Abstract
In this work, Cu2WS4 nanoparticles have been synthesized via a solvothermal decomposition approach using a heterobimetallic single source precursor, WCu2S4(PPh3)3. The single source precursor, WCu2S4(PPh3)3, has been characterized using multinuclear NMR spectroscopy, while Cu2WS4 nanoparticles have been characterized by powder X-ray diffraction (PXRD) for which Rietveld refinement has been performed to authenticate the lattice structure of the decomposed product, Cu2WS4. Furthermore, FESEM and EDAX analyses have been performed to assess the morphology and composition of Cu2WS4. An electrochemical study in acidic as well as basic media suggested that Cu2WS4 nanoparticles possess efficient bifunctional activity towards electrochemical hydrogen as well as oxygen evolution reactions. Linear sweep voltammetry (LSV) performed in 0.5 N H2SO4 indicates an onset potential for the HER of 462 mV and a Tafel slope of 140 mV dec−1. While LSV performed in 0.1 M KOH indicates an onset potential for the OER of 190 mV and a Tafel Slope of 117 mV dec−1.
1. Introduction
Electrocatalytic water splitting reactions have always been the center of attention amongst researchers as they ensure a green and sustainable approach to mitigating energy shortages, without adverse environmental impacts resulting from the unrestrained use of fossil fuels [1,2,3]. The total water splitting reaction results from the addition of half-cell reactions at the cathode (the hydrogen evolution reaction, or HER) and the anode (the oxygen evolution reaction, or OER). The HER involves a two-electron transfer and is associated with low overpotential and very high efficiency [4,5]. However, the overall rate of water splitting is limited by the OER, owing to its slow kinetics caused by the four-electron transfer involved in O–H bond cleavage and oxygen–oxygen bond formation occurring at a high overpotential [4,6,7,8,9,10,11]. Thus, there is an escalating demand for exploiting state-of-the-art electrocatalysts in order to alleviate the overpotentials associated with HERs as well as OERs [12]. Currently, Pt/C electrocatalysts are reported to be the best HER electrocatalyst [13,14], and RuO2 and IrO2 represent the most efficient electrocatalysts for OERs [6,7,15]. However, the commercial viability of these precious metal-based electrocatalysts is restricted due to their high price and scarcity. Therefore, significant efforts have been devoted to developing cost-effective transition metal-based catalysts viz. dichalogenide [16,17], phosphide [18,19], nitride [20] and carbide [21] which display superior HER activity. Phosphides [22,23,24], sulfides [25] and oxides/hydroxides [26,27,28,29] have exhibited exceptional OER activities. Commonly, most of the noble metal-free HER catalysts show activity in acidic media [30,31], while OER catalysts operate in alkaline media [2,32]. Thus, it is desirable to design a bifunctional electrocatalyst capable of catalyzing both HERs and OERs in both media to reduce the manufacturing cost of novel water splitting electrocatalysts and decrease the possibility of cross-contamination during long cycles [33]. In this context, multicomponent transition metal-based catalysts are thought to be the perfect candidate because of their variable and high redox states and better conducting properties compared to their single component counterparts [34,35]. For this reason, utilization of ternary transition metal chalcogenides (LTMCs) with layered frameworks as bifunctional electrocatalysts for overall splitting of water has emerged as a promising solution. However, the studies on this class of materials are relatively few, due to the difficulties encountered in their syntheses and thus in obtain high-quality materials [36].
In view of this, layered ternary copper tungsten sulfide (Cu2WS4), the first member of the LTMC class [37], possesses high electrical conductivity and a wide band gap, thereby making it suitable for electrocatalytic as well as photocatalytic hydrogen evolution reactions, visible light-assisted photocatalysis, electrochemical energy storage devices and biological applications [38,39,40,41,42,43,44,45,46,47,48,49]. Wu and co-workers have reported that single-layer Cu2WS4 exhibits efficient two-dimensional electrocatalytic activity towards HER [38]. Jing et al. have reported the photocatalytic HER activity of decahedral copper tungsten sulfide [39]. Ozel and colleagues demonstrated that Cu2WS4 nanocubes can efficiently catalyze a HER with the organic reducing agent decamethylferrocene (DMFc) in a water/1,2-dichloroethane (DCE) biphasic system [40]. For photocatalytic HER, Aslan et al. employed a system consisting of a Cu2WS4 catalyst and donor; the bridge-acceptor organic dye-sensitized TiO2 [41]. Additionally, Patir’s group have reported the use of Cu2WS4 as a co-catalyst for donor-π-acceptor dye-sensitized photoelectrochemical and photocatalytic HERs [42].
Despite several reports on the HER activity of Cu2WS4-based materials, there are few studies demonstrating their potential in OERs. For instance, Novak et al. reported that although pristine Cu2WS4 displays almost negligible activity towards OER, P-doped Cu2WS4 showed significantly improved OER performance [43]. In addition, recently, Sharma and co-workers have explored the use of porous Cu2WS4 as a negative electrode in supercapacitors [44]. Pazhamalai et al. have reported Cu2WS4 anchored on Ni foam to be a highly active negative asymmetrical supercapacitor electrode [45]. Furthermore, Gulen and co-workers employed Cu2WS4 nanocube inks as the catalyst at the counter electrode (CE) to act as a substitute for more expensive Pt-based CEs, to fabricate a cheap/high efficiency dye-sensitized solar cell (DSSC) [46]. In addition, Jia et al. reported that Cu2WS4 crystallites exhibited high efficiency towards the visible-light driven photocatalytic reduction of aqueous Cr(VI) [47]. Recently, Kannan et al. reported the excellent antibacterial activity of Cu2WS4 against harmful bacteria that cause skin cancer [48]. Furthermore, Shan et al. also demonstrated that Cu2WS4 nanocrystals can be potential antibacterial agents owing to their excellent antibacterial activity and biocompatibility [49]. New, cost-effective electrode materials for accelerating OERs are a target of research; therefore, the application of Cu2MoS4 and its composites as electrode materials to catalyze the OER [50], and the use of the Ni dithiolate anion and its composites with 2D materials as electrode materials for accelerating OER have both been reported [51].
Previous reports have indicated that Cu2WS4 and its derivatives/composites possess exceptional catalytic activity towards HERs, but its application towards OERs have been sporadically reported. This inspired us to explore its potential as a bi-functional catalyst to drive HERs in acidic media and OERs in alkaline media. In view of these noteworthy aspects associated with Cu2WS4, herein we describe the synthesis of Cu2WS4 (CWS), obtained via solvothermal decomposition of a heterobimetallic single source precursor, WCu2S4(PPh3)3 (SSP) [52]. Additionally, the characteristics of CWS as an electrocatalyst for HERs and OERs are described. To the best of our knowledge, this is the first report on the synthesis of Cu2WS4 using a single source precursor approach and the application of this material as a bifunctional catalyst for OERs and HERs. The pertinent outcomes of this entire investigation are presented herewith.
2. Results and Discussion
2.1. Synthesis of WCu2S4(PPh3)3 (SSP)
The precursor, WCu2S4(PPh3)3 (SSP) [50], was prepared by development of a new synthetic pathway by reacting diammonium tetrathiotungstate, (NH4)2WS4, with copper triphenylphosphine nitrate, Cu(PPh3)2NO3, in a stoichiometric ratio of 1:2 in a methanol–dichloromethane mixture (Scheme 1). The obtained SSP was air-stable and moisture-stable, and its purity and composition were assessed with the help of NMR spectroscopy.
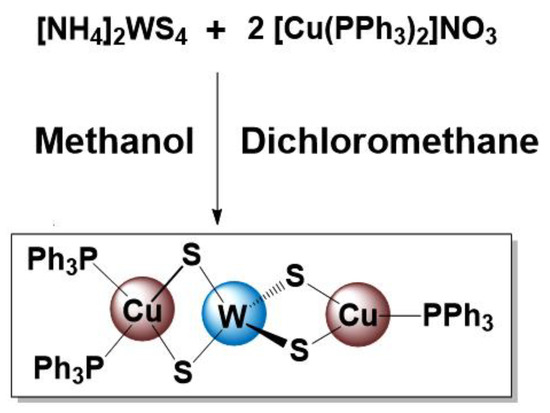
Scheme 1.
Synthetic routes for the preparation of SSP.
2.2. Spectroscopy
The 1H NMR spectrum of SSP displayed a broad multiplet ranging between δ 7.27–7.47 arising due to the aromatic ring protons of the triphenylphosphine ligands. In addition, the aromatic carbons of the triphenylphosphine ligands displayed signals in the range of δ 128.7–134.2 in the 13C NMR spectrum of SSP. The 31P{1H} NMR spectrum displays only one signal at δ 8.67.
2.3. PXRD and SEM
The phase formation and structure analysis of Cu2WS4 were carried out using powder X-ray diffraction (PXRD) data. The data were collected in 2θ range between 10 and 80° with a step size 0.02. The XRD pattern reveals formation of single-phase Cu2WS4 and the systematic absences in the diffraction pattern are consistent with the body-centered phase of Cu2WS4. For a detailed structural analysis, the Rietveld refinement of PXRD data was carried out using the tetragonal symmetry in the I2 m space group. Results of the Rietveld refinement of PXRD data of Cu2WS4 are shown in Figure 1. The fit between the observed and calculated diffraction profile is good, with an almost flat difference profile in the given 2θ range. The lattice parameters of Cu2WS4 obtained after the Rietveld analysis of PXRD were a = 5.4372(6) Å and c = 10.120(2) Å.
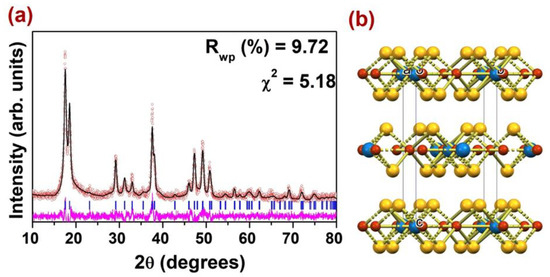
Figure 1.
(a) The PXRD plot for Cu2WS4; (b) Unit cell view of Cu2WS4.
Furthermore, SEM analysis revealed that the obtained Cu2WS4 possess nearly evenly distributed globule-like morphology with individual dimensions of globules ranging between ca. 25 and 30 nm (Figure 2). Additionally, EDAX analysis revealed that the composition of the materials was in agreement with the composition of Cu2WS4 (Figure S8).
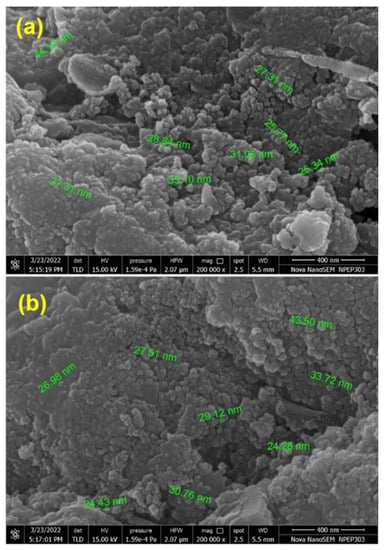
Figure 2.
FESEM images for Cu2WS4 in different region.
2.4. Electrochemical Study
To study the electrocatalytic behaviour of Cu2WS4 towards electrochemical water splitting, electrochemical measurements were performed in various media such as 0.1 M H2SO4, 0.5 M H2SO4 and 0.1 M KOH solution.
The LSV profile of Cu2WS4 in acidic media suggested that it exhibits moderate activity towards HER with an onset potential of 462 mV and a Tafel slope of 140 mV dec−1 (Figure 3).
H3O+ + e− + M → M-Hads + H2O
M-Hads + M-Hads → H2
M-Hads + H + e− → H2
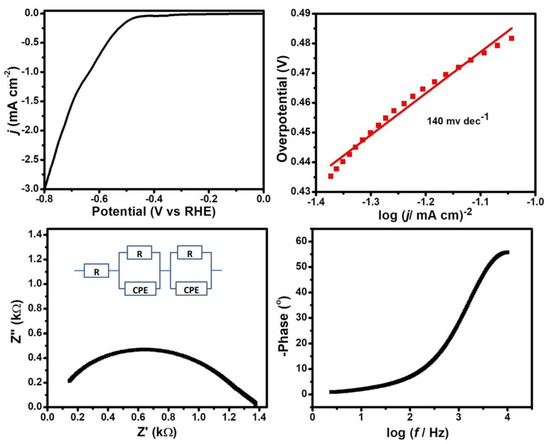
Figure 3.
(Top) LSV plot at 1 mv sec−1 scan rate and corresponding Tafel plot and (bottom) Nyquist (with equivalent circuit diagram) and Bode plots from the EIS study of Cu2WS4 in 0.5 M H2SO4 solution.
The Tafel slope is an important criterion to predict the HER mechanism which may proceed via the Volmer–Heyrovsky or the Tafel mechanism based on two possible desorption steps involved in acidic aqueous medium. Usually, a fast discharge reaction (Equation (1)) followed by a rate limiting combination reaction (Equation (2)) leads to a Tafel slope of ~30 mV dec−1. When Step 1 is fast and followed by a slow electrochemical desorption reaction, Step 3, a Tafel slope of ~40 mV dec−1 is obtained. If Equation (1) is rate-limiting or the surface coverage is close to one, the Tafel slope is ~120 mV dec−1. A high Tafel slope (>~120 mV dec−1) value indicates a Tafel mechanism for HER.
The LSV study at different concentrations of H2SO4 suggests there is no change in onset potential (potential where tangents corresponding to faradaic and non-faradaic zones intersect). However, the current density increases at higher H2SO4 concentrations, which is consistent with the first step as the rate limiting condition (Figure 3, Figures S1 and S2). The LSV profiles at different scan rates for both concentrations of H2SO4 indicate that current density differs with scan rate, but is not proportional to change in scan rate. Furthermore, the literature reports suggest that the HER mechanism is controlled by the Volmer, Heyrovsky or Tafel mechanisms if the Tafel slope is close to 120, 40 or 30 mV dec−1, respectively [53,54,55,56]. Hence, the Tafel slope value of 140 mV dec−1 indicates that the Tafel step is rate-controlling for HER reactions catalyzed by Cu2WS4. Furthermore, the impedance study suggests that the charge transfer resistance (Rct) is also dependent on the concentration of H2SO4 and is of the order of kiloohms (1.2 kΩ in 0.5 M H2SO4 and 7.1 in 0.1 M H2SO4 (Figure 3, Figures S3 and S4).
In addition to determining the possibility of the application of Cu2WS4 as an electrocatalyst for HERs, electrochemical measurements were also performed in 0.1 M KOH to check the activity of Cu2WS4 towards the electrochemical oxygen evolution reaction. The LSV profile of Cu2WS4 in alkaline medium suggested that the material exhibited activity towards the oxygen evolution reaction with an onset potential 190 mV and a Tafel slope of 117 mV dec−1 (Figure 4). Additionally, the impedance study suggests that the charge transfer resistance, Rct, is 700 kΩ. The magnitudes of overpotential, the Tafel slope and the Rct suggested that Butler–Volmer kinetics apply to this reaction. As reported earlier, the OER is expected to proceed on the catalyst surface by electrochemically produced metal cations as the active sites via the following steps:
M + OH− → M * − OH + e−
M * − OH + OH− → M * − O + H2O + e−
M * − O + OH− → M * − OOH + e−
M * − OOH + OH− → M * − OO + H2O + e−
M * − OO → M + O2
Overall reaction: 4OH− → O2 + 2H2O + 4e−
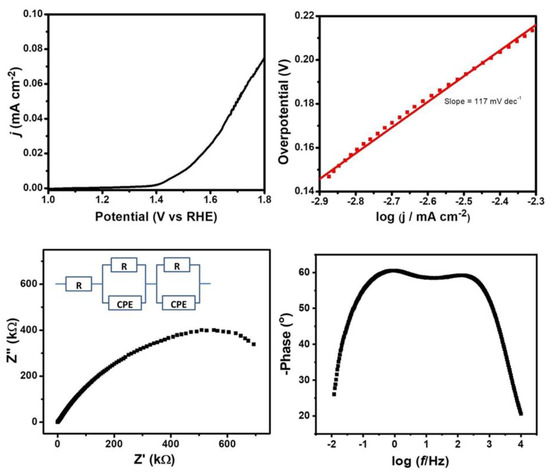
Figure 4.
(Top) LSV plot at 10 mv sec−1 scan rate and corresponding Tafel plot and (bottom) Nyquist (with equivalent circuit diagram) and Bode plots from the EIS study of Cu2WS4 in 0.1 M KOH solution.
LSV experiments performed at different scan rates in 0.1 M KOH indicated that the current density varies with scan rate; however, it is not proportional to the change in scan rate (Figure S5). A lower onset and lower Tafel slope for the OER reaction compared to the HER reaction suggest that the catalyst is prone to catalyze the OER reaction at lower onset potential compared to the HER. On the contrary, if we look at the current density and charge transfer resistance, the current density is lower and Rct is higher in the case of the OER compared to the HER. Hence, it can be concluded that although the onset potential for the the OER is lower compared to the HER, the turnover number for the OER is lower than the HER for a given overpotential above the onset potential.
Overall, the most important aspect of employing Cu2WS4 as a potential bifunctional catalyst for the electrochemical HER reaction in acidic media and the OER reaction in basic media is its very low onset potential. Hence, this material may be a good catalyst for the application of water as potential source of renewable energy in the form of hydrogen.
Furthermore, the stability of the prepared catalyst Cu2WS4 has been determined by a chronoamperometric study at potentiostatic conditions at different potentials. As shown in Figure S6 (Supplementary Materials), Cu2WS4 displays good stability with sustainable activity for 30 min at varied potentials. Hence, the catalyst may be used at various potentials for water splitting without change in activity.
Interestingly, in all the EIS spectra of Cu2WS4 in different media, i.e., 0.1 M H2SO4, 0.5 M H2SO4 and 0.1 M KOH, fitted well with R(QR)(QR) equivalent circuit (Figure 3 and Figure 4) (Where Q = constant phase element), with related fitting parameters shown in Table 1. The resistance of the solution decreases with increase in acid concentration due to the high mobility of H+ ions. Rc, Qc, Rct and Qct are resistance and capacitance due to physical barrier properties of the modified electrode (Layer) and charge transfer resistance and capacitance, respectively [57].

Table 1.
Parameters obtained by fitting EIS data with R(QR)(QR) equivalent circuit.
The OER performance of the electrocatalyst Cu2WS4 has been compared with the OER performance exhibited by previously reported electrocatalysts (Table 2). The results indicate that the reported synthesized electrocatalyst offers better performance than the previously reported electrocatalysts [43]. Additionally, the pristine Cu2WS4 material synthesized previously was found to be almost inactive in OERs. This suggests that the obtained material can be an apt electrocatalytic material for water splitting reactions.

Table 2.
Electrocatalytic OER performance of Cu2WS4 reported herein and previously reported analogous materials.
3. Conclusions
From this investigation it can be concluded that Cu2WS4 nanoparticles can be easily synthesized via a solvothermal approach using a heterobimetallic single source precursor, WCu2S4(PPh3)3. Electrochemical studies suggested that the prepared nanoparticle was appropriate for bifunctional activation of water to produce hydrogen and oxygen under suitable conditions. The electrochemical activity also suggested that the prepared catalyst possesses better activity towards OERs compared to HERs in terms of onset potential and the Tafel slope. However, it showed poor activity for OERs compared to HERs in terms of current density and charge transfer resistance. Such an investigation will create interest in creating similar strategies for the development of bifunctional catalysts with tungsten, copper and sulfur centers, that might be used to create cutting-edge bifunctional electrocatalysts effective concomitantly in HERs and OERs.
4. Experimental
4.1. Materials and Methods
All reagents and compounds did not require any additional purification beyond what was performed before use. Both (NH4)2WS4 [58] and Cu(PPh3)2NO3 [59] were synthesized using methods described in the literature. The 1H, 13C, and 31P NMR data were collected using a Bruker Avance IIIHD spectrometer. TMS was used as an internal standard for 1H and 13C NMR, whereas phosphoric acid was used for 31P NMR, and chemical shifts were reported in parts per million (ppm). Pananalytical X-ray diffraction (PXRD) phase identification of the decomposed material used Ni-filtered Cu Kα1 radiation (λ = 1.5405 Å). Glassy carbon electrodes (3 mm dia), Pt wire counter electrodes and Ag/AgCl reference electrodes were used in the electrochemical measurements. All of the electrochemical measurements were conducted on an Autolab 2.0 electrochemical workstation.
4.2. Syntheses
4.2.1. Synthesis of SSP (WCu2S4(PPh3)3)
A dichloromethane solution of Cu(PPh3)2NO3 (5.200 g, 8 mmol) was added dropwise to a methanolic solution of (NH4)2WS4 (1.392 g, 4 mmol) to yield a yellow precipitate. A yellow solution was obtained after the reaction mixture was stirred for 2 h in a nitrogen environment and then pump-dried. The triphenylphosphine impurities that were released during complex formation were removed by adding about 60 mL of diethyl ether to the yellow residue and stirring it for about an hour. Thereafter, the precipitate was filtered and vacuum-dried.
Characterization data: Yellow powder (yield 4.305 g, 87.80%); M. P. 238 °C, 1H NMR (CDCl3, 300 MHz, δ): δ 7.47, 7.35, 7.27 (m, C6H5). 13C NMR (75 MHz, CDCl3) δ: 134.2, 133.9 (p-C, C6H5), 130.1 (o-C, C6H5), 128.8, 128.7 (P-C, C6H5). 31P NMR (121.54 MHz), δ: 8.67.
4.2.2. Synthesis of Cu2WS4 Nanoparticles
Into a three-necked round-bottom flask attached to a condenser, 500 mg of SSP (WCu2S4(PPh3)3) was added to ~10 g hot octadecyl amine (ODA) solution at 350 °C under nitrogen, and an orange colored suspension was obtained after 60 min. After 120 min, the reaction was quenched and allowed to cool to room temperature by standing overnight under nitrogen. Excess of chloroform was added to the mixture to flocculate the Cu2WS4 nanoparticles. The ODA-capped copper tungsten sulfide nanoparticles were separated by centrifugation in chloroform four times and the material was further purified by washing several times with acetone.
4.3. Electrochemical Studies of Cu2WS4 Nanoparticles
Catalytically modified glassy carbon (GC) electrodes were used to take electrochemical readings in 0.1 M H2SO4, 0.5 M H2SO4 and 0.1 M KOH solutions. Typically, 5 mg of the decomposition product was dispersed in a 1 mL 4:1 v/v ethanol–water mixture, and then blended with 20 μL 5 wt.% Nafion. The mixture was sonicated for 10 min, thereafter, 10 μL of the resulting homogenous ink was drop-cast onto the surface of the GC electrode and left to dry at room temperature. The working electrode was catalytically modified GC, the counter electrode was Pt wire and the reference electrode was Ag/AgCl (saturated with KCl). The OER capabilities of the produced nanocatalysts were measured using a linear sweep voltammetry (LSV) approach with scan rates of 2, 5, 10 and 20 mV s−1. All reported potentials were calibrated against the reversible hydrogen electrode (RHE). The potential was converted from Ag/AgCl to RHE using Equation (10).
ERHE = EAg/AgCl + 0.059pH + EAg/AgCl0
(EAg/AgCl0 = +0.197V)
Using Equation (2), we determined the overpotential (η) for a variety of current densities (x).
ηx = ERHE − 1.23
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma16010299/s1, The Supporting Information contains figures related to the electrochemical study and band structure of Cu2WS4, as well as discussion related to DFT calculations with references. Additionally, it contains the crystallographic information file (cif) of Cu2WS4 obtained after Rietveld refinement. References [60,61] are cited in the Supplementary Materials.
Author Contributions
Methodology, M.A.; Software, M.A.; Validation, A.A.; Formal analysis, N.A.Y.A.; Data curation, W.S.S. and A.M.K.; Writing—original draft, M.A.; Writing—review & editing, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number IFKSURG-2-1498.
Conflicts of Interest
The authors announce no potential conflict of interest.
References
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhong, H.X.; Wang, Z.L.; Meng, F.L.; Zhang, X.B. Integrated three-dimensional carbon paper/carbon tubes/cobalt-sulfide sheets as a bifunctional electrode for overall water splitting. ACS Nano 2016, 10, 2342–2348. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, F.M.; Meng, X.Y.; Li, S.N.; Zeng, J.H.; Chen, Y. Ultrathin Co3O4 nanomeshes for the oxygen evolution reaction. ACS Catal. 2018, 8, 1913–1920. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.; Su, J.; Jiang, P.; Xia, G.; Chen, Q. Enhanced activity for hydrogen evolution reaction over CoFe catalysts by alloying with small amount of Pt. ACS Appl. Mater. Interfaces 2017, 9, 3596–3601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lai, W.; Cao, R. Energy-related small molecule activation reactions: Oxygen reduction and hydrogen and oxygen evolution reactions catalyzed by porphyrin-and corrole-based systems. Chem. Rev. 2017, 117, 3717–3797. [Google Scholar] [CrossRef]
- Aralekallu, S.; Sajjan, V.A.; Palanna, M.; Prabhu, C.P.K.; Hojamberdiev, M.; Sannegowda, L.K. Ni foam-supported azo linkage cobalt phthalocyanine as an efficient electrocatalyst for oxygen evolution reaction. J. Power Sources 2020, 449, 227516. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, R.; Cui, L.; Liu, D.; Hao, S.; Ma, Y.; Du, G.; Asiri, A.M.; Sun, X. High-performance electrolytic oxygen evolution in neutral media catalyzed by a cobalt phosphate nanoarray. Angew. Chem. Int. Ed. 2017, 56, 1064–1068. [Google Scholar] [CrossRef]
- Dong, B.; Zhao, X.; Han, G.Q.; Li, X.; Shang, X.; Liu, Y.R.; Hu, W.-H.; Chai, Y.-M.; Zhao, H.; Liu, C.-G. Two-step synthesis of binary Ni–Fe sulfides supported on nickel foam as highly efficient electrocatalysts for the oxygen evolution reaction. J. Mater. Chem. A 2016, 4, 13499–13508. [Google Scholar] [CrossRef]
- Shang, X.; Yan, K.L.; Rao, Y.; Dong, B.; Chi, J.Q.; Liu, Y.R.; Li, X.; Chai, Y.-M.; Liu, C.-G. In situ cathodic activation of V-incorporated Ni x S y nanowires for enhanced hydrogen evolution. Nanoscale 2017, 9, 12353–12363. [Google Scholar] [CrossRef]
- Shang, X.; Yan, K.L.; Lu, S.S.; Dong, B.; Gao, W.K.; Chi, J.Q.; Liu, Z.-Z.; Chai, Y.-M.; Liu, C.-G. Controlling electrodeposited ultrathin amorphous Fe hydroxides film on V-doped nickel sulfide nanowires as efficient electrocatalyst for water oxidation. J. Power Sources 2017, 363, 44–53. [Google Scholar] [CrossRef]
- Tian, G.L.; Zhao, M.Q.; Yu, D.; Kong, X.Y.; Huang, J.Q.; Zhang, Q.; Wei, F. Nitrogen-doped graphene/carbon nanotube hybrids: In situ formation on bifunctional catalysts and their superior electrocatalytic activity for oxygen evolution/reduction reaction. Small 2014, 10, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Gasteiger, H.A.; Shao-Horn, Y. Hydrogen oxidation and evolution reaction kinetics on platinum: Acid vs. alkaline electrolytes. J. Electrochem. Soc. 2010, 157, B1529–B1536. [Google Scholar] [CrossRef]
- Koper, M.T.M. A basic solution. Nat. Chem. 2013, 5, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, L.; Gao, G.; Chen, H.; Wang, B.; Zhou, J.; Soo, M.T.; Hong, M.; Yan, X.; Qian, G.; et al. A heterostructure coupling of exfoliated Ni–Fe hydroxide nanosheet and defective graphene as a bifunctional electrocatalyst for overall water splitting. Adv. Mater. 2017, 29, 1700017. [Google Scholar] [CrossRef]
- Wang, F.; Shifa, T.A.; Zhan, X.; Huang, Y.; Liu, K.; Cheng, Z.; Jiang, C.; He, J. Recent advances in transition-metal dichalcogenide based nanomaterials for water splitting. Nanoscale 2015, 7, 19764–19788. [Google Scholar] [CrossRef]
- Kong, D.; Cha, J.J.; Wang, H.; Lee, H.R.; Cui, Y. Recent advances in transition-metal dichalcogenide based nanomaterials for water splitting. Energy Environ. Sci. 2013, 6, 3553–3558. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, Q.; Asiri, A.M.; Sun, X.; Luo, Y. Self-supported FeP nanorod arrays: A cost-effective 3D hydrogen evolution cathode with high catalytic activity. ACS Catal. 2014, 4, 4065–4069. [Google Scholar] [CrossRef]
- Tian, L.; Yan, X.; Chen, X.; Liu, L.; Chen, X. One-pot, large-scale, simple synthesis of Co x P nanocatalysts for electrochemical hydrogen evolution. J. Mater. Chem. A 2016, 4, 13011–13016. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, B.; Xu, J.; Jia, G.; Chen, S.; Rawat, R.S.; Fan, H.J. Rapid synthesis of cobalt nitride nanowires: Highly efficient and low-cost catalysts for oxygen evolution. Angew. Chem. Int. Ed. 2016, 55, 8670–8674. [Google Scholar] [CrossRef]
- Wu, H.B.; Xia, B.Y.; Yu, L.; Yu, X.-Y.; Lou, X.W. Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production. Nat. Commun. 2015, 6, 6512–6519. [Google Scholar] [CrossRef] [PubMed]
- Stern, L.A.; Feng, L.; Song, F.; Hu, X. Ni2P as a Janus catalyst for water splitting: The oxygen evolution activity of Ni2P nanoparticles. Energy Environ. Sci. 2015, 8, 2347–2351. [Google Scholar] [CrossRef]
- Read, C.G.; Callejas, J.F.; Holder, C.F.; Schaak, R.E. General strategy for the synthesis of transition metal phosphide films for electrocatalytic hydrogen and oxygen evolution. ACS Appl. Mater. Interfaces 2016, 8, 12798–12803. [Google Scholar] [CrossRef]
- Liu, M.; Li, J. Cobalt phosphide hollow polyhedron as efficient bifunctional electrocatalysts for the evolution reaction of hydrogen and oxygen. ACS Appl. Mater. Interfaces 2016, 8, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wu, X.-J.; Cao, X.; Huang, X.; Tan, C.; Tian, J.; Liu, H.; Wang, J.; Zhang, H. Ni3S2 nanorods/Ni foam composite electrode with low overpotential for electrocatalytic oxygen evolution. Energy Environ. Sci. 2013, 6, 2921–2924. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, A.; Li, L.; Ai, L. Nickel–cobalt layered double hydroxide nanosheets as high-performance electrocatalyst for oxygen evolution reaction. J. Power Sources 2015, 278, 445–451. [Google Scholar] [CrossRef]
- Yu, L.; Yang, J.F.; Guan, B.Y.; Lu, Y.; Lou, X.W.D. Hierarchical Hollow Nanoprisms Based on Ultrathin Ni-Fe Layered Double Hydroxide Nanosheets with Enhanced Electrocatalytic Activity towards Oxygen Evolution. Angew. Chem. 2018, 130, 178–182. [Google Scholar] [CrossRef]
- Qiu, Z.; Ma, Y.; Edstr€om, K.; Niklasson, G.A.; Edvinsson, T. Controlled crystal growth orientation and surface charge effects in self-assembled nickel oxide nanoflakes and their activity for the oxygen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 28397–28407. [Google Scholar] [CrossRef]
- Stelmachowski, P.; Monteverde Videla, A.H.A.; Ciura, K.; Specchia, S. Oxygen evolution catalysis in alkaline conditions over hard templated nickel-cobalt based spinel oxides. Int. J. Hydrogen Energy 2017, 42, 27910–27918. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, J.; Cui, W.; Jiang, P.; Cheng, N.; Asiri, A.M.; Sun, X. Carbon Nanotubes Decorated with CoP Nanocrystals: A Highly Active Non-Noble-Metal Nanohybrid Electrocatalyst for Hydrogen Evolution. Angew. Chem. Int. Ed. 2014, 126, 6828–6832. [Google Scholar] [CrossRef]
- Fei, H.; Dong, J.; Arellano-Jimenez, M.J.; Ye, G.; Dong Kim, N.; Samuel, E.L.; Peng, Z.; Zhu, Z.; Qin, F.; Bao, J.; et al. Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat. Commun. 2015, 6, 8668–8675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, J.W.; Zhou, L.; Li, Z.H.; Shao, M.F.; Wei, M. Layer-by-layer assembly of exfoliated layered double hydroxide nanosheets for enhanced electrochemical oxidation of water. J. Mater. Chem. A 2016, 4, 11516–11523. [Google Scholar] [CrossRef]
- Siracusano, S.; Hodnik, N.; Jovanovic, P.; Ruiz-Zepeda, F.; Sala, M.; Baglio, V.; Arico, A.S. New insights into the stability of a high performance nanostructured catalyst for sustainable water electrolysis. Nano Energy 2017, 40, 618–632. [Google Scholar] [CrossRef]
- Liu, W.; Bao, J.; Guan, M.; Zhao, Y.; Lian, J.; Qiu, J.; Xu, L.; Huang, Y.; Qian, J.; Li, H. Nickel–cobalt-layered double hydroxide nanosheet arrays on Ni foam as a bifunctional electrocatalyst for overall water splitting. Dalton Trans. 2017, 46, 8372–8376. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xia, B.Y.; Zhao, B.; Wang, X. A review on noble-metal-free bifunctional heterogeneous catalysts for overall electrochemical water splitting. J. Mater. Chem. A 2016, 4, 17587–17603. [Google Scholar] [CrossRef]
- Ramasamy, K.; Sims, H.; Butler, W.H.; Gupta, A. Mono-, few-, and multiple layers of copper antimony sulfide (CuSbS2): A ternary layered sulfide. J. Am. Chem. Soc. 2014, 136, 1587–1598. [Google Scholar] [CrossRef]
- Pruss, E.A.; Snyder, B.S.; Stacy, A.M. A new layered ternary sulfide: Formation of Cu2WS4 by reaction of WS and Cu+ ions. Angew. Chem. Int. Ed. 1993, 32, 256–257. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, Y.; Peng, R.; Huang, B.; Dai, Y. Single-layer Cu2WS4 with promising electrocatalytic activity toward hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2019, 11, 45818–45824. [Google Scholar] [CrossRef]
- Jing, D.; Liu, M.; Chen, Q.; Guo, L. Efficient photocatalytic hydrogen production under visible light over a novel W-based ternary chalcogenide photocatalyst prepared by a hydrothermal process. Int. J. Hydrogen Energy 2010, 35, 8521–8527. [Google Scholar] [CrossRef]
- Ozel, F.; Aslan, E.; Sarilmaz, A.; Patir, I.H. Hydrogen evolution catalyzed by Cu2WS4 at liquid–liquid interfaces. ACS Appl. Mater. Interfaces 2016, 8, 25881–25887. [Google Scholar] [CrossRef]
- Aslan, E.; Gonce, M.K.; Yigit, M.Z.; Sarilmaz, A.; Stathatos, E.; Ozel, F.; Can, M.; Patir, I.H. Photocatalytic H2 evolution with a Cu2WS4 catalyst on a metal free D-π-A organic dye-sensitized TiO2. Appl. Catal. B Environ. 2017, 210, 320–327. [Google Scholar] [CrossRef]
- Patir, I.H.; Aslan, E.G.; Yanalak, M.; Karaman, A.; Sarilmaz, M.; Can, M.; Can, M.; Ozel, F. Donor-π-acceptor dye-sensitized photoelectrochemical and photocatalytic hydrogen evolution by using Cu2WS4 co-catalyst. Int J. Hydrogen Energy 2019, 44, 1441–1450. [Google Scholar] [CrossRef]
- Novak, T.G.; Prakash, O.; Tiwari, A.P.; Jeon, S. Solution-phase phosphorus substitution for enhanced oxygen evolution reaction in Cu2WS4. RSC Adv. 2019, 9, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Kim, S.J.; Kim, N.H.; Lee, J.H. All-solid-state asymmetric supercapacitor with MWCNT-based hollow NiCo2O4 positive electrode and porous Cu2WS4 negative electrode. Chem. Eng. J. 2021, 415, 128188. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Sahoo, S.; Mariappan, V.K.; Kim, S.J. Copper tungsten sulfide anchored on Ni-foam as a high-performance binder free negative electrode for asymmetric supercapacitor. Chem. Eng. J. 2019, 359, 409–418. [Google Scholar] [CrossRef]
- Gulen, M.; Sarilmaz, A.; Patir, I.H.; Ozel, F.; Sonmezoglu, S. Ternary copper-tungsten-disulfide nanocube inks as catalyst for highly efficient dye-sensitized solar cells. Electrochim. Acta 2018, 269, 119–127. [Google Scholar] [CrossRef]
- Jia, Q.; Zhang, Y.C.; Li, J.; Chen, Y.; Xu, B. Hydrothermal synthesis of Cu2WS4 as a visible-light-activated photocatalyst in the reduction of aqueous Cr (VI). Mater. Lett. 2014, 117, 24–27. [Google Scholar] [CrossRef]
- Kannan, S.; Vinitha, P.; Mohanraj, K.; Sivakumar, G. Antibacterial studies of novel Cu2WS4 ternary chalcogenide synthesized by hydrothermal process. J. Solid State Chem. 2018, 258, 376–382. [Google Scholar] [CrossRef]
- Shan, J.; Li, X.; Yang, K.; Xiu, W.; Wen, Q.; Zhang, Y.; Yuwen, L.; Weng, L.; Teng, Z.; Wang, L. Efficient Bacteria Killing by Cu2WS4 Nanocrystals with Enzyme-like Properties and Bacteria-Binding Ability. ACS Nano 2019, 13, 13797–13808. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.; Kociok-Köhn, G.; Bhimireddi, R.; Singh, A.; Singh, A.K.; Kumar, A.; Muddassir, M. Ternary copper molybdenum sulfide (Cu2MoS4) nanoparticles anchored on PANI/rGO as electrocatalysts for oxygen evolution reaction (OER). Appl. Organomet. Chem. 2022, 36, e6683. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.; Kociok-Köhn, G.; Molloy, K.C.; Singh, A.K.; Kumar, A.; Muddassir, M. Ni (ii) dithiolate anion composites with two-dimensional materials for electrochemical oxygen evolution reactions (OERs). New J. Chem. 2021, 45, 16264–16270. [Google Scholar] [CrossRef]
- Müller, A.; Bögge, H.; Schimanski, U. The preparation of different types of polynuclear transition metal sulfur compounds by thiometallates, including cubane-like ones. Crystal structures of {Cu3WS3Cl}(PPh3)3S, {Cu3WS3Cl}(PPh3)3O, {Cu3MoS3Cl}(PPh3)3S, {Cu3MoS3Cl}(PPh33O, (PPh3)3Cu2WS4·0.8CH2Cl2 and (PPh3)3Ag2MoS4·0.8CH2Cl2. Inorg. Chim. Acta 1983, 69, 5–16. [Google Scholar]
- Doyle, R.L.; Lyons, M.E.G. Photoelectrochem Solar Fuel Production; Springer: Cham, The Netherland, 2016; pp. 41–104. [Google Scholar]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, T.; Li, L.; Song, S.; Ding, R. Nickel-based electrodes as catalysts for hydrogen evolution reaction in alkaline media. Ionics 2018, 24, 1121. [Google Scholar] [CrossRef]
- Alobaid, A.; Wang, C.; Adomaitis, R.A. Mechanism and kinetics of HER and OER on NiFe LDH films in an alkaline electrolyte. J. Electrochem. Soc. 2018, 165, J3395. [Google Scholar] [CrossRef]
- Ding, Y.; Liang, J.; Liu, G.; Ni, W.; Shen, L. Preparation and anticorrosive property of soluble aniline tetramer. Coatings 2019, 9, 399. [Google Scholar] [CrossRef]
- Mcdonald, J.W.; Friesen, G.D.; Rosenhein, L.D.; Newton, W.E. Syntheses and characterization of ammonium and tetraalkylammonium thiomolybdates and thiotungstates. Inorg. Chim. Acta 1983, 72, 205–210. [Google Scholar] [CrossRef]
- Gysling, H.J.; Kubas, G.J. Coordination Complexes of Copper(I) Nitrate. In Inorganic Syntheses; Wiley: Hoboken, NJ, USA, 2007; Volume 19, pp. 93–94. [Google Scholar]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Buongiorno Nardelli, M.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter. 2017, 29, 465901. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. Quantum Espresso: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter. 2009, 21, 395502. Available online: http://www.quantum-espresso.org (accessed on 19 December 2022). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).