [33] |
| Chromium slag from Chemical Holdings Co., Ltd. (Fuzhou, China) during chromium salt production | in mg/kg: Cr(III) 112; Cr(VI) 464; Ca 26,600; Mg 3160; Fe 4550; Al 64.9; Cd 1.3; Ni 3.2; Cu 5.8; Mn 10.2; As 4.6; Co 1.5 | [34] |
| Precipitating Agents | Optimal pH | Max% of Cr(III) Removal | Ref. |
|---|---|---|---|
| CaCO3 | 8.9 | 99.95 | [42] |
| NaHCO3 | 8.3 | 99.97 | [42] |
| MgO | 8.9 | 99.98 | [42] |
| NaOH | 4–5 | 99.99 | [17,43] |
| CaO | 4–5 | 99.99 | [17] |
| Ca(OH)2 | >7 | 99.99 | [43] |
| Factor Affecting Adsorption | Effect on Adsorption |
|---|---|
| pH | Hydrogen (H+) and hydroxide (OH−) ions react with the activated sites of the adsorbent depending on the pH of the effluent |
| pH at the potential of zero-point charge (pHzpc) | The point of zero charge (PZC) or zeta potential analysis of the adsorbents determine the surface charge of the adsorbent at various pH values and affords information for the attraction and repulsion. When the pH value is lower than that of the PZC, the acidic water donates more protons than hydroxide groups, and, therefore, the surface of the bioadsorbent becomes positively charged (attracting anions). On the contrary, the surface is negatively charged (attracting cations/repelling anions) when the pH value is above the PZC |
| Adsorbent dosage | An increase in the number of active adsorption sites positively affects the efficiency of the removal of contaminants or pollutants; however, a dose that is too high reduces the total uptake of pollutants |
| Temperature | Increasing temperature reduces the viscosity of liquors, which enhances the mobility of contaminants from the bulk solution to the surface of the adsorbent |
| Pressure | Intensifies the adsorption until the process reaches equilibrium |
| Surface area | Small particles have a larger surface area compared to the large particles of adsorbent, allowing greater adsorption to be achieved |
| Coexisting ions | Fewer types of ions coexisting in the effluent increase efficiency of adsorption |
| Origin | Basic Process Parameters | Results | Ref. |
|---|---|---|---|
| Tannery industry | 2-compartment membrane (Nafion 117) electroflotation reactor, Anode: RuO2/TiO2-Ti, Cathode: Ti, Catholyte: spent liquor effluent, Anolyte: 0.01 N H2SO4 | Formation of an insoluble lipid–protein–Cr(OH)3 complex in the form of foam. The removal efficiency of Cr(III), lipid and protein = 98, 91 and 95%, respectively | [24] |
| RO and UF membrane system (polymeric membranes AFC 99, AFC 30, FB 200, PCI membrane), pH 3.5–12; feed flow rate 0.36–0.72 m3/h; TMP 25–40 bar | Total Cr removal efficiency (both Cr(VI) and Cr(III)) up to 99.99%, optimal pH 6.6; flow rate 0.62 m3/h, TMP 40 | [26] | |
| Chromium slag during chromium salt production | Bipolar membrane electrodialysis (BMED) with H2O2 (oxidative conversion of Cr(III) to Cr(VI) in alkaline solutions, where OH− form bipolar membrane) | Recovery of chromium up to 69%. During the purification process, chromium state conversion occurred, which contributed to its recovery | [34] |
| Rinse electroplating wastewater | Liquid membrane phase: palm oil as diluent, Span 80 as surfactant, methyltrioctylammonium chloride ([MTOA+][Cl−])) as an extractant; Strippant: 2.0 mol/dm3 thiourea in 2.0 mol/dm3 sulfuric acid | 100% and 82% of Cr are extracted and then removed. Extraction to membrane phase: Reduction in Cr(VI) in the internal phase: | [82] |
| Sewage wastewater | Pervaporation (PV) using polyvinyl alcohol (PVA)/sodium Y (NaY) zeolite membranes | The membrane allows for the selective separation of Cr(VI) and Cr(III). Cr(VI) was not detected in any permeates | [88] |
| Printing and dyeing factory | Forward osmosis (FO) with a TFC membrane, casting solution: 1.5 wt.% LiCl. Initial concentration in wastewater, in ppb total Cr 23.93, Sb 0.43, aniline 46.03 | Rejection of Cr, Sb, and aniline, after 10 h of FO operation, 99, 98, 99.5%, respectively. Cr was mainly as Cr(VI) | [89] |
| Microorganisms | Remarks | Ref. |
|---|---|---|
| Bioadsorption | ||
| Penicillium sp. (fungus) | 84% Cr(III) sorption achieved at pH 4.0, 35 °C with 1% (w/v) biomass of <150 μm size from a model tannery effluent, in g/dm3: 0.319 CaCl2; 0.962 MgCl2·6H2O; 0.234 Na2S; 6.205 Na2SO4·10H2O; 1.119 NaCl; 200 ppm Cr(III) | [92] |
| Escherichia coli (bacteria) immobilized on magnetic pellets | 2.38 mmol Cr/g cell, 88%, from a real tannery wastewater, in mg/dm3: 1580 Crtotal, 1380 Cl−, pH 4.25 | [93] |
| Kitasatosporia sp. (bacteria) | 99% Cr(VI) sorption from a tannery effluent pretreated after previous Cr(III) precipitation (composition presented in Table 1) | [29] |
| Bioaccumulation | ||
| Bacillus subtilis (bacteria) | Cr(III) from the tannery effluent in Vellore District (India) of various concentrations of metal ions (100 to 2000 mg/dm3), in 2760.023 Crtotal, 2760 Cr(III) | [94] |
| A native microalgae consortium (NMC) isolated from a wastewater treatment plant, containing Tetradesmus sp., Scenedesmus sp. and Ascomycota sp. (microalgae) | 99% Cr(III) sorption from a tannery effluent (composition presented in Table 1) | [28] |
| Sargassum wightii (microalgae) | 88% Cr(III) sorption in 5 stages (2 ppm level achieved in the liquor), 35% after the first stage, from a real tannery solution of 750 ppm, pH 3.5–3.8 | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staszak, K.; Kruszelnicka, I.; Ginter-Kramarczyk, D.; Góra, W.; Baraniak, M.; Lota, G.; Regel-Rosocka, M. Advances in the Removal of Cr(III) from Spent Industrial Effluents—A Review. Materials 2023, 16, 378. https://doi.org/10.3390/ma16010378
Staszak K, Kruszelnicka I, Ginter-Kramarczyk D, Góra W, Baraniak M, Lota G, Regel-Rosocka M. Advances in the Removal of Cr(III) from Spent Industrial Effluents—A Review. Materials. 2023; 16(1):378. https://doi.org/10.3390/ma16010378
Chicago/Turabian StyleStaszak, Katarzyna, Izabela Kruszelnicka, Dobrochna Ginter-Kramarczyk, Wojciech Góra, Marek Baraniak, Grzegorz Lota, and Magdalena Regel-Rosocka. 2023. "Advances in the Removal of Cr(III) from Spent Industrial Effluents—A Review" Materials 16, no. 1: 378. https://doi.org/10.3390/ma16010378
APA StyleStaszak, K., Kruszelnicka, I., Ginter-Kramarczyk, D., Góra, W., Baraniak, M., Lota, G., & Regel-Rosocka, M. (2023). Advances in the Removal of Cr(III) from Spent Industrial Effluents—A Review. Materials, 16(1), 378. https://doi.org/10.3390/ma16010378