The Thermodynamics and Kinetics of a Nitrogen Reaction in an Electric Arc Furnace Smelting Process
Abstract
1. Introduction
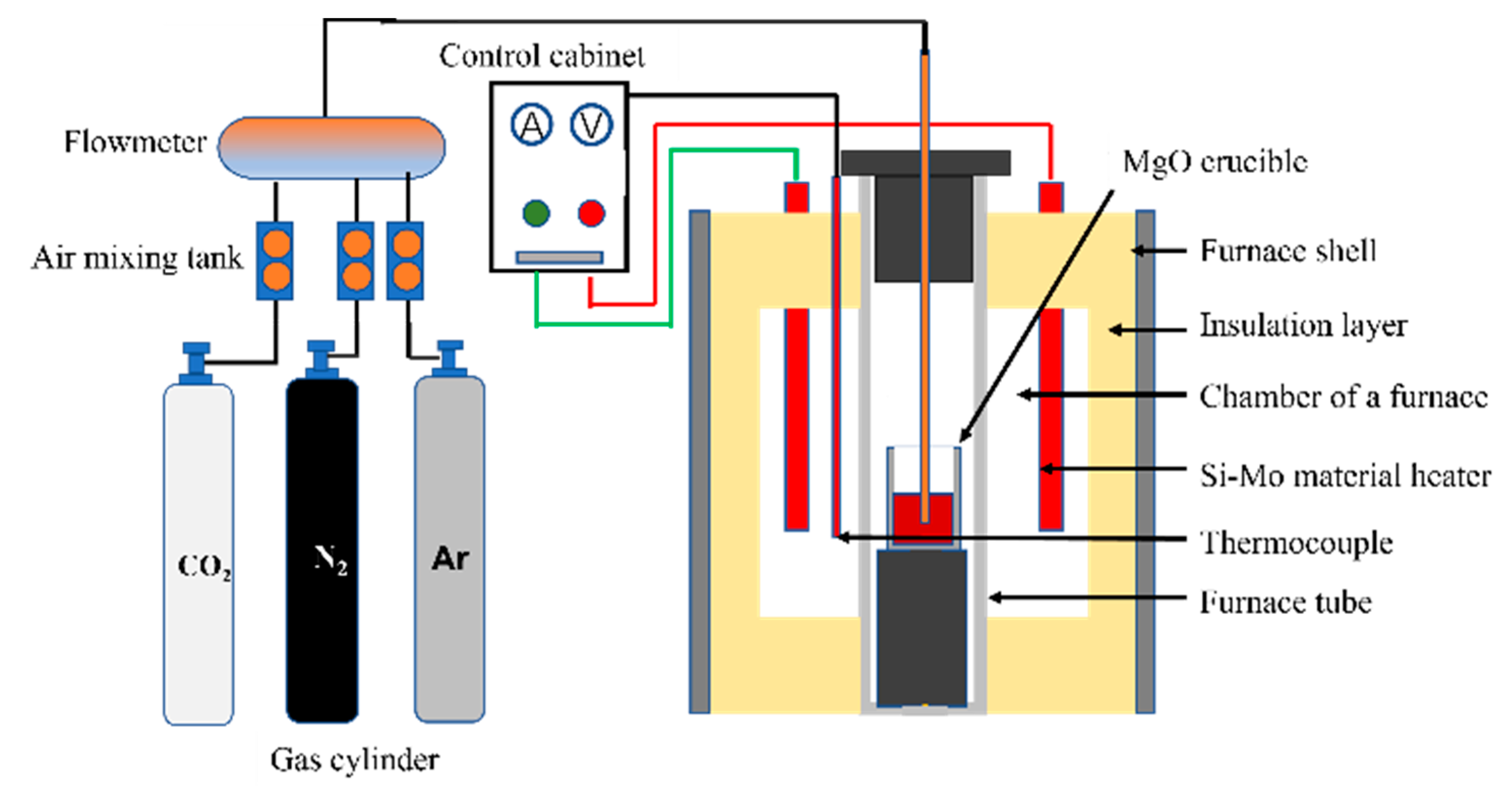
2. Materials and Methods
- (1)
- High-temperature thermocouples were used to correct the constant-temperature zone area, and the thickness of the bottom plate of the lower furnace of the crucible was adjusted to ensure that the thermocouple in the furnace could accurately display the temperature of the constant-temperature zone.
- (2)
- The composition of the molten steel was prepared according to the experimental scheme, and the MgO crucible was placed in the constant-temperature zone. Protective argon was introduced separately, and the flow was set to 600 mL/min for 30 min to purge air in the furnace tube.
- (3)
- The heating system was started. After the experimental temperature was reached, the atmosphere or Ar/N2 ratio was adjusted according to the experimental scheme, the partial pressure of N2 in the furnace was controlled, and nitrogen absorption/denitrification experiments were conducted.
- (4)
- A quartz tube was used to absorb the molten steel every 5 min and conduct water-cooling. Hydrogen, oxygen, and nitrogen gas analyzers were used to measure the nitrogen contents of the samples.
3. Results and Discussion
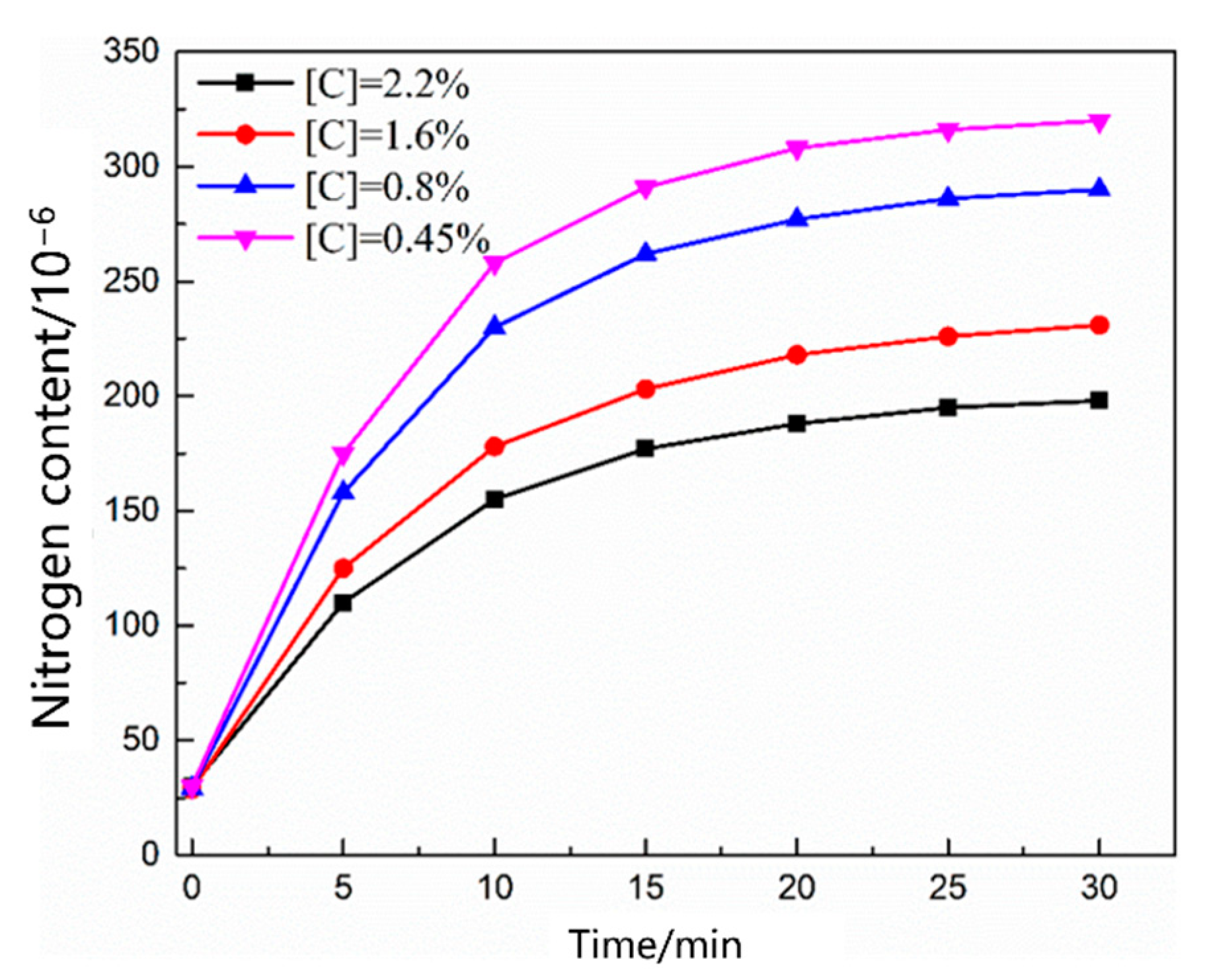
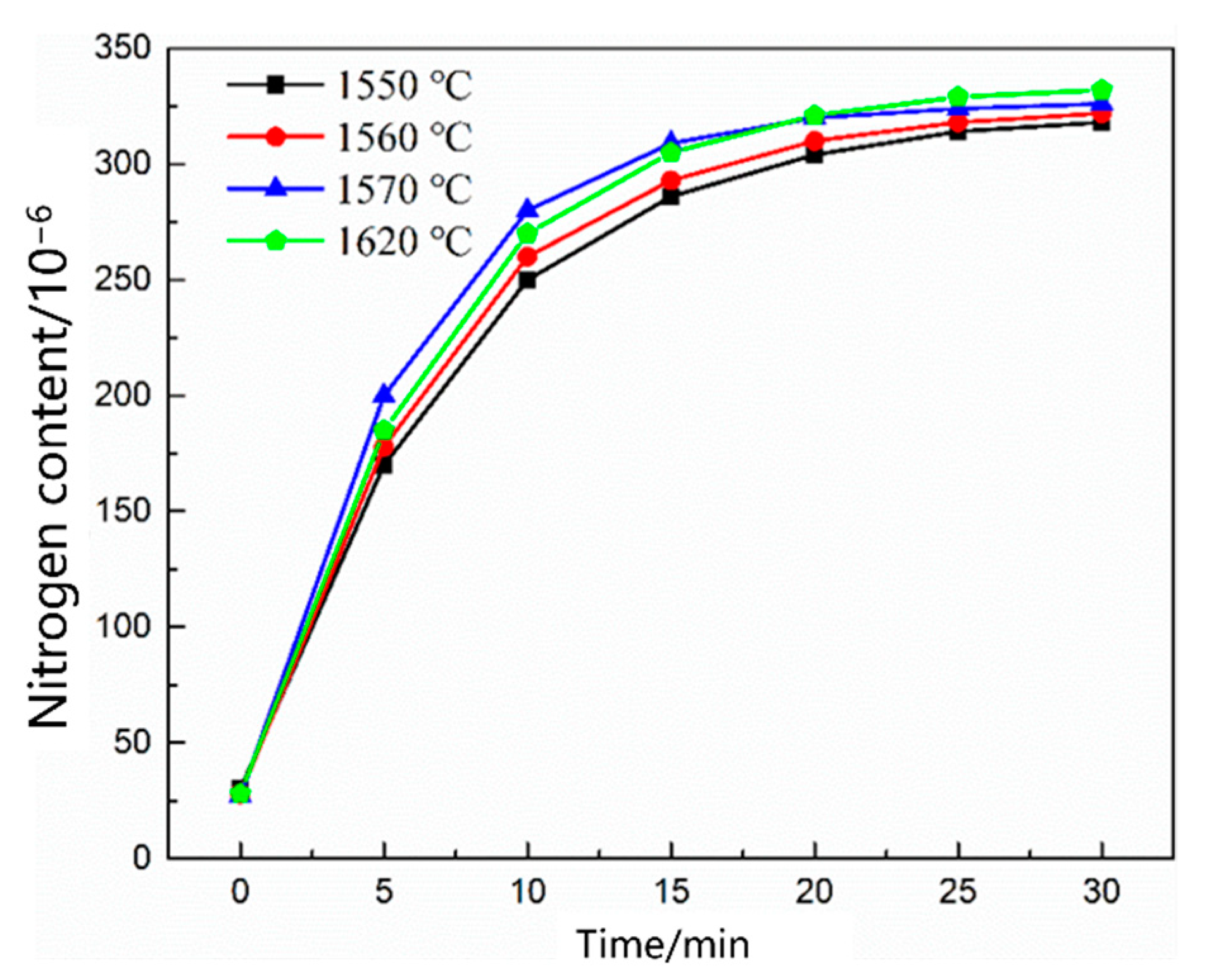
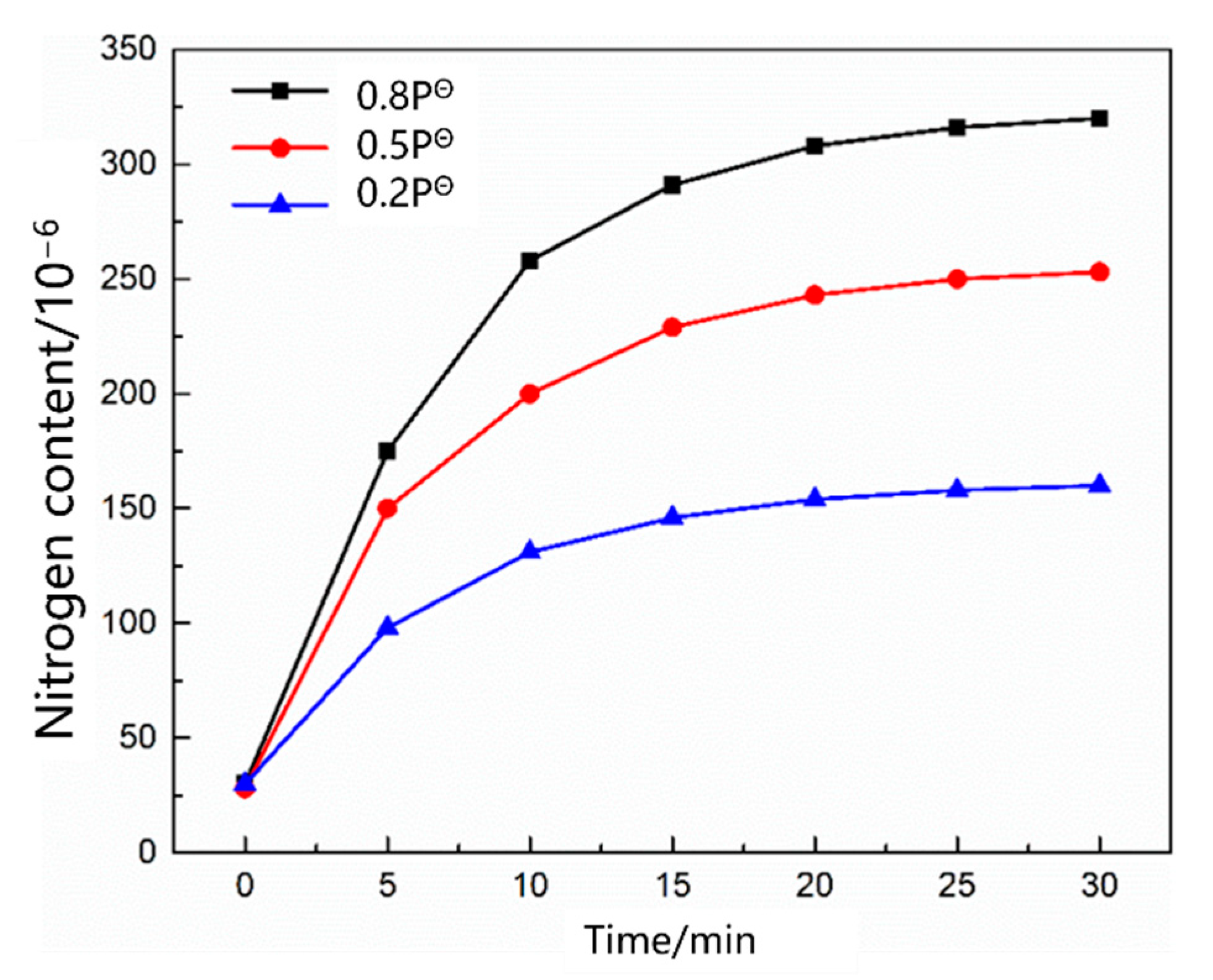
3.1. Thermodynamic and Kinetic Analyses of the Nitrogen Reaction in the Scrap Melting Stage
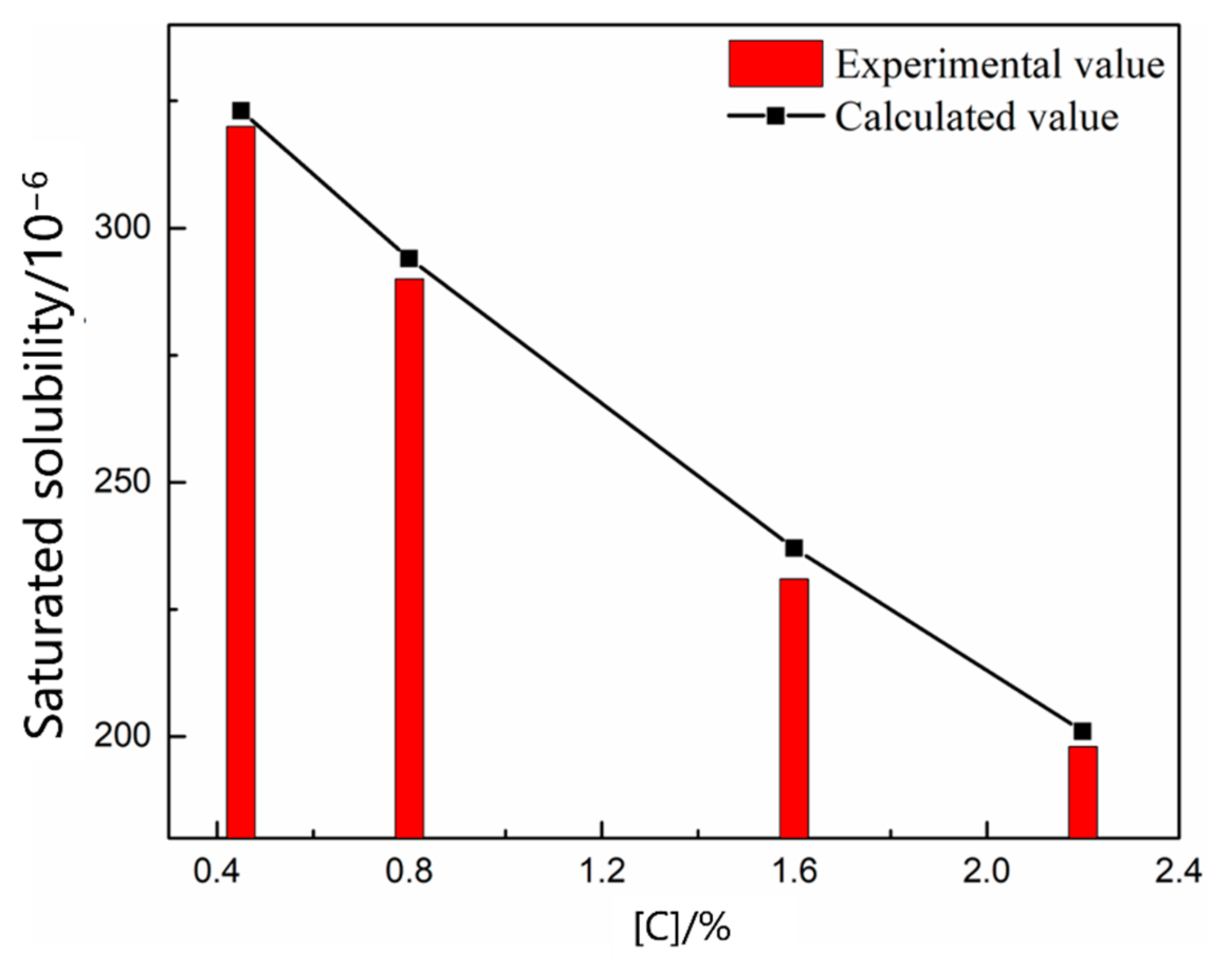
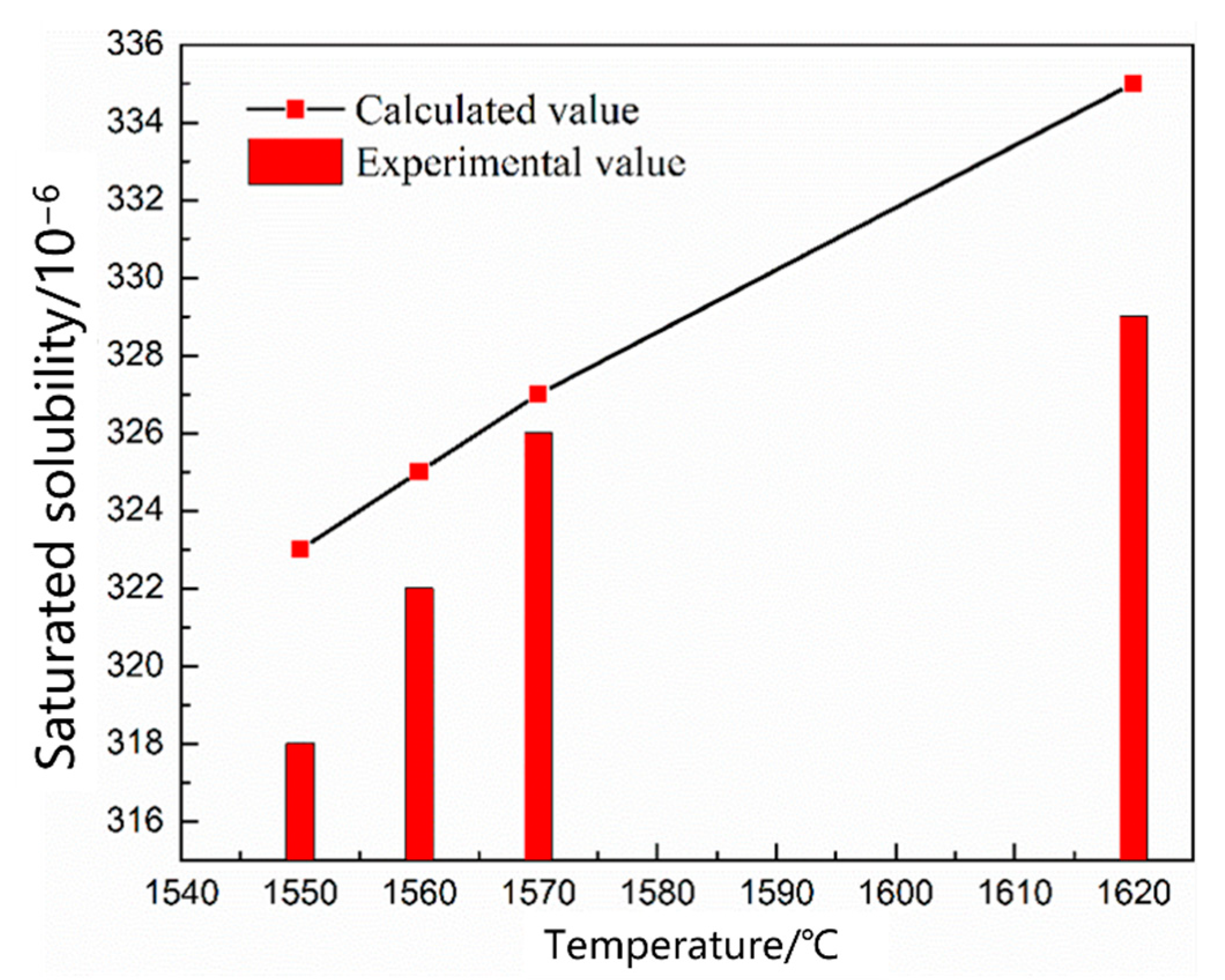
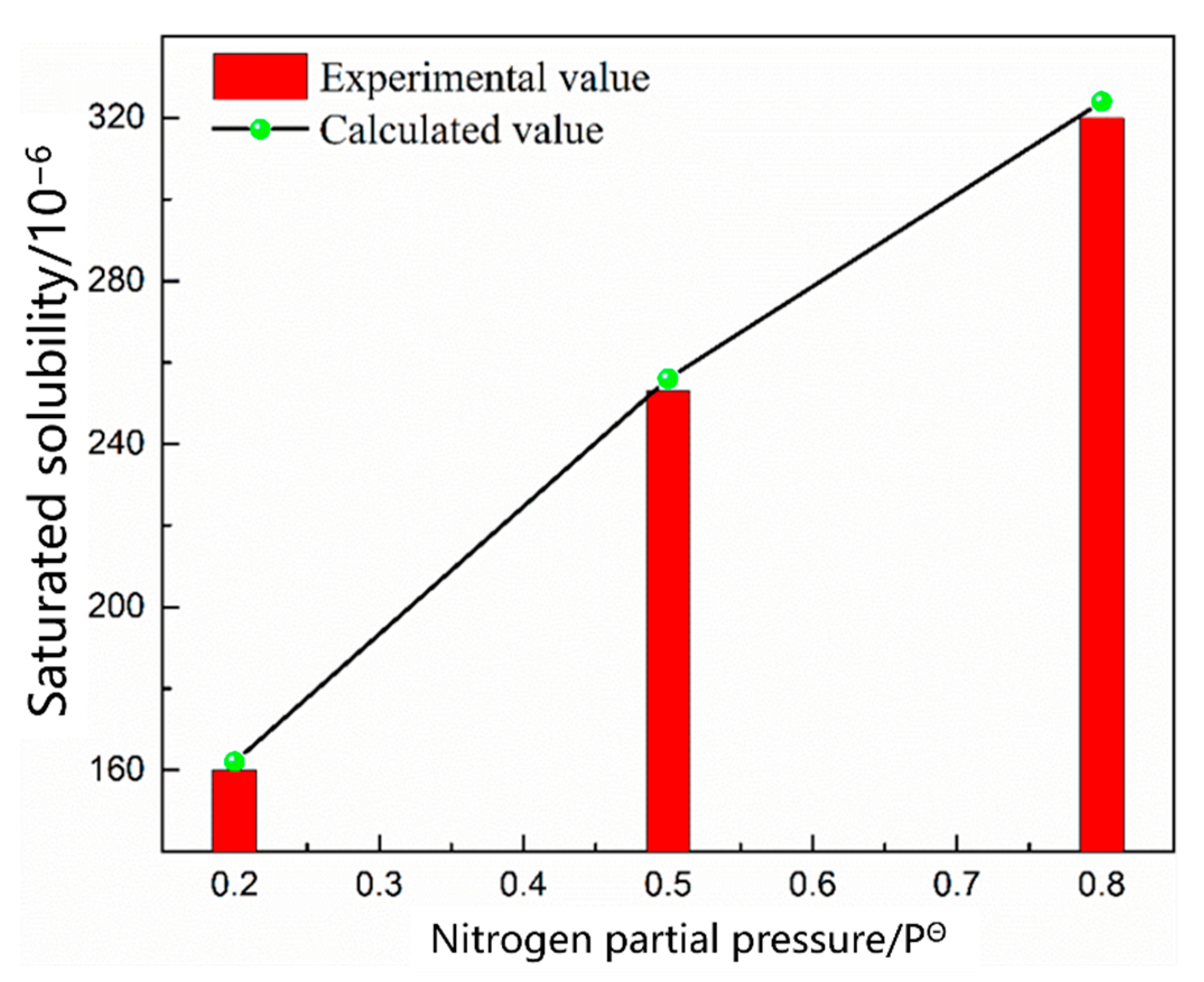
3.1.1. Thermodynamic Model of the Nitrogen Absorption Reaction during Scrap Melting
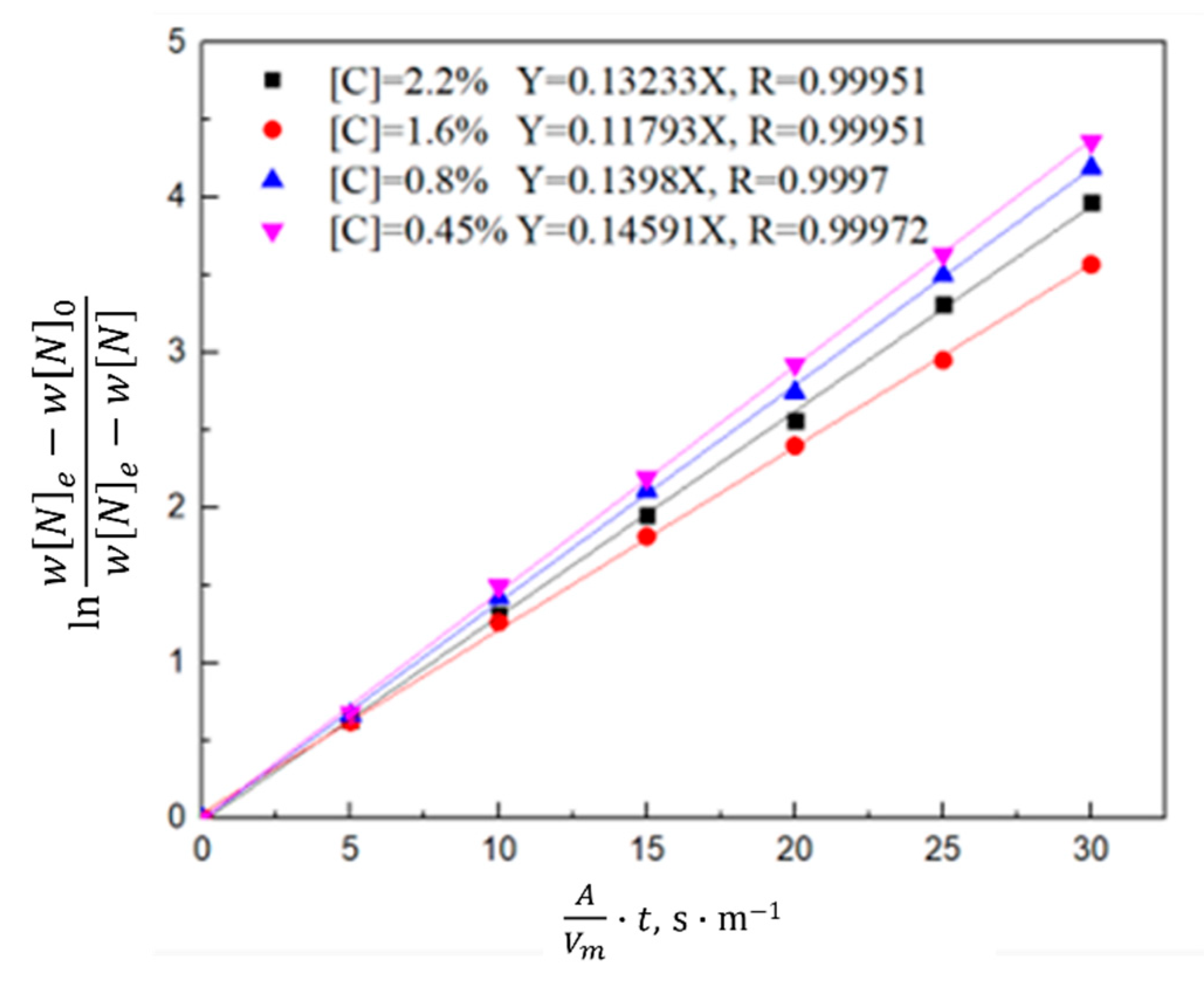
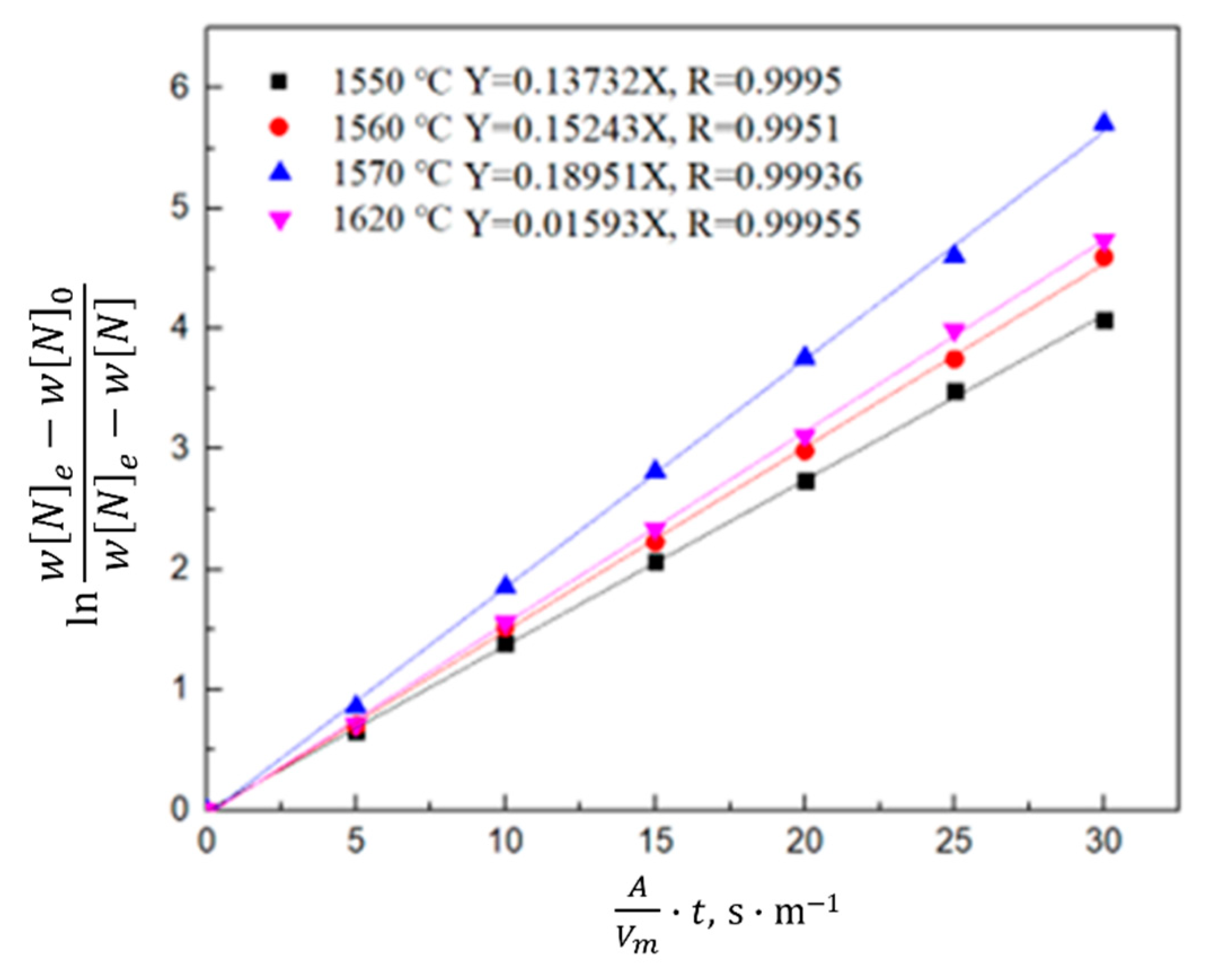
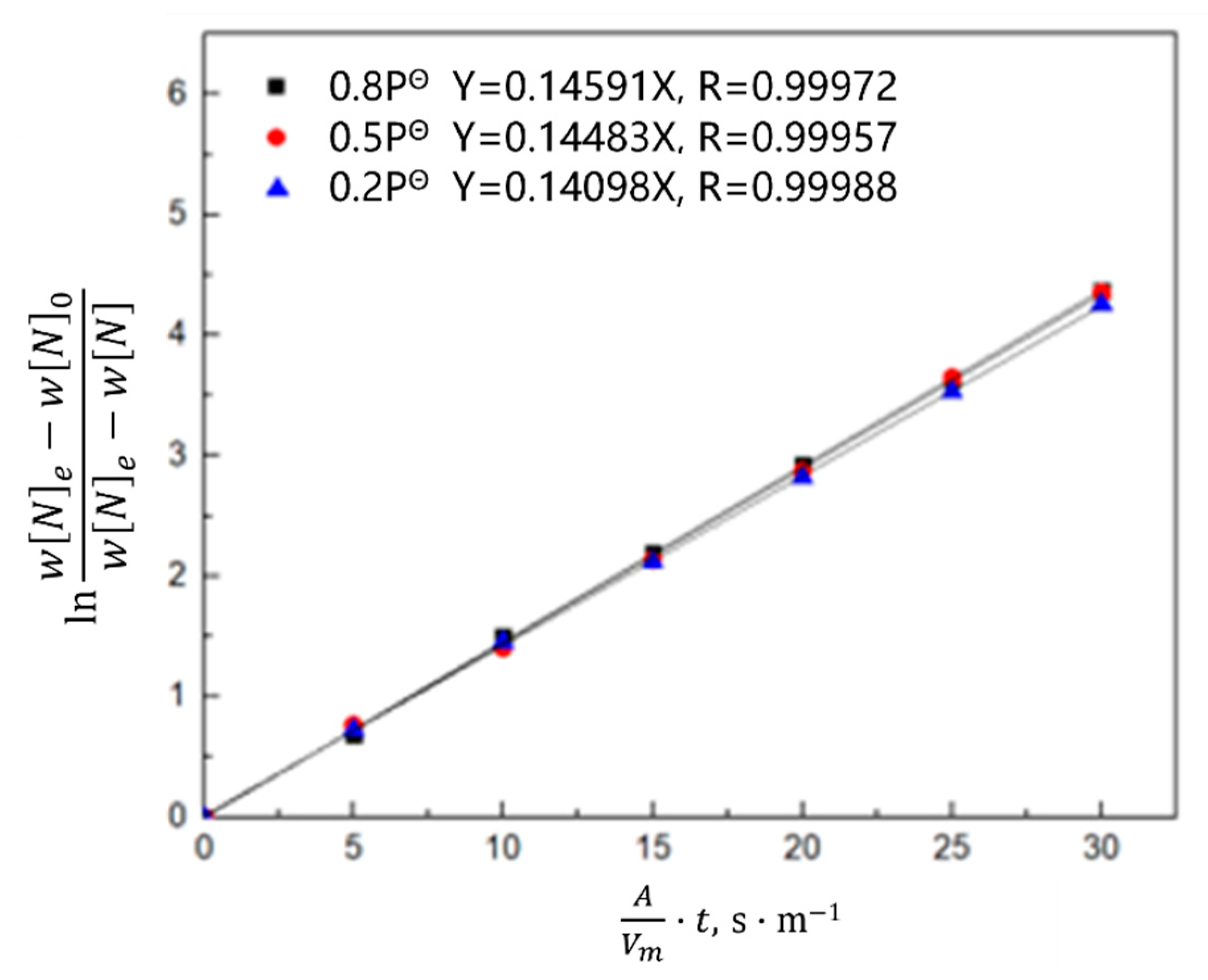
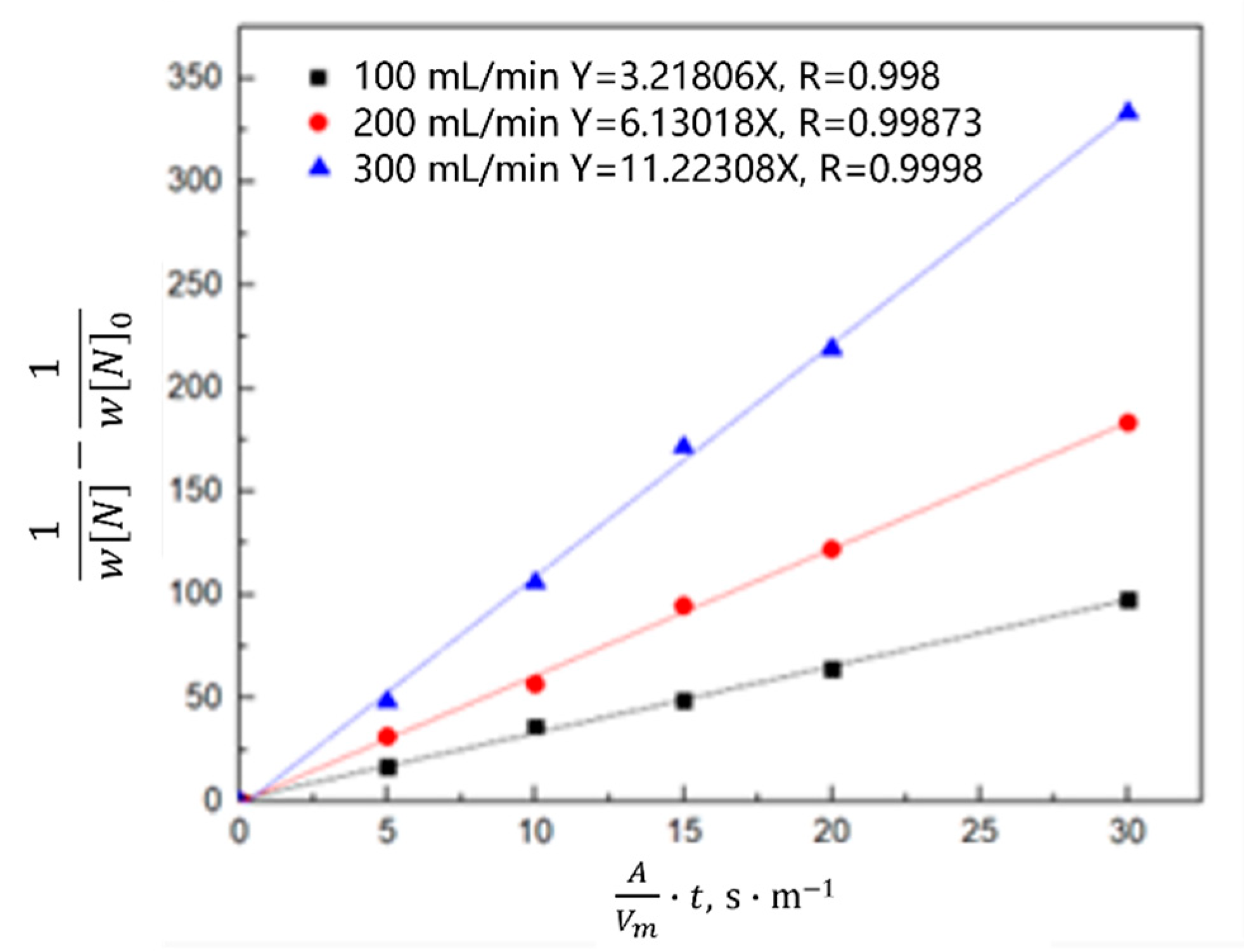
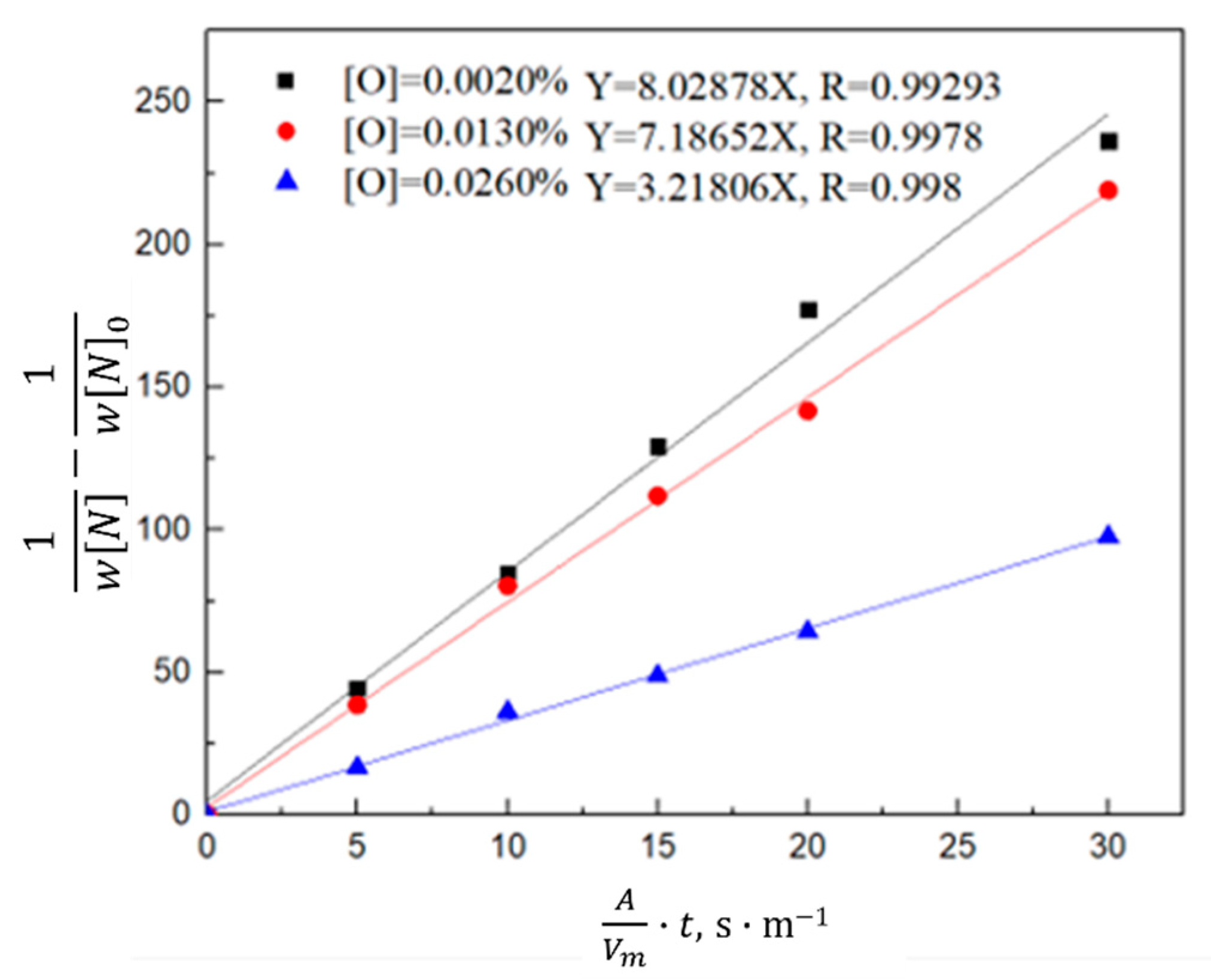
3.1.2. Kinetic Model of the Nitrogen Absorption Reaction during Scrap Melting
- Gas phase film mass transfer of nitrogen at the interface: . The expression for the correlation reaction rate V1 is shown in Equation (4).
- Chemical reaction at the gas–liquid interface: . The expression for the related reaction rate v2 is shown in Equation (5).
- Diffusion of nitrogen atoms in the molten steel: . The expression for the relevant reaction rate V3 is shown in Equation (6).
3.2. Thermodynamic and Kinetic Analyses of the Nitrogen Reaction in the Oxygen Blowing Decarbonization Stage
3.2.1. Thermodynamic Analysis of the Nitrogen Removal Reaction in the Oxygen Blowing Decarbonization Stage
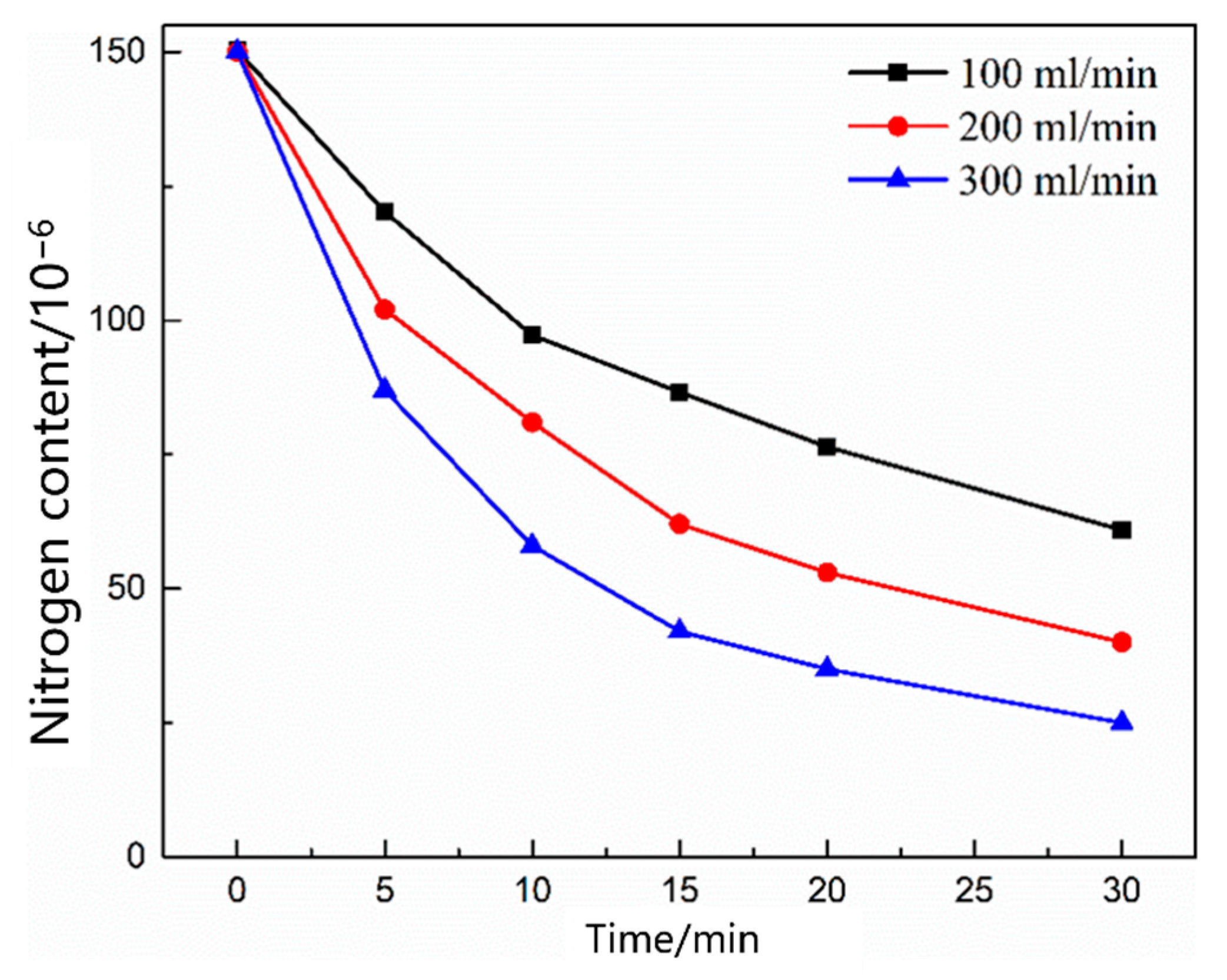
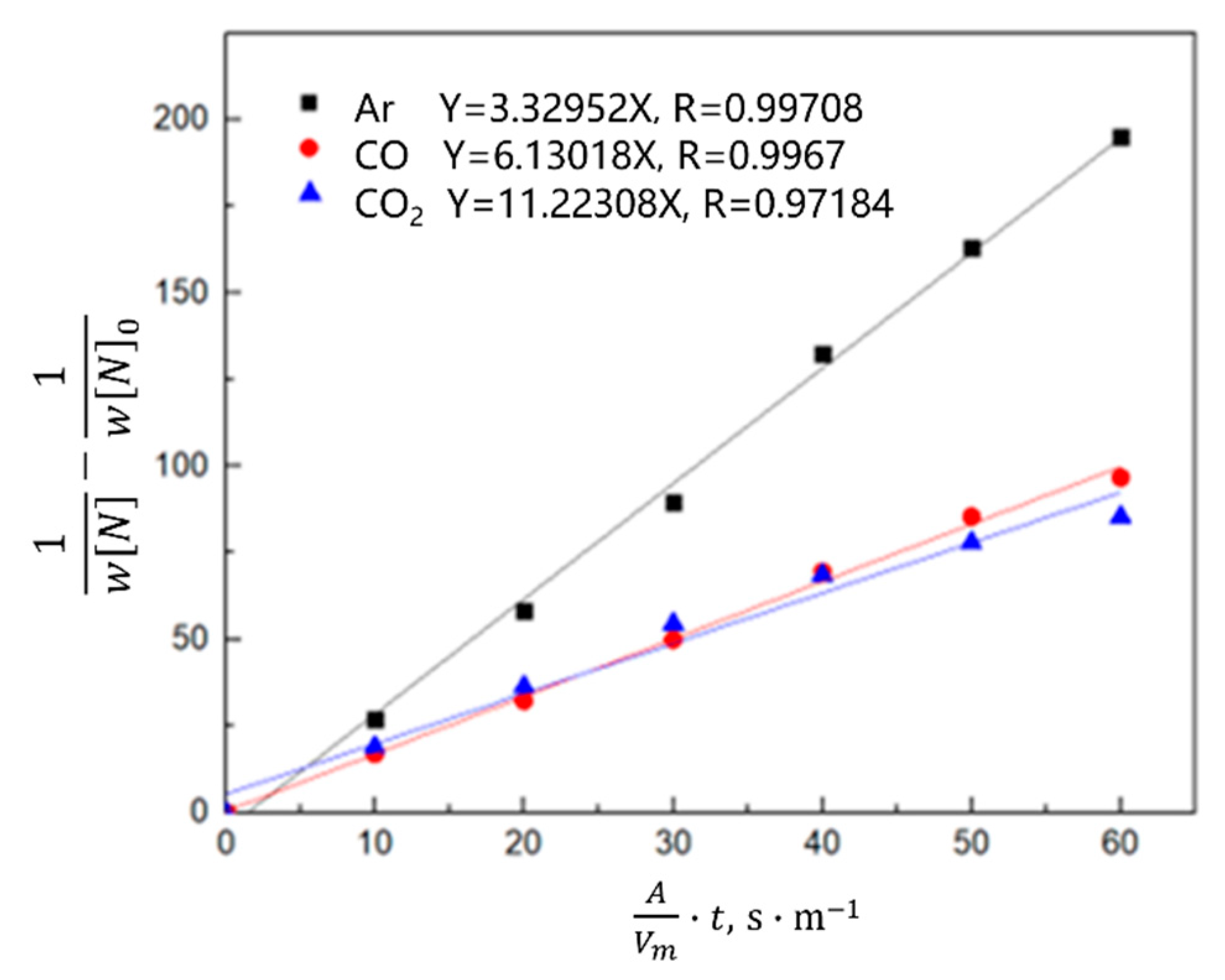
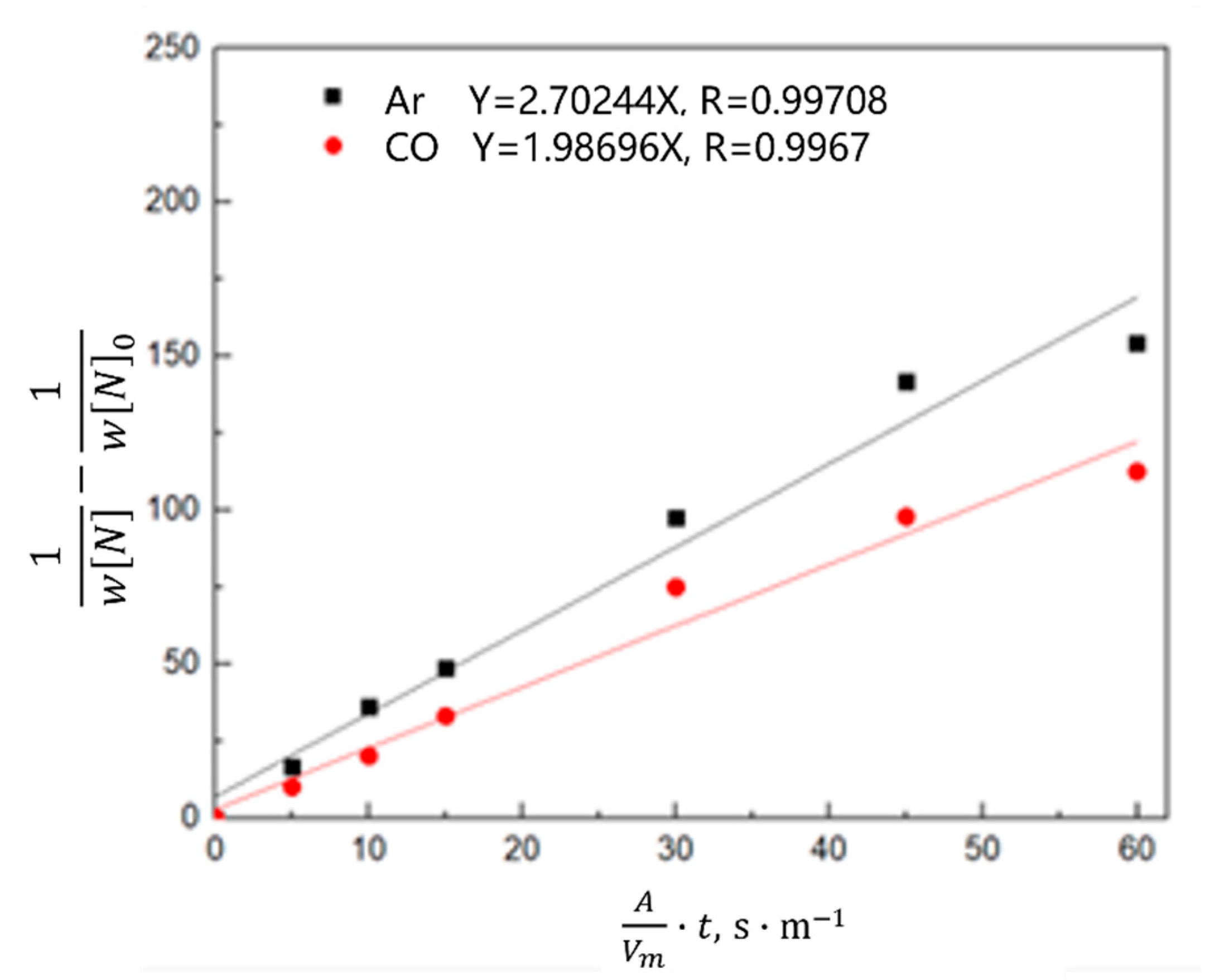
3.2.2. Kinetics of Denitrification in the Oxygen Blowing Decarburization Stage
3.3. Thermodynamic and Kinetic Analyses of the Nitrogen Reaction in the Rapid Heating Stage
3.3.1. Thermodynamic Analysis of the Nitrogen Reaction in the Heating Stage
3.3.2. Kinetic Analysis of the Nitrogen Reaction in the Heating Stage
3.4. Optimization of the Nitrogen Removal Process in EAF Smelting
- The nitrogen molecule N2 absorbs energy and becomes an activated molecule of an isomeric form, as shown in Equation (10).
- Active molecules dissolve into molten steel.
4. Conclusions
- (1)
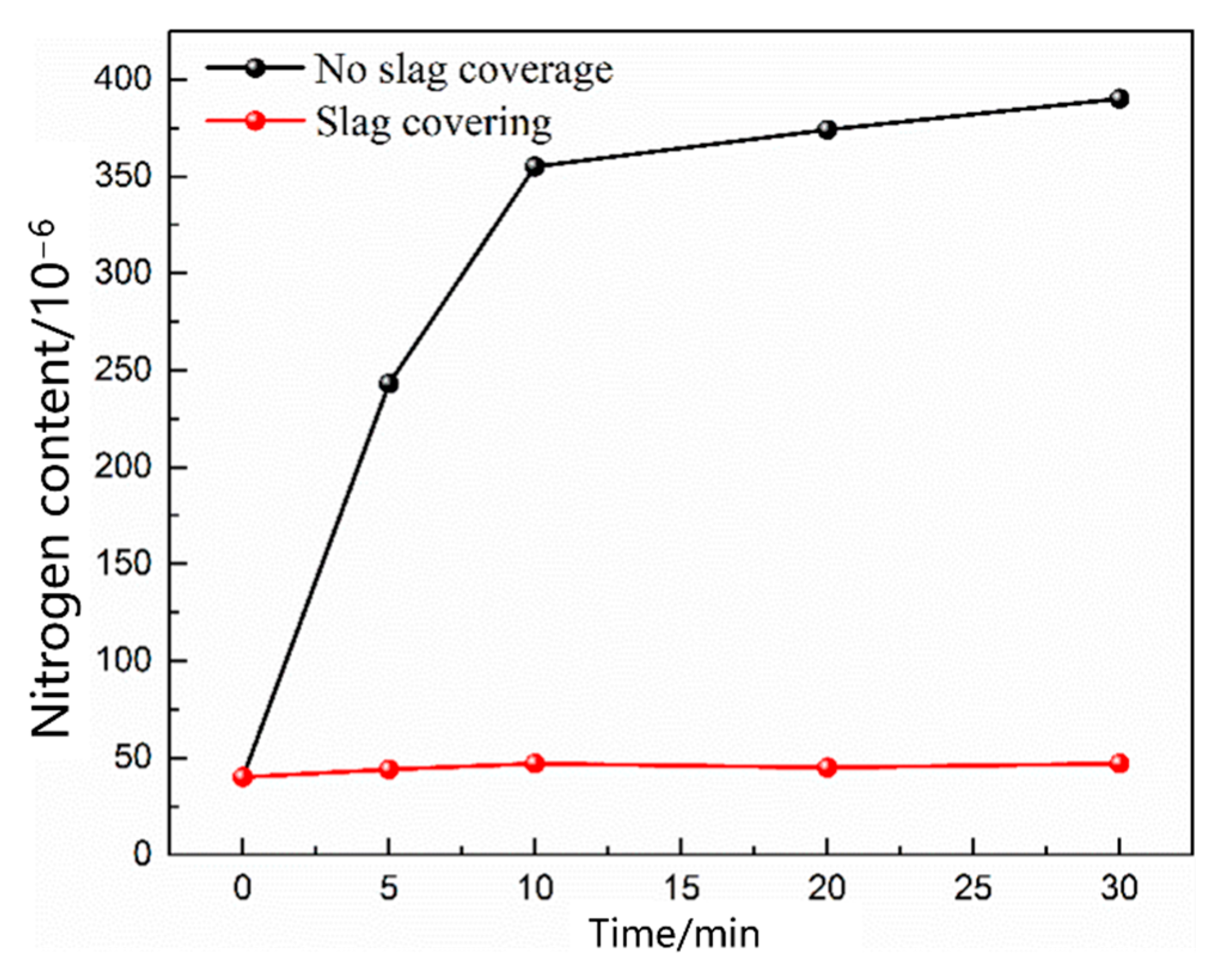
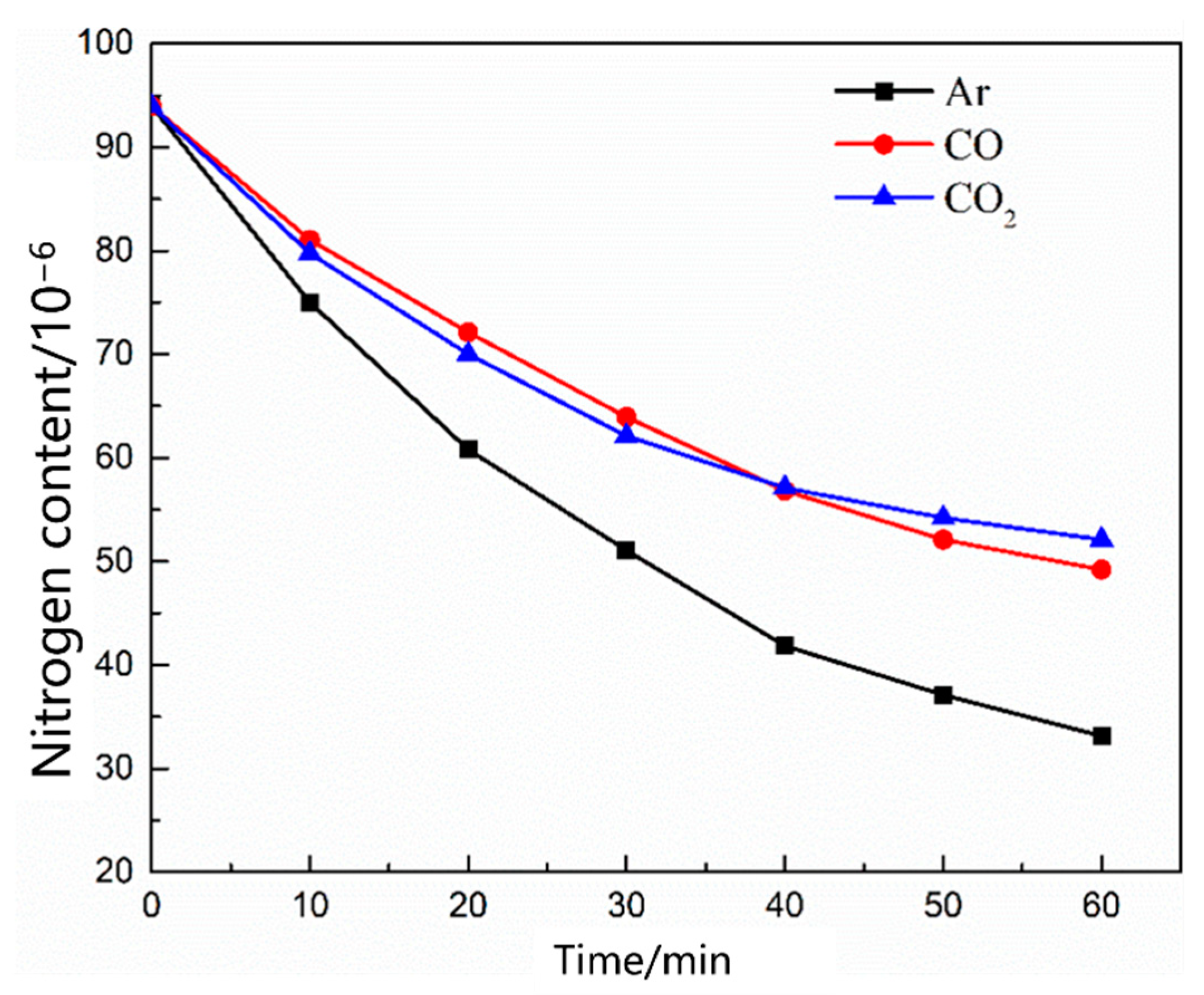
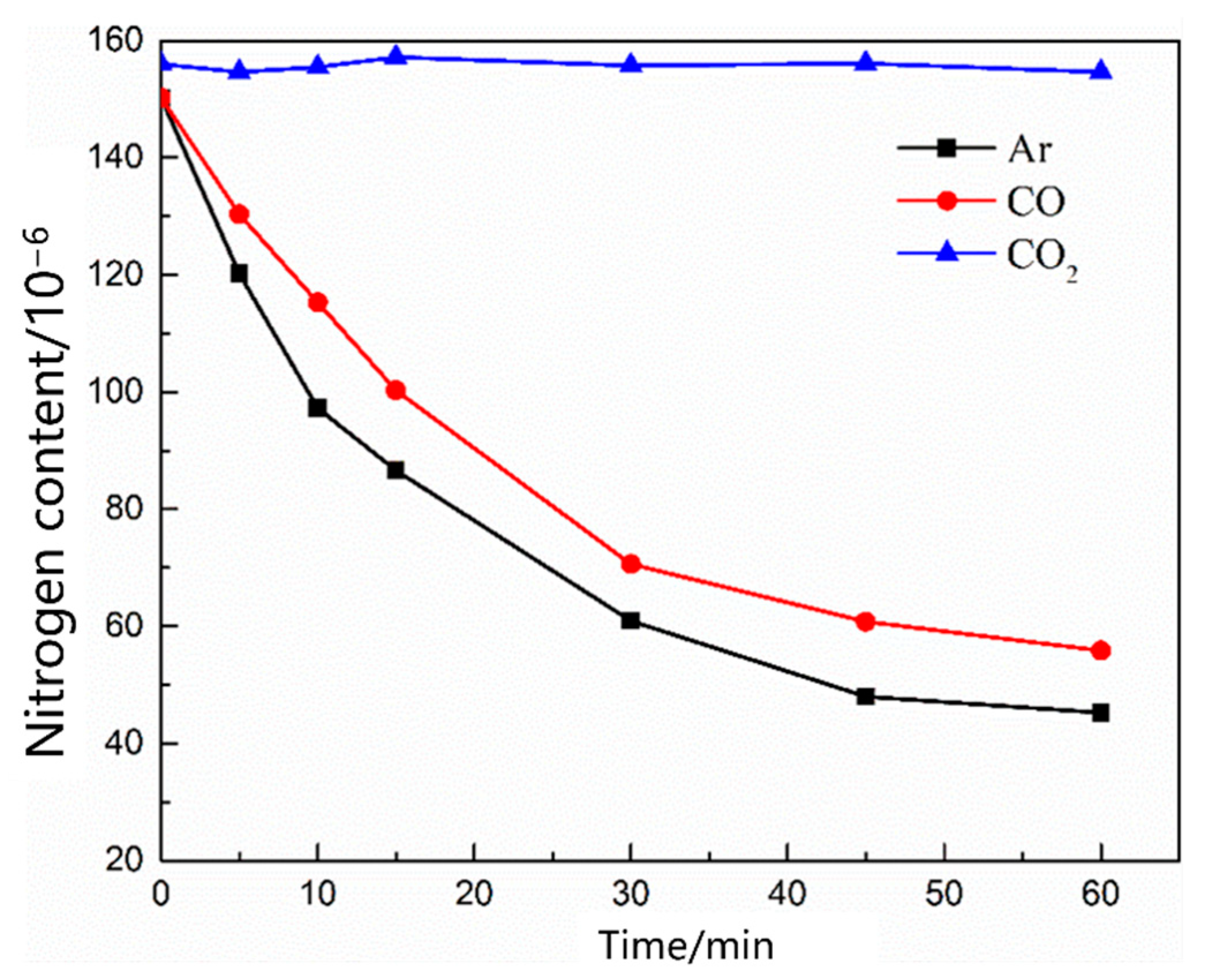
- During the scrap melting period, the nitrogen partial pressure in the upper part of the molten steel was high, and the upper part of the molten steel was not covered by liquid slag. The gas–liquid interface was in full contact, and the nitrogen molecules in the air were ionized by the arc heating zone to N2, N, N+, , N*, N−, and high-energy particles to improve the nitrogen reaction rate. The nitrogen content was close to saturation. The nitrogen absorption reaction is a first-order reaction, and the mass transfer of nitrogen atoms at the gas–liquid interface is a reaction-limiting step. Increasing the carbon content in the molten steel and reducing the temperature of the molten pool can reduce the solubility of nitrogen in the molten steel and reaction rate constant. Alternatively, advanced slagging, using the slag retention process with continuous feeding and isolating the nitrogen atmosphere on the steel surface, can effectively reduce the amount of nitrogen absorption.
- (2)
- During the oxygen blowing decarburization period, the nitrogen partial pressure in the upper part of the molten steel was low, and the liquid surface was well-covered with slag, which could effectively isolate the gas. The nitrogen reaction was characterized by denitrification. The denitrification reaction is a secondary reaction, and the chemical reaction at the gas–liquid interface is a limiting step. Controlling the oxygen content in molten steel and increasing gas generation can improve the denitrification reaction rate constant and nitrogen mass transfer coefficient in the molten steel.
- (3)
- During the heating stage, the oxygen content in the molten steel was high, which not only inhibited the gas–liquid interfacial reaction rate, but also reduced the mass transfer rate of nitrogen atoms in the molten steel. The limiting step of the denitrification reaction changed to the joint control by the nitrogen interfacial mass transfer and chemical reaction. When CO or CO2 was used as the denitrification gas, the gas partially dissolved into the molten steel, and the efficiency of the gas blowing denitrogenating at this stage was low.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fruenhan, R. Oxygen versus EAF steelmaking in the 21st century. Trans. Indian Inst. Met. 2006, 59, 607–617. [Google Scholar]
- Ma, G.; Zhu, R.; Dong, K. Development and application of electric arc furnace combined blowing technology. Ironmak. Steelmak. 2016, 43, 594–599. [Google Scholar] [CrossRef]
- Lee, B.; Sohn, I. Review of Innovative Energy Savings Technology for the Electric Arc Furnace. JOM 2014, 66, 1581–1594. [Google Scholar] [CrossRef]
- Marique, C.; Beyne, E.; Palmaers, A. Sources and control of nitrogen in oxygen steelmaking processes. Ironmak. Steelmak. 1988, 15, 38–42. [Google Scholar]
- Zhang, F.; Yang, S.; Li, J.; Liu, W.; Wang, T. Selection and key technologies of low-carbon steelmaking processes under the background of "Double Carbon". Chin. J. Eng. 2022, 44, 1483–1495. [Google Scholar]
- Harashima, K.; Mizoguchi, S.; Kajioka, H. Kinetics of Nitrogen Disorption from Low Nitrogen Liquid Iron by Blowing Ar and Reducing Gas Mixture or by Top Injection of Iron Ore Powder under Reduced Pressure. Tetsu-to-Hagané 2009, 74, 441–448. [Google Scholar] [CrossRef]
- Neuschütz, D.; Spirin, D. Nitrogen Removal and Arc Voltage Increase in EAF Steelmaking by Methane Injection into the Arc. Steel Res. Int. 2003, 74, 19–25. [Google Scholar] [CrossRef]
- Pal, J. Thermodynamic analysis of nitrogen removal in EAF by DRI fines injection. Ironmak. Steelmak. 2006, 33, 465–470. [Google Scholar] [CrossRef]
- Yi, C.; Zhu, R.; Chen, B.Y.; Wang, C.R.; Ke, J.X. Experimental Research on Reducing the Dust of BOF in CO2 and O2 Mixed Blowing Steelmaking Process. ISIJ Int. 2009, 49, 1694–1699. [Google Scholar] [CrossRef]
- Lv, M.; Zhu, R.; Wei, X.; Wang, H.; Bi, X. Research on Top and Bottom Mixed Blowing CO2 in Converter Steelmaking Process. Steel Res. Int. 2012, 83, 11–15. [Google Scholar] [CrossRef]
- Wang, H.; Yu, H.; Teng, L.; Seetharaman, S. Evaluation on material and heat balance of EAF processes with introduction of CO2. J. Min. Metall. Sect. B Metall. 2016, 52, 01–08. [Google Scholar] [CrossRef]
- Kawakami, M.; Ito, K.; Okuyama, M. Absorption and Desorption Rates of Nitrogen in Molten Iron by Bottom Injection of Gases. Tetsu-to-Hagané 2009, 73, 661–668. [Google Scholar] [CrossRef] [PubMed][Green Version]
- He, P.; Zhang, R.; Deng, K. Study on the Kinetics of Decarburization of Fe-C Melt by CO2. J. Iron Steel Res. 1988, 8, 107–113. [Google Scholar]
- Li, Z.; Zhu, R.; Liu, R.; Wang, X. Comparison of Smelting Effects by Bottom Blowing Different Gases. Iron Steel 2016, 51, 40–45. [Google Scholar]
- Wei, G.; Zhu, R.; Dong, K. Influence of bottom-blowing gas species on the nitrogen content in molten steel during the EAF steelmaking process. Ironmak. Steelmak. 2018, 45, 839–846. [Google Scholar] [CrossRef]
- Shalimov, A.G.; Nemenov, A.M. Production of electric steel with reduced nitrogen content. Metallurgist 2010, 54, 210–223. [Google Scholar] [CrossRef]
- Ji, H.; Ding, K.; Liu, K. Study on Controland Change of Nitrogen in the Process of Low Nitrogen Stainless Production. Ind. Heat. 2022, 51, 6–8. [Google Scholar]
- Tang, D.; Pistorius, P.C. Kinetics of Nitrogen Removal from Liquid Third Generation Advanced High-Strength Steel by Tank Degassing. Metall. Mater. Trans. B 2022, 53, 1383–1395. [Google Scholar] [CrossRef]
- Zhang, D.; Qiu, G.; Niu, B.; Jiang, Z.; Dong, T.; Yang, F.; Hu, Y.; Zhang, H. Thermodynamics and kinetics research of nitrogen dissolution in steel. Steelmaking 2015, 31, 7–11. [Google Scholar]
- Ishii, F.; Ban-ya, S.; Fuwa, T. Solubility of nitrogen in liquid iron and iron alloys containing the group VI a elements. Tetsu-to-Hagané 1982, 68, 946–955. [Google Scholar] [CrossRef]
- Inomoto, T.; Kitamura, S.; Yano, M. Kinetic Study of the Nitrogen Removal Rate from Molten Steel (Normal Steel and 17 mass% Cr Steel) under CO Boiling or Argon Gas Injection. Trans. Iron Steel Inst. Jpn. 2015, 55, 1822–1827. [Google Scholar] [CrossRef]
- Ito, K.; Amano, K.; Sakao, H. Kinetic Study on Nitrogen Absorption and Desorption of Molten Iron. Trans. Iron Steel Inst. Jpn. 2006, 28, 41–48. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, R.; Wang, H. Reaction mechanism of CO/CO2 with low-carbon aluminium-killed molten steel during the ladle furnace (LF) refining process. Ironmak. Steelmak. 2022, 49, 147–159. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, R.; Li, Z. Effect of smelting temperature and CO2 gas flow rate on decarburization kinetics between CO2 gas and liquid Fe-C alloy. Ironmak. Steelmak. 2021, 48, 852–859. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, R.; Li, Z. CO2 conversion and decarburization kinetics of CO2 gas and liquid Fe–C alloy at 1873 K. J. Iron Steel Res. Int. 2022, 29, 425–433. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, R.; Wei, G. Study on Final Equilibrium State and Process of CO2 Reacting with Fe–C Melt. Metall. Mater. Trans. B 2022, 53, 1396–1410. [Google Scholar] [CrossRef]
- Fu, J. Theory and Application of Modern Electric Furnace Steelmaking, 1st ed.; Metallurgical Industry Press: Beijing, China, 2008; pp. 135–137. [Google Scholar]
- Guo, H. Metallurgical Physical Chemistry, 2nd ed.; Metallurgical Industry Press: Beijing, China, 2006; pp. 154–159. [Google Scholar]

| Element | C | Si | Mn | S | N |
|---|---|---|---|---|---|
| Content/wt.% | 0.45 | 0.2 | 0.75 | 0.03 | 0.0045 |
| NO. | Carbon Content/% | Temperature/°C | Nitrogen Partial Pressure/PΘ |
|---|---|---|---|
| 1 | 0.45 | 1550 | 0.8 |
| 2 | 0.8 | ||
| 3 | 1.6 | ||
| 4 | 2.2 | ||
| 5 | 0.45 | 1550 | 0.8 |
| 6 | 1560 | ||
| 7 | 1570 | ||
| 8 | 1620 | ||
| 9 | 0.45 | 1550 | 0.2 |
| 10 | 0.5 | ||
| 11 | 0.8 |
| NO. | Gas Flow/mL/min | Gas Type | Slag |
|---|---|---|---|
| 1 | 100 | Ar | -- |
| 2 | 200 | ||
| 3 | 300 | ||
| 4 | 100 | Ar | -- |
| 5 | 100 | CO | |
| 6 | CO2 | ||
| 7 | N2 | Cover |
| NO. | Gas Type | Oxygen Content/% |
|---|---|---|
| 1 | Ar | 0.002 |
| 2 | 0.013 | |
| 3 | 0.026 | |
| 4 | Ar | 0.026 |
| 5 | CO | |
| 6 | CO2 |
| i | N | C | Si | Mn | S | N |
|---|---|---|---|---|---|---|
| 0.048 | 0.118 | 0.043 | −0.024 | 0.007 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Li, J.; Liu, W.; Jiao, A. The Thermodynamics and Kinetics of a Nitrogen Reaction in an Electric Arc Furnace Smelting Process. Materials 2023, 16, 33. https://doi.org/10.3390/ma16010033
Zhang F, Li J, Liu W, Jiao A. The Thermodynamics and Kinetics of a Nitrogen Reaction in an Electric Arc Furnace Smelting Process. Materials. 2023; 16(1):33. https://doi.org/10.3390/ma16010033
Chicago/Turabian StyleZhang, Fujun, Jingshe Li, Wei Liu, and Aoteng Jiao. 2023. "The Thermodynamics and Kinetics of a Nitrogen Reaction in an Electric Arc Furnace Smelting Process" Materials 16, no. 1: 33. https://doi.org/10.3390/ma16010033
APA StyleZhang, F., Li, J., Liu, W., & Jiao, A. (2023). The Thermodynamics and Kinetics of a Nitrogen Reaction in an Electric Arc Furnace Smelting Process. Materials, 16(1), 33. https://doi.org/10.3390/ma16010033






