Temperature Effect on Ionic Polymers Removal from Aqueous Solutions Using Activated Carbons Obtained from Biomass
Abstract
1. Introduction
2. Materials and Methods
2.1. Adsorbents and Adsorbates
2.2. Viscosity Measurements
2.3. Adsorption–Desorption Measurements
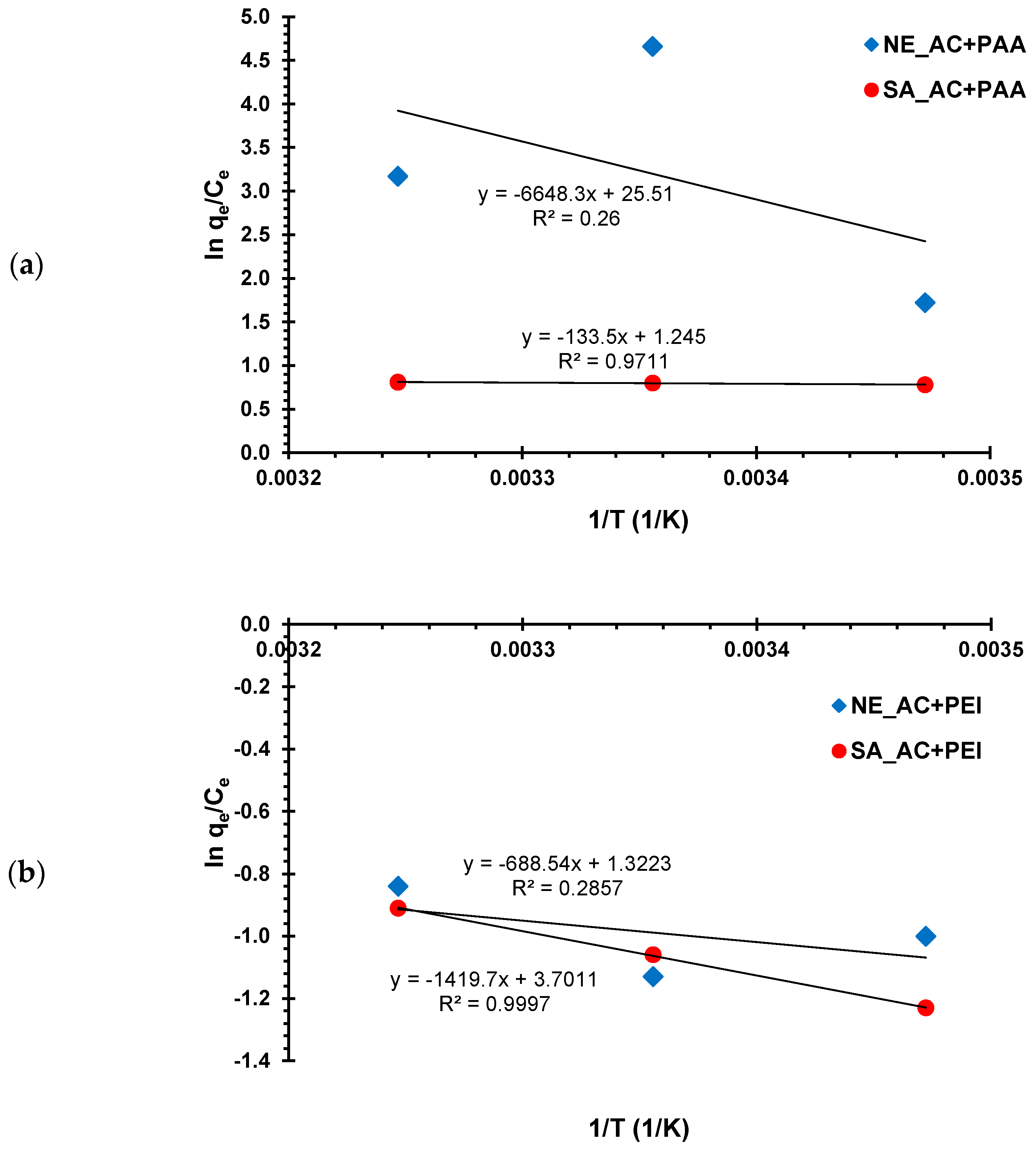
2.4. Thermodynamic Studies
3. Results
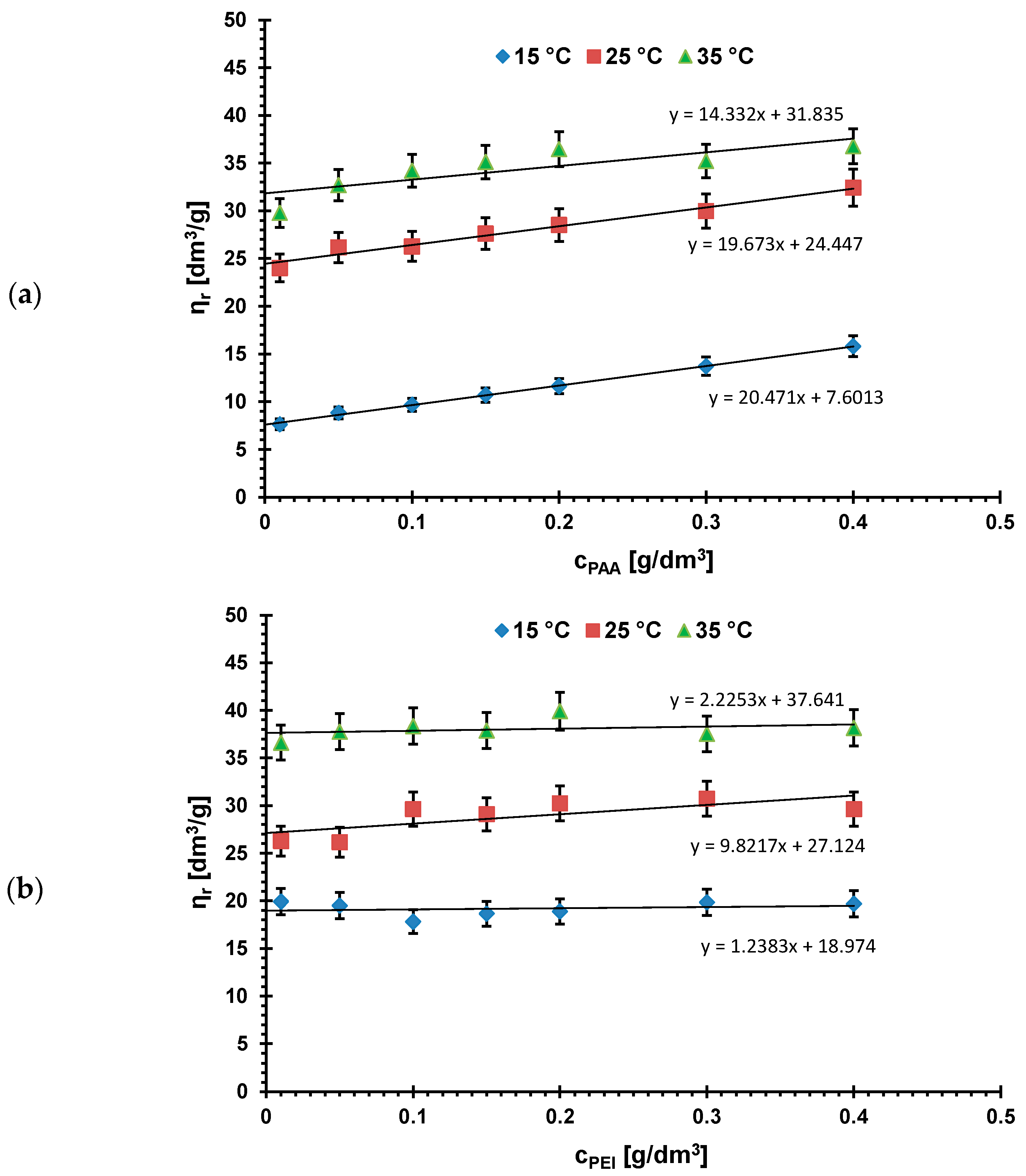
3.1. Linear Dimensions of Poly(Acrylic Acid) and Polyethyleneimine Chains in Aqueous Solutions at Different Temperatures
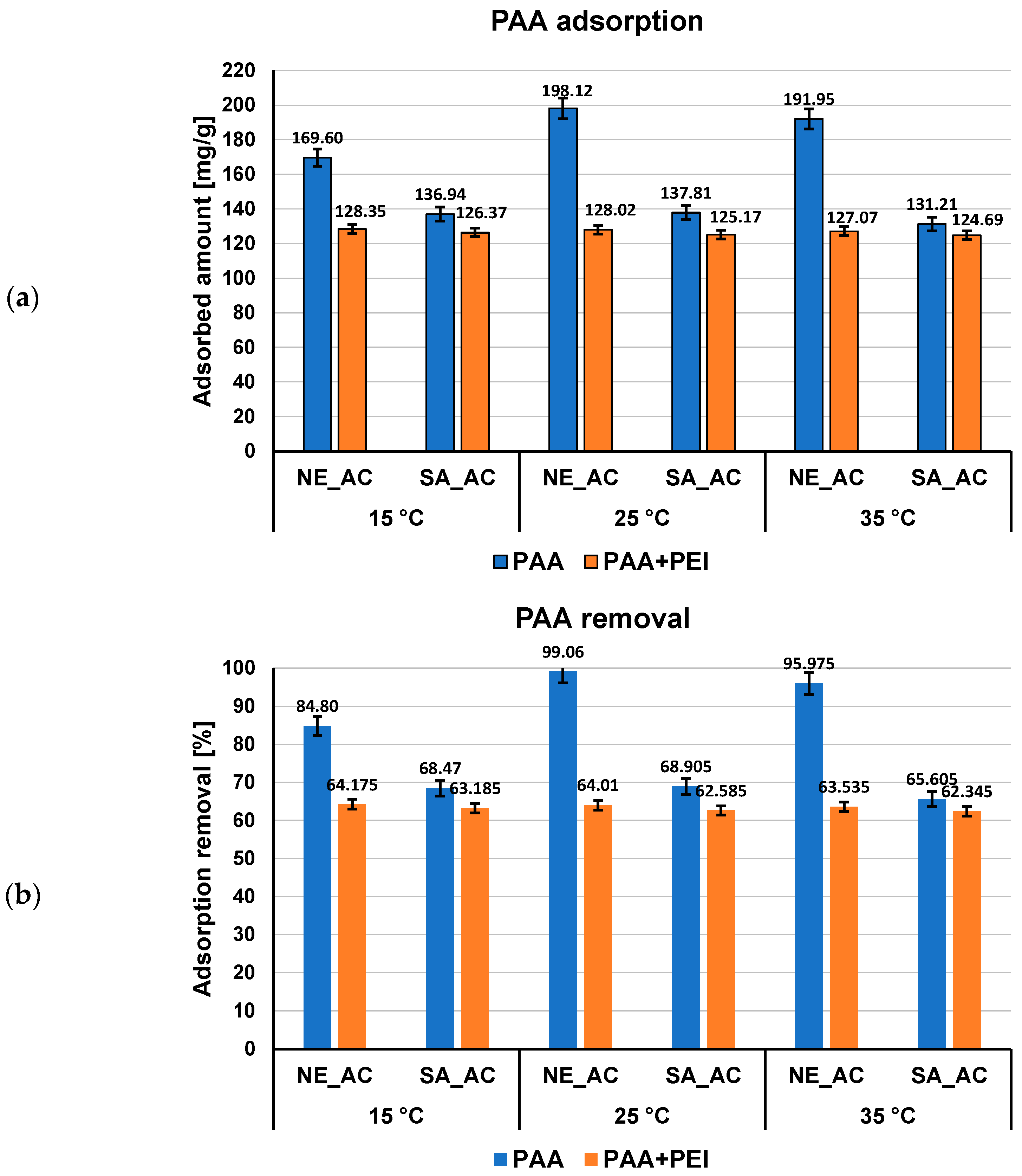
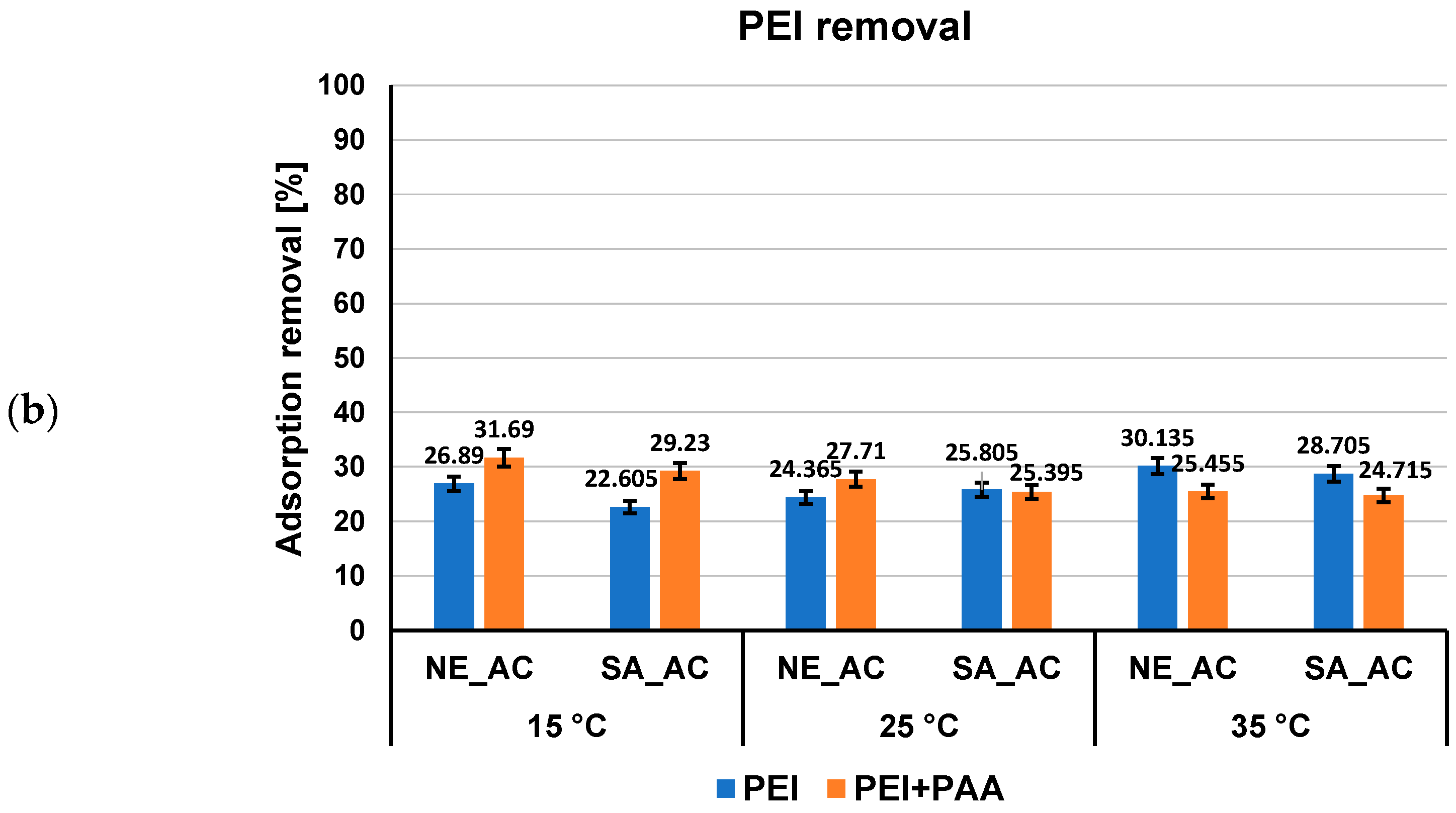
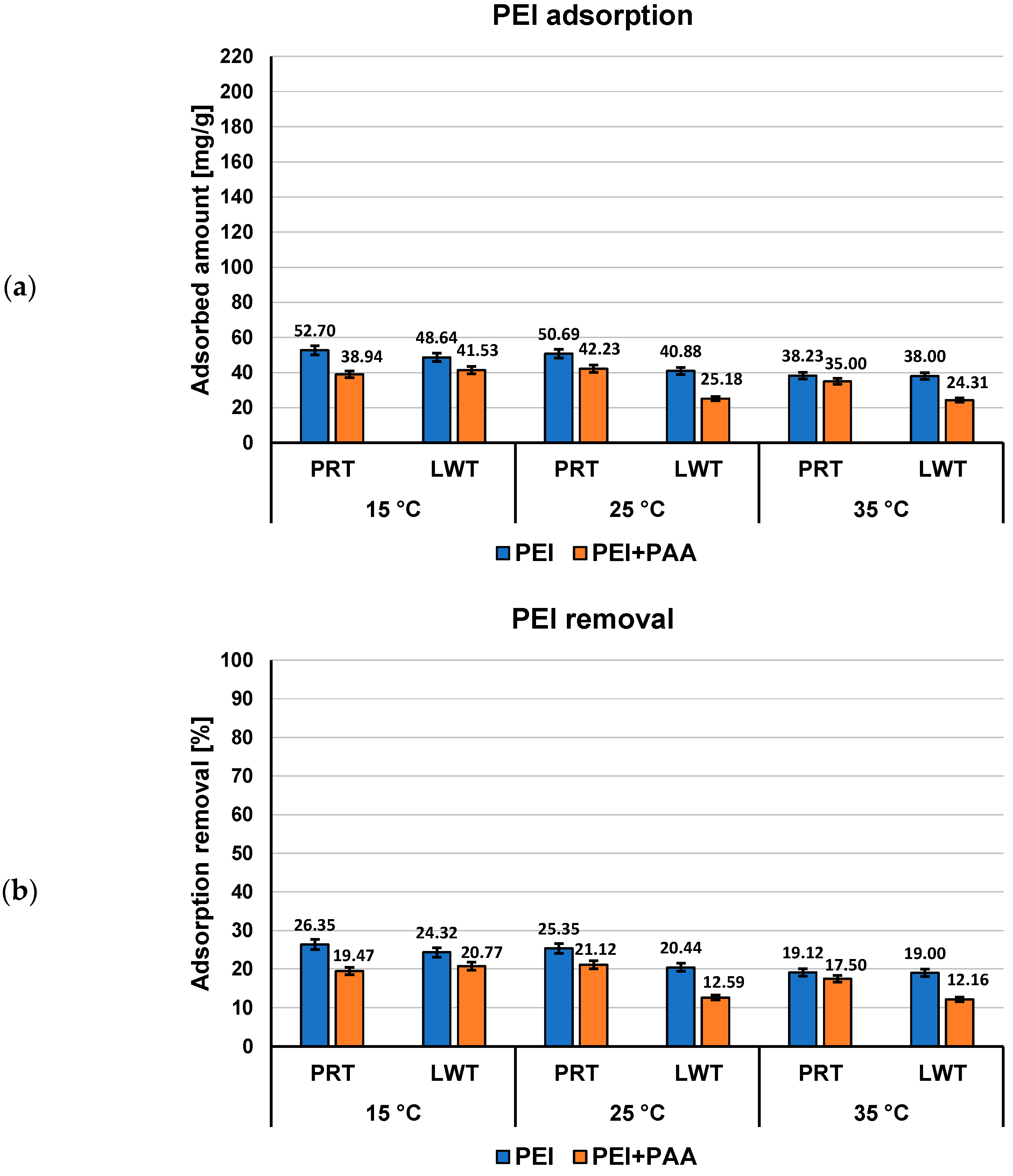
3.2. Adsorption–Desorption Properties of the Activated Carbons in Single and Mixed Solutions of Poly(Acrylic Acid) and Polethyleneimine at Different Temperatures
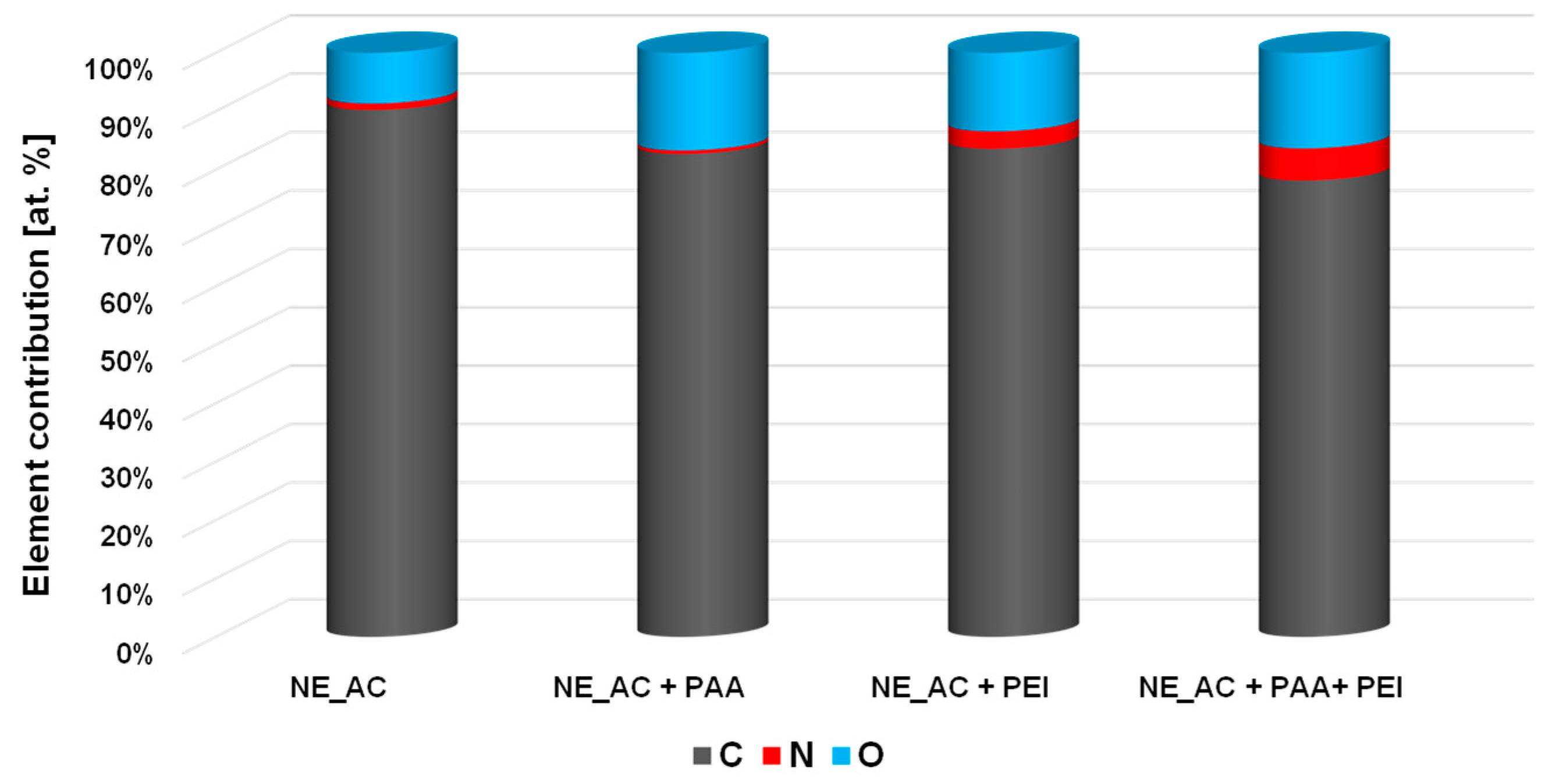
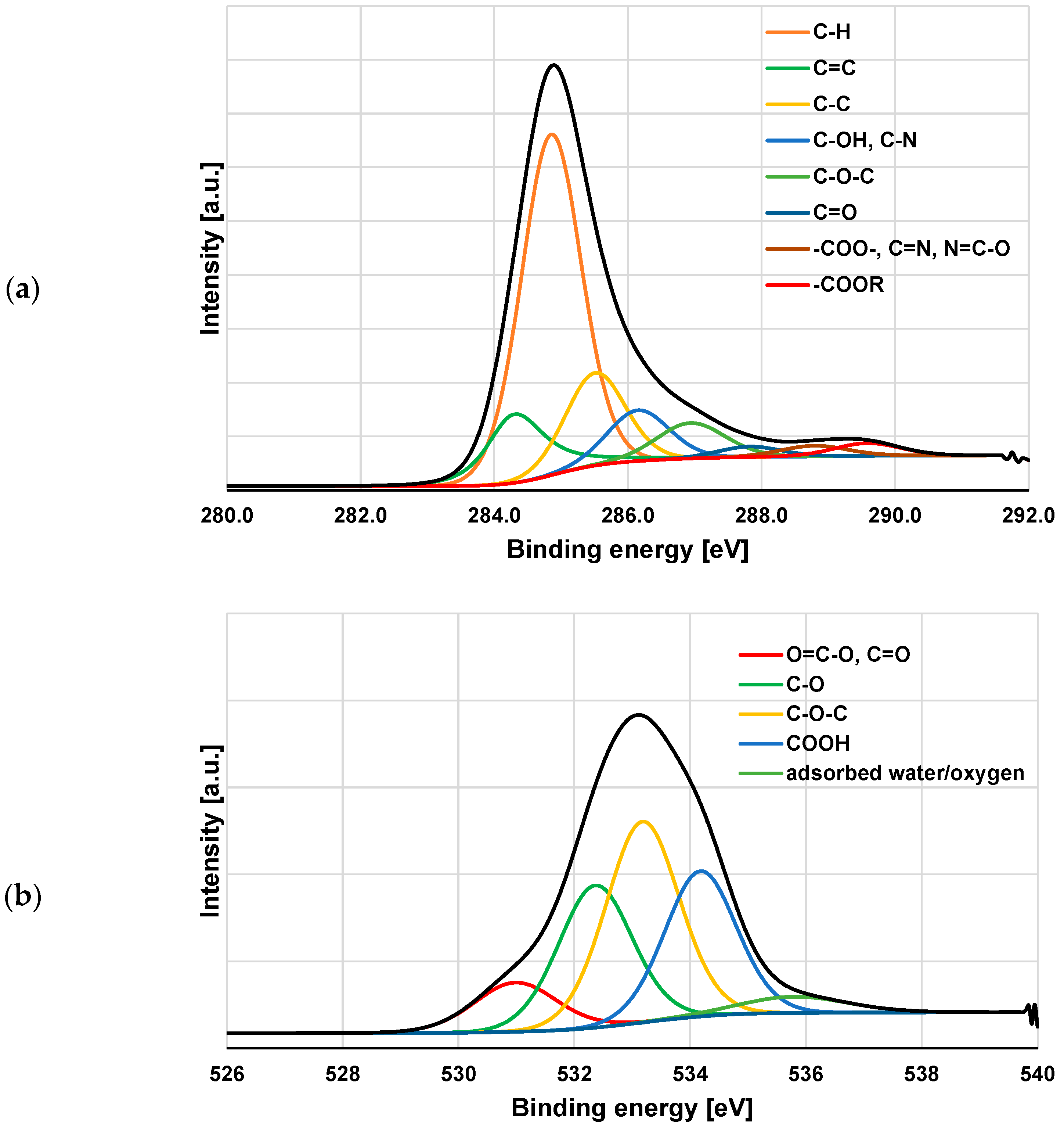
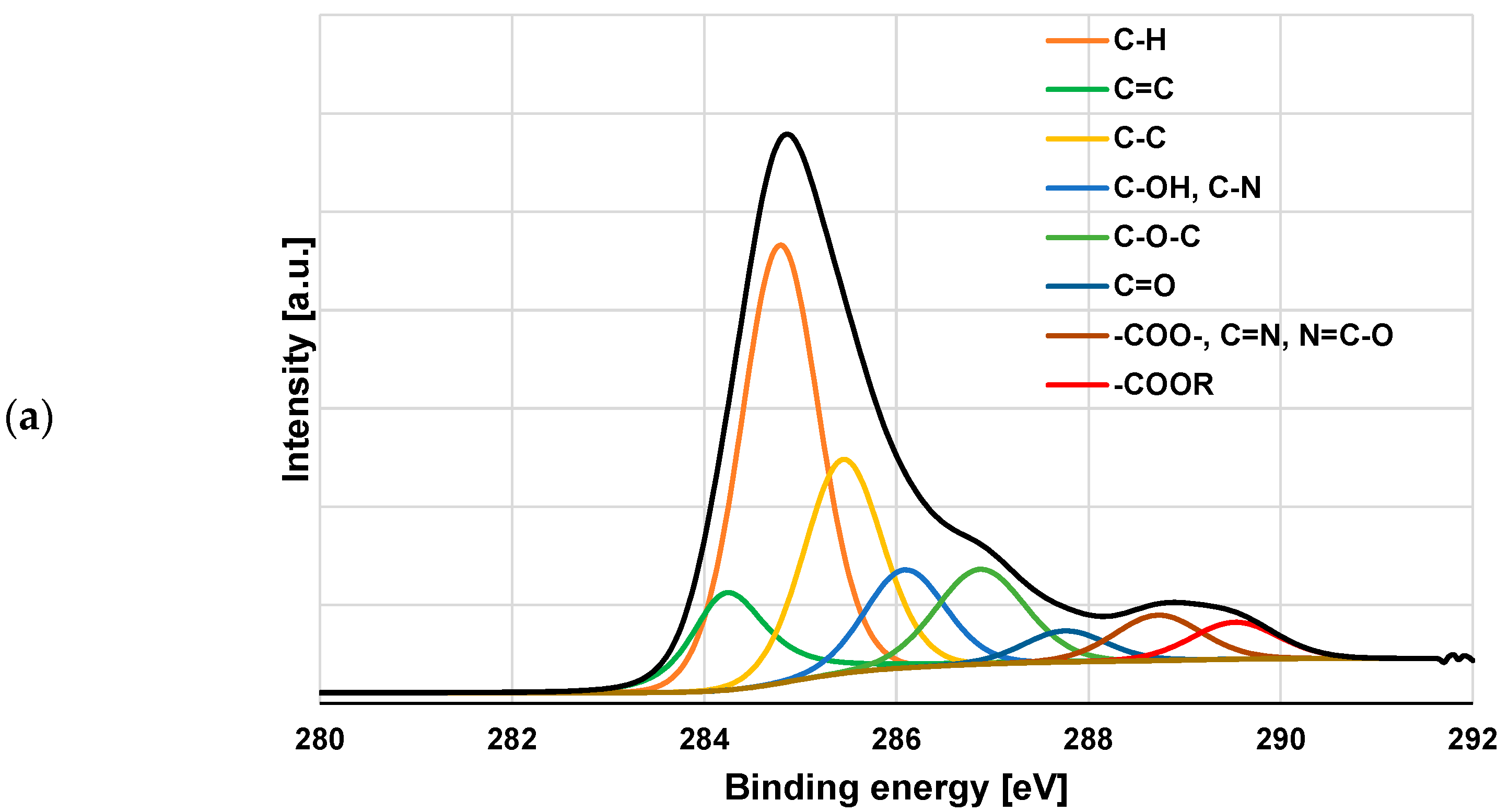
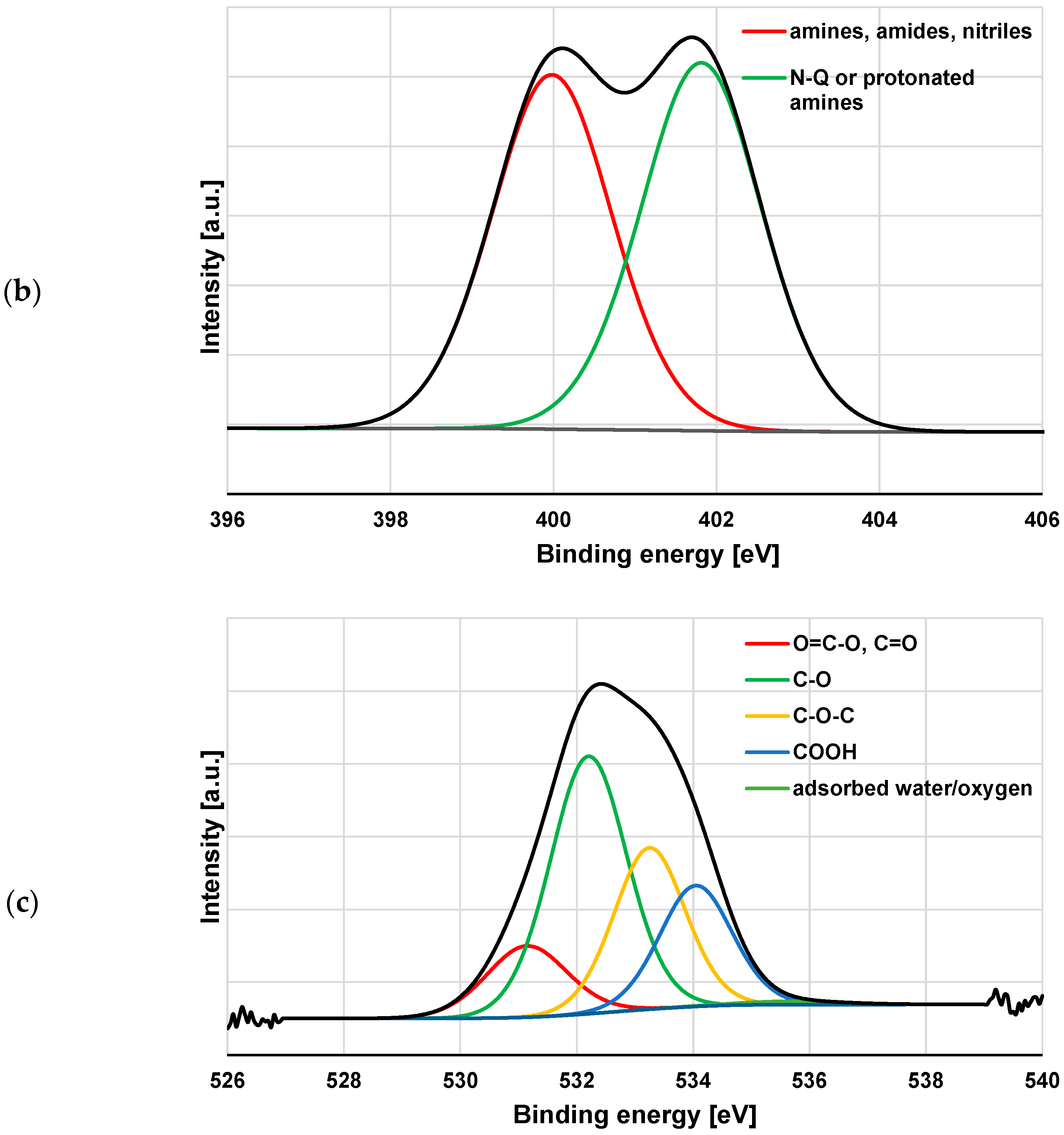
3.3. XPS Study of Polymeric Adsorption Layers Formed on the NA–AC Activated Carbons Surface at 25 °C
| Species | Binding Energy [eV] | NE_AC | NE_AC + PAA | NE_AC + PEI | NE_AC + PAA + PEI |
|---|---|---|---|---|---|
| C-H | 284.85 | 53.2 | 50.5 | 47.2 | 39.9 |
| C=C sp2 | 284.29 | 12.0 | 13.5 | 14.4 | 9.9 |
| C-C sp3 | 285.51 | 14.5 | 15.0 | 14.0 | 19.6 |
| C-OH, C-N | 286.15 | 8.2 | 6.9 | 5.8 | 9.2 |
| C-O-C | 286.94 | 6.4 | 8.3 | 8.4 | 10.0 |
| C=O | 287.82 | 1.7 | 2.1 | 1.8 | 3.0 |
| COO-, C=N, N=C-O | 288.79 | 1.9 | 2.0 | 4.3 | 4.6 |
| COOR | 289.59 | 2.2 | 1.7 | 4.2 | 3.8 |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, B.; Feng, L.; Zheng, J.; Zhang, Q.; Jiang, S.; Zhang, C.; Ding, Y.; Han, J.; Chen, W.; He, S. High performance supercapacitors based on wood-derived thick carbon electrodes synthesized via green activation process. Inorg. Chem. Front. 2022, 9, 6108–6123. [Google Scholar] [CrossRef]
- Lewoyehu, M. Comprehensive review on synthesis and application of activated carbon from agricultural residues for the remediation of venomous pollutants in wastewater. J. Anal. Appl. Pyrol. 2021, 159, 105279. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Zhang, H.; Liu, Y.; Xing, B. Adsorption of Pb(II) and Cd(II) by magnetic activated carbon and its mechanism. Sci. Total Environ. 2021, 757, 143910. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Khan, M.A.; Kameda, T.; Xu, H.; Wang, F.; Xia, M.; Yoshioka, T. New insights into the capture performance and mechanism of hazardous metals Cr3+ and Cd2+ onto an effective layered double hydroxide based material. J. Hazard. Mat. 2022, 426, 128062. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Yuan, X.; Zhao, Y.; Bai, J.; Li, Y.; Cao, Y.; Chen, Y.; Xiong, T. Adsorption behaviors and mechanisms of Fe/Mg layered double hydroxide loaded on bentonite on Cd (II) and Pb (II) removal. J. Colloid Interf. Sci. 2022, 612, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and application of Granular activated carbon from biomass waste materials for water treatment: A review. J. Biores. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Biores. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Sojka, R.E.; Bjorneberg, D.L.; Entry, J.A.; Lentz, R.D.; Orts, W.J. Polyacrylamide in agriculture and environmental land management. Adv. Agron. 2007, 92, 75–162. [Google Scholar] [CrossRef]
- Abidin, A.Z.; Puspasari, T.; Nugroho, W.A. Polymers for enhanced oil recovery technology. Proc. Chem. 2012, 4, 11–16. [Google Scholar] [CrossRef]
- Huang, D.L.; Wang, R.Z.; Liu, Y.G.; Zeng, G.M.; Lai, C.; Xu, P.; Lu, B.A.; Xu, J.J.; Wang, C.; Huang, C. Application of molecularly imprinted polymers in wastewater treatment: A review. Environ. Sci. Pollut. Res. 2015, 22, 963–977. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomat. Res. 2020, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics: Review. J. Mat. Sci. Mat. Med. 2020, 31, 50. [Google Scholar] [CrossRef] [PubMed]
- Jarpa-Parra, M.; Chen, L. Applications of Plant Polymer-Based Solid Foams: Current Trends in the Food Industry. Appl. Sci. 2021, 11, 9605. [Google Scholar] [CrossRef]
- Shen, Z.; Rajabi-Abhari, A.; Oh, K.; Lee, S.; Chen, J.; He, M.; Lee, H.L. The Effect of a Polymer-Stabilized Latex Cobinder on the Optical and Strength Properties of Pigment Coating Layers. Polymers 2021, 13, 568. [Google Scholar] [CrossRef]
- Bolto, B.; Xie, Z. The Use of Polymers in the Flotation Treatment of Wastewater. Processes 2019, 7, 374. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, S.; Lei, H.; Chen, R.; Yu, Q.; Li, H.L. Production of novel bioflocculant by Bacillus licheniformis X14 and its application to low temperature drinking water treatment. Biores. Tech. 2009, 100, 3650–3656. [Google Scholar] [CrossRef] [PubMed]
- Ang, T.; Kiatkittipong, K.; Kiatkittipong, W.; Chua, S.; Lim, J.W.; Show, P.; Bashir, M.J.K.; Ho, Y. Insight on extraction and characterisation of biopolymers as the green coagulants for microalgae harvesting. Water 2020, 12, 1388. [Google Scholar] [CrossRef]
- Wu, Q.; Dong, S.; Wang, L.; Li, X. Single and competitive adsorption behaviors of Cu2+, Pb2+ and Zn2+ on the biochar and magnetic biochar of pomelo peel in aqueous solution. Water 2021, 13, 868. [Google Scholar] [CrossRef]
- Maged, A.; Dissanayake, P.D.; Yang, X.; Pathirannahalage, C.; Bhatnagar, A.; Ok, Y.S. New mechanistic insight into rapid adsorption of pharmaceuticals from water utilizing activated biochar. Environ. Res. 2021, 202, 111693. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Nowicki, P. Simultaneous removal of lead(II) ions and poly(acrylic acid) macromolecules from liquid phase using of biocarbons obtained from corncob and peanut shell precursors. J. Molec. Liq. 2019, 296, 111806. [Google Scholar] [CrossRef]
- Zhu, S.; Ye, Z.; Liu, Z.; Chen, Z.; Li, J.; Xiang, Z. Adsorption Characteristics of Polymer Solutions on Media Surfaces and Their Main Influencing Factors. Polymers 2021, 13, 1774. [Google Scholar] [CrossRef] [PubMed]
- Chornaya, V.; Lipatov, Y.; Todosijchuk, T.; Menzheres, G. Effect of Temperature on the Structure of Adsorption Layers Formed by Adsorption of Polymers in the Transition Region from Dilute to Semidilute Solutions. J. Colloid Interf. Sci. 2002, 255, 36–43. [Google Scholar] [CrossRef]
- Wiśniewska, M. A Review of Temperature Influence on Adsorption Mechanism and Conformation of Water Soluble Polymers on the Solid Surface. J. Disp. Sci. Tech. 2011, 32, 1605–1623. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Brochard, F.; De Gennes, P.G. Dynamical scaling for polymers in theta solvents. Macromolecules 1997, 10, 1157–1161. [Google Scholar] [CrossRef]
- Zhao, D.; Schneider, D.; Fytas, G.; Kumar, S.K. Controlling the thermomechanical behavior of nanoparticle/polymer films. ACS Nano 2014, 8, 8163–8173. [Google Scholar] [CrossRef] [PubMed]
- Gęca, M.; Wiśniewska, M.; Nowicki, P. Simultaneous Removal of Polymers with Different Ionic Character from Their Mixed Solutions Using Herb-Based Biochars and Activated Carbons. Molecules 2022, 27, 7557. [Google Scholar] [CrossRef] [PubMed]
- Boehm, H.P.; Diehl, E.; Heck, W.; Sappok, R. Surface oxides of carbon. Angew. Chem. Int. Ed. 1964, 3, 669–677. (In English) [Google Scholar] [CrossRef]
- Wiśniewska, M. Study of the influence of temperature and the ionic strength of the solution on the adsorption and conformation of poly(acrylic acid) macromolecules on the ZrO2 surface. Ads. Sci. Tech. 2006, 24, 673–686. [Google Scholar] [CrossRef]
- Von Harpe, A.; Petersen, H.; Li, Y.; Kissel, T. Characterization of commercially available and synthesized polyethylenimines for gene delivery. J. Control. Release 2000, 69, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, J.N.; Nayak, P.L.; Lenka, S. Determination of unperturbated dimensions of polymers in binary solvent mixtures from viscosity measurements. Colloid Polym. Sci. 1987, 265, 982–985. [Google Scholar] [CrossRef]
- Garvey, M.J.; Tadros, T.F.; Vincent, B. A comparison of the volume occupied by macromolecules in the adsorbed state and in bulk solution: Adsorption of narrow molecular weight fractions of poly (vinyl alcohol) at the polystyrene/water interface. J. Colloid Interf. Sci. 1974, 49, 57–68. [Google Scholar] [CrossRef]
- Fox, T.G., Jr.; Fox, J.C.; Flory, P.J. The Effect of Rate of Shear on the Viscosity of Dilute Solutions of Polyisobutylene. J. Am. Chem. Soci. 1951, 73, 1901–1904. [Google Scholar] [CrossRef]
- Crummett, W.B.; Hummel, R.A. The determination of traces of polyacrylamides in water. J. Am. Water Works Assoc. 1963, 1, 55, 209–219. [Google Scholar] [CrossRef]
- Patkowski, J.; Myśliwiec, D.; Chibowski, S. Validation of a new method for spectrophotometric determination of polyethylenimine. Int. J. Polym. Anal. Charact. 2016, 21, 486–494. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Liu, Y. Is the free energy change of adsorption correctly calculated? J. Chem. Eng. Data 2009, 54, 1981–1982. [Google Scholar] [CrossRef]
- Muhammad, A.; Shah, A.H.A.; Bilal, S.; Rahman, G. Basic blue dye adsorption from water using polyaniline/magnetite (Fe3O4) composites: Kinetic and thermodynamic aspects. Materials 2019, 12, 1764. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, M.; Wawrzkiewicz, M.; Onyszko, M.; Medykowska, M.; Nosal-Wiercińska, A.; Bogatyrov, V. Carbon-Silica Composite as Adsorbent for Removal of Hazardous, C.I. Basic Yellow 2 and C.I. Basic Blue 3 Dyes. Materials 2021, 14, 3245. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Nowicki, P. Peat-based activated carbons as adsorbents for simultaneous separation of organic molecules from mixed solution of poly(acrylic acid) polymer and sodium dodecyl sulfate surfactant. Colloids Surf. A 2020, 585, 124179. [Google Scholar] [CrossRef]
- Chibowski, S.; Wiśniewska, M.; Opala Mazur, E. The effect of temperature on the adsorption and conformation of polyacrylic acid macromolecules at the ZrO2–polymer solution interface. Powder Tech. 2004, 141, 12–19. [Google Scholar] [CrossRef]
- Silberberg, A.; Eliassaf, J.; Katchalsky, A. Temperature-dependence of light scattering and intrinsic viscosity of hydrogen bonding polymers. J. Polym. Sci. 1957, 23, 259–284. [Google Scholar] [CrossRef]
- Flemming, P.; Münch, A.S.; Fery, A.; Uhlmann, P. Constrained thermoresponsive polymers–new insights into fundamentals and applications. Beilstein J. Org. Chem. 2021, 17, 2123–2163. [Google Scholar] [CrossRef]
- Gibson, F.W. Stabilization of Submicron Metal Oxide Particles in Aqueous Media. Ph.D. Thesis, Virginia Tech, Blacksburg, Virginia, 1998. [Google Scholar]
- Fukuda, Y.; Abe, D.; Tanaka, Y.; Uchida, J.; Suzuki, N.; Miyai, T.; Sasanuma, Y. Solution properties of poly(N-methylethylene imine), a highly hydrophilic polycation. Polym. J. 2016, 48, 1065–1072. [Google Scholar] [CrossRef]
- Yan, B.; Zheng, J.; Feng, L.; Du, C.; Jian, S.; Yang, W.; Wu, Y.A.; Jiang, S.; He, S.; Chen, W. Wood-derived biochar as thick electrodes for high-rate performance supercapacitors. Biochar 2022, 4, 50. [Google Scholar] [CrossRef]
- Kim, C.; Zhu, C.; Aoki, Y.; Habazaki, H. Heteroatom-doped porous carbon with tunable pore structure and high specific surface area for high performance supercapacitors. Electrochim. Acta 2019, 314, 173–187. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Chitin derived nitrogen-doped porous carbons with ultrahigh specific surface area and tailored hierarchical porosity for high performance supercapacitors. J. Bioresour. Bioprod. 2021, 6, 142–151. [Google Scholar] [CrossRef]
- Koinuma, M.; Tateishi, H.; Hatakeyama, K.; Miyamoto, S.; Ogata, C.; Funatsu, A.; Taniguchi, T.; Matsumoto, Y. Analysis of reduced graphene oxides by X-ray Photoelectron spectroscopy and electrochemical capacitance. Chem. Lett. 2013, 42, 924–926. [Google Scholar] [CrossRef]
- Teng, W.; Zhou, Q.; Wang, X.; Che, H.; Du, Y.; Hu, P.; Li, H.; Wang, J. Biotemplating preparation of N,O-codoped hierarchically porous carbon for high-performance supercapacitors. Appl. Surf. Sci. 2021, 566, 150613. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Ryzhkov, S.A.; Kirilenko, D.A.; Ulin, N.V.; Baidakova, M.V.; Shnitov, V.V.; Pavlov, S.I.; Chumakov, R.G.; Stolyarova, D.Y.; Besedina, N.A.; et al. From graphene oxide towards aminated graphene: Facile synthesis, its structure and electronic properties. Sci. Rep. 2020, 10, 6902. [Google Scholar] [CrossRef]
- Nowicki, P.; Pietrzak, R.; Wachowska, H. X-ray Photoelectron spectroscopy study of nitrogen-enriched active carbons obtained by ammoxidation and chemical activation of brown and bituminous coals. Energy Fuels 2010, 24, 1197–1206. [Google Scholar] [CrossRef]
- Radaelli, G.; Heredia-Guerrero, J.A.; Masood, M.T.; Ceseracciu, L.; Davis, A.; Carzino, R.; Prato, M.; Bayer, I.S.; Athanassiou, A. Highly Effective antiadhesive coatings from ph-modified water-dispersed perfluorinated acrylic copolymers: The case of vulcanizing rubber. Adv. Mater. Interf. 2016, 3, 1600069. [Google Scholar] [CrossRef]



| Adsorbent | Surface Area [m2/g] | Pore Volume [cm3/g] | Mean Pore Size [nm] | Acidic Groups [mmol/g] | Basic Groups [mmol/g] | Total Amount [mmol/g] | ||
|---|---|---|---|---|---|---|---|---|
| Total | Micropore | Total | Micropore | |||||
| NE–AC | 801 | 157 | 0.847 | 0.074 | 4.231 | 0.858 | 0.272 | 1.130 |
| SA–AC | 842 | 155 | 0.826 | 0.074 | 3.926 | 0.436 | 0.215 | 0.651 |
| Temperature | [η] [dm3/g] | [nm] | Rh [nm] |
|---|---|---|---|
| PAA | |||
| 15 °C | 7.601 | 1.96 | 0.53 |
| 25 °C | 24.447 | 2.85 | 0.77 |
| 35 °C | 31.835 | 3.16 | 0.85 |
| PEI | |||
| 15 °C | 18.974 | 2.66 | 0.72 |
| 25 °C | 27.124 | 3.00 | 0.81 |
| 35 °C | 37.641 | 3.35 | 0.90 |
| Calculated Parameters | Pseudo-First Order Model | Pseudo-Second Order Model | ||||
|---|---|---|---|---|---|---|
| qe [mg/g] | k1 [1/min] | R2 | qe [mg/g] | k2 [g/(mg·min)] | R2 | |
| PAA | ||||||
| 15 °C | 1.02881 | 8.65444 | 0.8313 | 94.3396 | 0.00185 | 0.9982 |
| 25 °C | 1.03624 | 7.91933 | 0.9420 | 102.041 | 0.00221 | 0.9999 |
| 35 °C | 1.03241 | 7.22797 | 0.9190 | 71.9424 | 0.00472 | 0.9854 |
| PEI | ||||||
| 15 °C | 1.02316 | 5.31486 | 0.9339 | 30.1205 | 0.00587 | 0.9989 |
| 25 °C | 1.01329 | 1.22266 | 0.6614 | 20.5339 | 0.02061 | 0.9995 |
| 35 °C | 1.02439 | 6.2257 | 0.8792 | 24.2718 | 0.00362 | 0.9949 |
| Desorption [%] | |||
|---|---|---|---|
| Desorption Agent | H2O | HNO3 | NaOH |
| PAA, 15 °C | |||
| NE_AC + PAA | 2.44 | 2.93 | 61.23 |
| NE_AC + PAA + PEI | 3.35 | 4.70 | 50.60 |
| SA_AC + PAA | 3.86 | 3.84 | 57.58 |
| SA_AC + PAA + PEI | 4.53 | 5.56 | 55.55 |
| PEI, 15 °C | |||
| NE_AC + PEI | 13.97 | - | - |
| NE_AC + PEI + PAA | 2.79 | - | - |
| SA_AC + PEI | 14.81 | - | - |
| SA_AC + PEI + PAA | 0.56 | - | - |
| PAA, 25 °C | |||
| NE_AC + PAA | 1.36 | 2.76 | 61.01 |
| NE_AC + PAA + PEI | 1.23 | 1.81 | 47.68 |
| SA_AC + PAA | 2.53 | 2.00 | 46.45 |
| SA_AC + PAA + PEI | 1.36 | 2.42 | 48.59 |
| PEI, 25 °C | |||
| NE_AC + PEI | 44.15 | - | - |
| NE_AC + PEI + PAA | 4.52 | - | - |
| SA_AC + PEI | 24.73 | - | - |
| SA_AC + PEI + PAA | 9.69 | - | - |
| PAA, 35 °C | |||
| NE_AC + PAA | 1.55 | 2.90 | 56.65 |
| NE_AC + PAA + PEI | 2.93 | 8.85 | 45.50 |
| SA_AC + PAA | 3.91 | 5.34 | 54.10 |
| SA_AC + PAA + PEI | 5.86 | 8.32 | 53.35 |
| PEI, 35 °C | |||
| NE_AC + PEI | 36.28 | - | - |
| NE_AC + PEI + PAA | 5.40 | - | - |
| SA_AC + PEI | 39.29 | - | - |
| SA_AC + PEI + PAA | 4.61 | - | - |
| Adsorbent | T (°C) | Kc (dm3/g) | ΔH° (kJ/mol) | ΔS° (kJ/(mol·K)) | ΔG° (kJ/mol) |
|---|---|---|---|---|---|
| PAA | |||||
| NE_AC | 15 | 5.58 | 55.4 | 212.4 | −16.8 |
| 25 | 105.38 | −24.7 | |||
| 35 | 23.84 | −21.7 | |||
| SA_AC | 15 | 2.17 | 1.1 | 10.3 | −14.5 |
| 25 | 2.22 | −15.1 | |||
| 35 | 2.24 | −15.6 | |||
| PEI | |||||
| NE_AC | 15 | 0.37 | 5.7 | 10.9 | |
| 25 | 0.32 | ||||
| 35 | 0.43 | ||||
| SA_AC | 15 | 0.29 | 11.8 | 30.9 | |
| 25 | 0.35 | ||||
| 35 | 0.40 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gęca, M.; Wiśniewska, M.; Urban, T.; Nowicki, P. Temperature Effect on Ionic Polymers Removal from Aqueous Solutions Using Activated Carbons Obtained from Biomass. Materials 2023, 16, 350. https://doi.org/10.3390/ma16010350
Gęca M, Wiśniewska M, Urban T, Nowicki P. Temperature Effect on Ionic Polymers Removal from Aqueous Solutions Using Activated Carbons Obtained from Biomass. Materials. 2023; 16(1):350. https://doi.org/10.3390/ma16010350
Chicago/Turabian StyleGęca, Marlena, Małgorzata Wiśniewska, Teresa Urban, and Piotr Nowicki. 2023. "Temperature Effect on Ionic Polymers Removal from Aqueous Solutions Using Activated Carbons Obtained from Biomass" Materials 16, no. 1: 350. https://doi.org/10.3390/ma16010350
APA StyleGęca, M., Wiśniewska, M., Urban, T., & Nowicki, P. (2023). Temperature Effect on Ionic Polymers Removal from Aqueous Solutions Using Activated Carbons Obtained from Biomass. Materials, 16(1), 350. https://doi.org/10.3390/ma16010350








