Assessment of Corrosion Protection Performance of FeOOH/Fe3O4/C Composite Coatings Formed In Situ on the Surface of Fe Metal in Air-Saturated 3.5 wt.% NaCl Solution
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Preparation of Coated Samples
2.2. Characterization
2.3. Electrochemical Corrosion Measurements
3. Results and Discussion
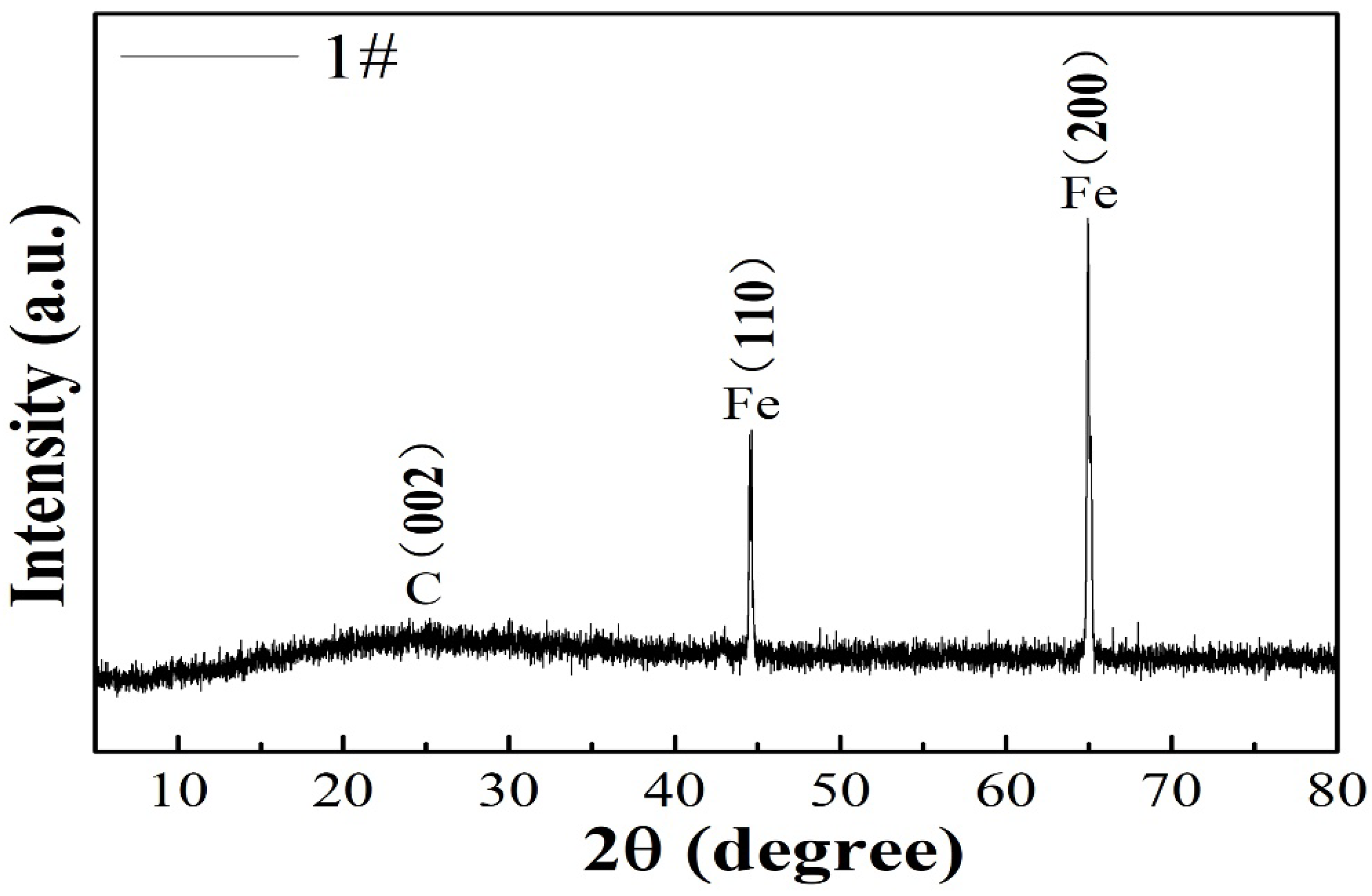
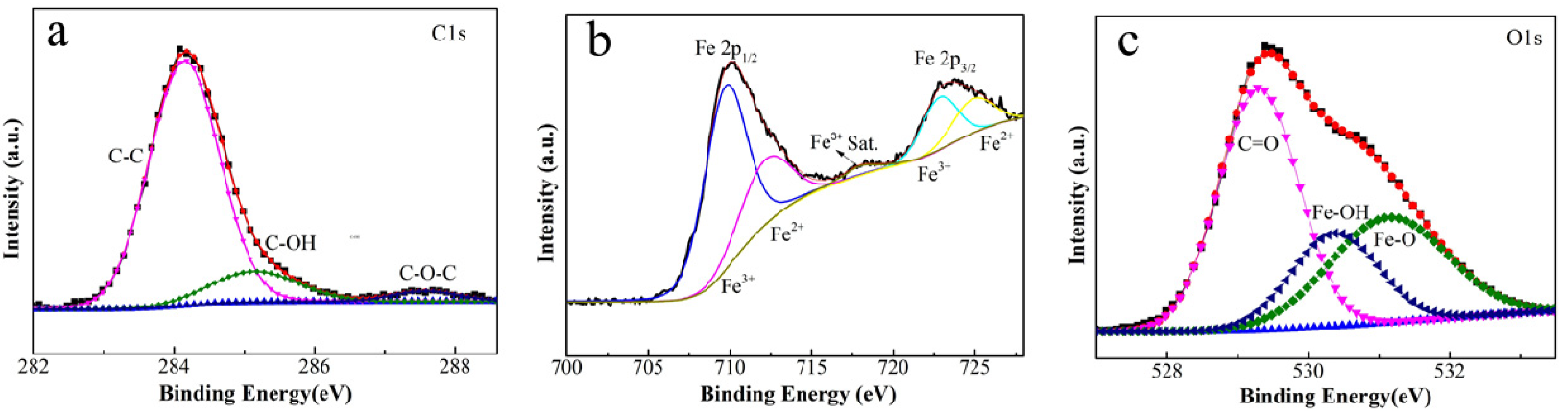
3.1. Characterizations of FeOOH/Fe3O4/C@Fe Composites
3.2. Corrosion Inhibition Capacity
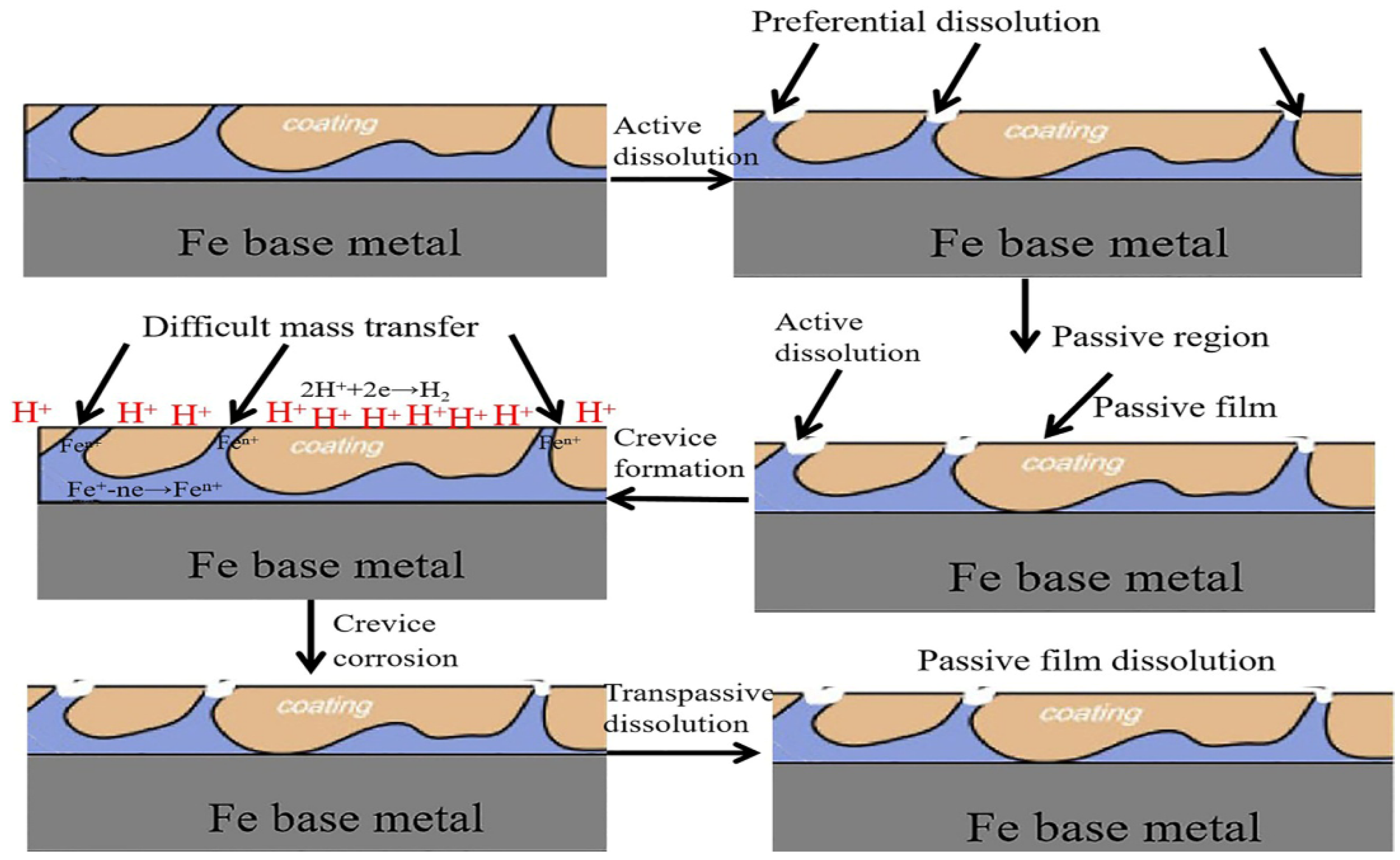
3.3. Mechanism of Improvement of Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, F.Y.; Liu, W.Q.; Wang, S.; Liu, C.H.; Shi, H.Y.; Liang, L.Y.; Pi, K. Surface functionalization of Ti3C2Tx and its application in aqueous polymer nanocomposites for reinforcing corrosion protection. Compos. Part B-Eng. 2021, 217, 108900. [Google Scholar] [CrossRef]
- Javidparvar, A.A.; Naderi, R.; Ramezanzadeh, B. Epoxy-polyamide nanocomposite coating with graphene oxide as cerium nanocontainer generating effective dual active/barrier corrosion protection. Compos. Part B-Eng. 2019, 172, 363–375. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.Y.; Wallinder, I.O.; Leygraf, C. The protective role of hydrozincite during initial corrosion of a Cu40Zn alloy in chloride-containing laboratory atmosphere. Corros. Sci. 2016, 103, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Qu, F.L.; Li, W.G.; Dong, W.K.; Tam, V.W.Y.; Yu, T. Durability deterioration of concrete under marine environment from material to structure: A critical review. J. Build. Eng. 2021, 35, 102074. [Google Scholar] [CrossRef]
- Wang, H.G.; Ren, H.S.; Jing, C.F.; Li, J.Z.; Zhou, Q.; Meng, F.B. Two birds with one stone: Graphene oxide@sulfonated polyaniline nanocomposites towards high-performance electromagnetic wave absorption and corrosion protection. Compos. Sci. Technol. 2021, 204, 108630. [Google Scholar] [CrossRef]
- Li, B.F.; Trueman, B.F.; Rahman, M.S.; Gagnon, G.A. Controlling lead release due to uniform and galvanic corrosion- An evaluation of silicate-base inhibitors. J. Build. Eng. 2021, 407, 124707. [Google Scholar]
- Amini, M.; Naderi, R.; Mahdavian, M.; Badiei, A. Release of lanthanum cations loaded into piperazin-modified SBA-15 to inhibit the mild steel corrosion. Microporous Mesoporous Mater. 2021, 315, 110908. [Google Scholar] [CrossRef]
- Haddadi, S.A.; Ghaderi, S.; Sadeghi, M.; Gorji, B.; Ahmadijokani, F.; Ramazani, S.A.A.; Mahdavian, M.; Arjmand, M. Enhanced active/barrier corrosion protective properties of epoxy coatings containing eco-friendly green inorganic/organic hybrid pigments based on zinc cations/Ferula Asafoetida leaves. J. Mol. Liq. 2021, 323, 114584. [Google Scholar] [CrossRef]
- Chiter, F.; Costa, D.; Maurice, V.; Marcus, P. DFT investigation of 2-mercaptobenzothiazole adsorption on model oxidized copper surfaces and relationship with corrosion inhibition. Appl. Surf. Sci. 2021, 537, 147802. [Google Scholar] [CrossRef]
- Barmatov, E.; Hughes, T.L. Effect of corrosion products and turbulent flow on inhibition efficiency of propargyl alcohol on AISI 1018 mild carbon steel in 4 M hydrochloric acid. Corros. Sci. 2017, 123, 170–181. [Google Scholar] [CrossRef]
- Hrimla, M.; Bahsis, L.; Boutouil, A.; Laamari, M.R.; Julve, M.; Stiriba, S.E. Corrosion inhibition performance of a structurally well-defined 1,2,3-trizazole derivative on mild steel-hydrochloric acid interface. J. Mol. Struct. 2021, 1231, 129895. [Google Scholar] [CrossRef]
- Asl, R.M.; Yousefpour, M.; Shanaghi, A. The investigation of corrosion behavior of ZrO2-Al2O3-inhitor/AA2024 nanocomposite thin film using sol-gel and AHP-TOPSIS method. Mater. Chem. Phys. 2021, 262, 124220. [Google Scholar] [CrossRef]
- Ma, L.; Qiang, Y.J.; Zhao, W.J. Designing novel organic inhibitor loaded MgAl-LDHs nanocontainer for enhanced corrosion resistance. Chem. Eng. J. 2021, 408, 127367. [Google Scholar] [CrossRef]
- Ren, X.L.; Xu, S.Y.; Gu, X.X.; Tan, B.C.; Hao, J.Y.; Feng, L.; Ren, W.H.; Gao, F.; Zhang, S.T.; Xiao, Y.R. Hyperbranched molecules having multiple functional groups as effective corrosion inhibitors for Al alloys in aqueous NaCl. J. Colloid Interface Sci. 2021, 585, 614–626. [Google Scholar] [CrossRef]
- Su, H.S.; Wu, Y.X.; Zhang, Y.M.; Jiang, Y.M.; Ding, Y.; Wang, L.; Zhang, J.L. Enhancing the long-term anti-corrosion property of Mg alloy by quaternary phosphonium salt: Integrated experimental and theoretical approaches. Corros. Sci. 2021, 178, 109010. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, S.; Jin, Z.Q.; Zhu, Q.J.; Zheng, M.; Jiang, Q.T.; Song, H.M.; Duan, J.Z. Fabrication of composite coatings with core-shell nanofibers and their mechanical properties, anticorrosive performance, and mechanism in seawater. Prog. Org. Coat. 2020, 149, 105893. [Google Scholar] [CrossRef]
- Kaghazchi, L.; Naderi, R.; Ramezanzadeh, B. Construction of a high-performance anti-corrosion film based on the green tannic acid molecules and zinc cations on steel: Electrochemical/Surface investigations. Constr. Build. Mater. 2020, 262, 120861. [Google Scholar] [CrossRef]
- Huang, H.J.; Fu, Y.; Mu, X.J.; Luo, Z.P.; Zhang, S.T.; Wang, Z.Y.; Li, H.R.; Gao, F. Molecular self-assembly of novel amphiphilic topological hyperbranched polymers for super protection of copper in extremely aggressive acid solution. Appl. Surf. Sci. 2020, 529, 147076. [Google Scholar] [CrossRef]
- Amiri, M.; Ghaffari, M.; Mirzaee, A.; Bahlakeh, G.; Saeb, M.R. Development and anti-corrosion performance of hyperbranched polyglycerol-decorated Fe3O4@SiO2 on mild steel in 1.0 M HCl. J. Mol. Liq. 2020, 314, 113597. [Google Scholar] [CrossRef]
- Qiang, Y.J.; Guo, L.; Li, H.; Lan, X.J. Fabrication of environmentally friendly Losartan potassium film for corrosion inhibition of mild steel in HCl medium. Chem. Eng. J. 2021, 406, 126863. [Google Scholar] [CrossRef]
- Mehta, H.; Kaur, G.; Chaudhary, G.R.; Prabhakar, N. Assessment of bio-corrosion inhibition ability of Hafnium based cationic metallosurfactant on iron surface. Corros. Sci. 2021, 179, 109101. [Google Scholar] [CrossRef]
- Wu, H.S.; Zhang, L.Y.; Zhang, Y.C.; Long, S.; Jie, X.H. Corrosion behavior of Mg-Al LDH film in-situ assembled with graphene on Mg alloy pre-sprayed Al layer. J. Alloys Compd. 2020, 834, 155107. [Google Scholar] [CrossRef]
- Hadzima, B.; Pastorek, F.; Borko, K.; Fintova, S.; Kajanek, D.; Bagherifard, S.; Gholami-Kermanshahi, M.; Trsko, L.; Pastorkova, J.; Brezina, J. Effect of phosphating time on protection properties of hurealite caoting: Differences between ground and shot peened HSLA steel surface. Surf. Coat. Technol. 2019, 375, 608–620. [Google Scholar] [CrossRef]
- Liu, A.M.; Ren, X.F.; Yang, Q.Y.; Sokolowski, J.; Guo, J.; Li, Y.Q.; Gao, L.G.; An, M.Z.; Wu, G. Theoretical and Experimental Studies of the Prevention Mechanism of Organic Inhibitors on Silver Anti-Tarnish. J. Electrochem. Soc. 2018, 165, H725–H732. [Google Scholar] [CrossRef]
- Unnisa, C.B.N.; Devi, G.N.; Hemapriya, V.; Chitra, S.; Chung, I.M.; Kim, S.H.; Prabakaran, M. Linear polyesters as effective corrosion inhibitors for steel rebars in chloride induced alkaline medium-An electrochemical approach. Constr. Build. Mater. 2018, 165, 866–876. [Google Scholar] [CrossRef]
- Asaldoust, S.; Hosseini, M.S.; Ramezanzadeh, B.; Bahlakeh, G. Construction of a unique anti-corrosion nanocomposite based on graphene oxide@Zn3PO4/epoxy, experimental characterization and detailed-theoretical quantum mechanics (QM) investigations. Constr. Build. Mater. 2020, 256, 119439. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Ramezanzadeh, B.; Mahdavian, M.; Bahlakeh, G. Development of metal-organic framework (MOF) decorated graphene oxide nanoplaforms for anti-corrosion epoxy coatings. Carbon 2020, 161, 231–251. [Google Scholar] [CrossRef]
- Vega, J.M.; Chimenti, S.; Garcia, E.; Grande, H.J.; Paulis, M.; Leiza, J.R. Impact of the in-situ phosphatization on the corrosion resistance of steel coated with fluorinated waterborne binders assessed by SKP and EIS. Prog. Org. Coat. 2020, 148, 105706. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.H.; Hu, W.B. Facile synthesis of novel hierarchical core@shell structural magnetic nanovehicle Fe3O4@ZnAlCe-MoO4-LDHs for carbon steel protection. J. Magn. Magn. Mater. 2021, 523, 167576. [Google Scholar] [CrossRef]
- Bouali, A.C.; Andre, N.M.; Campos, M.R.S.; Serdechnova, M.; dos Santos, J.F.; Amancio, S.T.; Zheludkevich, M.L. Influence of LDH conversion caotings on the adhesion and corrosion protection of friction spot-joined AA2024-T3/CF-PPS. J. Mater. Sci. Technol. 2021, 67, 197–210. [Google Scholar] [CrossRef]
- El Faydy, M.; About, H.; Benhiba, F.; Lakhrissi, B.; Guenbour, A.; Bentiss, F.; Warad, I.; Ebenso, E.E.; Zarrouk, A. The inhibitory effect of two 5-alkylthio-8-hydroxyquinoline salts on steel C22E in a molar electrolyte of hydrochloric acid: Experimental and theoretical studies. Surf. Interfaces 2020, 20, 100575. [Google Scholar] [CrossRef]
- Li, X.H.; Deng, S.D.; Lin, T.; Xie, X.G.; Xu, X. Inhibition action of triazolyl blue tetrazolium bromide on cold rolled steel corrosion in three chlorinated acetic acids. J. Mol. Liq. 2019, 274, 77–89. [Google Scholar] [CrossRef]
- Moschona, A.; Plesu, N.; Mezei, G.; Thoma, A.G.; Demadis, K.D. Corrosion protection of carbon steel by tetraphosphonates of systematically different molecular size. Corros. Sci. 2018, 145, 135–150. [Google Scholar] [CrossRef] [Green Version]
- Berrissoul, A.; Loukili, E.; Mechbal, N.; Benhiba, F.; Guenbour, A.; Dikici, B.; Zarrouk, A.; Dafali, A. Anticorrosion effect of a green sustainable inhibitor on mild steel in hydrochloric acid. J. Colloid. Interface Sci. 2020, 580, 740–752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.B.; Hua, Y.X. Effect of alkylimidazolium Ionic Liquids on the Corrosion Inhibition of Copper in Sulfuric Acid Solution. Acta Phys.-Chim. Sin. 2011, 27, 655–663. [Google Scholar]
- Ozcan, M.; Solmaz, R.; Kardas, G.; Dehri, I. Adsorption properties of barbiturates as green corrosion inhibitors on mild steel in phosphoric acid. Colloid Surface A 2008, 325, 57–63. [Google Scholar] [CrossRef]
- El Basiony, N.M.; Badr, E.E.; Baker, S.A.; El-Tabei, A.S. Experimental and theoretical (DFT&MC) studies for the adsorption of the synthesized Gemini cationic surfactant based on hydrazide moiety as X-65 steel acid corrosion inhibitor. Appl. Surf. Sci. 2021, 539, 148246. [Google Scholar]
- Kus, E.; Nealson, K.; Mansfeld, F. The effect of different exposure conditions on the biofilm/copper interface. Corros. Sci. 2007, 49, 3421–3427. [Google Scholar] [CrossRef]
- Arenas, M.A.; de Damborenea, J. Interference by cerium cations during the multi-step zinc dissolution process in a chloride-containing electrolyte. Corros. Sci. 2006, 48, 3196–3207. [Google Scholar] [CrossRef]
- Solomon, M.M.; Umoren, S.A.; Quraishi, M.A.; Salman, M. Myristic acid based imidazoline derivative as effective corrosion inhibitor for steel in 15% HCl medium. J. Colloid. Interface Sci. 2019, 551, 47–60. [Google Scholar] [CrossRef]
- Visa, A.; Plesu, N.; Maranescu, B.; Ilia, G.; Borota, A.; Crisan, L. Combined Experimental and Theoretical Insights into the Corrosion Inhibition Activity on Carbon Steel Iron of Phosphonic Acids. Molecules 2021, 26, 35. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Tang, R.H.; Peng, X.W.; Gao, A.G.; Han, Y.J. Corrosion behavior and mechanism of carbon steel influenced by interior deposit microflora of an in-service pipeline. Bioelectrochemistry 2020, 132, 107406. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.S.; Yang, W.Z.; Zhang, K.G.; Chen, Y.; Li, M.; Zuo, Y.W.; Yin, X.S.; Liu, Y. Effect of scale inhibitors on the structure and morphology of CaCO3 crystl electrochemically deposited on TA1 alloy. J. Colloid Interface Sci. 2020, 562, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Chahboun, N.; Veys-Renaux, D.; Rocca, E. Sealing mechanism of nanoporous alumina in fluorozirconate salt containing solutions. Appl. Surf. Sci. 2021, 541, 148459. [Google Scholar] [CrossRef]
- Ghaderi, S.; Ramazani, S.A.A.; Haddadi, S.A. Synthesis and characterization of highly hydrophilic self-associating terpolymers: Rheological, thermal, and corrosion protection studies. Chem. Eng. J. 2021, 405, 126939. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Mofidabadi, A.H.J. Construction of a high-potency anti-corrosive metal-organic film based on europium (III)-benzimidazole: Theoretical and electrochemical investigations. Constr. Build. Mater. 2021, 269, 121271. [Google Scholar] [CrossRef]
- Farhadian, A.; Rahimi, A.; Safaei, N.; Shaabani, A.; Sadeh, E.; Abdouss, M.; Alavi, A. Exploration of Sunflower Oil As a Renewable Biomass Source to Develop Scalable and Highly Effective Corrosion Inhibitors in a 15% HCl Medium at High Temperatures. ACS Appl. Mater. Interfaces 2021, 13, 3119–3138. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, Y.Y.; Hang, J.Z.; Zhang, Z.M.; Ma, Y.W.; Huang, H.L.; Yu, Q.J.; Wei, J.X. The effect of organic core-shell corrosion inhibitors on corrosion performance of the reinforcement in simulated concrete pore solution. Constr. Build. Mater. 2021, 267, 121011. [Google Scholar] [CrossRef]
- Onyeachu, I.B.; Solomon, M.M. Benzotrizole derivative as an effective corrosion inhibitor for low carbon steel in 1 M HCl and 1 M HCl+3.5wt% NaCl solutions. J. Mol. Liq. 2020, 313, 113536. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, R.; Sahoo, B. Graphene Oxide Coatings on Amino Acid Modified Fe Surfaces for Corrosion Inhibition. ACS Appl. Mater. Interfaces 2020, 3, 3540–3557. [Google Scholar] [CrossRef]
- Majidi, H.J.; Mirzaee, A.; Jafari, S.M.; Amiri, M.; Shahrousvand, M.; Babaei, A. Fabrication and characterization of graphene oxide-chitosan-zinc oxide ternary nano-hybrids for the corrosion inhibition of mild steel. Int. J. Biol. Macromol. 2020, 148, 1190–1200. [Google Scholar] [CrossRef]

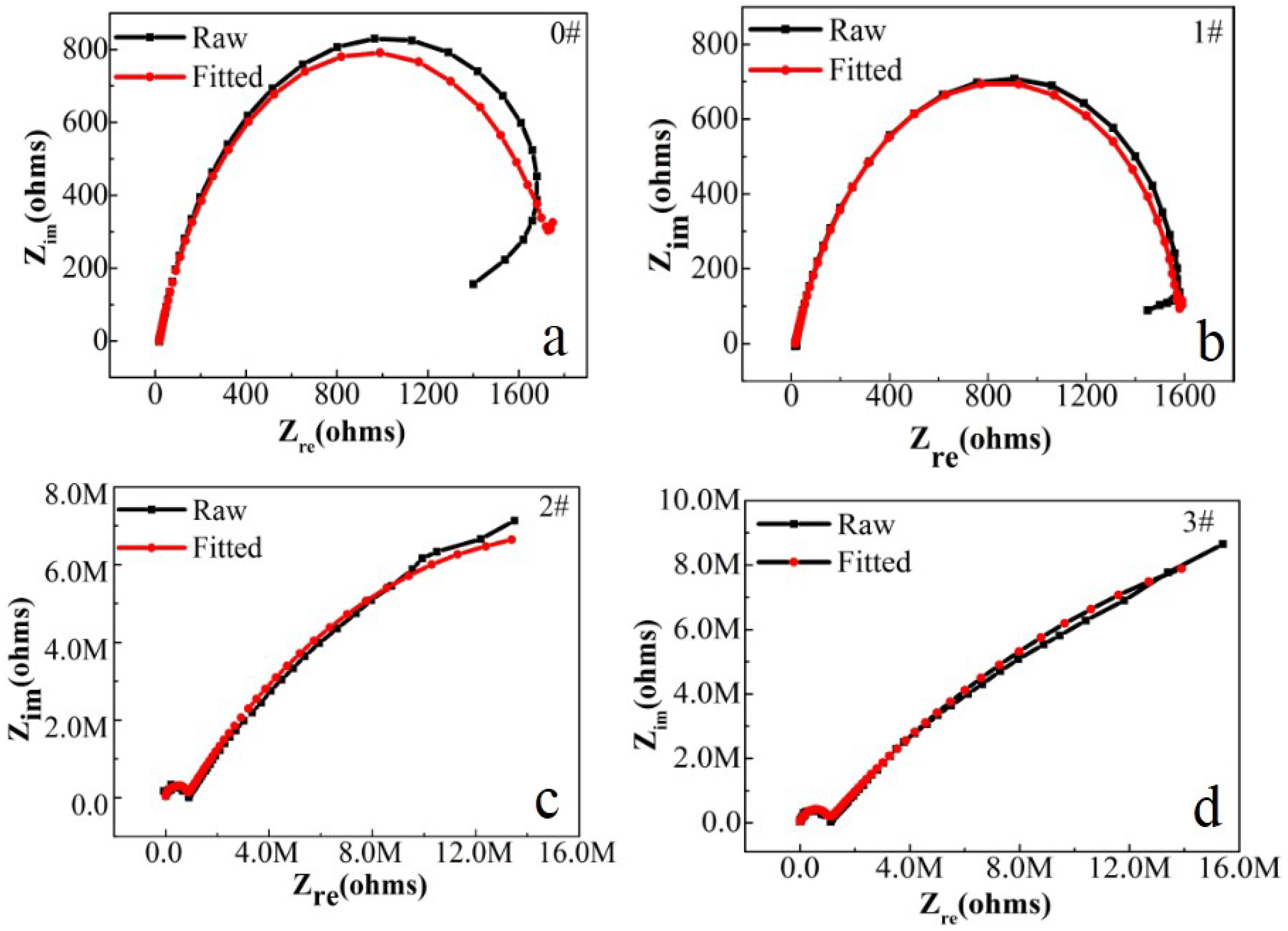
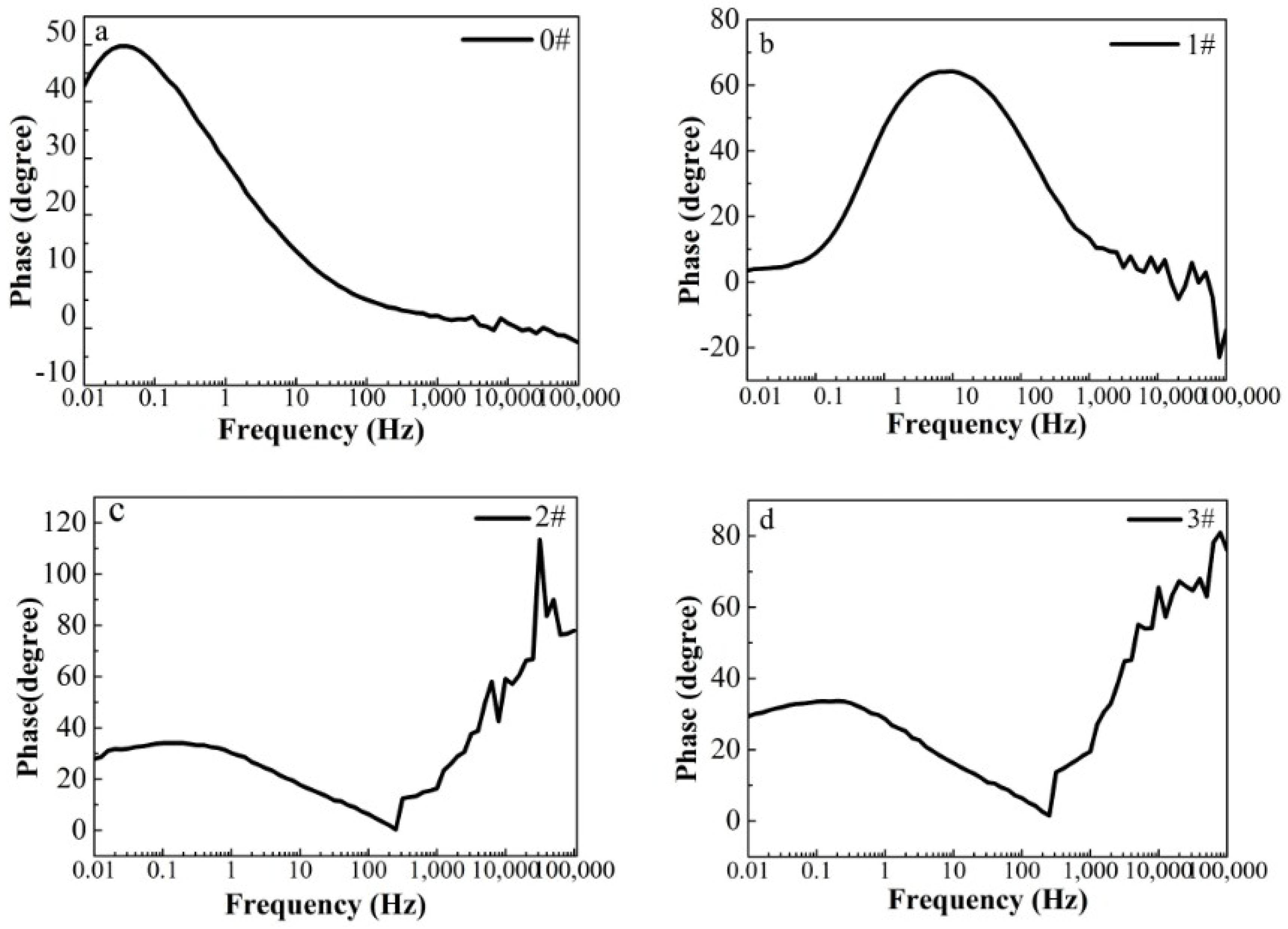

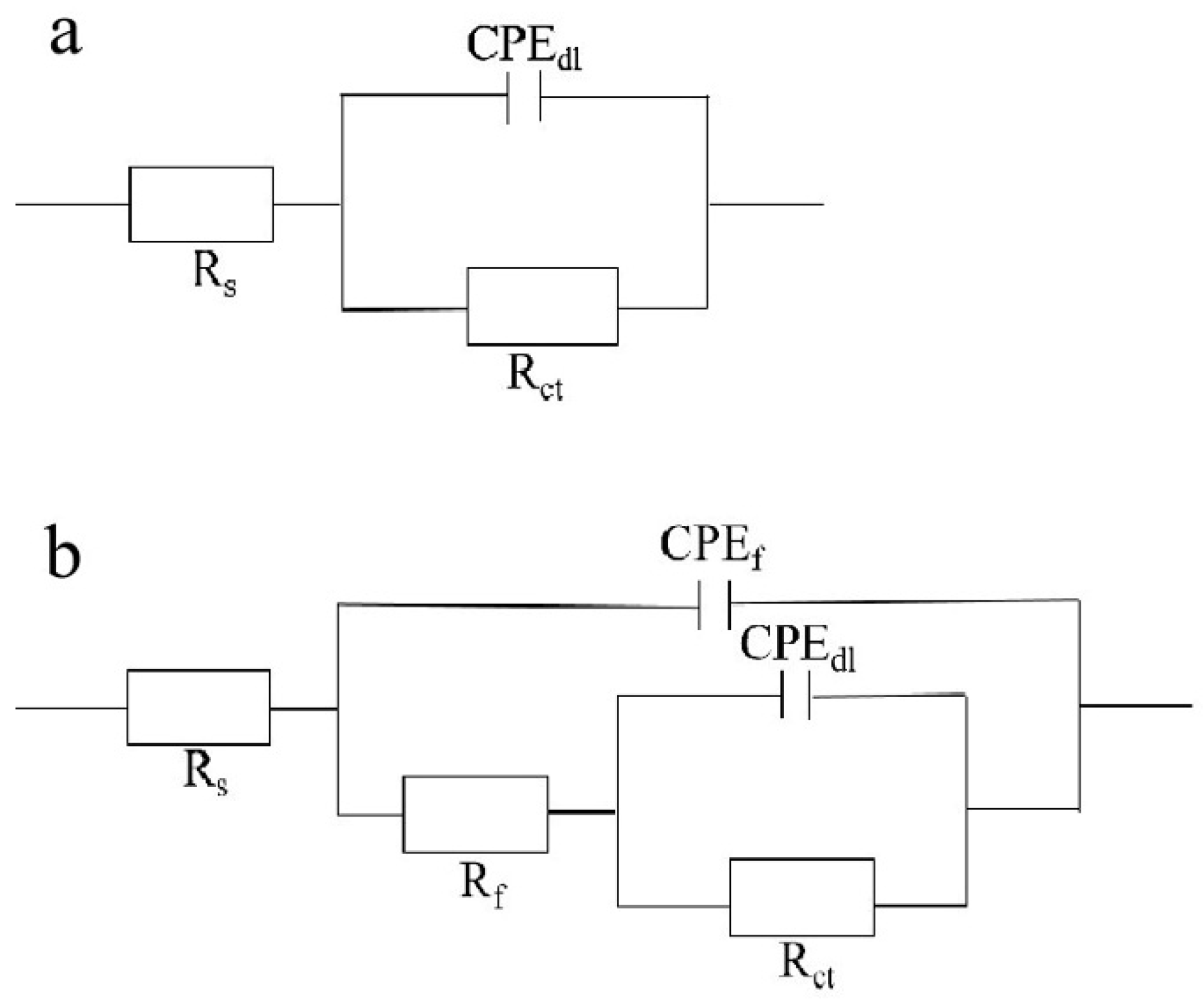
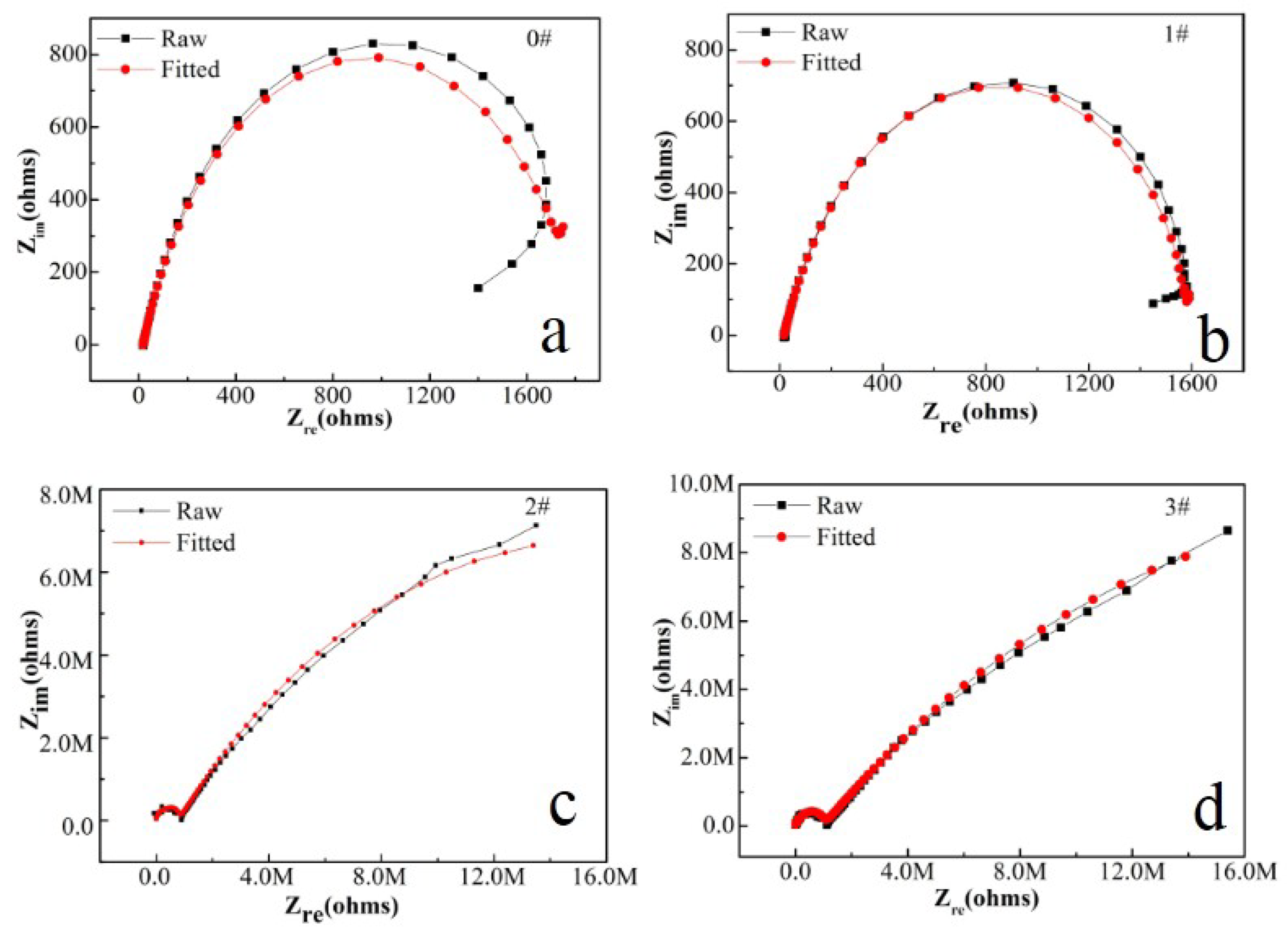
| Sample | Rs (Ω/cm2) | CPE-1 (S secn/cm2) | n-1 | Rcoat (Ω/cm2) | CPE-2 (S secn/cm2) | n-2 | Rct (Ω/cm2) |
|---|---|---|---|---|---|---|---|
| 1# | 19.8 | 1.03 × 10−4 | 0.8873 | 62.99 | 6.68 × 10−5 | 0.8427 | 1557 |
| 2# | 21.7 | 1.09 × 10−9 | 0.7437 | 1.09 × 106 | 1.56 × 10−7 | 0.4479 | 3.37 × 107 |
| 3# | 20.6 | 3.21 × 10−10 | 0.8335 | 1.01 × 106 | 1.95 × 10−7 | 0.5101 | 4.22 × 107 |
| Blank | 19.1 | / | / | / | 6.9 × 10−5 | 0.8538 | 1487 |
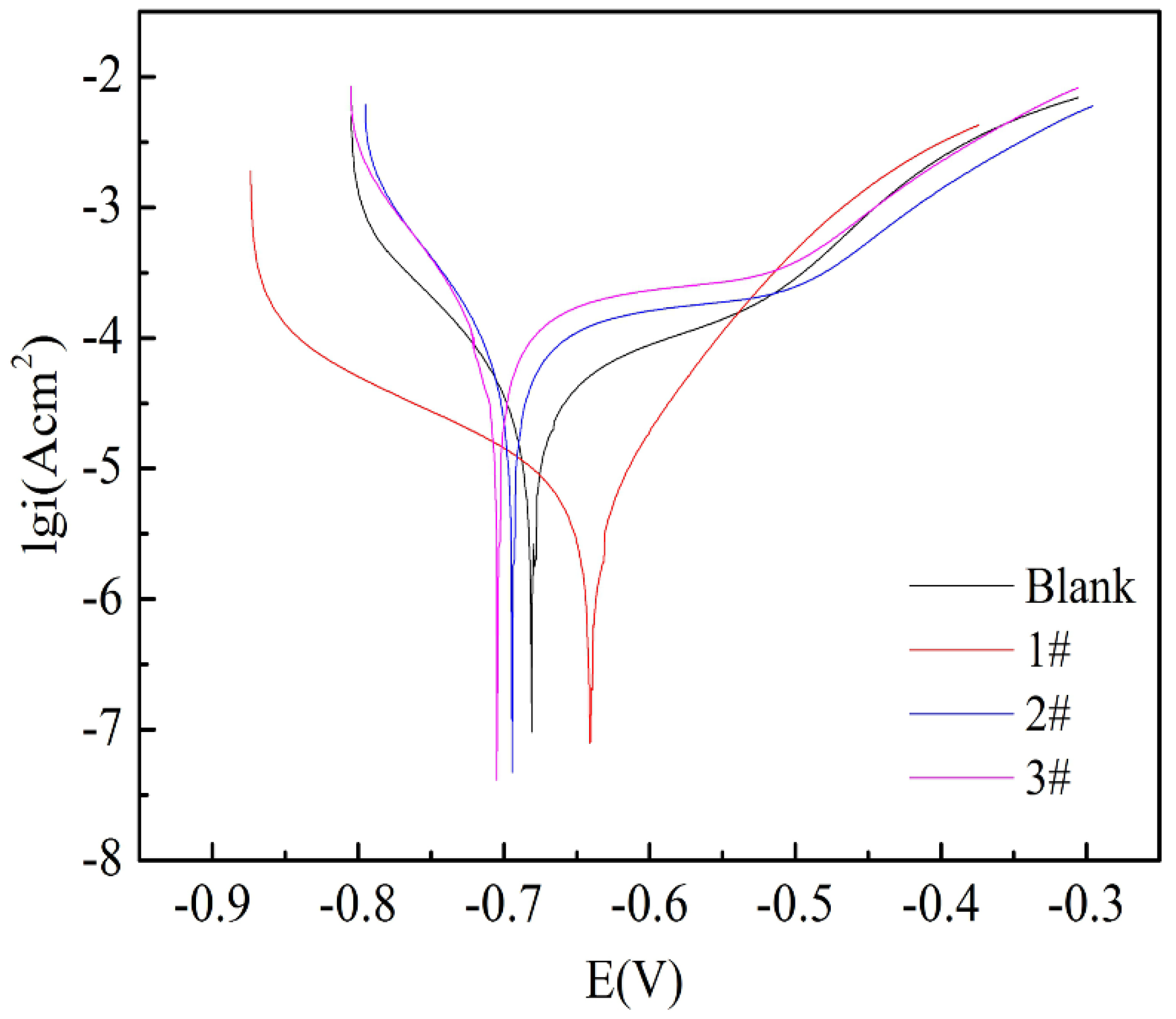
| Sample | Ecorr (V) | icorr (A/cm2) | βc (mV/dec) | βa (mV/dec) | Icorr Decrease Percentage (%) | η (%) |
|---|---|---|---|---|---|---|
| 1# | −0.6174 | 2.93 × 10−6 | −25.992 | 17.566 | 8.53 | 4.5 |
| 2# | −0.645 | 6.29 × 10−10 | 23.383 | 95.95 | 99.98 | 100 |
| 3# | −0.640 | 4.98 × 10−10 | 28.738 | 64.059 | 99.99 | 100 |
| Blank | −0.616 | 3.18 × 10−6 | −24.886 | 16.541 | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Luo, Q.; He, Y. Assessment of Corrosion Protection Performance of FeOOH/Fe3O4/C Composite Coatings Formed In Situ on the Surface of Fe Metal in Air-Saturated 3.5 wt.% NaCl Solution. Materials 2023, 16, 224. https://doi.org/10.3390/ma16010224
Huang L, Luo Q, He Y. Assessment of Corrosion Protection Performance of FeOOH/Fe3O4/C Composite Coatings Formed In Situ on the Surface of Fe Metal in Air-Saturated 3.5 wt.% NaCl Solution. Materials. 2023; 16(1):224. https://doi.org/10.3390/ma16010224
Chicago/Turabian StyleHuang, Lina, Qi Luo, and Yan He. 2023. "Assessment of Corrosion Protection Performance of FeOOH/Fe3O4/C Composite Coatings Formed In Situ on the Surface of Fe Metal in Air-Saturated 3.5 wt.% NaCl Solution" Materials 16, no. 1: 224. https://doi.org/10.3390/ma16010224
APA StyleHuang, L., Luo, Q., & He, Y. (2023). Assessment of Corrosion Protection Performance of FeOOH/Fe3O4/C Composite Coatings Formed In Situ on the Surface of Fe Metal in Air-Saturated 3.5 wt.% NaCl Solution. Materials, 16(1), 224. https://doi.org/10.3390/ma16010224






